The Effects of Pulsed Electromagnetic Field (PEMF) on Muscular Strength, Functional Performance and Depressive Symptoms in Elderly Adults with Sarcopenia: A Short-Term Intervention
Abstract
1. Introduction
2. Methods
2.1. Experimental Approach
2.2. Participants and Study Design
2.3. PEMF Therapy Protocol
2.4. Sample Characterization
2.5. Outcome Variables
2.6. Primary Outcome Measures
2.6.1. Evaluation of Lower-Limb Muscle Strength
2.6.2. Timed Up and Go Test (TUG)
2.7. Secondary Outcome Measures
2.7.1. Sarcopenia Assessment and Calf Perimetry
2.7.2. Yesavage Depression Scale
2.8. Size of Study
2.9. Bias
2.10. Statistics
3. Results
3.1. General Information
Adverse Effects and Treatment Adherence
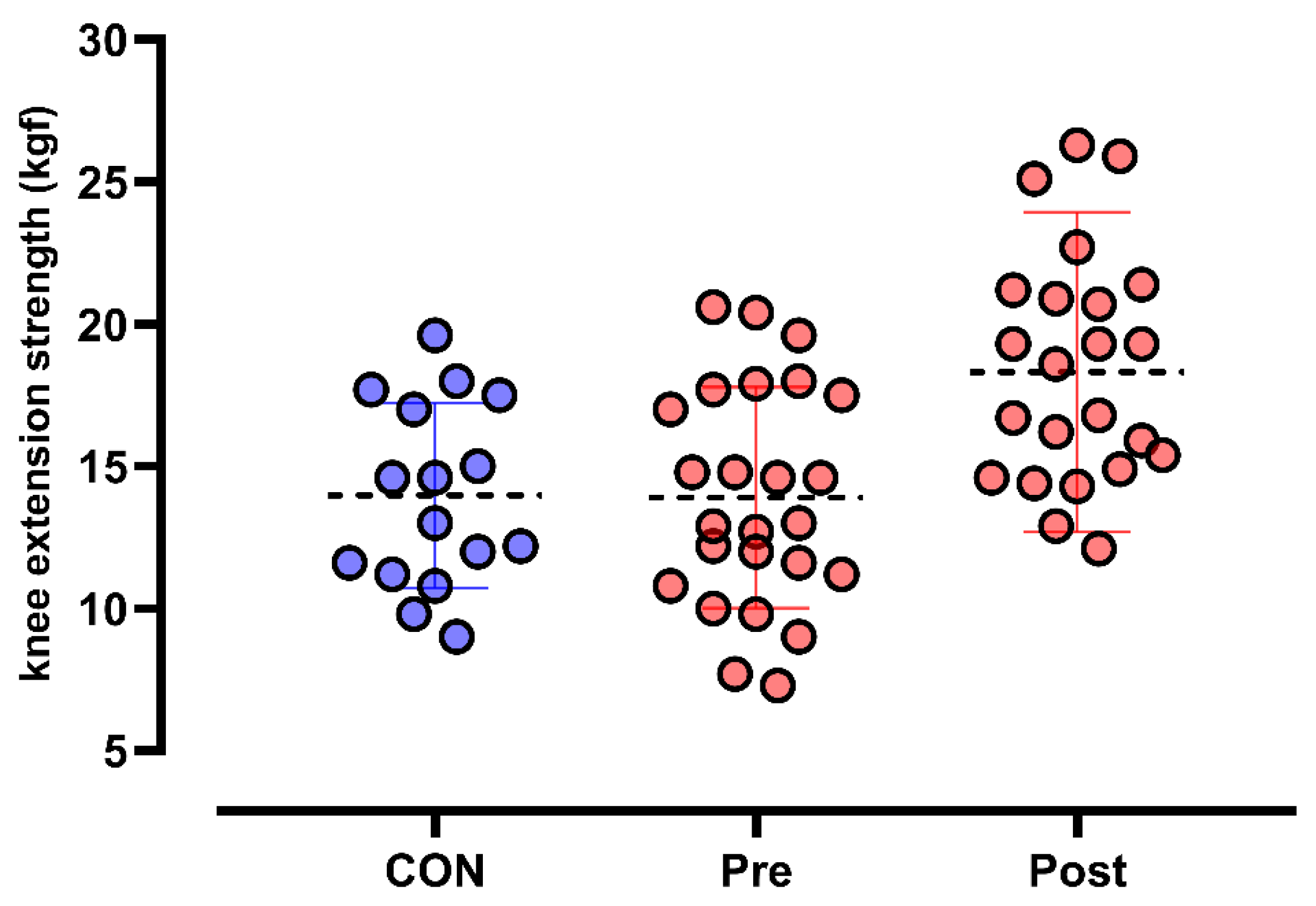
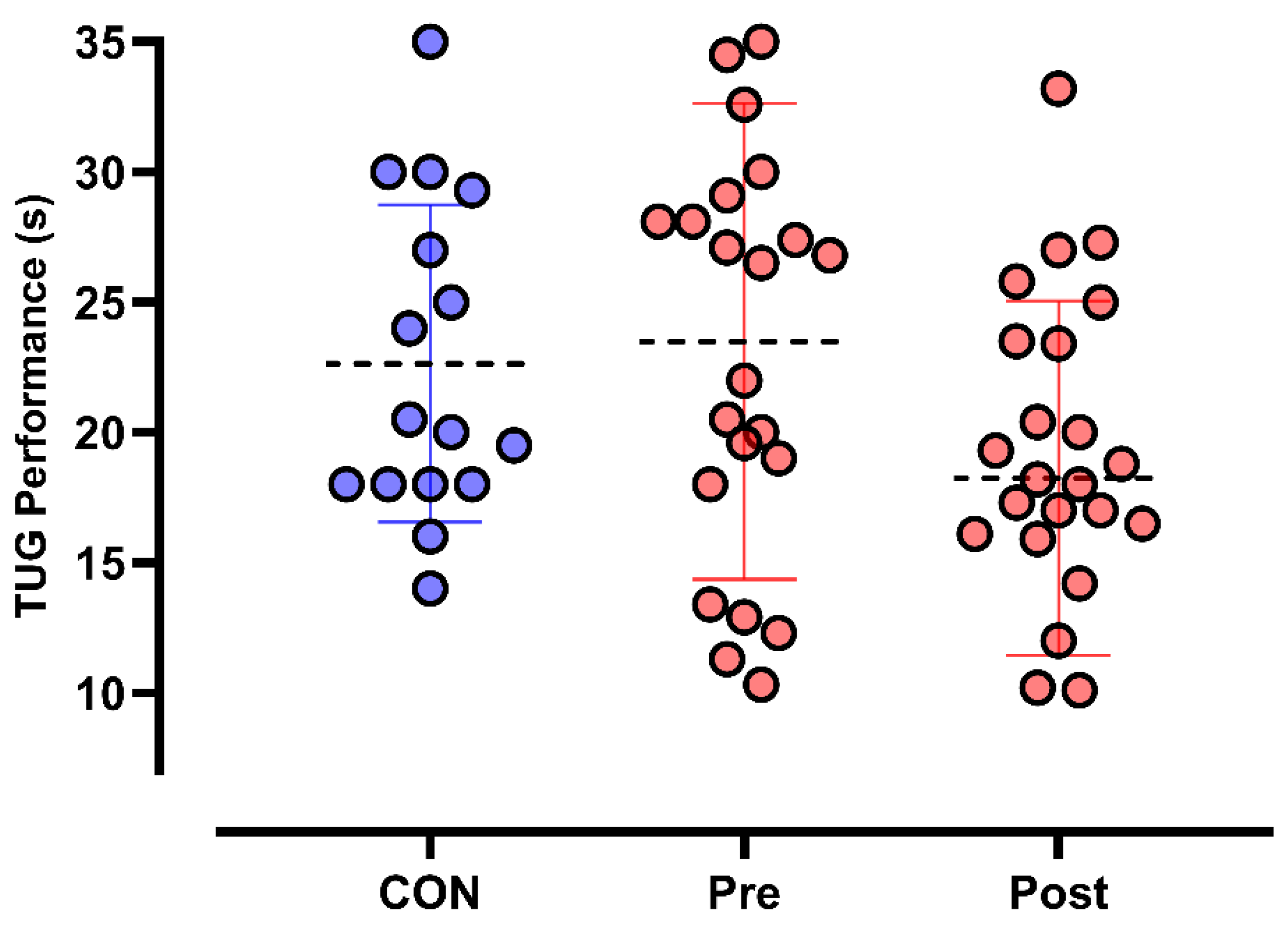
3.2. Primary Outcomes
3.2.1. Knee-Extension Dynamometry
3.2.2. TUG Test
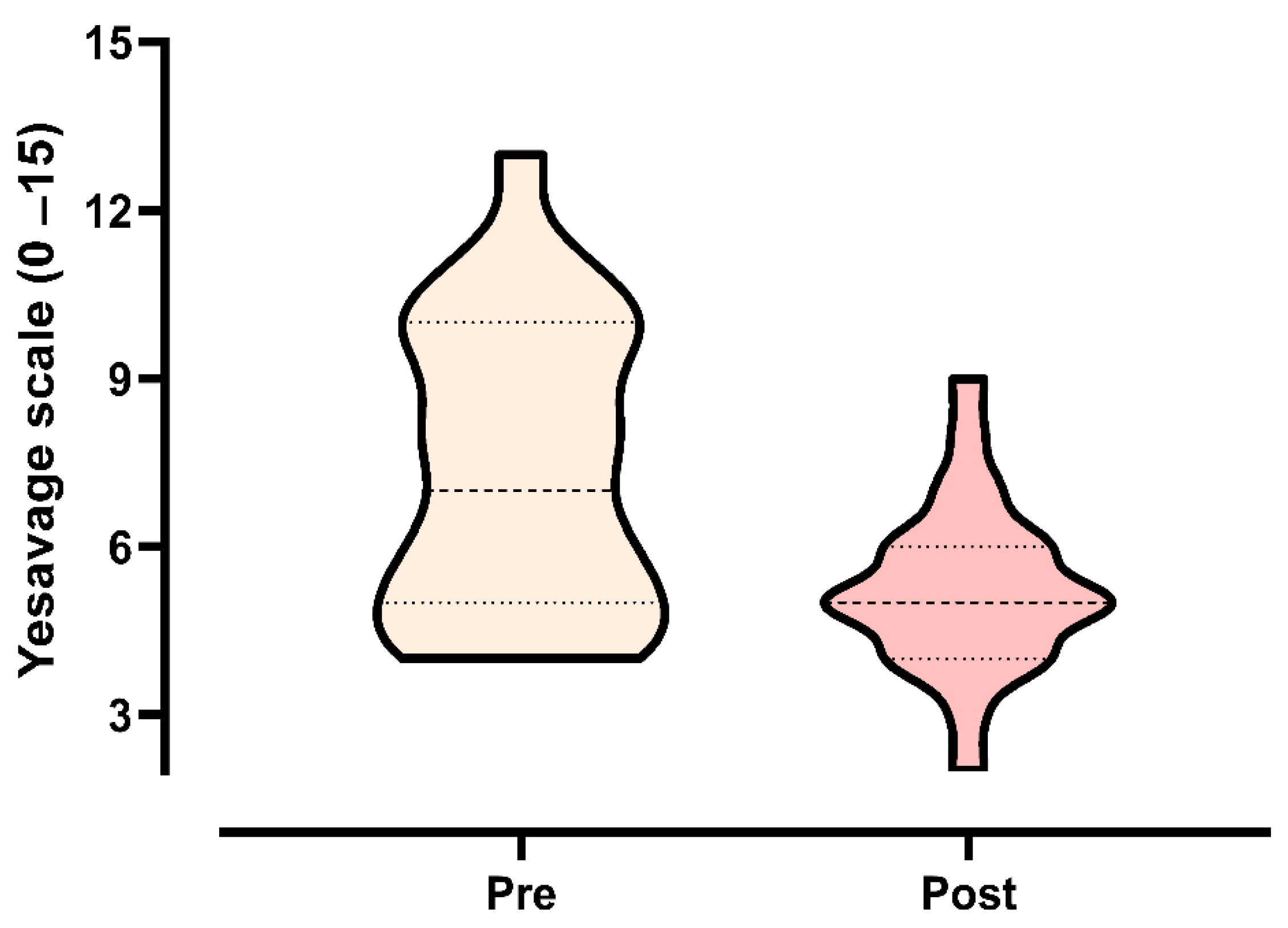
3.3. Secondary Outcomes
3.3.1. Calf Circumference
3.3.2. The SARC-F + CC Scale
3.3.3. Yesavage Depression Scale
4. Discussion
4.1. General Approach
4.2. Primary Outcome
4.3. Secondary Outcome
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bretan, O.; Silva Junior, J.E.; Ribeiro, O.R.; Corrente, J.E. Risk of falling among elderly persons living in the community: Assessment by the Timed up and go test. Braz. J. Otorhinolaryngol. 2013, 79, 18–21. [Google Scholar] [CrossRef] [PubMed]
- Churilov, I.; Churilov, L.; MacIsaac, R.J.; Ekinci, E.I. Systematic review and meta-analysis of prevalence of sarcopenia in post acute inpatient rehabilitation. Osteoporos. Int. 2018, 29, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Landi, F.; Schneider, S.M.; Zuniga, C.; Arai, H.; Boirie, Y.; Chen, L.K.; Fielding, R.A.; Martin, F.C.; Michel, J.P.; et al. Prevalence of and interventions for sarcopenia in ageing adults: A systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing 2014, 43, 748–759. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Sayer, A.A. Sarcopenia. Lancet 2019, 393, 2636–2646. [Google Scholar] [CrossRef] [PubMed]
- Landi, F.; Liperoti, R.; Russo, A.; Giovannini, S.; Tosato, M.; Capoluongo, E.; Bernabei, R.; Onder, G. Sarcopenia as a risk factor for falls in elderly individuals: Results from the ilSIRENTE study. Clin. Nutr. 2012, 31, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Antunes, A.C.; Araujo, D.A.; Verissimo, M.T.; Amaral, T.F. Sarcopenia and hospitalisation costs in older adults: A cross-sectional study. Nutr. Diet. 2017, 74, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Bruyère, O.; Beaudart, C.; Reginster, J.; Buckinx, F.; Schoene, D.; Hirani, V.; Cooper, C.; Kanis, J.A.; Rizzoli, R.; McCloskey, E.; et al. Assessment of muscle mass, muscle strength and physical performance in clinical practice: An international survey. Eur. Geriatr. Med. 2016, 7, 243–246. [Google Scholar] [CrossRef]
- Mijnarends, D.M.; Luiking, Y.C.; Halfens, R.J.G.; Evers, S.; Lenaerts, E.L.A.; Verlaan, S.; Wallace, M.; Schols, J.; Meijers, J.M.M. Muscle, Health and Costs: A Glance at their Relationship. J. Nutr. Health Aging 2018, 22, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.V.; Hsu, T.H.; Wu, W.T.; Huang, K.C.; Han, D.S. Is sarcopenia associated with depression? A systematic review and meta-analysis of observational studies. Age Ageing 2017, 46, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Chen, W.C.; Liu, C.W.; Huang, W.Y.; Lu, I.; Lin, C.W.; Huang, R.Y.; Chen, J.S.; Huang, C.H. Is moderate resistance training adequate for older adults with sarcopenia? A systematic review and network meta-analysis of RCTs. Eur. Rev. Aging Phys. Act. 2023, 20, 22. [Google Scholar] [CrossRef] [PubMed]
- Fragala, M.S.; Cadore, E.L.; Dorgo, S.; Izquierdo, M.; Kraemer, W.J.; Peterson, M.D.; Ryan, E.D. Resistance Training for Older Adults: Position Statement From the National Strength and Conditioning Association. J. Strength Cond. Res. 2019, 33, 2019–2052. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Shi, Q.; Nong, K.; Li, S.; Yue, J.; Huang, J.; Dong, B.; Beauchamp, M.; Hao, Q. Exercise for sarcopenia in older people: A systematic review and network meta-analysis. J. Cachexia Sarcopenia Muscle 2023, 14, 1199–1211. [Google Scholar] [CrossRef] [PubMed]
- Bernabei, R.; Landi, F.; Calvani, R.; Cesari, M.; Del Signore, S.; Anker, S.D.; Bejuit, R.; Bordes, P.; Cherubini, A.; Cruz-Jentoft, A.J.; et al. Multicomponent intervention to prevent mobility disability in frail older adults: Randomised controlled trial (SPRINTT project). BMJ 2022, 377, e068788. [Google Scholar] [CrossRef] [PubMed]
- Veen, J.; Montiel-Rojas, D.; Nilsson, A.; Kadi, F. Engagement in Muscle-Strengthening Activities Lowers Sarcopenia Risk in Older Adults Already Adhering to the Aerobic Physical Activity Guidelines. Int. J. Environ. Res. Public Health 2021, 18, 989. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, M.; Merchant, R.A.; Morley, J.E.; Anker, S.D.; Aprahamian, I.; Arai, H.; Aubertin-Leheudre, M.; Bernabei, R.; Cadore, E.L.; Cesari, M.; et al. International Exercise Recommendations in Older Adults (ICFSR): Expert Consensus Guidelines. J. Nutr. Health Aging 2021, 25, 824–853. [Google Scholar] [CrossRef] [PubMed]
- Kinney, B.M.; Lozanova, P. High intensity focused electromagnetic therapy evaluated by magnetic resonance imaging: Safety and efficacy study of a dual tissue effect based non-invasive abdominal body shaping. Lasers Surg. Med. 2019, 51, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Leonardo, P.S.; Cardoso, K.R.; Silva, B.O.; Silva, R.O.; Silva, H.C.; França, P.R.; Souza, B.N.; Moreira, I.L.; Coelho, C.F.; Oliveira, C.S.; et al. Evaluation of pulsed electromagnetic field therapy to improve muscle strength and functional aspects in the elderly: A pilot study. Man. Ther. Posturol. Rehabil. J. 2023, 21, 1–7. [Google Scholar] [CrossRef]
- Leonardo, P.S.; Cardoso, K.R.; Vieira, R.P.; Ruiz-Silva, C.; Coelho, C.; Martins, P.S.L.; Lopes-Martins, R.A.B. Applications of Pulsed Electromagnetic Field Therapy in Skeletal-Muscle System: An Integrative Review. Man. Ther. Posturol. Rehabil. J. 2023, 21, 1–11. [Google Scholar] [CrossRef]
- Petecchia, L.; Sbrana, F.; Utzeri, R.; Vercellino, M.; Usai, C.; Visai, L.; Vassalli, M.; Gavazzo, P. Electro-magnetic field promotes osteogenic differentiation of BM-hMSCs through a selective action on Ca(2+)-related mechanisms. Sci. Rep. 2015, 5, 13856. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; He, H.; Ye, W.; Perry, T.A.; He, C. Effects of Pulsed Electromagnetic Field Therapy on Pain, Stiffness, Physical Function, and Quality of Life in Patients With Osteoarthritis: A Systematic Review and Meta-Analysis of Randomized Placebo-Controlled Trials. Phys. Ther. 2020, 100, 1118–1131. [Google Scholar] [CrossRef] [PubMed]
- Yadollahpour, A.; Rashidi, S. Therapeutic Applications of Electromagnetic Fields in Musculoskeletal Disorders: A Review of Current Techniques and Mechanisms of Action. Biomed. Pharmacol. J. 2014, 7, 23–32. [Google Scholar] [CrossRef]
- Trofe, A.; Piras, A.; Muehsam, D.; Meoni, A.; Campa, F.; Toselli, S.; Raffi, M. Effect of Pulsed Electromagnetic Fields (PEMFs) on Muscular Activation during Cycling: A Single-Blind Controlled Pilot Study. Healthcare 2023, 11, 922. [Google Scholar] [CrossRef] [PubMed]
- Venugobal, S.; Tai, Y.K.; Goh, J.; Teh, S.; Wong, C.; Goh, I.; Maier, A.B.; Kennedy, B.K.; Franco-Obregon, A. Brief, weekly magnetic muscle therapy improves mobility and lean body mass in older adults: A Southeast Asia community case study. Aging 2023, 15, 1768–1790. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyere, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Christopher, A.; Kraft, E.; Olenick, H.; Kiesling, R.; Doty, A. The reliability and validity of the Timed Up and Go as a clinical tool in individuals with and without disabilities across a lifespan: A systematic review. Disabil. Rehabil. 2021, 43, 1799–1813. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Chou, M.Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e2. [Google Scholar] [CrossRef] [PubMed]
- Barreto de Lima, A.; Dos Santos Ribeiro, G.; Henriques-Neto, D.; Rubio Gouveia, E.; Baptista, F. Diagnostic performance of SARC-F and SARC-CalF in screening for sarcopenia in older adults in Northern Brazil. Sci. Rep. 2023, 13, 11698. [Google Scholar] [CrossRef] [PubMed]
- Borges, K.; Artacho, R.; Jodar-Graus, R.; Molina-Montes, E.; Ruiz-Lopez, M.D. Calf Circumference, a Valuable Tool to Predict Sarcopenia in Older People Hospitalized with Hip Fracture. Nutrients 2022, 14, 4255. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.E.; Chen, W.L. Calf circumference refines sarcopenia in correlating with mortality risk. Age Ageing 2022, 51, afab239. [Google Scholar] [CrossRef] [PubMed]
- Yesavage, J.A.; Brink, T.L.; Rose, T.L.; Lum, O.; Huang, V.; Adey, M.; Leirer, V.O. Development and validation of a geriatric depression screening scale: A preliminary report. J. Psychiatr. Res. 1982, 17, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Paradela, E.M.; Lourenco, R.A.; Veras, R.P. Validation of geriatric depression scale in a general outpatient clinic. Rev. Saude Publica 2005, 39, 918–923. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, L.; Cyrino, E.S.; Antunes, M.; Santos, D.A.; Sardinha, L.B. Sarcopenia and physical independence in older adults: The independent and synergic role of muscle mass and muscle function. J. Cachexia Sarcopenia Muscle 2017, 8, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Dent, E.; Morley, J.E.; Cruz-Jentoft, A.J.; Arai, H.; Kritchevsky, S.B.; Guralnik, J.; Bauer, J.M.; Pahor, M.; Clark, B.C.; Cesari, M.; et al. International Clinical Practice Guidelines for Sarcopenia (ICFSR): Screening, Diagnosis and Management. J. Nutr. Health Aging 2018, 22, 1148–1161. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Huang, W.Y.; Zhao, Y. Efficacy of Exercise on Muscle Function and Physical Performance in Older Adults with Sarcopenia: An Updated Systematic Review and Meta-Analysis. Int. J. Env. Res. Public Health 2022, 19, 8212. [Google Scholar] [CrossRef] [PubMed]
- Aagaard, P.; Simonsen, E.B.; Andersen, J.L.; Magnusson, P.; Dyhre-Poulsen, P. Neural adaptation to resistance training: Changes in evoked V-wave and H-reflex responses. J. Appl. Physiol. 2002, 92, 2309–2318. [Google Scholar] [CrossRef] [PubMed]
- Sale, D.G. Neural adaptation to resistance training. Med. Sci. Sports Exerc. 1988, 20, S135–S145. [Google Scholar] [CrossRef] [PubMed]
- Mithal, A.; Bonjour, J.P.; Boonen, S.; Burckhardt, P.; Degens, H.; El Hajj Fuleihan, G.; Josse, R.; Lips, P.; Morales Torres, J.; Rizzoli, R.; et al. Impact of nutrition on muscle mass, strength, and performance in older adults. Osteoporos. Int. 2013, 24, 1555–1566. [Google Scholar] [CrossRef] [PubMed]
- Carroll, T.J.; Riek, S.; Carson, R.G. Neural adaptations to resistance training: Implications for movement control. Sports Med. 2001, 31, 829–840. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, F.; Mayberg, H.S.; Wager, T.D.; Stohler, C.S.; Zubieta, J.K. Neurobiological mechanisms of the placebo effect. J. Neurosci. 2005, 25, 10390–10402. [Google Scholar] [CrossRef] [PubMed]
- Kamide, N.; Sato, H.; Sakamoto, M.; Shiba, Y. The effect of the interaction between fall-related self-efficacy and gait function on the occurrence of falls in community-dwelling older people. Aging Clin. Exp. Res. 2021, 33, 2715–2722. [Google Scholar] [CrossRef] [PubMed]
- Beauchet, O.; Fantino, B.; Allali, G.; Muir, S.W.; Montero-Odasso, M.; Annweiler, C. Timed Up and Go test and risk of falls in older adults: A systematic review. J. Nutr. Health Aging 2011, 15, 933–938. [Google Scholar] [CrossRef] [PubMed]
- Filippin, L.I.; Fernanda Miraglia, F.; Teixeira, V.N.; Boniatti, M.M. Timed Up and Go test as a sarcopenia screening tool in home-dwelling elderly persons. Rev. Bras. Geriatr. Gerontol. 2017, 20, 556–561. [Google Scholar] [CrossRef]
- Ortega-Bastidas, P.; Gomez, B.; Aqueveque, P.; Luarte-Martinez, S.; Cano-de-la-Cuerda, R. Instrumented Timed Up and Go Test (iTUG)-More Than Assessing Time to Predict Falls: A Systematic Review. Sensor 2023, 23, 3426. [Google Scholar] [CrossRef] [PubMed]
- Cadore, E.L.; Izquierdo, M. How to simultaneously optimize muscle strength, power, functional capacity, and cardiovascular gains in the elderly: An update. Age 2013, 35, 2329–2344. [Google Scholar] [CrossRef] [PubMed]
- Barbosa-Silva, T.G.; Menezes, A.M.; Bielemann, R.M.; Malmstrom, T.K.; Gonzalez, M.C.; Grupo de Estudos em Composição Corporal e Nutrição (COCONUT). Enhancing SARC-F: Improving Sarcopenia Screening in the Clinical Practice. J. Am. Med. Dir. Assoc. 2016, 17, 1136–1141. [Google Scholar] [CrossRef] [PubMed]
- Kiss, C.M.; Bertschi, D.; Beerli, N.; Berres, M.; Kressig, R.W.; Fischer, A.M. Calf circumference as a surrogate indicator for detecting low muscle mass in hospitalized geriatric patients. Aging Clin. Exp. Res. 2024, 36, 25. [Google Scholar] [CrossRef] [PubMed]
- Rolland, Y.; Lauwers-Cances, V.; Cournot, M.; Nourhashemi, F.; Reynish, W.; Riviere, D.; Vellas, B.; Grandjean, H. Sarcopenia, calf circumference, and physical function of elderly women: A cross-sectional study. J. Am. Geriatr. Soc. 2003, 51, 1120–1124. [Google Scholar] [CrossRef] [PubMed]
- Abe, T.; Loenneke, J.P.; Young, K.C.; Thiebaud, R.S.; Nahar, V.K.; Hollaway, K.M.; Stover, C.D.; Ford, M.A.; Bass, M.A.; Loftin, M. Validity of ultrasound prediction equations for total and regional muscularity in middle-aged and older men and women. Ultrasound Med. Biol. 2015, 41, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.; Cho, J.; Hong, H.; Jin, Y.; Kim, D.; Kang, H. Sarcopenia Is Associated with Cognitive Impairment and Depression in Elderly Korean Women. Iran. J. Public Health 2018, 47, 327–334. [Google Scholar] [PubMed]
- Yuan, T.F.; Paes, F.; Arias-Carrion, O.; Ferreira Rocha, N.B.; de Sa Filho, A.S.; Machado, S. Neural Mechanisms of Exercise: Anti-Depression, Neurogenesis, and Serotonin Signaling. CNS Neurol. Disord. Drug Targets 2015, 14, 1307–1311. [Google Scholar] [CrossRef] [PubMed]
- Sá Filho, A.; Cheniaux, E.; de Paula, C.C.; Murillo-Rodriguez, E.; Teixeira, D.; Monteiro, D.; Cid, L.; Yamamoto, T.; Telles-Correia, D.; Imperatori, C.; et al. Exercise is medicine: A new perspective for health promotion in bipolar disorder. Expert. Rev. Neurother. 2020, 20, 1099–1107. [Google Scholar] [CrossRef] [PubMed]
- Machado, S.; Filho, A.S.S.; Wilbert, M.; Barbieri, G.; Almeida, V.; Gurgel, A.; Rosa, C.V.; Lins, V.; Paixao, A.; Santana, K.; et al. Physical Exercise As Stabilizer For Alzheimer’S Disease Cognitive Decline: Current Status. Clin. Pract. Epidemiol. Ment. Health 2017, 13, 181–184. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sa Filho, A.S.; Barsanulfo, S.R.; Faria, S.S.; Inacio, P.A.; Ayatizadeh, F.; Machado, S. Exerkines: A Crosstalk between Lactate Production, Exercise and Mental Health. CNS Neurol. Disord. Drug Targets 2024, 23, 1057–1060. [Google Scholar] [CrossRef] [PubMed]
- de Souza Moura, A.M.; Lamego, M.K.; Paes, F.; Ferreira Rocha, N.B.; Simoes-Silva, V.; Rocha, S.A.; de Sa Filho, A.S.; Rimes, R.; Manochio, J.; Budde, H.; et al. Comparison Among Aerobic Exercise and Other Types of Interventions to Treat Depression: A Systematic Review. CNS Neurol. Disord. Drug Targets 2015, 14, 1171–1183. [Google Scholar] [CrossRef] [PubMed]
- de Sa Filho, A.S.; de Souza Moura, A.M.; Lamego, M.K.; Ferreira Rocha, N.B.; Paes, F.; Oliveira, A.C.; Lattari, E.; Rimes, R.; Manochio, J.; Budde, H.; et al. Potential Therapeutic Effects of Physical Exercise for Bipolar Disorder. CNS Neurol. Disord. Drug Targets 2015, 14, 1255–1259. [Google Scholar] [CrossRef] [PubMed]


| Age | Body Mass | Height | BMI | Lean Mass | Fat Mass | SARC-F + CC | |
|---|---|---|---|---|---|---|---|
| (Years) | (kg) | (m) | (kg·m−2) | (kg) | (kg) | (Score) | |
| PEMF | |||||||
| Mean | 76.6 | 72.1 | 1.60 | 31.3 | 47.4 | 30.4 | 11.6 |
| SD | 6.2 | 10.2 | 8.0 | 5.0 | 3.0 | 9.7 | 7.4 |
| CON | |||||||
| Mean | 73.8 | 70.5 | 1.63 | 30.4 | 45.4 | 32.1 | 8.9 |
| SD | 9.0 | 9.4 | 9.5 | 5.5 | 5.1 | 9.0 | 6.7 |
| SARC-F + CC < 10 (n = 16) | SARC-F + CC > 10 (n = 9) | p Value SARC | p Value Group | |||
|---|---|---|---|---|---|---|
| PEMF (n = 25) | Mean ± SD | 95% CI | Mean ± SD | 95% CI | ||
| Age (years) | 76.8 ± 5.9 | (73.7–80.0) | 76.0 ± 6.8 | (70.7–81.2) | 0.992 | 0.566 |
| TUG (s) | 22.6 ± 7.8 | (18.5–26.8) | 24.9 ± 11.4 | (16.0–33.7) | 0.912 | 0.876 |
| CC (cm) | 35.1 ± 4.0 | (32.9–37.3) | 32.0 ± 3.1 | (29.6–34.5) | 0.288 | 0.768 |
| Knee Extension (kgf) | 13.5 ± 3.8 | (11.4–15.6) | 14.5 ± 4.0 | (11.3 ± 17.6) | 0.992 | 0.993 |
| CON (n = 16) | (n = 8) | (n = 8) | ||||
| Age (years) | 74.1 ± 8.6 | (66.1 ± 82.1) | 75.1 ± 9.2 | (67.2 ± 82.7) | 0.921 | 0.939 |
| TUG (s) | 19.9 ± 6.2 | (14.0–25.7) | 21.3 ± 5.2 | (16.9–25.7) | 0.985 | 0.994 |
| CC (cm) | 28.7 ± 5.0 * | (24.0–33.3) | 33.4 ± 4.4 | (29.7–37.1) | 0.991 | 0.004 |
| Knee Extension (kgf) | 13.5 ± 3.3 | (10.4–16.5) | 13.9 ± 3.4 | (11.1–16.8) | 0.990 | 0.993 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leonardo, P.S.; Sá Filho, A.S.; Inacio, P.A.; França, P.R.; Aprigliano, V.; Teixeira, F.; Macedo, M.M.; Fonseca, D.F.; Lopes-Martins, P.S.L.; Costa, G.D.C.T.; et al. The Effects of Pulsed Electromagnetic Field (PEMF) on Muscular Strength, Functional Performance and Depressive Symptoms in Elderly Adults with Sarcopenia: A Short-Term Intervention. Life 2025, 15, 1111. https://doi.org/10.3390/life15071111
Leonardo PS, Sá Filho AS, Inacio PA, França PR, Aprigliano V, Teixeira F, Macedo MM, Fonseca DF, Lopes-Martins PSL, Costa GDCT, et al. The Effects of Pulsed Electromagnetic Field (PEMF) on Muscular Strength, Functional Performance and Depressive Symptoms in Elderly Adults with Sarcopenia: A Short-Term Intervention. Life. 2025; 15(7):1111. https://doi.org/10.3390/life15071111
Chicago/Turabian StyleLeonardo, Patrícia Sardinha, Alberto Souza Sá Filho, Pedro Augusto Inacio, Paulo Ricardo França, Vicente Aprigliano, Fernando Teixeira, Michel Monteiro Macedo, Douglas Farias Fonseca, Pedro Sardinha Leonardo Lopes-Martins, Gustavo De Conti Teixeira Costa, and et al. 2025. "The Effects of Pulsed Electromagnetic Field (PEMF) on Muscular Strength, Functional Performance and Depressive Symptoms in Elderly Adults with Sarcopenia: A Short-Term Intervention" Life 15, no. 7: 1111. https://doi.org/10.3390/life15071111
APA StyleLeonardo, P. S., Sá Filho, A. S., Inacio, P. A., França, P. R., Aprigliano, V., Teixeira, F., Macedo, M. M., Fonseca, D. F., Lopes-Martins, P. S. L., Costa, G. D. C. T., & Lopes-Martins, R. A. B. (2025). The Effects of Pulsed Electromagnetic Field (PEMF) on Muscular Strength, Functional Performance and Depressive Symptoms in Elderly Adults with Sarcopenia: A Short-Term Intervention. Life, 15(7), 1111. https://doi.org/10.3390/life15071111











