Integrating Ultrasound-Guided Injections and Peripheral Magnetic Stimulation in Chronic Myofascial/Lumbar Pain
Abstract
1. Introduction
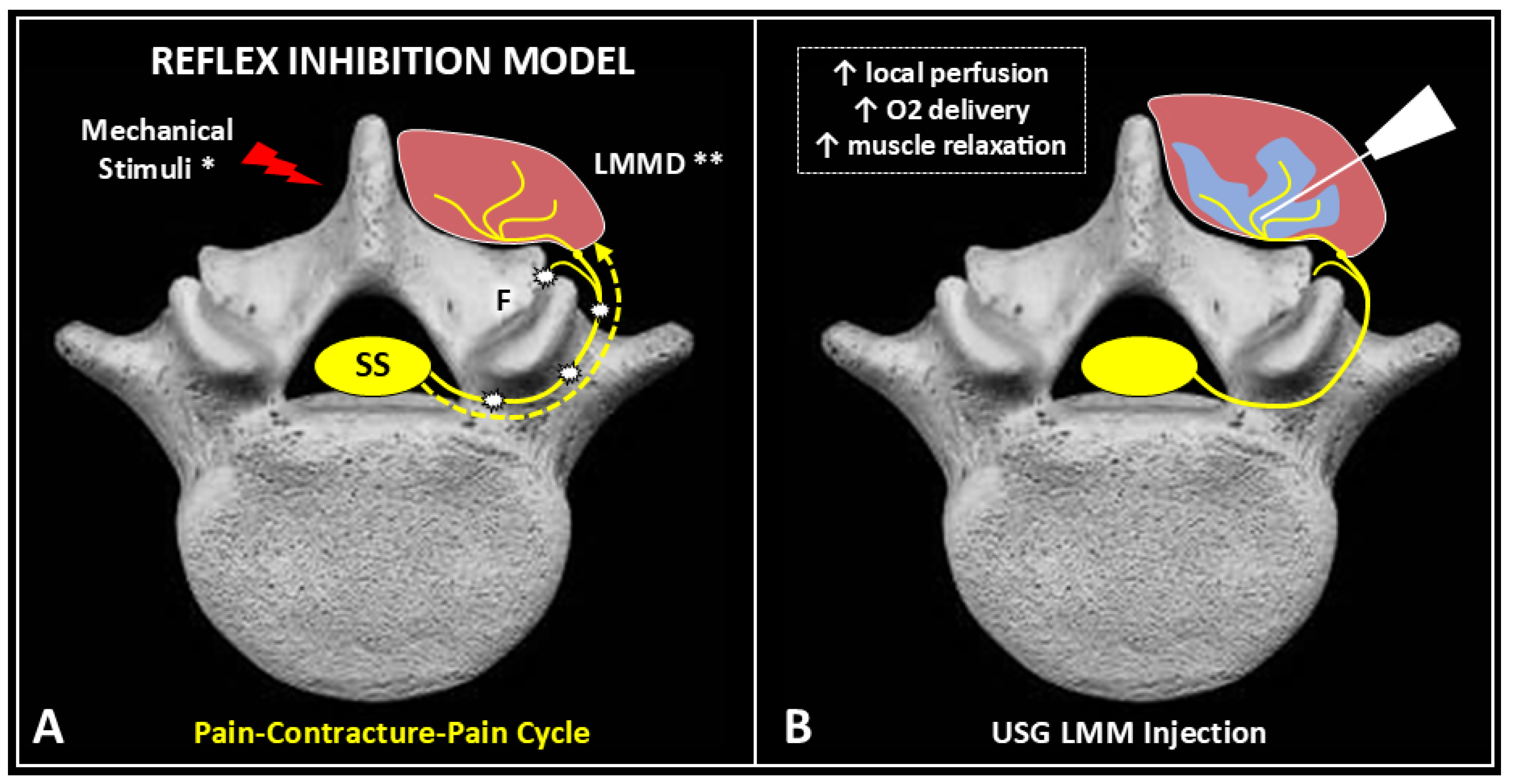
2. Pathophysiology of Myofascial Pain
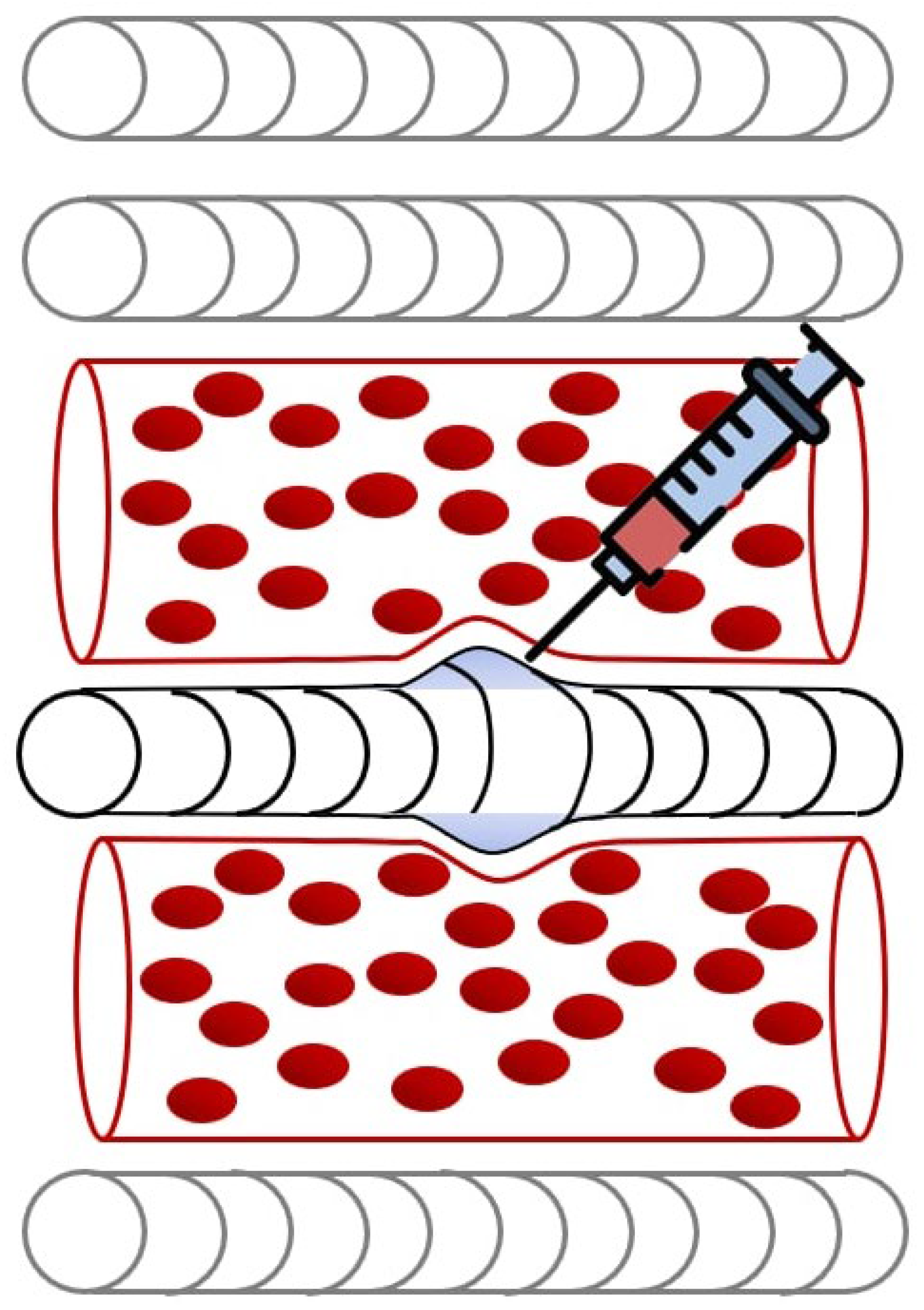
3. Effects of Ultrasound-Guided Injections
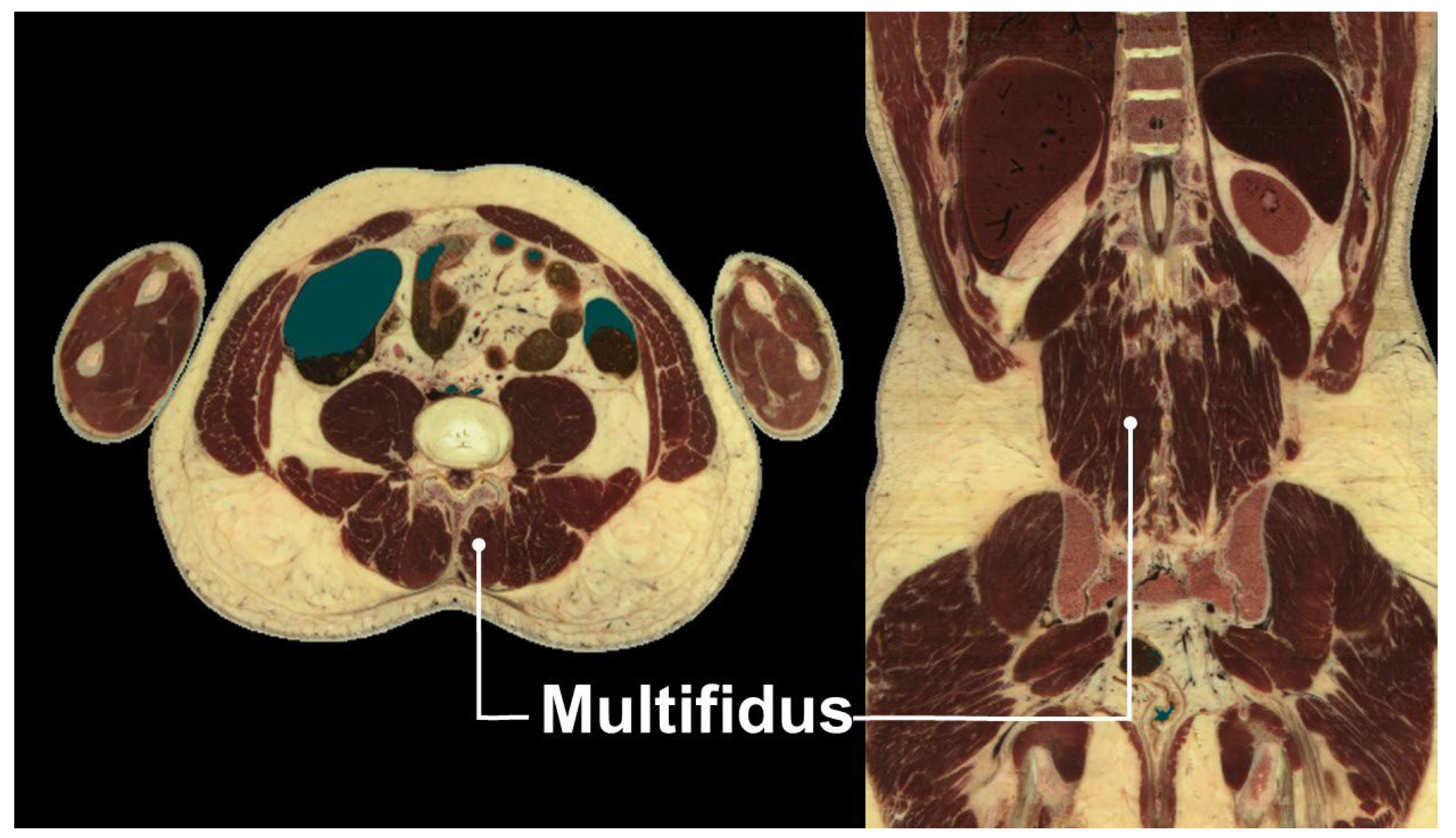
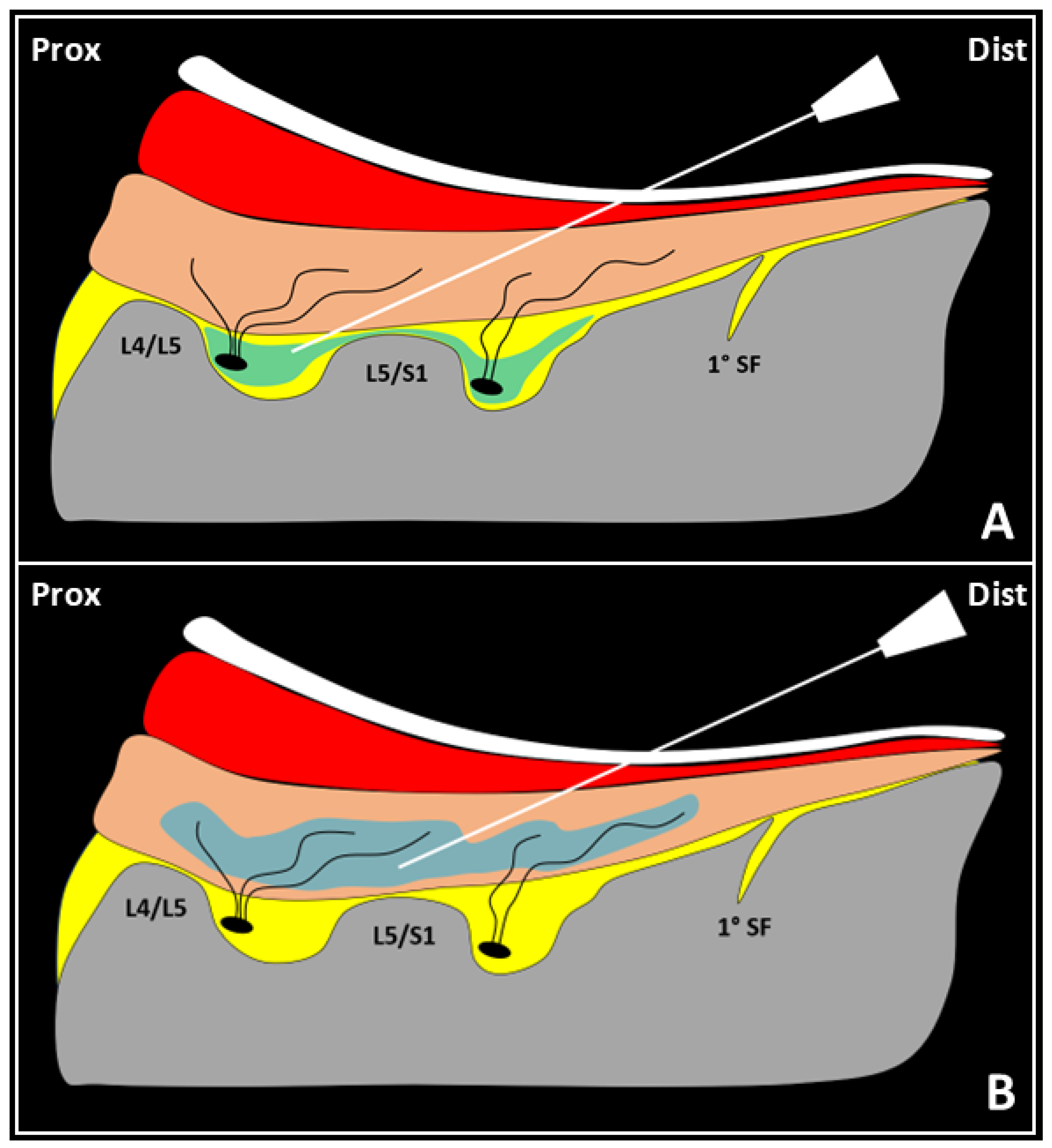
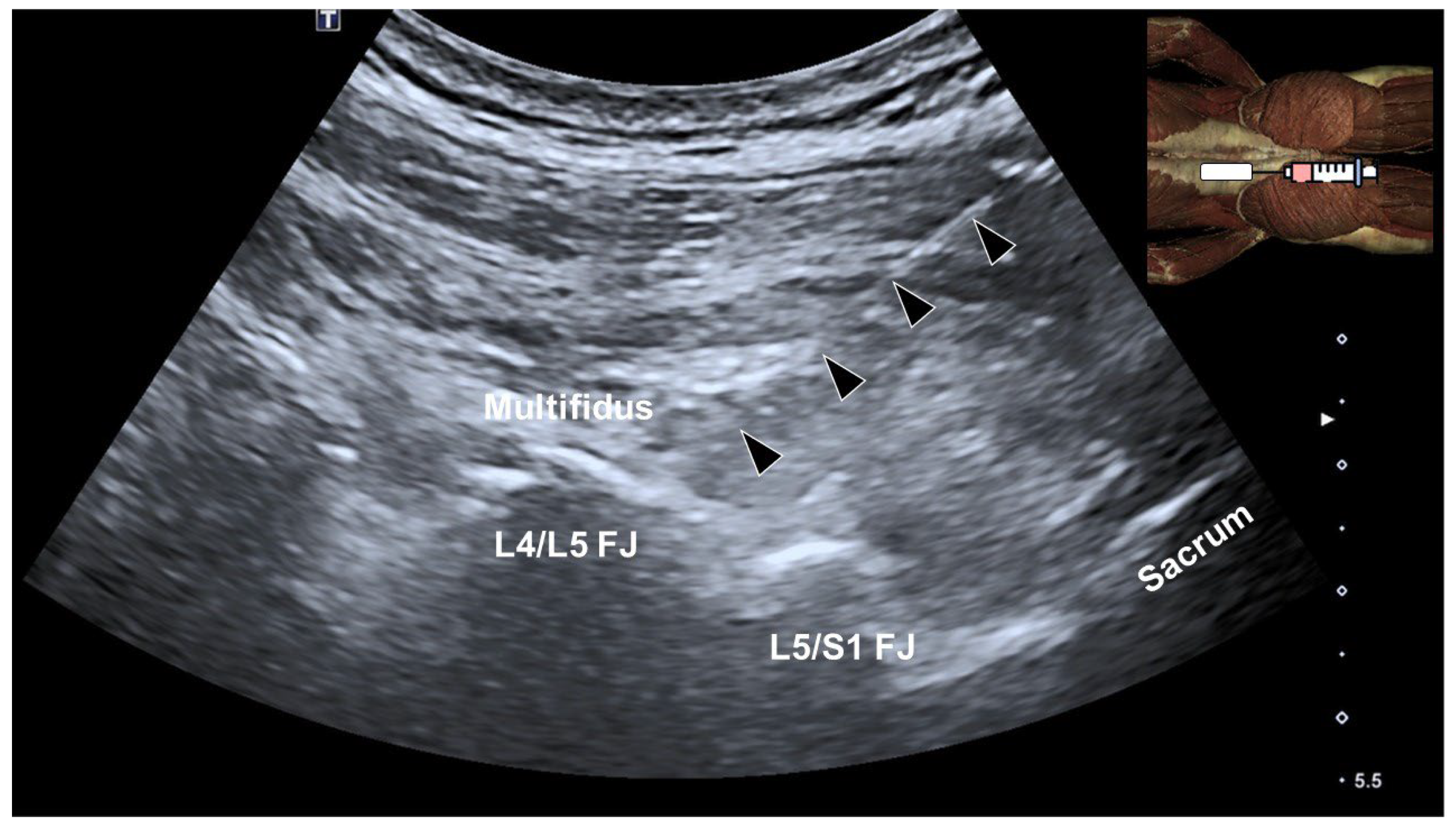
4. Multifidus Muscle and Low Back Pain
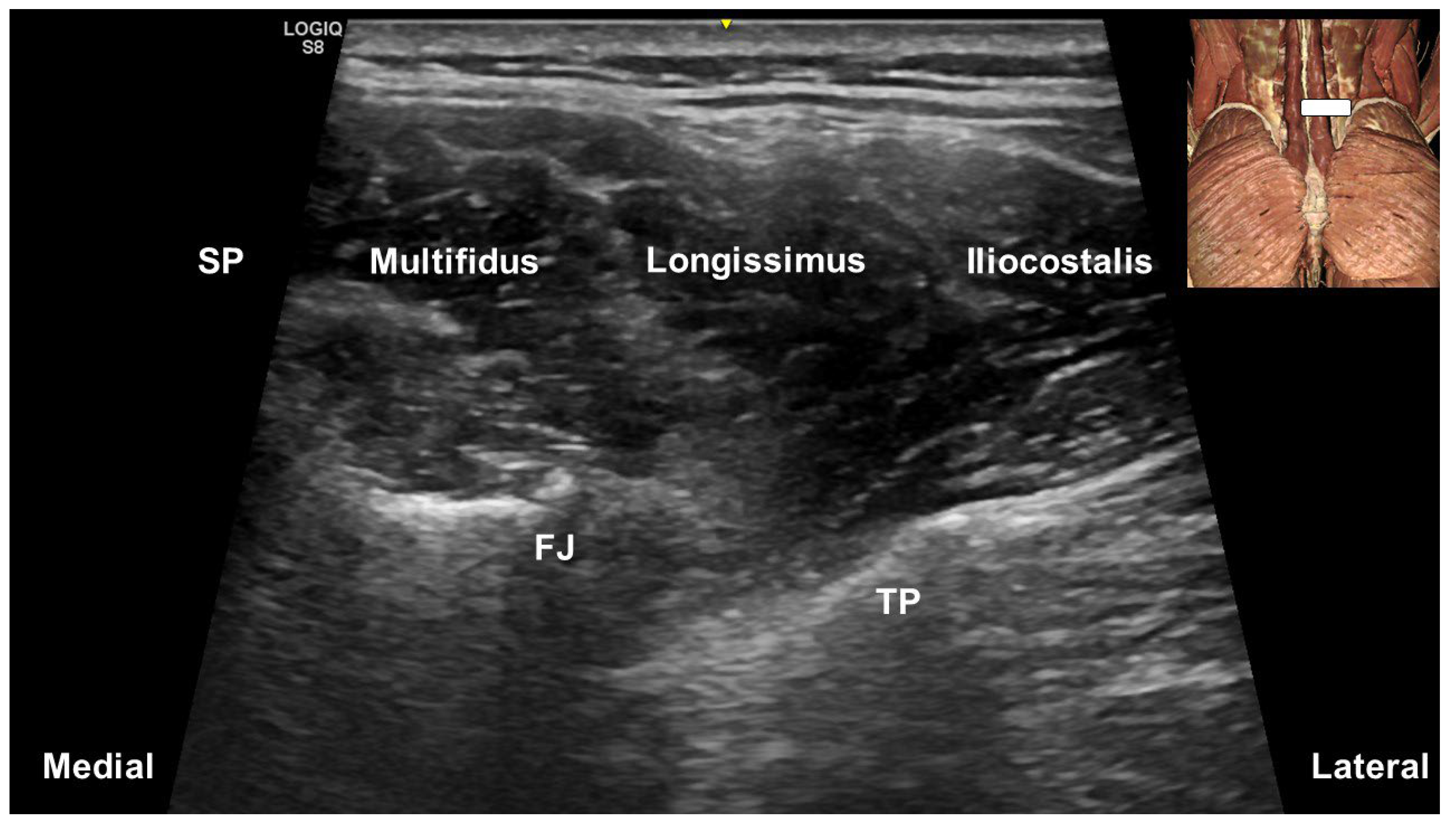
5. Ultrasound-Guided Injections Targeting the Multifidus Muscle
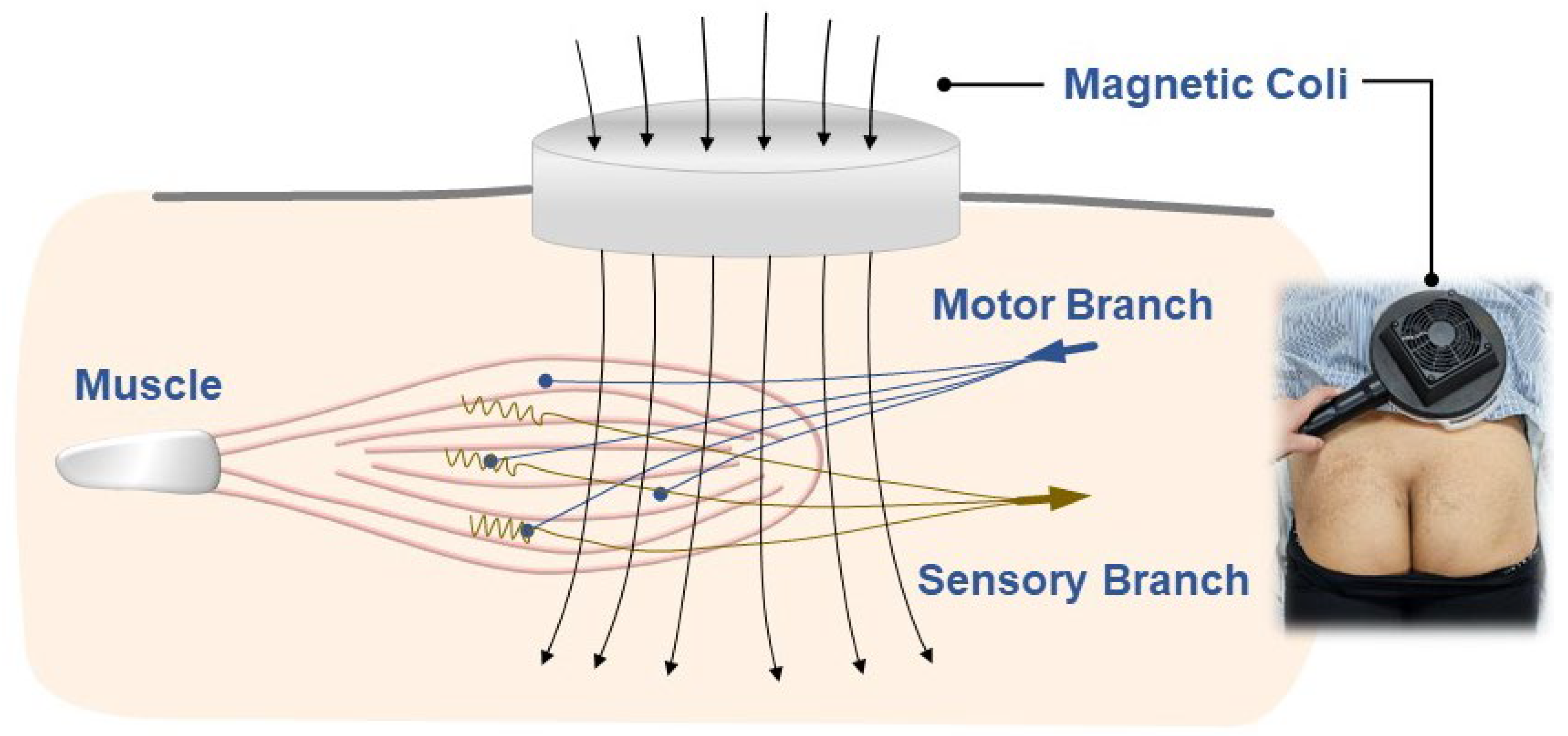
6. Repetitive Peripheral Magnetic Stimulation
6.1. Fundamental Principles
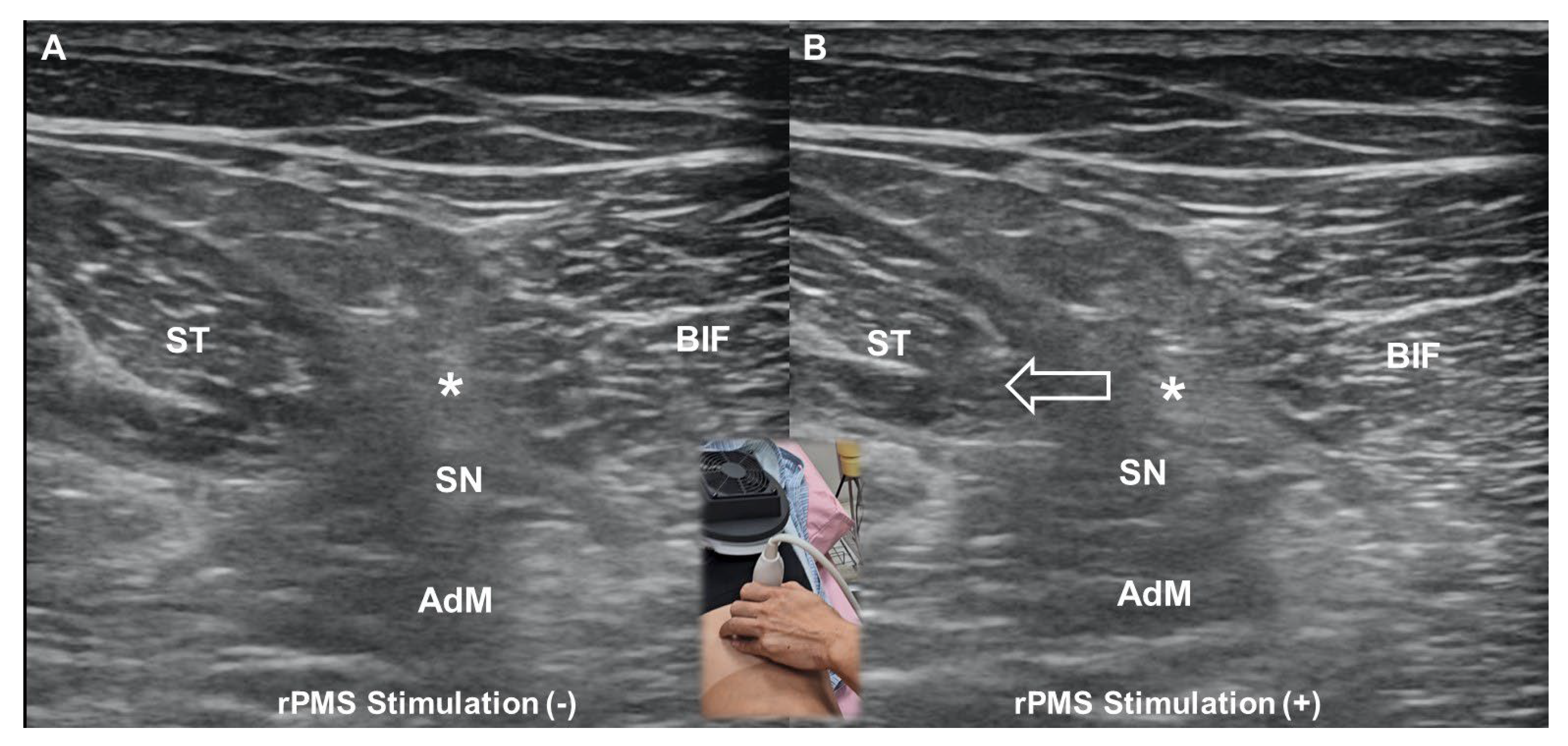
6.2. Mechanisms of Pain Relief
6.3. Machine Components
6.4. Comparison with Transcutaneous Electrical Nerve Stimulation
6.5. Optimizing Parameters
7. Combined Approach
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bonakdar, R.A. Chapter 62-Myofascial Pain Syndrome. In Integrative Medicine, 3rd ed.; Rakel, D., Ed.; W.B. Saunders: Philadelphia, PA, USA, 2012; pp. 579–587.e572. [Google Scholar]
- Ughreja, R.A.; Venkatesan, P.; Gopalakrishna, D.B.; Singh, Y.P. Effectiveness of myofascial release on pain, sleep, and quality of life in patients with fibromyalgia syndrome: A systematic review. Complement. Ther. Clin. Pract. 2021, 45, 101477. [Google Scholar] [CrossRef] [PubMed]
- Fernández-de-Las-Peñas, C.; Nijs, J.; Cagnie, B.; Gerwin, R.D.; Plaza-Manzano, G.; Valera-Calero, J.A.; Arendt-Nielsen, L. Myofascial Pain Syndrome: A Nociceptive Condition Comorbid with Neuropathic or Nociplastic Pain. Life 2023, 13, 694. [Google Scholar] [CrossRef] [PubMed]
- Dach, F.; Ferreira, K.S. Treating myofascial pain with dry needling: A systematic review for the best evidence-based practices in low back pain. Arq. Neuropsiquiatr. 2023, 81, 1169–1178. [Google Scholar] [CrossRef] [PubMed]
- Galasso, A.; Urits, I.; An, D.; Nguyen, D.; Borchart, M.; Yazdi, C.; Manchikanti, L.; Kaye, R.J.; Kaye, A.D.; Mancuso, K.F.; et al. A Comprehensive Review of the Treatment and Management of Myofascial Pain Syndrome. Curr. Pain Headache Rep. 2020, 24, 43. [Google Scholar] [CrossRef]
- Wu, W.T.; Chang, K.V.; Ricci, V.; Özçakar, L. Ultrasound imaging and guidance in the management of myofascial pain syndrome: A narrative review. J. Yeungnam Med. Sci. 2024, 41, 179–187. [Google Scholar] [CrossRef]
- Ricci, V.; Mezian, K.; Chang, K.V.; Tarantino, D.; Güvener, O.; Gervasoni, F.; Naňka, O.; Özçakar, L. Ultrasound Imaging and Guidance for Cervical Myofascial Pain: A Narrative Review. Int. J. Environ. Res. Public Health 2023, 20, 3838. [Google Scholar] [CrossRef]
- Kanjanapanang, N.; Chang, K.V. Peripheral Magnetic Stimulation. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2025. [Google Scholar]
- Jalali, F.; Nazari, M.A.; Bahrami, A.; Perrier, P.; Payan, Y. FIM: A fatigued-injured muscle model based on the sliding filament theory. Comput. Biol. Med. 2023, 164, 107367. [Google Scholar] [CrossRef]
- Jin, F.; Guo, Y.; Wang, Z.; Badughaish, A.; Pan, X.; Zhang, L.; Qi, F. The pathophysiological nature of sarcomeres in trigger points in patients with myofascial pain syndrome: A preliminary study. Eur. J. Pain 2020, 24, 1968–1978. [Google Scholar] [CrossRef]
- Jafri, M.S. Mechanisms of Myofascial Pain. Int. Sch. Res. Not. 2014, 2014, 523924. [Google Scholar] [CrossRef]
- Liu, Y.; Jin, F.; Zhou, L.; Li, X.; Li, X.; Chen, Q.; Yang, S.; Sun, J.; Qi, F. Platelet-derived Growth Factor Receptor-α Induces Contraction Knots and Inflammatory Pain-like Behavior in a Rat Model of Myofascial Trigger Points. Anesthesiology 2024, 141, 929–945. [Google Scholar] [CrossRef]
- Silva, A.B.; Malheiro, N.; Oliveira, B.; Pereira, D.; Antunes, F.; Borges, J.; Cunha, A.C. Efficacy of ultrasound-guided infiltration with levobupivacaine and triamcinolone for myofascial pain syndrome of the quadratus lumborum: A retrospective observational study. Braz. J. Anesthesiol. 2023, 73, 718–724. [Google Scholar] [CrossRef]
- Moussavi, R.S.; Carson, P.J.; Boska, M.D.; Weiner, M.W.; Miller, R.G. Nonmetabolic fatigue in exercising human muscle. Neurology 1989, 39, 1222–1226. [Google Scholar] [CrossRef] [PubMed]
- Macintosh, J.E.; Valencia, F.; Bogduk, N.; Munro, R.R. The morphology of the human lumbar multifidus. Clin. Biomech. 1986, 1, 196–204. [Google Scholar] [CrossRef]
- Freeman, M.D.; Woodham, M.A.; Woodham, A.W. The role of the lumbar multifidus in chronic low back pain: A review. PM R 2010, 2, 142–146. [Google Scholar] [CrossRef]
- Hung, C.Y.; Wang, B.; Chang, H.C.; Wu, W.T.; Liu, P.T.; Chang, K.V.; Su, D.C.; Mezian, K.; Ricci, V.; Özçakar, L. Pictorial Essay on Ultrasound and Magnetic Resonance Imaging of Paraspinal Muscles for Myofascial Pain Syndrome. Life 2024, 14, 499. [Google Scholar] [CrossRef]
- Suputtitada, A.; Chen, J.L.; Wu, C.K.; Peng, Y.N.; Yen, T.Y.; Chen, C.P.C. Determining the Most Suitable Ultrasound-Guided Injection Technique in Treating Lumbar Facet Joint Syndrome. Biomedicines 2023, 11, 3308. [Google Scholar] [CrossRef]
- Francio, V.T.; Westerhaus, B.D.; Carayannopoulos, A.G.; Sayed, D. Multifidus dysfunction and restorative neurostimulation: A scoping review. Pain Med. 2023, 24, 1341–1354. [Google Scholar] [CrossRef]
- Gabel, C.P.; Mokhtarinia, H.R.; Melloh, M.; Mateo, S. Slacklining as therapy to address non-specific low back pain in the presence of multifidus arthrogenic muscle inhibition. World J. Orthop. 2021, 12, 178–196. [Google Scholar] [CrossRef]
- Saslow, W.M. Chapter 12-Faraday’s Law of Electromagnetic Induction. In Electricity, Magnetism, and Light; Saslow, W.M., Ed.; Academic Press: San Diego, CA, USA, 2002; pp. 505–558. [Google Scholar]
- Melzack, R. Gate control theory: On the evolution of pain concepts. Pain Forum. 1996, 5, 128–138. [Google Scholar] [CrossRef]
- Lim, Y.H.; Song, J.M.; Choi, E.H.; Lee, J.W. Effects of Repetitive Peripheral Magnetic Stimulation on Patients With Acute Low Back Pain: A Pilot Study. Ann. Rehabil. Med. 2018, 42, 229–238. [Google Scholar] [CrossRef]
- Leonard, G.; Goffaux, P.; Marchand, S. Deciphering the role of endogenous opioids in high-frequency TENS using low and high doses of naloxone. Pain 2010, 151, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.L.; Sanada, L.S.; Rakel, B.A.; Sluka, K.A. Increasing Intensity of TENS Prevents Analgesic Tolerance in Rats. J. Pain 2012, 13, 884–890. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.T.; Chang, K.V.; Mezian, K.; Ricci, V.; Özçakar, L. “Sono-vision” of muscle contractions during peripheral magnetic stimulation. Med. Ultrason. 2025, 27, 106–107. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.I. Resolving Long-Standing Uncertainty about the Clinical Efficacy of Transcutaneous Electrical Nerve Stimulation (TENS) to Relieve Pain: A Comprehensive Review of Factors Influencing Outcome. Medicina 2021, 57, 378. [Google Scholar] [CrossRef]
- Diao, Y.; Pan, J.; Xie, Y.; Liao, M.; Wu, D.; Liu, H.; Liao, L. Effect of Repetitive Peripheral Magnetic Stimulation on Patients With Low Back Pain: A Meta-analysis of Randomized Controlled Trials. Arch. Phys. Med. Rehabil. 2023, 104, 1526–1538. [Google Scholar] [CrossRef]
- Zschorlich, V.R.; Hillebrecht, M.; Tanjour, T.; Qi, F.; Behrendt, F.; Kirschstein, T.; Köhling, R. Repetitive Peripheral Magnetic Nerve Stimulation (rPMS) as Adjuvant Therapy Reduces Skeletal Muscle Reflex Activity. Front. Neurol. 2019, 10, 930. [Google Scholar] [CrossRef]
- Ke, J.; Wei, J.; Zheng, B.; Tan, T.; Zhou, W.; Zou, X.; Zou, H.; Zeng, H.; Zhou, G.; Chen, L.; et al. Effect of High-Frequency Repetitive Peripheral Magnetic Stimulation on Motor Performance in Intracerebral Haemorrhage: A Clinical Trial. J. Stroke Cerebrovasc. Dis. 2022, 31, 106446. [Google Scholar] [CrossRef]
- Wu, W.T.; Chang, K.V.; Özçakar, L. Integrating Ultrasound-Guided Multifidus Injections with Repeated Peripheral Magnetic Stimulation for Low Back Pain: A Feasibility Study. J. Pain Res. 2024, 17, 2873–2880. [Google Scholar] [CrossRef]
- Shen, P.C.; Lin, T.Y.; Wu, W.T.; Özçakar, L.; Chang, K.V. Comparison of ultrasound- vs. landmark-guided injections for musculoskeletal pain: An umbrella review. J. Rehabil. Med. 2024, 56, jrm40679. [Google Scholar] [CrossRef]
- Carette, S.; Marcoux, S.; Truchon, R.; Grondin, C.; Gagnon, J.; Allard, Y.; Latulippe, M. A controlled trial of corticosteroid injections into facet joints for chronic low back pain. N. Engl. J. Med. 1991, 325, 1002–1007. [Google Scholar] [CrossRef]
- Oliveira, C.B.; Maher, C.G.; Ferreira, M.L.; Hancock, M.J.; Oliveira, V.C.; McLachlan, A.J.; Koes, B.W.; Ferreira, P.H.; Cohen, S.P.; Pinto, R.Z. Epidural corticosteroid injections for lumbosacral radicular pain. Cochrane Database Syst. Rev. 2020, 2020, Cd013577. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, W.-T.; Chang, K.-V.; Mezian, K.; Ricci, V.; Özçakar, L. Integrating Ultrasound-Guided Injections and Peripheral Magnetic Stimulation in Chronic Myofascial/Lumbar Pain. Life 2025, 15, 563. https://doi.org/10.3390/life15040563
Wu W-T, Chang K-V, Mezian K, Ricci V, Özçakar L. Integrating Ultrasound-Guided Injections and Peripheral Magnetic Stimulation in Chronic Myofascial/Lumbar Pain. Life. 2025; 15(4):563. https://doi.org/10.3390/life15040563
Chicago/Turabian StyleWu, Wei-Ting, Ke-Vin Chang, Kamal Mezian, Vincenzo Ricci, and Levent Özçakar. 2025. "Integrating Ultrasound-Guided Injections and Peripheral Magnetic Stimulation in Chronic Myofascial/Lumbar Pain" Life 15, no. 4: 563. https://doi.org/10.3390/life15040563
APA StyleWu, W.-T., Chang, K.-V., Mezian, K., Ricci, V., & Özçakar, L. (2025). Integrating Ultrasound-Guided Injections and Peripheral Magnetic Stimulation in Chronic Myofascial/Lumbar Pain. Life, 15(4), 563. https://doi.org/10.3390/life15040563






