Centella asiatica: Advances in Extraction Technologies, Phytochemistry, and Therapeutic Applications
Abstract
1. Introduction
Literature Search Strategy
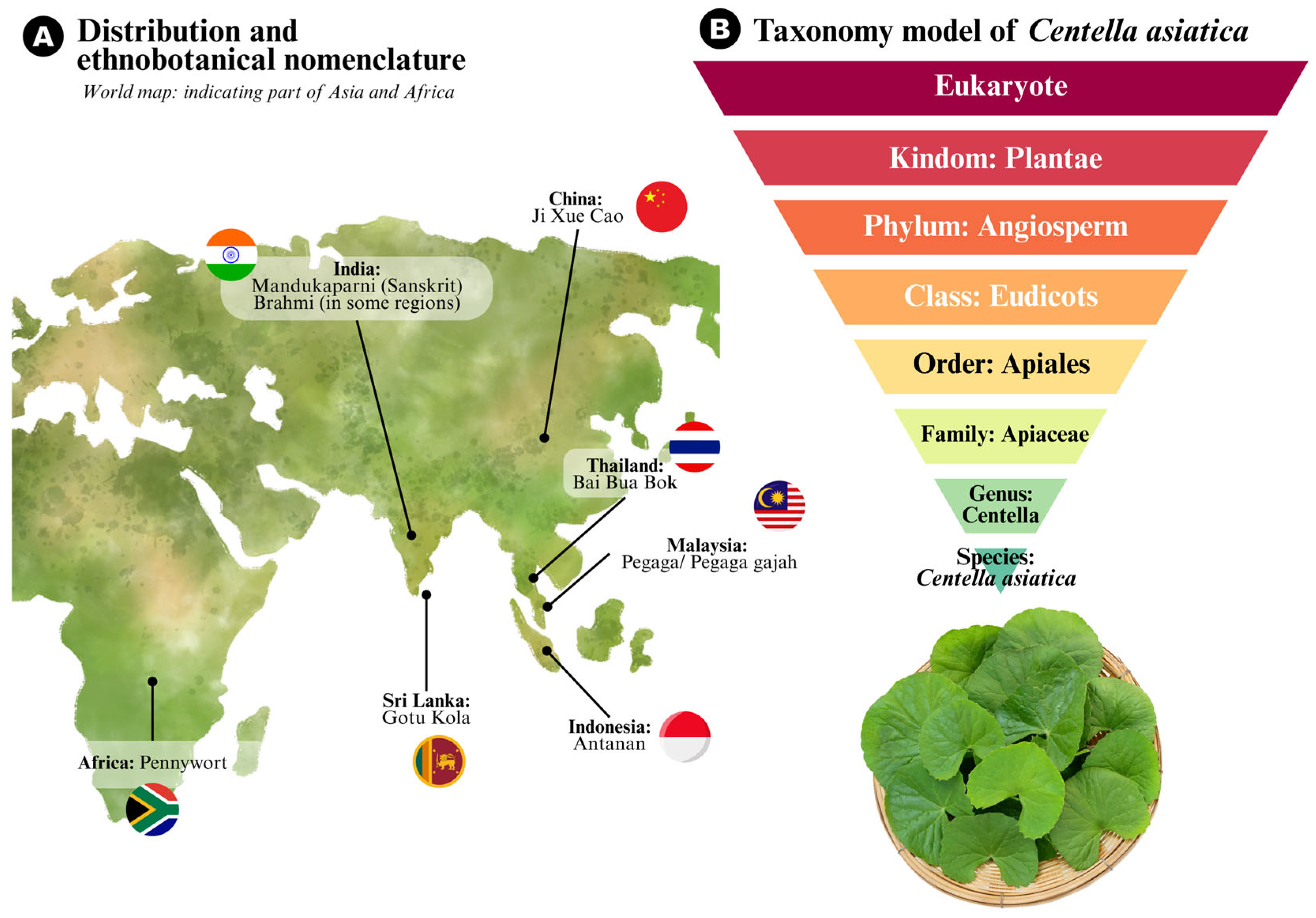
2. Global Distribution, Ecological Adaptability, and Cultural Significance
3. Taxonomy and Botanical Description
4. Conservation Status
5. Phytochemistry of C. asiatica
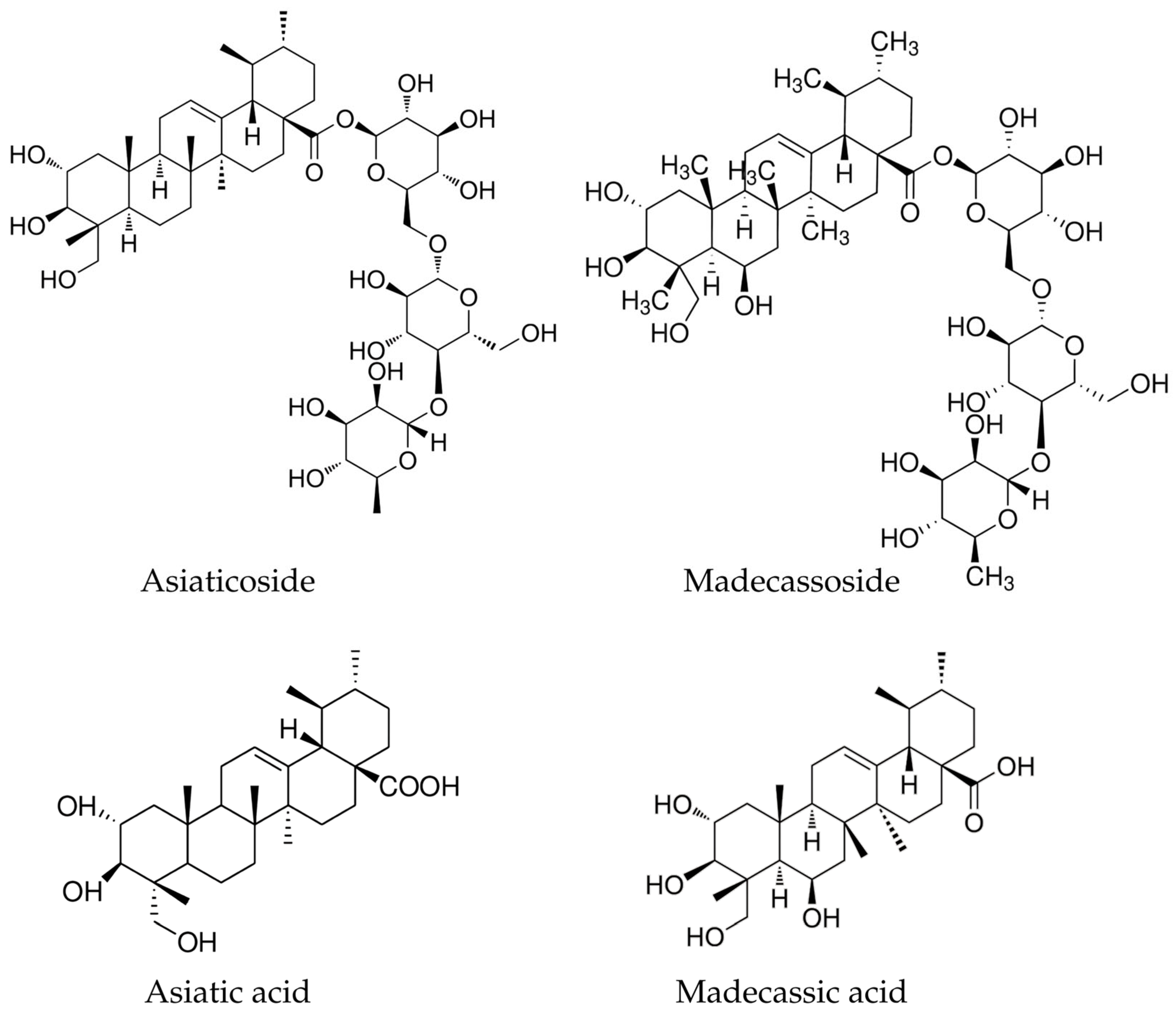
5.1. Triterpenoid Saponins: Signature Phytochemicals
5.1.1. Asiaticoside
5.1.2. Madecassoside
5.1.3. Asiatic Acid and Madecassic Acid
5.2. Flavonoids and Other Polyphenols
5.3. Alkaloids
5.4. Essential Oils and Volatile Compounds
5.5. Phytosterols
5.6. Polyacetylenes
5.7. Biosynthesis and Biotechnological Perspectives
5.8. Chemotypic Variation and Standardization Challenges
6. Advanced Extraction Techniques
6.1. Supercritical Fluid Extraction (SFE)
6.2. Microwave-Assisted Extraction (MAE)
6.3. Ultrasound-Assisted Extraction (UAE)
6.4. Enzyme-Assisted Extraction (EAE)
6.5. Pressurized Liquid Extraction (PLE)
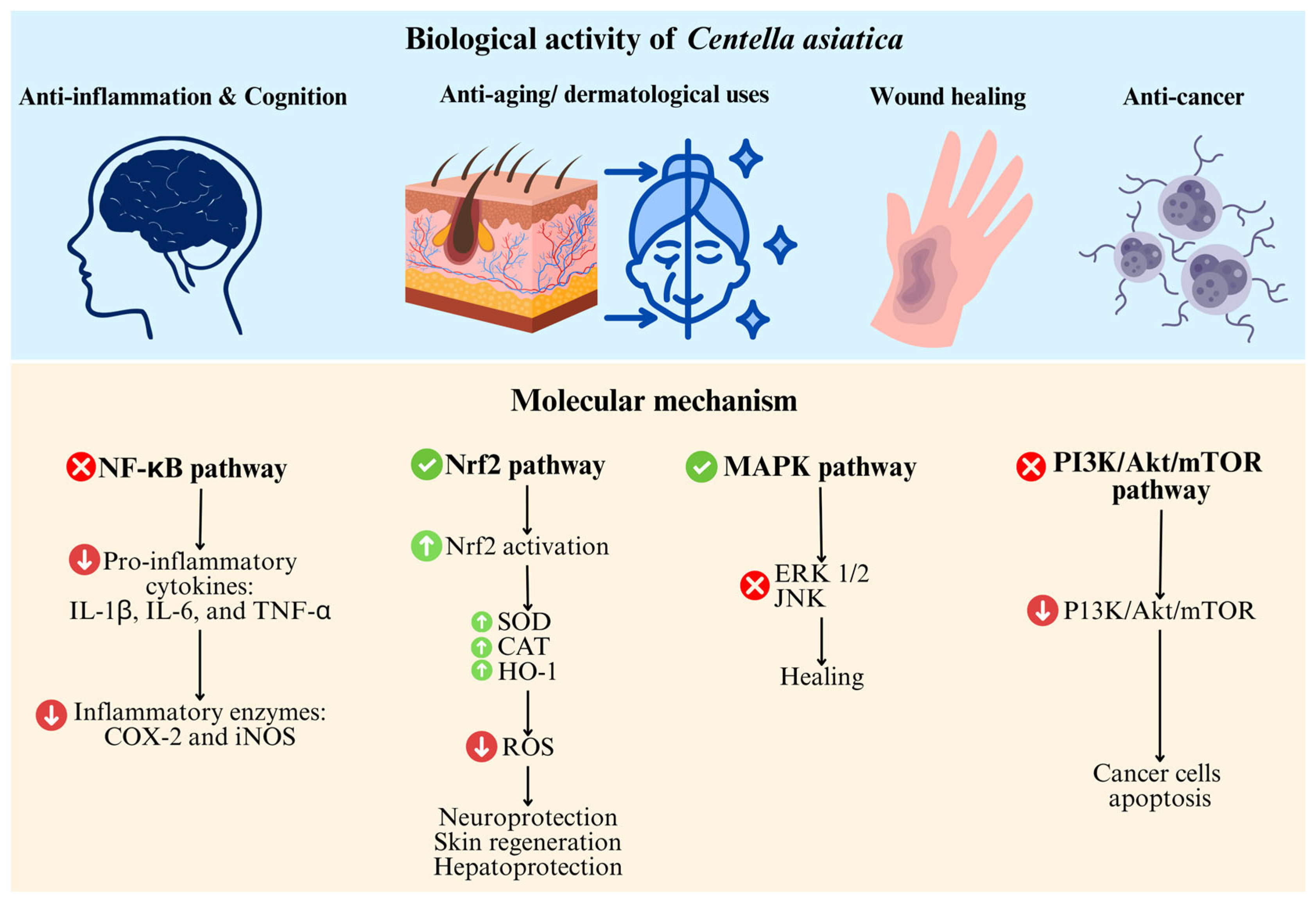
7. Therapeutic Applications
7.1. Molecular Mechanisms Underpinning Therapeutic Actions
7.2. Clinical Evidence and Meta-Analyses
7.3. Pharmacokinetics and Dosage Considerations
7.4. Limitations and Controversies
7.5. Safety and Drug-Herb Interactions
7.6. Regulatory Perspectives and Quality Control
8. Healthcare and Commercial Applications
9. Future Prospects
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AFLP | Amplified fragment length polymorphism |
| BDNF | Brain-derived neurotrophic factor |
| CAT | Catalase |
| CBD | Convention on Biological Diversity |
| CO2 | Carbon dioxide |
| COX-2 | Cyclooxygenase-2 |
| EAE | Enzyme-assisted extraction |
| ERK1/2 | Extracellular signal-regulated kinases 1 or 2 |
| FGF | Fibroblast growth factor |
| GACP | Good Agricultural and Collection Practices |
| GC-MS | Gas chromatography-Mass spectrometry |
| GMP | Good Manufacturing Practices |
| HO-1 | Heme oxygenase-1 |
| HPLC | High-performance liquid chromatography |
| IL-1β | Interleukin-1β |
| iNOS | Inducible nitric oxide synthase |
| IUCN | International Union for Conservation of Nature |
| JNK | c-Jun N-terminal kinases |
| LC-MS | Liquid chromatography-Mass spectrometry |
| MAE | Microwave-assisted extraction |
| MAPK | Mitogen-activated protein kinases |
| MMP | Matrix metalloproteinase |
| MS | Mass spectrometry |
| mTOR | Mammalian target of rapamycin |
| NF-κB | Nuclear factor kappa B |
| NMR | Nuclear magnetic resonance |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| NSAIDs | Nonsteroidal anti-inflammatory drugs |
| OH | Hydroxyl |
| PI3K | Phosphatidylinositol 3-kinase |
| PI3K/Akt | Phosphatidylinositol 3-kinase/protein kinase B |
| PLE | Pressurized liquid extraction |
| RAPD | Randomly amplified polymorphic DNA |
| ROS | Reactive oxygen species |
| SFE | Supercritical fluid extraction |
| SOD | Superoxide dismutase |
| SSR | Simple sequence repeats |
| TGF-β1 | Transforming growth factor beta 1 |
| TNF-α | Tumor necrosis factor alpha |
| UAE | Ultrasound-assisted extraction |
| UPLC | ultra-performance liquid chromatography |
| VEGF | Vascular endothelial growth factor |
| WHO | World Health Organization |
References
- Orhan, I.E. Centella asiatica (L.) Urban: From Traditional Medicine to Modern Medicine with Neuroprotective Potential. Evid.-Based Complement. Altern. Med. 2012, 2012, 946259. [Google Scholar] [CrossRef] [PubMed]
- Gbolahan, B.; Abiola, A.; Kamaldin, J.; Ahmad, M.; Atanassova, M. Accession in Centella asiatica; Current Understanding and Future Knowledge. J. Pure Appl. Microbiol. 2016, 10, 2485–2494. [Google Scholar] [CrossRef]
- Bandopadhyay, S.; Mandal, S.; Ghorai, M.; Jha, N.K.; Kumar, M.; Radha; Ghosh, A.; Proćków, J.; de la Lastra, J.M.P.; Dey, A. Therapeutic properties and pharmacological activities of asiaticoside and madecassoside: A review. J. Cell. Mol. Med. 2023, 27, 593–608. [Google Scholar] [CrossRef]
- Wright, K.M.; McFerrin, J.; Magaña, A.A.; Roberts, J.; Caruso, M.; Kretzschmar, D.; Stevens, J.F.; Maier, C.S.; Quinn, J.F.; Soumyanath, A. Developing a Rational, Optimized Product of Centella asiatica for Examination in Clinical Trials: Real World Challenges. Front. Nutr. 2022, 8, 799137. [Google Scholar] [CrossRef] [PubMed]
- Idris, F.N.; Nadzir, M.M. Comparative Studies on Different Extraction Methods of Centella asiatica and Extracts Bioactive Compounds Effects on Antimicrobial Activities. Antibiotics 2021, 10, 457. [Google Scholar] [CrossRef]
- Pillai, A.R.S.; Bhosale, Y.K.; Roy, S.; Parise, A. Extraction of Bioactive Compounds From Centella asiatica and Enlightenment of Its Utilization Into Food Packaging: A Review. Int. J. Food Sci. 2024, 2024, 1249553. [Google Scholar] [CrossRef] [PubMed]
- Gray, N.E.; Magana, A.A.; Lak, P.; Wright, K.M.; Quinn, J.; Stevens, J.F.; Maier, C.S.; Soumyanath, A. Centella asiatica: Phytochemistry and mechanisms of neuroprotection and cognitive enhancement. Phytochem. Rev. 2018, 17, 161–194. [Google Scholar] [CrossRef]
- Yang, L.; Marney, L.; Magana, A.A.; Choi, J.; Wright, K.; Mcferrin, J.; Gray, N.E.; Soumyanath, A.; Stevens, J.F.; Maier, C.S. Quantification of Caffeoylquinic Acids and Triterpenes as Targeted Bioactive Compounds of Centella asiatica in Extracts and Formulations by Liquid Chromatography Mass Spectrometry. J. Chromatogr. Open 2023, 4, 100091. [Google Scholar] [CrossRef]
- Kandasamy, A.; Aruchamy, K.; Rangasamy, P.; Varadhaiyan, D.; Gowri, C.; Oh, T.H.; Ramasundaram, S.; Athinarayanan, B. Phytochemical Analysis and Antioxidant Activity of Centella asiatica Extracts: An Experimental and Theoretical Investigation of Flavonoids. Plants 2023, 12, 3547. [Google Scholar] [CrossRef]
- Xie, D.-F.; Xie, C.; Ren, T.; Song, B.-N.; Zhou, S.-D.; He, X.-J. Plastid phylogenomic insights into relationships, divergence, and evolution of Apiales. Planta 2022, 256, 1–19. [Google Scholar] [CrossRef]
- Theerawitaya, C.; Praseartkul, P.; Taota, K.; Tisarum, R.; Samphumphuang, T.; Singh, H.P.; Cha-Um, S. Investigating high throughput phenotyping based morpho-physiological and biochemical adaptations of indian pennywort (Centella asiatica L. urban) in response to different irrigation regimes. Plant Physiol. Biochem. 2023, 202, 107927. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, N.; Ibrahim, M.H.; Izad, A.A.; Zainal, B.; Zain, N.A.M. Morphology, Leaf Gas Exchange and Quality of Pegaga (Centella asiatica) under Different Nitrogen Fertilization Rates. Annu. Res. Rev. Biol. 2018, 28, 1–11. [Google Scholar] [CrossRef]
- Liew, K.Y.; Hafiz, F.; Chong, Y.J.; Harith, H.H.; Israf, D.A.; Tham, C.L.; Wilkinson, J.M. A Review of Malaysian Herbal Plants and Their Active Constituents with Potential Therapeutic Applications in Sepsis. Evid.-Based Complement. Altern. Med. 2020, 2020, 8257817. [Google Scholar] [CrossRef]
- Rahajanirina, V.; Raoseta, S.O.R.; Roger, E.; Razafindrazaka, H.; Pirotais, S.; Boucher, M.; Danthu, P. The Influence of Certain Taxonomic and Environmental Parameters on Biomass Production and Triterpenoid Content in the Leaves of Centella asiatica (L.) Urb. from Madagascar. Chem. Biodivers. 2012, 9, 298–308. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, D.; Luo, J.; Yao, S.; Chen, J.; Li, L.; Geng, J.; Mo, Y.; Ming, R.; Liu, J. The chromosome-level genome of Centella asiatica provides insights into triterpenoid biosynthesis. Plant Physiol. Biochem. 2025, 222, 109710. [Google Scholar] [CrossRef]
- Ho, T.H.N.; Rasphone, S.; Chanthanousone, H.; Nguyen, B.L.Q.; Le, M.H.D.; Truong, H.T.H. Genetic diversity analysis of Centella asiatica L. Urban in Vietnam with RAPD marker. Hue Univ. J. Sci. Nat. Sci. 2023, 132, 101–112. [Google Scholar] [CrossRef]
- Prasad, A.; Dhawan, S.S.; Mathur, A.K.; Prakash, O.; Gupta, M.M.; Verma, R.K.; Lal, R.K.; Mathur, A. Morphological, Chemical and Molecular Characterization of Centella asiatica Germplasms for Commercial Cultivation in the Indo-Gangetic Plains. Nat. Prod. Commun. 2014, 9, 779–784. [Google Scholar] [CrossRef]
- Alqahtani, A.; Cho, J.-L.; Wong, K.H.; Li, K.M.; Razmovski-Naumovski, V.; Li, G.Q. Differentiation of Three Centella Species in Australia as Inferred from Morphological Characteristics, ISSR Molecular Fingerprinting and Phytochemical Composition. Front. Plant Sci. 2017, 8, 1980. [Google Scholar] [CrossRef]
- Prasad, A.; Singh, M.; Yadav, N.P.; Mathur, A.K.; Mathur, A. Molecular, chemical and biological stability of plants derived from artificial seeds of Centella asiatica (L.) Urban—An industrially important medicinal herb. Ind. Crop. Prod. 2014, 60, 205–211. [Google Scholar] [CrossRef]
- Nishteswar, K. Depleting medicinal plant resources: A threat for survival of Ayurveda. Ayu 2014, 35, 349–350. [Google Scholar] [CrossRef]
- Chen, S.-L.; Yu, H.; Luo, H.-M.; Wu, Q.; Li, C.-F.; Steinmetz, A. Conservation and sustainable use of medicinal plants: Problems, progress, and prospects. Chin. Med. 2016, 11, 37. [Google Scholar] [CrossRef] [PubMed]
- Rawat, A.K.S.; Tewari, S.K. Quality Assurance of Medicinal and Aromatic Plants-Good Agricultural and Collection Practices (GAP & GCP). In Medicinal and Aromatic Plants of the World; Máthé, Á., Ed.; Springer: Dordrecht, The Netherlands, 2015; Volume 1. [Google Scholar] [CrossRef]
- Yang, G.; Xu, J.; Chi, X.L.; Zang, C.X.; Que, L. Influence of Nagoya Protocol on traditional Chinese medicine. Zhong Guo Zhong Yao Za Zhi 2018, 43, 396–400. (In Chinese) [Google Scholar] [CrossRef]
- Coolsaet, B.; Dedeurwaerdere, T.; Pitseys, J. The Challenges for Implementing the Nagoya Protocol in a Multi-Level Governance Context: Lessons from the Belgian Case. Resources 2013, 2, 555–580. [Google Scholar] [CrossRef]
- James, J.T.; Dubery, I.A. Pentacyclic triterpenoids from the medicinal herb, Centella asiatica (L.) Urban. Molecules 2009, 14, 3922–3941. [Google Scholar] [CrossRef] [PubMed]
- Songvut, P.; Chariyavilaskul, P.; Khemawoot, P.; Tansawat, R. Pharmacokinetics and metabolomics investigation of an orally modified formula of standardized Centella asiatica extract in healthy volunteers. Sci. Rep. 2021, 11, 1–13. [Google Scholar] [CrossRef]
- Yuan, Y.; Qiao, Y.; Zheng, X.; Yu, X.; Dong, Y.; Wang, H.; Sun, L. Simultaneous determination of four active compounds in Centella asiatica by supramolecular solvent-based extraction coupled with high performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2023, 1708, 464298. [Google Scholar] [CrossRef]
- Zakaria, F.; Akhtar, M.T.; Norhamidah, W.I.W.; Noraini, A.B.; Muhamad, A.; Shohaimi, S.; Maulidiani; Ahmad, H.; Ismail, I.S.; Ismail, N.H.; et al. Centella asiatica (L.) Urb. Extract ameliorates branched-chain amino acid (BCAA) metabolism in acute reserpine-induced stress zebrafish model via 1H Nuclear Magnetic Resonance (NMR)-based metabolomics approach. Comp. Biochem. Physiol. Part. C: Toxicol. Pharmacol. 2023, 264, 109501. [Google Scholar] [CrossRef]
- Ondeko, D.A.; Juma, B.F.; Baraza, L.D.; Nyongesa, P.K. LC-ESI/MS and GC-MS methanol extract analysis, phytochemical and antimicrobial activity studies of Centella asiatica. Asian J. Chem. Sci. 2020, 8, 32–51. [Google Scholar] [CrossRef]
- Hashim, P.; Sidek, H.; Helan, M.H.M.; Sabery, A.; Palanisamy, U.D.; Ilham, M. Triterpene Composition and Bioactivities of Centella asiatica. Molecules 2011, 16, 1310–1322. [Google Scholar] [CrossRef]
- Shukla, A.; Rasik, A.; Jain, G.; Shankar, R.; Kulshrestha, D.; Dhawan, B. In vitro and in vivo wound healing activity of asiaticoside isolated from Centella asiatica. J. Ethnopharmacol. 1999, 65, 1–11. [Google Scholar] [CrossRef]
- He, Z.; Hu, Y.; Niu, Z.; Zhong, K.; Liu, T.; Yang, M.; Ji, L.; Hu, W. A review of pharmacokinetic and pharmacological properties of asiaticoside, a major active constituent of Centella asiatica (L.) Urb. J. Ethnopharmacol. 2023, 302, 115865. [Google Scholar] [CrossRef] [PubMed]
- Zhou, A.; Feng, H.-Y.; Fan, C.-N.; Wang, J.; Yuan, Z.-Y.; Xu, G.-H.; Li, C.-F.; Huang, W.-F.; Yi, L.-T. Asiaticoside attenuates chronic restraint stress-induced hippocampal CA1 neuronal ferroptosis via activating BDNF/Nrf2/GPX4 signaling pathway. Drug Des. Dev. Ther. 2025, 19, 793–810. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Das, T.; Nandy, S.; Ghorai, M.; Saha, S.C.; Gopalakrishnan, A.V.; Kumar, M.; Radha; Ghosh, A.; Mukerjee, N.; et al. Biotechnological and endophytic-mediated production of centellosides in Centella asiatica. Appl. Microbiol. Biotechnol. 2023, 107, 473–489. [Google Scholar] [CrossRef] [PubMed]
- Won, J.-H.; Shin, J.-S.; Park, H.-J.; Jung, H.-J.; Koh, D.-J.; Jo, B.-G.; Lee, J.-Y.; Yun, K.; Lee, K.-T. Anti-inflammatory effects of madecassic acid via the suppression of NF-κB pathway in LPS-induced RAW 264.7 macrophage cells. Planta Medica 2010, 76, 251–257. [Google Scholar] [CrossRef]
- Jung, E.; Lee, J.-A.; Shin, S.; Roh, K.-B.; Kim, J.-H.; Park, D. Madecassoside inhibits melanin synthesis by blocking ultraviolet-induced inflammation. Molecules 2013, 18, 15724–15736. [Google Scholar] [CrossRef]
- Lu, W.; Luo, D.; Chen, D.; Zhang, S.; Chen, X.; Zhou, H.; Liu, Q.; Chen, S.; Liu, W. Systematic study of paeonol/madecassoside co-delivery nanoemulsion transdermal delivery system for enhancing barrier repair and anti-inflammatory efficacy. Molecules 2023, 28, 5275. [Google Scholar] [CrossRef]
- Tan, S.C.; Bhattamisra, S.K.; Chellappan, D.K.; Candasamy, M. Actions and therapeutic potential of madecassoside and other major constituents of Centella asiatica: A review. Appl. Sci. 2021, 11, 8475. [Google Scholar] [CrossRef]
- Razali, N.N.M.; Ng, C.T.; Fong, L.Y. Cardiovascular protective effects of Centella asiatica and its triterpenes: A review. Planta Medica 2019, 85, 1203–1215. [Google Scholar] [CrossRef]
- Kraft, O.; Hartmann, A.-K.; Hoenke, S.; Serbian, I.; Csuk, R. Madecassic acid—A new scaffold for highly cytotoxic agents. Int. J. Mol. Sci. 2022, 23, 4362. [Google Scholar] [CrossRef]
- Ganie, I.B.; Ahmad, Z.; Shahzad, A.; Zaushintsena, A.; Neverova, O.; Ivanova, S.; Wasi, A.; Tahseen, S. Biotechnological Intervention and Secondary Metabolite Production in Centella asiatica L. Plants 2022, 11, 2928. Plants 2022, 11, 2928. [Google Scholar] [CrossRef]
- Lim, J.; Lee, H.; Hong, S.; Lee, J.; Kim, Y. comparison of the antioxidant potency of four triterpenes of Centella asiatica against oxidative stress. Antioxidants 2024, 13, 483. [Google Scholar] [CrossRef] [PubMed]
- Sul, O.-J.; Ra, S.W. Quercetin prevents LPS-induced oxidative stress and inflammation by modulating NOX2/ROS/NF-kB in lung epithelial cells. Molecules 2021, 26, 6949. [Google Scholar] [CrossRef] [PubMed]
- Domitrović, R.; Jakovac, H.; Marchesi, V.V.; Vladimir-Knežević, S.; Cvijanović, O.; Tadić, Ž.; Romić, Ž.; Rahelić, D. Differential hepatoprotective mechanisms of rutin and quercetin in CCl4-intoxicated BALB/cN mice. Acta Pharmacol. Sin. 2012, 33, 1260–1270. [Google Scholar] [CrossRef]
- Khan, H.; Ullah, H.; Aschner, M.; Cheang, W.S.; Akkol, E.K. Neuroprotective effects of quercetin in alzheimer’s disease. Biomolecules 2020, 10, 59. [Google Scholar] [CrossRef]
- Chatterjee, J.; Atmuri, A.; Raichur, E.J.; Anil, G. Asiatic acid, quercetin, and kaempferol from Centella asiatica as potential inhibitors of alpha-1-antichymotrypsin in Alzheimer’s disease. Nat. Prod. Commun. 2024, 19, 1934578X241264637. [Google Scholar] [CrossRef]
- Michalak, M. Plant-derived antioxidants: Significance in skin health and the ageing process. Int. J. Mol. Sci. 2022, 23, 585. [Google Scholar] [CrossRef]
- Peres, D.A.; de Oliveira, C.A.; Da Costa, M.S.; Tokunaga, V.K.; Mota, J.P.; Rosado, C.F.; Consiglieri, V.O.; Kaneko, T.M.; Velasco, M.V.R.; Baby, A.R. Rutin increases critical wavelength of systems containing a single UV filter and with good skin compatibility. Ski. Res. Technol. 2016, 22, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.-H.; Jaremko, M. Important flavonoids and their role as a therapeutic agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef]
- Lokanathan, Y.; Omar, N.; Puzi, N.N.A.; Saim, A.; Idrus, R.H. Recent updates in neuroprotective and neuroregenerative potential of Centella asiatica. Malays. J. Med. Sci. 2016, 23, 4–14. [Google Scholar]
- Rashid, H.-O.; Akter, M.M.; Uddin, J.; Islam, S.; Rahman, M.; Jahan, K.; Sarker, M.M.R.; Sadik, G. Antioxidant, cytotoxic, antibacterial and thrombolytic activities of Centella asiatica L.: Possible role of phenolics and flavonoids. Clin. Phytoscience 2023, 9, 1–9. [Google Scholar] [CrossRef]
- Kunjumon, R.; Johnson, A.J.; Baby, S. Centella asiatica: Secondary metabolites, biological activities and biomass sources. Phytomedicine Plus 2022, 2, 100176. [Google Scholar] [CrossRef]
- Hafiz, Z.Z.; Amin, M.‘.M.; James, R.M.J.; Teh, L.K.; Salleh, M.Z.; Adenan, M.I. Inhibitory effects of raw-extract Centella asiatica (RECA) on acetylcholinesterase, inflammations, and oxidative stress activities via in vitro and in vivo. Molecules 2020, 25, 892. [Google Scholar] [CrossRef] [PubMed]
- Murray, A.P.; Faraoni, M.B.; Castro, M.J.; Alza, N.P.; Cavallaro, V. Natural AChE inhibitors from plants and their contribution to alzheimer’s disease therapy. Curr. Neuropharmacol. 2013, 11, 388–413. [Google Scholar] [CrossRef]
- Joshi, V.P.; Kumar, N.; Singh, B.; Chamoli, R.P. Chemical composition of the essential oil of Centella asiatica (L.) Urb. from Western Himalaya. Nat. Prod. Commun. 2007, 2, 1934578X0700200515. [Google Scholar] [CrossRef]
- Retnaningtyas, E.; Susatia, B.; Khotimah, H.; Rudijanto, A.; Abousouh, A.A.A.; Setiawan, A. Centella asiatica transfersomes and Bergamot essential oil nanoemulsion combined in gel exhibited anti-photoaging effects on UVB-radiated BALB/c mice. J. King Saud. Univ.-Sci. 2024, 36, 103207. [Google Scholar] [CrossRef]
- Miszczuk, E.; Bajguz, A.; Kiraga, Ł.; Crowley, K.; Chłopecka, M. Phytosterols and the digestive system: A review study from insights into their potential health benefits and safety. Pharmaceuticals 2024, 17, 557. [Google Scholar] [CrossRef]
- Feng, S.; Dai, Z.; Liu, A.B.; Huang, J.; Narsipur, N.; Guo, G.; Kong, B.; Reuhl, K.; Lu, W.; Luo, Z.; et al. Intake of stigmasterol and β-sitosterol alters lipid metabolism and alleviates NAFLD in mice fed a high-fat western-style diet. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2018, 1863, 1274–1284. [Google Scholar] [CrossRef]
- Shen, M.; Yuan, L.; Zhang, J.; Wang, X.; Zhang, M.; Li, H.; Jing, Y.; Zeng, F.; Xie, J. Phytosterols: Physiological functions and potential application. Foods 2024, 13, 1754. [Google Scholar] [CrossRef]
- Hu, Q.; Zhuo, Z.; Fang, S.; Zhang, Y.; Feng, J. Phytosterols improve immunity and exert anti-inflammatory activity in weaned piglets. J. Sci. Food Agric. 2017, 97, 4103–4109. [Google Scholar] [CrossRef]
- Scherer, S.S.; Pietramaggiori, G.; Matthews, J.; Perry, S.; Assmann, A.; Carothers, A.; Demcheva, M.; Muise-Helmericks, R.C.; Seth, A.; Vournakis, J.N.; et al. Poly-N-Acetyl Glucosamine Nanofibers: A new bioactive material to enhance diabetic wound healing by cell migration and angiogenesis. Ann. Surg. 2009, 250, 322–330. [Google Scholar] [CrossRef]
- Li, R.; Liu, K.; Huang, X.; Li, D.; Ding, J.; Liu, B.; Chen, X. Bioactive materials promote wound healing through modulation of cell behaviors. Adv. Sci. 2022, 9, e2105152. [Google Scholar] [CrossRef] [PubMed]
- Divekar, P.A.; Narayana, S.; Divekar, B.A.; Kumar, R.; Gadratagi, B.G.; Ray, A.; Singh, A.K.; Rani, V.; Singh, V.; Singh, A.K.; et al. Plant secondary metabolites as defense tools against herbivores for sustainable crop protection. Int. J. Mol. Sci. 2022, 23, 2690. [Google Scholar] [CrossRef]
- Minto, R.E.; Blacklock, B.J. Biosynthesis and function of polyacetylenes and allied natural products. Prog. Lipid Res. 2008, 47, 233–306. [Google Scholar] [CrossRef]
- Wan, L.; Huang, Q.; Li, C.; Yu, H.; Tan, G.; Wei, S.; El-Sappah, A.H.; Sooranna, S.; Zhang, K.; Pan, L.; et al. Integrated metabolome and transcriptome analysis identifies candidate genes involved in triterpenoid saponin biosynthesis in leaves of Centella asiatica (L.) Urban. Front. Plant Sci. 2024, 14, 1295186. [Google Scholar] [CrossRef]
- Baek, S.; Han, J.-E.; Ho, T.-T.; Park, S.-Y. Development of hairy root cultures for biomass and triterpenoid production in Centella asiatica. Plants 2022, 11, 148. [Google Scholar] [CrossRef] [PubMed]
- Confalonieri, M.; Carelli, M.; Gianoglio, S.; Moglia, A.; Biazzi, E.; Tava, A. CRISPR/Cas9-mediated targeted mutagenesis of CYP93E2 modulates the triterpene saponin biosynthesis in Medicago truncatula. Front. Plant Sci. 2021, 12, 690231. [Google Scholar] [CrossRef] [PubMed]
- Sneha, V.; Pooja, A.; Amrita, D.; Divya, K.; Viswanatha, C.K. Antimicrobial and Antioxidant Activities in the Root, Stem and Leaf Extracts of Centella asiatica. Adv. Biotechnol. Microbiol. 2017, 3, 555618. [Google Scholar] [CrossRef]
- Sabaragamuwa, R.; Perera, C.O. Total triterpenes, polyphenols, flavonoids, and antioxidant activity of bioactive phytochemicals of Centella asiatica by different extraction techniques. Foods 2023, 12, 3972. [Google Scholar] [CrossRef]
- Kumari, K.; Menghani, E. Standardization and validation: A tool for identification and generation of features for herbal for-mulations. Afr. J. Biol. Sci. 2024, 6, 3013–3029. [Google Scholar] [CrossRef]
- Uwineza, P.A.; Waśkiewicz, A. Recent advances in supercritical fluid extraction of natural bioactive compounds from natural plant materials. Molecules 2020, 25, 3847. [Google Scholar] [CrossRef]
- Wrona, O.; Rafińska, K.; Możeński, C.; Buszewski, B. Supercritical fluid extraction of bioactive compounds from plant materials. J. AOAC Int. 2017, 100, 1624–1635. [Google Scholar] [CrossRef] [PubMed]
- Phaisan, S.; Makkliang, F.; Putalun, W.; Sakamoto, S.; Yusakul, G. Development of a colorless Centella asiatica (L.) Urb. extract using a natural deep eutectic solvent (NADES) and microwave-assisted extraction (MAE) optimized by response surface methodology. RSC Adv. 2021, 11, 8741–8750. [Google Scholar] [CrossRef]
- Akhtar, I.; Javad, S.; Yousaf, Z.; Iqbal, S.; Jabeen, K. Review: Microwave assisted extraction of phytochemicals an efficient and modern approach for botanicals and pharmaceuticals. Pak. J. Pharm. Sci. 2019, 32, 223–230. [Google Scholar] [PubMed]
- Thong-On, W.; Pathomwichaiwat, T.; Boonsith, S.; Koo-Amornpattana, W.; Prathanturarug, S. Green extraction optimization of triterpenoid glycoside-enriched extract from Centella asiatica (L.) Urban using response surface methodology (RSM). Sci. Rep. 2021, 11, 22026. [Google Scholar] [CrossRef]
- Kumar, K.; Srivastav, S.; Sharanagat, V.S. Ultrasound assisted extraction (UAE) of bioactive compounds from fruit and vegetable processing by-products: A review. Ultrason. Sonochem. 2021, 70, 105325. [Google Scholar] [CrossRef]
- Łubek-Nguyen, A.; Ziemichód, W.; Olech, M. Application of enzyme-assisted extraction for the recovery of natural bioactive compounds for nutraceutical and pharmaceutical applications. Appl. Sci. 2022, 12, 3232. [Google Scholar] [CrossRef]
- Streimikyte, P.; Viskelis, P.; Viskelis, J. Enzymes-assisted extraction of plants for sustainable and functional applications. Int. J. Mol. Sci. 2022, 23, 2359. [Google Scholar] [CrossRef]
- Wen, L.; Zhang, Z.; Sun, D.-W.; Sivagnanam, S.P.; Tiwari, B.K. Combination of emerging technologies for the extraction of bioactive compounds. Crit. Rev. Food Sci. Nutr. 2020, 60, 1826–1841. [Google Scholar] [CrossRef]
- Perez-Vazquez, A.; Carpena, M.; Barciela, P.; Cassani, L.; Simal-Gandara, J.; Prieto, M.A. Pressurized liquid extraction for the recovery of bioactive compounds from seaweeds for food industry application: A review. Antioxidants 2023, 12, 612. [Google Scholar] [CrossRef]
- Ruksiriwanich, W.; Khantham, C.; Sringarm, K.; Sommano, S.; Jantrawut, P. Depigmented Centella asiatica extraction by pretreated with supercritical carbon dioxide fluid for wound healing application. Processes 2020, 8, 277. [Google Scholar] [CrossRef]
- Sun, B.; Wu, L.; Wu, Y.; Zhang, C.; Qin, L.; Hayashi, M.; Kudo, M.; Gao, M.; Liu, T. Therapeutic potential of Centella asiatica and its triterpenes: A review. Front. Pharmacol. 2020, 11, 568032. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Choi, J.Y.; Son, D.J.; Park, E.K.; Song, M.J.; Hellström, M.; Hong, J.T. Anti-inflammatory effect of titrated extract of Centella asiatica in phthalic anhydride-induced allergic dermatitis animal model. Int. J. Mol. Sci. 2017, 18, 738. [Google Scholar] [CrossRef]
- Sukketsiri, W.; Tanasawet, S.; Moolsap, F.; Tantisira, M.H.; Hutamekalin, P.; Tipmanee, V. ECa 233 suppresses LPS-induced proinflammatory responses in macrophages via suppressing ERK1/2, p38 MAPK and Akt pathways. Biol. Pharm. Bull. 2019, 42, 1358–1365. [Google Scholar] [CrossRef]
- Sasmita, A.O.; Ling, A.P.K.; Voon, K.G.L.; Koh, R.Y.; Wong, Y.P. Madecassoside activates anti-neuroinflammatory mechanisms by inhibiting lipopolysaccharide-induced microglial inflammation. Int. J. Mol. Med. 2018, 41, 3033–3040. [Google Scholar] [CrossRef] [PubMed]
- Giribabu, N.; Karim, K.; Kilari, E.K.; Nelli, S.R.; Salleh, N. Oral administration of Centella asiatica (L.) Urb leave aqueous extract ameliorates cerebral oxidative stress, inflammation, and apoptosis in male rats with type-2 diabetes. Inflammopharmacology 2020, 28, 1599–1622. [Google Scholar] [CrossRef] [PubMed]
- Buranasudja, V.; Rani, D.; Malla, A.; Kobtrakul, K.; Vimolmangkang, S. Insights into antioxidant activities and anti-skin-aging potential of callus extract from Centella asiatica (L.). Sci. Rep. 2021, 11, 13459. [Google Scholar] [CrossRef]
- Lv, J.; Sharma, A.; Zhang, T.; Wu, Y.; Ding, X. Pharmacological review on asiatic acid and its derivatives: A potential compound. JALA J. Assoc. Lab. Autom. 2018, 23, 111–127. [Google Scholar] [CrossRef]
- Wiciński, M.; Fajkiel-Madajczyk, A.; Kurant, Z.; Gajewska, S.; Kurant, D.; Kurant, M.; Sousak, M. Can asiatic acid from Centella asiatica be a potential remedy in cancer therapy?—A review. Cancers 2024, 16, 1317. [Google Scholar] [CrossRef]
- Hao, Y.; Huang, J.; Ma, Y.; Chen, W.; Fan, Q.; Sun, X.; Shao, M.; Cai, H. Asiatic acid inhibits proliferation, migration and induces apoptosis by regulating Pdcd4 via the PI3K/Akt/mTOR/p70S6K signaling pathway in human colon carcinoma cells. Oncol. Lett. 2018, 15, 8223–8230. [Google Scholar] [CrossRef]
- Puttarak, P.; Dilokthornsakul, P.; Saokaew, S.; Dhippayom, T.; Kongkaew, C.; Sruamsiri, R.; Chuthaputti, A.; Chaiyakunapruk, N. Effects of Centella asiatica (L.) Urb. on cognitive function and mood related outcomes: A Systematic Review and Meta-analysis. Sci. Rep. 2017, 7, 10646. [Google Scholar] [CrossRef]
- Varada, S.; Chamberlin, S.R.; Bui, L.; Brandes, M.S.; Gladen-Kolarsky, N.; Harris, C.J.; Gray, N.E. Oral Asiatic Acid Improves Cognitive Function and Modulates Antioxidant and Mitochondrial Pathways in Female 5xFAD Mice. Nutrients 2025, 17, 729. [Google Scholar] [CrossRef] [PubMed]
- Lawal, O.M.; Wakel, F.; Dekker, M. Consumption of fresh Centella asiatica improves short term alertness and contentedness in healthy females. J. Funct. Foods 2021, 77, 104337. [Google Scholar] [CrossRef]
- Arribas-López, E.; Zand, N.; Ojo, O.; Snowden, M.J.; Kochhar, T. A Systematic Review of the Effect of Centella asiatica on Wound Healing. Int. J. Environ. Res. Public. Heal. 2022, 19, 3266. [Google Scholar] [CrossRef]
- Liu, Y.-Q.; Zhang, D.; Deng, J.; Liu, Y.; Li, W.; Nie, X.; Haque, A. Preparation and Safety Evaluation of Centella asiatica Total Glycosides Nitric Oxide Gel and Its Therapeutic Effect on Diabetic Cutaneous Ulcers. Evid.-Based Complement. Altern. Med. 2022, 2022, 1419146. [Google Scholar] [CrossRef]
- Zhao, C.; Wu, S.; Wang, H. Medicinal Plant Extracts Targeting UV-Induced Skin Damage: Molecular Mechanisms and Therapeutic Potential. Int. J. Mol. Sci. 2025, 26, 2278. [Google Scholar] [CrossRef]
- Kuo, C.-W.; Chiu, Y.-F.; Wu, M.-H.; Li, M.-H.; Wu, C.-N.; Chen, W.-S.; Huang, C.-H. Gelatin/Chitosan Bilayer Patches Loaded with Cortex Phellodendron amurense/Centella asiatica Extracts for Anti-Acne Application. Polymers 2021, 13, 579. [Google Scholar] [CrossRef]
- Utami, A.T.; Muzaahim, A.; Harrats, A.H.; Imroon, M.A. Systematic Review and Meta-Analysis: Efficacy of Centella asiatica in Treating Acne Vulgaris. Biomed. J. Sci. Tech. Res. 2024, 58, 50034–50037. [Google Scholar] [CrossRef]
- Boonyarattanasoonthorn, T.; Kijtawornrat, A.; Songvut, P.; Nuengchamnong, N.; Buranasudja, V.; Khemawoot, P. Increase water solubility of Centella asiatica extract by indigenous bioenhancers could improve oral bioavailability and disposition kinetics of triterpenoid glycosides in beagle dogs. Sci. Rep. 2022, 12, 2909. [Google Scholar] [CrossRef] [PubMed]
- Vishnumukkala, T.; Kalerammana, P.; Karikalan, B.; Thomas, W.; Jagadeesan, S.; Chiroma, S.M.; Nor, N.H.M.; Moklas, M.A.M. Centella asiatica ameliorates AlCl3 and D-galactose induced nephrotoxicity in rats via modulation of oxidative stress. Bioinformation 2024, 20, 508–514. [Google Scholar] [CrossRef]
- Rao, B.V.; Heggade, R.H.B.; Jagannath, H.H. Ethanolic leaf extract of Centella asiatica L. possesses nephroprotection in an animal model of nephrotoxicity. Indian. J. Pharm. Educ. Res. 2024, 58, s274–s281. [Google Scholar] [CrossRef]
- Vishnumukkala, T.; Gopalakrishna, P.K.; Karikalan, B.; Jagadeesan, S.; Baharuldin, M.T.H.B.; Thomas, W.; Moklas, M.A.M. Protective effect of Centella asiatica on AlCl3 and D-galactose induced hepatotoxicity in rats through the alleviation of oxidative stress as demonstrated by histological changes in liver. Int. J. Anat. Res. 2023, 11, 8740–8747. [Google Scholar] [CrossRef]
- Choi, M.-J.; Zheng, H.-M.; Kim, J.M.; Lee, K.W.; Park, Y.H.; Lee, D.H. Protective effects of Centella asiatica leaf extract on dimethylnitrosamine-induced liver injury in rats. Mol. Med. Rep. 2016, 14, 4521–4528. [Google Scholar] [CrossRef]
- Hong, W.; Hwang-Bo, J.; Jeon, H.; Ko, M.; Choi, J.; Jeong, Y.-J.; Park, J.-H.; Kim, I.; Kim, T.-W.; Kim, H.; et al. A Comparative Study of the Hepatoprotective Effect of Centella asiatica Extract (CA-HE50) on Lipopolysaccharide/d-galactosamine-Induced Acute Liver Injury in C57BL/6 Mice. Nutrients 2021, 13, 4090. [Google Scholar] [CrossRef]
- Cheng, C.L.; Koo, M.W.L. Effects of Centella asiatica on ethanol induced gastric mucosal lesions in rats. Life Sci. 2000, 67, 2647–2653. [Google Scholar] [CrossRef]
- Zheng, H.-M.; Choi, M.-J.; Kim, J.M.; Cha, K.H.; Lee, K.W.; Park, Y.H.; Hong, S.-S.; Lee, D.H. Centella asiatica leaf extract protects against indomethacin-induced gastric mucosal injury in rats. J. Med. Food 2016, 19, 38–46. [Google Scholar] [CrossRef]
- Wattanathorn, J.; Mator, L.; Muchimapura, S.; Tongun, T.; Pasuriwong, O.; Piyawatkul, N.; Yimtae, K.; Sripanidkulchai, B.; Singkhoraard, J. Positive modulation of cognition and mood in the healthy elderly volunteer following the administration of Centella asiatica. J. Ethnopharmacol. 2008, 116, 325–332. [Google Scholar] [CrossRef]
- Somboonwong, J.; Kankaisre, M.; Tantisira, B.; Tantisira, M.H. Wound healing activities of different extracts of Centella asiatica in incision and burn wound models: An experimental animal study. BMC Complement. Altern. Med. 2012, 12, 103. [Google Scholar] [CrossRef] [PubMed]
- Ramsis, T.; Selim, H.M.R.M.; Elseedy, H.; Fayed, E.A. The role of current synthetic and possible plant and marine phytochemical compounds in the treatment of acne. RSC Adv. 2024, 14, 24287–24321. [Google Scholar] [CrossRef] [PubMed]
- Anukunwithaya, T.; Tantisira, M.H.; Tantisira, B.; Khemawoot, P. Pharmacokinetics of a Standardized Extract of Centella asiatica ECa 233 in Rats. Planta Medica 2017, 83, 710–717. [Google Scholar] [CrossRef]
- Farhana, K.M.; Malueka, R.G.; Wibowo, S.; Gofir, A.; Sali, A. Effectiveness of Gotu Kola Extract 750 mg and 1000 mg Compared with Folic Acid 3 mg in Improving Vascular Cognitive Impairment after Stroke. Evid.-Based Complement. Altern. Med. 2016, 2016, 2795915. [Google Scholar] [CrossRef]
- Diniz, L.R.L.; Calado, L.L.; Duarte, A.B.S.; de Sousa, D.P. Centella asiatica and Its Metabolite Asiatic Acid: Wound Healing Effects and Therapeutic Potential. Metabolites 2023, 13, 276. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Peng, Q.; Guo, Y.; Wang, X.; Zhang, L. Preparation and in vitro and in vivo Study of Asiaticoside-Loaded Nanoemulsions and Nanoemulsions-Based Gels for Transdermal Delivery. Int. J. Nanomed. 2020, 15, 3123–3136. [Google Scholar] [CrossRef] [PubMed]
- Nastiti, C.M.R.R.; Ponto, T.; Abd, E.; Grice, J.E.; Benson, H.A.E.; Roberts, M.S. Topical Nano and Microemulsions for Skin Delivery. Pharmaceutics 2017, 9, 37. [Google Scholar] [CrossRef] [PubMed]
- Kindler, S.; Schuster, M.; Seebauer, C.; Rutkowski, R.; Hauschild, A.; Podmelle, F.; Metelmann, C.; Metelmann, B.; Müller-Debus, C.; Metelmann, H.-R.; et al. Triterpenes for Well-Balanced Scar Formation in Superficial Wounds. Molecules 2016, 21, 1129. [Google Scholar] [CrossRef]
- Park, K.S. Pharmacological Effects of Centella asiatica on Skin Diseases: Evidence and Possible Mechanisms. Evid.-Based Complement. Altern. Med. 2021, 2021, 5462633. [Google Scholar] [CrossRef]
- Belcaro, G.; Maquart, F.-X.; Scoccianti, M.; Dugall, M.; Hosoi, M.; Cesarone, M.R.; Luzzi, R.; Cornelli, U.; Ledda, A.; Feragalli, B. TECA (Titrated Extract of Centella asiatica): New microcirculatory, biomolecular, and vascular application in preventive and clinical medicine. A status paper. Panminerva Med. 2011, 53, 105–118. [Google Scholar]
- Lingling, G.; Yuan, Z.; Weigen, L. Preparation, optimization, characterization and in vivo pharmacokinetic study of asiatic acid tromethamine salt-loaded solid lipid nanoparticles. Drug Dev. Ind. Pharm. 2016, 42, 1325–1333. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, H.; Sun, F.; Sun, S.; Zhu, Z.; Chai, Y. Biopharmaceutical and pharmacokinetic characterization of asiatic acid in Centella asiatica as determined by a sensitive and robust HPLC–MS method. J. Ethnopharmacol. 2015, 163, 31–38. [Google Scholar] [CrossRef]
- Gohil, K.J.; Patel, J.A.; Gajjar, A.K. Pharmacological review on Centella asiatica: A potential herbal cure-all. Indian J. Pharm. Sci. 2010, 72, 546–556. [Google Scholar] [CrossRef]
- Johnson, W.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; et al. Safety Assessment of Centella asiatica-derived ingredients as used in cosmetics. Int. J. Toxicol. 2023, 42, 5S–22S. [Google Scholar] [CrossRef]
- Caesar, L.K.; Cech, N.B. Synergy and antagonism in natural product extracts: When 1 + 1 does not equal 2. Nat. Prod. Rep. 2019, 36, 869–888. [Google Scholar] [CrossRef] [PubMed]
- Vaou, N.; Stavropoulou, E.; Voidarou, C.; Tsakris, Z.; Rozos, G.; Tsigalou, C.; Bezirtzoglou, E. Interactions between medical plant-derived bioactive compounds: Focus on antimicrobial combination effects. Antibiotics 2022, 11, 1014. [Google Scholar] [CrossRef] [PubMed]
- Jorge, O.A.; Jorge, A.D. Hepatotoxicity associated with the ingestion of Centella asiatica. Rev. Esp. Enfermedades Dig. 2005, 97, 115–124. [Google Scholar] [CrossRef]
- Bylka, W.; Znajdek-Awiżeń, P.; Studzińska-Sroka, E.; Brzezińska, M. Centella asiatica in cosmetology. Adv. Dermatol. Allergol. 2013, 1, 46–49. [Google Scholar] [CrossRef]
- Gruenwald, J.; Brendler, T.; Jaenicke, C. PDR for Herbal Medicines™, 3rd ed.; Medical Economics Company: Montvale, NJ, USA, 2004. [Google Scholar]
- Thirza, S.Q.; Pratiwi, M.D.; Noviardi, D.E.P.P.; Sriepinndonta, P.M.; Fitriani, F.N.; Kalsum, U.; Khotimah, H.; Mintaroem, K.; Norahmawati, E. Anti-inflammatory effects of Centella asiatica ethanolic extracts towards indomethacin-induced gastric ulcer model in rats by altering COX-2 expression. AIP Conf. Proc. 2021, 2353, 030062. [Google Scholar] [CrossRef]
- Ceremuga, T.E.; Valdivieso, D.; Kenner, C.; Lucia, A.; Lathrop, K.; Stailey, O.; Bailey, H.; Criss, J.; Linton, J.; Fried, J.; et al. Evaluation of the anxiolytic and antidepressant effects of asiatic acid, a compound from Gotu kola or Centella asiatica, in the male Sprague Dawley rat. AANA J. 2015, 83, 91–98. [Google Scholar]
- Government of India, Ministry of Health and Family Welfare, Department of AYUSH. The Ayurvedic Pharmacopoeia of India; Part I; The Controller of Publications: New Delhi, India, 2004; Volume IV. [Google Scholar]
- Gatt, A.R.; Bonanno, P.V.; Zammit, R. Ethical considerations in the regulation and use of herbal medicines in the European Union. Front. Med. Technol. 2024, 6, 1358956. [Google Scholar] [CrossRef]
- Upton, R.; Agudelo, I.; Cabrera, Y.; Caceres, A.; Calderón, A.; Calzada, F.; Camacho, R.; da Costa, F.; Dobrecky, C.; Enciso, R.; et al. A U.S. Pharmacopeia (USP) overview of Pan American botanicals used in dietary supplements and herbal medicines. Front. Pharmacol. 2024, 15, 1426210. [Google Scholar] [CrossRef] [PubMed]
- Jiso, A.; Khemawoot, P.; Techapichetvanich, P.; Soopairin, S.; Phoemsap, K.; Damrongsakul, P.; Wongwiwatthananukit, S.; Vivithanaporn, P. Drug-herb interactions among thai herbs and anticancer drugs: A scoping review. Pharmaceuticals 2022, 15, 146. [Google Scholar] [CrossRef]
- Lee, B.; Choi, Y.; Kim, P.-W.; Yang, C.; Lee, M.S. Regulation and status of herbal medicine clinical trials in Korea: A narrative review. Integr. Med. Res. 2021, 10, 100688. [Google Scholar] [CrossRef]
- Razif, R.; Fadilah, N.I.M.; Ahmad, H.; Hao, D.L.Q.; Maarof, M.; Fauzi, M.B. Asiaticoside-loaded multifunctional bioscaffolds for enhanced hyperglycemic wound healing. Biomedicines 2025, 13, 277. [Google Scholar] [CrossRef]
- Nazari, M.; Shokoohizadeh, L.; Taheri, M. Natural products in the treatment of diabetic foot infection. Eur. J. Med. Res. 2025, 30, 1–17. [Google Scholar] [CrossRef] [PubMed]
- World Intellectual Property Organization. Bacopa Monnieri Complexes with Phosphatidylserine and Phosphoryl Ethers and Use Thereof for Enhancing Cognitive Functions and Memory. WO Patent 2024208561A1, 10 October 2024.
- Nathan, P.J.; Tanner, S.; Lloyd, J.; Harrison, B.; Curran, L.; Oliver, C.; Stough, C. Effects of a combined extract of Ginkgo biloba and Bacopa monniera on cognitive function in healthy humans. Hum. Psychopharmacol. Clin. Exp. 2004, 19, 91–96. [Google Scholar] [CrossRef]
- DataIntelo. Centella asiatica Extract Market Report|Global Forecast From 2025 to 2033. 2024. Available online: https://dataintelo.com/report/centella-asiatica-extract-market (accessed on 25 April 2025).
- Cognitive Market Research. Nutraceuticals and Dietary Supplements Market Research Reports. 2023. Available online: https://www.cognitivemarketresearch.com/list/food-%26-beverages/nutraceuticals-and-dietary-supplements (accessed on 25 April 2025).
- Mahmood, A.; Tiwari, A.K.; ŞAHİN, K.; Küçük, Ö.; Ali, S. Triterpenoid saponin-rich fraction of Centella asiatica decreases IL-1β andNF-κB, and augments tissue regeneration and excision wound repair. Turk. J. Biol. 2016, 40, 399–409. [Google Scholar] [CrossRef]
- Kang, M.S.; Park, K.C.; Nam, S.M. A split-face study of moisturizer containing Centella asiatica extract after ablative fractional carbon dioxide laser resurfacing. Arch. Aesthetic Plast. Surg. 2021, 27, 56–60. [Google Scholar] [CrossRef]
- Wang, W.; Lu, K.-J.; Yu, C.-H.; Huang, Q.-L.; Du, Y.-Z. Nano-drug delivery systems in wound treatment and skin regeneration. J. Nanobiotechnol. 2019, 17, 82. [Google Scholar] [CrossRef] [PubMed]
- Abboud, H.A.; Zelkó, R.; Kazsoki, A. A systematic review of liposomal nanofibrous scaffolds as a drug delivery system: A decade of progress in controlled release and therapeutic efficacy. Drug Deliv. 2025, 32, 2445259. [Google Scholar] [CrossRef]
- Limón, D.; Gil-Lianes, P.; Rodríguez-Cid, L.; Alvarado, H.L.; Díaz-Garrido, N.; Mallandrich, M.; Baldomà, L.; Calpena, A.C.; Domingo, C.; Aliaga-Alcalde, N.; et al. Supramolecular Hydrogels Consisting of Nanofibers Increase the Bioavailability of Curcuminoids in Inflammatory Skin Diseases. ACS Appl. Nano Mater. 2022, 5, 13829–13839. [Google Scholar] [CrossRef]
- Sharma, N.; Tan, M.A.; An, S.S.A. Phytosterols: Potential metabolic modulators in neurodegenerative diseases. Int. J. Mol. Sci. 2021, 22, 12255. [Google Scholar] [CrossRef]
- Park, J.; Beck, B.R.; Kim, H.H.; Lee, S.; Kang, K. A brief review of machine learning-based bioactive compound research. Appl. Sci. 2022, 12, 2906. [Google Scholar] [CrossRef]
- Ejiofor, I.I. Chapter 3-Computational phytochemistry, databases, and tools. In Drug Discovery Update, Phytochemistry, Computational Tools and Databases in Drug Discovery; Egbuna, C., Rudrapal, M., Tijjani, H., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 39–55. [Google Scholar] [CrossRef]
- Sadee, W.; Wang, D.; Hartmann, K.; Toland, A.E. Pharmacogenomics: Driving personalized medicine. Pharmacol. Rev. 2023, 75, 789–814. [Google Scholar] [CrossRef] [PubMed]
- Sowmya, C.; Anand, M.; Rani, C.I.; Amuthaselvi, G.; Janaki, P. Recent developments and inventive approaches in vertical farming. Front. Sustain. Food Syst. 2024, 8, 1400787. [Google Scholar] [CrossRef]
- Csambalik, L.; Divéky-Ertsey, A.; Gál, I.; Madaras, K.; Sipos, L.; Székely, G.; Pusztai, P. Sustainability perspectives of organic farming and plant factory systems—From divergences towards synergies. Horticulturae 2023, 9, 895. [Google Scholar] [CrossRef]
- Feliziani, G.; Bordoni, L.; Gabbianelli, R. Regenerative organic agriculture and human health: The interconnection between soil, food quality, and nutrition. Antioxidants 2025, 14, 530. [Google Scholar] [CrossRef]


| Method | Yield | Selectivity | Energy Consumption | Eco-Friendliness | Scale-Up Feasibility |
|---|---|---|---|---|---|
| SFE [71,72] | High | High | Moderate | Excellent | Moderate |
| MAE [73,74] | Moderate to High | Moderate | High | Good | High |
| UAE [75,76] | High | Moderate | Low | Very Good | High |
| EAE [77,78,79] | Moderate | High | Low | Excellent | Moderate |
| PLE [79,80] | High | Moderate to High | Moderate | Good | High |
| Parameter | Extract/Compound | Dosage Range | Pharmacokinetic Characteristics | Clinical Notes |
|---|---|---|---|---|
| Cognitive enhancement [107,110,111] | Standardized extract (whole plant) | 300–750 mg/day orally | Low oral bioavailability, limited systemic exposure, and unclear metabolites | Administered in divided doses; effects observed after 4–12 weeks of use |
| Wound healing (topical) [95,108,112] | Madecassoside or asiaticoside cream | 1% w/w, applied twice daily | Localized dermal penetration; avoids first-pass metabolism | Demonstrated enhanced collagen synthesis and reduced scar formation in diabetic ulcers |
| Skin repair/ Cosmeceutical [113,114,115] | Triterpenoid-enriched cream/gel | 0.5–1% w/w topical application | Improved penetration with nanoemulsion/ liposomes | Used in anti-aging, post-laser recovery, and acne-prone skin |
| Anti-inflammatory (oral) [83,116,117] | TECA (Titrated Extract of C. asiatica) | 60–120 mg/day (standardized) | Rapid first-pass metabolism, plasma half-life < 2 h | Variable responses: chronic dosing recommended for vascular and inflammatory conditions |
| Bioavailability studies [118,119] | Asiatic acid, madecassic acid | 30–50 mg/kg (preclinical) | Poor absorption, high hepatic metabolism, and low peak plasma levels | Nanocarrier and phytosome formulations under development to enhance systemic delivery |
| Topical formulations [120,121] | Whole plant extract | Depends on the formulation matrix | Primarily local activity, systemic absorption is minimal | High safety margin; limited systemic toxicity |
| Application Domain | Therapeutic Indication | Formulation Type | Clinical Evidence | Commercial Examples/Products |
|---|---|---|---|---|
| Pharmaceutical | Wound healing (e.g., diabetic ulcers) | Creams, ointments, and hydrogels with madecassoside | RCTs show improved re-epithelialization, collagen synthesis, and reduced scarring | Madecassol®, Emdecassol, Tiger Balm |
| Venous insufficiency | Oral tablets/topical gels | Clinical trials report reduced edema and capillary permeability | Centelase, Titrated Extract of C. asiatica (TECA) | |
| Anti-inflammatory and neuroprotection | Standardized extracts | Preclinical and limited clinical trials show NF-κB modulation and antioxidant effects | Under research—potential NCE (new chemical entity) | |
| Nutraceutical | Cognitive support, stress adaptation | Capsules, soft gels, nootropic blends | Systematic reviews suggest modest improvement in memory and anxiety | NeuroGain®, Himalaya Mentat, Focus Factor |
| General wellness and anti-aging | Oral powders, adaptogenic blends | Observational studies support antioxidant effects; clinical trials are ongoing | Nature’s Answer, Organic India | |
| Cosmeceutical | Skin repair and anti-aging | Creams, serums, masks with asiaticoside/madecassoside | Controlled studies confirm improved skin texture, elasticity, and hydration | La Roche-Posay Cicaplast, Innisfree Cica Line |
| Acne, sensitive skin | Gels, post-laser treatments | Meta-analysis shows a reduction in acne lesions, redness, and skin irritation | SNP Cica Repair, Etude House Madecassoside Gel |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hein, Z.M.; Gopalakrishna, P.K.; Kanuri, A.K.; Thomas, W.; Hussan, F.; Naik, V.R.; Shantakumari, N.; Che Ramli, M.D.; Mohd Moklas, M.A.; Che Mohd Nassir, C.M.N.; et al. Centella asiatica: Advances in Extraction Technologies, Phytochemistry, and Therapeutic Applications. Life 2025, 15, 1081. https://doi.org/10.3390/life15071081
Hein ZM, Gopalakrishna PK, Kanuri AK, Thomas W, Hussan F, Naik VR, Shantakumari N, Che Ramli MD, Mohd Moklas MA, Che Mohd Nassir CMN, et al. Centella asiatica: Advances in Extraction Technologies, Phytochemistry, and Therapeutic Applications. Life. 2025; 15(7):1081. https://doi.org/10.3390/life15071081
Chicago/Turabian StyleHein, Zaw Myo, Prarthana Kalerammana Gopalakrishna, Anil Kumar Kanuri, Warren Thomas, Farida Hussan, Venkatesh R. Naik, Nisha Shantakumari, Muhammad Danial Che Ramli, Mohamad Aris Mohd Moklas, Che Mohd Nasril Che Mohd Nassir, and et al. 2025. "Centella asiatica: Advances in Extraction Technologies, Phytochemistry, and Therapeutic Applications" Life 15, no. 7: 1081. https://doi.org/10.3390/life15071081
APA StyleHein, Z. M., Gopalakrishna, P. K., Kanuri, A. K., Thomas, W., Hussan, F., Naik, V. R., Shantakumari, N., Che Ramli, M. D., Mohd Moklas, M. A., Che Mohd Nassir, C. M. N., & Vishnumukkala, T. (2025). Centella asiatica: Advances in Extraction Technologies, Phytochemistry, and Therapeutic Applications. Life, 15(7), 1081. https://doi.org/10.3390/life15071081






