Low-Dose Quercetin Dephosphorylates AKT and Suppresses Proteins Related to Migration in Human Metastatic Uveal Melanoma Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Line and Culturing Conditions
2.2. Reagents and Cell Viability Determination by MTT Assay
2.3. RNA Isolation
2.4. Quantitative Real-Time PCR (qRT-PCR) Analysis
2.5. Protein Isolation
2.6. Western Blot
2.7. Proteome Profiler Human XL Oncology Array
2.8. Statistical Analysis
3. Results
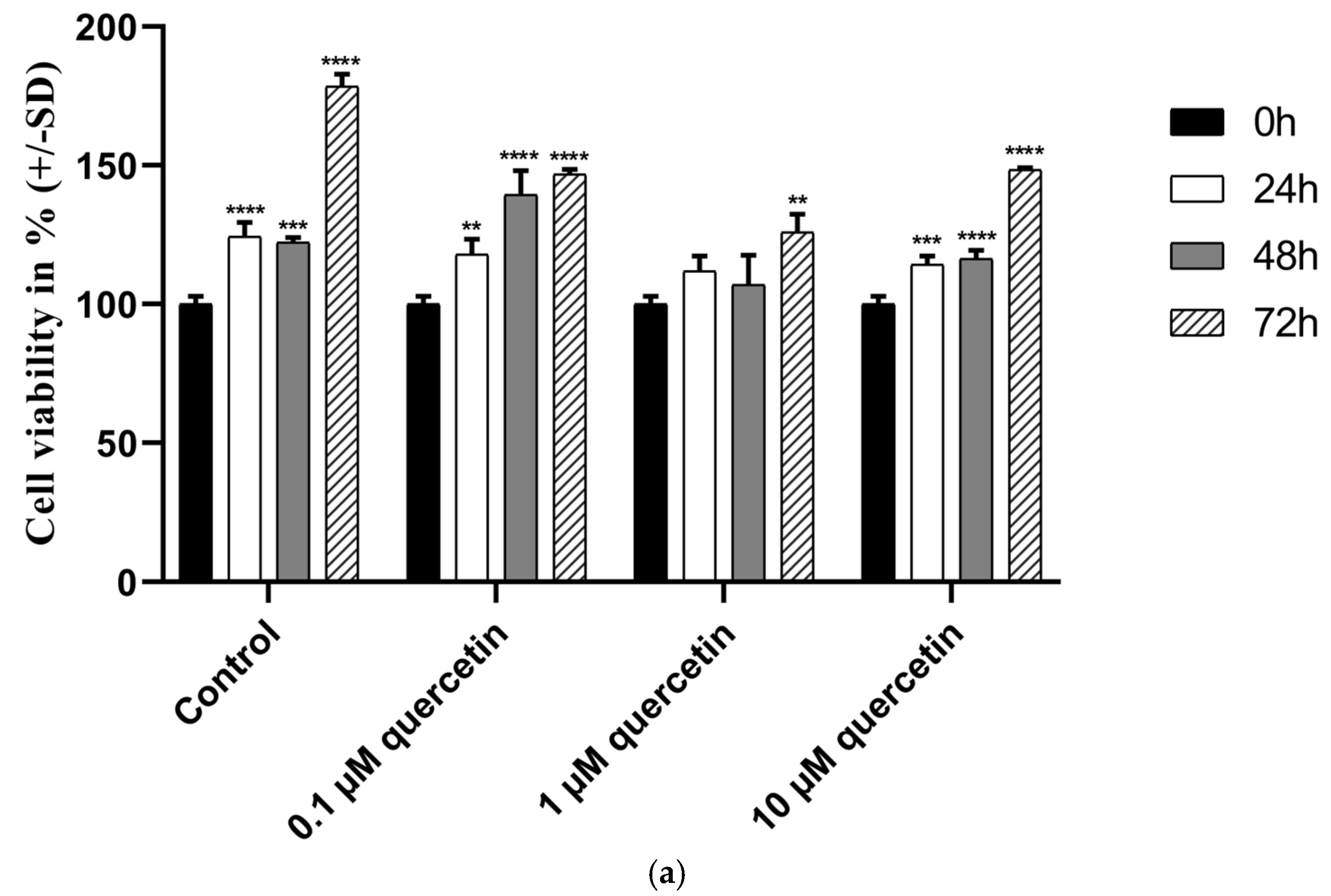
3.1. Effect of Quercetin on the Cell Viability of MM28 Metastatic UM Cells
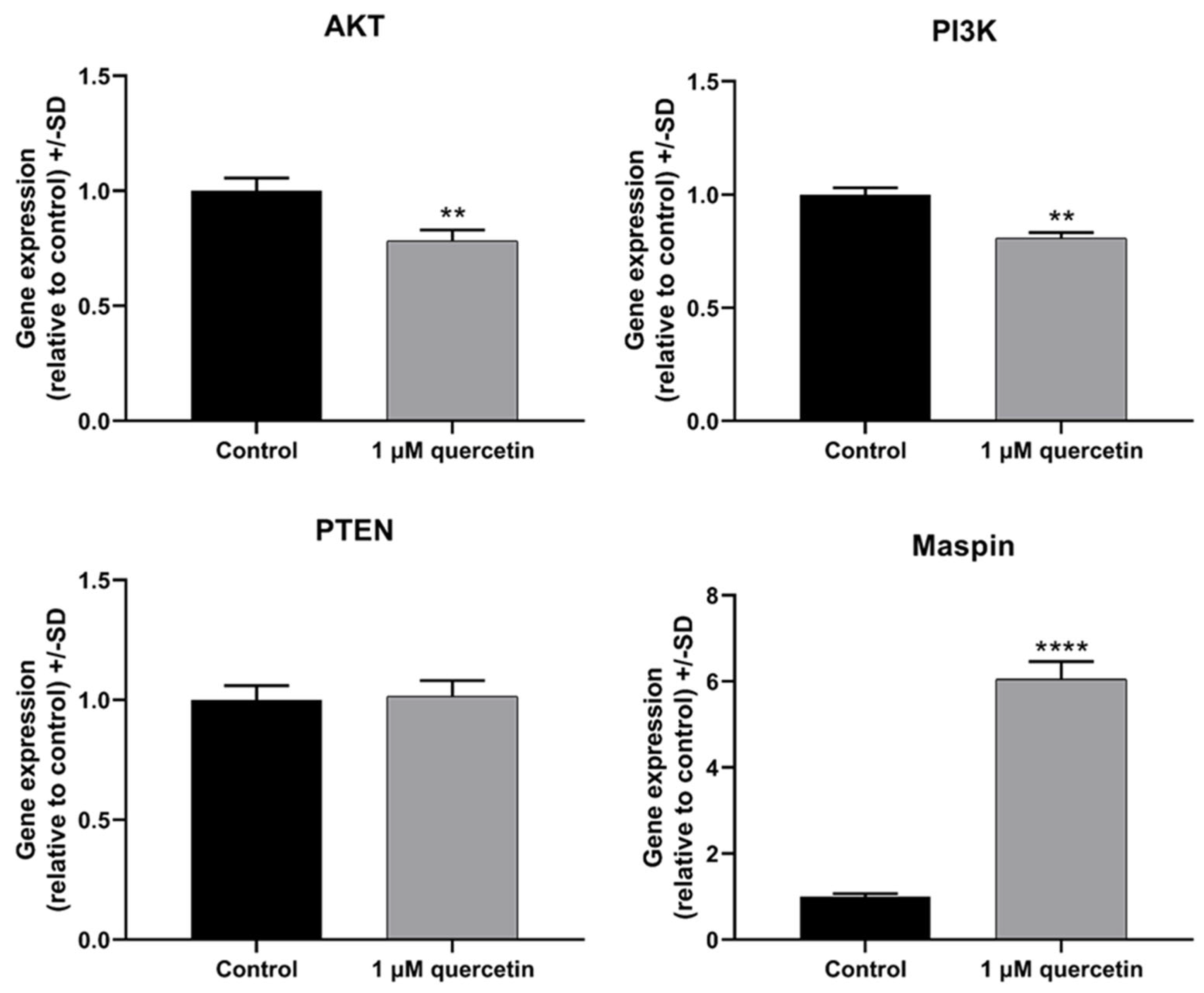
3.2. Effect of Quercetin on Genes Related to Proliferation and Migration of MM28 Metastatic UM Cells
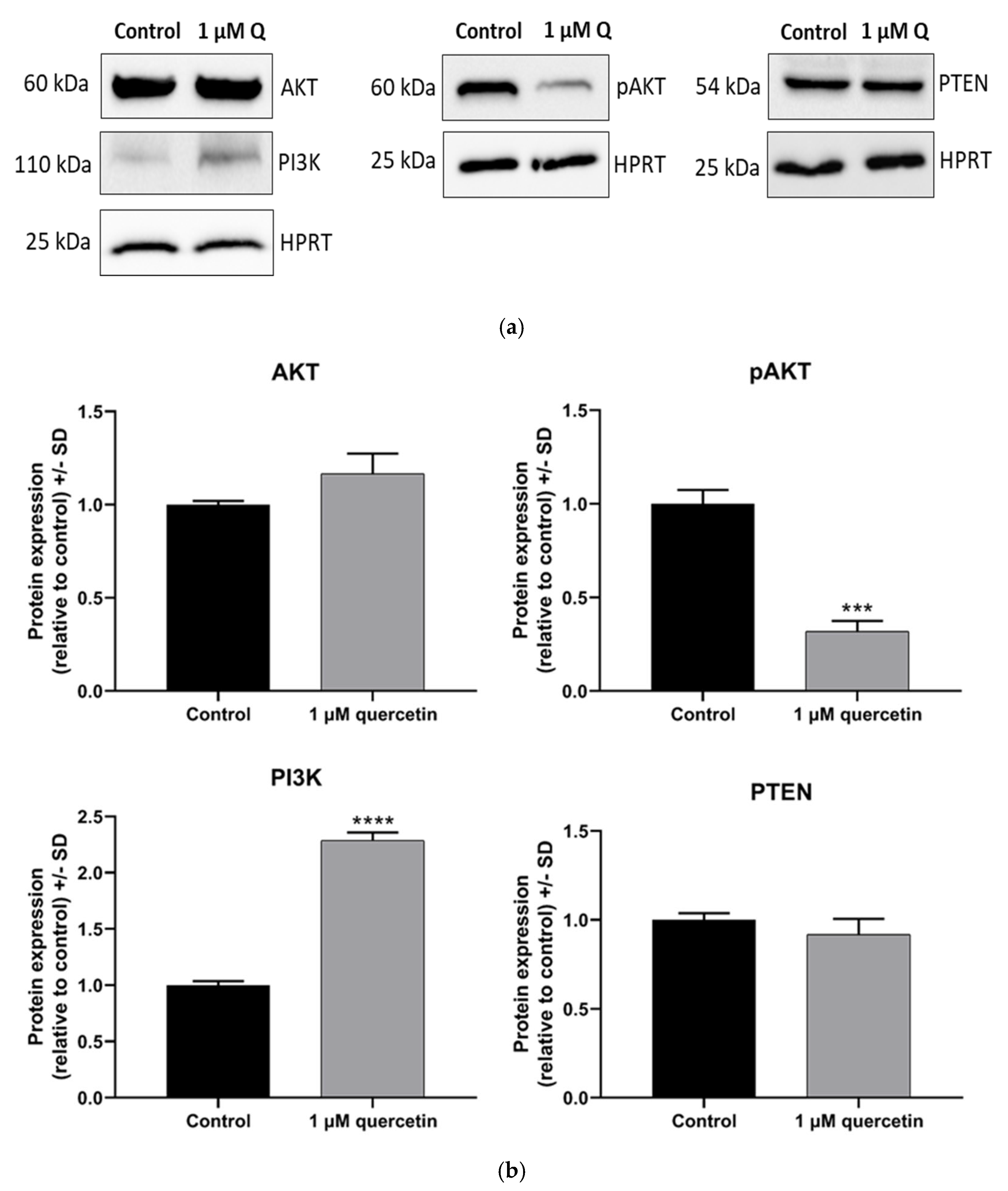
3.3. Dephosphorylation Effect of Quercetin on AKT Protein in MM28 Cells
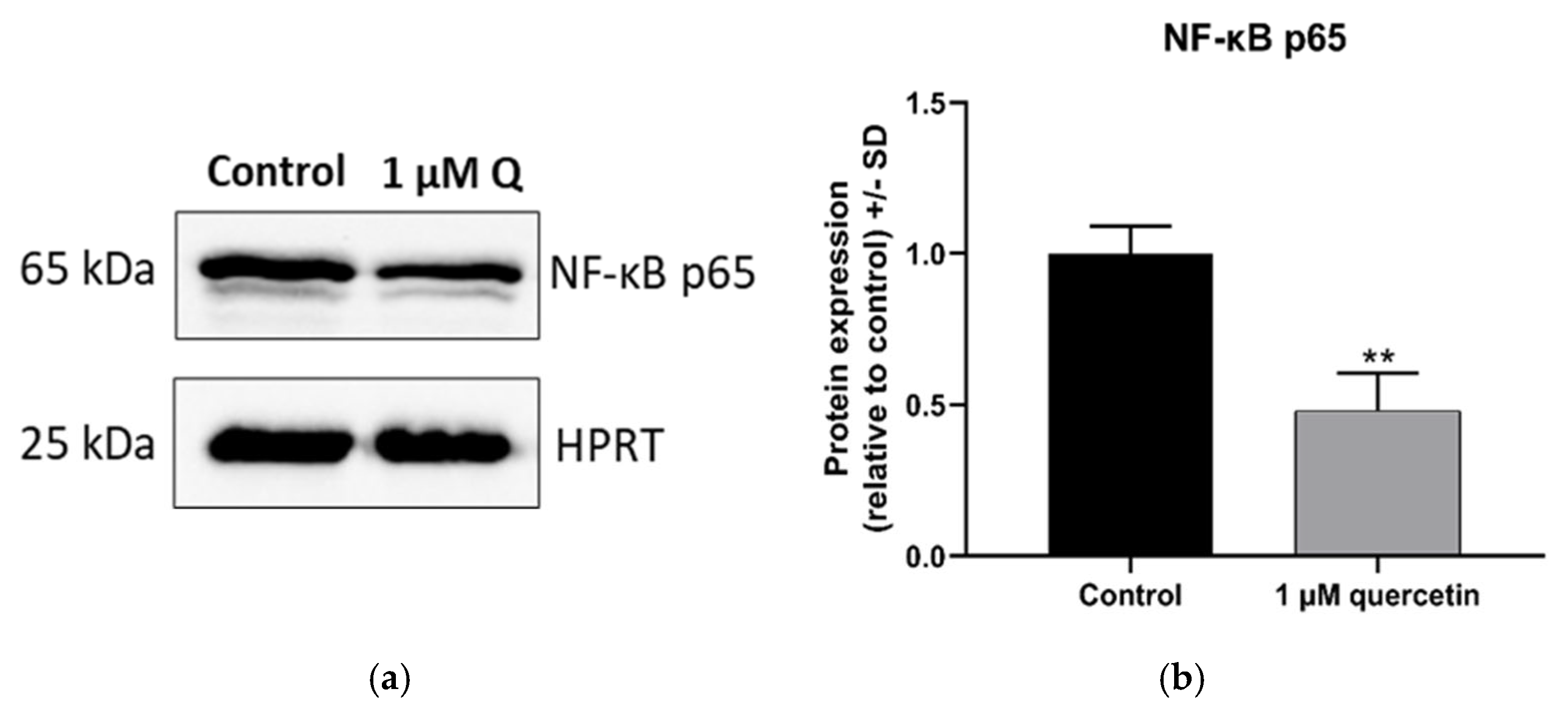
3.4. Effect of Quercetin on the Expression of NF-κB Protein
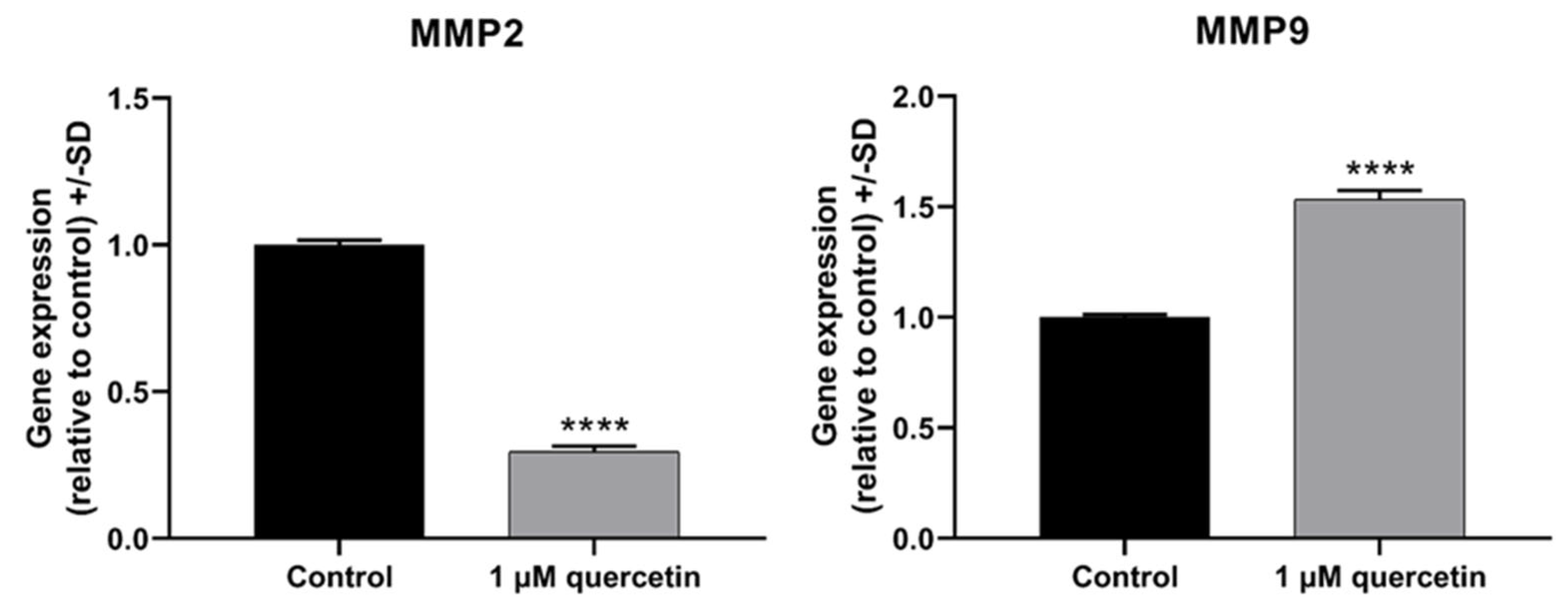
3.5. Effect of Quercetin on Matrix Metalloproteinase Proteins
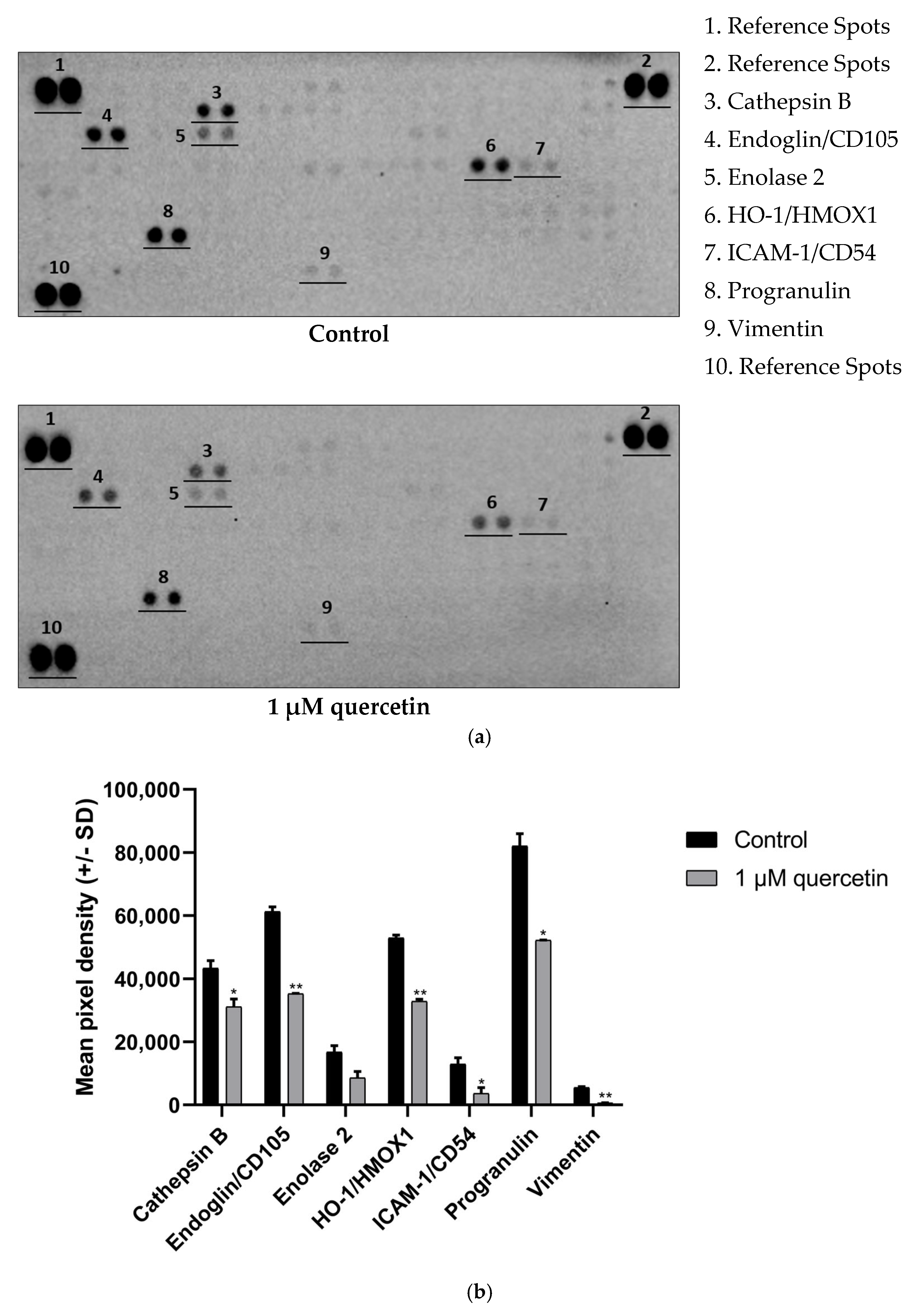
3.6. Effect of Quercetin Treatment on Proteins Related to EMT
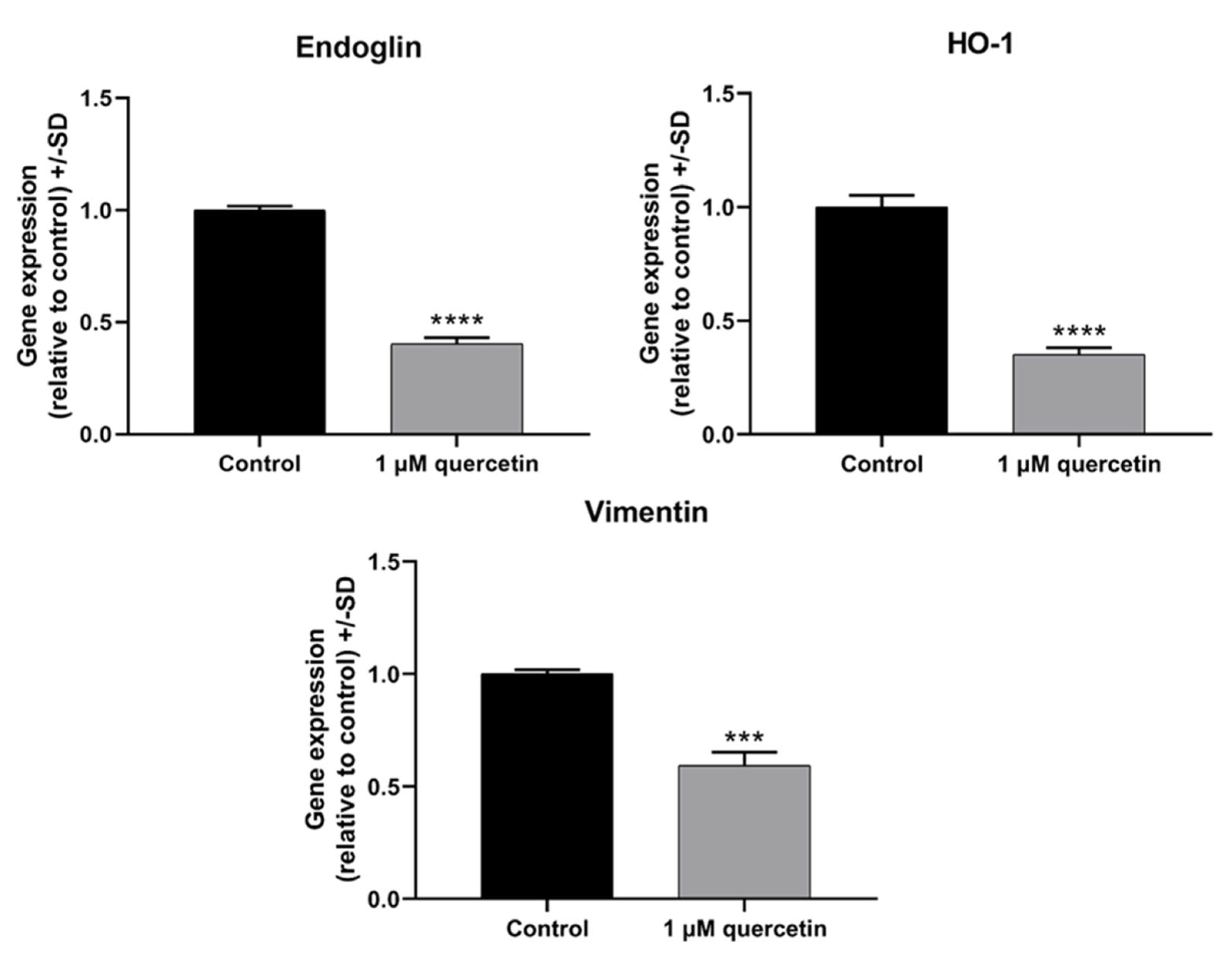
3.7. Effect of Quercetin Treatment on Genes Related to EMT
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MDPI | Multidisciplinary Digital Publishing Institute |
| DOAJ | Directory of open access journals |
| TLA | Three letter acronym |
| LD | Linear dichroism |
| ABC | ATP-binding cassette |
| AKT | Protein Kinase B |
| BAP1 | BRCA-1-associated protein 1 |
| BRAF | B-Raf Proto-Oncogene |
| ccRCC | Clear cell renal cell carcinoma |
| ECM | Extracellular matrix |
| ENO2 | Enolase 2 |
| GNA11 | G Protein Subunit Alpha 11 |
| GNAQ | G Protein Subunit Alpha Q |
| HO-1 | Heme Oxygenase 1 |
| ICAM-1 | Intercellular Adhesion Molecule 1 |
| ICR | Institute of Cancer Research |
| MMP | Matrix metalloproteinase |
| MALAT1 | Metastasis Associated Lung Adenocarcinoma Transcript 1 |
| NF-κB | Nuclear factor-kappa B |
| pAKT | Phosphorylated Akt |
| PDAC | Pancreatic ductal adenocarcinoma |
| PI3K | Phosphatidylinositol 3-Kinase |
| PI3K/AKT | Phosphatidylinositol 3-Kinase/AKT |
| PTEN | Phosphatase and Tensin Homolog |
| ROS | Reactive Oxygen Species |
| Twist | Twist Family BHLH Transcription Factor 1 |
| UM | Uveal melanoma |
References
- Carvajal, R.D.; Sacco, J.J.; Jager, M.J.; Eschelman, D.J.; Olofsson Bagge, R.; Harbour, J.W.; Chieng, N.D.; Patel, S.P.; Joshua, A.M.; Piperno-Neumann, S. Advances in the clinical management of uveal melanoma. Nat. Rev. Clin. Oncol. 2023, 20, 99–115. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Vidal, C.; Fernandez-Diaz, D.; Fernandez-Marta, B.; Lago-Baameiro, N.; Pardo, M.; Silva, P.; Paniagua, L.; Blanco-Teijeiro, M.J.; Piñeiro, A.; Bande, M. Treatment of metastatic uveal melanoma: Systematic review. Cancers 2020, 12, 2557. [Google Scholar] [CrossRef] [PubMed]
- Kivelä, T. The epidemiological challenge of the most frequent eye cancer: Retinoblastoma, an issue of birth and death. Br. J. Ophthalmol. 2009, 93, 1129–1131. [Google Scholar] [CrossRef] [PubMed]
- Nayman, T.; Bostan, C.; Logan, P.; Burnier, M.N., Jr. Uveal melanoma risk factors: A systematic review of meta-analyses. Curr. Eye Res. 2017, 42, 1085–1093. [Google Scholar] [CrossRef] [PubMed]
- Damato, B. Treatment of primary intraocular melanoma. Expert. Rev. Anticancer Ther. 2006, 6, 493–506. [Google Scholar] [CrossRef]
- Kujala, E.; Makitie, T.; Kivelä, T. Very long-term prognosis of patients with malignant uveal melanoma. Investig. Ophthalmol. Vis. Sci. 2003, 44, 4651–4659. [Google Scholar] [CrossRef]
- Ramos, A.M.; Aller, P. Quercetin decreases intracellular GSH content and potentiates the apoptotic action of the antileukemic drug arsenic trioxide in human leukemia cell lines. Biochem. Pharmacol. 2008, 75, 1912–1923. [Google Scholar] [CrossRef]
- Walle, T.; Vincent, T.S.; Walle, U.K. Evidence of covalent binding of the dietary flavonoid quercetin to DNA and protein in human intestinal and hepatic cells. Biochem. Pharmacol. 2003, 65, 1603–1610. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, K.; Zhang, Q.; Mei, J.; Chen, C.-J.; Feng, Z.-Z.; Yu, D.-H. Effects of quercetin on the apoptosis of the human gastric carcinoma cells. Toxicol. Vitro 2012, 26, 221–228. [Google Scholar] [CrossRef]
- Hayashi, Y.; Matsushima, M.; Nakamura, T.; Shibasaki, M.; Hashimoto, N.; Imaizumi, K.; Shimokata, K.; Hasegawa, Y.; Kawabe, T. Quercetin protects against pulmonary oxidant stress via heme oxygenase-1 induction in lung epithelial cells. Biochem. Biophys. Res. Commun. 2012, 417, 169–174. [Google Scholar] [CrossRef]
- Kim, Y.-H.; Lee, D.-H.; Jeong, J.-H.; Guo, Z.S.; Lee, Y.J. Quercetin augments TRAIL-induced apoptotic death: Involvement of the ERK signal transduction pathway. Biochem. Pharmacol. 2008, 75, 1946–1958. [Google Scholar] [CrossRef]
- Djuric, Z.; Severson, R.K.; Kato, I. Association of dietary quercetin with reduced risk of proximal colon cancer. Nutr. Cancer 2012, 64, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Dilek, B.; Meltem, Ö. Quercetin suppresses cell proliferation using the apoptosis pathways in MCF-7 and MDA-MB-231 human breast carcinoma cells in monolayer and spheroid model cultures. S. Afr. J. Bot. 2023, 162, 259–270. [Google Scholar] [CrossRef]
- Jeong, J.H.; An, J.Y.; Kwon, Y.T.; Rhee, J.G.; Lee, Y.J. Effects of low dose quercetin: Cancer cell-specific inhibition of cell cycle progression. J. Cell Biochem. 2009, 106, 73–82. [Google Scholar] [CrossRef]
- Singh, K.; Tarapcsak, S.; Gyongy, Z.; Ritter, Z.; Batta, G.; Bosire, R.; Remenyik, J.; Goda, K. Effects of Polyphenols on P-Glycoprotein (ABCB1) Activity. Pharmaceutics 2021, 13, 2062. [Google Scholar] [CrossRef]
- Li, S.; Yuan, S.; Zhao, Q.; Wang, B.; Wang, X.; Li, K. Quercetin enhances chemotherapeutic effect of doxorubicin against human breast cancer cells while reducing toxic side effects of it. Biomed. Pharmacother. 2018, 100, 441–447. [Google Scholar] [CrossRef]
- Rascio, F.; Spadaccino, F.; Rocchetti, M.T.; Castellano, G.; Stallone, G.; Netti, G.S.; Ranieri, E. The Pathogenic Role of PI3K/AKT Pathway in Cancer Onset and Drug Resistance: An Updated Review. Cancers 2021, 13, 3949. [Google Scholar] [CrossRef]
- Chen, W.J.; Tsai, J.H.; Hsu, L.S.; Lin, C.L.; Hong, H.M.; Pan, M.H. Quercetin blocks the aggressive phenotype of triple-negative breast cancer by inhibiting IGF1/IGF1R-mediated EMT program. J. Food Drug Anal. 2021, 29, 98–112. [Google Scholar] [CrossRef]
- Pan, H.C.; Jiang, Q.; Yu, Y.; Mei, J.P.; Cui, Y.K.; Zhao, W.J. Quercetin promotes cell apoptosis and inhibits the expression of MMP-9 and fibronectin via the AKT and ERK signalling pathways in human glioma cells. Neurochem. Int. 2015, 80, 60–71. [Google Scholar] [CrossRef]
- Bhat, F.A.; Sharmila, G.; Balakrishnan, S.; Arunkumar, R.; Elumalai, P.; Suganya, S.; Raja Singh, P.; Srinivasan, N.; Arunakaran, J. Quercetin reverses EGF-induced epithelial to mesenchymal transition and invasiveness in prostate cancer (PC-3) cell line via EGFR/PI3K/Akt pathway. J. Nutr. Biochem. 2014, 25, 1132–1139. [Google Scholar] [CrossRef]
- Lu, J.; Wang, Z.; Li, S.; Xin, Q.; Yuan, M.; Li, H.; Song, X.; Gao, H.; Pervaiz, N.; Sun, X. Quercetin inhibits the migration and invasion of HCCLM3 cells by suppressing the expression of p-Akt1, matrix metalloproteinase (MMP) MMP-2, and MMP-9. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2018, 24, 2583. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Li, X.; Wu, L.; Zhou, D.; Song, Y.; Zhang, L.; Wu, Q.; He, Q.; Wang, G.; Liu, X.; et al. Quercetin Suppresses Human Glioblastoma Migration and Invasion via GSK3beta/beta-catenin/ZEB1 Signaling Pathway. Front. Pharmacol. 2022, 13, 963614. [Google Scholar] [CrossRef]
- Cao, H.H.; Tse, A.K.; Kwan, H.Y.; Yu, H.; Cheng, C.Y.; Su, T.; Fong, W.F.; Yu, Z.L. Quercetin exerts anti-melanoma activities and inhibits STAT3 signaling. Biochem. Pharmacol. 2014, 87, 424–434. [Google Scholar] [CrossRef]
- Chen, S.F.; Nien, S.; Wu, C.H.; Liu, C.L.; Chang, Y.C.; Lin, Y.S. Reappraisal of the anticancer efficacy of quercetin in oral cancer cells. J. Chin. Med. Assoc. 2013, 76, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Ye, T.; Xiang, Y.; Shi, Z.; Zhang, J.; Lou, B.; Zhang, F.; Chen, B.; Zhou, M. Quercetin inhibits epithelial-mesenchymal transition, decreases invasiveness and metastasis, and reverses IL-6 induced epithelial-mesenchymal transition, expression of MMP by inhibiting STAT3 signaling in pancreatic cancer cells. OncoTargets Ther. 2017, 10, 4719–4729. [Google Scholar] [CrossRef]
- Roshanazadeh, M.; Babaahmadi Rezaei, H.; Rashidi, M. Quercetin synergistically potentiates the anti-metastatic effect of 5-fluorouracil on the MDA-MB-231 breast cancer cell line. Iran. J. Basic Med. Sci. 2021, 24, 928–934. [Google Scholar] [CrossRef]
- Dolcet, X.; Llobet, D.; Pallares, J.; Matias-Guiu, X. NF-kB in development and progression of human cancer. Virchows Arch. 2005, 446, 475–482. [Google Scholar] [CrossRef]
- Xia, L.; Tan, S.; Zhou, Y.; Lin, J.; Wang, H.; Oyang, L.; Tian, Y.; Liu, L.; Su, M.; Wang, H.; et al. Role of the NFκB-signaling pathway in cancer. OncoTargets Ther. 2018, 11, 2063–2073. [Google Scholar] [CrossRef] [PubMed]
- Chekalina, N.; Burmak, Y.; Petrov, Y.; Borisova, Z.; Manusha, Y.; Kazakov, Y.; Kaidashev, I. Quercetin reduces the transcriptional activity of NF-kB in stable coronary artery disease. Indian Heart J. 2018, 70, 593–597. [Google Scholar] [CrossRef]
- Ruiz, P.A.; Braune, A.; Holzlwimmer, G.; Quintanilla-Fend, L.; Haller, D. Quercetin inhibits TNF-induced NF-κB transcription factor recruitment to proinflammatory gene promoters in murine intestinal epithelial cells. J. Nutr. 2007, 137, 1208–1215. [Google Scholar] [CrossRef]
- Uttarawichien, T.; Kamnerdnond, C.; Inwisai, T.; Suwannalert, P.; Sibmooh, N.; Payuhakrit, W. Quercetin Inhibits Colorectal Cancer Cells Induced-Angiogenesis in Both Colorectal Cancer Cell and Endothelial Cell through Downregulation of VEGF-A/VEGFR2. Sci. Pharm. 2021, 89, 23. [Google Scholar] [CrossRef]
- Nieto, M.A.; Huang, R.Y.; Jackson, R.A.; Thiery, J.P. Emt: 2016. Cell 2016, 166, 21–45. [Google Scholar] [CrossRef] [PubMed]
- Lambert, A.W.; Pattabiraman, D.R.; Weinberg, R.A. Emerging Biological Principles of Metastasis. Cell 2017, 168, 670–691. [Google Scholar] [CrossRef]
- Jolly, M.K.; Boareto, M.; Huang, B.; Jia, D.; Lu, M.; Ben-Jacob, E.; Onuchic, J.N.; Levine, H. Implications of the Hybrid Epithelial/Mesenchymal Phenotype in Metastasis. Front. Oncol. 2015, 5, 155. [Google Scholar] [CrossRef]
- Beigi, Y.Z.; Lanjanian, H.; Fayazi, R.; Salimi, M.; Hoseyni, B.H.M.; Noroozizadeh, M.H.; Masoudi-Nejad, A. Heterogeneity and molecular landscape of melanoma: Implications for targeted therapy. Mol. Biomed. 2024, 5, 17. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, D.; Gupta, P.K.; Banerjee, S.; Kulkarni, S. Quercetin induces proteolysis of mesenchymal marker vimentin through activation of caspase-3, and decreases cancer stem cell population in human papillary thyroid cancer cell line. Phytomed. Plus 2021, 1, 100108. [Google Scholar] [CrossRef]
- Kim, S.R.; Lee, E.Y.; Kim, D.J.; Kim, H.J.; Park, H.R. Quercetin Inhibits Cell Survival and Metastatic Ability via the EMT-mediated Pathway in Oral Squamous Cell Carcinoma. Molecules 2020, 25, 757. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Chen, D.; Yang, F.; Xing, N. Quercetin Inhibits Epithelial-to-Mesenchymal Transition (EMT) Process and Promotes Apoptosis in Prostate Cancer via Downregulating lncRNA MALAT1. Cancer Manag. Res. 2020, 12, 1741–1750. [Google Scholar] [CrossRef]
- Kiraly, J.; Szabo, E.; Fodor, P.; Vass, A.; Choudhury, M.; Gesztelyi, R.; Szasz, C.; Flasko, T.; Dobos, N.; Zsebik, B.; et al. Expression of hsa-miRNA-15b, -99b, -181a and Their Relationship to Angiogenesis in Renal Cell Carcinoma. Biomedicines 2024, 12, 1441. [Google Scholar] [CrossRef]
- He, Y.; Sun, M.M.; Zhang, G.G.; Yang, J.; Chen, K.S.; Xu, W.W.; Li, B. Targeting PI3K/Akt signal transduction for cancer therapy. Signal Transduct. Target. Ther. 2021, 6, 425. [Google Scholar] [CrossRef]
- Hu, D.N.; Rosen, R.B.; Chan, C.C.; Yang, W.E.; Yang, S.F. Uveal melanocytes express high constitutive levels of MMP-8 which can be upregulated by TNF-alpha via the MAPK pathway. Exp. Eye Res. 2018, 175, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Juurikka, K.; Butler, G.S.; Salo, T.; Nyberg, P.; Astrom, P. The Role of MMP8 in Cancer: A Systematic Review. Int. J. Mol. Sci. 2019, 20, 4506. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Fernandez, A.; Fueyo, A.; Folgueras, A.R.; Garabaya, C.; Pennington, C.J.; Pilgrim, S.; Edwards, D.R.; Holliday, D.L.; Jones, J.L.; Span, P.N.; et al. Matrix metalloproteinase-8 functions as a metastasis suppressor through modulation of tumor cell adhesion and invasion. Cancer Res. 2008, 68, 2755–2763. [Google Scholar] [CrossRef]
- Elumalai, P.; Ezhilarasan, D.; Raghunandhakumar, S. Quercetin Inhibits the Epithelial to Mesenchymal Transition through Suppressing Akt Mediated Nuclear Translocation of beta-Catenin in Lung Cancer Cell Line. Nutr. Cancer 2022, 74, 1894–1906. [Google Scholar] [CrossRef]
- Rantala, E.S.; Hernberg, M.M.; Piperno-Neumann, S.; Grossniklaus, H.E.; Kivela, T.T. Metastatic uveal melanoma: The final frontier. Prog. Retin. Eye Res. 2022, 90, 101041. [Google Scholar] [CrossRef]
- Tura, A.; Herfs, V.; Maassen, T.; Zuo, H.; Vardanyan, S.; Prasuhn, M.; Ranjbar, M.; Kakkassery, V.; Grisanti, S. Quercetin Impairs the Growth of Uveal Melanoma Cells by Interfering with Glucose Uptake and Metabolism. Int. J. Mol. Sci. 2024, 25, 4292. [Google Scholar] [CrossRef]
- Gao, Y.; Li, C.; Xue, T.; Lin, C.; Hou, R.; Xia, Q.; Ding, D.; Li, J.; Wang, D.; Feng, Y. Quercetin Mediated TET1 Expression Through MicroRNA-17 Induced Cell Apoptosis in Melanoma Cells. Biochem. Genet. 2023, 61, 762–777. [Google Scholar] [CrossRef] [PubMed]
- Moses, L.B.; Abu Bakar, M.F.; Mamat, H.; Aziz, Z.A. Unfermented Freeze-Dried Leaf Extract of Tongkat Ali (Eurycoma longifolia Jack.) Induced Cytotoxicity and Apoptosis in MDA-MB-231 and MCF-7 Breast Cancer Cell Lines. Evid. Based Complement. Altern. Med. 2021, 2021, 8811236. [Google Scholar] [CrossRef]
- Peeva, M.I.; Georgieva, M.G.; Balacheva, A.A.; Pavlov, A.; Tzvetkov, N.T. In Vitro Investigation of the Cytotoxic and Antiproliferative Effects of Haberlea rhodopensis Total Extract: A Comparative Study. Cosmetics 2024, 11, 46. [Google Scholar] [CrossRef]
- Russo, G.L.; Ungaro, P. Epigenetic Mechanisms of Quercetin and Other Flavonoids in Cancer Therapy and Prevention. In Epigenetics of Cancer Prevention; Academic Press: Cambridge, MA, USA, 2019; pp. 187–202. [Google Scholar]
- Xiang, X.; Hoang, H.D.; Gilchrist, V.H.; Langlois, S.; Alain, T.; Cowan, K.N. Quercetin induces pannexin 1 expression via an alternative transcript with a translationally active 5′ leader in rhabdomyosarcoma. Oncogenesis 2022, 11, 9. [Google Scholar] [CrossRef]
- Cervellera, C.; Russo, M.; Dotolo, S.; Facchiano, A.; Russo, G.L. STL1, a New AKT Inhibitor, Synergizes with Flavonoid Quercetin in Enhancing Cell Death in A Chronic Lymphocytic Leukemia Cell Line. Molecules 2021, 26, 5810. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.; Spagnuolo, C.; Volpe, S.; Tedesco, I.; Bilotto, S.; Russo, G.L. ABT-737 resistance in B-cells isolated from chronic lymphocytic leukemia patients and leukemia cell lines is overcome by the pleiotropic kinase inhibitor quercetin through Mcl-1 down-regulation. Biochem. Pharmacol. 2013, 85, 927–936. [Google Scholar] [CrossRef]
- Zhang, X.A.; Zhang, S.; Yin, Q.; Zhang, J. Quercetin induces human colon cancer cells apoptosis by inhibiting the nuclear factor-kappa B Pathway. Pharmacogn. Mag. 2015, 11, 404–409. [Google Scholar] [CrossRef]
- Vidya Priyadarsini, R.; Senthil Murugan, R.; Maitreyi, S.; Ramalingam, K.; Karunagaran, D.; Nagini, S. The flavonoid quercetin induces cell cycle arrest and mitochondria-mediated apoptosis in human cervical cancer (HeLa) cells through p53 induction and NF-kappaB inhibition. Eur. J. Pharmacol. 2010, 649, 84–91. [Google Scholar] [CrossRef]
- Chou, Y.C.; Sheu, J.R.; Chung, C.L.; Chen, C.Y.; Lin, F.L.; Hsu, M.J.; Kuo, Y.H.; Hsiao, G. Nuclear-targeted inhibition of NF-kappaB on MMP-9 production by N-2-(4-bromophenyl) ethyl caffeamide in human monocytic cells. Chem. Biol. Interact. 2010, 184, 403–412. [Google Scholar] [CrossRef]
- Jiang, H.; Li, H. Prognostic values of tumoral MMP2 and MMP9 overexpression in breast cancer: A systematic review and meta-analysis. BMC Cancer 2021, 21, 149. [Google Scholar] [CrossRef]
- El-Shabrawi, Y.; Ardjomand, N.; Radner, H.; Ardjomand, N. MMP-9 is predominantly expressed in epithelioid and not spindle cell uveal melanoma. J. Pathol. 2001, 194, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Saragusti, A.C.; Ortega, M.G.; Cabrera, J.L.; Estrin, D.A.; Marti, M.A.; Chiabrando, G.A. Inhibitory effect of quercetin on matrix metalloproteinase 9 activity molecular mechanism and structure-activity relationship of the flavonoid-enzyme interaction. Eur. J. Pharmacol. 2010, 644, 138–145. [Google Scholar] [CrossRef]
- Chen, B.; He, T.; Xing, Y.; Cao, T. Effects of quercetin on the expression of MCP-1, MMP-9 and VEGF in rats with diabetic retinopathy. Exp. Ther. Med. 2017, 14, 6022–6026. [Google Scholar] [CrossRef] [PubMed]
- Dostal, Z.; Modriansky, M. The effect of quercetin on microRNA expression: A critical review. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 2019, 163, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Li, X.; Li, Y.; Wang, R.; Wang, C.; Li, Y. New molecular mechanisms of quercetin in improving recurrent spontaneous abortion based on in-depth network pharmacology and molecular docking. Front. Chem. 2024, 12, 1407667. [Google Scholar] [CrossRef]
- Gondi, C.S.; Rao, J.S. Cathepsin B as a cancer target. Expert. Opin. Ther. Targets 2013, 17, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Kedhari Sundaram, M.; Raina, R.; Afroze, N.; Bajbouj, K.; Hamad, M.; Haque, S.; Hussain, A. Quercetin modulates signaling pathways and induces apoptosis in cervical cancer cells. Biosci. Rep. 2019, 39, BSR20190720. [Google Scholar] [CrossRef]
- Yang, S.; Zhou, H.; Wang, G.; Zhong, X.H.; Shen, Q.L.; Zhang, X.J.; Li, R.Y.; Chen, L.H.; Zhang, Y.H.; Wan, Z. Quercetin is protective against short-term dietary advanced glycation end products intake induced cognitive dysfunction in aged ICR mice. J. Food Biochem. 2020, 44, e13164. [Google Scholar] [CrossRef] [PubMed]
- Vijayababu, M.R.; Arunkumar, A.; Kanagaraj, P.; Venkataraman, P.; Krishnamoorthy, G.; Arunakaran, J. Quercetin downregulates matrix metalloproteinases 2 and 9 proteins expression in prostate cancer cells (PC-3). Mol. Cell Biochem. 2006, 287, 109–116. [Google Scholar] [CrossRef]
- Chuang, C.H.; Yeh, C.L.; Yeh, S.L.; Lin, E.S.; Wang, L.Y.; Wang, Y.H. Quercetin metabolites inhibit MMP-2 expression in A549 lung cancer cells by PPAR-gamma associated mechanisms. J. Nutr. Biochem. 2016, 33, 45–53. [Google Scholar] [CrossRef]
- Treps, L.; Faure, S.; Clere, N. Vasculogenic mimicry, a complex and devious process favoring tumorigenesis—Interest in making it a therapeutic target. Pharmacol. Ther. 2021, 223, 107805. [Google Scholar] [CrossRef]
- Sabazade, S.; Gill, V.; Herrspiegel, C.; Stalhammar, G. Vasculogenic mimicry correlates to presenting symptoms and mortality in uveal melanoma. J. Cancer Res. Clin. Oncol. 2022, 148, 587–597. [Google Scholar] [CrossRef]
- Schoonderwoerd, M.J.A.; Goumans, M.T.H.; Hawinkels, L. Endoglin: Beyond the Endothelium. Biomolecules 2020, 10, 289. [Google Scholar] [CrossRef]
- Zheng, Y.; Wu, C.; Yang, J.; Zhao, Y.; Jia, H.; Xue, M.; Xu, D.; Yang, F.; Fu, D.; Wang, C.; et al. Insulin-like growth factor 1-induced enolase 2 deacetylation by HDAC3 promotes metastasis of pancreatic cancer. Signal Transduct. Target. Ther. 2020, 5, 53. [Google Scholar] [CrossRef]
- Leiherer, A.; Stoemmer, K.; Muendlein, A.; Saely, C.H.; Kinz, E.; Brandtner, E.M.; Fraunberger, P.; Drexel, H. Quercetin Impacts Expression of Metabolism- and Obesity-Associated Genes in SGBS Adipocytes. Nutrients 2016, 8, 282. [Google Scholar] [CrossRef]
- Zhu, X.; Huang, S.; Zeng, L.; Ma, J.; Sun, S.; Zeng, F.; Kong, F.; Cheng, X. HMOX-1 inhibits TGF-beta-induced epithelial-mesenchymal transition in the MCF-7 breast cancer cell line. Int. J. Mol. Med. 2017, 40, 411–417. [Google Scholar] [CrossRef]
- Hsu, F.F.; Yeh, C.T.; Sun, Y.J.; Chiang, M.T.; Lan, W.M.; Li, F.A.; Lee, W.H.; Chau, L.Y. Signal peptide peptidase-mediated nuclear localization of heme oxygenase-1 promotes cancer cell proliferation and invasion independent of its enzymatic activity. Oncogene 2015, 34, 2360–2370. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Cai, F.; Yu, L.; An, R.; Wei, B.; Li, M. Quercetin inhibits ferroptosis through the SIRT1/Nrf2/HO-1 signaling pathway and alleviates asthma disease. Transl. Pediatr. 2024, 13, 1747–1759. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Wu, C.; Fu, Z.; Liu, S. ICAM1 promotes bone metastasis via integrin-mediated TGF-beta/EMT signaling in triple-negative breast cancer. Cancer Sci. 2022, 113, 3751–3765. [Google Scholar] [CrossRef] [PubMed]
- Ying, B.; Yang, T.; Song, X.; Hu, X.; Fan, H.; Lu, X.; Chen, L.; Cheng, D.; Wang, T.; Liu, D.; et al. Quercetin inhibits IL-1 beta-induced ICAM-1 expression in pulmonary epithelial cell line A549 through the MAPK pathways. Mol. Biol. Rep. 2009, 36, 1825–1832. [Google Scholar] [CrossRef]
- Dong, T.; Yang, D.; Li, R.; Zhang, L.; Zhao, H.; Shen, Y.; Zhang, X.; Kong, B.; Wang, L. PGRN promotes migration and invasion of epithelial ovarian cancer cells through an epithelial mesenchymal transition program and the activation of cancer associated fibroblasts. Exp. Mol. Pathol. 2016, 100, 17–25. [Google Scholar] [CrossRef]
- Usman, S.; Waseem, N.H.; Nguyen, T.K.N.; Mohsin, S.; Jamal, A.; Teh, M.T.; Waseem, A. Vimentin Is at the Heart of Epithelial Mesenchymal Transition (EMT) Mediated Metastasis. Cancers 2021, 13, 4985. [Google Scholar] [CrossRef]
- Chang, W.W.; Hu, F.W.; Yu, C.C.; Wang, H.H.; Feng, H.P.; Lan, C.; Tsai, L.L.; Chang, Y.C. Quercetin in elimination of tumor initiating stem-like and mesenchymal transformation property in head and neck cancer. Head Neck 2013, 35, 413–419. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fodor, P.; Király, J.; Szabó, Z.; Goda, K.; Zsebik, B.; Halmos, G. Low-Dose Quercetin Dephosphorylates AKT and Suppresses Proteins Related to Migration in Human Metastatic Uveal Melanoma Cells. Life 2025, 15, 979. https://doi.org/10.3390/life15060979
Fodor P, Király J, Szabó Z, Goda K, Zsebik B, Halmos G. Low-Dose Quercetin Dephosphorylates AKT and Suppresses Proteins Related to Migration in Human Metastatic Uveal Melanoma Cells. Life. 2025; 15(6):979. https://doi.org/10.3390/life15060979
Chicago/Turabian StyleFodor, Petra, József Király, Zsuzsanna Szabó, Katalin Goda, Barbara Zsebik, and Gábor Halmos. 2025. "Low-Dose Quercetin Dephosphorylates AKT and Suppresses Proteins Related to Migration in Human Metastatic Uveal Melanoma Cells" Life 15, no. 6: 979. https://doi.org/10.3390/life15060979
APA StyleFodor, P., Király, J., Szabó, Z., Goda, K., Zsebik, B., & Halmos, G. (2025). Low-Dose Quercetin Dephosphorylates AKT and Suppresses Proteins Related to Migration in Human Metastatic Uveal Melanoma Cells. Life, 15(6), 979. https://doi.org/10.3390/life15060979







