HIV Protein TAT Dysregulates Multiple Pathways in Human iPSCs-Derived Microglia
Abstract
1. Introduction
2. Materials and Methods
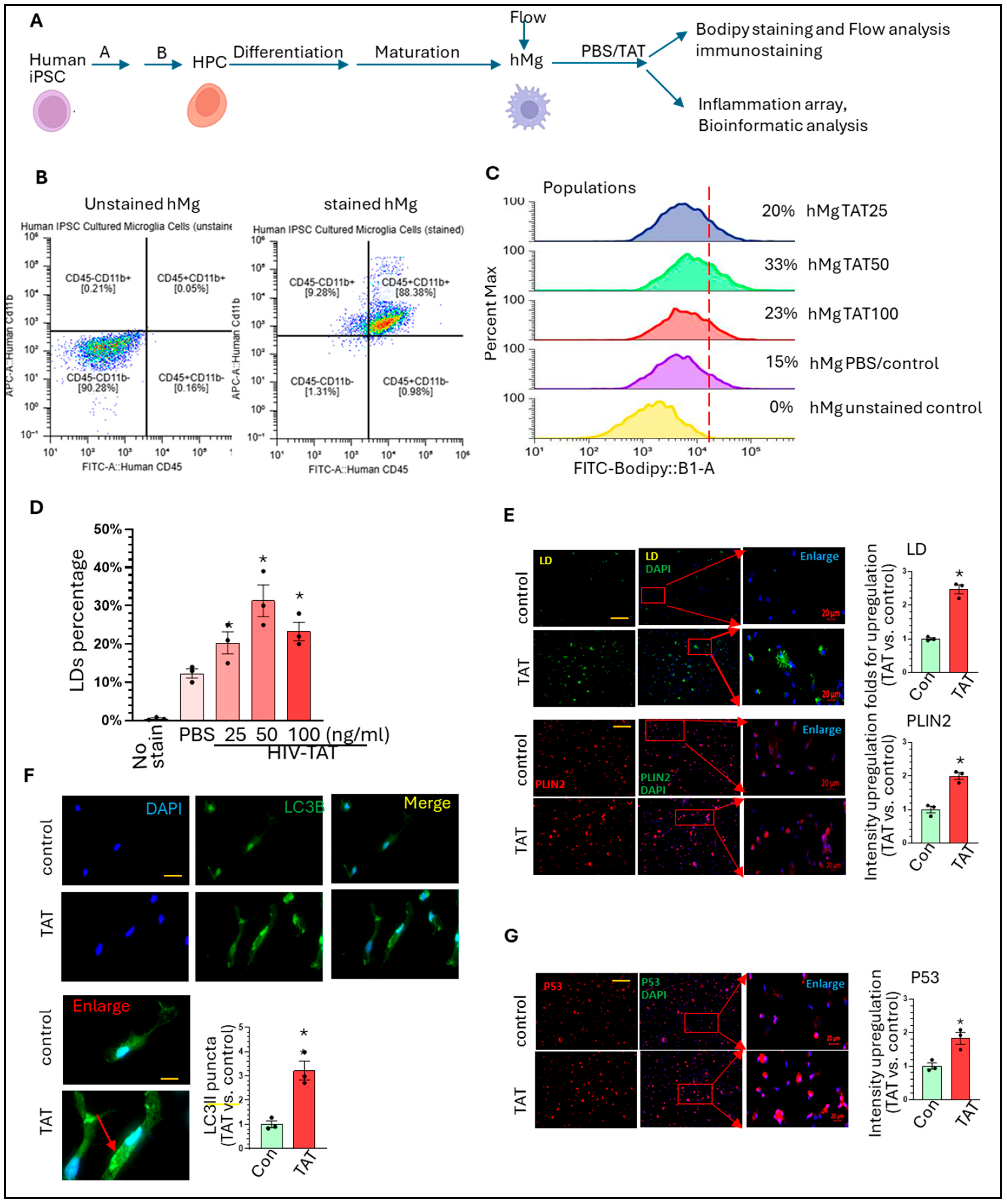
3. Results and Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siddiqi, A.E.; Hall, H.I.; Hu, X.; Song, R. Population-Based Estimates of Life Expectancy After HIV Diagnosis: United States 2008–2011. J. Acquir. Immune Defic. Syndr. 2016, 72, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Wing, E.J. HIV and aging. Int. J. Infect. Dis. 2016, 53, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.K.; Kuhn, T.B.; Chen, J.; Bamburg, J.R. HIV Associated Neurodegenerative Disorders: A New Perspective on the Role of Lipid Rafts in Gp120-Mediated Neurotoxicity. Curr. HIV Res. 2018, 16, 258–269. [Google Scholar] [CrossRef] [PubMed]
- Eggers, C.; Arendt, G.; Hahn, K.; Husstedt, I.W.; Maschke, M.; Neuen-Jacob, E.; Obermann, M.; Rosenkranz, T.; Schielke, E.; Straube, E.; et al. HIV-1-associated neurocognitive disorder: Epidemiology, pathogenesis, diagnosis, and treatment. J. Neurol. 2017, 264, 1715–1727. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Sarkar, A.; Mitsuya, H. HIV-Associated Neurocognitive Disorder (HAND) and the Prospect of Brain-Penetrating Protease Inhibitors for Antiretroviral Treatment. Med. Res. Arch. 2017, 5, 1113. [Google Scholar]
- Gustafson, D.R.; McFarlane, S.I. Obesity, Vascular Disease and Frailty in Aging Women with HIV. Adv. Geriatr. Med. Res. 2021, 3, e210014. [Google Scholar] [CrossRef]
- Filgueira, L.; Larionov, A.; Lannes, N. The Influence of Virus Infection on Microglia and Accelerated Brain Aging. Cells 2021, 10, 1836. [Google Scholar] [CrossRef]
- Singh, S.; Deshetty, U.M.; Ray, S.; Oladapo, A.; Horanieh, E.; Buch, S.; Periyasamy, P. Non-Coding RNAs in HIV Infection, NeuroHIV, and Related Comorbidities. Cells 2024, 13, 898. [Google Scholar] [CrossRef]
- Thompson, L.J.; Genovese, J.; Hong, Z.; Singh, M.V.; Singh, V.B. HIV-Associated Neurocognitive Disorder: A Look into Cellular and Molecular Pathology. Int. J. Mol. Sci. 2024, 25, 4697. [Google Scholar] [CrossRef]
- Mirarchi, A.; Albi, E.; Arcuri, C. Microglia Signatures: A Cause or Consequence of Microglia-Related Brain Disorders? Int. J. Mol. Sci. 2024, 25, 10951. [Google Scholar] [CrossRef]
- Tremblay, M.E.; Verkhratsky, A. General Pathophysiology of Microglia. Adv. Neurobiol. 2024, 37, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Spurgat, M.S.; Tang, S.J. Single-Cell RNA-Sequencing: Astrocyte and Microglial Heterogeneity in Health and Disease. Cells 2022, 11, 2021. [Google Scholar] [CrossRef]
- Sun, J.; Song, Y.; Chen, Z.; Qiu, J.; Zhu, S.; Wu, L.; Xing, L. Heterogeneity and Molecular Markers for CNS Glial Cells Revealed by Single-Cell Transcriptomics. Cell. Mol. Neurobiol. 2022, 42, 2629–2642. [Google Scholar] [CrossRef] [PubMed]
- De Biase, L.M.; Schuebel, K.E.; Fusfeld, Z.H.; Jair, K.; Hawes, I.A.; Cimbro, R.; Zhang, H.Y.; Liu, Q.R.; Shen, H.; Xi, Z.X.; et al. Local Cues Establish and Maintain Region-Specific Phenotypes of Basal Ganglia Microglia. Neuron 2017, 95, 341–356.e6. [Google Scholar] [CrossRef]
- Cserep, C.; Posfai, B.; Denes, A. Shaping Neuronal Fate: Functional Heterogeneity of Direct Microglia-Neuron Interactions. Neuron 2021, 109, 222–240. [Google Scholar] [CrossRef]
- Bennett, J.P., Jr.; Keeney, P.M.; Brohawn, D.G. RNA Sequencing Reveals Small and Variable Contributions of Infectious Agents to Transcriptomes of Postmortem Nervous Tissues From Amyotrophic Lateral Sclerosis, Alzheimer’s Disease and Parkinson’s Disease Subjects, and Increased Expression of Genes From Disease-Activated Microglia. Front. Neurosci. 2019, 13, 235. [Google Scholar] [CrossRef]
- Xu, Y.J.; Au, N.P.B.; Ma, C.H.E. Functional and Phenotypic Diversity of Microglia: Implication for Microglia-Based Therapies for Alzheimer’s Disease. Front. Aging Neurosci. 2022, 14, 896852. [Google Scholar] [CrossRef]
- Keren-Shaul, H.; Spinrad, A.; Weiner, A.; Matcovitch-Natan, O.; Dvir-Szternfeld, R.; Ulland, T.K.; David, E.; Baruch, K.; Lara-Astaiso, D.; Toth, B.; et al. A Unique Microglia Type Associated with Restricting Development of Alzheimer’s Disease. Cell 2017, 169, 1276–1290.e17. [Google Scholar] [CrossRef]
- Krasemann, S.; Madore, C.; Cialic, R.; Baufeld, C.; Calcagno, N.; El Fatimy, R.; Beckers, L.; O’Loughlin, E.; Xu, Y.; Fanek, Z.; et al. The TREM2-APOE Pathway Drives the Transcriptional Phenotype of Dysfunctional Microglia in Neurodegenerative Diseases. Immunity 2017, 47, 566–581.e9. [Google Scholar] [CrossRef]
- Li, Q.; Cheng, Z.; Zhou, L.; Darmanis, S.; Neff, N.F.; Okamoto, J.; Gulati, G.; Bennett, M.L.; Sun, L.O.; Clarke, L.E.; et al. Developmental Heterogeneity of Microglia and Brain Myeloid Cells Revealed by Deep Single-Cell RNA Sequencing. Neuron 2019, 101, 207–223.e10. [Google Scholar] [CrossRef]
- Afridi, R.; Lee, W.H.; Suk, K. Microglia Gone Awry: Linking Immunometabolism to Neurodegeneration. Front. Cell. Neurosci. 2020, 14, 246. [Google Scholar] [CrossRef]
- Tobeh, N.S.; Bruce, K.D. Emerging Alzheimer’s disease therapeutics: Promising insights from lipid metabolism and microglia-focused interventions. Front. Aging Neurosci. 2023, 15, 1259012. [Google Scholar] [CrossRef]
- Marschallinger, J.; Iram, T.; Zardeneta, M.; Lee, S.E.; Lehallier, B.; Haney, M.S.; Pluvinage, J.V.; Mathur, V.; Hahn, O.; Morgens, D.W.; et al. Lipid-droplet-accumulating microglia represent a dysfunctional and proinflammatory state in the aging brain. Nat. Neurosci. 2020, 23, 194–208. [Google Scholar] [CrossRef]
- Haney, M.S.; Palovics, R.; Munson, C.N.; Long, C.; Johansson, P.; Yip, O.; Dong, W.; Rawat, E.; West, E.; Schlachetzki, J.C.; et al. APOE4/4 is linked to damaging lipid droplets in Alzheimer’s microglia. bioRxiv 2023. [Google Scholar] [CrossRef]
- Filipello, F.; You, S.F.; Mirfakhar, F.S.; Mahali, S.; Bollman, B.; Acquarone, M.; Korvatska, O.; Marsh, J.A.; Sivaraman, A.; Martinez, R.; et al. Defects in lysosomal function and lipid metabolism in human microglia harboring a TREM2 loss of function mutation. Acta Neuropathol. 2023, 145, 749–772. [Google Scholar] [CrossRef]
- Rim, C.; You, M.J.; Nahm, M.; Kwon, M.S. Emerging role of senescent microglia in brain aging-related neurodegenerative diseases. Transl. Neurodegener. 2024, 13, 10. [Google Scholar] [CrossRef]
- Shafqat, A.; Khan, S.; Omer, M.H.; Niaz, M.; Albalkhi, I.; AlKattan, K.; Yaqinuddin, A.; Tchkonia, T.; Kirkland, J.L.; Hashmi, S.K. Cellular senescence in brain aging and cognitive decline. Front. Aging Neurosci. 2023, 15, 1281581. [Google Scholar] [CrossRef]
- Gabande-Rodriguez, E.; Perez-Canamas, A.; Soto-Huelin, B.; Mitroi, D.N.; Sanchez-Redondo, S.; Martinez-Saez, E.; Venero, C.; Peinado, H.; Ledesma, M.D. Lipid-induced lysosomal damage after demyelination corrupts microglia protective function in lysosomal storage disorders. EMBO J. 2019, 38, e99553. [Google Scholar] [CrossRef]
- Jadhav, V.S.; Krause, K.H.; Singh, S.K. HIV-1 Tat C modulates NOX2 and NOX4 expressions through miR-17 in a human microglial cell line. J. Neurochem. 2014, 131, 803–815. [Google Scholar] [CrossRef]
- Singh, S.; Thangaraj, A.; Chivero, E.T.; Guo, M.L.; Periyasamy, P.; Buch, S. Role of Dysregulated Autophagy in HIV Tat, Cocaine, and cART Mediated NLRP3 Activation in Microglia. J. Neuroimmune Pharmacol. 2023, 18, 327–347. [Google Scholar] [CrossRef]
- Thangaraj, A.; Chivero, E.T.; Tripathi, A.; Singh, S.; Niu, F.; Guo, M.L.; Pillai, P.; Periyasamy, P.; Buch, S. HIV TAT-mediated microglial senescence: Role of SIRT3-dependent mitochondrial oxidative stress. Redox Biol. 2021, 40, 101843. [Google Scholar] [CrossRef]
- Chivero, E.T.; Guo, M.L.; Periyasamy, P.; Liao, K.; Callen, S.E.; Buch, S. HIV-1 Tat Primes and Activates Microglial NLRP3 Inflammasome-Mediated Neuroinflammation. J. Neurosci. 2017, 37, 3599–3609. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.L.; Cheng, Y.; Pineda, D.M.; Dempsey, R.E.; Yang, L. Lipid Droplets Accumulation in the Brain of HIV Transgenic Rat: Implication in the Accelerated Aging of HIV Infected Individuals. Aging Dis. 2024, 16, 454–468. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Jung, J.; Guo, L.; Shuboni-Mulligan, D.D.; Chen, J.F.; Hu, W.; Guo, M.L. HIV-TAT dysregulates microglial lipid metabolism through SREBP2/miR-124 axis: Implication of lipid droplet accumulation microglia in NeuroHIV. Brain Behav. Immun. 2025, 123, 108–122. [Google Scholar] [CrossRef]
- Fujiwara, R.; Yoda, E.; Tukey, R.H. Species differences in drug glucuronidation: Humanized UDP-glucuronosyltransferase 1 mice and their application for predicting drug glucuronidation and drug-induced toxicity in humans. Drug Metab. Pharmacokinet. 2018, 33, 9–16. [Google Scholar] [CrossRef]
- Ahmed, S.H. Individual decision-making in the causal pathway to addiction: Contributions and limitations of rodent models. Pharmacol. Biochem. Behav. 2018, 164, 22–31. [Google Scholar] [CrossRef]
- Shen, H.W.; Jiang, X.L.; Gonzalez, F.J.; Yu, A.M. Humanized transgenic mouse models for drug metabolism and pharmacokinetic research. Curr. Drug Metab. 2011, 12, 997–1006. [Google Scholar]
- Smith, A.M.; Gibbons, H.M.; Oldfield, R.L.; Bergin, P.M.; Mee, E.W.; Curtis, M.A.; Faull, R.L.; Dragunow, M. M-CSF increases proliferation and phagocytosis while modulating receptor and transcription factor expression in adult human microglia. J. Neuroinflammation 2013, 10, 85. [Google Scholar] [CrossRef]
- Marshall, G.P., 2nd; Demir, M.; Steindler, D.A.; Laywell, E.D. Subventricular zone microglia possess a unique capacity for massive in vitro expansion. Glia 2008, 56, 1799–1808. [Google Scholar] [CrossRef]
- O’Keefe, G.M.; Nguyen, V.T.; Benveniste, E.N. Class II transactivator and class II MHC gene expression in microglia: Modulation by the cytokines TGF-beta, IL-4, IL-13 and IL-10. Eur. J. Immunol. 1999, 29, 1275–1285. [Google Scholar] [CrossRef]
- Smith, A.M.; Graham, E.S.; Feng, S.X.; Oldfield, R.L.; Bergin, P.M.; Mee, E.W.; Faull, R.L.; Curtis, M.A.; Dragunow, M. Adult human glia, pericytes and meningeal fibroblasts respond similarly to IFNy but not to TGFbeta1 or M-CSF. PLoS ONE 2013, 8, e80463. [Google Scholar] [CrossRef]
- Carpentier, P.A.; Duncan, D.S.; Miller, S.D. Glial toll-like receptor signaling in central nervous system infection and autoimmunity. Brain Behav. Immun. 2008, 22, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.S.; Arbour, N.; Manusow, J.; Montgrain, V.; Blain, M.; McCrea, E.; Shapiro, A.; Antel, J.P. TLR signaling tailors innate immune responses in human microglia and astrocytes. J. Immunol. 2005, 175, 4320–4330. [Google Scholar] [PubMed]
- Ding, M.; St Pierre, B.A.; Parkinson, J.F.; Medberry, P.; Wong, J.L.; Rogers, N.E.; Ignarro, L.J.; Merrill, J.E. Inducible nitric-oxide synthase and nitric oxide production in human fetal astrocytes and microglia. A kinetic analysis. J. Biol. Chem. 1997, 272, 11327–11335. [Google Scholar]
- Colasanti, M.; Persichini, T.; Di Pucchio, T.; Gremo, F.; Lauro, G.M. Human ramified microglial cells produce nitric oxide upon Escherichia coli lipopolysaccharide and tumor necrosis factor alpha stimulation. Neurosci. Lett. 1995, 200, 144–146. [Google Scholar]
- Pocock, J.M.; Piers, T.M. Modelling microglial function with induced pluripotent stem cells: An update. Nat. Rev. Neurosci. 2018, 19, 445–452. [Google Scholar] [CrossRef]
- Brownjohn, P.W.; Smith, J.; Solanki, R.; Lohmann, E.; Houlden, H.; Hardy, J.; Dietmann, S.; Livesey, F.J. Functional Studies of Missense TREM2 Mutations in Human Stem Cell-Derived Microglia. Stem Cell Rep. 2018, 10, 1294–1307. [Google Scholar] [CrossRef]
- Garcia-Reitboeck, P.; Phillips, A.; Piers, T.M.; Villegas-Llerena, C.; Butler, M.; Mallach, A.; Rodrigues, C.; Arber, C.E.; Heslegrave, A.; Zetterberg, H.; et al. Human Induced Pluripotent Stem Cell-Derived Microglia-Like Cells Harboring TREM2 Missense Mutations Show Specific Deficits in Phagocytosis. Cell Rep. 2018, 24, 2300–2311. [Google Scholar] [CrossRef]
- Lin, Y.T.; Seo, J.; Gao, F.; Feldman, H.M.; Wen, H.L.; Penney, J.; Cam, H.P.; Gjoneska, E.; Raja, W.K.; Cheng, J.; et al. APOE4 Causes Widespread Molecular and Cellular Alterations Associated with Alzheimer’s Disease Phenotypes in Human iPSC-Derived Brain Cell Types. Neuron 2018, 98, 1141–1154.e7. [Google Scholar] [CrossRef]
- Westendorp, M.O.; Frank, R.; Ochsenbauer, C.; Stricker, K.; Dhein, J.; Walczak, H.; Debatin, K.M.; Krammer, P.H. Sensitization of T cells to CD95-mediated apoptosis by HIV-1 Tat and gp120. Nature 1995, 375, 497–500. [Google Scholar] [CrossRef]
- Goldstein, G. HIV-1 Tat protein as a potential AIDS vaccine. Nat. Med. 1996, 2, 960–964. [Google Scholar] [PubMed]
- Xiao, H.; Neuveut, C.; Tiffany, H.L.; Benkirane, M.; Rich, E.A.; Murphy, P.M.; Jeang, K.T. Selective CXCR4 antagonism by Tat: Implications for in vivo expansion of coreceptor use by HIV-1. Proc. Natl. Acad. Sci. USA 2000, 97, 11466–11471. [Google Scholar] [CrossRef] [PubMed]
- Falnes, P.O.; Sandvig, K. Penetration of protein toxins into cells. Curr. Opin. Cell Biol. 2000, 12, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Fawell, S.; Seery, J.; Daikh, Y.; Moore, C.; Chen, L.L.; Pepinsky, B.; Barsoum, J. Tat-mediated delivery of heterologous proteins into cells. Proc. Natl. Acad. Sci. USA 1994, 91, 664–668. [Google Scholar] [CrossRef]
- Lin, S.; Cheng, H.; Yang, G.; Wang, C.; Leung, C.K.; Zhang, S.; Tan, Y.; Zhang, H.; Wang, H.; Miao, L.; et al. NRF2 Antagonizes HIV-1 Tat and Methamphetamine-Induced BV2 Cell Ferroptosis by Regulating SLC7A11. Neurotox. Res. 2023, 41, 398–407. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, Y.; Guo, H.; Li, Q.; Yan, C.; Li, Y.; He, S.; Wang, N.; Wang, Q. Impaired lipophagy induced-microglial lipid droplets accumulation contributes to the buildup of TREM1 in diabetes-associated cognitive impairment. Autophagy 2023, 19, 2639–2656. [Google Scholar] [CrossRef]
- Xu, Y.; Propson, N.E.; Du, S.; Xiong, W.; Zheng, H. Autophagy deficiency modulates microglial lipid homeostasis and aggravates tau pathology and spreading. Proc. Natl. Acad. Sci. USA 2021, 118, e2023418118. [Google Scholar] [CrossRef]
- Zhu, X.L.; Zhang, H.W.; Peng, W.J.; Gao, S.; Yang, Z.L.; Zhang, J.Q.; Liu, X.S. Autophagy impairment is involved in midazolam-induced lipid droplet accumulation and consequent phagocytosis decrease in BV2 cells. Biochem. Biophys. Res. Commun. 2023, 643, 147–156. [Google Scholar] [CrossRef]
- Thangaraj, A.; Periyasamy, P.; Liao, K.; Bendi, V.S.; Callen, S.; Pendyala, G.; Buch, S. HIV-1 TAT-mediated microglial activation: Role of mitochondrial dysfunction and defective mitophagy. Autophagy 2018, 14, 1596–1619. [Google Scholar] [CrossRef]
- Lei, J.; Yin, X.; Shang, H.; Jiang, Y. IP-10 is highly involved in HIV infection. Cytokine 2019, 115, 97–103. [Google Scholar] [CrossRef]
- Evans, V.A.; Khoury, G.; Saleh, S.; Cameron, P.U.; Lewin, S.R. HIV persistence: Chemokines and their signalling pathways. Cytokine Growth Factor. Rev. 2012, 23, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.Y.; Huang, H.H.; Zhen, C.; Chen, S.Y.; Song, B.; Cao, W.J.; Shen, L.L.; Zhou, M.J.; Zhang, X.C.; Xu, R.; et al. Distinct inflammation-related proteins associated with T cell immune recovery during chronic HIV-1 infection. Emerg. Microbes Infect. 2023, 12, 2150566. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Zhang, S.; Liu, M.; Liu, J. Cardiovascular disease and its risk factors among people living with HIV: A systematic review and meta-analysis. J. Infect. Public. Health 2025, 18, 102654. [Google Scholar] [CrossRef] [PubMed]
- Okello, S.; Ueda, P.; Kanyesigye, M.; Byaruhanga, E.; Kiyimba, A.; Amanyire, G.; Kintu, A.; Fawzi, W.W.; Muyindike, W.R.; Danaei, G. Association between HIV and blood pressure in adults and role of body weight as a mediator: Cross-sectional study in Uganda. J. Clin. Hypertens. 2017, 19, 1181–1191. [Google Scholar] [CrossRef]
- Meseguer-Donlo, J.; Soldado-Folgado, J.; Du, J.; Gonzalez-Mena, A.; Blasco-Hernando, F.; Canas-Ruano, E.; Nogues, X.; Knobel, H.; Garcia-Giralt, N.; Guerri-Fernandez, R. HIV infection is associated with upregulated circulating levels of the inflammaging miR-21-5p. J. Microbiol. Immunol. Infect. 2023, 56, 931–938. [Google Scholar] [CrossRef]
- Narouei, A.; Rouzbahani, N.H.; Ghanbari, M.; Taj, L.; Mohraz, M.; Gholami, M.; Baesi, K. Expression of mir-221, mir-29a, mir-155 and mir-146a in Peripheral Blood Mononuclear Cell (PBMC) in HIV-1 Infected Patients. Infect. Disord. Drug Targets 2021, 21, e270421188776. [Google Scholar] [CrossRef]
- Masip, J.; Gasca-Capote, C.; Jimenez-Leon, M.R.; Peraire, J.; Perez-Gomez, A.; Alba, V.; Malo, A.I.; Leal, L.; Martin, C.R.; Rallon, N.; et al. Differential miRNA plasma profiles associated with the spontaneous loss of HIV-1 control: miR-199a-3p and its potential role as a biomarker for quick screening of elite controllers. Clin. Transl. Med. 2021, 11, e474. [Google Scholar] [CrossRef]
- Yuan, S.; Wu, Q.; Wang, Z.; Che, Y.; Zheng, S.; Chen, Y.; Zhong, X.; Shi, F. miR-223: An Immune Regulator in Infectious Disorders. Front. Immunol. 2021, 12, 781815. [Google Scholar] [CrossRef]
- Yuan, S.; Tang, C.; Chen, D.; Li, F.; Huang, M.; Ye, J.; He, Z.; Li, W.; Chen, Y.; Lin, X.; et al. miR-98 Modulates Cytokine Production from Human PBMCs in Systemic Lupus Erythematosus by Targeting IL-6 mRNA. J. Immunol. Res. 2019, 2019, 9827574. [Google Scholar] [CrossRef]
- Parker, M.I.; Palladino, M.A. MicroRNAs downregulated following immune activation of rat testis. Am. J. Reprod. Immunol. 2017, 77, e12673. [Google Scholar] [CrossRef]
- Kong, W.; Frouard, J.; Xie, G.; Corley, M.J.; Helmy, E.; Zhang, G.; Schwarzer, R.; Montano, M.; Sohn, P.; Roan, N.R.; et al. Neuroinflammation generated by HIV-infected microglia promotes dysfunction and death of neurons in human brain organoids. Proc. Natl. Acad. Sci. USA 2024, 3, 179. [Google Scholar] [CrossRef]
- Boreland, A.J.; Stillitano, A.C.; Lin, H.C.; Abbo, Y.; Hart, R.P.; Jiang, P.; Pang, Z.P.; Rabson, A.B. Sustained type I interferon signaling after human immunodeficiency virus type 1 infection of human iPSC derived microglia and cerebral organoids. iScience 2024, 27, 109628. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Liu, J.B.; Wang, X.; Meng, F.Z.; Xiao, Q.H.; Liu, L.; Zhu, J.; Hu, W.H.; Ho, W.Z. Activation of Toll-like receptor 3 inhibits HIV infection of human iPSC-derived microglia. J. Med. Virol. 2023, 95, e29217. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, L.L.; Jiang, R.; Cheng, Y.; Russell, B.; Sanders, Y.Y.; Guo, M.-L. HIV Protein TAT Dysregulates Multiple Pathways in Human iPSCs-Derived Microglia. Life 2025, 15, 1082. https://doi.org/10.3390/life15071082
Guo LL, Jiang R, Cheng Y, Russell B, Sanders YY, Guo M-L. HIV Protein TAT Dysregulates Multiple Pathways in Human iPSCs-Derived Microglia. Life. 2025; 15(7):1082. https://doi.org/10.3390/life15071082
Chicago/Turabian StyleGuo, Liam Liyang, Robert Jiang, Yan Cheng, Brooke Russell, Yan Y. Sanders, and Ming-Lei Guo. 2025. "HIV Protein TAT Dysregulates Multiple Pathways in Human iPSCs-Derived Microglia" Life 15, no. 7: 1082. https://doi.org/10.3390/life15071082
APA StyleGuo, L. L., Jiang, R., Cheng, Y., Russell, B., Sanders, Y. Y., & Guo, M.-L. (2025). HIV Protein TAT Dysregulates Multiple Pathways in Human iPSCs-Derived Microglia. Life, 15(7), 1082. https://doi.org/10.3390/life15071082





