Influence of Insert Brand and Culture Method on Ciliary Activity and Epithelial Cell Types in Human Nasal Air–Liquid Interface Cell Cultures
Abstract
1. Introduction
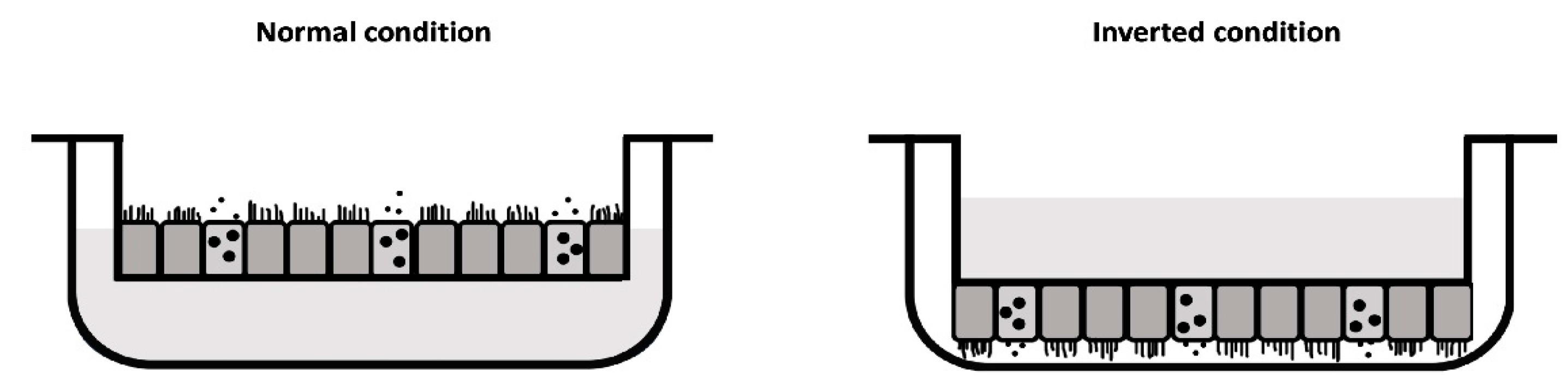
2. Materials and Methods
2.1. Human Nasal Epithelial Cell (hNEC) Cultures
2.2. High-Speed Video Microscopy (HSVM) for Analysis of Ciliary Activity
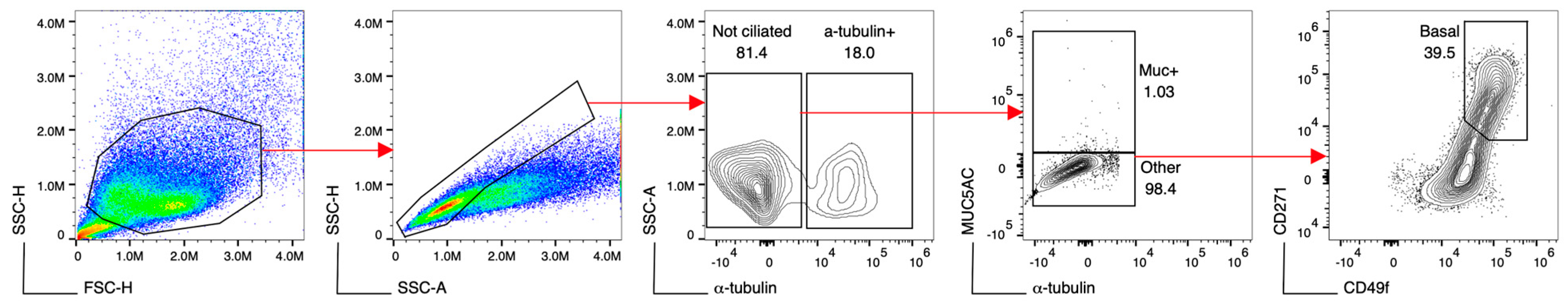
2.3. Flow Cytometry for Analysis of Cell Culture Composition
2.4. Statistical Analysis
3. Results
3.1. Study Population
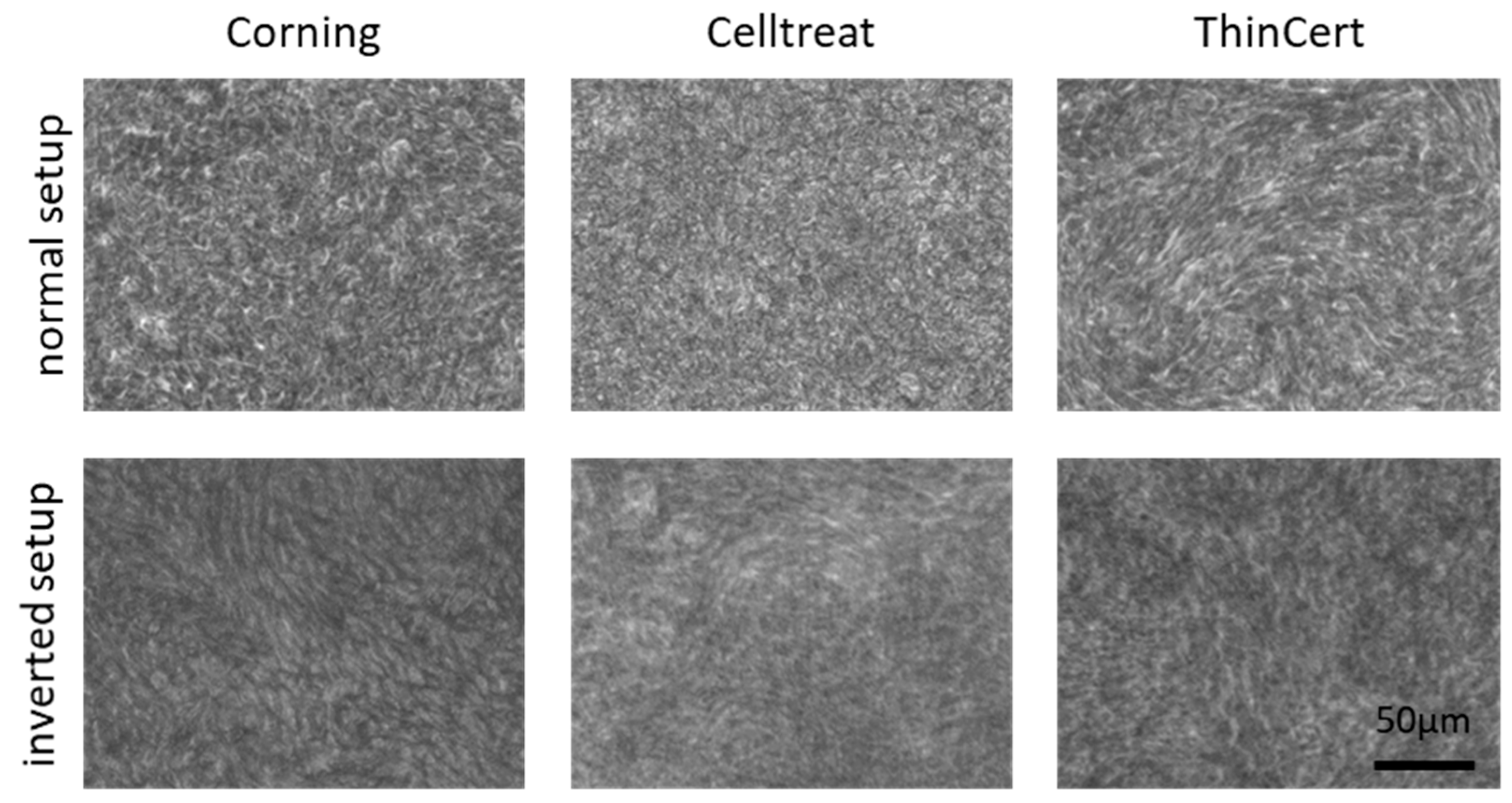
3.2. No Morphological Differences Between Insert Types and Culture Conditions
3.3. Comparison of Physical Characteristics of Insert Types
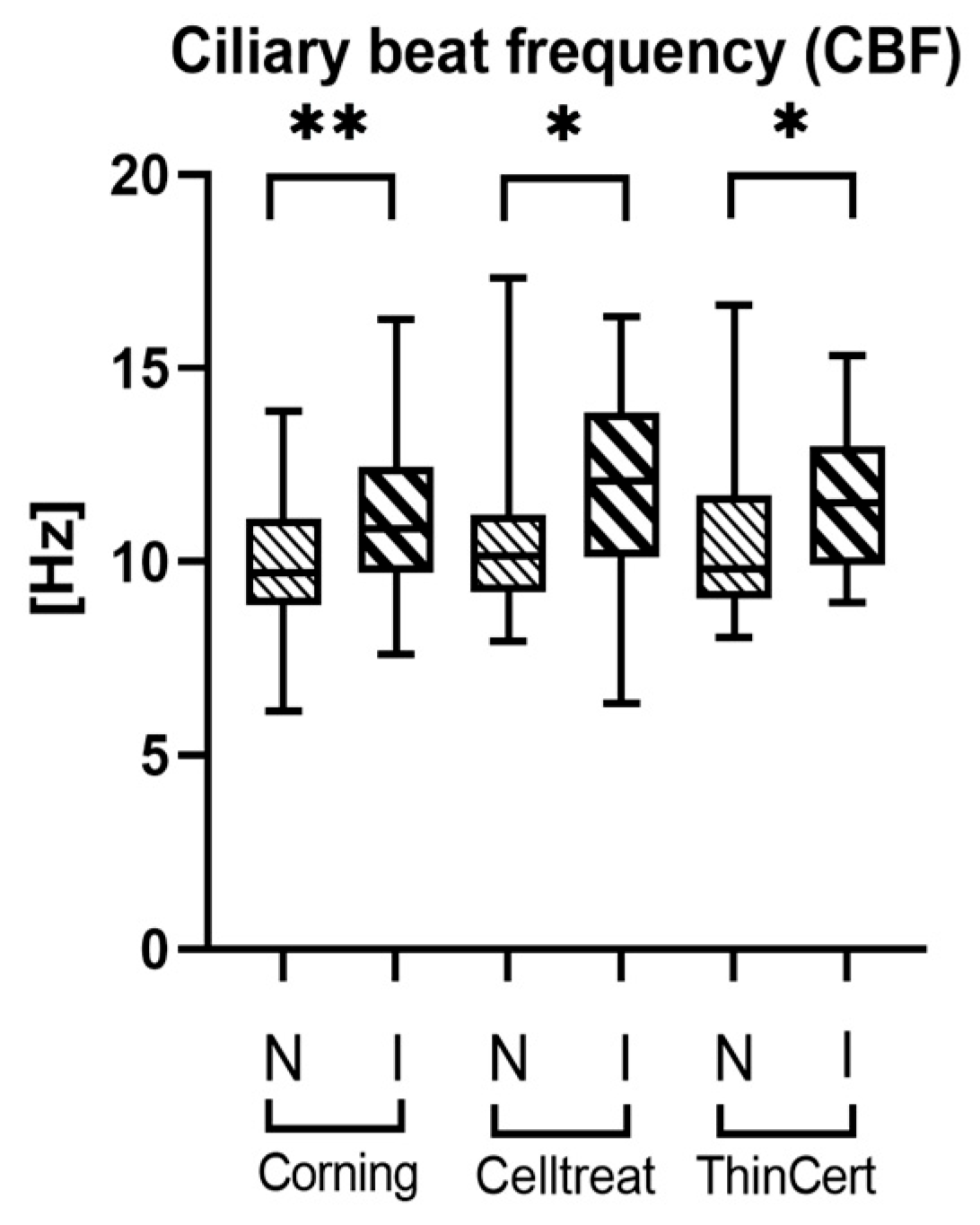
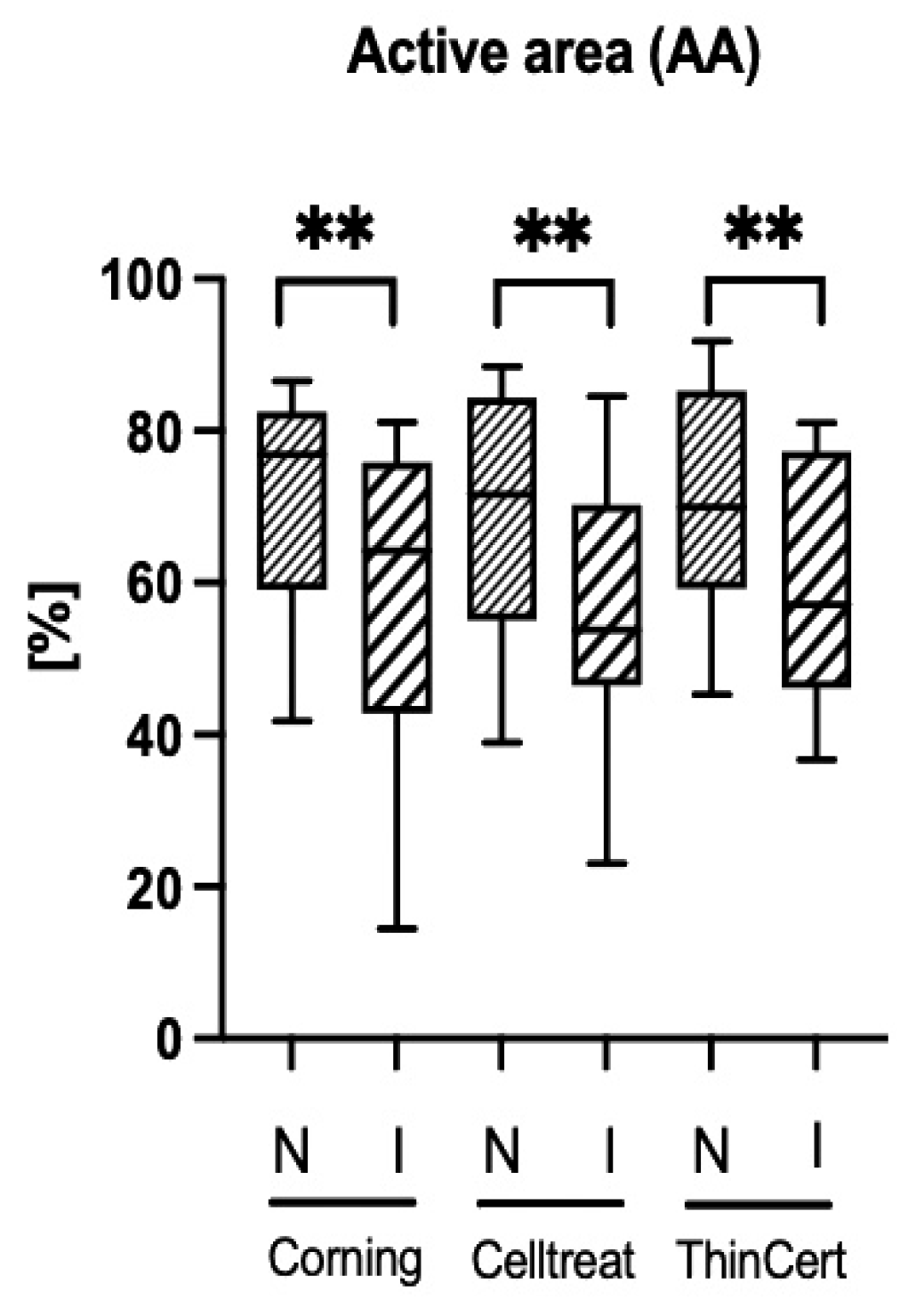
3.4. Ciliary Activity Differs Between Culture Conditions, but Not Between Insert Brands
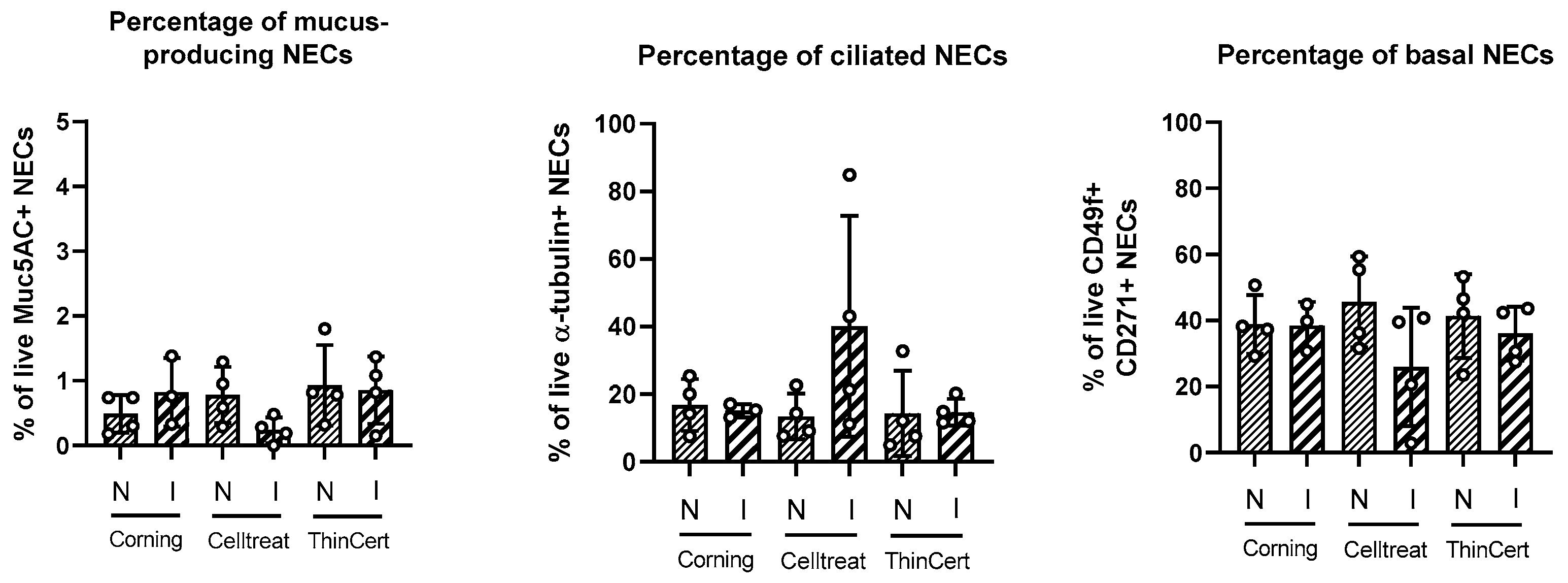
3.5. No Differences in Cell Culture Composition
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kuek, L.E.; Lee, R.J. First Contact: The Role of Respiratory Cilia in Host-Pathogen Interactions in the Airways. Am. J. Physiol. Lung Cell. Mol. Physiol. 2020, 319, L603–L619. [Google Scholar] [CrossRef] [PubMed]
- Crystal, R.G.; Randell, S.H.; Engelhardt, J.F.; Voynow, J.; Sunday, M.E. Airway Epithelial Cells: Current Concepts and Challenges. Proc. Am. Thorac. Soc. 2008, 5, 772–777. [Google Scholar] [CrossRef] [PubMed]
- Whitsett, J.A. Airway Epithelial Differentiation and Mucociliary Clearance. Ann. Am. Thorac. Soc. 2018, 15, S143–S148. [Google Scholar] [CrossRef] [PubMed]
- Ochs, M.; Weibel, E.R. Functional design of the human lung for gas exchange. In Fishman’s Pulmonary Diseases and Disorders; Fishman, A.P., Elias, J.A., Fishman, J.A., Grippi, M.A., Senior, R.M., Pack, A.I., Eds.; Mc Graw Hill: New York, NY, USA, 2008; pp. 23–69. [Google Scholar]
- Bustamante-Marin, X.M.; Ostrowski, L.E. Cilia and Mucociliary Clearance. Cold Spring Harb. Perspect. Biol. 2017, 9, a028241. [Google Scholar] [CrossRef]
- Button, B.; Cai, L.-H.; Ehre, C.; Kesimer, M.; Hill, D.B.; Sheehan, J.K.; Boucher, R.C.; Rubinstein, M. A Periciliary Brush Promotes the Lung Health by Separating the Mucus Layer from Airway Epithelia. Science 2012, 337, 937–941. [Google Scholar] [CrossRef]
- Lucas, J.S.; Burgess, A.; Mitchison, H.M.; Moya, E.; Williamson, M.; Hogg, C. Diagnosis and management of primary ciliary dyskinesia. Arch. Dis. Child. 2014, 99, 850–856. [Google Scholar] [CrossRef]
- Peabody, J.E.; Shei, R.-J.; Bermingham, B.M.; Phillips, S.E.; Turner, B.; Rowe, S.M.; Solomon, G.M. Seeing Cilia: Imaging Modalities for Ciliary Motion and Clinical Connections. Am. J. Physiol. Lung Cell. Mol. Physiol. 2018, 314, L909–L921. [Google Scholar] [CrossRef]
- Baldassi, D.; Gabold, B.; Merkel, O.M. Air−Liquid Interface Cultures of the Healthy and Diseased Human Respiratory Tract: Promises, Challenges, and Future Directions. Adv. NanoBiomed Res. 2021, 1, 2000111. [Google Scholar] [CrossRef]
- Silva, S.; Bicker, J.; Falcão, A.; Fortuna, A. Air-Liquid Interface (ALI) Impact on Different Respiratory Cell Cultures. Eur. J. Pharm. Biopharm. 2023, 184, 62–82. [Google Scholar] [CrossRef]
- Müller, L.; Brighton, L.E.; Carson, J.L.; Fischer, W.A.; Jaspers, I. Culturing of Human Nasal Epithelial Cells at the Air Liquid Interface. J. Vis. Exp. 2013, 80, e50646. [Google Scholar] [CrossRef]
- Yaqub, N.; Wayne, G.; Birchall, M.; Song, W. Recent Advances in Human Respiratory Epithelium Models for Drug Discovery. Biotechnol. Adv. 2021, 54, 107832. [Google Scholar] [CrossRef] [PubMed]
- Michi, A.N.; Proud, D. A Toolbox for Studying Respiratory Viral Infections Using Air-Liquid Interface Cultures of Human Airway Epithelial Cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2021, 321, L263–L279. [Google Scholar] [CrossRef]
- Lenz, A.-G.; Stoeger, T.; Cei, D.; Schmidmeir, M.; Semren, N.; Burgstaller, G.; Lentner, B.; Eickelberg, O.; Meiners, S.; Schmid, O. Efficient Bioactive Delivery of Aerosolized Drugs to Human Pulmonary Epithelial Cells Cultured in Air–Liquid Interface Conditions. Am. J. Respir. Cell Mol. Biol. 2014, 51, 526–535. [Google Scholar] [CrossRef] [PubMed]
- Verstraelen, S.; Jacobs, A.; Laer, J.V.; Hollanders, K.; Deun, M.V.; Bertels, D.; Brabers, R.; Witters, H.; Remy, S.; Geerts, L.; et al. An in Vitro Air-Liquid Interface Inhalation Platform for Petroleum Substances and Constituents. ALTEX—Altern. Anim. Exp. 2021, 38, 550–564. [Google Scholar] [CrossRef]
- Jaspers, I.; Ciencewicki, J.M.; Zhang, W.; Brighton, L.E.; Carson, J.L.; Beck, M.A.; Madden, M.C. Diesel exhaust enhances influenza virus infections in respiratory epithelial cells. Toxicol. Sci. 2005, 85, 990–1002. [Google Scholar] [CrossRef] [PubMed]
- Brocke, S.A.; Billings, G.T.; Taft-Benz, S.; Alexis, N.E.; Heise, M.T.; Jaspers, I. Woodsmoke particle exposure prior to SARS-CoV-2 infection alters antiviral response gene expression in human nasal epithelial cells in a sex-dependentmanner. American Journal of Physiology. Lung Cell. Mol. Physiol. 2022, 322, L479–L494. [Google Scholar] [CrossRef]
- Müller, L.; Savas, S.T.; Tschanz, S.A.; Stokes, A.; Escher, A.; Nussbaumer, M.; Bullo, M.; Kuehni, C.E.; Blanchon, S.; Jung, A.; et al. A Comprehensive Approach for the Diagnosis of Primary Ciliary Dyskinesia-Experiences from the First 100 Patients of the PCD-UNIBE Diagnostic Center. Diagnostics 2021, 11, 1540. [Google Scholar] [CrossRef]
- Redman, E.; Fierville, M.; Cavard, A.; Plaisant, M.; Arguel, M.-J.; Ruiz Garcia, S.; McAndrew, E.M.; Girard-Riboulleau, C.; Lebrigand, K.; Magnone, V.; et al. Cell Culture Differentiation and Proliferation Conditions Influence the in Vitro Regeneration of the Human Airway Epithelium. Am. J. Respir. Cell Mol. Biol. 2024, 71, 267–281. [Google Scholar] [CrossRef]
- Luengen, A.E.; Kniebs, C.; Buhl, E.M.; Cornelissen, C.G.; Schmitz-Rode, T.; Jockenhoevel, S.; Thiebes, A.L. Choosing the Right Differentiation Medium to Develop Mucociliary Phenotype of Primary Nasal Epithelial Cells in Vitro. Sci. Rep. 2020, 10, 6963. [Google Scholar] [CrossRef]
- Saint-Criq, V.; Delpiano, L.; Casement, J.; Onuora, J.C.; Lin, J.; Gray, M.A. Choice of Differentiation Media Significantly Impacts Cell Lineage and Response to CFTR Modulators in Fully Differentiated Primary Cultures of Cystic Fibrosis Human Airway Epithelial Cells. Cells 2020, 9, 2137. [Google Scholar] [CrossRef]
- Brocke, S.A.; Speen, A.M.; Masood, S.; Worden, C.P.; Jaspers, I. Comparison of Permeable Cell Culture Inserts for Use in Culture of a Human in Vitro Air-Liquid Interface Model System. Physiol. Rep. 2024, 12, e15921. [Google Scholar] [CrossRef] [PubMed]
- Escher, A.; Kieninger, E.; Groof, S.D.; Savas, S.T.; Schneiter, M.; Tschanz, S.A.; Frenz, M.; Latzin, P.; Casaulta, C.; Müller, L. In Vitro Effect of Combined Hypertonic Saline and Salbutamol on Ciliary Beating Frequency and Mucociliary Transport in Human Nasal Epithelial Cells of Healthy Volunteers and Patients with Cystic Fibrosis. J. Aerosol Med. Pulm. Drug Deliv. 2023, 36, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Schneiter, M.; Tschanz, S.A.; Escher, A.; Müller, L.; Frenz, M. The Cilialyzer—A Freely Available Open-Source Software for the Analysis of Mucociliary Activity in Respiratory Cells. Comput. Methods Programs Biomed. 2023, 241, 107744. [Google Scholar] [CrossRef]
- Yonker, L.M.; Mou, H.; Chu, K.K.; Pazos, M.A.; Leung, H.; Cui, D.; Ryu, J.-H.; Hibbler, R.M.; Eaton, A.D.; Ford, T.E.; et al. Development of a Primary Human Co-Culture Model of Inflamed Airway Mucosa. Sci. Rep. 2017, 7, 8182. [Google Scholar] [CrossRef]
- Delmotte, P.; Sanderson, M.J. Ciliary Beat Frequency Is Maintained at a Maximal Rate in the Small Airways of Mouse Lung Slices. Am. J. Respir. Cell Mol. Biol. 2006, 35, 110–117. [Google Scholar] [CrossRef] [PubMed]
| Corning® Transwell® | CELLTREAT® | ThinCert® | |
|---|---|---|---|
| Material | Polyethylene terephthalate | Polyethylene | Polyethylene terephthalate |
| Pore diameter | 0.4 µm | 0.4 µm | 0.4 µm |
| Culture area | 1.12 cm2 | 1.11 cm2 * | 1.131 cm2 |
| Pore density | 4 × 106/cm2 | not provided | 2 × 106/cm2 |
| Membrane diameter | 12 mm | 11.91 mm * | 12.07 mm |
| Sex (Male/Female) | Mean Age (Years) | Age Range (Years) | |
|---|---|---|---|
| CBF/AA analysis | 10/7 | 34.8 | 24–60 |
| Cell culture composition | 2/2 | 33.8 | 25–46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Celkova, P.; Seydoux, E.; De Groof, S.; Müller, L. Influence of Insert Brand and Culture Method on Ciliary Activity and Epithelial Cell Types in Human Nasal Air–Liquid Interface Cell Cultures. Life 2025, 15, 958. https://doi.org/10.3390/life15060958
Celkova P, Seydoux E, De Groof S, Müller L. Influence of Insert Brand and Culture Method on Ciliary Activity and Epithelial Cell Types in Human Nasal Air–Liquid Interface Cell Cultures. Life. 2025; 15(6):958. https://doi.org/10.3390/life15060958
Chicago/Turabian StyleCelkova, Patricia, Emilie Seydoux, Susan De Groof, and Loretta Müller. 2025. "Influence of Insert Brand and Culture Method on Ciliary Activity and Epithelial Cell Types in Human Nasal Air–Liquid Interface Cell Cultures" Life 15, no. 6: 958. https://doi.org/10.3390/life15060958
APA StyleCelkova, P., Seydoux, E., De Groof, S., & Müller, L. (2025). Influence of Insert Brand and Culture Method on Ciliary Activity and Epithelial Cell Types in Human Nasal Air–Liquid Interface Cell Cultures. Life, 15(6), 958. https://doi.org/10.3390/life15060958






