Bladder p75NTR-Mediated Anti-Inflammatory Response via the TLR4/TRAF6/NF-κB Axis
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Cell Culture
2.3. In Vivo Transurethral Infection
2.4. Bladder Physiological Recordings
2.5. Western Blotting
2.6. RTqPCR
2.7. NF-kB Nuclear Translocation
2.8. Immunoprecipitation
2.9. Histology
2.10. Immunohistochemistry
2.11. Nitric Oxide Assay
2.12. Elisa Kit
2.13. Data Analysis
3. Results
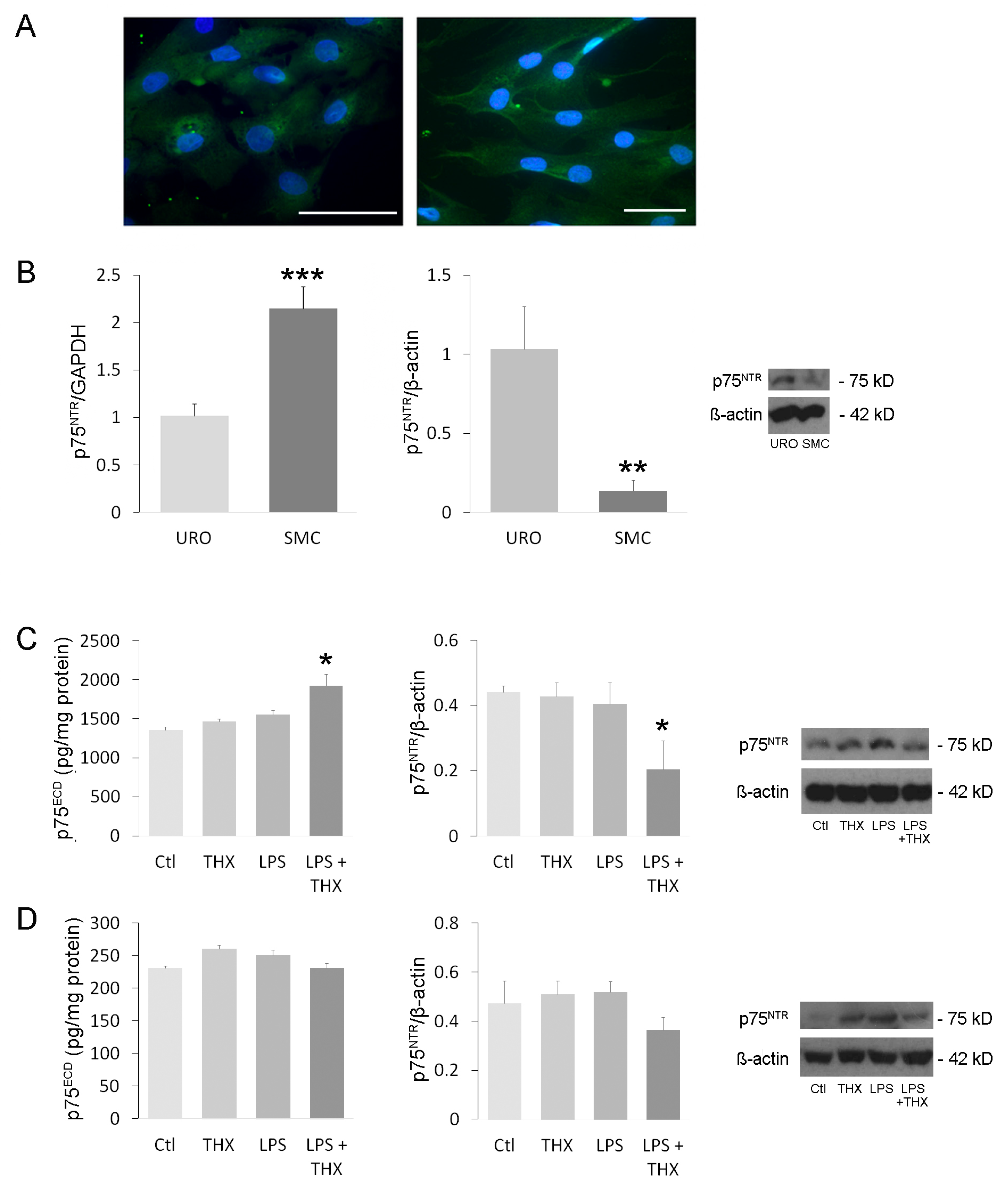
3.1. Expression and Activation of Receptor p75NTR in Bladder Cells
3.2. TNF-α Cell Content and Nitric Oxide (NO) Secretion in the Presence of LPS and THX-B
3.3. Inflammatory and Survival Pathways in Bladder Cells During LPS Incubation
3.4. LPS on Tight Junctions
3.5. Assessment of In Vivo Inflammation
3.6. Organ Bath Recordings
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CCAC | Canadian Council for Animal Care |
| DMEM | Dulbecco’s Modified Eagle Medium |
| EFS | Electrical Field Stimulation |
| ERK | Extracellular Signal-Regulated Kinase |
| FBS | Fetal Bovine Serum |
| H&E | Hematoxylin and Eosin |
| IC/BPS | Interstitial Cystitis or Bladder Pain Syndrome |
| iNOS | Inducible Nitric Oxide Synthase |
| JNK | c-Jun N-terminal Kinase |
| LPS | Lipopolysaccharide |
| NEDD | N-(1-Naphthyl)ethylenediamine dihydrochloride |
| NF-κB | Nuclear Factor Kappa B |
| NO | Nitric Oxide |
| p38MAPK | p38 Mitogen-Activated Protein Kinase |
| p75NTR | p75 Neurotrophin Receptor |
| PBS | Phosphate-Buffered Saline |
| PKC | Protein Kinase C |
| proBDNF | Proform of Brain-Derived Neurotrophic Factor |
| proNGF | Proform of the Nerve Growth Factor |
| SD | Sprague Dawley |
| SMC | Smooth Muscle Cell |
| TBST | Tris-Buffered Saline with Tween 20 |
| THX-B | pharmacological antagonist of p75NTR |
| TLR4 | Toll-Like Receptor 4 |
| TNF-α | Tumor Necrosis Factor alpha |
| TRAF6 | Tumor Necrosis Factor Receptor-Associated Factor 6 |
| UTI | Urinary Tract Infection |
| Y27623 | Rho inhibitor 4-((1R)-1-aminoethyl)-N-pyridin-4-ylcyclohexane-1-carboxamide |
| ZO-1 | Zonula Occludens 1 |
References
- Foxman, B. Urinary Tract Infection Syndromes: Occurrence, Recurrence, Bacteriology, Risk Factors, and Disease Burden. Infect. Dis. Clin. 2014, 28, 1–13. [Google Scholar]
- O’Brien, V.P.; Hannan, T.J.; Nielsen, H.V.; Hultgren, S.J. Drug and Vaccine Development for the Treatment and Prevention of Urinary Tract Infections. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.W.; Chlebicki, M.P. Urinary Tract Infections in Adults. Singapore Med. J. 2016, 57, 485. [Google Scholar] [CrossRef]
- Mustafa, S.; Cagri, K.; Selcuk, G. Recurrent Bladder Cystitis: Who Takes the Role? World J. Urol. 2020, 38, 2755–2760. [Google Scholar]
- Paul, R. State of the Globe: Rising Antimicrobial Resistance of Pathogens in Urinary Tract Infection. J. Glob. Infect. Dis. 2018, 10, 117. [Google Scholar] [CrossRef]
- Kaye, K.S.; Gupta, V.; Mulgirigama, A.; Joshi, A.V.; Scangarella-Oman, N.E.; Yu, K.; Ye, G.; Mitrani-Gold, F.S. Antimicrobial Re-Sistance Trends in Urine Escherichia Coli Isolates from Adult and Adolescent Females in the United States from 2011 to 2019: Rising ESBL Strains and Impact on Patient Management. Clin. Infect. Dis. 2021, 73, 1992–1999. [Google Scholar] [CrossRef] [PubMed]
- Chung, H. The Association between Chronic Inflammation and Recurrent Cystitis in Women. Urogenit. Tract Infect. 2016, 11, 86–92. [Google Scholar] [CrossRef]
- Arinzon, Z.; Shabat, S.; Peisakh, A.; Berner, Y. Clinical Presentation of Urinary Tract Infection (UTI) Differs with Aging in Wom-En. Arch. Gerontol. Geriatr. 2012, 55, 145–147. [Google Scholar] [CrossRef]
- Lu, J.-L.; Xia, Q.-D.; Sun, Y.; Xun, Y.; Hu, H.-L.; Liu, C.-Q.; Sun, J.-X.; Xu, J.-Z.; Hu, J.; Wang, S.-G. Toll-like Receptor 4 as a Favorable Prognostic Marker in Bladder Cancer: A Multi-Omics Analysis. Front. Cell Dev. Biol. 2021, 9, 1077. [Google Scholar] [CrossRef]
- Mossa, A.H.; Galan, A.; Cammisotto, P.G.; Velasquez Flores, M.; Shamout, S.; Barcelona, P.; Saragovi, H.U.; Campeau, L. Antagonism of proNGF or Its Receptor p75NTR Reverses Remodelling and Improves Bladder Function in a Mouse Model of Diabetic Voiding Dysfunction. Diabetologia 2020, 63, 1932–1946. [Google Scholar] [CrossRef]
- Elshaer, S.L.; Alwhaibi, A.; Mohamed, R.; Lemtalsi, T.; Coucha, M.; Longo, F.M.; El-Remessy, A.B. Modulation of the P75 Neuro-Trophin Receptor Using LM11A-31 Prevents Diabetes-Induced Retinal Vascular Permeability in Mice via Inhibition of Inflam-Mation and the RhoA Kinase Pathway. Diabetologia 2019, 62, 1488–1500. [Google Scholar] [CrossRef] [PubMed]
- Zabbarova, I.V.; Ikeda, Y.; Carder, E.J.; Wipf, P.; Wolf-Johnston, A.S.; Birder, L.A.; Yoshimura, N.; Getchell, S.E.; Almansoori, K.; Tyagi, P. Targeting P75 Neurotrophin Receptors Ameliorates Spinal Cord Injury-induced Detrusor Sphincter Dyssynergia in Mice. Neurourol. Urodyn. 2018, 37, 2452–2461. [Google Scholar] [CrossRef]
- Ciesielska, A.; Matyjek, M.; Kwiatkowska, K. TLR4 and CD14 Trafficking and Its Influence on LPS-Induced pro-Inflammatory Signaling. Cell. Mol. Life Sci. 2021, 78, 1233–1261. [Google Scholar] [CrossRef]
- Volosin, M.; Trotter, C.; Cragnolini, A.; Kenchappa, R.S.; Light, M.; Hempstead, B.L.; Carter, B.D.; Friedman, W.J. Induction of Proneurotrophins and Activation of P75NTR -Mediated Apoptosis via Neurotrophin Receptor-Interacting Factor in Hippocampal Neurons after Seizures. J. Neurosci. 2008, 28, 9870–9879. [Google Scholar] [CrossRef]
- Lu, Y.-C.; Yeh, W.-C.; Ohashi, P.S. LPS/TLR4 Signal Transduction Pathway. Cytokine 2008, 42, 145–151. [Google Scholar] [CrossRef]
- Charalampopoulos, I.; Vicario, A.; Pediaditakis, I.; Gravanis, A.; Simi, A.; Ibáñez, C.F. Genetic Dissection of Neurotrophin Sig-Naling through the P75 Neurotrophin Receptor. Cell Rep. 2012, 2, 1563–1570. [Google Scholar] [CrossRef] [PubMed]
- Mysona, B.A.; Al-Gayyar, M.M.; Matragoon, S.; Abdelsaid, M.A.; El-Azab, M.F.; Saragovi, H.U.; El-Remessy, A.B. Modulation of p75NTR Prevents Diabetes-and proNGF-Induced Retinal Inflammation and Blood–Retina Barrier Breakdown in Mice and Rats. Diabetologia 2013, 56, 2329–2339. [Google Scholar] [CrossRef]
- Lee, S.; Mattingly, A.; Lin, A.; Sacramento, J.; Mannent, L.; Castel, M.-N.; Canolle, B.; Delbary-Gossart, S.; Ferzaz, B.; Mor-ganti, J.M. A Novel Antagonist of p75NTR Reduces Peripheral Expansion and CNS Trafficking of Pro-Inflammatory Monocytes and Spares Function after Traumatic Brain Injury. J. Neuroinflamm. 2016, 13, 88. [Google Scholar] [CrossRef] [PubMed]
- Hamouda, A.; Sirmakesyan, S.; Hajj, A.; Cammisotto, P.G.; Saragovi, H.U.; Campeau, L. p75NTR Antagonist THX-B Increases Mature Nerve Growth Factor Secretion by Bladder Cells through Decreased Activity of Matrix Metalloproteinase-9. Mol. Cell. Endocrinol. 2025, 599, 112487. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2− ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Mossa, A.H.; Abdaem, J.; Cammisotto, P.; Campeau, L. Deleterious Impact of Nerve Growth Factor Precursor (proNGF) on Bladder Urothelial and Smooth Muscle Cells. Cell Signal. 2021, 81, 109936. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhang, X.; Broderick, M.; Fein, H. Measurement of Nitric Oxide Production in Biological Systems by Using Griess Reac-Tion Assay. Sensors 2003, 3, 276–284. [Google Scholar] [CrossRef]
- Amura, C.R.; Chen, L.-C.; Hirohashi, N.; Lei, M.-G.; Morrison, D.C. Two Functionally Independent Pathways for Lipopolysac-Charide-Dependent Activation of Mouse Peritoneal Macrophages. J. Immunol. 1997, 159, 5079–5083. [Google Scholar] [CrossRef]
- Mumtaz, F.; Khan, M.; Thompson, C.; Morgan, R.; Mikhailidis, D. Nitric Oxide in the Lower Urinary Tract: Physiological and Pathological Implications. BJU Int. 2000, 85, 567–578. [Google Scholar] [CrossRef]
- Poljakovic, M.; Svensson, M.-L.; Svanborg, C.; Johansson, K.; Larsson, B.; Persson, K. Escherichia Coli-Induced Inducible Nitric Oxide Synthase and Cyclooxygenase Expression in the Mouse Bladder and Kidney. Kidney Int. 2001, 59, 893–904. [Google Scholar] [CrossRef] [PubMed]
- Toullec, D.; Pianetti, P.; Coste, H.; Bellevergue, P.; Grand-Perret, T.; Ajakane, M.; Baudet, V.; Boissin, P.; Boursier, E.; Loriolle, F. The Bisindolylmaleimide GF 109203X Is a Potent and Selective Inhibitor of Protein Kinase C. J. Biol. Chem. 1991, 266, 15771–15781. [Google Scholar] [CrossRef] [PubMed]
- Khursigara, G.; Orlinick, J.R.; Chao, M.V. Association of the P75 Neurotrophin Receptor with TRAF6. J. Biol. Chem. 1999, 274, 2597–2600. [Google Scholar] [CrossRef]
- Inouye, B.M.; Hughes, F.M., Jr.; Sexton, S.J.; Purves, J.T. The Emerging Role of Inflammasomes as Central Mediators in Inflamma-Tory Bladder Pathology. Curr. Urol. 2018, 11, 57–72. [Google Scholar] [CrossRef]
- Yura, R.E.; Bradley, S.G.; Antonetti, D.; Reeves, W.B.; Bond, J.S. Meprin Metalloproteases Play A Role in Host Response to Urinary Tract Infection; Wiley Online Library: Hoboken, NJ, USA, 2007. [Google Scholar]
- Liu, H.-T.; Shie, J.-H.; Chen, S.-H.; Wang, Y.-S.; Kuo, H.-C. Differences in Mast Cell Infiltration, E-Cadherin, and Zonula Oc-Cludens-1 Expression between Patients with Overactive Bladder and Interstitial Cystitis/Bladder Pain Syndrome. Urology 2012, 80, 13–225. [Google Scholar] [CrossRef]
- Fanning, A.S.; Jameson, B.J.; Jesaitis, L.A.; Anderson, J.M. The Tight Junction Protein ZO-1 Establishes a Link between the Trans-Membrane Protein Occludin and the Actin Cytoskeleton. J. Biol. Chem. 1998, 273, 29745–29753. [Google Scholar] [CrossRef]
- Tunggal, J.A.; Helfrich, I.; Schmitz, A.; Schwarz, H.; Günzel, D.; Fromm, M.; Kemler, R.; Krieg, T.; Niessen, C.M. E-cadherin Is Es-Sential for in Vivo Epidermal Barrier Function by Regulating Tight Junctions. EMBO J. 2005, 24, 1146–1156. [Google Scholar] [CrossRef] [PubMed]
- Maake, C.; Landman, M.; Wang, X.; Schmid, D.; Ziegler, U.; John, H. Expression of Smoothelin in the Normal and the Overactive Human Bladder. J. Urol. 2006, 175, 1152–1157. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, M.S.; Fahnestock, M. ProNGF, but Not NGF, Switches from Neurotrophic to Apoptotic Activity in Response to Reduc-Tions in TrkA Receptor Levels. Int. J. Mol. Sci. 2017, 18, 599. [Google Scholar] [CrossRef]
- Girard, B.M.; Malley, S.E.; Vizzard, M.A. Neurotrophin/Receptor Expression in Urinary Bladder of Mice with Overexpression of NGF in Urothelium. Am. J. Physiol.-Ren. Physiol. 2011, 300, 345–355. [Google Scholar] [CrossRef]
- Frade, J.M. Nuclear Translocation of the P75 Neurotrophin Receptor Cytoplasmic Domain in Response to Neurotrophin Binding. J. Neurosci. 2005, 25, 1407–1411. [Google Scholar] [CrossRef]
- Kraemer, B.R.; Snow, J.P.; Vollbrecht, P.; Pathak, A.; Valentine, W.M.; Deutch, A.Y.; Carter, B.D. A Role for the P75 Neurotrophin Receptor in Axonal Degeneration and Apoptosis Induced by Oxidative Stress. J. Biol. Chem. 2014, 289, 21205–21216. [Google Scholar] [CrossRef]
- Duan, L.; Chen, B.-Y.; Sun, X.-L.; Luo, Z.-J.; Rao, Z.-R.; Wang, J.-J.; Chen, L.-W. LPS-Induced proNGF Synthesis and Release in the N9 and BV2 Microglial Cells: A New Pathway Underling Microglial Toxicity in Neuroinflammation. PLoS ONE 2013, 8, 73768. [Google Scholar] [CrossRef] [PubMed]
- Mossa, A.; Flores, M.V.; Nguyen, H.; Cammisotto, P.G.; Campeau, L. Beta-3 Adrenoceptor Signaling Pathways in Urothelial and Smooth Muscle Cells in the Presence of Succinate. J. Pharmacol. Exp. Ther. 2018, 367, 252–259. [Google Scholar] [CrossRef]
- Anastasia, A.; Barker, P.A.; Chao, M.V.; Hempstead, B.L. Detection of p75NTR Trimers: Implications for Receptor Stoichiometry and Activation. J. Neurosci. 2015, 35, 11911–11920. [Google Scholar] [CrossRef]
- Lebrun-Julien, F.; Bertrand, M.J.; Backer, O.; Stellwagen, D.; Morales, C.R.; Polo, A.; Barker, P.A. ProNGF Induces TNFα-Dependent Death of Retinal Ganglion Cells through a p75NTR Non-Cell-Autonomous Signaling Pathway. Proc. Natl. Acad. Sci. USA 2010, 107, 3817–3822. [Google Scholar] [CrossRef]
- Bai, Y.; Dergham, P.; Nedev, H.; Xu, J.; Galan, A.; Rivera, J.C.; ZhiHua, S.; Mehta, H.M.; Woo, S.B.; Sarunic, M.V. Chronic and Acute Models of Retinal Neurodegeneration TrkA Activity Are Neuroprotective Whereas p75NTR Activity Is Neurotoxic through a Paracrine Mechanism. J. Biol. Chem. 2010, 285, 39392–39400. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-D.; Lee, M.-H. Decreased Expression of Zonula Occludens-1 and Occludin in the Bladder Urothelium of Patients with Interstitial Cystitis/Painful Bladder Syndrome. J. Formos. Med. Assoc. 2014, 113, 17–22. [Google Scholar] [CrossRef]
- Qin, L.-H.; Huang, W.; Mo, X.-A.; Chen, Y.-L.; Wu, X.-H. LPS Induces Occludin Dysregulation in Cerebral Microvascular Endothelial Cells via MAPK Signaling and Augmenting MMP-2 Levels. Oxid. Med. Cell. Longev. 2015, 2015, 120641. [Google Scholar] [CrossRef] [PubMed]
- Azadzoi, K.M. Effect of Chronic Ischemia on Bladder Structure and Function, Bladder Disease, Part A. Res. Concepts Clin. Appl. 2003, 539, 271–280. [Google Scholar]
- Chung, C.-W.; Zhang, Q.L.; Qiao, L.-Y. Endogenous Nerve Growth Factor Regulates Collagen Expression and Bladder Hyper-Trophy through Akt and MAPK Pathways during Cystitis 2. J. Biol. Chem. 2010, 285, 4206–4212. [Google Scholar] [CrossRef]
- Malik, S.C.; Sozmen, E.G.; Baeza-Raja, B.; Le Moan, N.; Akassoglou, K.; Schachtrup, C. In Vivo Functions of p75NTR: Challenges and Opportunities for an Emerging Therapeutic Target. Trends Pharmacol. Sci. 2021, 42, 772–788. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Covarrubias, C.; Mossa, A.H.; Yan, L.R.; Desormeau, B.; Cammisotto, P.G.; Saragovi, H.U.; Campeau, L. Bladder p75NTR-Mediated Anti-Inflammatory Response via the TLR4/TRAF6/NF-κB Axis. Life 2025, 15, 957. https://doi.org/10.3390/life15060957
Covarrubias C, Mossa AH, Yan LR, Desormeau B, Cammisotto PG, Saragovi HU, Campeau L. Bladder p75NTR-Mediated Anti-Inflammatory Response via the TLR4/TRAF6/NF-κB Axis. Life. 2025; 15(6):957. https://doi.org/10.3390/life15060957
Chicago/Turabian StyleCovarrubias, Claudia, Abubakr H. Mossa, Laura R. Yan, Benjamin Desormeau, Philippe G. Cammisotto, H. Uri Saragovi, and Lysanne Campeau. 2025. "Bladder p75NTR-Mediated Anti-Inflammatory Response via the TLR4/TRAF6/NF-κB Axis" Life 15, no. 6: 957. https://doi.org/10.3390/life15060957
APA StyleCovarrubias, C., Mossa, A. H., Yan, L. R., Desormeau, B., Cammisotto, P. G., Saragovi, H. U., & Campeau, L. (2025). Bladder p75NTR-Mediated Anti-Inflammatory Response via the TLR4/TRAF6/NF-κB Axis. Life, 15(6), 957. https://doi.org/10.3390/life15060957








