Trophoblast Extracellular Vesicles as Modulators of Keratinocyte Stress Response and Senescence
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Cultures
2.2. TEV Enrichment
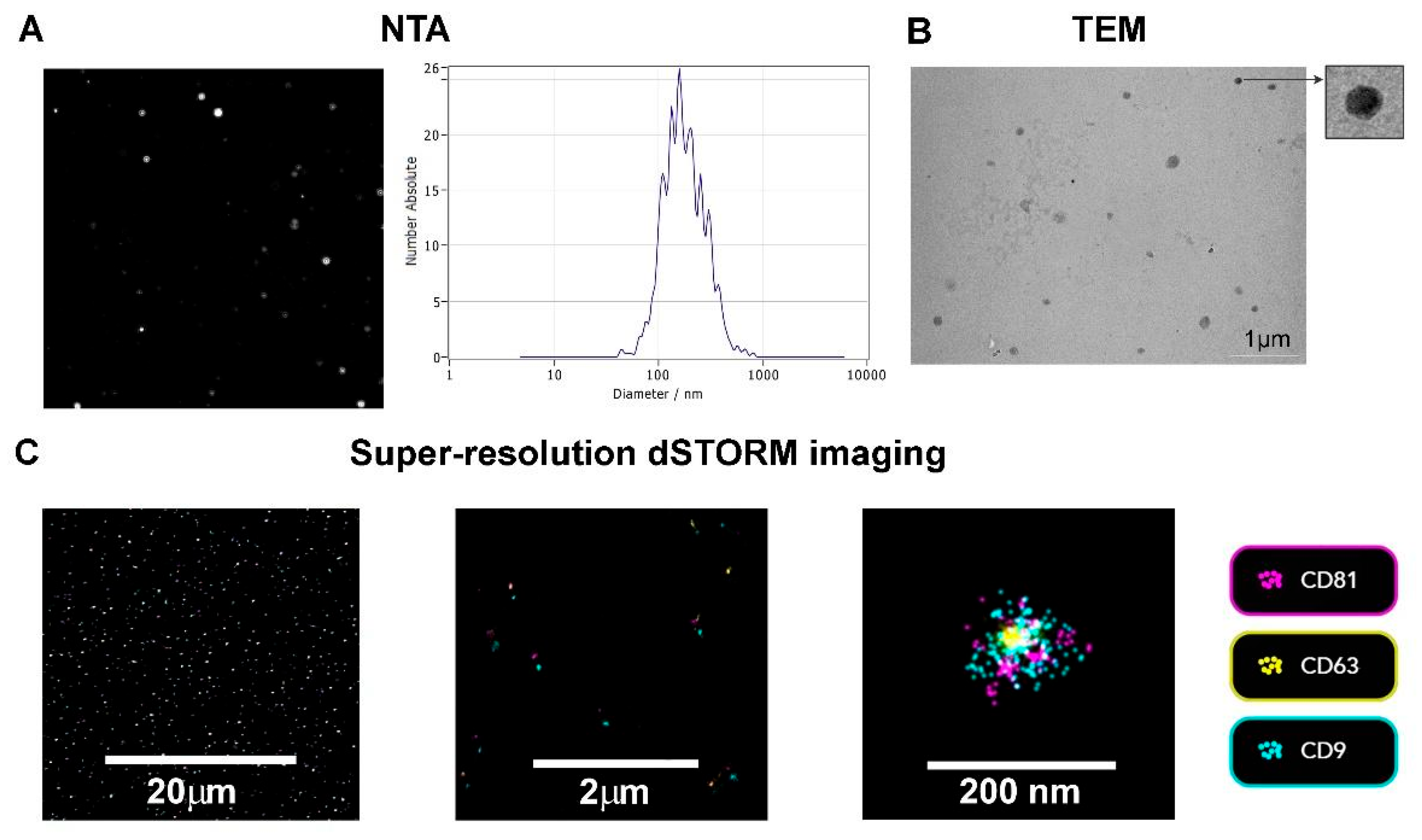
2.3. Nanoparticle Tracking Analysis (NTA)
2.4. Transmission Electron Microscopy (TEM)
2.5. TEV Super-Resolution Imaging
2.6. Experimental Design
2.7. Cell-Based ELISA Assay (cELISA)
2.8. 2′,7′–Dichlorofluorescin Diacetate (DCFH-DA) Assay
2.9. Senescence-Associated β-Galactosidase (SA-β-Gal) Staining
2.10. Alkaline Comet Assay
2.11. Flow Cytometry
2.12. qPCR Analysis
2.13. Wound Healing Assay
2.14. Statistical Analysis
3. Results
3.1. TEV Characterization
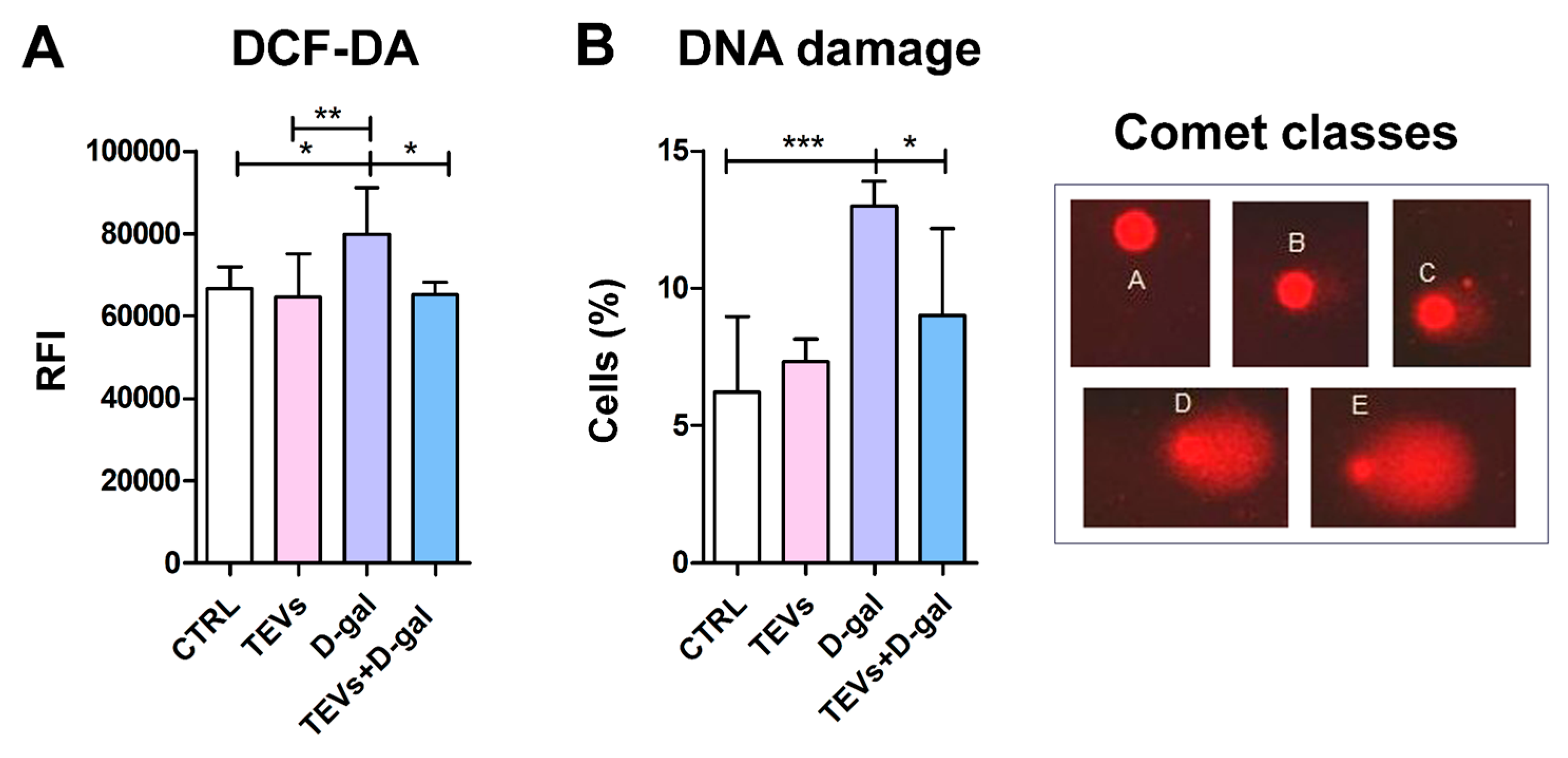
3.2. TEV Pretreatment Alleviates D-Gal-Induced OS and DNA Damage in Keratinocytes
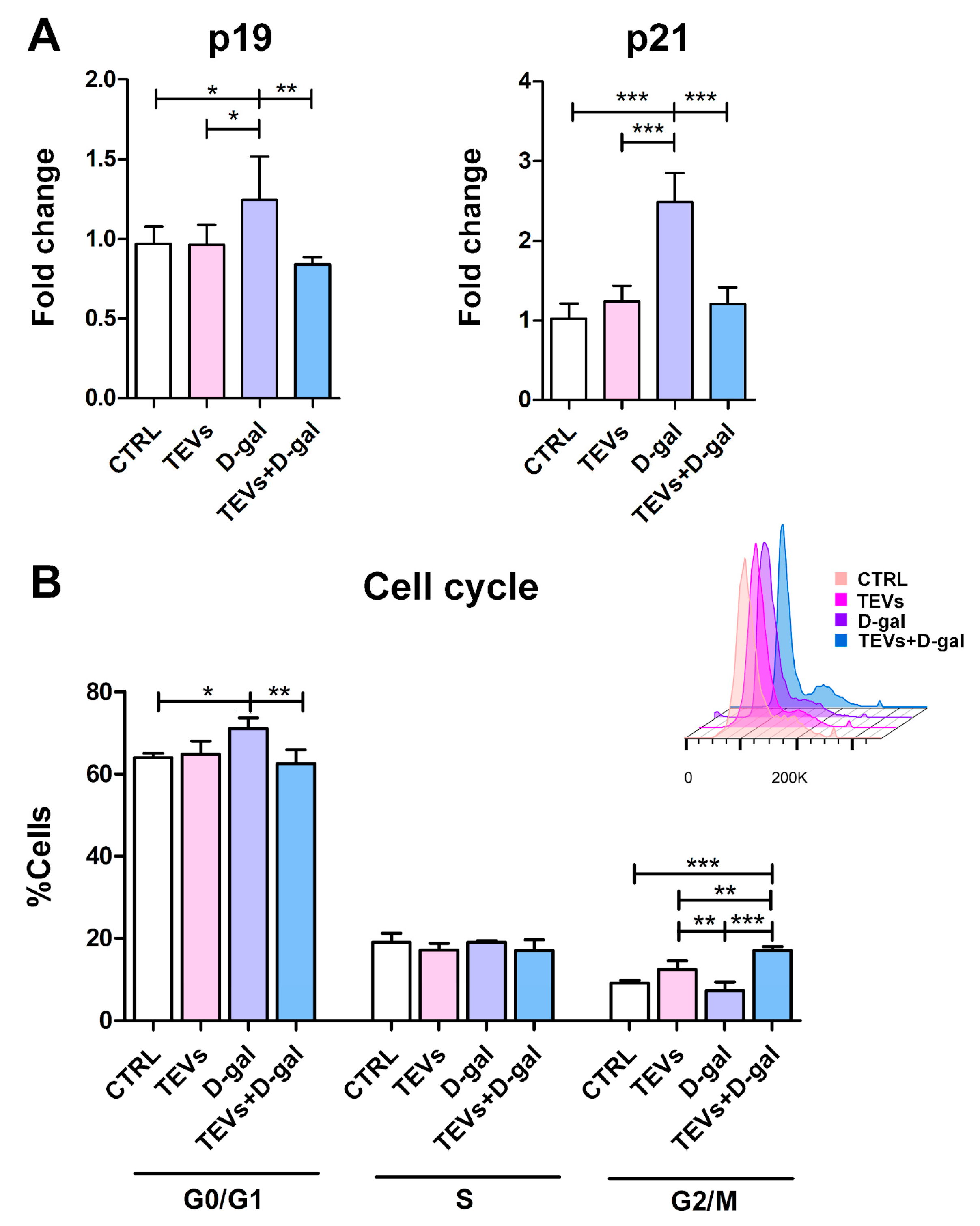
3.3. TEV Pretreatment Mitigates D-Gal-Induced Upregulation of Senescence Markers in Keratinocytes
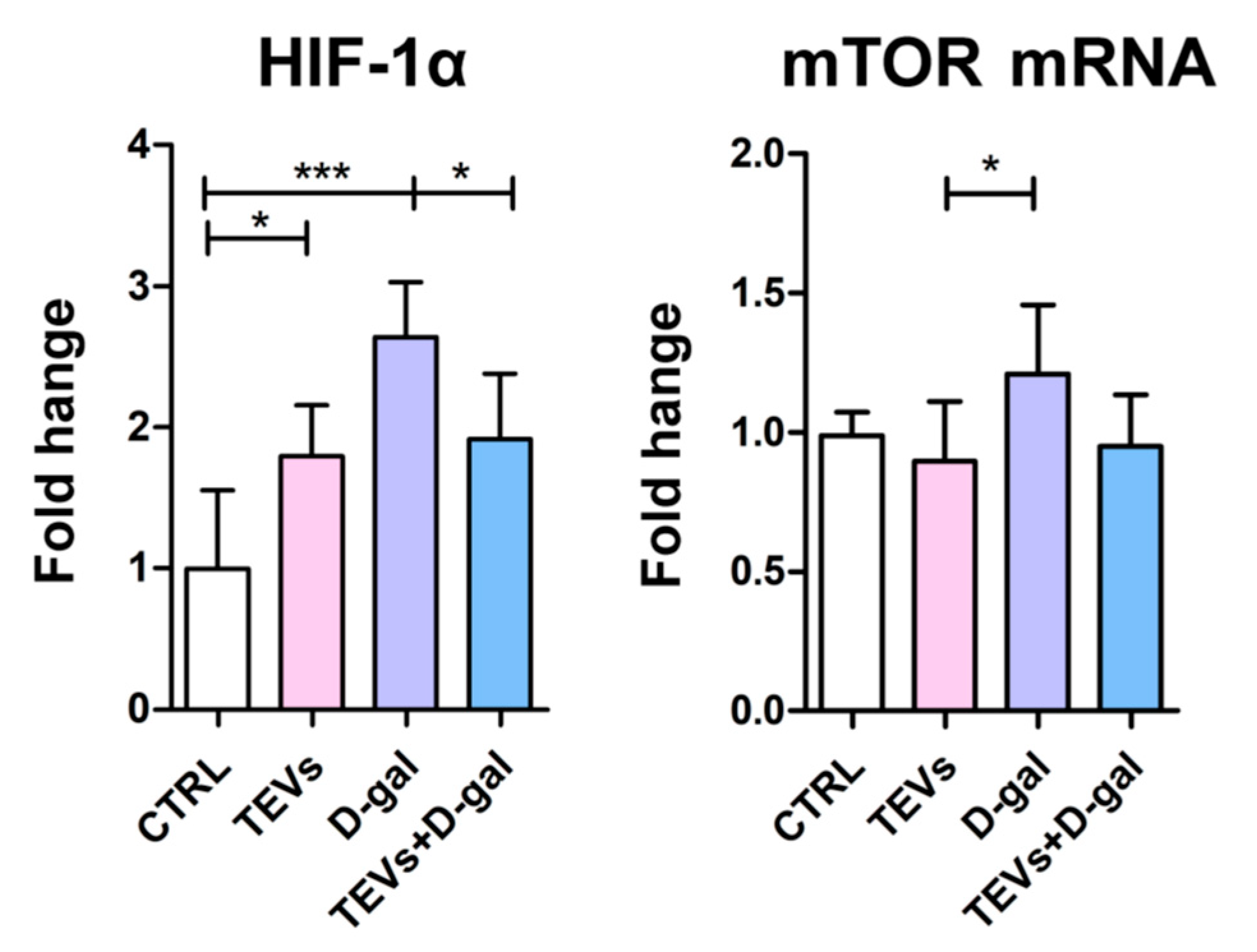
3.4. TEV Pretreatment Downregulates HIF-1α and mTOR Expression in D-Gal-Exposed Keratinocytes
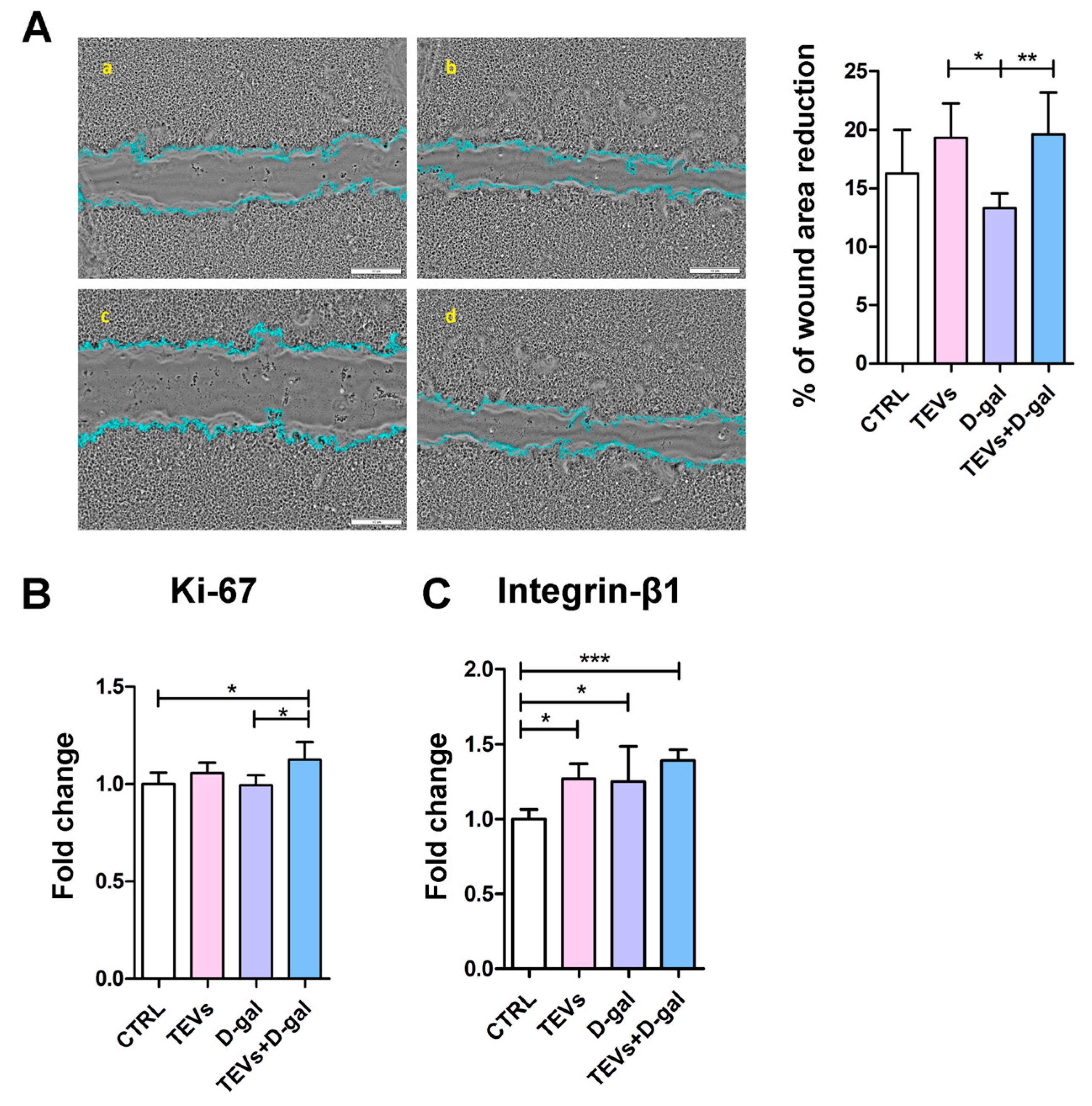
3.5. TEV Pretreatment Increases Keratinocyte Proliferation Rate, Migration, and Integrin-β1 Subunit Expression After D-Gal Exposure
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kanitakis, J. Anatomy, Histology and Immunohistochemistry of Normal Human Skin. Eur. J. Dermatol. 2002, 12, 390–399; quiz 400–401. [Google Scholar] [PubMed]
- Wong, Q.Y.A.; Chew, F.T. Defining Skin Aging and Its Risk Factors: A Systematic Review and Meta-Analysis. Sci. Rep. 2021, 11, 22075. [Google Scholar] [CrossRef] [PubMed]
- Kuang, L.; Li, C. ΔNp63α-Mediated Epigenetic Regulation in Keratinocyte Senescence. Epigenetics 2023, 18, 2173931. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.P.; Katta, R. Sugar Sag: Glycation and the Role of Diet in Aging Skin. Ski. Ther. Lett. 2015, 20, 1–5. [Google Scholar]
- Danby, F.W. Nutrition and Aging Skin: Sugar and Glycation. Clin. Dermatol. 2010, 28, 409–411. [Google Scholar] [CrossRef]
- Umbayev, B.; Askarova, S.; Almabayeva, A.; Saliev, T.; Masoud, A.-R.; Bulanin, D. Galactose-Induced Skin Aging: The Role of Oxidative Stress. Oxid. Med. Cell. Longev. 2020, 2020, 7145656. [Google Scholar] [CrossRef]
- Dworkin, J.P.; Miller, S.L. A Kinetic Estimate of the Free Aldehyde Content of Aldoses. Carbohydr. Res. 2000, 329, 359–365. [Google Scholar] [CrossRef]
- Acosta, P.B.; Gross, K.C. Hidden Sources of Galactose in the Environment. Eur. J. Pediatr. 1995, 154, S87–S92. [Google Scholar] [CrossRef]
- Williams, C.A. Galactose. In Encyclopedia of Food Sciences and Nutrition; Caballero, B., Ed.; Academic Press: Cambridge, MA, USA, 2003; pp. 2843–2846. [Google Scholar]
- Delwing-de Lima, D.; Hennrich, S.B.; Delwing-Dal Magro, D.; Aurélio, J.G.M.; Serpa, A.P.; Augusto, T.W.; Pereira, N.R. The Effect of D-Galactose Induced Oxidative Stress on in Vitro Redox Homeostasis in Rat Plasma and Erythrocytes. Biomed. Pharmacother. 2017, 86, 686–693. [Google Scholar] [CrossRef]
- Li, J.; Zhang, C.-X.; Liu, Y.-M.; Chen, K.-L.; Chen, G. A Comparative Study of Anti-Aging Properties and Mechanism: Resveratrol and Caloric Restriction. Oncotarget 2017, 8, 65717–65729. [Google Scholar] [CrossRef]
- Chen, J.; Li, Y.; Zhu, Q.; Li, T.; Lu, H.; Wei, N.; Huang, Y.; Shi, R.; Ma, X.; Wang, X.; et al. Anti-Skin-Aging Effect of Epigallocatechin Gallate by Regulating Epidermal Growth Factor Receptor Pathway on Aging Mouse Model Induced by d-Galactose. Mech. Ageing Dev. 2017, 164, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sukoyan, G.; Tsivtsivadze, E.; Golovach, V.; Kezeli, T.; Demina, N. Anti-Aging Effect of Cynara Cardunculus L. var. Cynara Scolymus L. Extract in D-Galactose-Induced Skin Aging Model in Rats. Pharmacol. Pharm. 2018, 9, 428–439. [Google Scholar] [CrossRef]
- Chin, T.; Lee, X.E.; Ng, P.Y.; Lee, Y.; Dreesen, O. The Role of Cellular Senescence in Skin Aging and Age-Related Skin Pathologies. Front. Physiol. 2023, 14, 1297637. [Google Scholar] [CrossRef]
- Pogozhykh, O.; Prokopyuk, V.; Figueiredo, C.; Pogozhykh, D. Placenta and Placental Derivatives in Regenerative Therapies: Experimental Studies, History, and Prospects. Stem Cells Int. 2018, 2018, 4837930. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.A.; Baba, S.K.; Sadida, H.Q.; Marzooqi, S.A.; Jerobin, J.; Altemani, F.H.; Algehainy, N.; Alanazi, M.A.; Abou-Samra, A.-B.; Kumar, R.; et al. Extracellular Vesicles as Tools and Targets in Therapy for Diseases. Signal Transduct. Target. Ther. 2024, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Godakumara, K.; Ord, J.; Lättekivi, F.; Dissanayake, K.; Viil, J.; Boggavarapu, N.R.; Faridani, O.R.; Jääger, K.; Velthut-Meikas, A.; Jaakma, Ü.; et al. Trophoblast Derived Extracellular Vesicles Specifically Alter the Transcriptome of Endometrial Cells and May Constitute a Critical Component of Embryo-Maternal Communication. Reprod. Biol. Endocrinol. 2021, 19, 115. [Google Scholar] [CrossRef]
- Manni, G.; Buratta, S.; Pallotta, M.T.; Chiasserini, D.; Di Michele, A.; Emiliani, C.; Giovagnoli, S.; Pascucci, L.; Romani, R.; Bellezza, I.; et al. Extracellular Vesicles in Aging: An Emerging Hallmark? Cells 2023, 12, 527. [Google Scholar] [CrossRef]
- Atay, S.; Gercel-Taylor, C.; Kesimer, M.; Taylor, D.D. Morphologic and Proteomic Characterization of Exosomes Released by Cultured Extravillous Trophoblast Cells. Exp. Cell Res. 2011, 317, 1192–1202. [Google Scholar] [CrossRef]
- Su, Y.; Li, Q.; Zhang, Q.; Li, Z.; Yao, X.; Guo, Y.; Xiao, L.; Wang, X.; Ni, H. Exosomes Derived from Placental Trophoblast Cells Regulate Endometrial Epithelial Receptivity in Dairy Cows during Pregnancy. J. Reprod. Dev. 2022, 68, 21–29. [Google Scholar] [CrossRef]
- Salomon, C.; Yee, S.; Scholz-Romero, K.; Kobayashi, M.; Vaswani, K.; Kvaskoff, D.; Illanes, S.E.; Mitchell, M.D.; Rice, G.E. Extravillous Trophoblast Cells-Derived Exosomes Promote Vascular Smooth Muscle Cell Migration. Front. Pharmacol. 2014, 5, 175. [Google Scholar] [CrossRef]
- Ćujić, D.; Kosanović, M.; Jovanović Krivokuća, M.; Vićovac, L.; Janković, M. Extracellular Presence/Release of Galectins from HTR-8/SVneo Extravillous Trophoblast Cells. Turk. J. Biol. 2017, 41, 843–848. [Google Scholar] [CrossRef]
- Panjwani, N. Role of Galectins in Re-Epithelialization of Wounds. Ann. Transl. Med. 2014, 2, 89. [Google Scholar] [PubMed]
- Hedlund, M.; Stenqvist, A.-C.; Nagaeva, O.; Kjellberg, L.; Wulff, M.; Baranov, V.; Mincheva-Nilsson, L. Human Placenta Expresses and Secretes NKG2D Ligands via Exosomes That Down-Modulate the Cognate Receptor Expression: Evidence for Immunosuppressive Function. J. Immunol. 2009, 183, 340–351. [Google Scholar] [CrossRef] [PubMed]
- Salomon, C.; Ryan, J.; Sobrevia, L.; Kobayashi, M.; Ashman, K.; Mitchell, M.; Rice, G.E. Exosomal Signaling during Hypoxia Mediates Microvascular Endothelial Cell Migration and Vasculogenesis. PLoS ONE 2013, 8, e68451. [Google Scholar] [CrossRef]
- Ouyang, Y.; Bayer, A.; Chu, T.; Tyurin, V.A.; Kagan, V.E.; Morelli, A.E.; Coyne, C.B.; Sadovsky, Y. Isolation of Human Trophoblastic Extracellular Vesicles and Characterization of Their Cargo and Antiviral Activity. Placenta 2016, 47, 86–95. [Google Scholar] [CrossRef]
- Schürer, N.; Köhne, A.; Schliep, V.; Barlag, K.; Goerz, G. Lipid Composition and Synthesis of HaCaT Cells, an Immortalized Human Keratinocyte Line, in Comparison with Normal Human Adult Keratinocytes. Exp. Dermatol. 1993, 2, 179–185. [Google Scholar] [CrossRef]
- Micallef, L.; Belaubre, F.; Pinon, A.; Jayat-Vignoles, C.; Delage, C.; Charveron, M.; Simon, A. Effects of Extracellular Calcium on the Growth-Differentiation Switch in Immortalized Keratinocyte HaCaT Cells Compared with Normal Human Keratinocytes. Exp. Dermatol. 2009, 18, 143–151. [Google Scholar] [CrossRef]
- Neupane, Y.R.; Handral, H.K.; Alkaff, S.A.; Chng, W.H.; Venkatesan, G.; Huang, C.; Lee, C.K.; Wang, J.-W.; Sriram, G.; Dienzo, R.A.; et al. Cell-Derived Nanovesicles from Mesenchymal Stem Cells as Extracellular Vesicle-Mimetics in Wound Healing. Acta Pharm. Sin. B 2023, 13, 1887–1902. [Google Scholar] [CrossRef]
- Zhao, B.; Zhang, Y.; Han, S.; Zhang, W.; Zhou, Q.; Guan, H.; Liu, J.; Shi, J.; Su, L.; Hu, D. Exosomes Derived from Human Amniotic Epithelial Cells Accelerate Wound Healing and Inhibit Scar Formation. J. Mol. Histol. 2017, 48, 121–132. [Google Scholar] [CrossRef]
- Yang, L.; Shi, J.; Wang, X.; Zhang, R. Curcumin Alleviates D-Galactose-Induced Cardiomyocyte Senescence by Promoting Autophagy via the SIRT1/AMPK/MTOR Pathway. Evid. Based. Complement. Alternat. Med. 2022, 2022, 2990843. [Google Scholar] [CrossRef]
- Xu, W.; Xiang, X.; Lin, L.; Gong, Z.-H.; Xiao, W.-J. L-Theanine Delays d-Galactose-Induced Senescence by Regulating the Cell Cycle and Inhibiting Apoptosis in Rat Intestinal Cells. J. Sci. Food Agric. 2024, 104, 2073–2084. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Arnedo, A.; Torres Figueroa, F.; Clavijo, C.; Arbeláez, P.; Cruz, J.C.; Muñoz-Camargo, C. An Image J Plugin for the High Throughput Image Analysis of in Vitro Scratch Wound Healing Assays. PLoS ONE 2020, 15, e0232565. [Google Scholar] [CrossRef] [PubMed]
- Rubinstein, E.; Théry, C.; Zimmermann, P. Tetraspanins Affect Membrane Structures and the Trafficking of Molecular Partners: What Impact on Extracellular Vesicles? Biochem. Soc. Trans. 2025, 53, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Cadet, J.; Davies, K.J.A.; Medeiros, M.H.; Di Mascio, P.; Wagner, J.R. Formation and Repair of Oxidatively Generated Damage in Cellular DNA. Free Radic. Biol. Med. 2017, 107, 13–34. [Google Scholar] [CrossRef]
- Singh, N.P.; McCoy, M.T.; Tice, R.R.; Schneider, E.L. A Simple Technique for Quantitation of Low Levels of DNA Damage in Individual Cells. Exp. Cell Res. 1988, 175, 184–191. [Google Scholar] [CrossRef]
- Lee, B.Y.; Han, J.A.; Im, J.S.; Morrone, A.; Johung, K.; Goodwin, E.C.; Kleijer, W.J.; DiMaio, D.; Hwang, E.S. Senescence-Associated Beta-Galactosidase Is Lysosomal Beta-Galactosidase. Aging Cell 2006, 5, 187–195. [Google Scholar] [CrossRef]
- Liu, L.; Xie, H.; Chen, X.; Shi, W.; Xiao, X.; Lei, D.; Li, J. Differential Response of Normal Human Epidermal Keratinocytes and HaCaT Cells to Hydrogen Peroxide-Induced Oxidative Stress. Clin. Exp. Dermatol. 2012, 37, 772–780. [Google Scholar] [CrossRef]
- Yang, N.-C.; Hu, M.-L. The Limitations and Validities of Senescence Associated-Beta-Galactosidase Activity as an Aging Marker for Human Foreskin Fibroblast Hs68 Cells. Exp. Gerontol. 2005, 40, 813–819. [Google Scholar] [CrossRef]
- Yegorov, Y.E.; Akimov, S.S.; Hass, R.; Zelenin, A.V.; Prudovsky, I.A. Endogenous Beta-Galactosidase Activity in Continuously Nonproliferating Cells. Exp. Cell Res. 1998, 243, 207–211. [Google Scholar] [CrossRef]
- Untergasser, G.; Gander, R.; Rumpold, H.; Heinrich, E.; Plas, E.; Berger, P. TGF-Beta Cytokines Increase Senescence-Associated Beta-Galactosidase Activity in Human Prostate Basal Cells by Supporting Differentiation Processes, but Not Cellular Senescence. Exp. Gerontol. 2003, 38, 1179–1188. [Google Scholar] [CrossRef]
- Chung, M.G.; Kim, Y.; Cha, Y.K.; Park, T.H.; Kim, Y. Bitter Taste Receptors Protect against Skin Aging by Inhibiting Cellular Senescence and Enhancing Wound Healing. Nutr. Res. Pract. 2022, 16, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kumari, R.; Jat, P. Mechanisms of Cellular Senescence: Cell Cycle Arrest and Senescence Associated Secretory Phenotype. Front. Cell Dev. Biol. 2021, 9, 645593. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Nepovimova, E.; Heger, Z.; Valko, M.; Wu, Q.; Kuca, K.; Adam, V. Role of Hypoxia in Cellular Senescence. Pharmacol. Res. 2023, 194, 106841. [Google Scholar] [CrossRef]
- Hong, W.X.; Hu, M.S.; Esquivel, M.; Liang, G.Y.; Rennert, R.C.; McArdle, A.; Paik, K.J.; Duscher, D.; Gurtner, G.C.; Lorenz, H.P.; et al. The Role of Hypoxia-Inducible Factor in Wound Healing. Adv. Wound Care 2014, 3, 390–399. [Google Scholar] [CrossRef]
- Cho, Y.-S.; Bae, J.-M.; Chun, Y.-S.; Chung, J.-H.; Jeon, Y.-K.; Kim, I.-S.; Kim, M.-S.; Park, J.-W. HIF-1alpha Controls Keratinocyte Proliferation by up-Regulating P21(WAF1/Cip1). Biochim. Biophys. Acta 2008, 1783, 323–333. [Google Scholar] [CrossRef]
- Sakamoto, T.; Weng, J.S.; Hara, T.; Yoshino, S.; Kozuka-Hata, H.; Oyama, M.; Seiki, M. Hypoxia-Inducible Factor 1 Regulation through Cross Talk between MTOR and MT1-MMP. Mol. Cell. Biol. 2014, 34, 30–42. [Google Scholar] [CrossRef]
- Xu, C.; Huang, X.; Tong, Y.; Feng, X.; Wang, Y.; Wang, C.; Jiang, Y. Icariin Modulates the Sirtuin/NF-κB Pathway and Exerts Anti-aging Effects in Human Lung Fibroblasts. Mol. Med. Rep. 2020, 22, 3833–3839. [Google Scholar] [CrossRef]
- Thedieck, K.; Holzwarth, B.; Prentzell, M.T.; Boehlke, C.; Kläsener, K.; Ruf, S.; Sonntag, A.G.; Maerz, L.; Grellscheid, S.-N.; Kremmer, E.; et al. Inhibition of MTORC1 by Astrin and Stress Granules Prevents Apoptosis in Cancer Cells. Cell 2013, 154, 859–874. [Google Scholar] [CrossRef]
- Sun, X.; Kaufman, P.D. Ki-67: More than a Proliferation Marker. Chromosoma 2018, 127, 175–186. [Google Scholar] [CrossRef]
- Borghesan, M.; O’Loghlen, A. Integrins in Senescence and Aging. Cell Cycle 2017, 16, 909–910. [Google Scholar] [CrossRef]
- Levy, L.; Broad, S.; Diekmann, D.; Evans, R.D.; Watt, F.M. Beta1 Integrins Regulate Keratinocyte Adhesion and Differentiation by Distinct Mechanisms. Mol. Biol. Cell 2000, 11, 453–466. [Google Scholar] [CrossRef] [PubMed]
- Lü, D.; Li, Z.; Gao, Y.; Luo, C.; Zhang, F.; Zheng, L.; Wang, J.; Sun, S.; Long, M. Β1 Integrin Signaling in Asymmetric Migration of Keratinocytes under Mechanical Stretch in a Co-Cultured Wound Repair Model. Biomed. Eng. Online 2016, 15, 130. [Google Scholar] [CrossRef] [PubMed]
- Yudintceva, N.; Bobkov, D.; Sulatsky, M.; Mikhailova, N.; Oganesyan, E.; Vinogradova, T.; Muraviov, A.; Remezova, A.; Bogdanova, E.; Garapach, I.; et al. Mesenchymal Stem Cells-Derived Extracellular Vesicles for Therapeutics of Renal Tuberculosis. Sci. Rep. 2024, 14, 4495. [Google Scholar] [CrossRef] [PubMed]
- Borosch, S.; Dahmen, E.; Beckers, C.; Stoppe, C.; Buhl, E.M.; Denecke, B.; Goetzenich, A.; Kraemer, S. Characterization of Extracellular Vesicles Derived from Cardiac Cells in an in Vitro Model of Preconditioning. J. Extracell. Vesicles 2017, 6, 1390391. [Google Scholar] [CrossRef]
- Jeppesen, D.K.; Fenix, A.M.; Franklin, J.L.; Higginbotham, J.N.; Zhang, Q.; Zimmerman, L.J.; Liebler, D.C.; Ping, J.; Liu, Q.; Evans, R.; et al. Reassessment of Exosome Composition. Cell 2019, 177, 428–445.e18. [Google Scholar] [CrossRef]
- Mathieu, M.; Névo, N.; Jouve, M.; Valenzuela, J.I.; Maurin, M.; Verweij, F.J.; Palmulli, R.; Lankar, D.; Dingli, F.; Loew, D.; et al. Specificities of Exosome versus Small Ectosome Secretion Revealed by Live Intracellular Tracking of CD63 and CD9. Nat. Commun. 2021, 12, 4389. [Google Scholar] [CrossRef]
- Barranco, I.; Padilla, L.; Parrilla, I.; Álvarez-Barrientos, A.; Pérez-Patiño, C.; Peña, F.J.; Martínez, E.A.; Rodriguez-Martínez, H.; Roca, J. Extracellular Vesicles Isolated from Porcine Seminal Plasma Exhibit Different Tetraspanin Expression Profiles. Sci. Rep. 2019, 9, 11584. [Google Scholar] [CrossRef]
- Vornic, I.; Buciu, V.; Furau, C.G.; Gaje, P.N.; Ceausu, R.A.; Dumitru, C.-S.; Barb, A.C.; Novacescu, D.; Cumpanas, A.A.; Latcu, S.C.; et al. Oxidative Stress and Placental Pathogenesis: A Contemporary Overview of Potential Biomarkers and Emerging Therapeutics. Int. J. Mol. Sci. 2024, 25, 12195. [Google Scholar] [CrossRef]
- Duan, J.; Liu, X.; Shen, S.; Tan, X.; Wang, Y.; Wang, L.; Kang, L.; Wang, K.; Wei, Z.; Qi, Y.; et al. Trophoblast Stem-Cell-Derived Exosomes Alleviate Cardiotoxicity of Doxorubicin via Improving Mfn2-Mediated Mitochondrial Fusion. Cardiovasc. Toxicol. 2023, 23, 23–31. [Google Scholar] [CrossRef]
- Wang, T.; Jian, Z.; Baskys, A.; Yang, J.; Li, J.; Guo, H.; Hei, Y.; Xian, P.; He, Z.; Li, Z.; et al. MSC-Derived Exosomes Protect against Oxidative Stress-Induced Skin Injury via Adaptive Regulation of the NRF2 Defense System. Biomaterials 2020, 257, 120264. [Google Scholar] [CrossRef]
- Lei, X.; He, N.; Zhu, L.; Zhou, M.; Zhang, K.; Wang, C.; Huang, H.; Chen, S.; Li, Y.; Liu, Q.; et al. Mesenchymal Stem Cell-Derived Extracellular Vesicles Attenuate Radiation-Induced Lung Injury via MiRNA-214-3p. Antioxid. Redox Signal. 2021, 35, 849–862. [Google Scholar] [CrossRef] [PubMed]
- Platas, J.; Guillén, M.I.; Pérez Del Caz, M.D.; Gomar, F.; Castejón, M.A.; Mirabet, V.; Alcaraz, M.J. Paracrine Effects of Human Adipose-Derived Mesenchymal Stem Cells in Inflammatory Stress-Induced Senescence Features of Osteoarthritic Chondrocytes. Aging 2016, 8, 1703–1717. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.O.; Reshi, Q.U.A.; Godakumara, K.; Kodithuwakku, S.; Fazeli, A. Extracellular Vesicles as Mediators of Stress Response in Embryo-Maternal Communication. Front. Cell Dev. Biol. 2024, 12, 1440849. [Google Scholar] [CrossRef] [PubMed]
- Du, F.; Zhu, L.; Qian, Z.-M.; Wu, X.-M.; Yung, W.-H.; Ke, Y. Hyperthermic Preconditioning Protects Astrocytes from Ischemia/Reperfusion Injury by up-Regulation of HIF-1 Alpha Expression and Binding Activity. Biochim. Biophys. Acta 2010, 1802, 1048–1053. [Google Scholar] [CrossRef]
- Fafián-Labora, J.; Morente-López, M.; Sánchez-Dopico, M.J.; Arntz, O.J.; van de Loo, F.A.J.; De Toro, J.; Arufe, M.C. Influence of Mesenchymal Stem Cell-Derived Extracellular Vesicles in Vitro and Their Role in Ageing. Stem Cell Res. Ther. 2020, 11, 13. [Google Scholar] [CrossRef]
- Jiang, L.; Zeng, H.; Ni, L.; Qi, L.; Xu, Y.; Xia, L.; Yu, Y.; Liu, B.; Yang, H.; Hao, H.; et al. HIF-1α Preconditioning Potentiates Antioxidant Activity in Ischemic Injury: The Role of Sequential Administration of Dihydrotanshinone I and Protocatechuic Aldehyde in Cardioprotection. Antioxid. Redox Signal. 2019, 31, 227–242. [Google Scholar] [CrossRef]
- Heyman, S.N.; Leibowitz, D.; Mor-Yosef Levi, I.; Liberman, A.; Eisenkraft, A.; Alcalai, R.; Khamaisi, M.; Rosenberger, C. Adaptive Response to Hypoxia and Remote Ischaemia Pre-Conditioning: A New Hypoxia-Inducible Factors Era in Clinical Medicine. Acta Physiol. 2016, 216, 395–406. [Google Scholar] [CrossRef]
- Muhandiram, S.; Dissanayake, K.; Orro, T.; Godakumara, K.; Kodithuwakku, S.; Fazeli, A. Secretory Proteomic Responses of Endometrial Epithelial Cells to Trophoblast-Derived Extracellular Vesicles. Int. J. Mol. Sci. 2023, 24, 11924. [Google Scholar] [CrossRef]
- Han, H.; Liu, Z.; Yin, J.; Gao, J.; He, L.; Wang, C.; Hou, R.; He, X.; Wang, G.; Li, T.; et al. D-Galactose Induces Chronic Oxidative Stress and Alters Gut Microbiota in Weaned Piglets. Front. Physiol. 2021, 12, 634283. [Google Scholar] [CrossRef]
- Wortzel, I.; Dror, S.; Kenific, C.M.; Lyden, D. Exosome-Mediated Metastasis: Communication from a Distance. Dev. Cell 2019, 49, 347–360. [Google Scholar] [CrossRef]
- Go, Y.Y.; Lee, C.M.; Ju, W.M.; Chae, S.-W.; Song, J.-J. Extracellular Vesicles (Secretomes) from Human Trophoblasts Promote the Regeneration of Skin Fibroblasts. Int. J. Mol. Sci. 2021, 22, 6959. [Google Scholar] [CrossRef]
- Wu, D.; Kang, L.; Tian, J.; Wu, Y.; Liu, J.; Li, Z.; Wu, X.; Huang, Y.; Gao, B.; Wang, H.; et al. Exosomes Derived from Bone Mesenchymal Stem Cells with the Stimulation of Fe3O4 Nanoparticles and Static Magnetic Field Enhance Wound Healing Through Upregulated MiR-21-5p. Int. J. Nanomed. 2020, 15, 7979–7993. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Ohkuchi, A.; Kuwata, T.; Usui, R.; Baba, Y.; Suzuki, H.; Chaw Kyi, T.T.; Matsubara, S.; Saito, S.; Takizawa, T. Endogenous and Exogenous MiR-520c-3p Modulates CD44-Mediated Extravillous Trophoblast Invasion. Placenta 2017, 50, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Cabral-Pacheco, G.A.; Garza-Veloz, I.; Castruita-De la Rosa, C.; Ramirez-Acuña, J.M.; Perez-Romero, B.A.; Guerrero-Rodriguez, J.F.; Martinez-Avila, N.; Martinez-Fierro, M.L. The Roles of Matrix Metalloproteinases and Their Inhibitors in Human Diseases. Int. J. Mol. Sci. 2020, 21, 9739. [Google Scholar] [CrossRef] [PubMed]
- Rasekh, M.; Arshad, M.S.; Ahmad, Z. Advances in Drug Delivery Integrated with Regenerative Medicine: Innovations, Challenges, and Future Frontiers. Pharmaceutics 2025, 17, 456. [Google Scholar] [CrossRef]
- Gupta, D.; Zickler, A.M.; El Andaloussi, S. Dosing Extracellular Vesicles. Adv. Drug Deliv. Rev. 2021, 178, 113961. [Google Scholar] [CrossRef]
- Hildebrand, D.G.; Lehle, S.; Borst, A.; Haferkamp, S.; Essmann, F.; Schulze-Osthoff, K. α-Fucosidase as a Novel Convenient Biomarker for Cellular Senescence. Cell Cycle 2013, 12, 1922–1927. [Google Scholar] [CrossRef]
- Cruz, A.M.; Gonçalves, M.C.; Marques, M.S.; Veiga, F.; Paiva-Santos, A.C.; Pires, P.C. In Vitro Models for Anti-Aging Efficacy Assessment: A Critical Update in Dermocosmetic Research. Cosmetics 2023, 10, 66. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nacka-Aleksić, M.; Pirković, A.; Vilotić, A.; Kosanović, M.; Dekanski, D.; Legner, J.; Jovanović Krivokuća, M. Trophoblast Extracellular Vesicles as Modulators of Keratinocyte Stress Response and Senescence. Life 2025, 15, 918. https://doi.org/10.3390/life15060918
Nacka-Aleksić M, Pirković A, Vilotić A, Kosanović M, Dekanski D, Legner J, Jovanović Krivokuća M. Trophoblast Extracellular Vesicles as Modulators of Keratinocyte Stress Response and Senescence. Life. 2025; 15(6):918. https://doi.org/10.3390/life15060918
Chicago/Turabian StyleNacka-Aleksić, Mirjana, Andrea Pirković, Aleksandra Vilotić, Maja Kosanović, Dragana Dekanski, Janko Legner, and Milica Jovanović Krivokuća. 2025. "Trophoblast Extracellular Vesicles as Modulators of Keratinocyte Stress Response and Senescence" Life 15, no. 6: 918. https://doi.org/10.3390/life15060918
APA StyleNacka-Aleksić, M., Pirković, A., Vilotić, A., Kosanović, M., Dekanski, D., Legner, J., & Jovanović Krivokuća, M. (2025). Trophoblast Extracellular Vesicles as Modulators of Keratinocyte Stress Response and Senescence. Life, 15(6), 918. https://doi.org/10.3390/life15060918






