Obstacle Circumvention and Motor Daily Dual Task During a Simulation of Street Crossing by Individuals with Parkinson’s Disease
Abstract
1. Introduction
2. Methods
2.1. Participants
2.2. Experimental Design and Clinical Evaluations
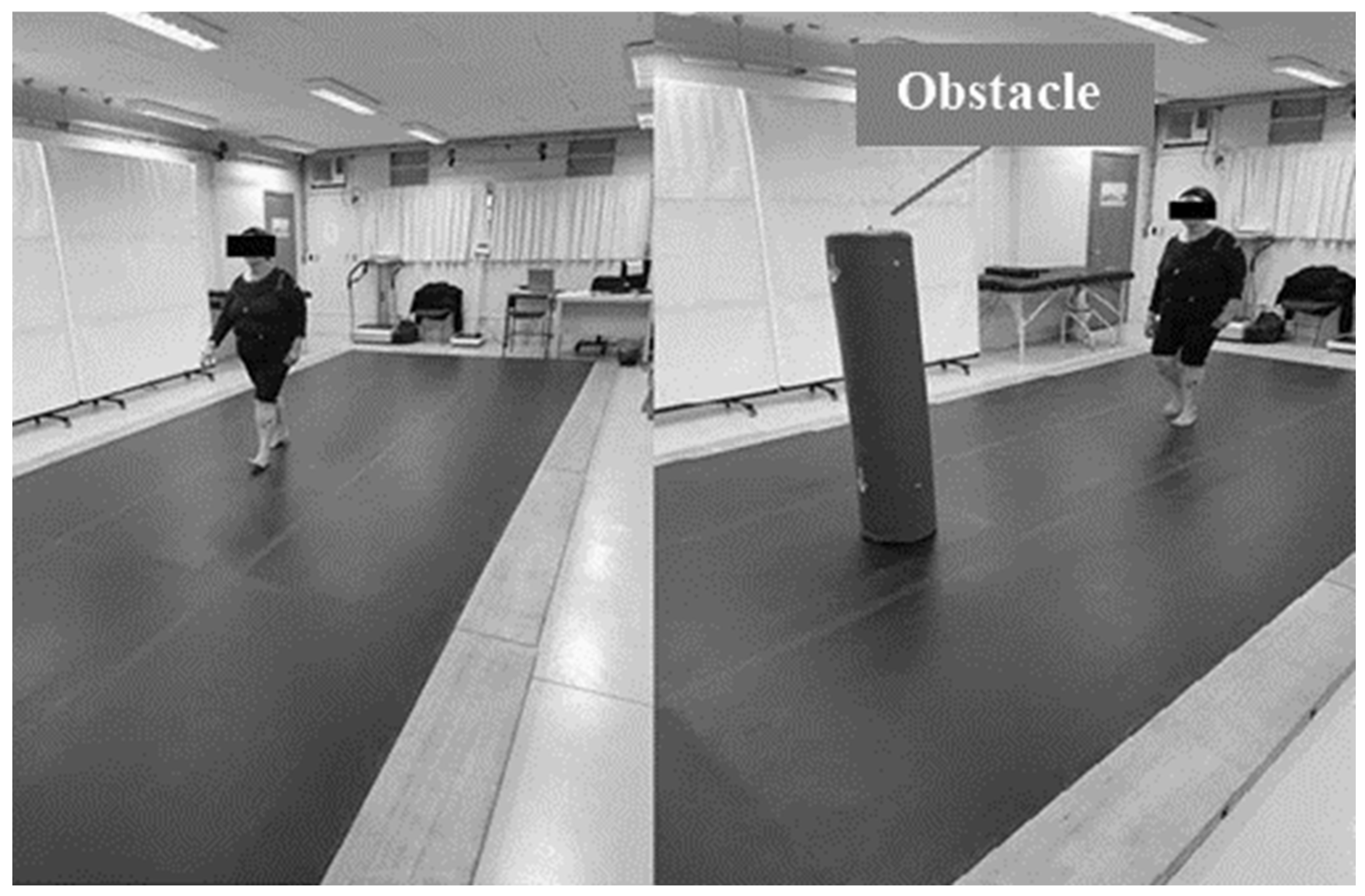
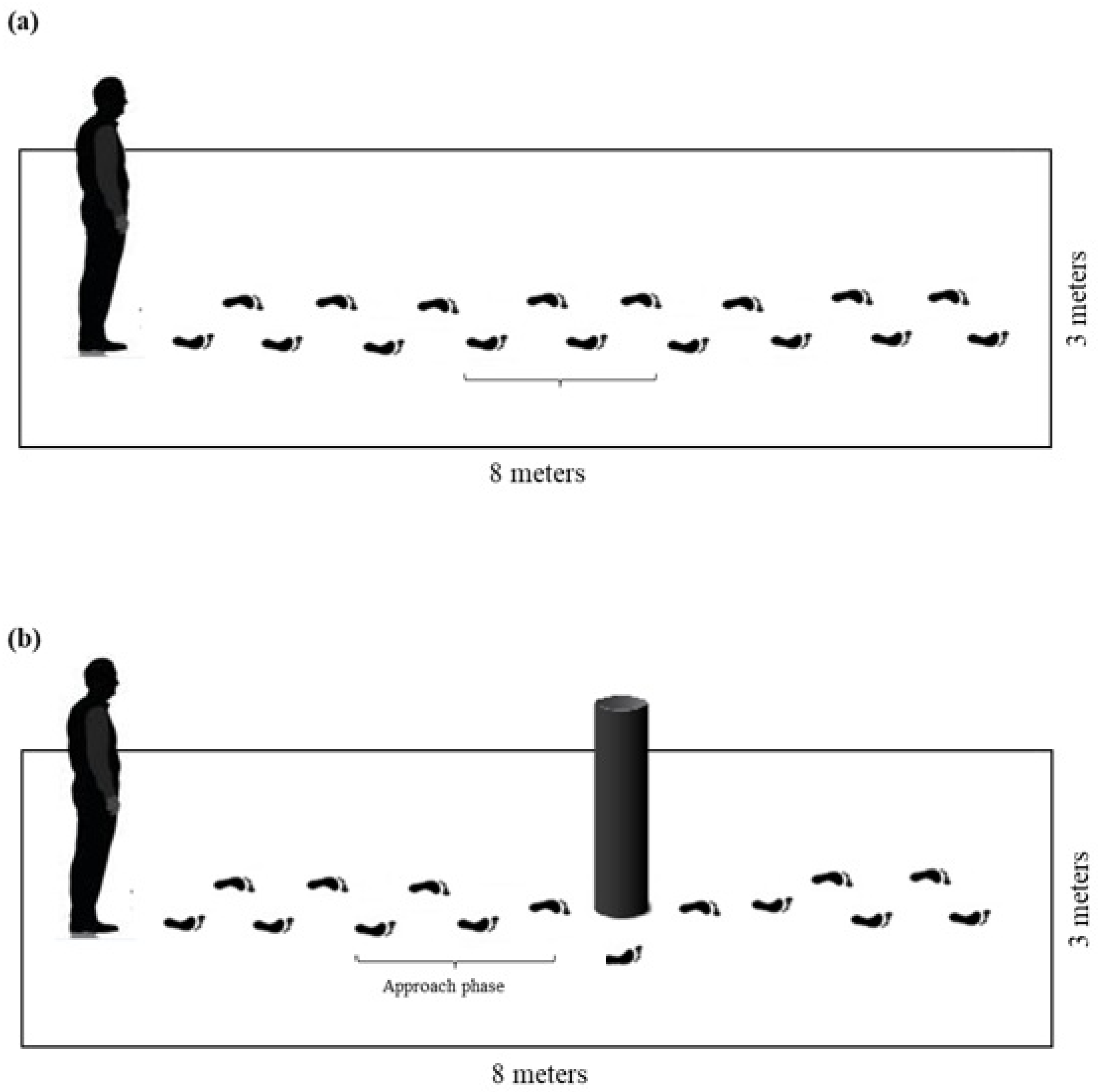
2.3. Gait Assessment Under Different Street-Crossing Conditions
2.4. Data Processing and Analysis
2.5. Statistical Analysis
3. Results
3.1. Clinical Parameters
3.2. Spatiotemporal Parameters of All Gait Conditions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dommes, A.; Lay, T.L.; Vienne, F.; Dang, N.T.; Cavallo, V. Towards an Explanation of Age-Related Difficulties in Crossing a Two-Way Street. Accid. Anal. Prev. 2015, 85, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Wiczorek, R.; Protzak, J. The Impact of Visual and Cognitive Dual-Task Demands on Traffic Perception During Road Crossing of Older and Younger Pedestrians. Front. Psychol. 2022, 13, 775165. [Google Scholar] [CrossRef] [PubMed]
- Fritz, N.E.; Cheek, F.M.; Nichols-Larsen, D.S. Motor-Cognitive Dual-Task Training in Neurologic Disorders: A Systematic Review. J. Neurol. Phys. Ther. 2015, 39, 142–153. [Google Scholar] [CrossRef]
- Butler, A.A.; Lord, S.R.; Fitzpatrick, R.C. Perceptions of Speed and Risk: Experimental Studies of Road Crossing by Older People. PLoS ONE 2016, 11, e0152617. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, H.; Gourlet, Y. Fatal Pedestrian Accidents in France: A Typological Analysis. Accid. Anal. Prev. 1997, 29, 303–312. [Google Scholar] [CrossRef]
- Fernandes, C.M.; Boing, A.C. Pedestrian Mortality in Road Traffic Accidents in Brazil: Time Trend Analysis, 1996–2015. Epidemiol. Serv. Saude 2019, 28, e2018079. [Google Scholar]
- Dommes, A.; Cavallo, V.; Dubuisson, J.B.; Tournier, I.; Vienne, F. Crossing a Two-Way Street: Comparison of Young and Old Pedestrians. J. Saf. Res. 2014, 50, 27–34. [Google Scholar] [CrossRef]
- Samii, A.; Nutt, J.G.; Ransom, B.R. Parkinson’s disease. Lancet 2004, 363, 1783–1793. [Google Scholar] [CrossRef]
- Kalia, L.V.; Lang, A.E. Parkinson’s disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef]
- Bloem, B.R.; Okun, M.S.; Klein, C. Parkinson’s disease. Lancet 2021, 397, 2284–2303. [Google Scholar] [CrossRef]
- Lew, M. Overview of Parkinson’s Disease. Pharmacotherapy 2007, 27 Pt 2, 155S–160S. [Google Scholar] [CrossRef] [PubMed]
- Opara, J.A.; Malecki, A.; Malecka, E.; Socha, T. Motor assessment in Parkinson’s disease. Ann. Agric. Environ. Med. 2017, 24, 411–415. [Google Scholar] [CrossRef]
- Creaby, M.W.; Cole, M.H. Gait characteristics and falls in Parkinson’s disease: A systematic review and meta-analysis. Parkinsonism Relat. Disord. 2018, 57, 1–8. [Google Scholar] [CrossRef]
- Ford, K.J.; Joop, A.; Memon, R.; Rowe, J.B.; Husain, M. Pedestrian Safety in Patients with Parkinson’s Disease: A Case-Control Study. Mov. Disord. 2017, 32, 1748–1755. [Google Scholar] [CrossRef]
- Amaral-Felipe, K.M.; Yamada, P.A.; Abreu, D.C.C.; Barbieri, F.A. Kinematic Gait Parameters for Older Adults with Parkinson’s Disease During Street Crossing Simulation. Hum. Mov. Sci. 2020, 70, 102599. [Google Scholar] [CrossRef] [PubMed]
- Caronni, A.; Amadei, M.; Diana, L.; Sangalli, G.; Scarano, S.; Perucca, L.; Rota, V.; Bolognini, N. In Parkinson’s Disease, Dual-Tasking Reduces Gait Smoothness During the Straight-Walking and Turning-While-Walking Phases of the Timed Up and Go Test. BMC Sports Sci. Med. Rehabil. 2025, 17, 42. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.P.; Lin, I.I.; Chiou, W.D.; Huang, Y.Z.; Chuang, L.L.; Lien, H.Y. The Executive-Function-Related Cognitive-Motor Dual Task Walking Performance and Task Prioritizing Effect on People with Parkinson’s Disease. Healthcare 2023, 11, 567. [Google Scholar] [CrossRef]
- Raffegeau, T.E.; Krehbiel, L.M.; Kang, N.; Altmann, L.J.P.; Cauraugh, J.H.; Hass, C.J. A Meta-Analysis: Parkinson’s Disease and Dual-Task Walking. Parkinsonism Relat. Disord. 2019, 62, 28–35. [Google Scholar] [CrossRef]
- DeLong, M.R.; Wichmann, T. Circuits and Circuit Disorders of the Basal Ganglia. Arch. Neurol. 2007, 64, 20–24. [Google Scholar] [CrossRef]
- Heimer, G.; Rivlin, M.; Israel, Z.; Bergman, H. Synchronizing Activity of Basal Ganglia and Pathophysiology of Parkinson’s Disease. J. Neural Transm. Suppl. 2006, 70, 17–20. [Google Scholar]
- O’Shea, S.; Morris, M.E.; Iansek, R. Dual Task Interference During Gait in People with Parkinson’s Disease: Effects of Motor versus Cognitive Secondary Tasks. Phys. Ther. 2002, 82, 888–897. [Google Scholar] [CrossRef] [PubMed]
- Beauchet, O.B.G. Marche et Double Tâche: Définition, Intérêts et Perspectives. Psychol. Neuropsychiatr. Vieil. 2006, 4, 215–225. [Google Scholar] [PubMed]
- Brauer, S.; Woollacott, M.H.; Lamont, R.; Clewett, S. Single and Dual Task Gait Training in People with Parkinson’s Disease: A Protocol for a Randomised Controlled Trial. BMC Neurol. 2011, 11, 90. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Hallett, M. Neural Correlates of Dual Task Performance in Patients with Parkinson’s Disease. J. Neurol. Neurosurg. Psychiatry 2008, 79, 760–766. [Google Scholar] [CrossRef]
- Hoehn, M.M.; Yahr, M.D. Parkinsonism: Onset, Progression, and Mortality. Neurology 1967, 17, 427–442. [Google Scholar] [CrossRef]
- Abbud, G.A.C.; Li, K.Z.H.; Demont, R.G. Attentional Requirements of Walking According to the Gait Phase and Onset of Auditory Stimuli. Gait Posture 2009, 30, 227–232. [Google Scholar] [CrossRef]
- Hahn, M.E.; Lee, H.J.; Chou, L.S. Increased Muscular Challenge in Older Adults During Obstructed Gait. Gait Posture 2005, 22, 356–361. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-Mental State”: A Practical Method for Grading the Cognitive State of Patients for the Clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Espay, A.J.; Fasano, A.; van Nuenen, B.F.L.; Payne, M.; Snijders, A.H.; Bloem, B.R. “On” State Freezing of Gait in Parkinson Disease: A Paradoxical Levodopa-Induced Complication. Neurology 2012, 78, 454–457. [Google Scholar] [CrossRef]
- Vieira, E.R.; Lim, H.H.; Brunt, D.; Vankoski, S. Temporo-Spatial Gait Parameters During Street Crossing Conditions: A Comparison Between Younger and Older Adults. Gait Posture 2015, 41, 510–515. [Google Scholar] [CrossRef]
- Simieli, L.; Vitório, R.; Rodrigues, S.T.; Gobbi, L.T.B.; Barbieri, F.A. Gaze and Motor Behavior of People with PD During Obstacle Circumvention. Gait Posture 2017, 58, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Delzenne, J.; Bourelly, A.; Garnier, C.; Zory, R. How Do Healthy Older Pedestrians Walk When They Cross the Street? Comput. Methods Biomech. Biomed. Engin. 2014, 17 (Suppl. 1), 98–99. [Google Scholar] [CrossRef] [PubMed]
- Sarbaz, Y.; Banaie, M.; Pooyan, M.; Nazarpour, K. Modeling the Gait of Normal and Parkinsonian Persons for Improving the Diagnosis. Neurosci. Lett. 2012, 509, 72–75. [Google Scholar] [CrossRef]
- Penedo, T.; Kalva-Filho, C.A.; Cursiol, J.A.; Faria, M.H.; Coelho, D.B.; Barbieri, F.A. Spatial-Temporal Parameters During Unobstructed Walking in People with Parkinson’s Disease and Healthy Older People: A Public Data Set. Front. Aging Neurosci. 2024, 16, 1354738. [Google Scholar] [CrossRef] [PubMed]
- Bokura, H.; Yamaguchi, S.; Kobayashi, S. Event-Related Potentials for Response Inhibition in Parkinson’s Disease. Neuropsychologia 2005, 43, 967–975. [Google Scholar] [CrossRef]
- Yarnall, A.; Rochester, L.; Burn, D.J. The Interplay of Cholinergic Function, Attention, and Falls in Parkinson’s Disease. Mov. Disord. 2011, 26, 2496–2503. [Google Scholar] [CrossRef]
- Hausdorff, J.M.; Balash, J.; Giladi, N. Effects of Cognitive Challenge on Gait Variability in Patients with Parkinson’s Disease. J. Geriatr. Psychiatry Neurol. 2003, 16, 53–58. [Google Scholar] [CrossRef]
- Lin, C.H.; Ou, Y.K.; Wu, R.M.; Liu, Y.C. Predictors of Road Crossing Safety in Pedestrians with Parkinson’s Disease. Accid. Anal. Prev. 2013, 51, 202–207. [Google Scholar] [CrossRef]
- Piet, A.; Geritz, J.; Garcia, P.; Erb, J.; Spalek, K.; Protzak, J. Predicting Executive Functioning from Walking Features in Parkinson’s Disease Using Machine Learning. Sci. Rep. 2024, 14, 29522. [Google Scholar] [CrossRef]
- Di Filippo, F.; De Biasi, G.; Russo, M.; Santilli, L.; Ricciardi, C.; Mirarchi, L.; Russo, A. Dual-Task-Related Gait Patterns as Possible Marker of Precocious and Subclinical Cognitive Alterations in Parkinson Disease. Sci. Rep. 2025, 15, 3371. [Google Scholar] [CrossRef]
- Conselho Nacional de Trânsito. Manual Brasileiro de Sinalização de Trânsito: Sinalização Semafórica; CONTRAN: Brasília, Brazil, 2023; Volume 5, p. 46. [Google Scholar]
- Monteiro, E.P.; Wild, L.B.; Martinez, F.G.; Lirani, L.S.; Wutzki, L.L.; Netto, F.O.; Carvalho, A.R.; Lacerda, A.C.R. Biomechanical Aspects of Locomotion in People with Parkinson’s Disease: Review Study. Rev. Bras. Ciênc. Esporte 2017, 39, 450–457. [Google Scholar] [CrossRef]
- Barnes, M.P. Spasticity: A Rehabilitation Challenge in the Elderly. Gerontology 2001, 47, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Pistacchi, M.; Gioulis, M.; Giovannini, E.D.; Sanson, F.; Filippi, G. Gait Analysis and Clinical Correlations in Early Parkinson’s Disease. Funct. Neurol. 2017, 32, 28–34. [Google Scholar] [CrossRef]
- Williams, D.S.; Martin, A.E. Gait Modification When Decreasing Double Support Percentage. J. Biomech. 2019, 92, 76–83. [Google Scholar] [CrossRef]
- Oxley, J.; Fildes, B.; Ihsen, E.; Charlton, J. Differences in Traffic Judgements Between Young and Old Adult Pedestrians. Accid. Anal. Prev. 1997, 29, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.W.; Duncan, M.J.; Oxford, S.W.; Eyre, E.L.J.; Nevill, A.M.; Clarke, N.D. Effects of External Loads on Postural Sway During Quiet Stance in Adults Aged 20–80 Years. Appl. Ergon. 2018, 66, 64–69. [Google Scholar] [CrossRef]
- Lee, J.M.; Koh, S.B.; Chae, S.W.; Seo, W.K.; Kim, J.H.; Park, K.W. Postural Instability and Cognitive Dysfunction in Early Parkinson’s Disease. Can. J. Neurol. Sci. 2012, 39, 473–482. [Google Scholar] [CrossRef]
- Instituto de Pesquisa Econômica Aplicada (IPEA). Impactos Socioeconômicos dos Acidentes de Transporte no Brasil no Período de 2007 a 2018; Nota Técnica 75; IPEA: Brasília, Brazil, 2020. [Google Scholar]

| CG (n = 18) | PG (n = 18) | p Values | |
|---|---|---|---|
| Sex (M/F) | 3 M/15 F | 12 M/6 F | - |
| Age (years) | 67.05 ± 6.46 | 68.72 ± 8.04 | 0.49 |
| Body Mass (kg) | 75.66 ± 17.15 | 65.94 ± 17.37 | 0.11 |
| Height (m) | 1.56 ± 0.07 | 1.60 ± 0.10 | 0.14 |
| BMI (kg/m2) | 30.95 ± 5.75 | 25.96 ± 5.20 | 0.01 * |
| MMSE (points) | 27.39 ± 2.40 | 27.56 ± 2.20 | 0.82 |
| UPDRS (points) | - | 26.50 ± 10.62 | - |
| H&Y | - | 2.31 ± 0.48 | - |
| Diagnostic Time (years) | - | 5.91 ± 3.78 | - |
| Number of falls (last year) | 0.38 ± 0.84 | 0.61 ± 0.97 | 0.47 |
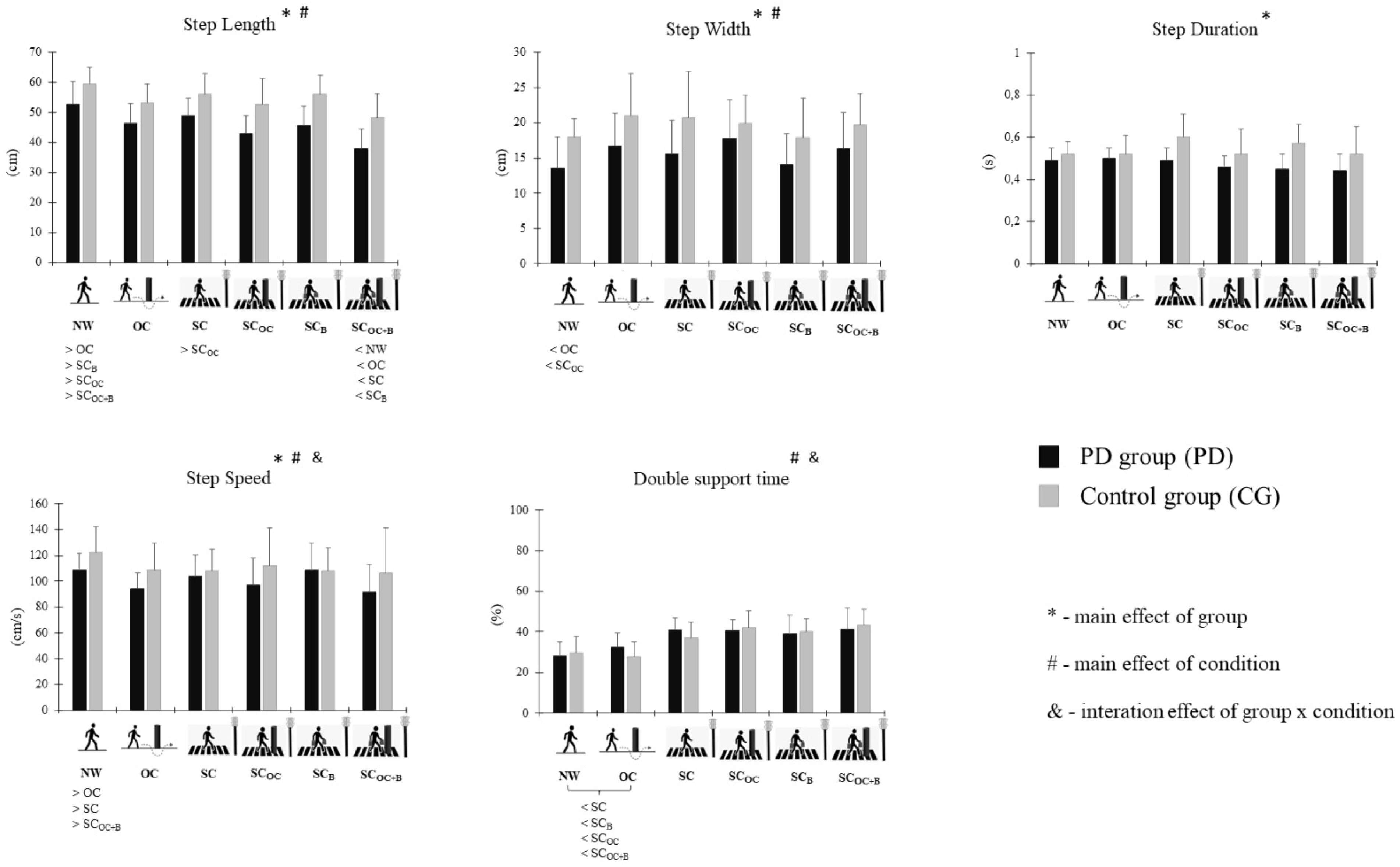
| Variables | Conditions | Groups | Main Effects (F(1,17) and p Values) | |||
|---|---|---|---|---|---|---|
| CG (n = 18) | PG (n = 18) | Group | Conditions | Group × Conditions | ||
| Step length (cm) | NW | 59.36 (5.70) | 52.61 (7.73) | F = 63.668 p ≤ 0.001 | F = 22.943 p ≤ 0.001 | F = 0.485 p = 0.781 |
| SC | 56.03 (6.76) | 49.00 (5.65) | ||||
| SCB | 56.01 (6.43) | 45.61 (6.62) | ||||
| OC | 53.2 (6.20) | 46.21 (6.77) | ||||
| SCOC | 52.59 (8.66) | 42.83 (6.03) | ||||
| SCOC+B | 48.18 (8.18) | 37.86 (6.58) | ||||
| Step width (cm) | NW | 18.02 (2.61) | 13.54 (4.47) | F = 11.776 p = 0.003 | F = 5.858 p = 0.005 | F = 0.854 p = 0.537 |
| SC | 20.66 (6.66) | 15.57 (4.73) | ||||
| SCB | 17.84 (5.65) | 14.10 (4.38) | ||||
| OC | 21.02 (5.92) | 16.69 (4.68) | ||||
| SCOC | 19.90 (4.09) | 17.83 (5.49) | ||||
| SCOC+B | 19.64 (4.52) | 16.37 (5.10) | ||||
| Step duration (s) | NW | 0.52 (0.06) | 0.49 (0.06) | F = 20.362 p ≤ 0.001 | F = 1.839 p = 0.174 | F = 2.945 p = 0.054 |
| SC | 0.60 (0.11) | 0.49 (0.06) | ||||
| SCB | 0.57 (0.09) | 0.45 (0.07) | ||||
| OC | 0.52 (0.09) | 0.50 (0.05) | ||||
| SCOC | 0.52 (0.12) | 0.46 (0.05) | ||||
| SCOC+B | 0.52 (0.13) | 0.44 (0.08) | ||||
| Step speed (cm/s) | NW | 122.09 (20.53) | 108.82 (12.59) | F = 7.624 p = 0.013 | F = 5.921 p = 0.005 | F = 3.819 p = 0.024 |
| SC | 108.29 (16.45) | 104.17 (16.49) | ||||
| SCB | 108.04 (18.12) | 108.99 (20.54) | ||||
| OC | 108.82 (20.74) | 94.29 (12.11) | ||||
| SCOC | 111.79 (29.46) | 97.47 (20.45) | ||||
| SCOC+B | 106.51 (34.48) | 91.88 (20.89) | ||||
| Double support time (%) | NW | 29.58 (8.26) | 28.22 (6.86) | F = 0.130 p = 0.723 | F = 21.014 p ≤ 0.001 | F = 3.267 p = 0.040 |
| SC | 37.09 (7.86) | 40.86 (5.95) | ||||
| SCB | 40.13 (6.11) | 39.10 (9.12) | ||||
| OC | 27.59 (7.66) | 32.30 (6.99) | ||||
| SCOC | 42.31 (8.00) | 40.49 (5.42) | ||||
| SCOC+B | 43.34 (7.83) | 41.47 (10.45) | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soares, C.F.; Silveira-Ciola, A.P.; Simieli, L.; Yamada, P.d.A.; Barbieri, F.A.; Faganello-Navega, F.R. Obstacle Circumvention and Motor Daily Dual Task During a Simulation of Street Crossing by Individuals with Parkinson’s Disease. Life 2025, 15, 900. https://doi.org/10.3390/life15060900
Soares CF, Silveira-Ciola AP, Simieli L, Yamada PdA, Barbieri FA, Faganello-Navega FR. Obstacle Circumvention and Motor Daily Dual Task During a Simulation of Street Crossing by Individuals with Parkinson’s Disease. Life. 2025; 15(6):900. https://doi.org/10.3390/life15060900
Chicago/Turabian StyleSoares, Carolina Favarin, Aline Prieto Silveira-Ciola, Lucas Simieli, Patrícia de Aguiar Yamada, Fábio Augusto Barbieri, and Flávia Roberta Faganello-Navega. 2025. "Obstacle Circumvention and Motor Daily Dual Task During a Simulation of Street Crossing by Individuals with Parkinson’s Disease" Life 15, no. 6: 900. https://doi.org/10.3390/life15060900
APA StyleSoares, C. F., Silveira-Ciola, A. P., Simieli, L., Yamada, P. d. A., Barbieri, F. A., & Faganello-Navega, F. R. (2025). Obstacle Circumvention and Motor Daily Dual Task During a Simulation of Street Crossing by Individuals with Parkinson’s Disease. Life, 15(6), 900. https://doi.org/10.3390/life15060900








