Cerebral Near-Infrared Spectroscopy and Electrical Cardiometry During Endotracheal Suction in Ventilated Infants Following Surgery: A Feasibility Study
Abstract
1. Introduction
2. Patients and Methods
2.1. Patients and Design
2.2. Near-Infrared Spectroscopy
2.3. Electrical Cardiometry
2.4. Respiratory Procedures
2.5. Statistical Analysis
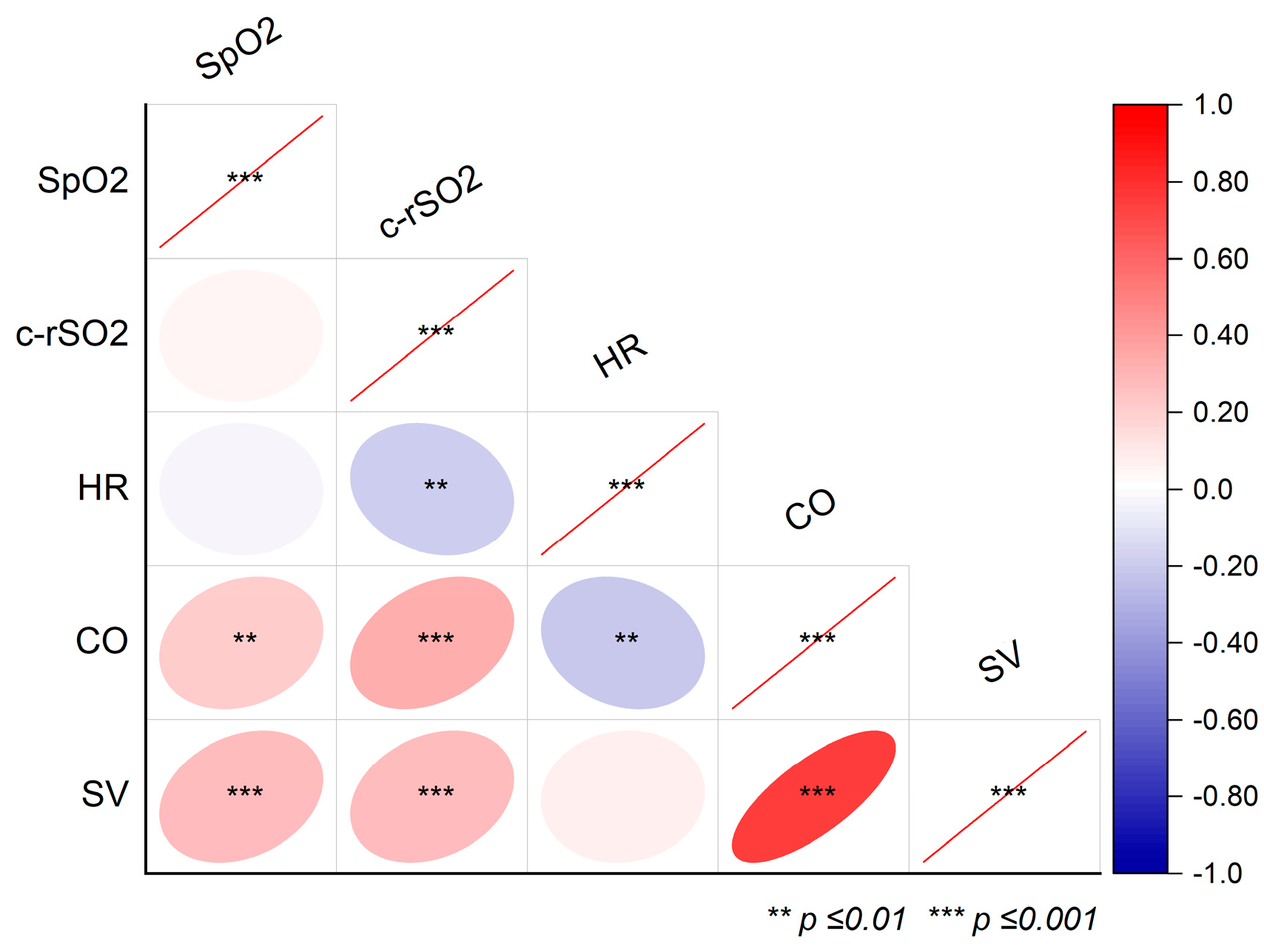
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| CO | Cardiac output |
| CPAP | Continuous positive airway pressure |
| c-rSO2 | Cerebral regional oxygenation |
| EC | Electrical Cardiometry |
| ETS | Endotracheal suction |
| HFNC | High-flow nasal cannula |
| HR | Heart rate |
| Hz | Hertz |
| MV | Mechanical ventilation |
| NIRS | Near-Infrared Spectroscopy |
| SIMV | Synchronized intermittent mandatory ventilation |
| SpO2 | Peripheral oxygen saturation |
| SQI | Signal quality index |
| SV | Stroke volume |
| T1–3 | Timepoints 1–3 (before, during, and after ETS) |
References
- Chakkarapani, A.A.; Adappa, R.; Ali, S.K.M.; Gupta, S.; Soni, N.B.; Chicoine, L.; Hummler, H.D. “Current Concepts of Mechanical Ventilation in Neonates”—Part 1. Int. J. Pediatr. Adolesc. Med. 2020, 7, 15–20. [Google Scholar] [CrossRef]
- Shekerdemian, L.; Bohn, D. Cardiovascular Effects of Mechanical Ventilation. Arch. Dis. Child. 1999, 80, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, A.; Ruoss, J.L.; Stanford, A.H.; Lakshminrusimha, S.; McNamara, P.J. Hemodynamic Consequences of Respiratory Interventions in Preterm Infants. J. Perinatol. 2022, 42, 1153–1160. [Google Scholar] [CrossRef] [PubMed]
- American Association for Respiratory Care. AARC Clinical Practice Guidelines. Endotracheal Suctioning of Mechanically Ventilated Patients with Artificial Airways 2010. Respir. Care 2010, 55, 758–764. [Google Scholar]
- Skov, L.; Ryding, J.; Pryds, O.; Greisen, G. Changes in Cerebral Oxygenation and Cerebral Blood Volume during Endotracheal Suctioning in Ventilated Neonates. Acta Paediatr. 1992, 81, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Stolwijk, L.J.; Keunen, K.; de Vries, L.S.; Groenendaal, F.; van der Zee, D.C.; van Herwaarden, M.Y.A.; Lemmers, P.M.A.; Benders, M.J.N.L. Neonatal Surgery for Noncardiac Congenital Anomalies: Neonates at Risk of Brain Injury. J. Pediatr. 2017, 182, 335–341.e1. [Google Scholar] [CrossRef]
- Selvanathan, T.; Au-Young, S.H.; Guo, T.; Chau, V.; Branson, H.M.; Synnes, A.; Ly, L.; Kelly, E.N.; Grunau, R.E.; Miller, S.P. Major Surgery, Brain Injury, and Neurodevelopmental Outcomes in Very Preterm Infants. Neurology 2023, 101, 952–957. [Google Scholar] [CrossRef]
- Gardner, D.; Shirland, L. Evidence-Based Guideline for Suctioning the Intubated Neonate and Infant. Neonatal Netw. 2009, 28, 281–302. [Google Scholar] [CrossRef]
- Clifton-Koeppel, R. Endotracheal Tube Suctioning in the Newborn: A Review of the Literature. Newborn Infant. Nurs. Rev. 2006, 6, 94–99. [Google Scholar] [CrossRef]
- Kooi, E.M.W.; Mintzer, J.P.; Rhee, C.J.; Ergenekon, E.; Schwarz, C.E.; Pichler, G.; de Boode, W.P.; Alarcón, A.; Alderliesten, T.; Austin, T.; et al. Neonatal Somatic Oxygenation and Perfusion Assessment Using Near-Infrared Spectroscopy. Pediatr. Res. 2024, 96, 1180–1194. [Google Scholar] [CrossRef]
- Pellicer, A.; de Boode, W.; Dempsey, E.; Greisen, G.; Mintzer, J.; Naulaers, G.; Pichler, G.; Roehr, C.C.; Roll, C.; Schwarz, C.; et al. Cerebral Near-Infrared Spectroscopy Guided Neonatal Intensive Care Management for the Preterm Infant. Pediatr. Res. 2024. [Google Scholar] [CrossRef]
- Levy, P.T.; Pellicer, A.; Schwarz, C.E.; Neunhoeffer, F.; Schuhmann, M.U.; Breindahl, M.; Fumagelli, M.; Mintzer, J.; de Boode, W.; Alarcon, A.; et al. Near-Infrared Spectroscopy for Perioperative Assessment and Neonatal Interventions. Pediatr. Res. 2024, 96, 922–932. [Google Scholar] [CrossRef]
- Nasr, V.G.; Bergersen, L.T.; Lin, H.-M.; Benni, P.B.; Bernier, R.S.; Anderson, M.E.; Kussman, B.D. Validation of a Second-Generation Near-Infrared Spectroscopy Monitor in Children with Congenital Heart Disease. Anesth. Analg. 2019, 128, 661–668. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, R.; Dempsey, E.M.; Garvey, A.A.; Schwarz, C.E. Non-Invasive Cardiac Output Monitoring in Neonates. Front. Pediatr. 2021, 8, 614585. [Google Scholar] [CrossRef]
- Soaida, S.; Hanna, M.; Mahmoud, A.; Elmetwally, S. Electrical Velocimetry (ICON Cardiometry) Assessment of Hemodynamic Changes Associated with Different Inflation Pressures during Pediatric Thoracoscopic Surgery: A Pilot Study. Egypt. J. Cardiothorac. Anesth. 2021, 15, 3. [Google Scholar] [CrossRef]
- Rachel, M.; Jan, M.; Heather, C.; Jana, S. Non-Invasive Cardiac Output Monitoring before and after Baby Extubation—A Feasibility Study (NICOMBabe Study). Early Hum. Dev. 2022, 170, 105605. [Google Scholar] [CrossRef]
- Boet, A.; Jourdain, G.; Capderou, A.; Grollmuss, O.; Labrune, P.; De Luca, D.; Demontoux, S. PO-0488 Non-Invasive Haemodynamic Monitoring Using Electrical Cardiometry in Neonates During Respiratory Procedures. Arch. Dis. Child. 2014, 99, A407–A408. [Google Scholar] [CrossRef][Green Version]
- Gil-Anton, J.; Redondo, S.; Urabayen, D.G.; Faza, M.N.; Sanz, I.; Pilar, J. Combined Cerebral and Renal Near-Infrared Spectroscopy After Congenital Heart Surgery. Pediatr. Cardiol. 2015, 36, 1173–1178. [Google Scholar] [CrossRef]
- Marin, T.; Moore, J. Understanding Near-Infrared Spectroscopy. Adv. Neonatal Care 2011, 11, 382–388. [Google Scholar] [CrossRef]
- Noori, S.; Drabu, B.; Soleymani, S.; Seri, I. Continuous Non-Invasive Cardiac Output Measurements in the Neonate by Electrical Velocimetry: A Comparison with Echocardiography. Arch. Dis. Child. Fetal Neonatal Ed. 2012, 97, F340–F343. [Google Scholar] [CrossRef]
- van Wyk, L.; Austin, T.; Barzilay, B.; Bravo, M.C.; Breindahl, M.; Czernik, C.; Dempsey, E.; de Boode, W.-P.; de Vries, W.; Eriksen, B.H.; et al. A Recommendation for the Use of Electrical Biosensing Technology in Neonatology. Pediatr. Res. 2024, 97, 510–523. [Google Scholar] [CrossRef] [PubMed]
- Merter, O.S.; Dertli, S.; Taskin, E.; Aydin, M.; Benli, S. Effects of Endotracheal Suctioning Duration Cerebral Oxygenation in Preterm Infants. J. Clin. Nurs. 2024, 34, 1063–1071. [Google Scholar] [CrossRef] [PubMed]
- Roll, C.; Horsch, S.; Knief, J.; Hüsing, J.; Hanssler, L. Vergleich Der Effekte von Endotrachealem Absaugen Und Surfactantapplikation Auf Hämodynamik Und Oxygenierung Frühgeborener—Eine Nahinfrarotspektroskopie-Studie1. Z. Geburtshilfe Neonatol. 2001, 205, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Kohlhauser, C.; Bernert, G.; Hermon, M.; Popow, C.; Seidl, R.; Pollak, A. Effects of Endotracheal Suctioning in High-Frequency Oscillatory and Conventionally Ventilated Low Birth Weight Neonates on Cerebral Hemodynamics Observed by near Infrared Spectroscopy (NIRS). Pediatr. Pulmonol. 2000, 29, 270–275. [Google Scholar] [CrossRef]
- Bernert, G.; von Siebenthal, K.; Seidl, R.; Vanhole, C.; Devlieger, H.; Casaer, P. The Effect of Behavioural States on Cerebral Oxygenation during Endotracheal Suctioning of Preterm Babies. Neuropediatrics 1997, 28, 111–115. [Google Scholar] [CrossRef]
- Shah, A.R.; Kurth, C.D.; Gwiazdowski, S.G.; Chance, B.; Delivoria-Papadopoulos, M. Fluctuations in Cerebral Oxygenation and Blood Volume during Endotracheal Suctioning in Premature Infants. J. Pediatr. 1992, 120, 769–774. [Google Scholar] [CrossRef]
- Bucher, H.U.; Blum-Gisler, M.; Duc, G. Changes in Cerebral Blood Volume during Endotracheal Suctioning. J. Pediatr. 1993, 122, 324. [Google Scholar] [CrossRef]
- Limperopoulos, C.; Gauvreau, K.K.; O’Leary, H.; Moore, M.; Bassan, H.; Eichenwald, E.C.; Soul, J.S.; Ringer, S.A.; Di Salvo, D.N.; du Plessis, A.J. Cerebral Hemodynamic Changes During Intensive Care of Preterm Infants. Pediatrics 2008, 122, e1006–e1013. [Google Scholar] [CrossRef]
- Misirlioglu, M.; Horoz, O.O.; Yildizdas, D.; Ekinci, F.; Yontem, A.; Menemencioglu, A.; Salva, G. The Effects of Endotracheal Suctioning on Hemodynamic Parameters and Tissue Oxygenation in Pediatric Intensive Care Unit. J. Pediatr. Intensive Care 2022, 11, 349–354. [Google Scholar] [CrossRef]
- Chegondi, M.; Francis, T.; Lin, W.-C.; Naqvi, S.; Raszynski, A.; Totapally, B.R. Effects of Closed Endotracheal Suctioning on Systemic and Cerebral Oxygenation and Hemodynamics in Children. Pediatr. Crit. Care Med. 2018, 19, e23–e30. [Google Scholar] [CrossRef]
- Mosca, F.A.; Colnaghi, M.; Lattanzio, M.; Bray, M.; Pugliese, S.; Fumagalli, M. Closed versus Open Endotracheal Suctioning in Preterm Infants: Effects on Cerebral Oxygenation and Blood Volume. Biol. Neonate 1997, 72, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Vesoulis, Z.A.; Mintzer, J.P.; Chock, V.Y. Neonatal NIRS Monitoring: Recommendations for Data Capture and Review of Analytics. J. Perinatol. 2021, 41, 675–688. [Google Scholar] [CrossRef] [PubMed]
- Sakuma, S.; Inamoto, K.; Higuchi, N.; Ariji, Y.; Nakayama, M.; Izumi, M. Experimental Pain in the Gingiva and its Impact on Prefrontal Cortical Hemodynamics: A Functional near-Infrared Spectroscopy Study. Neurosci. Lett. 2014, 575, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Yücel, M.A.; Aasted, C.M.; Petkov, M.P.; Borsook, D.; Boas, D.A.; Becerra, L. Specificity of Hemodynamic Brain Responses to Painful Stimuli: A Functional Near-Infrared Spectroscopy Study. Sci. Rep. 2015, 5, 9469. [Google Scholar] [CrossRef]
- Becerra, L.; Aasted, C.M.; Boas, D.A.; George, E.; Yücel, M.A.; Kussman, B.D.; Kelsey, P.; Borsook, D. Brain Measures of Nociception Using Near-Infrared Spectroscopy in Patients Undergoing Routine Screening Colonoscopy. Pain 2016, 157, 840–848. [Google Scholar] [CrossRef]
- Lanning, K.M.; Ylikauma, L.A.; Erkinaro, T.M.; Ohtonen, P.P.; Vakkala, M.A.; Kaakinen, T.I. Changes in Transcranial Near-Infrared Spectroscopy Values Reflect Changes in Cardiac Index during Cardiac Surgery. Acta Anaesthesiol. Scand. 2023, 67, 599–605. [Google Scholar] [CrossRef]
- Truong, L.; Kim, J.H.; Katheria, A.C.; Finer, N.N.; Marc-Aurele, K. Haemodynamic Effects of Premedication for Neonatal Intubation: An Observational Study. Arch. Dis. Child. Fetal Neonatal Ed. 2020, 105, 123–127. [Google Scholar] [CrossRef]
- Martini, S.; Frabboni, G.; Rucci, P.; Czosnyka, M.; Smielewski, P.; Galletti, S.; Cimatti, A.G.; Faldella, G.; Corvaglia, L.; Austin, T. Cardiovascular and Cerebrovascular Responses to Cardio-Respiratory Events in Preterm Infants during the Transitional Period. J. Physiol. 2020, 598, 4107–4119. [Google Scholar] [CrossRef]
- Forman, E.; Breatnach, C.R.; Ryan, S.; Semberova, J.; Miletin, J.; Foran, A.; EL-Khuffash, A. Noninvasive Continuous Cardiac Output and Cerebral Perfusion Monitoring in Term Infants with Neonatal Encephalopathy: Assessment of Feasibility and Reliability. Pediatr. Res. 2017, 82, 789–795. [Google Scholar] [CrossRef]
- Marino, B.S.; Tabbutt, S.; MacLaren, G.; Hazinski, M.F.; Adatia, I.; Atkins, D.L.; Checchia, P.A.; DeCaen, A.; Fink, E.L.; Hoffman, G.M.; et al. Cardiopulmonary Resuscitation in Infants and Children with Cardiac Disease. Circulation 2018, 137, E691–E782. [Google Scholar] [CrossRef]
- Hansen, M.L.; Hyttel-Sørensen, S.; Jakobsen, J.C.; Gluud, C.; Kooi, E.M.W.; Mintzer, J.; de Boode, W.P.; Fumagalli, M.; Alarcon, A.; Alderliesten, T.; et al. Cerebral Near-Infrared Spectroscopy Monitoring (NIRS) in Children and Adults: A Systematic Review with Meta-Analysis. Pediatr Res 2024, 96, 856–867. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Xing, D.; Zhou, S.; Fang, F.; Fu, Y.; Xu, F. Electrical Bioimpedance Measurement and Near-Infrared Spectroscopy in Pediatric Postoperative Neurocritical Care: A Prospective Observational Study. Front Neurol 2023, 14. [Google Scholar] [CrossRef] [PubMed]
- Van Loo, L.; Cools, B.; Dereymaeker, A.; Jansen, K. Neuromonitoring Modalities Predicting Neurological Impairment in Pediatric Congenital Heart Disease: A Systematic Review. Front Neurol 2024, 15. [Google Scholar] [CrossRef] [PubMed]
- Pardo, A.C.; Carrasco, M.; Wintermark, P.; Nunes, D.; Chock, V.Y.; Sen, S.; Wusthoff, C.J.; Bonifacio, S.; Aly, H.; Chau, V.; et al. Neuromonitoring Practices for Neonates with Congenital Heart Disease: A Scoping Review. Pediatr Res 2024. [Google Scholar] [CrossRef]
| Patient’s ID | 01 | 02 | 03 | 04 | 05 | 06 | 07 | Σ |
|---|---|---|---|---|---|---|---|---|
| Gender (n; f/m) | f | f | f | m | m | m | f | 4/3 |
| Gestational age (weeks) | 34 | 26 | 42 | 28 | 31 | 26 | 33 | 31 (26–42) |
| Birth weight (kg) | 1.94 | 0.40 | 3.45 | 1.18 | 1.70 | 0.71 | 1.08 | 1.2 (0.4–3.5) |
| Postnatal age (d) | 6 | 149 | 1 | 34 | 16 | 44 | 1 | 16 (1–149) |
| Weight (kg) | 1.94 | 3.79 | 3.1 | 1.95 | 2.14 | 1.38 | 1.08 | 2.0 (1.1–3.8) |
| Length (cm) | 46 | 48 | 55 | 45 | 41 | 37 | 36 | 44 (36–55) |
| Type of surgery | Lap/iOC | Lap/ileus * | Tho/EA | Lap/NEC | Lap/NEC | Lap/NEC | Lap/DA | |
| Comorbidities | - | BPD, PVL | VSD | BPD | - | MMC | VSD | |
| Respiratory data | ||||||||
| ETT type | Vygon | Portex | Vygon | Vigon | Rüsch | Vigon | Portex | |
| ETT inner diameter (mm) | 3.0 | 3.5 | 3.0 | 2.5 | 3.0 | 2.5 | 2.5 | |
| Vent. support before surgery (y/n) / type | n | n | n | y/CPAP | y/SIMV | y/SIMV | n | |
| ET-intubation time (hrs) | 26 | 13.5 | 56 | 65 | 150 | 506 | 94 | 77 (13.5–506) |
| Endotracheal Suction | Overall Model Characteristics | Pairwise Post Hoc Testing (p-Values) | |||||
|---|---|---|---|---|---|---|---|
| T1 (before) | T2 (during) | T3 (after) | T1 vs. T2 | T1 vs. T3 | T2 vs. T3 | ||
| SpO2 (%) | 97 (95–99) | 96 (95–99) | 97 (95–99) | x2 (2) = 0.55 (p = 0.76) | 1 | 1 | 1 |
| HR (1/min) | 144 ± 3 | 136 ± 3 | 142 ± 2 | F (2,68) = 7.87 (p < 0.001) | <0.001 | 0.39 | 0.032 |
| c-rSO2 (%) | 76 (68–81) | 75 (65–81) | 79 (70–82) | x2 (2) = 19.1 (p < 0.001) | 0.09 | 0.09 | <0.001 |
| SV (mL/beat) | 3.3 (2.7–4.1) | 3.4 (3–4.2) | 3.5 (3.2–4.5) | x2 (2) = 10.5 (p = 0.005) | 0.46 | 0.004 | 0.22 |
| CO (L/min) | 0.48 (0.43–0.57) | 0.48 (0.38–0.56) | 0.5 (0.45–0.58) | x2 (2) = 8.9 (p < 0.013) | 0.70 | 0.25 | 0.01 |
| Reference | Present Study | Merter et al. [22] | Misirlioglu et al. [29] | Chegondi et al. [30] | Limperopoulos et al. [28] | Roll et al. [23] | Kohlhauser et al. [24] | Bernert et al. [25] | Mosca et al. [31] | Skov et al. [5] | Shah et al. [26] | Bucher et al. [27] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study year | 2025 | 2024 | 2022 | 2017 | 2008 | 2001 | 2000 | 1997 | 1997 | 1992 | 1992 | 1989 |
| Patients (n) | 6 | 51 | 20 | 19 | 82 | 12 | 15 | 13 | 11 | 29 | 12 | 11 |
| ETS events (n) | 38 | 51 | 70 | 287 | 96 | 12 | 26 | 20 | 66 | 58 | ≤36 | n.s. |
| GA (weeks) | 31 | 32 | n.a. | n.a. | 26 | 28 | 28.5 | 30 | 29 | 31 | 30 | “prematures” |
| Postnatal age | 16 d | 5 d | 59 m | 5.7 y | 11.5 h | 12 h | 9.7/4.6 d * | 2 d | 3 d | 36 h | 11 d | n.s. |
| NIRS Device | Invos 5100C | Invos 5100C | Invos 5100C | INVOS (n.s.) | NIRO-500 | Kritikon Redox 2020 | NIRO-500 | NIR1000 | Niro-500 | NIR (n.s.) | Linear Instruments | NIR1000 |
| Mean arterial pressure | n.a. | n.a. | d&a↔ | a↑ | d↔ | d&a↔ | d↔ | d↑(n = 5), ↓(n = 3), ↔(n = 12) | a↔ | d↔ | d↑ | n.a. |
| Heart rate | d↓, a↔ | d↓, a↔ | d↑, a ↔ | a↓ | n.a. | d↓ | d↓ | d↓ | a↓ | n.a. | d↓ | n.a. |
| Oxygen saturation | d&a↔ | d↓, a↑ | d↓, a↔ | a↓ | d↓ | d↓ | d↓ | d↓ | a↓ | d↓ | d↓ | n.a. |
| Cerebral NIRS | d↓(trend), a↑ | d↓, a↔ | d↔ | a↑ | d↑ | d&a↓ | d↓ | d↓ | a↔ | d↓ | d↓ | d↓ |
| Stroke volume | d↔, a↑ | - | - | - | - | - | - | - | - | - | - | - |
| Cardiac output | d↔, a↑ | - | - | - | - | - | - | - | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nissen, M.; Tröbs, R.-B. Cerebral Near-Infrared Spectroscopy and Electrical Cardiometry During Endotracheal Suction in Ventilated Infants Following Surgery: A Feasibility Study. Life 2025, 15, 901. https://doi.org/10.3390/life15060901
Nissen M, Tröbs R-B. Cerebral Near-Infrared Spectroscopy and Electrical Cardiometry During Endotracheal Suction in Ventilated Infants Following Surgery: A Feasibility Study. Life. 2025; 15(6):901. https://doi.org/10.3390/life15060901
Chicago/Turabian StyleNissen, Matthias, and Ralf-Bodo Tröbs. 2025. "Cerebral Near-Infrared Spectroscopy and Electrical Cardiometry During Endotracheal Suction in Ventilated Infants Following Surgery: A Feasibility Study" Life 15, no. 6: 901. https://doi.org/10.3390/life15060901
APA StyleNissen, M., & Tröbs, R.-B. (2025). Cerebral Near-Infrared Spectroscopy and Electrical Cardiometry During Endotracheal Suction in Ventilated Infants Following Surgery: A Feasibility Study. Life, 15(6), 901. https://doi.org/10.3390/life15060901






