DuoStim Shows Comparable Efficacy but Better Efficiency than Two Conventional Stimulations in Poor/Suboptimal Responders Undergoing Vitrified Oocyte Accumulation for PGT-A
Abstract
1. Introduction
2. Materials and Methods
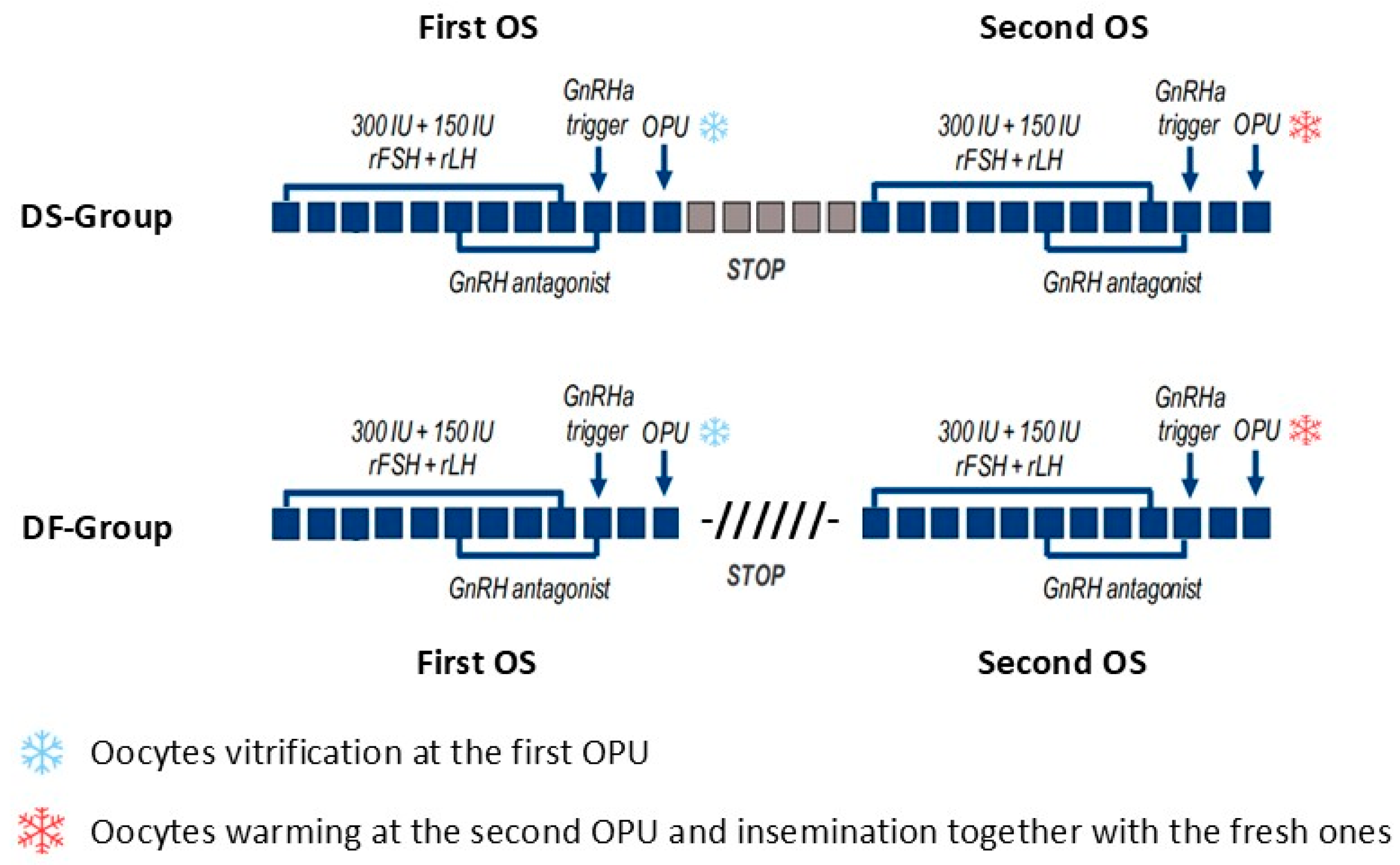
2.1. Study Design and Patient Population
2.2. Ovarian Stimulation (OS), IVF and PGT-A
2.3. Statistical Analysis
3. Results
3.1. DuoStim and Two Conventional Stimulations Show Similar Efficacy, with Comparable Oocyte Yield, Euploid Blastocysts, and CLBR
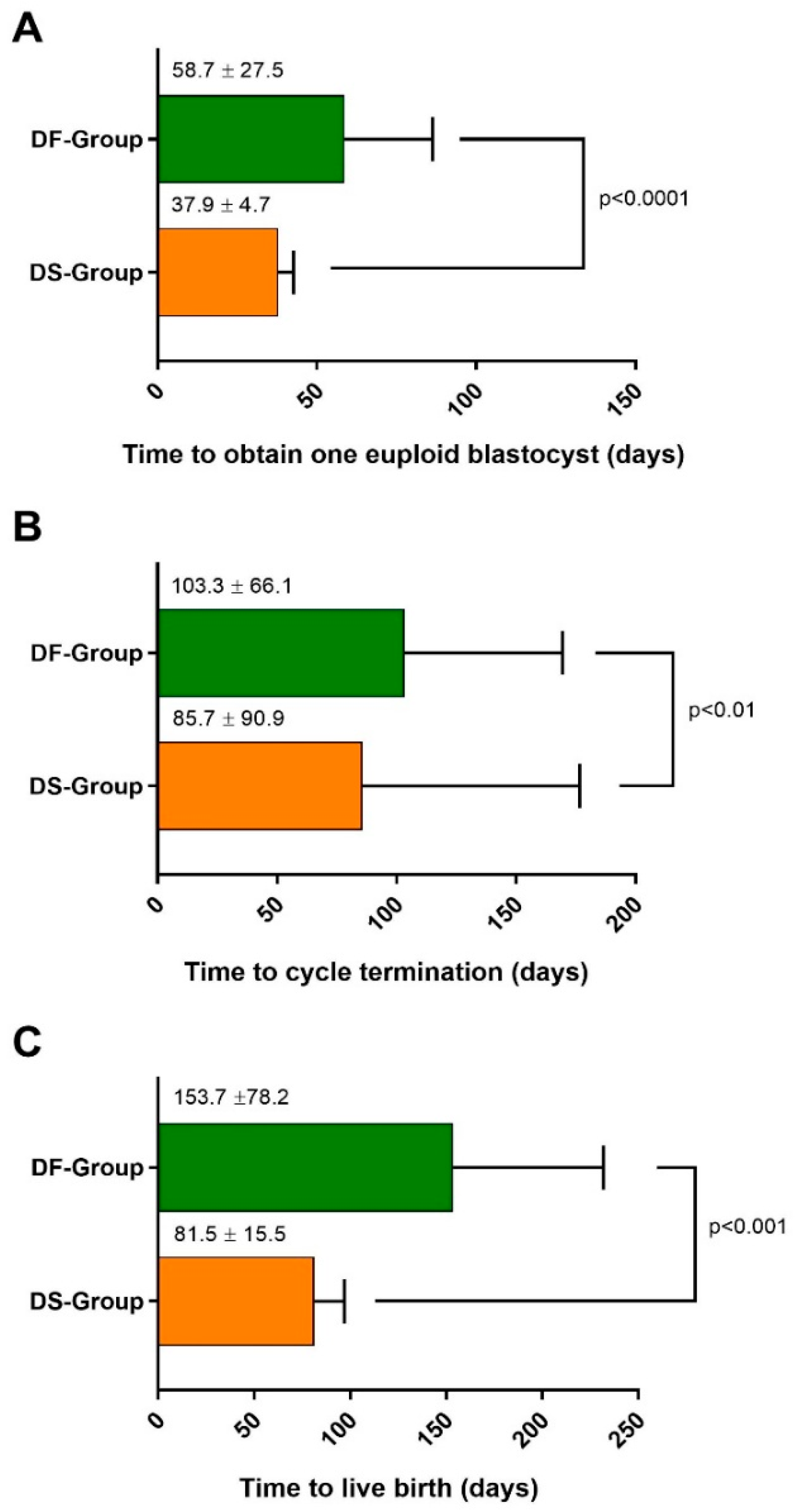
3.2. DuoStim Shows Better Efficiency, with a Shorter Time to Obtain Euploid Blastocysts, Achieve a Live Birth, and Conclude the Cycle
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BMI | Body Mass Index |
| FSH | Follicle-Stimulating Hormone |
| LH | Luteinizing Hormone |
| AMH | Anti-Müllerian Hormone |
| MII | Metaphase II |
| 2PN | 2 Pronuclei |
| OS | Ovarian Stimulation |
| IVF | In Vitro Fertilization |
| OPU | Oocyte Pick-up |
| ET | Embryo Transfer |
| ITT | Intention To Treat |
| CLBR | Cumulative Live Birth Rate |
| PGT-A | Preimplantation Genetic Test for Aneuploidies |
| TTEB | Time To Obtain One Euploid Blastocyst |
| TTLB | Time To Live Birth |
| TTCT | Time To Cycle Termination |
References
- Baerwald, A.R.; Adams, G.P.; Pierson, R.A. A new model for ovarian follicular development during the human menstrual cycle. Fertil Steril. 2003, 80, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Baerwald, A.R.; Adams, G.P.; Pierson, R.A. Characterization of ovarian follicular wave dynamics in women. Biol. Reprod. 2003, 69, 1023–1031. [Google Scholar] [CrossRef] [PubMed]
- Moffat, R.; Pirtea, P.; Gayet, V.; Wolf, J.P.; Chapron, C.; de Ziegler, D. Dual ovarian stimulation is a new viable option for enhancing the oocyte yield when the time for assisted reproductive technnology is limited. Reprod. Biomed. Online 2014, 29, 659–661. [Google Scholar] [CrossRef]
- Kuang, Y.; Chen, Q.; Hong, Q.; Lyu, Q.; Ai, A.; Fu, Y.; Shoham, Z. Double stimulations during the follicular and luteal phases of poor responders in IVF/ICSI programmes (Shanghai protocol). Reprod. Biomed. Online 2014, 29, 684–691. [Google Scholar] [CrossRef] [PubMed]
- Ubaldi, F.M.; Capalbo, A.; Vaiarelli, A.; Cimadomo, D.; Colamaria, S.; Alviggi, C.; Trabucco, E.; Venturella, R.; Vajta, G.; Rienzi, L. Follicular versus luteal phase ovarian stimulation during the same menstrual cycle (DuoStim) in a reduced ovarian reserve population results in a similar euploid blastocyst formation rate: New insight in ovarian reserve exploitation. Fertil Steril. 2016, 105, 1488–1495.e1. [Google Scholar] [CrossRef]
- Cimadomo, D.; Vaiarelli, A.; Colamaria, S.; Trabucco, E.; Alviggi, C.; Venturella, R.; Alviggi, E.; Carmelo, R.; Rienzi, L.; Ubaldi, F.M. Luteal phase anovulatory follicles result in the production of competent oocytes: Intra-patient paired case-control study comparing follicular versus luteal phase stimulations in the same ovarian cycle. Hum. Reprod. 2018, 33, 1442–1448. [Google Scholar] [CrossRef]
- Vaiarelli, A.; Cimadomo, D.; Alviggi, E.; Sansone, A.; Trabucco, E.; Dusi, L.; Buffo, L.; Barnocchi, N.; Fiorini, F.; Colamaria, S.; et al. The euploid blastocysts obtained after luteal phase stimulation show the same clinical, obstetric and perinatal outcomes as follicular phase stimulation-derived ones: A multicenter study. Hum. Reprod. 2020, 35, 2598–2608. [Google Scholar] [CrossRef]
- Vaiarelli, A.; Cimadomo, D.; Conforti, A.; Schimberni, M.; Giuliani, M.; D’Alessandro, P.; Colamaria, S.; Alviggi, C.; Rienzi, L.; Ubaldi, F.M. Luteal phase after conventional stimulation in the same ovarian cycle might improve the management of poor responder patients fulfilling the Bologna criteria: A case series. Fertil Steril. 2020, 113, 121–130. [Google Scholar] [CrossRef]
- Sighinolfi, G.; Sunkara, S.K.; La Marca, A. New strategies of ovarian stimulation based on the concept of ovarian follicular waves: From conventional to random and double stimulation. Reprod. Biomed. Online 2018, 37, 489–497. [Google Scholar] [CrossRef]
- Cecchino, G.N.; Roque, M.; Cerrillo, M.; Filho, R.D.R.; Chiamba, F.D.S.; Hatty, J.H.; García-Velasco, J.A. DuoStim cycles potentially boost reproductive outcomes in poor prognosis patients. Gynecol. Endocrinol. 2021, 37, 519–522. [Google Scholar] [CrossRef]
- Cobo, A.; Garrido, N.; Crespo, J.; José, R.; Pellicer, A. Accumulation of oocytes: A new strategy for managing low-responder patients. Reprod. Biomed. Online 2012, 24, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Kuwayama, M.; Vajta, G.; Kato, O.; Leibo, S.P. Highly efficient vitrification method for cryopreservation of human oocytes. Reprod. Biomed. Online 2005, 11, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Cobo, A.; Kuwayama, M.; Pérez, S.; Ruiz, A.; Pellicer, A.; Remohí, J. Comparison of concomitant outcome achieved with fresh and cryopreserved donor oocytes vitrified by the Cryotop method. Fertil Steril. 2008, 89, 1657–1664. [Google Scholar] [CrossRef] [PubMed]
- Cobo, A.; Meseguer, M.; Remohí, J.; Pellicer, A. Use of cryo-banked oocytes in an ovum donation programme: A prospective, randomized, controlled, clinical trial. Hum. Reprod. 2010, 25, 2239–2246. [Google Scholar] [CrossRef]
- Chamayou, S.; Sicali, M.; Alecci, C.; Ragolia, C.; Liprino, A.; Nibali, D.; Storaci, G.; Cardea, A.; Guglielmino, A. The accumulation of vitrified oocytes is a strategy to increase the number of euploid available blastocysts for transfer after preimplantation genetic testing. J. Assist. Reprod. Genet. 2017, 34, 479–486. [Google Scholar] [CrossRef]
- Massin, N.; Abdennebi, I.; Porcu-Buisson, G.; Chevalier, N.; Descat, E.; Piétin-Vialle, C.; Goro, S.; Brussieux, M.; Pinto, M.; Pasquier, M.; et al. The BISTIM study: A randomized controlled trial comparing dual ovarian stimulation (duostim) with two conventional ovarian stimulations in poor ovarian responders undergoing IVF. Hum. Reprod. 2023, 2, dead038. [Google Scholar] [CrossRef]
- Cerrillo, M.; Cecchino, G.N.; Toribio, M.; García-Rubio, M.J.; García-Velasco, J.A. A randomized, non-inferiority trial on the DuoStim strategy in PGT-A cycles. Reprod. Biomed. Online 2023, 46, 536–542. [Google Scholar] [CrossRef]
- Fatemi, H.M.; Polyzos, N.P.; van Vaerenbergh, I.; Bourgain, C.; Blockeel, C.; Alsbjerg, B.; Papanikolaou, E.G.; Humaidan, P. Early luteal phase endocrine profile is affected by the mode of triggering final oocyte maturation and the luteal phase support used in recombinant follicle-stimulating hormone-gonadotropin-releasing hormone antagonist in vitro fertilization cycles. Fertil Steril. 2013, 100, 742–747. [Google Scholar] [CrossRef]
- La Marca, A.; Grisendi, V.; Spada, E.; Argento, C.; Milani, S.; Plebani, M.; Seracchioli, R.; Volpe, A. Reference values in ovarian response to controlled ovarian stimulation throughout the reproductive period. Gynecol. Endocrinol. 2014, 30, 66–69. [Google Scholar] [CrossRef]
- Esteves, S.C.; Alviggi, C.; Humaidan, P.; Fischer, R.; Andersen, C.Y.; Conforti, A.; Bühler, K.; Sunkara, S.K.; Polyzos, N.P.; Galliano, D.; et al. The POSEIDON Criteria and Its Measure of Success Through the Eyes of Clinicians and Embryologists. Front. Endocrinol. 2019, 10, 814. [Google Scholar] [CrossRef]
- Gica, C.; Maxim, B.G.; Botezatu, R.; Peltecu, G.; Panaitescu, A.M.; Iordachescu, D.; Gica, N. Double Ovarian Stimulation in the Same Ovarian Cycle. Maedica 2021, 16, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Polat, M.; Mumusoglu, S.; Yarali Ozbek, I.; Bozdag, G.; Yarali, H. Double or dual stimulation in poor ovarian responders: Where do we stand? Ther. Adv. Reprod. Health. 2021, 15, 26334941211024172. [Google Scholar] [CrossRef] [PubMed]
- Glujovsky, D.; Pesce, R.; Miguens, M.; Sueldo, C.E.; Lattes, K.; Ciapponi, A. How effective are the non-conventional ovarian stimulation protocols in ART? A systematic review and meta-analysis. J. Assist. Reprod. Genet. 2020, 37, 2913–2928. [Google Scholar] [CrossRef] [PubMed]
- Sfakianoudis, K.; Pantos, K.; Grigoriadis, S.; Rapani, A.; Maziotis, E.; Tsioulou, P.; Giannelou, P.; Kontogeorgi, A.; Pantou, A.; Vlahos, N.; et al. What is the true place of a double stimulation and double oocyte retrieval in the same cycle for patients diagnosed with poor ovarian reserve? A systematic review including a meta-analytical approach. J. Assist. Reprod. Genet. 2020, 37, 181–204. [Google Scholar] [CrossRef]
- Alpha Scientists In Reproductive Medicine. The Alpha consensus meeting on cryopreservation key performance indicators and benchmarks: Proceedings of an expert meeting. Reprod. Biomed. Online 2012, 25, 146–167. [Google Scholar] [CrossRef]
- Forman, E.J.; Li, X.; Ferry, K.M.; Scott, K.; Treff, N.R.; Scott, R.T., Jr. Oocyte vitrification does not increase the risk of embryonic aneuploidy or diminish the implantation potential of blastocysts created after intracytoplasmic sperm injection: A novel, paired randomized controlled trial using DNA fingerprinting. Fertil Steril. 2012, 98, 644–649. [Google Scholar] [CrossRef]
- Goldman, K.N.; Kramer, Y.; Hodes-Wertz, B.; Noyes, N.; McCaffrey, C.; Grifo, J.A. Long-term cryopreservation of human oocytes does not increase embryonic aneuploidy. Fertil Steril. 2015, 103, 662–668. [Google Scholar] [CrossRef]
- Canosa, S.; Cimadomo, D.; Conforti, A.; Maggiulli, R.; Giancani, A.; Tallarita, A.; Golia, F.; Fabozzi, G.; Vaiarelli, A.; Gennarelli, G.; et al. The effect of extended cryo-storage following vitrification on embryo competence: A systematic review and meta-analysis. J. Assist. Reprod. Genet. 2022, 39, 873–882. [Google Scholar] [CrossRef]
- Moutzouroulia, A.; Asimakopoulou, Z.; Tzavara, C.; Asimakopoulos, K.; Adonakis, G.; Kaponis, A. The impact of infertility on the mental health of women undergoing in vitro fertilization treatment. Sex Reprod. Healthc. 2025, 43, 101072. [Google Scholar] [CrossRef]
- Bergenheim, S.; Saupstad, M.; Colombo, C.; Møller, J.E.; Bogstad, J.W.; Freiesleben, N.C.; Behrendt-Møller, I.; Prætorius, L.; Oxlund, B.; Nøhr, B.; et al. Psychosocial and physical wellbeing in women and male partners undergoing immediate versus postponed modified natural cycle frozen embryo transfer after ovarian stimulation and oocyte pick-up: A sub-study of a randomized controlled trial. Hum. Reprod. 2025, 40, 96–109. [Google Scholar] [CrossRef]
- Cimadomo, D.; Taggi, M.; Cimadomo, V.; Innocenti, F.; Albricci, L.; Colamaria, S.; Argento, C.; Giuliani, M.; Ferrero, S.; Borini, A.; et al. Value of PGT-A when only one or two blastocysts are obtained: Propensity-score matching and cost-effectiveness study. Ultrasound Obstet. Gynecol. 2025, 65, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, D.A.; Leung, A.; Resetkova, N.; Ruthazer, R.; Penzias, A.S.; Sakkas, D.; Alper, M.M. How many oocytes are optimal to achieve multiple live births with one stimulation cycle? The one-and-done approach. Fertil Steril. 2017, 107, 397–404.e3. [Google Scholar] [CrossRef] [PubMed]
- Vaiarelli, A.; Cimadomo, D.; Blancafort, C.; Trabucco, E.; Alviggi, E.; Vallefuoco, R.; Livi, C.; Benini, F.; Canosa, S.; Llácer, J.; et al. A multi-cycle approach via DuoStim is beneficial to treat couples indicated to PGT-M plus PGT-A. A propensity score matching-based case series. Eur. J. Obstet. Gynecol. Reprod. Biol. 2024, 303, 272–278. [Google Scholar] [CrossRef] [PubMed]
| DS-Group (n = 66) | DF-Group (n = 40) | p-Value | |
|---|---|---|---|
| Woman’s age (years) | 39.9 ± 2.5 | 40.1 ± 2.7 | 0.71 |
| Partner’s age (years) | 41.7 ± 4.9 | 42.6 ± 5.8 | 0.48 |
| Previous IVF cycles (n) | 0.7 ± 1.2 | 1.0 ± 1.6 | 0.29 |
| BMI (kg/m2) | 21.9 ± 3.5 | 22.1 ± 3.5 | 0.73 |
| Basal FSH (IU/I) | 9.9 ± 3.4 | 10.3 ± 3.0 | 0.18 |
| Basal LH (IU/I) | 5.1 ± 1.9 | 5.1 ± 2.0 | 0.99 |
| AMH (ng/mL) | 1.1 ± 1.3 | 0.9 ± 0.7 | 0.72 |
| Antral Follicle Count (n) | 9.7 ± 5.6 | 8.7 ± 4.0 | 0.47 |
| Duration of OS (days, range) | First: 10.4 ± 2.3 (5–17) Second: 12.1 ± 2.5 (6–18) | First: 11.1 ± 2.6 (7–19) Second: 11.6 ± 2.5 (7–17) | 0.31 0.42 |
| Time between first and second OPU (days, range) | 18.6 ± 2.8 (12–25) | 40.9 ± 25.5 (17–103) | <0.0001 |
| Time between the first OPU and the start of the second OS (days, range) | 5 (-) | 27.7 ± 25.5 (8–90) | <0.0001 |
| Retrieved oocytes (n) | 8.4 ± 3.9 | 8.2 ± 4.0 | 0.80 |
| Oocyte survival rate after vitrification/warming (%) | 87.6 ± 27.3% | 87.3 ± 29.8% | 0.65 |
| MII oocytes after two OS (n) | 5.9 ± 3.3 | 5.7 ± 3.1 | 0.65 |
| 2PN zygotes (n) | 4.9 ± 2.7 | 5.0 ± 2.9 | 0.97 |
| Blastocysts (n) | 2.1 ± 1.8 | 2.3 ± 2.1 | 0.82 |
| Euploid Blastocysts (n) | 0.9 ± 1.2 | 1.1 ± 1.1 | 0.37 |
| Euploid blastocysts/oocyte (%) | 9.8 ± 11.9% | 12.3 ± 12.6% | 0.32 |
| Euploid blastocysts/MII oocyte (%) | 12.8 ± 14.6% | 16.3 ± 15.2% | 0.23 |
| Euploid blastocysts/2PN zygote (%) | 15.9 ± 18.7% | 17.3 ± 15.9% | 0.37 |
| Euploid blastocysts/biopsied (%) | 28.9 ± 32.4% | 34.1 ± 34.5% | 0.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Canosa, S.; Revelli, A.; Cimadomo, D.; Vaiarelli, A.; Gennarelli, G.; Guidetti, D.; Carosso, A.R.; Rienzi, L.; Ubaldi, F.M.; Bongioanni, F. DuoStim Shows Comparable Efficacy but Better Efficiency than Two Conventional Stimulations in Poor/Suboptimal Responders Undergoing Vitrified Oocyte Accumulation for PGT-A. Life 2025, 15, 899. https://doi.org/10.3390/life15060899
Canosa S, Revelli A, Cimadomo D, Vaiarelli A, Gennarelli G, Guidetti D, Carosso AR, Rienzi L, Ubaldi FM, Bongioanni F. DuoStim Shows Comparable Efficacy but Better Efficiency than Two Conventional Stimulations in Poor/Suboptimal Responders Undergoing Vitrified Oocyte Accumulation for PGT-A. Life. 2025; 15(6):899. https://doi.org/10.3390/life15060899
Chicago/Turabian StyleCanosa, Stefano, Alberto Revelli, Danilo Cimadomo, Alberto Vaiarelli, Gianluca Gennarelli, Daniela Guidetti, Andrea Roberto Carosso, Laura Rienzi, Filippo Maria Ubaldi, and Francesca Bongioanni. 2025. "DuoStim Shows Comparable Efficacy but Better Efficiency than Two Conventional Stimulations in Poor/Suboptimal Responders Undergoing Vitrified Oocyte Accumulation for PGT-A" Life 15, no. 6: 899. https://doi.org/10.3390/life15060899
APA StyleCanosa, S., Revelli, A., Cimadomo, D., Vaiarelli, A., Gennarelli, G., Guidetti, D., Carosso, A. R., Rienzi, L., Ubaldi, F. M., & Bongioanni, F. (2025). DuoStim Shows Comparable Efficacy but Better Efficiency than Two Conventional Stimulations in Poor/Suboptimal Responders Undergoing Vitrified Oocyte Accumulation for PGT-A. Life, 15(6), 899. https://doi.org/10.3390/life15060899









