Early Urine Output in the Emergency Room as a Prognostic Indicator for Critically Ill Patients Undergoing Continuous Renal Replacement
Abstract
1. Introduction
2. Methods
2.1. Research Design
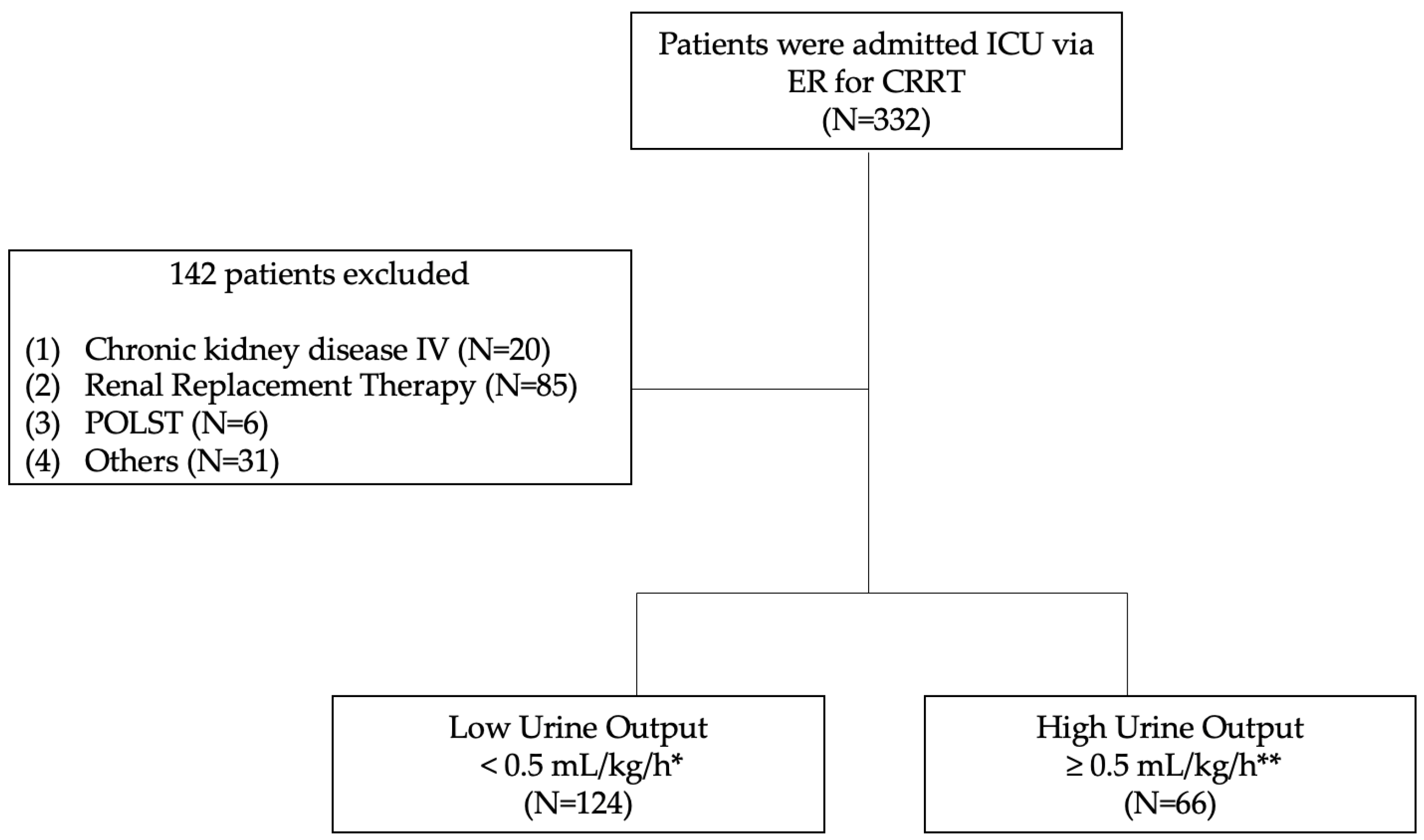
2.2. Inclusion and Exclusion Criteria
2.3. Definitions
2.4. Initiation of CRRT
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
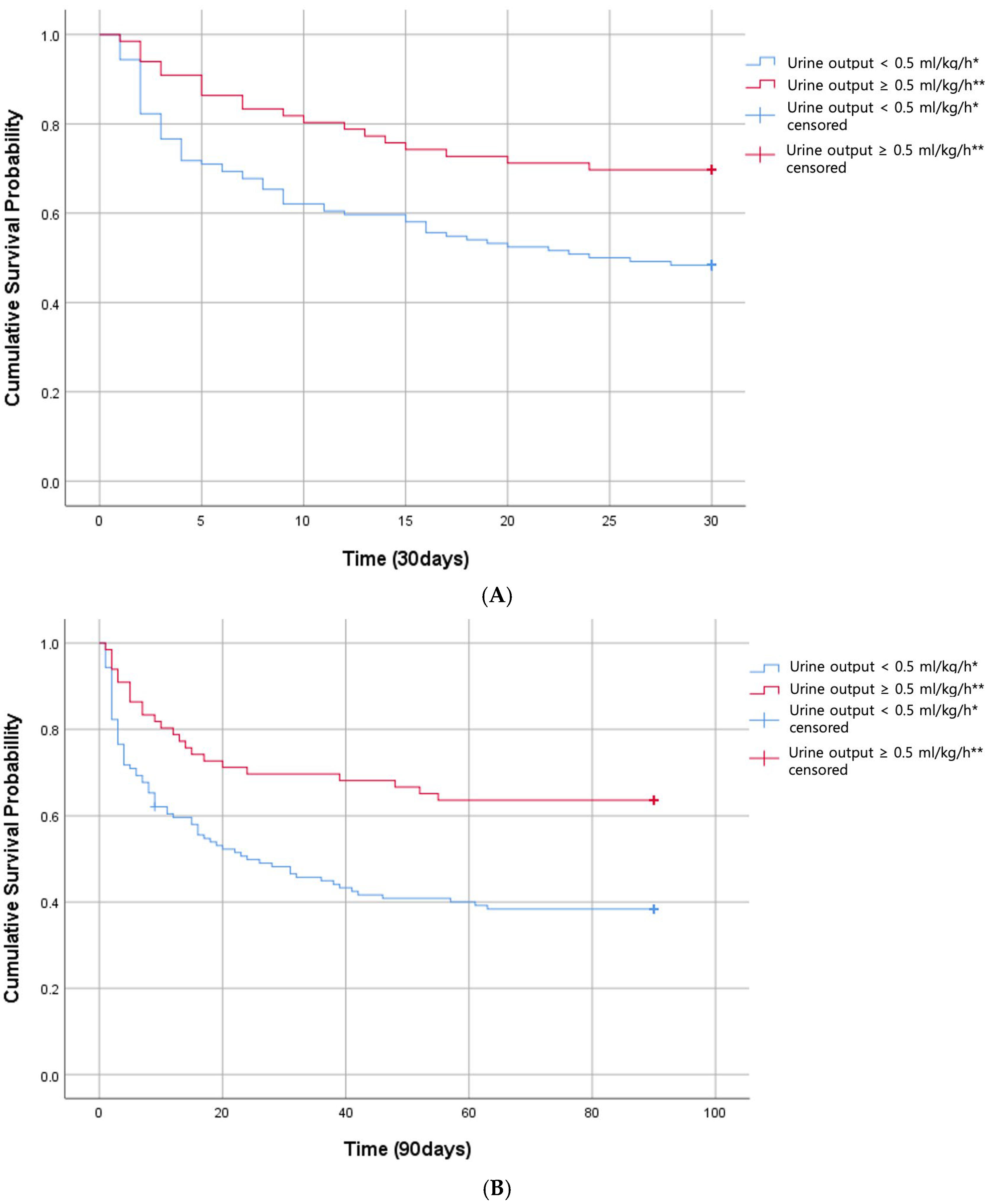
3.2. 30-Day and 90-Day Mortality
3.3. RRT-Free Days
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Makris, K.; Spanou, L. Acute Kidney Injury: Definition, Pathophysiology and Clinical Phenotypes. Clin. Biochem. Rev. 2016, 37, 85–98. [Google Scholar] [PubMed]
- Rewa, O.; Bagshaw, S.M. Acute kidney injury-epidemiology, outcomes and economics. Nat. Rev. Nephrol. 2014, 10, 193–207. [Google Scholar] [CrossRef] [PubMed]
- Siew, E.D.; Davenport, A. The growth of acute kidney injury: A rising tide or just closer attention to detail? Kidney Int. 2015, 87, 46–61. [Google Scholar] [CrossRef] [PubMed]
- Chawla, L.S.; Amdur, R.L.; Amodeo, S.; Kimmel, P.L.; Palant, C.E. The severity of acute kidney injury predicts progression to chronic kidney disease. Kidney Int. 2011, 79, 1361–1369. [Google Scholar] [CrossRef]
- An, J.N.; Hwang, J.H.; Kim, D.K.; Lee, H.; Ahn, S.Y.; Kim, S.; Park, J.T.; Kang, S.W.; Oh, Y.K.; Kim, Y.S.; et al. Chronic Kidney Disease After Acute Kidney Injury Requiring Continuous Renal Replacement Therapy and Its Impact on Long-Term Outcomes: A Multicenter Retrospective Cohort Study in Korea. Crit. Care Med. 2017, 45, 47–57. [Google Scholar] [CrossRef]
- Tandukar, S.; Palevsky, P.M. Continuous Renal Replacement Therapy: Who, When, Why, and How. Chest 2019, 155, 626–638. [Google Scholar] [CrossRef]
- Schaffer, P.; Chowdhury, R.; Jordan, K.; DeWitt, J.; Elliott, J.; Schroeder, K. Outcomes of Continuous Renal Replacement Therapy in a Community Health System. J. Intensive Care Med. 2022, 37, 1043–1048. [Google Scholar] [CrossRef]
- Huang, A.F.; Lin, L.; Ling, P.; Liao, P.H. Association Between the Central Venous Pressure and All-Cause Mortality in Critically Ill Patients with Acute Kidney Injury. Int. J. Gen. Med. 2021, 14, 8019–8027. [Google Scholar] [CrossRef]
- Sadaka, F.; Cytron, M.; Fowler, K.; Javaux, V.; O’Brien, J. A Comparison of Apache II and Apache III Scores in Predicting Mortality of Patients with Sepsis. Crit. Care Med. 2014, 42, A1600. [Google Scholar] [CrossRef]
- Ferreira, F.L.; Bota, D.P.; Bross, A.; Melot, C.; Vincent, J.L. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA—J. Am. Med. Assoc. 2001, 286, 1754–1758. [Google Scholar] [CrossRef]
- Le Gall, J.R.; Lemeshow, S.; Saulnier, F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA 1993, 270, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Friedericksen, D.V.; van der Merwe, L.; Hattingh, T.L.; Nel, D.G.; Moosa, M.R. Acute renal failure in the medical ICU still predictive of high mortality. S. Afr. Med. J. 2009, 99, 873–875. [Google Scholar] [PubMed]
- Huang, H.Q.; Bai, X.H.; Ji, F.T.; Xu, H.; Fu, Y.N.; Cao, M.H. Early-Phase Urine Output and Severe-Stage Progression of Oliguric Acute Kidney Injury in Critical Care. Front. Med. 2021, 8, 711717. [Google Scholar] [CrossRef]
- Engoren, M.; Maile, M.D.; Heung, M.; Jewell, E.S.; Vahabzadeh, C.; Haft, J.W.; Kheterpal, S. The Association Between Urine Output, Creatinine Elevation, and Death. Ann. Thorac. Surg. 2017, 103, 1229–1238. [Google Scholar] [CrossRef] [PubMed]
- Heffernan, A.J.; Judge, S.; Petrie, S.M.; Godahewa, R.; Bergmeir, C.; Pilcher, D.; Nanayakkara, S. Association Between Urine Output and Mortality in Critically Ill Patients: A Machine Learning Approach. Crit. Care Med. 2022, 50, e263–e271. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, H.Y.; Bae, E.H.; Kim, S.W.; Ma, S.K. Biomarkers Predicting Survival of Sepsis Patients Treated with Continuous Renal Replacement Therapy. Chonnam Med. J. 2017, 53, 64–68. [Google Scholar] [CrossRef]
- Shannon, M.; Kim, B.S.; Stephens, R.S.; Metkus, T. Calculated Plasma Volume Status Is Associated with Mortality in the Acute Respiratory Distress Syndrome. Crit. Care Explor. 2024, 3, e0534. [Google Scholar] [CrossRef]
- Jaber, S.; Paugam, C.; Futier, E. Sodium bicarbonate therapy for patients with severe metabolic acidaemia in the intensive care unit (BICAR-ICU): A multicentre, open-label, randomised controlled, phase 3 trial. Lancet 2018, 392, 2440. [Google Scholar] [CrossRef]
- Kapp, M.B. Overcoming Legal Impediments to Physician Orders for Life-Sustaining Treatment. AMA J. Ethics 2016, 18, 861–868. [Google Scholar] [CrossRef]
- Khwaja, A. KDIGO Clinical Practice Guidelines for Acute Kidney Injury. Nephron Clin. Pract. 2012, 120, C179–C184. [Google Scholar] [CrossRef]
- Albert, C.; Zapf, A.; Haase, M.; Rover, C.; Pickering, J.W.; Albert, A.; Bellomo, R.; Breidthardt, T.; Camou, F.; Chen, Z.Q.; et al. Neutrophil Gelatinase-Associated Lipocalin Measured on Clinical Laboratory Platforms for the Prediction of Acute Kidney Injury and the Associated Need for Dialysis Therapy: A Systematic Review and Meta-analysis. Am. J. Kidney Dis. 2020, 76, 826–841. [Google Scholar] [CrossRef] [PubMed]
- An, J.N.; Kim, S.G.; Song, Y.R. When and why to start continuous renal replacement therapy in critically ill patients with acute kidney injury. Kidney Res. Clin. Pract. 2021, 40, 566–577. [Google Scholar] [CrossRef]
- Yu, Y.; Xu, H.; Jiang, S.; Gao, C. Predictors for mortality and recovery in patients with acute renal injury receiving continuous renal replacement therapy. Int. J. Artif. Organs 2022, 45, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Sheng, S.; Zhang, Y.H.; Ma, H.K.; Huang, Y. Albumin levels predict mortality in sepsis patients with acute kidney injury undergoing continuous renal replacement therapy: A secondary analysis based on a retrospective cohort study. BMC Nephrol. 2022, 23, 52. [Google Scholar] [CrossRef] [PubMed]
- Cho, A.Y.; Yoon, H.J.; Lee, K.Y.; Sun, I.O. Clinical characteristics of sepsis-induced acute kidney injury in patients undergoing continuous renal replacement therapy. Ren. Fail. 2018, 40, 403–409. [Google Scholar] [CrossRef]
- Perez-Fernandez, X.; Sabater-Riera, J.; Sileanu, F.E.; Vazquez-Reveron, J.; Ballus-Noguera, J.; Cardenas-Campos, P.; Betbese-Roig, A.; Kellum, J.A. Clinical variables associated with poor outcome from sepsis-associated acute kidney injury and the relationship with timing of initiation of renal replacement therapy. J. Crit. Care 2017, 40, 154–160. [Google Scholar] [CrossRef]
- Duda, I.; Krzych, L. Plasma Neutrophil Gelatinase-Associated Lipocalin Is Useful for Predicting Mortality in Critically Ill Patients. J. Clin. Med. 2021, 10, 2576. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, Y.; Zhu, L.; Cui, K.; Zhang, S.; Xu, Y.; Jiang, Y. Plasma proenkephalin and neutrophil gelatinase-associated lipocalin predict mortality in ICU patients with acute kidney injury. BMC Nephrol. 2024, 25, 181. [Google Scholar] [CrossRef]
- Tornblom, S.; Nisulal, S.; Petaja, L.; Vaara, S.T.; Haapio, M.; Pesonen, E.; Pettila, V.; Grp, F.S. Urine NGAL as a biomarker for septic AKI: A critical appraisal of clinical utility-data from the observational FINNAKI study. Ann. Intensive Care 2020, 10, 51. [Google Scholar] [CrossRef]
- Daniels, L.B.; Barrett-Connor, E.; Clopton, P.; Laughlin, G.A.; Ix, J.H.; Maisel, A.S. Plasma neutrophil gelatinase-associated lipocalin is independently associated with cardiovascular disease and mortality in community-dwelling older adults: The Rancho Bernardo Study. J. Am. Coll. Cardiol. 2012, 59, 1101–1109. [Google Scholar] [CrossRef]
- de Roquetaillade, C.; Chousterman, B.G.; Dépret, F.; Mebazaa, A.; Deniau, B. Plasmatic NGAL Monitoring in Cardiogenic Shock: Deciphering the Past, the Present, and the Future. Shock 2022, 57, 467–468. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.Y.; Kim, J.W.; Paik, J.H.; Jung, H.M.; Baek, K.J.; Park, S.O.; Lee, K.R. Value of plasma neutrophil gelatinase-associated lipocalin in predicting the mortality of patients with sepsis at the emergency department. Clin. Chim. Acta 2016, 452, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Voth, M.; Verboket, R.; Henrich, D.; Marzi, I. L-FABP and NGAL are novel biomarkers for detection of abdominal injury and hemorrhagic shock. Injury 2023, 54, 1246–1256. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.D.; Cui, L.F.; Ye, P.P.; Li, J.J.; Wu, S.L.; Luo, Y. Change of Kidney Function Is Associated with All-Cause Mortality and Cardiovascular Diseases: Results from the Kailuan Study. J. Am. Heart Assoc. 2018, 7, e010596. [Google Scholar] [CrossRef]
- Haas, L.; Eckart, A.; Haubitz, S.; Mueller, B.; Schuetz, P.; Segerer, S. Estimated glomerular filtration rate predicts 30-day mortality in medical emergency departments: Results of a prospective multi-national observational study. PLoS ONE 2020, 15, e0230998. [Google Scholar] [CrossRef]
| Characteristic | Low Urine Output (n = 124) | High Urine Output (n = 66) | Total (n = 190) | p |
|---|---|---|---|---|
| Demographic data | ||||
| Age, mean (SD) (yr) | 66.8 ± 15.2 | 70.2 ± 11.6 | 68.0 ± 14.1 | 0.094 |
| Sex (male, %) | 81 (65.3) | 39 (59.1) | 120 (63.1) | |
| SBP (mmHg) | 94.5 ± 28.5 | 120.3 ± 36.0 | 103.5 ± 33.6 | <0.001 |
| DBP (mmHg) | 55.3 ± 18.0 | 67.1 ± 18.3 | 59.4 ± 18.9 | <0.001 |
| BMI (kg/m2) | 21.45 ± 7.22 | 22.39 ± 7.05 | 21.78 ± 7.15 | 0.392 |
| Laboratory parameters | ||||
| Hemoglobin (g/dL) | 11.07 ± 3.12 | 11.40 ± 2.59 | 11.18 ± 2.94 | 0.464 |
| Glucose (mg/dL) | 209.77 ± 177.69 | 208.39 ± 148.48 | 209.29 ± 168.10 | 0.947 |
| Serum albumin (g/dL) | 2.83 ± 0.63 | 2.98 ± 0.60 | 2.89 ± 0.62 | 0.104 |
| Lactic acid (mmol/L) | 7.80 ± 5.18 | 5.07 ± 4.54 | 6.85 ± 5.12 | <0.001 |
| CRP (mg/dL) | 9.29 ± 10.69 | 10.00 ± 11.34 | 9.53 ± 10.89 | 0.666 |
| NGAL (ng/mL) | 1066.91 ± 1006.39 | 511.34 ± 598.10 | 873.92 ± 923.32 | <0.001 |
| BUN (mg/dL) | 47.73 ± 29.52 | 36.95 ± 25.50 | 43.98 ± 28.59 | 0.010 |
| Serum creatinine (mg/dL) | 3.23 ± 2.38 | 2.10 ± 1.52 | 3.29 ± 5.81 | <0.001 |
| eGFR (mL/min/1.73 m2) | 30.52 ± 24.95 | 44.94 ± 33.96 | 35.53 ± 29.14 | 0.001 |
| Potassium (mEq/L) | 4.73 ± 1.18 | 4.42 ± 1.09 | 4.62 ± 1.16 | 0.075 |
| Bicarbonate (mEq/L) | 15.04 ± 6.82 | 18.06 ± 6.45 | 16.09 ± 6.83 | 0.003 |
| UPCR (mg/mg) | 1.45 ± 1.18 | 1.44 ± 1.18 | 1.45 ± 1.18 | 0.075 |
| Urine hematuria (%) | 66 (53) | 29 (44) | 95 (47.8) | 0.003 |
| FV (mL) | 490.7 ± 294.2 | 525.2 ± 342.2 | 502.7 ± 311.2 | 0.460 |
| UV (mL) | 60.3 ± 54.6 | 653.7 ± 390.3 | 266.4 ± 366.9 | <0.001 |
| SOFA score | 7.47 ± 2.47 | 6.61 ± 2.55 | 7.17 ± 2.53 | 0.027 |
| CRRT start (h) * | 14.32 ± 3.79 | 17.35 ± 5.5 | 15.37 ± 4.67 | <0.001 |
| Cause of CRRT (%) | ||||
| Septic shock (%) | 53 (43) | 38 (57) | 91 (48) | 0.051 |
| ** Cardiologic problem (%) | 30 (24) | 12 (18) | 42 (22) | 0.036 |
| *** Hypovolemic problem (%) | 17 (14) | 3 (0.5) | 20 (11) | 0.050 |
| **** Others (%) | 24 (19) | 13 (20) | 37 (19) | 0.955 |
| Comorbidities (%) | ||||
| Hypertension (%) | 73 (58.9) | 39 (59.1) | 112 (58.9) | 0.217 |
| Diabetes mellitus(%) | 58 (46.8) | 40 (60.6) | 98 (51.6) | 0.069 |
| Heart failure (%) | 17 (13.7) | 8 (12.1) | 25 (13.2) | 0.758 |
| Cardiovascular disease (%) | 15 (12.1) | 6 (9.1) | 21 (11.1) | 0.529 |
| 30-Day Mortality | 90-Day Mortality | |||||
|---|---|---|---|---|---|---|
| Significant Variable | HR | 95% CI | p | HR | 95% CI | p |
| Urine < 0.5 mL/kg/h * | 1.88 | 1.10–3.25 | 0.023 | 2.07 | 1.26–3.42 | 0.004 |
| SBP (mmHg) | 1.01 | 0.99–1.02 | 0.453 | 1.01 | 1.00–1.02 | 0.143 |
| DBP (mmHg) | 0.98 | 0.96–1.00 | 0.101 | 0.98 | 0.96–1.00 | 0.053 |
| NGAL (ng/mL) | 1.00 | 1.00–1.00 | 0.755 | 1.00 | 1.00–1.00 | 0.604 |
| eGFR (mL/min/1.73 m2) | 0.99 | 0.98–1.01 | 0.293 | 0.99 | 0.98–1.00 | 0.138 |
| BUN (mg/dL) | 1.00 | 0.98–1.01 | 0.513 | 0.99 | 0.98–10.1 | 0.292 |
| Creatinine (mg/dL) | 0.91 | 0.76–1.10 | 0.324 | 0.92 | 0.78–1.10 | 0.351 |
| Albumin (g/dL) | 0.79 | 0.56–1.17 | 0.236 | 0.74 | 0.51–1.06 | 0.097 |
| HCO3 (mEq/L) | 0.98 | 0.95–1.02 | 0.346 | 0.98 | 0.95–1.02 | 0.313 |
| SOFA score | 1.26 | 1.09–1.46 | 0.002 | 1.21 | 1.07–1.37 | 0.003 |
| LUO (n = 124) | HUO (n = 66) | Mean Difference (95% CI) | p | |
|---|---|---|---|---|
| RRT-free days through day 30 mean ± SD | 7.6 ± 12.1 | 15.1 ± 14.0 | −7.33 (−11.36 to −3.29) | 0.000 |
| RRT-free days through day 90 mean ± SD | 27.0 ± 39.0 | 47.1 ± 42.5 | 0.002 | |
| eGFR < 15 mL/min/1.73 m2 (n = 42) | eGFR ≥ 15 mL/min/1.73 m2 (n = 148) | Mean Difference (95% CI) | p | |
| RRT-free days through day 30 mean ± SD | 7.1 ± 11.4 | 11.2± 13.6 | −4.05 (−8.20 to 0.11) | 0.056 |
| RRT-free days through day 90 mean ± SD | 25.9 ± 38.1 | 36.3± 41.9 | −10.41 (−23.99 to 3.17) | 0.131 |
| eGFR < 30 mL/min/1.73 m2 (n = 98) | eGFR ≥ 30 mL/min/1.73 m2 (n = 92) | Mean Difference (95% CI) | p | |
| RRT-free days through day 30 mean ± SD | 7.6 ± 11.9 | 13.1 ± 14.0 | −5.50 (−9.23 to −1.77) | 0.004 |
| RRT-free days through day 90 mean ± SD | 26.2 ± 38.3 | 42.3 ± 42.8 | −16.14 (−27.80 to −4.49) | 0.007 |
| eGFR < 60 mL/min/1.73 m2 (n = 165) | eGFR ≥ 60 mL/min/1.73 m2 (n = 25) | Mean Difference (95% CI) | p | |
| RRT-free days through day 30 mean ± SD | 9.5 ± 12.9 | 15.8 ± 14.4 | −6.39 (−12.61 to −0.16) | 0.045 |
| RRT-free days through day 90 mean ± SD | 31.7 ± 40.6 | 49.4 ± 43.0 | −17.75 (−35.06 to −0.43) | 0.045 |
| NGAL < 364 ng/mL (n = 76) | NGAL ≥ 364 ng/mL (n = 114) | Mean Difference (95% CI) | p | |
| RRT-free days through day 30 mean ± SD | 11.7 ± 14.0 | 9.3 ± 12.7 | 2.38 (−1.56 to 6.32) | 0.234 |
| RRT-free days through day 90 mean ± SD | 36.6 ± 42.6 | 32.3 ± 40.4 | 4.36 (−7.70 to 16.42) | 0.477 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, S.H.; Kang, C.; Park, H.; Lee, E.J.; Ham, Y.R.; Na, K.R.; Park, J.S.; Choi, D.E. Early Urine Output in the Emergency Room as a Prognostic Indicator for Critically Ill Patients Undergoing Continuous Renal Replacement. Life 2025, 15, 866. https://doi.org/10.3390/life15060866
Han SH, Kang C, Park H, Lee EJ, Ham YR, Na KR, Park JS, Choi DE. Early Urine Output in the Emergency Room as a Prognostic Indicator for Critically Ill Patients Undergoing Continuous Renal Replacement. Life. 2025; 15(6):866. https://doi.org/10.3390/life15060866
Chicago/Turabian StyleHan, Soo Hyun, Changshin Kang, Hyerim Park, Eu Jin Lee, Young Rok Ham, Ki Ryang Na, Jung Soo Park, and Dae Eun Choi. 2025. "Early Urine Output in the Emergency Room as a Prognostic Indicator for Critically Ill Patients Undergoing Continuous Renal Replacement" Life 15, no. 6: 866. https://doi.org/10.3390/life15060866
APA StyleHan, S. H., Kang, C., Park, H., Lee, E. J., Ham, Y. R., Na, K. R., Park, J. S., & Choi, D. E. (2025). Early Urine Output in the Emergency Room as a Prognostic Indicator for Critically Ill Patients Undergoing Continuous Renal Replacement. Life, 15(6), 866. https://doi.org/10.3390/life15060866






