The Role of Artificial Intelligence in Predicting the Progression of Intraocular Hypertension to Glaucoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Group
- An input layer;
- One or more hidden layers;
- An output layer;
- Computation occurs only in the hidden and output layers;
- Input signals are propagated forward through each layer of the network.
- Mean Squared Error (MSE);
- Correlation coefficient (r2);
- Percentage error (Ep%).
2.2. Statistical Analysis
3. Results
3.1. Statistical Analysis Results
3.2. Neural Network Modeling Result
3.2.1. The First Dataset Consisted of 75 Entries, Which Were Randomly Divided into 63 Entries for the Training Stage and 12 for the Testing Stage
3.2.2. The Second Dataset of Patients with Treated Ocular Hypertension Comprises 70 Datasets
3.2.3. The Third Database (Control Group) Contains 89 Datasets Randomly Divided into 77 for Training and 12 for Validation
4. Discussions
4.1. Limitations and Challenges
4.2. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MDPI | Multidisciplinary Digital Publishing Institute |
| DOAJ | Directory of open access journals |
| TLA | Three-letter acronym |
| LD | Linear dichroism |
References
- Donkor, R.; Jammal, A.A.; Greenfield, D.S. Relationship between Blood Pressure and Rates of Glaucomatous Visual Field. Progression The Vascular Imaging in Glaucoma Study. Ophthalmology 2025, 132, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Jan, C.; He, M.; Vingrys, A.; Zhu, Z.; Stafford, R.S. Diagnosing glaucoma in primary eye care and the role of Artifcial Intelligence applications for reducing the prevalence of undetected glaucoma in Australia. Eye 2024, 38, 2003–2013. [Google Scholar] [CrossRef]
- Zhu, Y.; Salowe, R.; Chow, C.; Li, S.; Bastani, O.; O’Brien, J.M. Advancing Glaucoma Care: Integrating Artificial Intelligence in Diagnosis, Management, and Progression Detection. Bioengineering 2024, 11, 122. [Google Scholar] [CrossRef]
- Lemij, H.G.; de Vente, C.; Sánchez, C.I.; Vermeer, K.A. Characteristics of a Large, Labeled Data Set for the training of artificial intelligence for glaucoma screening with fundus photographs. Ophthalmol. Sci. 2023, 3, 100300. [Google Scholar] [CrossRef]
- Anton, N.; Doroftei, B.; Curteanu, S.; Catãlin, L.; Ilie, O.D.; Târcoveanu, F.; Bogdănici, C.M. Comprehensive Review on the Use of Artificial Intelligence in Ophthalmology and Future Research Directions. Diagnostics 2022, 13, 100. [Google Scholar] [CrossRef] [PubMed]
- Schuman, J.S.; Cadena, M.D.L.A.R.; McGee, R.; Al-Aswad, L.A.; Medeiros, F.A. For the Collaborative Community on Ophthalmic Imaging Executive Committee and Glaucoma Workgroup. A Case for the Use of Artificial Intelligence in Glaucoma Assessment. Ophthalmol. Glaucoma 2022, 5, e3–e13. [Google Scholar] [CrossRef]
- Chen, H.S.-L.; Chen, G.-A.; Syu, J.-Y.; Chuang, L.-H.; Su, W.-W.; Wu, W.-C.; Liu, J.-H.; Chen, J.-R.; Huang, S.-C.; Kang, E.Y.-C. Early Glaucoma Detection by Using Style Transfer to Predict Retinal Nerve Fiber Layer Thickness Distribution on the Fundus Photograph. Ophthalmol. Sci. 2022, 2, 00180. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.; Pinto, A.; Alves, V.; Silva, C.A. Brain Tumor Segmentation Using Convolutional Neural Networks in MRI Images. IEEE Trans. Med. Imaging 2016, 35, 1240–1251. [Google Scholar] [CrossRef]
- Choi, E.; Bahadori, M.T.; Schuetz, A.; Stewart, W.F.; Sun, J. Doctor AI: Predicting Clinical Events via Recurrent Neural Networks. JMLR Work. Conf. Proc. 2016, 56, 301–318. [Google Scholar]
- Bottaci, L.; Drew, P.J.; Hartley, J.E.; Hadfield, M.B.; Farouk, R.; Lee, P.W.; Mc Macintyre, I.; Duthie, G.S.; Monson, J.R. Artificial neural networks applied to outcome prediction for colorectal cancer patients in separate institutions. Lancet 1997, 350, 469–472. [Google Scholar] [CrossRef]
- Burke, H.B.; Hoang, A.; Iglehart, J.D.; Marks, J.R. Predicting response to adjuvant and radiation therapy in patients with early stage breast carcinoma. Cancer 1998, 82, 874–877. [Google Scholar] [CrossRef]
- Marchevsky, A.M.; Patel, S.; Wiley, K.J.; Stephenson, M.A.; Gondo, M.; Brown, R.W.; Yi, E.S.; Benedict, W.F.; Anton, R.C.; Cagle, P.T. Artificial neural networks and logistic regression as tools for pre-diction of survival in patients with stages I and II non-small cell lung cancer. Mod. Pathol. 1998, 11, 618–625. [Google Scholar] [PubMed]
- Han, M.; Snow, P.B.; Epstein, C.T.Y.; Jones, K.A.; Walsh, P.C.; Partin, A.W. A neural network predicts progression for men with Gleason score 3+4 ver-sus 4+3 tumors after radical prostatectomy. Urology 2000, 56, 994–999. [Google Scholar] [CrossRef]
- Iftikhar, P.; Kuijpers, M.V.; Khayyat, A.; Iftikhar, A.; De Sa, M.D. Artificial Intelligence: A New Paradigm in Obstetrics and Gynecology Research and Clinical Practice. Cureus 2020, 12, e7124. [Google Scholar] [CrossRef] [PubMed]
- Manna, C.; Nanni, L.; Lumini, A.; Pappalardo, S. Artificial intelligence techniques for embryo and oocyte classification. Reprod. Biomed. Online 2012, 26, 42–49. [Google Scholar] [CrossRef]
- Guh, R.-S.; Wu, T.-C.J.; Weng, S.-P. Integrating genetic algorithm and decision tree learning for assistance in predicting in vitro fertilization outcomes. Expert. Syst. Appl. 2011, 38, 4437–4449. [Google Scholar] [CrossRef]
- Bivoleanu, A.; Gheorghe, L.; Doroftei, B.; Scripcariu, I.-S.; Vasilache, I.-A.; Harabor, V.; Adam, A.-M.; Adam, G.; Mun-teanu, I.V.; Susanu, C.; et al. Predicting Adverse Neurodevelopmental Outcomes in Premature Neonates with Intrau-terine Growth Restriction Using a Three Layered Neural Network. Diagnostics 2025, 15, 111. [Google Scholar] [CrossRef]
- Vasilache, I.A.; Scripcariu, I.S.; Doroftei, B.; Bernad, R.L.; Cărăuleanu, A.; Socolov, D.; Melinte-Popescu, A.S.; Vicoveanu, P.; Harabor, V.; Mihalceanu, E.; et al. Prediction of Intrauterine Growth Restriction and Preeclampsia Using Machine Learning-Based Algorithms: A Prospective Study. Diagnostics 2024, 14, 453. [Google Scholar] [CrossRef]
- Anton, N.; Dragoi, E.N.; Tarcoveanu, F.; Ciuntu, R.E.; Lisa, C.; Curteanu, S.; Doroftei, B.; Ciuntu, B.M.; Chiseli¸tă, D.; Bogdănici, C.M. Assessing Changes in Diabetic Retinopathy Caused by Diabetes Mellitus and Glaucoma Using Support Vector Machines in Combination with Differential Evolution Algorithm. Appl. Sci. 2021, 11, 3944. [Google Scholar] [CrossRef]
- Tarcoveanu, F.; Leon, F.; Curteanu, S.; Chiselita, D.; Bogdanici, C.M.; Anton, N. Classification Algorithms Used in Predicting Glaucoma Progression. Healthcare 2022, 10, 1831. [Google Scholar] [CrossRef]
- Anton, N.; Lisa, C.; Doroftei, B.; Curteanu, S.; Bogdanici, C.M.; Chiselita, D.; Branisteanu, D.C.; Nechita-Dumitriu, I.; Ilie, O.-D.; Ciuntu, R.E. Use of Artificial Neural Networks to Predict the Progression of Glaucoma in Patients with Sleep Apnea. Appl. Sci. 2022, 12, 6061. [Google Scholar] [CrossRef]
- Ting, D.S.W.; Pasquale, L.R.; Peng, L.; Campbell, J.P.; Lee, A.Y.; Raman, R.; Tan, G.S.W.; Schmetterer, L.; Keane, P.A.; Wong, T.Y. Artificial Intelligence and Deep Learning in Ophthalmology. Br. J. Ophthalmol. 2019, 103, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Djulbegovic, M.B.; Bair, H.; Gonzalez, D.J.T.; Ishikawa, H.; Wollstein, G.; Schuman, J.S. Artificial intelligence for optical coherence tomography in glaucoma. Transl. Vis. Sci. Technol. 2025, 14, 27. [Google Scholar] [CrossRef]
- Martucci, A.; Gallo Afflitto, G.; Pocobelli, G.; Aiello, F.; Mancino, R.; Nucci, C. Lights and Shadows onArtificial Intelligence in Glaucoma: Transforming Screening, Monitoring, and Prognosis. J. Clin. Med. 2025, 14, 2139. [Google Scholar] [CrossRef]
- Prahs, P.; Märker, D.; Mayer, C.; Helbig, H. Deep learning to support therapy decisions for intravitreal injections. Ophthalmology 2018, 115, 722–727. [Google Scholar]
- Schlegl, T.; Waldstein, S.M.; Bogunovic, H.; Endstraßer, F.; Sadeghipour, A.; Philip, A.-M.; Podkowinski, D.; Gerendas, B.S.; Langs, G.; Schmidt-Erfurth, U. Fully Automated Detection and Quantification of Macular Fluid in OCT Using Deep Learning. Ophthalmology 2018, 125, 549–558. [Google Scholar] [CrossRef]
- Liu, Y.; Holekamp, N.M.; Heier, J.S. Prospective, Longitudinal Study: Daily Self-Imaging with Home OCT for Neovas-cular Age-Related Macular Degeneration. Ophthalmol. Retin. 2022, 6, 575–585. [Google Scholar] [CrossRef]
- Bowd, C.; Chan, K.; Zangwill, L.M.; Goldbaum, M.H.; Lee, T.W.; Sejnowski, T.J.; Weinreb, R.N. Comparing neural networks and linear discriminant functions for glaucoma detection using confocal scanning laser ophthalmoscopy of the optic disc. Investig. Ophthalmol. Vis. Sci. 2002, 43, 3444–3454. [Google Scholar]
- Grewal, D.S.; Jain, R.; Grewal, S.P.S.; Rihani, V. Artificial Neural Network-Based Glaucoma Diagnosis Using Retinal Nerve Fiber Layer Analysis. Eur. J. Ophthalmol. 2008, 18, 915–921. [Google Scholar] [CrossRef]
- Simon, M.A.; Alonso, L.; Alfonso, A.A. A hybrid visual field classifier to support early glaucoma diagnosis. Intel. Artif. 2005, 9, 9–17. [Google Scholar]
- Girard, M.J.A.; Schmetterer, L. Artificial intelligence and deep learning in glaucoma: Current state and future prospects. Prog. Brain Res. 2020, 257, 37–64. [Google Scholar] [PubMed]
- Ji, P.X.; Ramalingam, V.; Balas, M.; Pickel, L.; Mathew, D.J. Artificial Intelligence in Glaucoma: A New Landscape of Diagnosis and Management. J. Clin. Transl. Ophthalmol. 2024, 2, 47–63. [Google Scholar] [CrossRef]
- Yousefi, S. Clinical Applications of Artificial Intelligence in Glaucoma. J. Ophthalmic Vis. Res. 2023, 18, 97–112. [Google Scholar] [CrossRef]
- Târcoveanu, F.; Leon, F.; Lisa, C.; Curteanu, S.; Feraru, A.; Ali, K.; Anton, N. The use of ar-tificial neural networks in studying the progression of glaucoma. Sci. Rep. 2024, 14, 19597. [Google Scholar] [CrossRef]
- Wen, J.C.; Lee, C.S.; Keane, P.A.; Xiao, S.; Rokem, A.S.; Chen, P.P.; Wu, Y.; Lee, A.Y. Forecasting future Humphrey visual fields using deep learning. PLoS ONE 2019, 14, e0214875. [Google Scholar] [CrossRef] [PubMed]
- Thakur, S.; Dinh, L.L.; Lavanya, R.; Quek, T.C.; Liu, Y.; Cheng, C.Y. Use of artificial intelligence in forecasting glaucoma pro-gression. Taiwan. J. Ophthalmol. 2023, 13, 168–183. [Google Scholar] [CrossRef]
- Huang, X.; Kong, X.; Shen, Z.; Ouyang, J.; Li, Y.; Jin, K.; Ye, J. GRAPE: A multi-modal dataset of longitudinal follow-up visual field and fundus images for glaucoma management. Sci. Data 2023, 10, 520. [Google Scholar] [CrossRef]


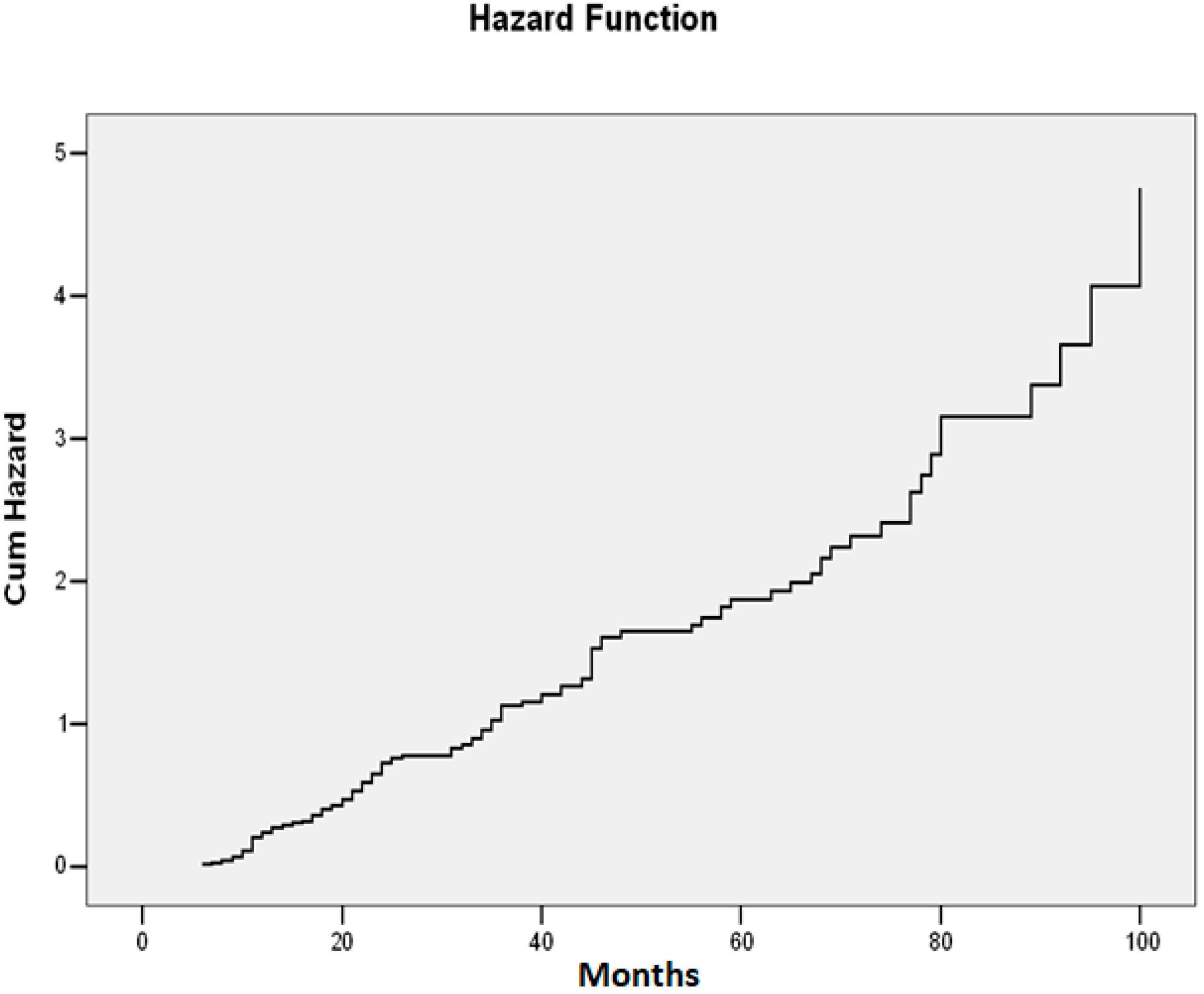
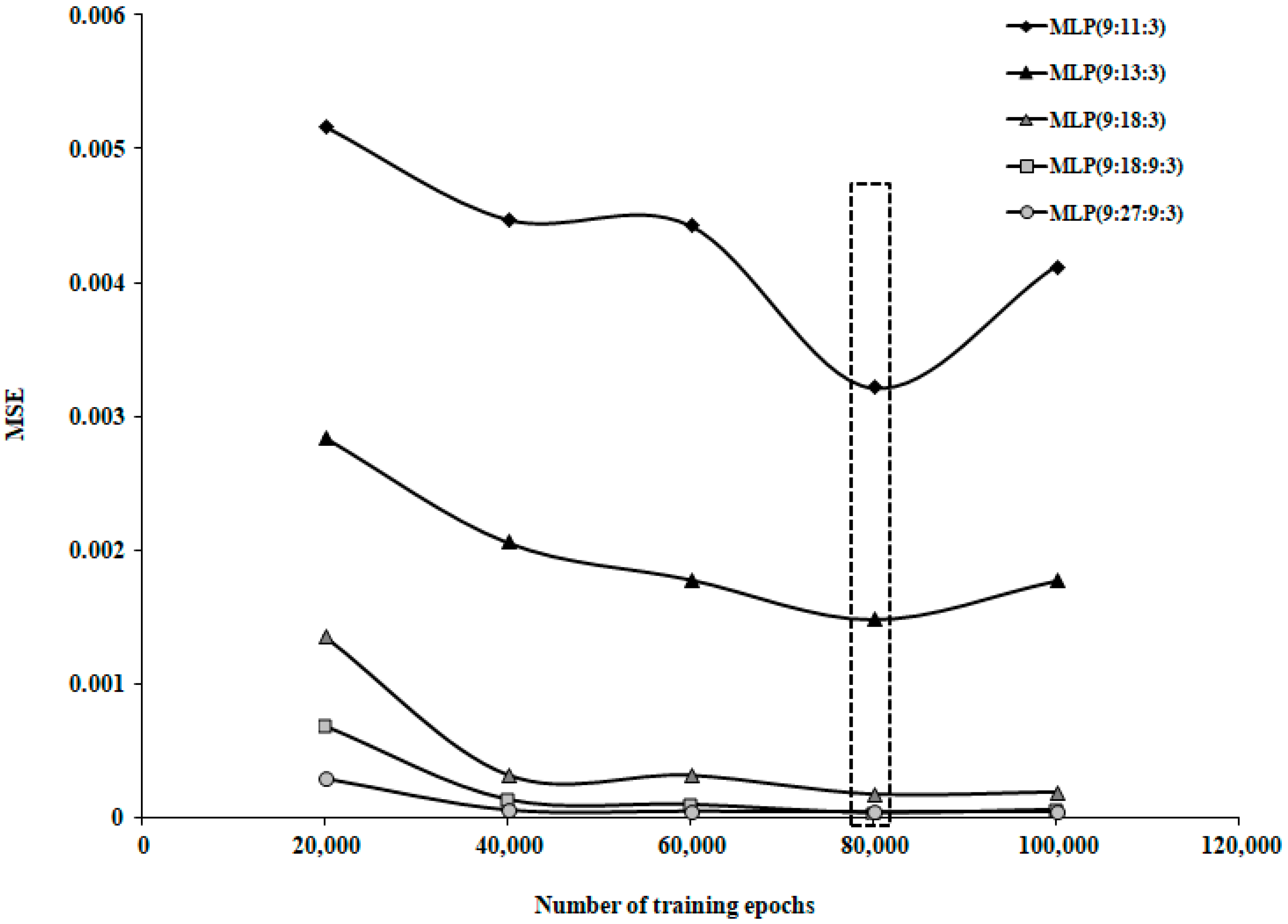
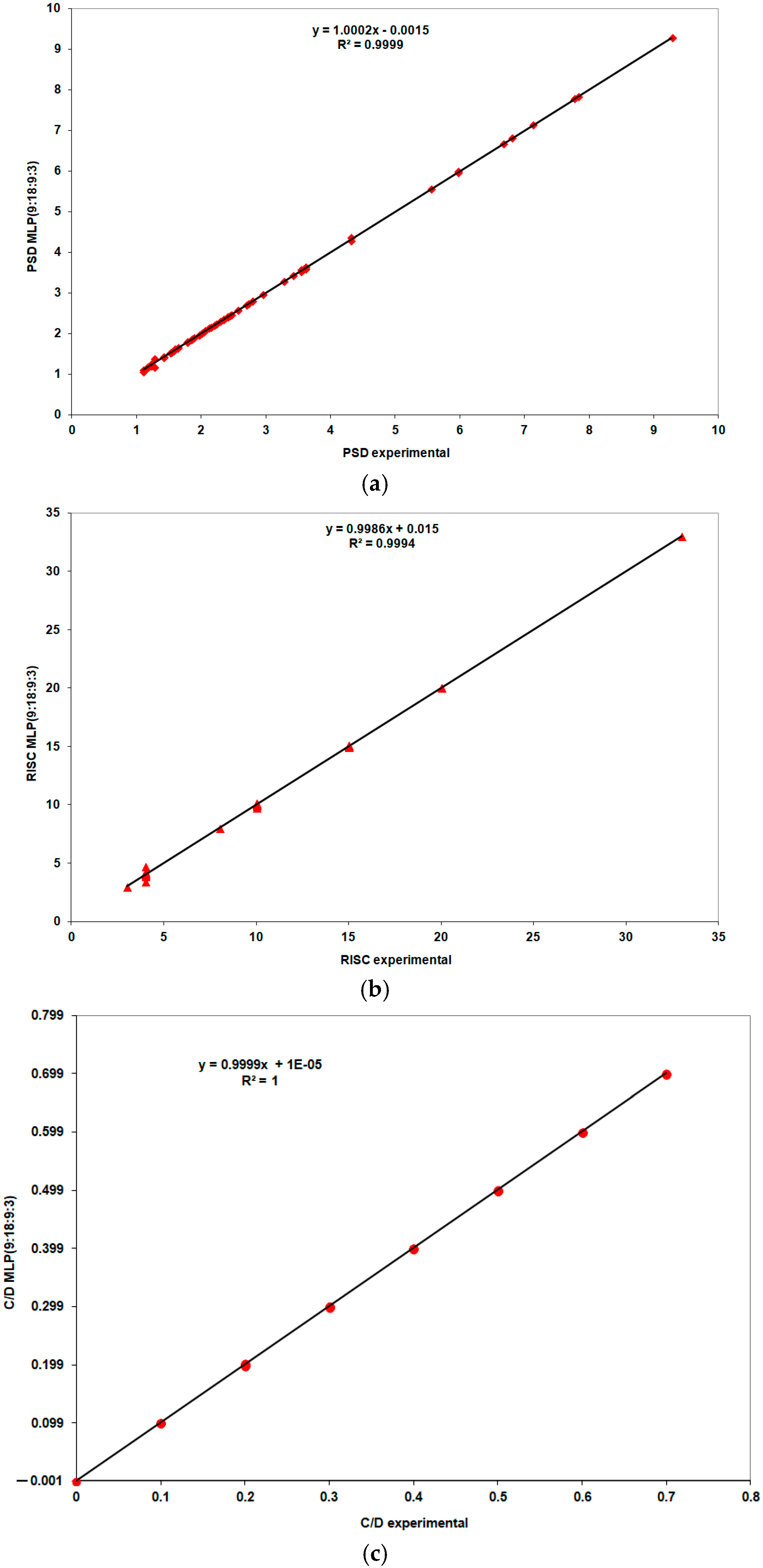
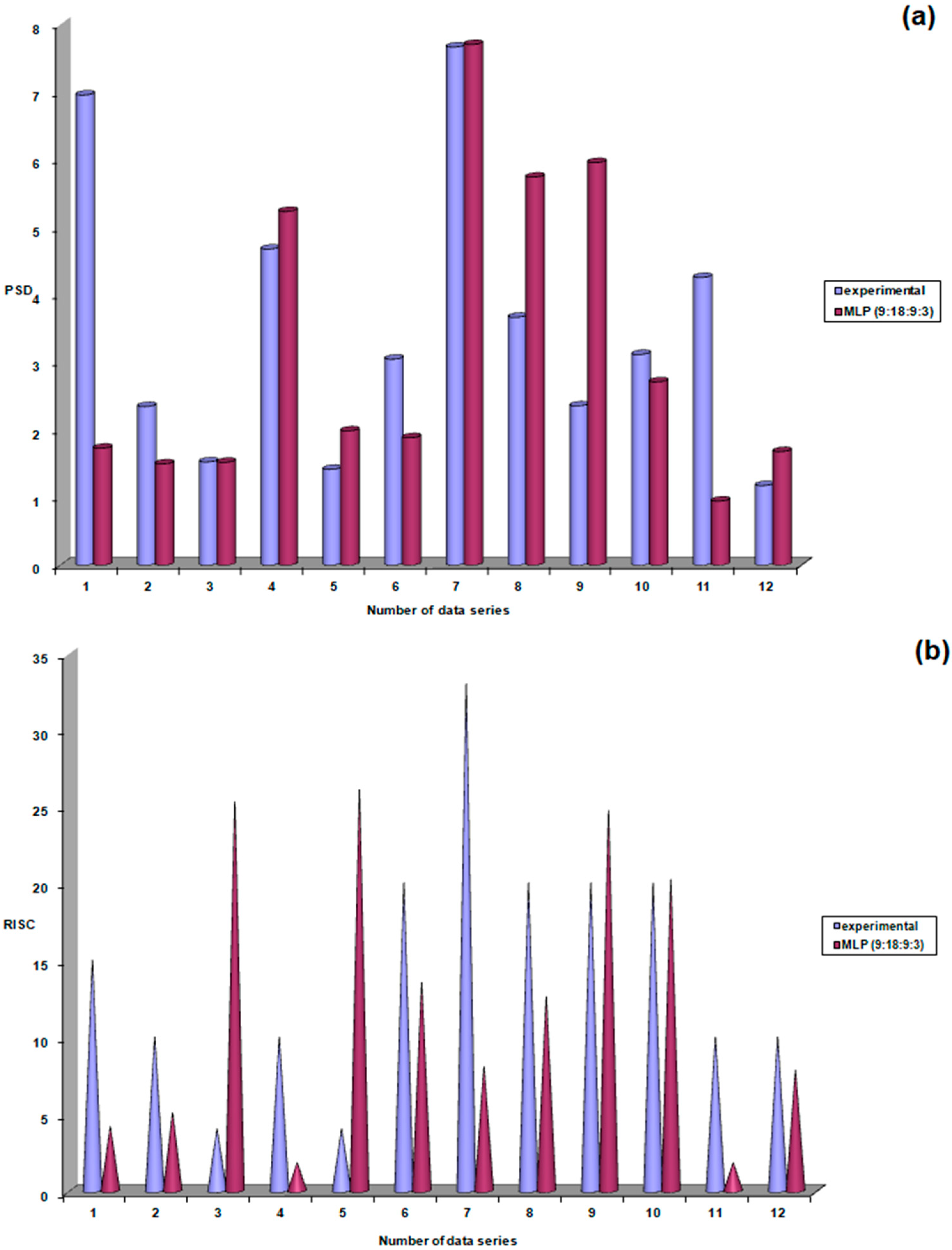
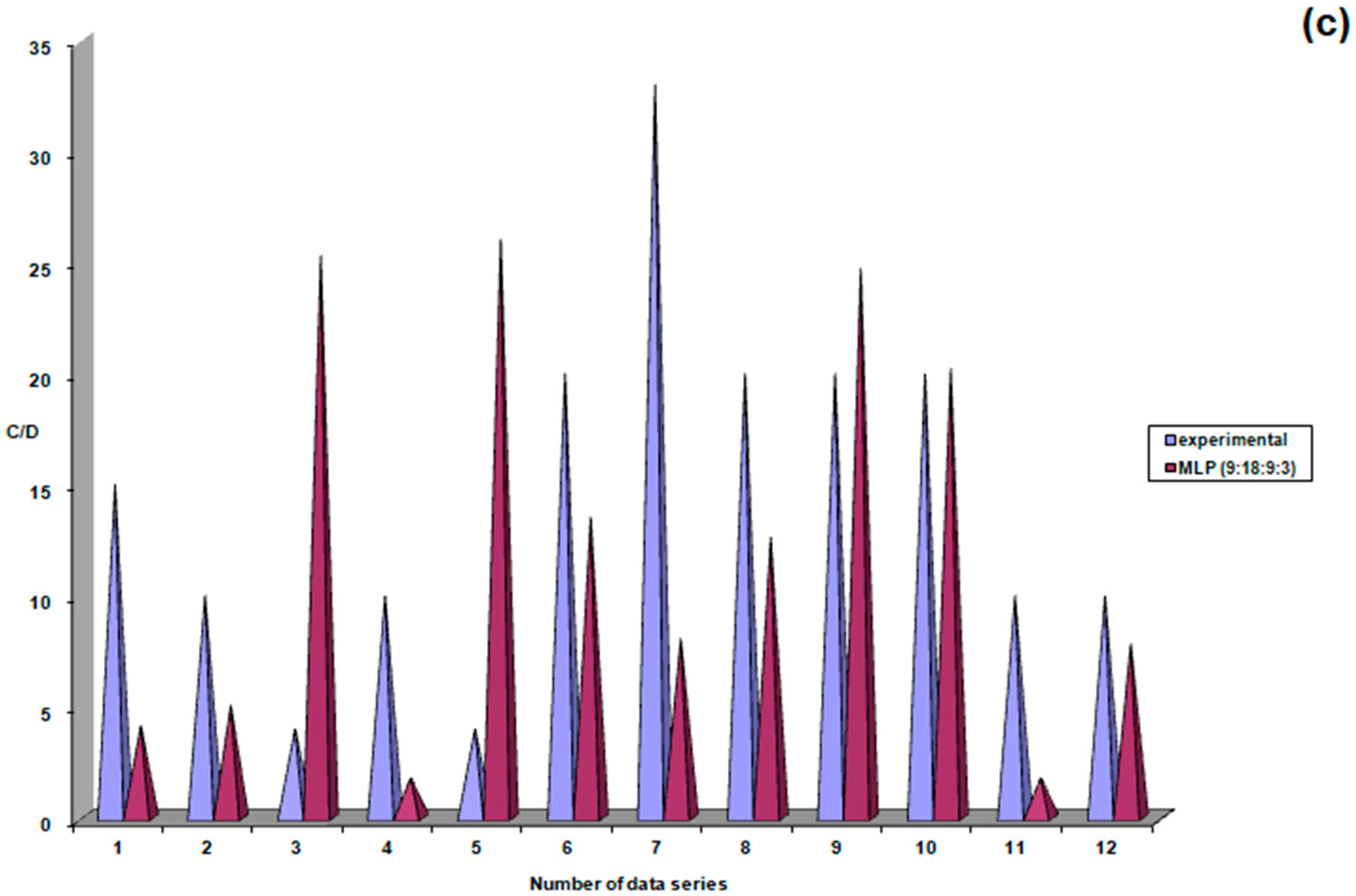
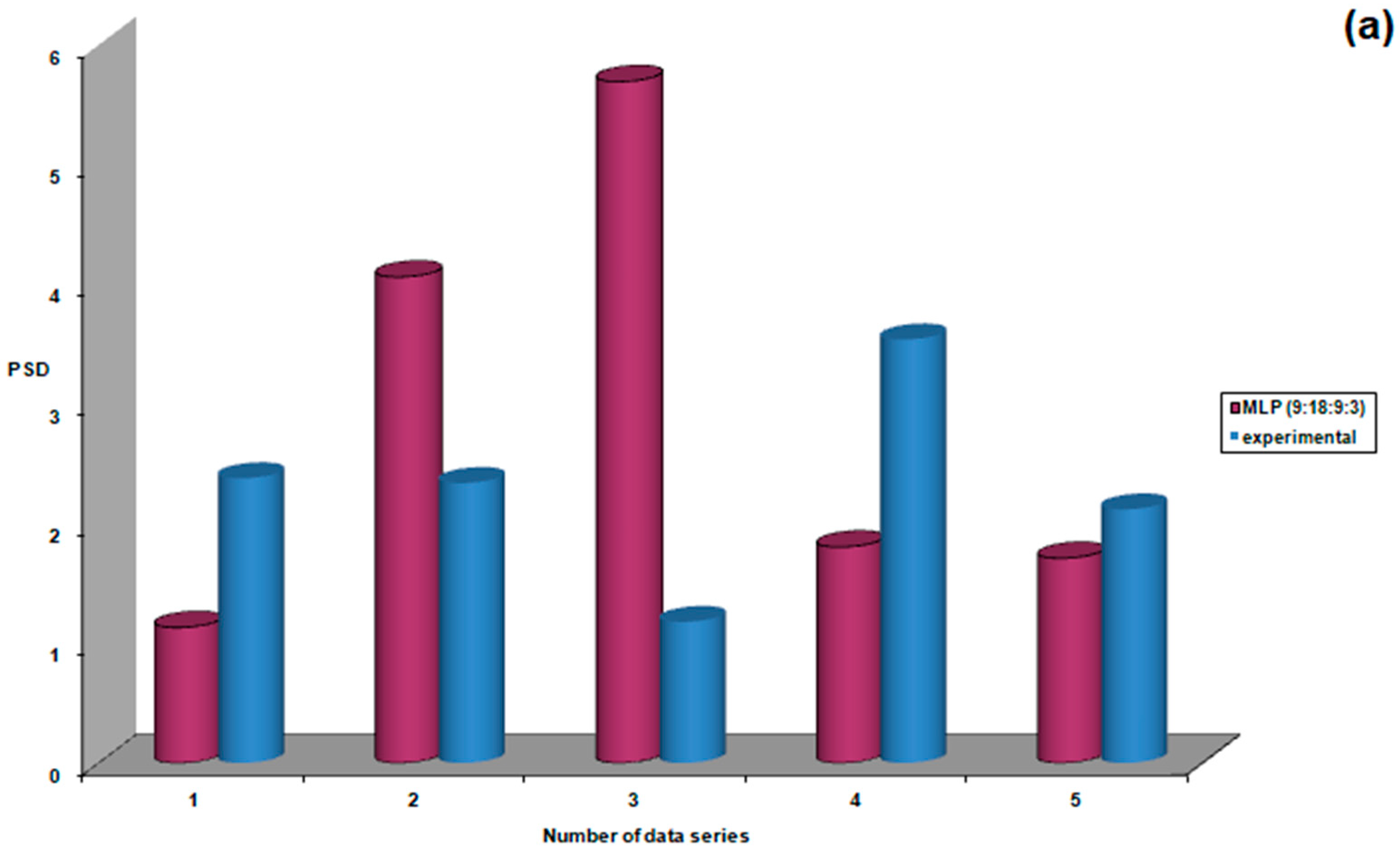
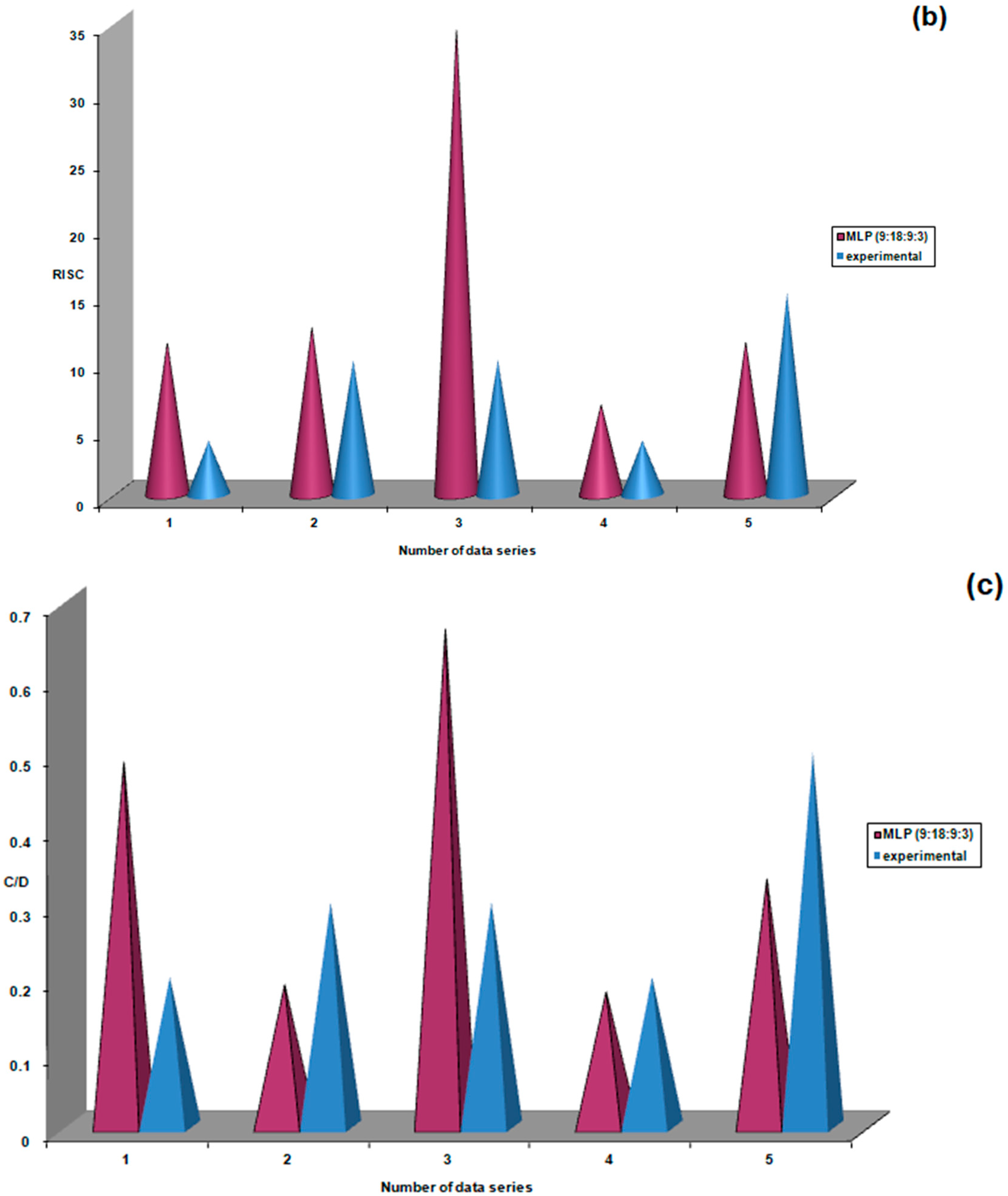






| Study Group | N | Mean | Standard Deviation | Standard Error | Confidence Interval 95% | Min | Max | FANOVA p Test | |
|---|---|---|---|---|---|---|---|---|---|
| −95%CI | +95%CI | ||||||||
| IOP maximum | |||||||||
| Group I | 75 | 22.89 | 4.23 | 0.49 | 21.92 | 23.87 | 15 | 34 | 0.001 |
| Group II | 70 | 20.70 | 6.19 | 0.74 | 19.22 | 22.18 | 10 | 48 | |
| Group III | 89 | 19.71 | 3.48 | 0.37 | 18.97 | 20.44 | 10 | 33 | |
| Total | 234 | 21.03 | 4.84 | 0.32 | 20.40 | 21.65 | 10 | 48 | |
| IOP minimum | |||||||||
| Group I | 75 | 17.32 | 3.46 | 0.40 | 16.52 | 18.12 | 10 | 28 | 0.002 |
| Group II | 70 | 17.17 | 5.01 | 0.60 | 15.98 | 18.37 | 11 | 38 | |
| Group III | 89 | 15.39 | 3.24 | 0.34 | 14.71 | 16.08 | 9 | 30 | |
| Total | 234 | 16.54 | 4.00 | 0.26 | 16.03 | 17.06 | 9 | 38 | |
| Study Group | N | Mean | Standard Deviation | Standard Error | Confidence Interval 95% | Min | Max | Test FANOVA p | |
|---|---|---|---|---|---|---|---|---|---|
| −95%CI | +95%CI | ||||||||
| Whole Group | 234 | 13.66 | 8.57 | 0.56 | 12.55 | 14.76 | 3 | 33 | - |
| Group I | 75 | 16.83 | 9.80 | 1.04 | 14.77 | 18.89 | 4 | 33 | 0.001 |
| Group II | 70 | 12.21 | 7.86 | 0.94 | 10.34 | 14.09 | 4 | 33 | |
| Group III | 89 | 11.24 | 6.29 | 0.73 | 9.79 | 12.69 | 3 | 33 | |
| Model | R | R Square | Adjusted R Square | Std. Error of the Estimate | Change Statistics | ||||
|---|---|---|---|---|---|---|---|---|---|
| R Square Change | F Change | df1 | df2 | Sig. F Change | |||||
| 1 | 0.106 (a) | 0.011 | 0.007 | 8.542 | 0.011 | 2.637 | 1 | 232 | 0.106 |
| 2 | 0.309 (b) | 0.096 | 0.088 | 8.187 | 0.084 | 21.537 | 1 | 231 | 0.001 |
| 3 | 0.313 (c) | 0.098 | 0.086 | 8.193 | 0.003 | 0.675 | 1 | 230 | 0.412 |
| 4 | 0.314 (d) | 0.099 | 0.083 | 8.208 | 0.001 | 0.169 | 1 | 229 | 0.681 |
| 5 | 0.492 (e) | 0.242 | 0.225 | 7.544 | 0.143 | 43.055 | 1 | 228 | 0.001 |
| 6 | 0.492 (f) | 0.242 | 0.222 | 7.561 | 0.000 | 0.012 | 1 | 227 | 0.914 |
| 7 | 0.614 (g) | 0.377 | 0.358 | 6.868 | 0.135 | 49.073 | 1 | 226 | 0.001 |
| 8 | 0.768 (h) | 0.590 | 0.575 | 5.585 | 0.213 | 116.768 | 1 | 225 | 0.001 |
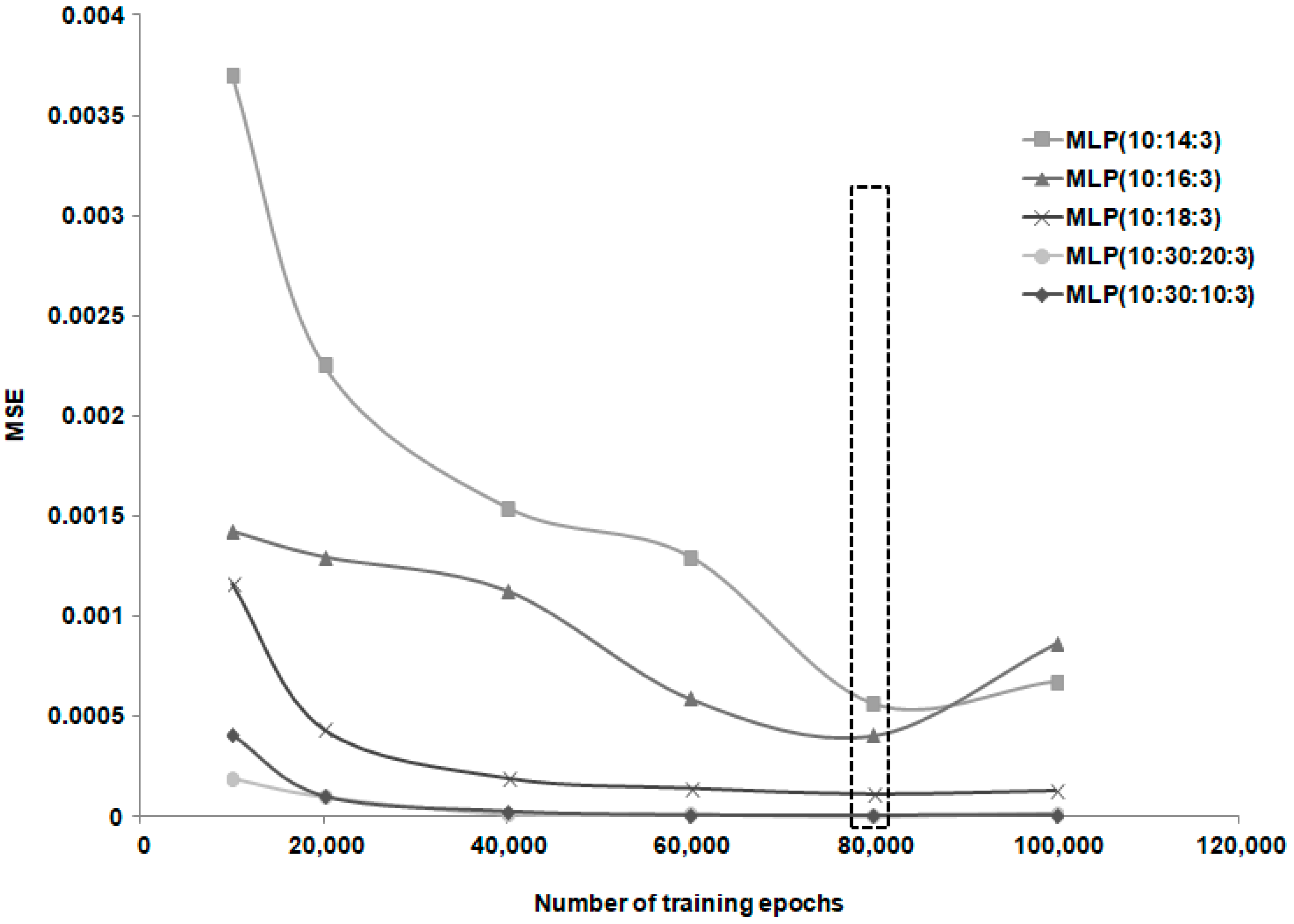
| No. | Network Topology | MSE | NMSE | r2 | Ep (%) | Training Phase Length (Minutes) |
|---|---|---|---|---|---|---|
| 1. | MLP(9:9:3) | 0.008268 | 0.047467 | 0.974388 | 12.58 | 2.57 |
| 2. | MLP (9:11:3) | 0.003215 | 0.018456 | 0.988966 | 8.39 | 4.36 |
| 3. | MLP (9:13:3) | 0.001481 | 0.008502 | 0.994451 | 5.14 | 4.37 |
| 4. | MLP (9:15:3) | 0.001156 | 0.006640 | 0.996061 | 4.87 | 4.36 |
| 5. | MLP (9:17:3) | 0.000183 | 0.001048 | 0.999359 | 1.94 | 5.12 |
| 6. | MLP (9:18:3) | 0.000175 | 0.001002 | 0.999431 | 1.87 | 4.23 |
| 7. | MLP (9:27:3) | 0.000048 | 0.000273 | 0.999818 | 0.73 | 5.12 |
| 8. | MLP (9:18:9:3) | 0.000031 | 0.000176 | 0.999981 | 0.59 | 4.38 |
| 9. | MLP (9:27:9:3) | 0.000035 | 0.000185 | 0.999890 | 0.62 | 7.58 |
| No. | Network Topology | MSE | NMSE | r2 | Ep(%) | Training Phase Length (Minutes) |
|---|---|---|---|---|---|---|
| 1. | MLP(10:10:3) | 0.001849 | 0.009333 | 0.994498 | 5.26 | 2.23 |
| 2. | MLP (10:12:3) | 0.000620 | 0.003130 | 0.998483 | 2.22 | 2.12 |
| 3. | MLP (10:14:3) | 0.000309 | 0.001560 | 0.999258 | 1.77 | 1.77 |
| 4. | MLP (10:16:3) | 0.000080 | 0.000405 | 0.999851 | 1.01 | 3.16 |
| 5. | MLP (10:18:3) | 0.000009 | 0.000045 | 0.999977 | 0.21 | 3.19 |
| 6. | MLP (10:20:3) | 0.000085 | 0.000428 | 0.999821 | 0.56 | 3.16 |
| 7. | MLP (10:20:10:3) | 0.000008 | 0.000040 | 0.999984 | 0.20 | 5.02 |
| 8. | MLP (10:30:10:3) | 0.000014 | 0.000069 | 0.999974 | 0.21 | 4.52 |
| No. | Network Topology | MSE | NMSE | r2 | Ep (%) | Training Phase Length (Minutes) |
|---|---|---|---|---|---|---|
| 1. | MLP(10:10:3) | 0.005721 | 0.023217 | 0.986456 | 8.62 | 4.10 |
| 2. | MLP (10:12:3) | 0.001885 | 0.007649 | 0.995992 | 5.54 | 4.04 |
| 3. | MLP (10:14:3) | 0.000563 | 0.002283 | 0.998824 | 2.80 | 5.24 |
| 4. | MLP (10:16:3) | 0.000406 | 0.001649 | 0.999310 | 2.22 | 3.98 |
| 5. | MLP (10:18:3) | 0.000111 | 0.000452 | 0.999795 | 1.24 | 5.18 |
| 6. | MLP (10:20:3) | 0.000105 | 0.000424 | 0.999821 | 1.03 | 5.38 |
| 7. | MLP (10:30:3) | 0.000010 | 0.000041 | 0.999981 | 0.31 | 5.07 |
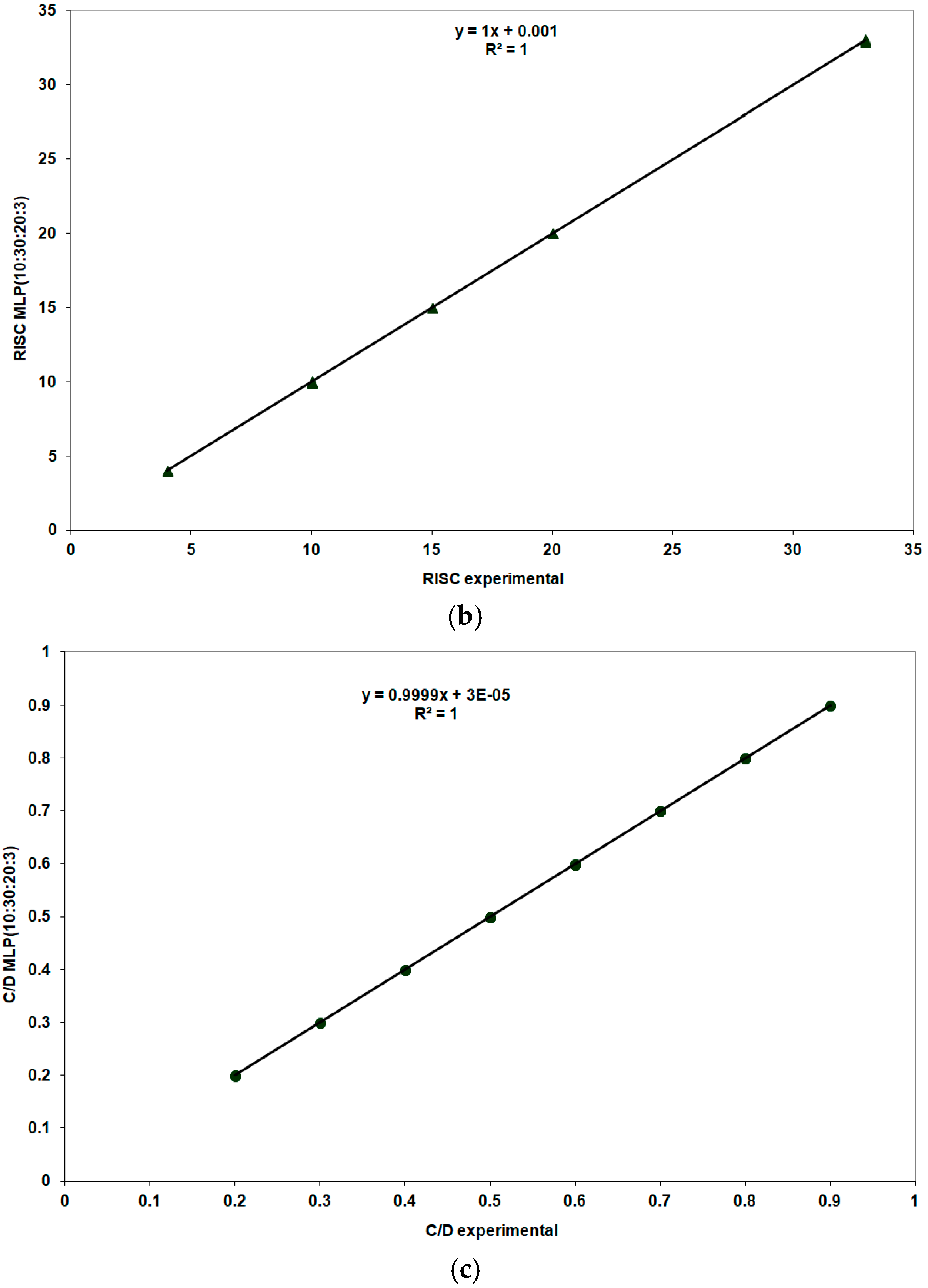
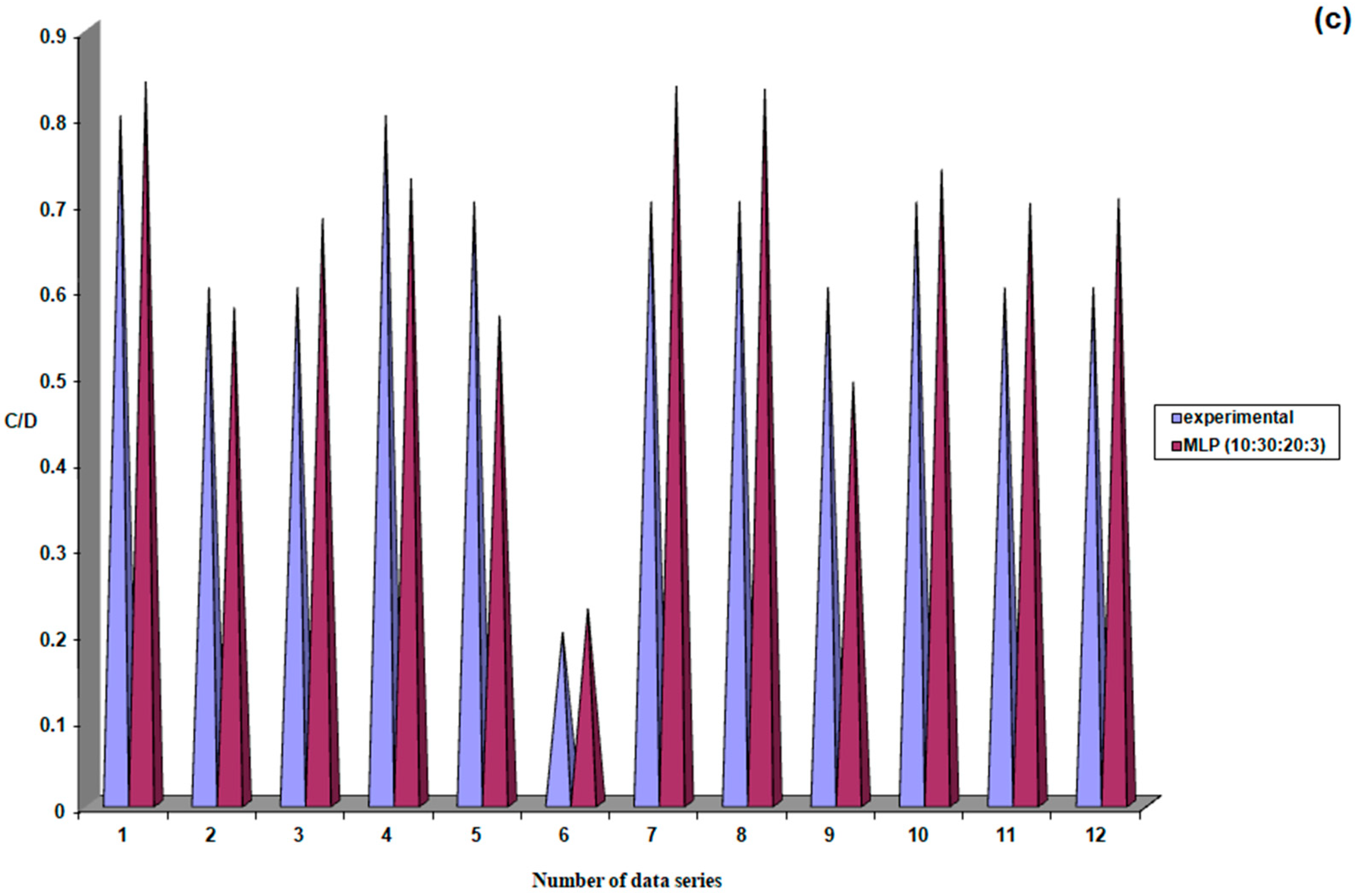
| 8. | MLP (10:30:20:3) | 0.000001 | 0.000004 | 0.999999 | 0.06 | 5.30 |
| 9. | MLP (10:30:10:3) | 0.000007 | 0.000029 | 0.999985 | 0.22 | 5.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anton, N.; Lisa, C.; Doroftei, B.; Pîrvulescu, R.A.; Barac, R.I.; Lungu, I.I.; Bogdănici, C.M. The Role of Artificial Intelligence in Predicting the Progression of Intraocular Hypertension to Glaucoma. Life 2025, 15, 865. https://doi.org/10.3390/life15060865
Anton N, Lisa C, Doroftei B, Pîrvulescu RA, Barac RI, Lungu II, Bogdănici CM. The Role of Artificial Intelligence in Predicting the Progression of Intraocular Hypertension to Glaucoma. Life. 2025; 15(6):865. https://doi.org/10.3390/life15060865
Chicago/Turabian StyleAnton, Nicoleta, Cătălin Lisa, Bogdan Doroftei, Ruxandra Angela Pîrvulescu, Ramona Ileana Barac, Ionuț Iulian Lungu, and Camelia Margareta Bogdănici. 2025. "The Role of Artificial Intelligence in Predicting the Progression of Intraocular Hypertension to Glaucoma" Life 15, no. 6: 865. https://doi.org/10.3390/life15060865
APA StyleAnton, N., Lisa, C., Doroftei, B., Pîrvulescu, R. A., Barac, R. I., Lungu, I. I., & Bogdănici, C. M. (2025). The Role of Artificial Intelligence in Predicting the Progression of Intraocular Hypertension to Glaucoma. Life, 15(6), 865. https://doi.org/10.3390/life15060865









