Comparative Analysis of Physiological Parameters, Antioxidant Defense, Ion Regulation, and Gene Expression in Two Distinct Maize Hybrids Under Salt Stress at Seedling Stage
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Na+ and K+ Concentration Measurements
2.3. Chlorophyll and Photosynthetic Parameter Measurements
2.4. Relative Water Content (RWC) Measurement
2.5. Proline Measurement
2.6. Membranes Stability Index (MSI) and Malondialdehyde (MDA)
2.7. Antioxidant Enzyme Identification (CAT, SOD, and POD)
2.8. Analysis of Gene Expression
2.9. Determination of Growth Parameters
2.10. Data Analysis
3. Results
3.1. Na+ and K+ Concentrations and Na+ to K+ Ratio
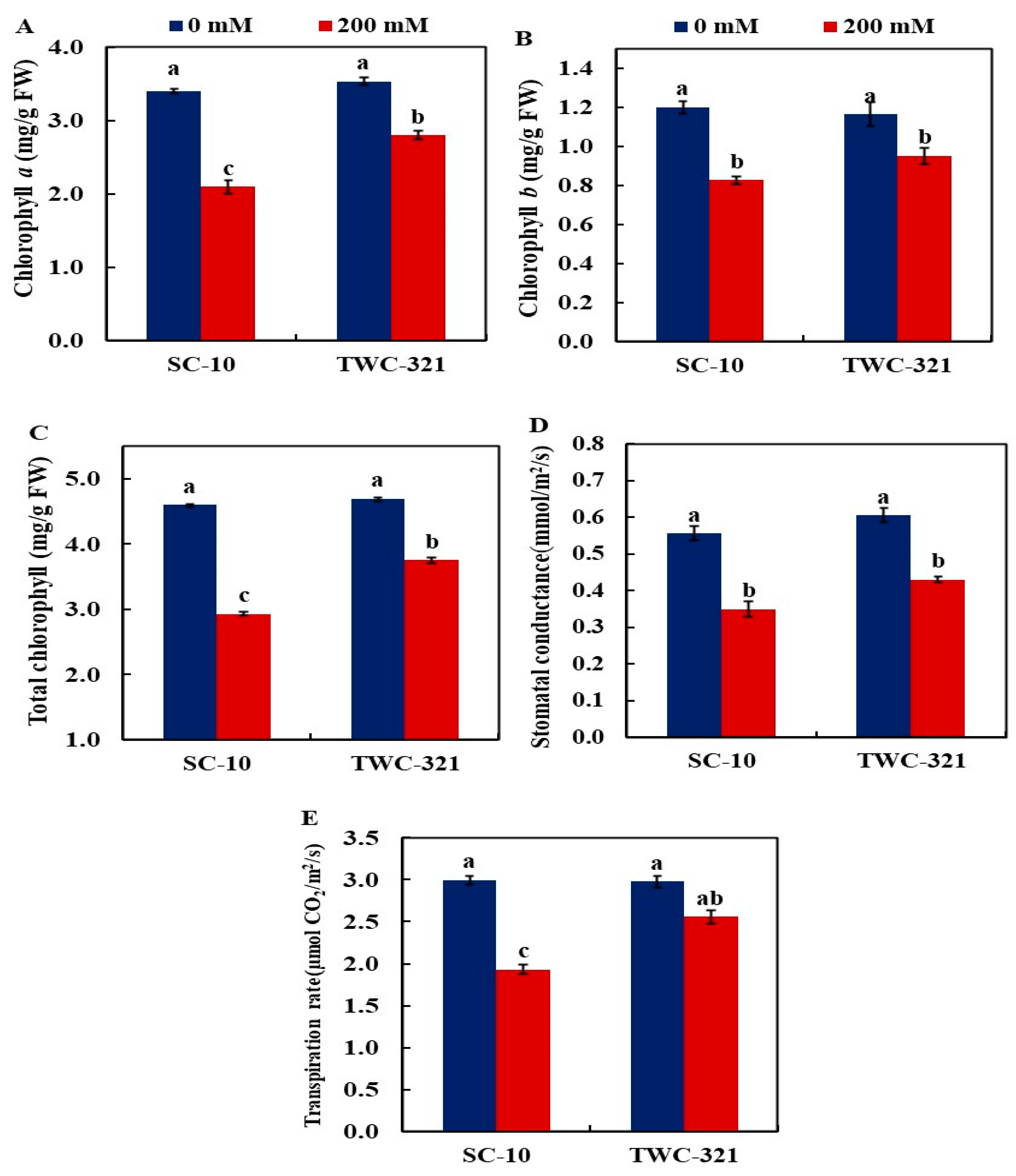
3.2. Chlorophyll Content and Photosynthetic Efficiency
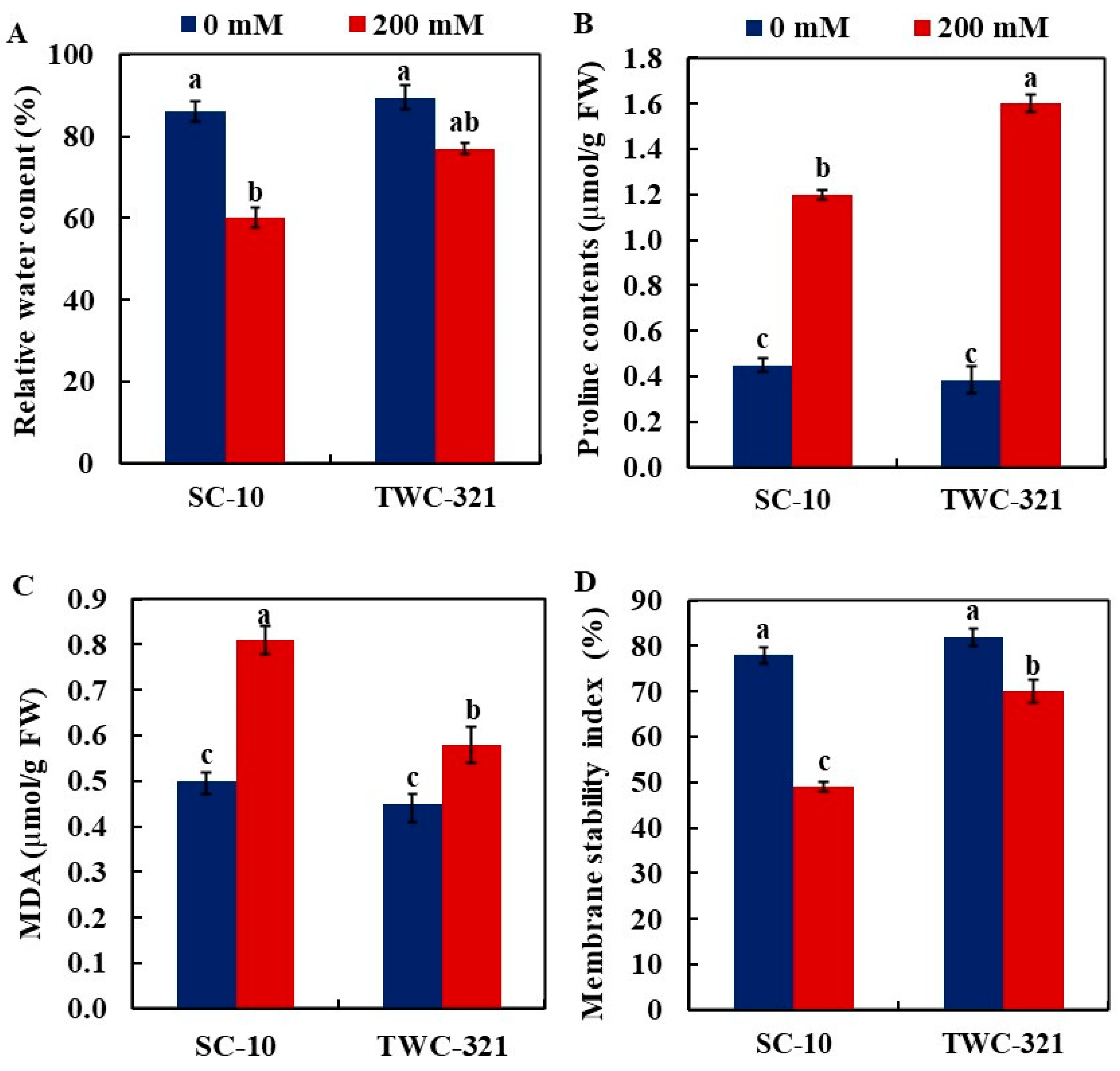
3.3. Relative Water Content, Proline Accumulation, Malondialdehyde, and Membrane Stability Index
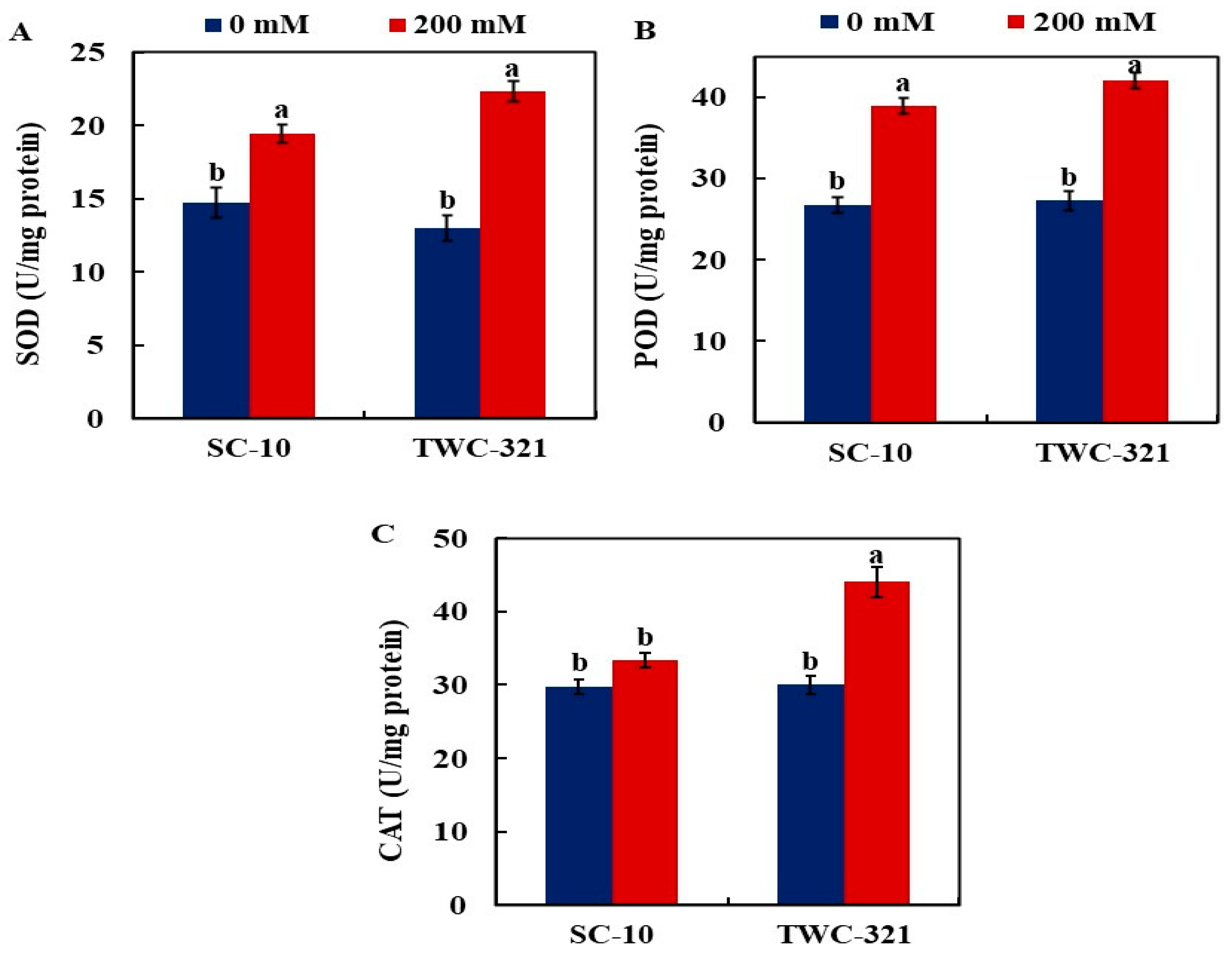
3.4. Activity of Antioxidant Enzymes
3.5. Gene Expression Analysis
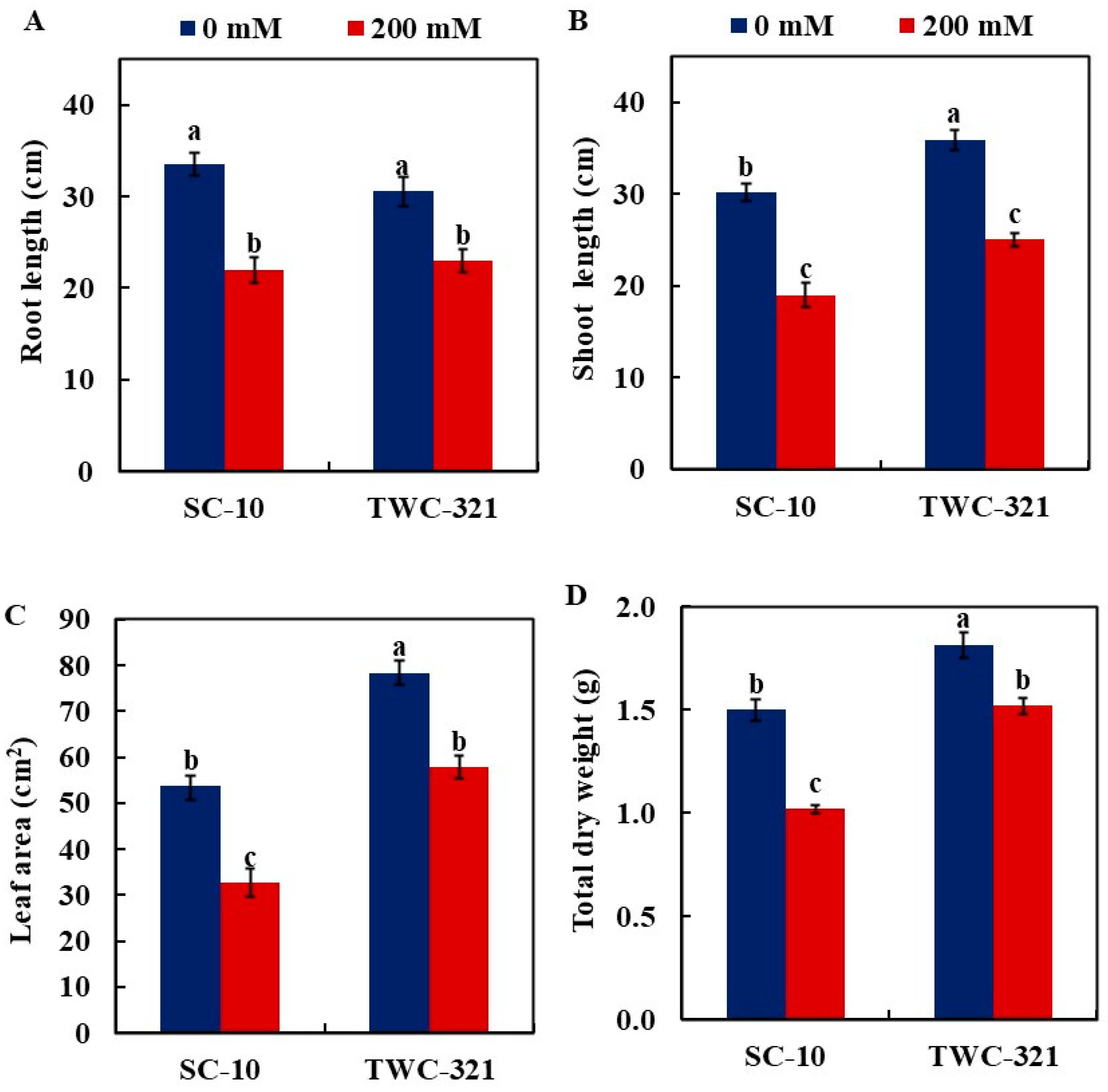
3.6. Plant Growth
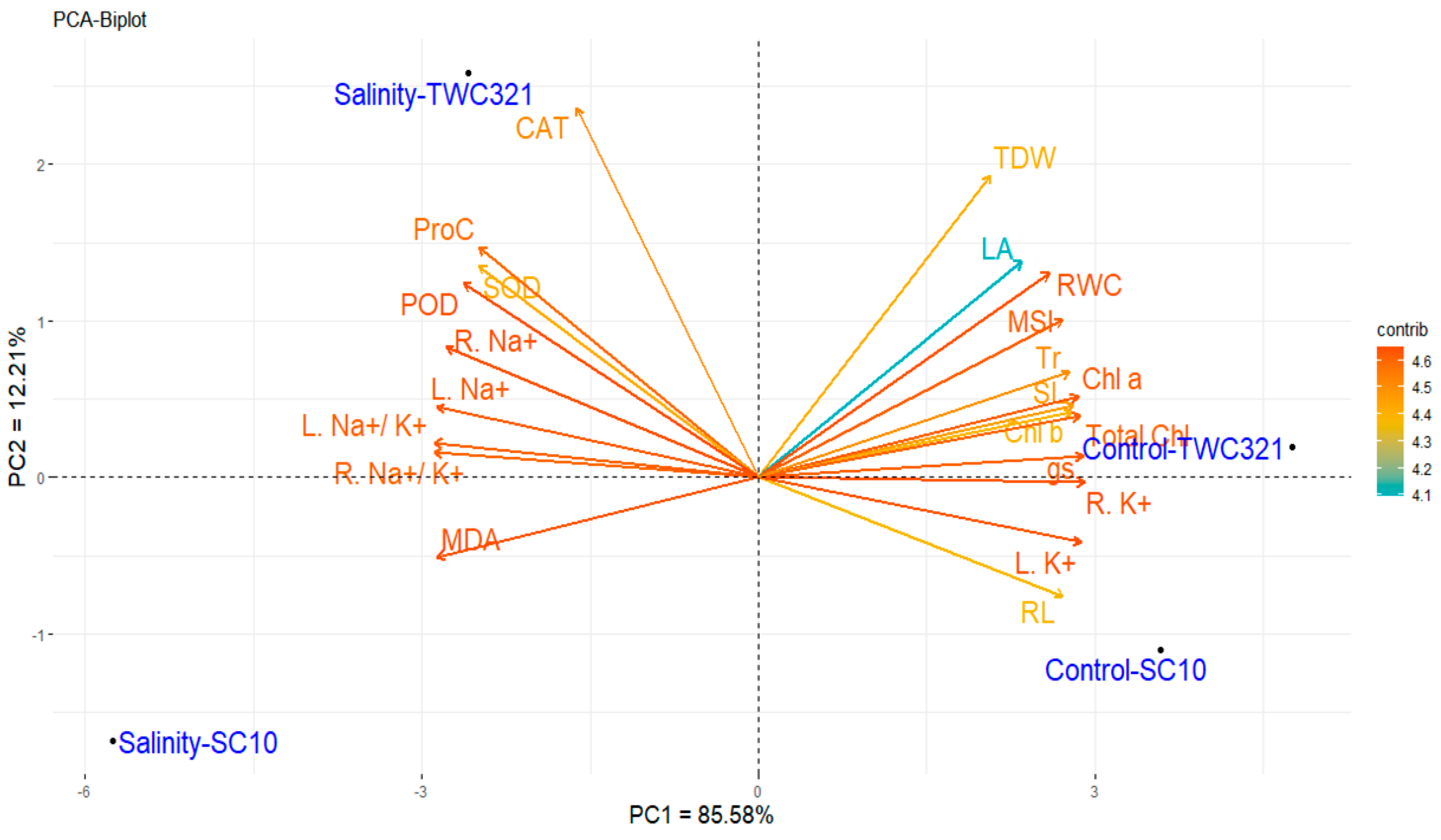
3.7. Principal Component and Heat Map Analyses
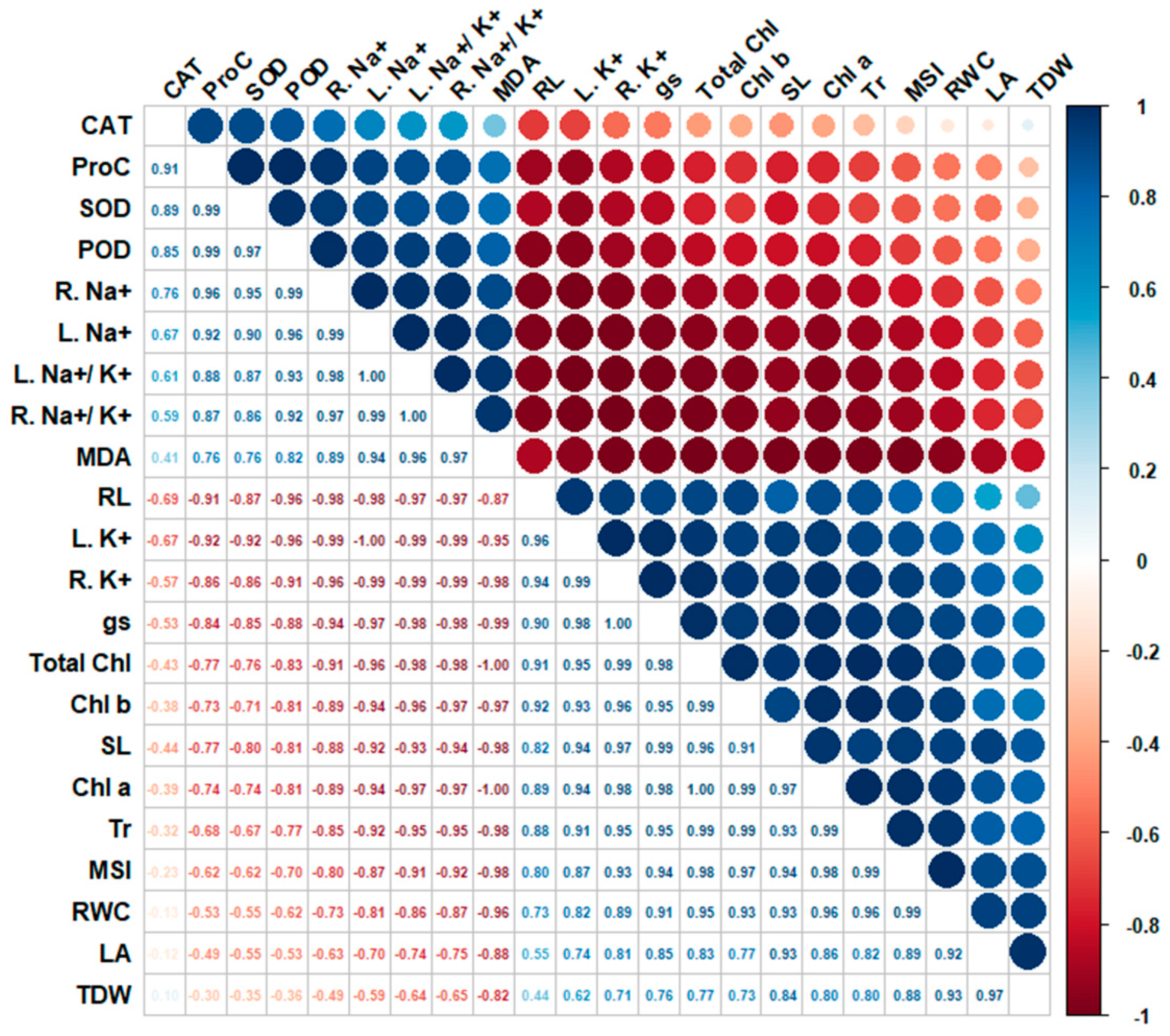
3.8. Correlation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, M.; Kong, X.; Xu, X.; Li, C.; Tian, H.; Ding, Z. Comparative transcriptome profiling of the maize primary, crown and seminal root in response to salinity stress. PLoS ONE 2015, 10, e0121222. [Google Scholar] [CrossRef] [PubMed]
- Mansour, E.; Moustafa, E.S.; Abdul-Hamid, M.I.; Ash-shormillesy, S.M.; Merwad, A.-R.M.; Wafa, H.A.; Igartua, E. Field responses of barley genotypes across a salinity gradient in an arid Mediterranean environment. Agric. Water Manag. 2021, 258, 107206. [Google Scholar] [CrossRef]
- Sahbeni, G.; Ngabire, M.; Musyimi, P.K.; Székely, B. Challenges and opportunities in remote sensing for soil salinization mapping and monitoring: A review. Remote Sens. 2023, 15, 2540. [Google Scholar] [CrossRef]
- Liu, W.; Jiang, L.; Jiapaer, G.; Wu, G.; Li, Q.; Yang, J. Monitoring the salinization of agricultural land and assessing its drivers in the Altay region. Ecol. Indic. 2024, 167, 112678. [Google Scholar] [CrossRef]
- Moustafa, E.S.; Ali, M.M.; Kamara, M.M.; Awad, M.F.; Hassanin, A.A.; Mansour, E. Field screening of wheat advanced lines for salinity tolerance. Agronomy 2021, 11, 281. [Google Scholar] [CrossRef]
- ElSayed, A.I.; Mohamed, A.H.; Rafudeen, M.S.; Omar, A.A.; Awad, M.F.; Mansour, E. Polyamines mitigate the destructive impacts of salinity stress by enhancing photosynthetic capacity, antioxidant defense system and upregulation of calvin cycle-related genes in rapeseed (Brassica napus L.). Saudi J. Biol. Sci. 2022, 29, 3675–3686. [Google Scholar] [CrossRef]
- Moustafa, E.S.; El-Sobky, E.-S.E.; Farag, H.I.; Yasin, M.A.; Attia, A.; Rady, M.O.; Awad, M.F.; Mansour, E. Sowing date and genotype influence on yield and quality of dual-purpose barley in a salt-affected arid region. Agronomy 2021, 11, 717. [Google Scholar] [CrossRef]
- Zhang, E.; Zhu, X.; Wang, W.; Sun, Y.; Tian, X.; Chen, Z.; Mou, X.; Zhang, Y.; Wei, Y.; Fang, Z. Metabolomics reveals the response of hydroprimed maize to mitigate the impact of soil salinization. Front. Plant Sci. 2023, 14, 1109460. [Google Scholar] [CrossRef]
- Huang, S.; Gill, S.; Ramzan, M.; Ahmad, M.Z.; Danish, S.; Huang, P.; Al Obaid, S.; Alharbi, S.A. Uncovering the impact of AM fungi on wheat nutrient uptake, ion homeostasis, oxidative stress, and antioxidant defense under salinity stress. Sci. Rep. 2023, 13, 8249. [Google Scholar]
- Almeida, D.M.; Oliveira, M.M.; Saibo, N.J.M. Regulation of Na+ and K+ homeostasis in plants: Towards improved salt stress tolerance in crop plants. Genet. Mol. Biol. 2017, 40, 326–345. [Google Scholar]
- Munns, R.; Passioura, J.B.; Colmer, T.D.; Byrt, C.S. Osmotic adjustment and energy limitations to plant growth in saline soil. New Phytol. 2020, 225, 1091–1096. [Google Scholar]
- Farooq, M.; Hussain, M.; Wakeel, A.; Siddique, K.H.M. Salt stress in maize: Effects, resistance mechanisms, and management. A review. Agron. Sustain. Dev. 2015, 35, 461–481. [Google Scholar]
- Desoky, E.-S.M.; Merwad, A.-R.M.; Abo El-Maati, M.F.; Mansour, E.; Arnaout, S.M.; Awad, M.F.; Ramadan, M.F.; Ibrahim, S.A. Physiological and biochemical mechanisms of exogenously applied selenium for alleviating destructive impacts induced by salinity stress in bread wheat. Agronomy 2021, 11, 926. [Google Scholar] [CrossRef]
- Zhang, M.; Cao, Y.; Wang, Z.; Wang, Z.q.; Shi, J.; Liang, X.; Song, W.; Chen, Q.; Lai, J.; Jiang, C. A retrotransposon in an HKT1 family sodium transporter causes variation of leaf Na+ exclusion and salt tolerance in maize. New Phytol. 2018, 217, 1161–1176. [Google Scholar] [PubMed]
- Rizk, M.S.; Assaha, D.V.M.; Mekawy, A.M.M.; Shalaby, N.E.; Ramadan, E.A.; El-Tahan, A.M.; Ibrahim, O.M.; Metwelly, H.I.F.; Okla, M.K.; Maridueña-Zavala, M.G. Comparative analysis of salinity tolerance mechanisms in two maize genotypes: Growth performance, ion regulation, and antioxidant responses. BMC Plant Biol. 2024, 24, 818. [Google Scholar]
- Mansour, E.; Moustafa, E.S.; Desoky, E.-S.M.; Ali, M.M.; Yasin, M.A.; Attia, A.; Alsuhaibani, N.; Tahir, M.U.; El-Hendawy, S. Multidimensional evaluation for detecting salt tolerance of bread wheat genotypes under actual saline field growing conditions. Plants 2020, 9, 1324. [Google Scholar] [CrossRef]
- Rizk, M.S.; Mekawy, A.M.M.; Assaha, D.V.M.; Chuamnakthong, S.; Shalaby, N.E.; Ueda, A. Regulation of Na+ and K+ transport and oxidative stress mitigation reveal differential salt tolerance of two Egyptian maize (Zea mays L.) hybrids at the seedling stage. J. Plant Growth Regul. 2021, 40, 1629–1639. [Google Scholar] [CrossRef]
- Li, J.; Zhu, Q.; Jiao, F.; Yan, Z.; Zhang, H.; Zhang, Y.; Ding, Z.; Mu, C.; Liu, X.; Li, Y. Research progress on the mechanism of salt tolerance in maize: A classic field that needs new efforts. Plants 2023, 12, 2356. [Google Scholar] [CrossRef]
- Kamara, M.M.; Mansour, E.; Khalaf, A.E.A.; Eid, M.A.M.; Hassanin, A.A.; Abdelghany, A.M.; Kheir, A.M.S.; Galal, A.A.; Behiry, S.I.; Silvar, C. Molecular diversity and combining ability in newly developed maize inbred lines under low-nitrogen conditions. Life 2024, 14, 641. [Google Scholar] [CrossRef]
- Kamara, M.M.; Safhi, F.A.; Al Aboud, N.M.; Aljabri, M.; Alharbi, S.A.; Ghazzawy, H.S.; Alshaharni, M.O.; Fayad, E.; Felemban, W.F.; Abd El-Moneim, D. Genetic diversity and combining ability of developed maize lines to realize heterotic and high yielding hybrids for arid conditions. Phyton-Int. J. Exp. Bot. 2024, 93, 3465–3485. [Google Scholar] [CrossRef]
- Ahmad, U.; Hussain, M.; Ahmad, W.; Javed, J.; Arshad, Z.; Akram, Z. Impact of global climate change on maize (Zea mays): Physiological responses and modern breeding techniques. Trends Biotech. Plant Sci. 2024, 2, 62–77. [Google Scholar]
- Vennam, R.R.; Bheemanahalli, R.; Reddy, K.R.; Dhillon, J.; Zhang, X.; Adeli, A. Early-Season Maize Responses to Salt Stress: Morpho-Physiological, Leaf Reflectance, and Mineral Composition. J. Agric. Food Res. 2024, 15, 100994. [Google Scholar] [CrossRef]
- Liang, W.; Ma, X.; Wan, P.; Liu, L. Plant salt-tolerance mechanism: A review. Biochem. Biophys. Res. Commun. 2018, 495, 286–291. [Google Scholar] [CrossRef]
- Selem, E.; Hassan, A.A.; Awad, M.F.; Mansour, E.; Desoky, E.-S.M. Impact of exogenously sprayed antioxidants on physio-biochemical, agronomic, and quality parameters of potato in salt-affected soil. Plants 2022, 11, 210. [Google Scholar] [CrossRef]
- Hanin, M.; Ebel, C.; Ngom, M.; Laplaze, L.; Masmoudi, K. New insights on plant salt tolerance mechanisms and their potential use for breeding. Front. Plant Sci. 2016, 7, 1787. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Zhou, X.; Song, H.; Zhang, M.; Jiang, C. Advances in deciphering salt tolerance mechanism in maize. Crop J. 2023, 11, 1001–1010. [Google Scholar] [CrossRef]
- Xiao, F.; Zhou, H. Plant salt response: Perception, signaling, and tolerance. Front. Plant Sci. 2023, 13, 1053699. [Google Scholar] [CrossRef]
- Cao, Y.; Liang, X.; Yin, P.; Zhang, M.; Jiang, C. A domestication-associated reduction in K+-preferring HKT transporter activity underlies maize shoot K+ accumulation and salt tolerance. New Phytol. 2019, 222, 301–317. [Google Scholar] [CrossRef]
- Assaha, D.V.M.; Mekawy, A.M.M.; Liu, L.; Noori, M.S.; Kokulan, K.S.; Ueda, A.; Nagaoka, T.; Saneoka, H. Na+ retention in the root is a key adaptive mechanism to low and high salinity in the glycophyte, Talinum paniculatum (Jacq.) Gaertn.(Portulacaceae). J. Agron. Crop Sci. 2017, 203, 56–67. [Google Scholar] [CrossRef]
- Gondim, F.A.; Miranda, R.d.S.; Gomes-Filho, E.; Prisco, J.T. Enhanced salt tolerance in maize plants induced by H2O2 leaf spraying is associated with improved gas exchange rather than with non-enzymatic antioxidant system. Theor. Exp. Plant Physiol. 2013, 25, 251–260. [Google Scholar] [CrossRef]
- Arzani, A.; Kumar, S.; Mansour, M.M.F. Salt tolerance in plants: Molecular and functional adaptations. Front. Plant Sci. 2023, 14, 1280788. [Google Scholar] [CrossRef]
- Atta, K.; Mondal, S.; Gorai, S.; Singh, A.P.; Kumari, A.; Ghosh, T.; Roy, A.; Hembram, S.; Gaikwad, D.J.; Mondal, S. Impacts of salinity stress on crop plants: Improving salt tolerance through genetic and molecular dissection. Front. Plant Sci. 2023, 14, 1241736. [Google Scholar]
- Hussain, S.; Rasheed, M.; Ahmed, Z.; Jilani, G. Characterizing maize genotypes for salt tolerance using morphological and ionic traits at seedling stage. Int. Lett. Nat. Sci. 2023, 86, 1–24. [Google Scholar]
- Khare, T.; Kumar, V.; Kishor, P.B.K. Na+ and Cl− ions show additive effects under NaCl stress on induction of oxidative stress and the responsive antioxidative defense in rice. Protoplasma 2015, 252, 1149–1165. [Google Scholar] [PubMed]
- Wang, J.; Luo, Y.; Ye, F.; Ding, Z.J.; Zheng, S.J.; Qiao, S.; Wang, Y.; Guo, J.; Yang, W.; Su, N. Structures and ion transport mechanisms of plant high-affinity potassium transporters. Mol. Plant 2024, 17, 409–422. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Song, G.; Shan, X.; Wei, Z.; Liu, Y.; Jiang, C.; Jiang, Y.; Jin, F.; Li, Y. Association analysis and identification of ZmHKT1; 5 variation with salt-stress tolerance. Front. Plant Sci. 2018, 9, 1485. [Google Scholar] [CrossRef]
- Pitann, B.; Mohamed, A.K.; Neubert, A.B.; Schubert, S. Tonoplast Na+/H+ antiporters of newly developed maize (Zea mays) hybrids contribute to salt resistance during the second phase of salt stress. J. Plant Nutr. Soil Sci. 2013, 176, 148–156. [Google Scholar]
- Chevilly, S.; Dolz-Edo, L.; Morcillo, L.; Vilagrosa, A.; López-Nicolás, J.M.; Yenush, L.; Mulet, J.M. Identification of distinctive physiological and molecular responses to salt stress among tolerant and sensitive cultivars of broccoli (Brassica oleracea var. Italica). BMC Plant Biol. 2021, 21, 488. [Google Scholar]
- Mheni, N.T.; Kilasi, N.; Quiloy, F.A.; Heredia, M.C.; Bilaro, A.; Meliyo, J.; Dixit, S.; Nchimbi Msolla, S. Breeding rice for salinity tolerance and salt-affected soils in Africa: A review. Cogent Food Agric. 2024, 10, 2327666. [Google Scholar]
- Maimaiti, A.; Gu, W.; Yu, D.; Guan, Y.; Qu, J.; Qin, T.; Wang, H.; Ren, J.; Zheng, H.; Wu, P. Dynamic molecular regulation of salt stress responses in maize (Zea mays L.) seedlings. Front. Plant Sci. 2025, 16, 1535943. [Google Scholar]
- El-Metwally, E.A.; El-Deeb, A.A.; Safina, S.A.; Rabbani, B.G. Behavior of some maize hybrids cultivated with different plant densities. J. Plant Prod. 2011, 2, 479–490. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Calif. Agric. Exp. Stn. Circ. 1950, 347, 1–39. [Google Scholar]
- Wolf, B. A comprehensive system of leaf analyses and its use for diagnosing crop nutrient status. Commun. Soil Sci. Plant Anal. 1982, 13, 1035–1059. [Google Scholar]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and carotenoids: Measurement and characterization by UV-VIS spectroscopy. Curr. Prot. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Barrs, H.D.; Weatherley, P.E. A re-examination of the relative turgidity technique for estimating water deficits in leaves. Aust. J. Biol. Sci. 1962, 15, 413–428. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.A.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Du, Z.; Bramlage, W.J. Modified thiobarbituric acid assay for measuring lipid oxidation in sugar-rich plant tissue extracts. J. Agric. Food Chem. 1992, 40, 1566–1570. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar]
- Bergmeyer, H.-U. Methods of Enzymatic Analysis; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Hammerschmidt, R.; Nuckles, E.M.; Kuć, J. Association of enhanced peroxidase activity with induced systemic resistance of cucumber to Colletotrichum lagenarium. Physiol. Plant Pathol. 1982, 20, 73–82. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. Biochem. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Gupta, S.; Schillaci, M.; Walker, R.; Smith, P.M.; Watt, M.; Roessner, U. Alleviation of salinity stress in plants by endophytic plant-fungal symbiosis: Current knowledge, perspectives and future directions. Plant Soil 2021, 461, 219–244. [Google Scholar]
- Huqe, M.A.S.; Haque, M.S.; Sagar, A.; Uddin, M.N.; Hossain, M.A.; Hossain, A.K.M.Z.; Rahman, M.M.; Wang, X.; Al-Ashkar, I.; Ueda, A. Characterization of maize hybrids (Zea mays L.) for detecting salt tolerance based on morpho-physiological characteristics, ion accumulation and genetic variability at early vegetative stage. Plants 2021, 10, 2549. [Google Scholar] [CrossRef]
- Mansour, M.M.F. Role of vacuolar membrane transport systems in plant salinity tolerance. J. Plant Growth Regul. 2023, 42, 1364–1401. [Google Scholar] [CrossRef]
- Shabala, L.; Zhang, J.; Pottosin, I.; Bose, J.; Zhu, M.; Fuglsang, A.T.; Velarde-Buendia, A.; Massart, A.; Hill, C.B.; Roessner, U. Cell-type-specific H+-ATPase activity in root tissues enables K+ retention and mediates acclimation of barley (Hordeum vulgare) to salinity stress. Plant Physiol. 2016, 172, 2445–2458. [Google Scholar]
- Wang, M.; Zheng, Q.; Shen, Q.; Guo, S. The critical role of potassium in plant stress response. Int. J. Mol. Sci. 2013, 14, 7370–7390. [Google Scholar] [CrossRef]
- Kholova, J.; Sairam, R.; Meena, R.; Srivastava, G. Response of maize genotypes to salinity stress in relation to osmolytes and metal-ions contents, oxidative stress and antioxidant enzymes activity. Biol. Plant. 2009, 53, 249–256. [Google Scholar]
- Shabala, S.; Munns, R. Salinity stress: Physiological constraints and adaptive mechanisms. In Plant Stress Physiology; CABI: Wallingford, UK, 2017; pp. 24–63. [Google Scholar]
- de Azevedo Neto, A.D.; Prisco, J.T.; Enéas-Filho, J.; de Abreu, C.E.B.; Gomes-Filho, E. Effect of salt stress on antioxidative enzymes and lipid peroxidation in leaves and roots of salt-tolerant and salt-sensitive maize genotypes. Environ. Exp. Bot. 2006, 56, 87–94. [Google Scholar]
- Abdelaziz, M.N.; Xuan, T.D.; Mekawy, A.M.M.; Wang, H.; Khanh, T.D. Relationship of salinity tolerance to Na+ exclusion, proline accumulation, and antioxidant enzyme activity in rice seedlings. Agriculture 2018, 8, 166. [Google Scholar] [CrossRef]
- Elsawy, H.I.A.; Mekawy, A.M.M.; Elhity, M.A.; Abdel-Dayem, S.M.; Abdelaziz, M.N.; Assaha, D.V.M.; Ueda, A.; Saneoka, H. Differential responses of two Egyptian barley (Hordeum vulgare L.) cultivars to salt stress. Plant Physiol. Biochem. 2018, 127, 425–435. [Google Scholar]
- Acosta-Motos, J.R.; Ortuño, M.F.; Bernal-Vicente, A.; Diaz-Vivancos, P.; Sanchez-Blanco, M.J.; Hernandez, J.A. Plant responses to salt stress: Adaptive mechanisms. Agronomy 2017, 7, 18. [Google Scholar] [CrossRef]
- Zamani, E.; Bakhtari, B.; Razi, H.; Hildebrand, D.; Moghadam, A.; Alemzadeh, A. Comparative morphological, physiological, and biochemical traits in sensitive and tolerant maize genotypes in response to salinity and pb stress. Sci. Rep. 2024, 14, 31036. [Google Scholar]
- Ashraf, M.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar]
- ElShamey, E.A.; Sakran, R.M.; ElSayed, M.A.; Aloufi, S.; Alharthi, B.; Alqurashi, M.; Mansour, E.; Abd El-Moneim, D. Heterosis and combining ability for floral and yield characters in rice using cytoplasmic male sterility system. Saudi J. Biol. Sci. 2022, 29, 3727–3738. [Google Scholar]
- Salem, T.; Rabie, H.; Mowafy, S.; Eissa, A.; Mansour, E. Combining ability and genetic components of Egyptian cotton for earliness, yield, and fiber quality traits. Sabrao J. Breed. Genet. 2020, 52, 369–389. [Google Scholar]
- Swailam, M.; Mowafy, S.; El-Naggar, N.; Mansour, E. Agronomic responses of diverse bread wheat genotypes to phosphorus levels and nitrogen forms in a semiarid environment. Sabrao J. Breed. Genet. 2021, 53, 592–608. [Google Scholar]
- Singh, A.; Kumar, A.; Kumar, R.; Sheoran, P.; Yadav, R.K.; Sharma, P.C. Multivariate analyses discern shared and contrasting eco-physiological responses to salinity stress of Ziziphus rootstocks and budded trees. S. Afr. J. Bot. 2022, 146, 573–584. [Google Scholar]
- Omar, M.; Rabie, H.A.; Mowafi, S.A.; Othman, H.T.; El-Moneim, D.A.; Alharbi, K.; Mansour, E.; Ali, M.M. Multivariate analysis of agronomic traits in newly developed maize hybrids grown under different agro-environments. Plants 2022, 11, 1187. [Google Scholar] [CrossRef]
- Habibullah, M.; Sarkar, S.; Islam, M.M.; Ahmed, K.U.; Rahman, M.Z.; Awad, M.F.; ElSayed, A.I.; Mansour, E.; Hossain, M.S. Assessing the response of diverse sesame genotypes to waterlogging durations at different plant growth stages. Plants 2021, 10, 2294. [Google Scholar] [CrossRef]
- Patwa, N.; Pandey, V.; Gupta, O.P.; Yadav, A.; Meena, M.R.; Ram, S.; Singh, G. Unravelling wheat genotypic responses: Insights into salinity stress tolerance in relation to oxidative stress, antioxidant mechanisms, osmolyte accumulation and grain quality parameters. BMC Plant Biol. 2024, 24, 875. [Google Scholar]



| Genes | Gene ID | Forward | Reverse |
|---|---|---|---|
| zmHKT2 | XM_008646809 | 5′-GCTCAACTTCTCCACGTTCA-3′ | 5′-CACCACCCAGAGAAGCTGTA-3′ |
| zmHKT1;5 | NM_001175105 | 5′-TCAACTTCAGCGTCCTCAACA-3′ | 5′-GAATCCCACGTTGCCATACG-3′ |
| zmNHX1 | AY270036 | 5′-ATGCAGGGTTCCAAGTGAAG-3′ | 5′-AATATTGCCCCAAGTGCAAG-3′ |
| zmActin | NM_001155179 | 5′-GACCTCACCGACCACCTAATG -3′ | 5′-CTGAACCTTTCTGACCCAATG-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rehan, M.; Kamara, M.M.; Barakat, H. Comparative Analysis of Physiological Parameters, Antioxidant Defense, Ion Regulation, and Gene Expression in Two Distinct Maize Hybrids Under Salt Stress at Seedling Stage. Life 2025, 15, 591. https://doi.org/10.3390/life15040591
Rehan M, Kamara MM, Barakat H. Comparative Analysis of Physiological Parameters, Antioxidant Defense, Ion Regulation, and Gene Expression in Two Distinct Maize Hybrids Under Salt Stress at Seedling Stage. Life. 2025; 15(4):591. https://doi.org/10.3390/life15040591
Chicago/Turabian StyleRehan, Medhat, Mohamed M. Kamara, and Hassan Barakat. 2025. "Comparative Analysis of Physiological Parameters, Antioxidant Defense, Ion Regulation, and Gene Expression in Two Distinct Maize Hybrids Under Salt Stress at Seedling Stage" Life 15, no. 4: 591. https://doi.org/10.3390/life15040591
APA StyleRehan, M., Kamara, M. M., & Barakat, H. (2025). Comparative Analysis of Physiological Parameters, Antioxidant Defense, Ion Regulation, and Gene Expression in Two Distinct Maize Hybrids Under Salt Stress at Seedling Stage. Life, 15(4), 591. https://doi.org/10.3390/life15040591








