Comparative Analysis of the ELISIO-HX and Xevonta-Hi Dialyzers in Standard Hemodialysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion Criteria
2.2. Dialysis Procedure
2.3. Laboratory Analysis
2.4. Statistical Analysis
3. Results
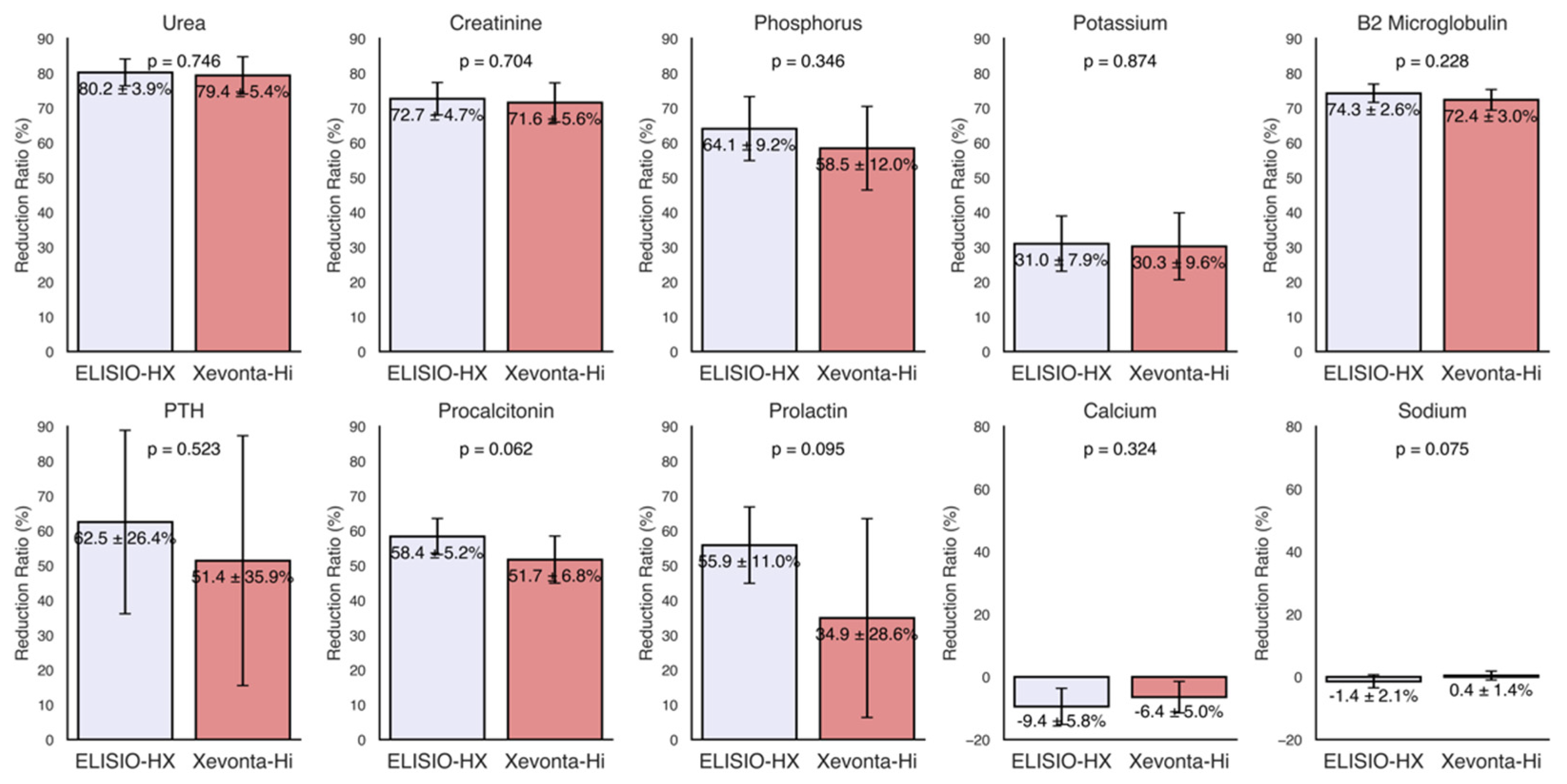
3.1. Small Molecules
3.2. Middle Molecules
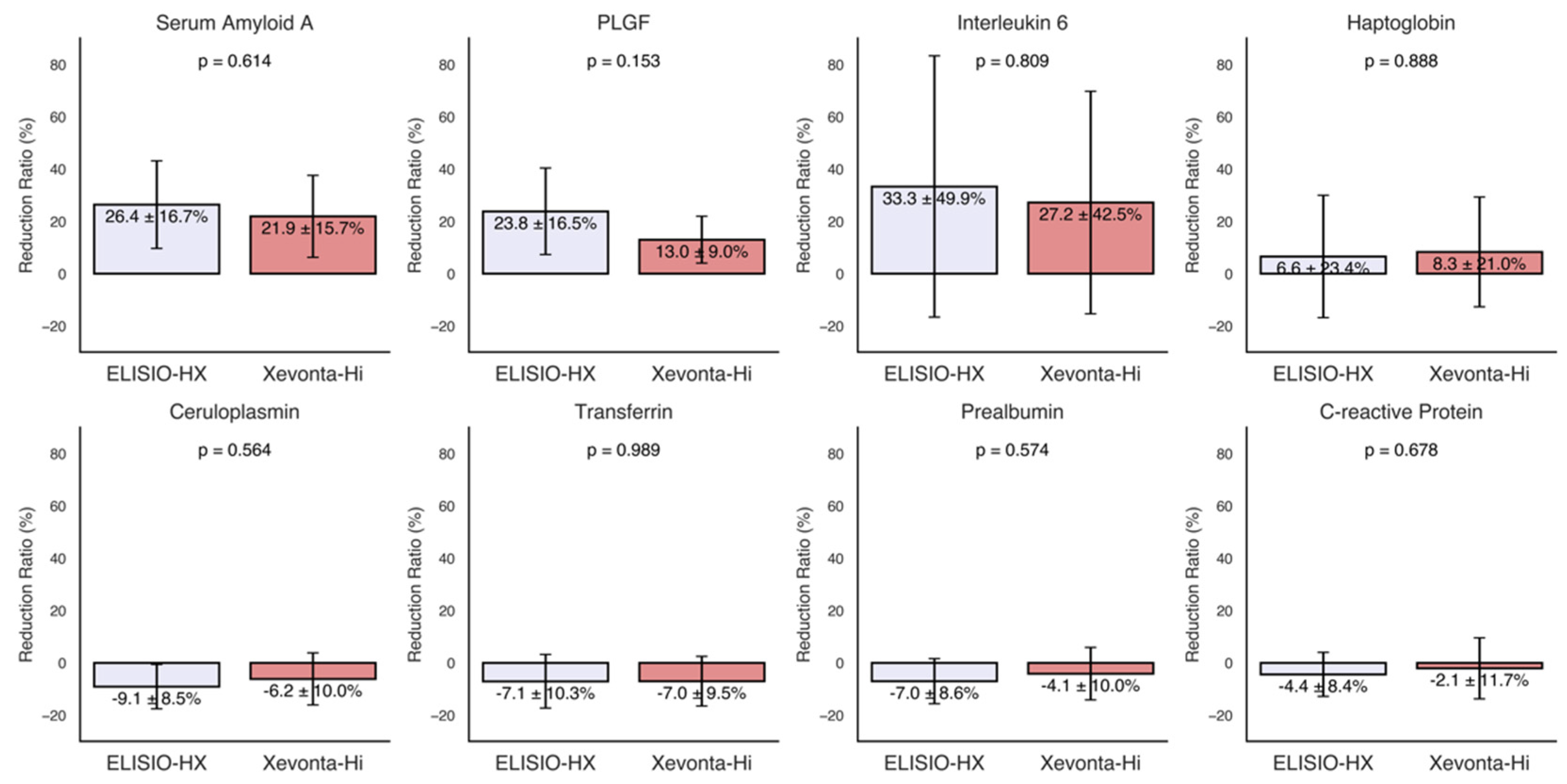
3.3. Inflammatory Markers
3.4. Albumin and Total Protein Loss
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| β2M | β2-Microglobulin |
| CKD | Chronic kidney disease |
| CRP | C-reactive protein |
| HDx | Expanded hemodialysis |
| IL-6 | Interleukin-6 |
| MCO | Medium cutoff |
| PTH | Parathyroid hormone |
| PLGF | Placental-like growth factor |
| OL-HDF | Online hemodiafiltration |
References
- Francis, A.; Harhay, M.N.; Ong, A.C.M.; Tummalapalli, S.L.; Fogo, A.B.; Fliser, D.; Roy-Chaudhury, P.; Fontana, M.; Nangaku, M.; Wanner, C.; et al. Chronic kidney disease and the global public health agenda: An international consensus. Nat. Rev. Nephrol. 2024, 20, 473–485. [Google Scholar] [PubMed]
- Bowry, S.K.; Chazot, C. The scientific principles and technological determinants of haemodialysis membranes. Clin. Kidney J. 2021, 14, i5–i16. [Google Scholar] [CrossRef] [PubMed]
- Basile, C.; Davenport, A.; Mitra, S.; Pal, A.; Stamatialis, D.; Chrysochou, C.; Kirmizis, D. Frontiers in hemodialysis: Innovations and technological advances. Artif. Organs 2021, 45, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Nazari, S.; Abdelrasoul, A. Impact of membrane modification and surface immobilization techniques on the hemocompatibility of hemodialysis membranes: A critical review. Membranes 2022, 12, 1063. [Google Scholar] [CrossRef]
- Blankestijn, P.J.; Vernooij, R.W.M.; Hockham, C.; Strippoli, G.F.; Canaud, B.; Hegbrant, J.; Barth, C.; Covic, A.; Cromm, K.; Cucui, A.; et al. Effect of hemodiafiltration or hemodialysis on mortality in kidney failure. N. Engl. J. Med. 2023, 389, 700–709. [Google Scholar]
- See, E.J.; Hedley, J.; Agar, J.W.M.; Hawley, C.M.; Johnson, D.W.; Kelly, P.J.; Lee, V.W.; Mac, K.; Polkinghorne, K.R.; Rabindranath, K.S.; et al. Patient survival on haemodiafiltration and haemodialysis: A cohort study using the Australia and New Zealand Dialysis and Transplant Registry. Nephrol. Dial. Transplant. 2019, 34, 326–338. [Google Scholar] [CrossRef]
- Maduell, F.; Broseta, J.J.; Rodas, L.; Montagud-Marrahi, E.; Rodriguez-Espinosa, D.; Hermida, E.; Arias-Guillén, M.; Fontseré, N.; Vera, M.; Gómez, M.; et al. Comparison of Solute Removal Properties Between High-Efficient Dialysis Modalities in Low Blood Flow Rate. Ther. Apher. Dial. 2020, 24, 387–392. [Google Scholar]
- Torreggiani, M.; Piccoli, G.B.; Moio, M.R.; Conte, F.; Magagnoli, L.; Ciceri, P.; Cozzolino, M. Choice of the dialysis modality: Practical considerations. J. Clin. Med. 2023, 12, 3328. [Google Scholar] [CrossRef]
- Canaud, B.; Köhler, K.; Sichart, J.-M.; Möller, S. Global prevalent use, trends and practices in haemodiafiltration. Nephrol. Dial. Transplant. 2020, 35, 398–407. [Google Scholar]
- Zweigart, C.; Boschetti-de-Fierro, A.; Hulko, M.; Nilsson, L.-G.; Beck, W.; Storr, M.; Krause, B. Medium cut-off membranes—Closer to the natural kidney removal function. Int. J. Artif. Organs 2017, 40, 328–334. [Google Scholar]
- Molano, A.P.; Hutchison, C.A.; Sanchez, R.; Rivera, A.S.; Buitrago, G.; Dazzarola, M.P.; Munevar, M.; Guerrero, M.; Vesga, J.I.; Sanabria, M. Medium Cutoff Versus High-Flux Hemodialysis Membranes and Clinical Outcomes: A Cohort Study Using Inverse Probability Treatment Weighting. Kidney Med. 2022, 4, 100431. [Google Scholar] [PubMed]
- Ronco, C. The rise of expanded hemodialysis. Blood Purif. 2017, 44, I–VIII. [Google Scholar] [CrossRef]
- Jonny, J.; Teressa, M. Expanded hemodialysis: A new concept of renal replacement therapy. J. Investig. Med. 2023, 71, 38–41. [Google Scholar]
- Mares, J.; Kielberger, L.; Klaboch, J. Fp529comparative performance and biocompatibility assessment study of a new high-flux dialyzer xevonta®. Nephrol. Dial. Transplant. 2015, 30, iii249. [Google Scholar]
- Maduell, F.; Broseta, J.J.; Rodríguez-Espinosa, D.; Del Risco-Zevallos, J.; Gómez, M.; Rodas, L.M.; Arias-Guillén, M.; Vera, M.; Fontseré, N.; Salgado, M.d.C.; et al. Efficacy and Safety of the Medium Cut-Off ELISIO-HX Dialyzer. Blood Purif. 2023, 52, 68–74. [Google Scholar]
- Santos García, A.; Macías Carmona, N.; Vega Martínez, A.; Estébanez, S.A.; Grávalos, T.L.; Sauco, I.A.; Guzmán, U.V.; González, N.P.; Vega, L.C.; Gómez, J.M.L. Removal capacity of different high-flux dialyzers during postdilution online hemodiafiltration. Hemodial. Int. 2019, 23, 50–57. [Google Scholar]
- Maduell, F.; Rodas, L.; Broseta, J.J.; Gomez, M.; Xipell, M.; Guillen, E.; Montagud-Marrahi, E.; Arias-Guillén, M.; Fontseré, N.; Vera, M.; et al. Medium Cut-Off Dialyzer versus Eight Hemodiafiltration Dialyzers: Comparison Using a Global Removal Score. Blood Purif. 2019, 48, 167–174. [Google Scholar]
- Formanowicz, D.; Formanowicz, P. Transferrin changes in haemodialysed patients. Int. Urol. Nephrol. 2012, 44, 907–919. [Google Scholar]
- Panichi, V.; Taccola, D.; Rizza, G.M.; Consani, C.; Migliori, M.; Filippi, C.; Paoletti, S.; Sidoti, A.; Borracelli, D.; Panicucci, E.; et al. Ceruloplasmin and acute phase protein levels are associated with cardiovascular disease in chronic dialysis patients. J. Nephrol. 2004, 17, 715–720. [Google Scholar]
- Minović, I.; Eisenga, M.F.; Riphagen, I.J.; van den Berg, E.; Kootstra-Ros, J.; Frenay, A.-R.S.; Van Goor, H.; Rimbach, G.; Esatbeyoglu, T.; Levy, A.P.; et al. Circulating haptoglobin and metabolic syndrome in renal transplant recipients. Sci. Rep. 2017, 7, 14264. [Google Scholar]
- Chrysostomou, S.; Stathakis, C.; Petrikkos, G.; Daikos, G.; Gompou, A.; Perrea, D. Assessment of prealbumin in hemodialysis and renal-transplant patients. J. Ren. Nutr. 2010, 20, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Honda, H.; Qureshi, A.R.; Heimbürger, O.; Barany, P.; Wang, K.; Pecoits-Filho, R.; Stenvinkel, P.; Lindholm, B. Serum albumin, C-reactive protein, interleukin 6, and fetuin a as predictors of malnutrition, cardiovascular disease, and mortality in patients with ESRD. Am. J. Kidney Dis. 2006, 47, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Zakiyanov, O.; Kalousová, M.; Zima, T.; Tesař, V. Placental growth factor in patients with decreased renal function. Ren. Fail. 2011, 33, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Honkanen, E.; Grönhagen-Riska, C.; Teppo, A.M.; Maury, C.; Meri, S. Acute-phase proteins during hemodialysis: Correlations with serum interleukin-1 beta levels and different dialysis membranes. Nephron 1991, 57, 283–287. [Google Scholar] [CrossRef]
- Hulko, M.; Haug, U.; Gauss, J.; Boschetti-De-Fierro, A.; Beck, W.; Krause, B. Requirements and pitfalls of dialyzer sieving coefficients comparisons. Artif. Organs 2018, 42, 1164–1173. [Google Scholar] [CrossRef]
- Kirsch, A.H.; Lyko, R.; Nilsson, L.-G.; Beck, W.; Amdahl, M.; Lechner, P.; Schneider, A.; Wanner, C.; Rosenkranz, A.R.; Krieter, D.H. Performance of hemodialysis with novel medium cut-off dialyzers. Nephrol. Dial. Transplant. 2017, 32, 165–172. [Google Scholar] [CrossRef]
- Belmouaz, M.; Bauwens, M.; Hauet, T.; Bossard, V.; Jamet, P.; Joly, F.; Chikhi, E.; Joffrion, S.; Gand, E.; Bridoux, F. Comparison of the removal of uraemic toxins with medium cut-off and high-flux dialysers: A randomized clinical trial. Nephrol. Dial. Transplant. 2020, 35, 328–335. [Google Scholar] [CrossRef]
- Vega-Vega, O.; Caballero-Islas, A.E.; Del Toro-Cisneros, N.; Hernandez-Ordoñez, S.Ó.; Arvizu-Hernández, M.; Martínez-Rueda, A.; Camacho-Colin, D.; Gómez-Correa, L.L.; Correa-Rotter, R. Improved β2-Microglobulin and Phosphorous Removal with Expanded Hemodialysis and Online Hemodiafiltration versus High-Flux Hemodialysis: A Cross-Over Randomized Clinical Trial. Blood Purif. 2023, 52, 712–720. [Google Scholar] [CrossRef]
- Himmelfarb, J. Uremic toxicity, oxidative stress, and hemodialysis as renal replacement therapy. Semin. Dial. 2009, 22, 636–643. [Google Scholar] [CrossRef]
- Ling, X.C.; Kuo, K.-L. Oxidative stress in chronic kidney disease. Ren. Replace. Ther. 2018, 4, 53. [Google Scholar] [CrossRef]
- Zickler, D.; Schindler, R.; Willy, K.; Martus, P.; Pawlak, M.; Storr, M.; Hulko, M.; Boehler, T.; Glomb, M.A.; Liehr, K.; et al. Medium Cut-Off (MCO) Membranes Reduce Inflammation in Chronic Dialysis Patients-A Randomized Controlled Clinical Trial. PLoS ONE 2017, 12, e0169024. [Google Scholar]
- Lim, J.-H.; Jeon, Y.; Yook, J.-M.; Choi, S.-Y.; Jung, H.-Y.; Choi, J.-Y.; Park, S.-H.; Kim, C.-D.; Kim, Y.-L.; Cho, J.-H. Medium cut-off dialyzer improves erythropoiesis stimulating agent resistance in a hepcidin-independent manner in maintenance hemodialysis patients: Results from a randomized controlled trial. Sci. Rep. 2020, 10, 16062. [Google Scholar]
- Ficheux, A.; Gayrard, N.; Szwarc, I.; Andress, D.; Soullier, S.; Duny, Y.; Goubert, G.; Thomas, M.; Bismuth-Mondolfo, J.; Daures, J.-P.; et al. The use of SDS-PAGE scanning of spent dialysate to assess uraemic toxin removal by dialysis. Nephrol. Dial. Transplant. 2011, 26, 2281–2289. [Google Scholar] [CrossRef]
- Potier, J.; Queffeulou, G.; Bouet, J. Are all dialyzers compatible with the convective volumes suggested for postdilution online hemodiafiltration? Int. J. Artif. Organs 2016, 39, 460–470. [Google Scholar] [CrossRef]

| Patient | Age | Sex | CKD Etiology | DM | HT | ND |
|---|---|---|---|---|---|---|
| 1 | 64 | M | IgA Nephropathy | Yes | Yes | No |
| 2 | 55 | F | ANCA + Vasculitis | No | Yes | No |
| 3 | 40 | M | Not determined | No | Yes | No |
| 4 | 54 | F | Alport syndrome | No | Yes | No |
| 5 | 37 | F | Not determined | No | Yes | No |
| 6 | 19 | F | Goodpasture syndrome | No | Yes | No |
| 7 | 67 | F | Amyloidosis | No | Yes | No |
| Week | Patient | Duration | Heparin Dosage | Kt/v | Vascular Access Flow Rate | Vascular Access Type |
|---|---|---|---|---|---|---|
| 1 | 1 | 4 h | Enoxaparin 20 mg | 1.38 | 300 mL/min | Arteriovenous Fistula |
| 1 | 2 | 3:30 h | Enoxaparin 40 mg | 1.64 | 340 mL/min | Arteriovenous Fistula |
| 1 | 3 | 4 h | Enoxaparin 20 mg | 1.13 | 350 mL/min | Central Venous Catheter |
| 1 | 4 | 4 h | Enoxaparin 40 mg | 1.26 | 330 mL/min | Prosthetic |
| 1 | 5 | 4 h | Enoxaparin 40 mg | 1.52 | 340 mL/min | Central Venous Catheter |
| 1 | 6 | 4 h | Enoxaparin 20 mg | 1.65 | 330 mL/min | Central Venous Catheter |
| 1 | 7 | 4 h | Sodium heparin 10 mg + 1 × 5 mg | 2.04 | 350 mL/min | Central Venous Catheter |
| 2 | 1 | 4 h | Enoxaparin 20 mg | 1.53 | 340 mL/min | Arteriovenous Fistula |
| 2 | 2 | 3:30 h | Enoxaparin 40 mg | 1.68 | 340 mL/min | Arteriovenous Fistula |
| 2 | 3 | 4 h | Enoxaparin 20 mg | 1.19 | 350 mL/min | Central Venous Catheter |
| 2 | 4 | 4 h | Enoxaparin 20 mg | 1.26 | 330 mL/min | Prosthetic |
| 2 | 5 | 4 h | Enoxaparin 40 mg | 1.63 | 340 mL/min | Central Venous Catheter |
| 2 | 6 | 4 h | Enoxaparin 20 mg | 1.65 | 340 mL/min | Central Venous Catheter |
| 2 | 7 | 4 h | Sodium heparin 10 mg + 1 × 5 mg | 1.91 | 350 mL/min | Central Venous Catheter |
| 3 | 1 | 4 h | Enoxaparin 20 mg | 1.43 | 340 mL/min | Arteriovenous Fistula |
| 3 | 2 | 3:30 h | Enoxaparin 40 mg | 1.71 | 340 mL/min | Arteriovenous Fistula |
| 3 | 3 | 4 h | Enoxaparin 20 mg | 1.39 | 340 mL/min | Central Venous Catheter |
| 3 | 4 | 4 h | Enoxaparin 20 mg | 1.54 | 340 mL/min | Prosthetic |
| 3 | 5 | 3:50 h | Enoxaparin 40 mg | 1.62 | 340 mL/min | Central Venous Catheter |
| 3 | 6 | 4 h | Enoxaparin 20 mg | 1.65 | 340 mL/min | Central Venous Catheter |
| 3 | 7 | 4 h | Sodium heparin 10 mg + 3 × 5 mg | 1.86 | 340 mL/min | Central Venous Catheter |
| 4 | 1 | 4 h | Enoxaparin 20 mg | 1.4 | 300 mL/min | Arteriovenous Fistula |
| 4 | 2 | 3:30 h | Enoxaparin 40 mg | 1.62 | 320 mL/min | Arteriovenous Fistula |
| 4 | 3 | 4 h | Enoxaparin 20 mg | 1.39 | 340 mL/min | Central Venous Catheter |
| 4 | 4 | 4 h | Enoxaparin 20 mg | 1.47 | 320 mL/min | Prosthetic |
| 4 | 5 | 4 h | Enoxaparin 40 mg | 1.68 | 340 mL/min | Central Venous Catheter |
| 4 | 6 | 4 h | Enoxaparin 20 mg | 1.62 | 340 mL/min | Central Venous Catheter |
| 4 | 7 | 4 h | Sodium heparin 10 mg + 1 × 5 mg | 1.89 | 340 mL/min | Central Venous Catheter |
| Characteristic | ELISIO-HX 21 | Xevonta-Hi 20 |
|---|---|---|
| Membrane Material | Polynephron™ (Polyethersulfone, BPA-free) | Amembris™ (Polysulfone) |
| Surface Area (m2) | 2.1 | 2.0 |
| Inner Fiber Diameter (µm) | 200 | 195 |
| Membrane Thickness (µm) | 40 | 35 |
| Ultrafiltration Coefficient (mL/h/mmHg) | 82 | 111 |
| Sterilization Method | Dry, oxygen-free gamma | Dry, oxygen-free gamma |
| Parameter | ELISIO-HX Pre Avg ± SD | Xevonta-Hi Pre Avg ± SD | p-Value Mann–Whitney’s U test | Interpretation 95% CI |
|---|---|---|---|---|
| Urea (mg/dL) | 118.00 ± 17.00 | 127.07 ± 28.13 | 0.6540 | ns |
| Creatinine (mg/dL) | 10.14 ± 1.19 | 10.26 ± 1.12 | 0.8048 | ns |
| Phosphorus (mg/dL) | 4.82 ± 0.93 | 4.11 ± 1.23 | 0.3374 | ns |
| Potassium (mEq/L) | 4.90 ± 0.57 | 4.69 ± 0.55 | 0.5649 | ns |
| Sodium (mEq/L) | 136.36 ± 3.08 | 136.57 ± 2.88 | 1.0000 | ns |
| Calcium (mg/dL) | 9.23 ± 0.32 | 9.20 ± 0.43 | 0.9489 | ns |
| B2 Microglobulin (mg/L) | 25.74 ± 5.73 | 26.52 ± 5.64 | 0.8048 | ns |
| PTH (pg/mL) | 751.55 ± 822.07 | 400.24 ± 186.40 | 0.5350 | ns |
| Procalcitonin (ng/mL) | 0.42 ± 0.26 | 0.53 ± 0.29 | 0.4052 | ns |
| Prolactin (ng/mL) | 59.58 ± 96.25 | 42.44 ± 65.91 | 0.5350 | ns |
| Serum Amyloid A (mg/L) | 21.66 ± 30.58 | 44.31 ± 63.20 | 0.6200 | ns |
| PLGF (pg/mL) | 31.00 ± 7.52 | 33.51 ± 9.68 | 0.7104 | ns |
| IL-6 (pg/mL) | 13.60 ± 19.09 | 12.75 ± 19.20 | 0.6200 | ns |
| Haptoglobin (mg/dL) | 110.43 ± 62.21 | 118.00 ± 68.23 | 0.9015 | ns |
| Ceruloplasmin (mg/dL) | 21.59 ± 5.01 | 21.13 ± 4.17 | 0.8478 | ns |
| Transferrin (mg/dL) | 181.93 ± 32.94 | 185.71 ± 36.61 | 0.9015 | ns |
| Pre-Albumin (mg/dL) | 28.66 ± 7.20 | 29.86 ± 7.03 | 0.7981 | ns |
| CRP (mg/L) | 10.58 ± 15.43 | 9.69 ± 11.36 | 1.0000 | ns |
| Albumin (g/dL) | 3.81 ± 0.50 | 3.97 ± 0.57 | 0.6085 | ns |
| Total Protein (g/dL) | 6.23 ± 0.59 | 6.44 ± 0.68 | 0.4817 | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villacorta Linaza, B.; Román Cabezas, M.; Sánchez-Pozo, M.C.; Alcaide Lara, M.P.; Cabra-Rodríguez, R.; Toro Prieto, F.J. Comparative Analysis of the ELISIO-HX and Xevonta-Hi Dialyzers in Standard Hemodialysis. Life 2025, 15, 596. https://doi.org/10.3390/life15040596
Villacorta Linaza B, Román Cabezas M, Sánchez-Pozo MC, Alcaide Lara MP, Cabra-Rodríguez R, Toro Prieto FJ. Comparative Analysis of the ELISIO-HX and Xevonta-Hi Dialyzers in Standard Hemodialysis. Life. 2025; 15(4):596. https://doi.org/10.3390/life15040596
Chicago/Turabian StyleVillacorta Linaza, Blanca, Mario Román Cabezas, María Cristina Sánchez-Pozo, María Paz Alcaide Lara, Rocío Cabra-Rodríguez, and Francisco Javier Toro Prieto. 2025. "Comparative Analysis of the ELISIO-HX and Xevonta-Hi Dialyzers in Standard Hemodialysis" Life 15, no. 4: 596. https://doi.org/10.3390/life15040596
APA StyleVillacorta Linaza, B., Román Cabezas, M., Sánchez-Pozo, M. C., Alcaide Lara, M. P., Cabra-Rodríguez, R., & Toro Prieto, F. J. (2025). Comparative Analysis of the ELISIO-HX and Xevonta-Hi Dialyzers in Standard Hemodialysis. Life, 15(4), 596. https://doi.org/10.3390/life15040596






