Gut Microbiota: A New Challenge in Mood Disorder Research
Abstract
1. Introduction
2. Gut Microbiota and Mental Health
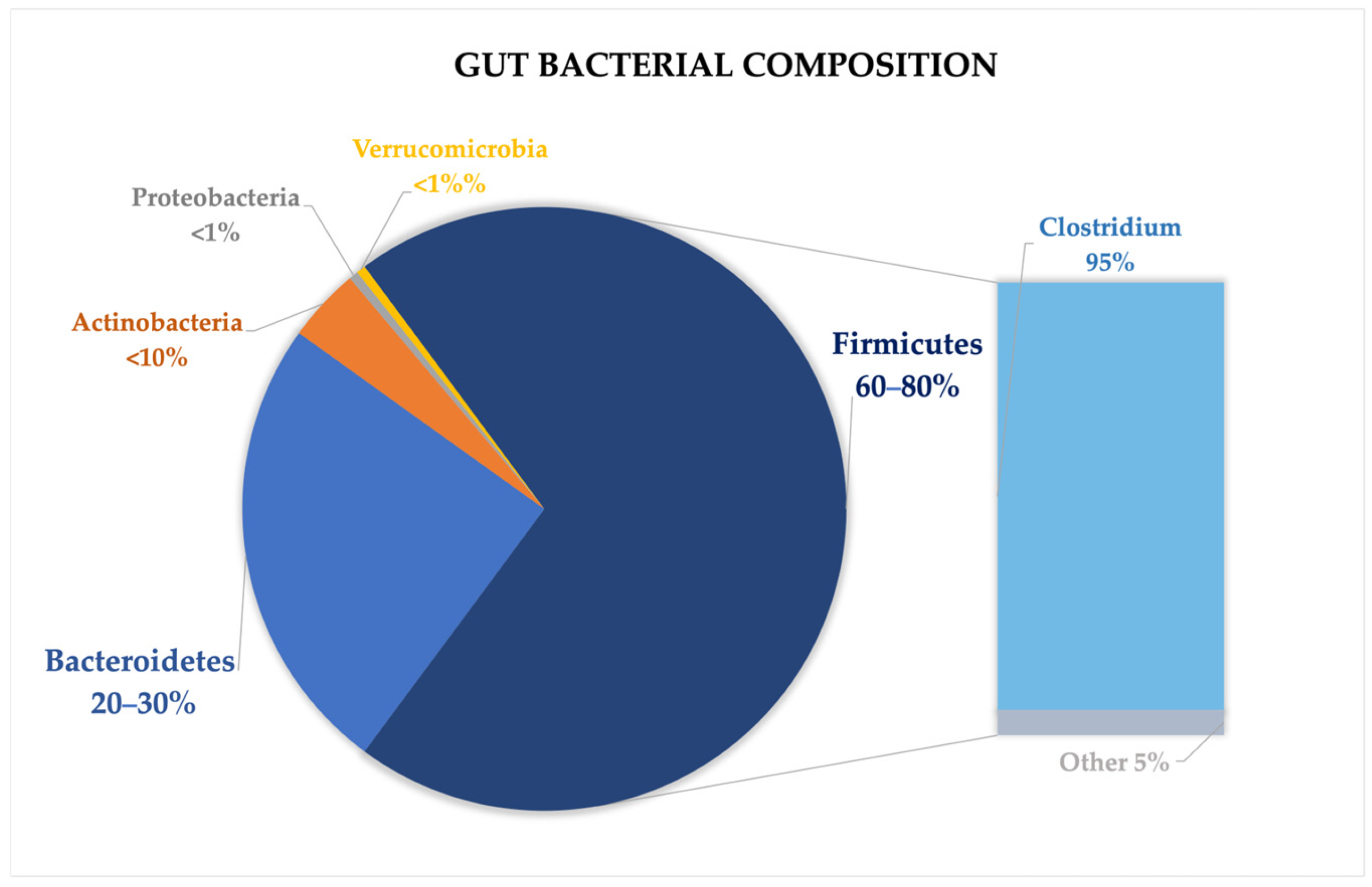
2.1. Gut Microbiota Composition
2.2. Microbiota Inhabiting Different Segments of Gastrointestinal Tract
2.3. Lifestyle and Diet
2.4. Delivery and Milk Feeding
2.5. Lifetime Changes
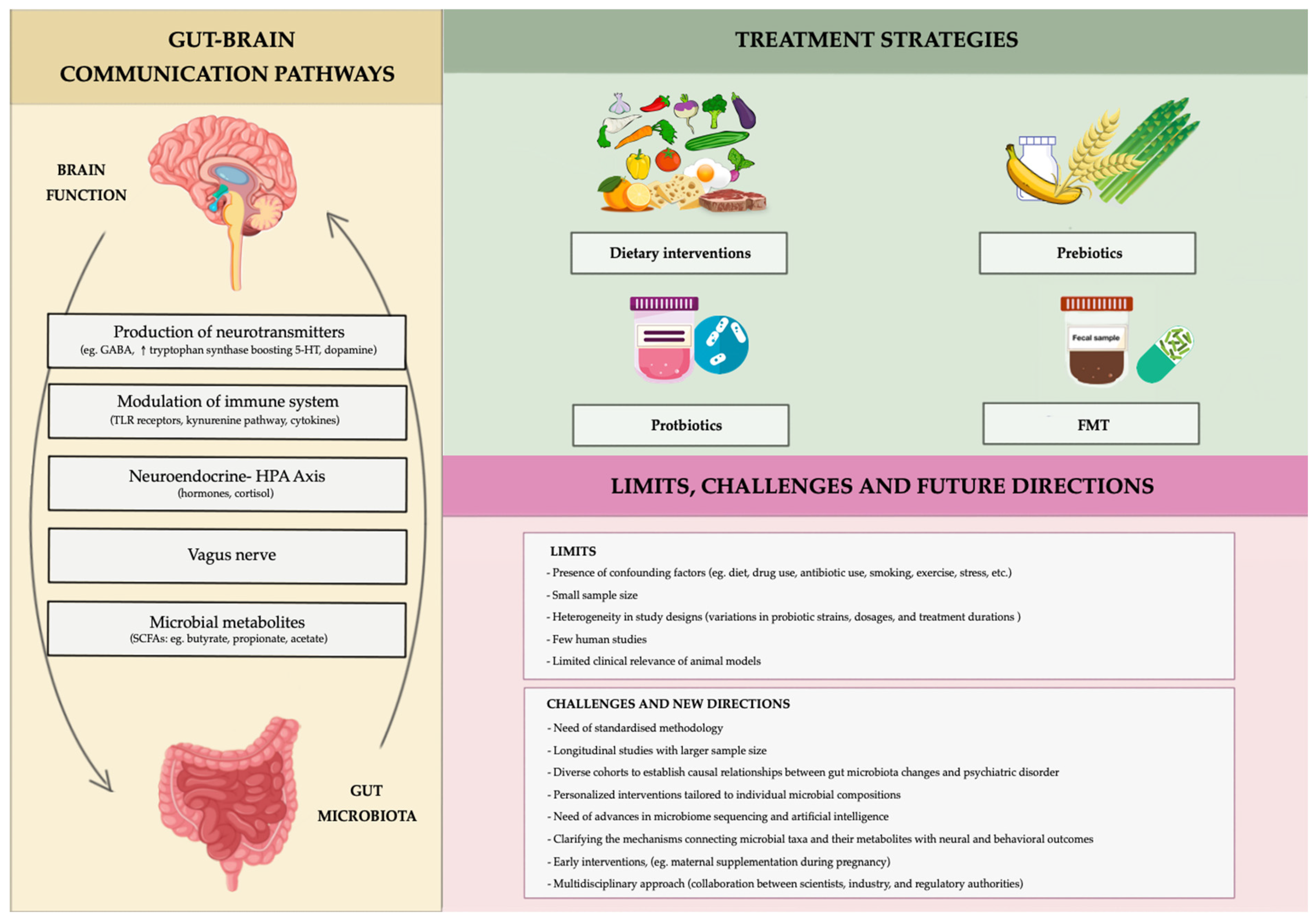
3. The Role of the Microbiota in Regulating the Gut–Brain Axis
4. Modulating Mood Through the Gut: The Role of the Microbiota
5. Therapeutic Approaches
5.1. Diet and Prebiotics
5.2. Psychobiotics
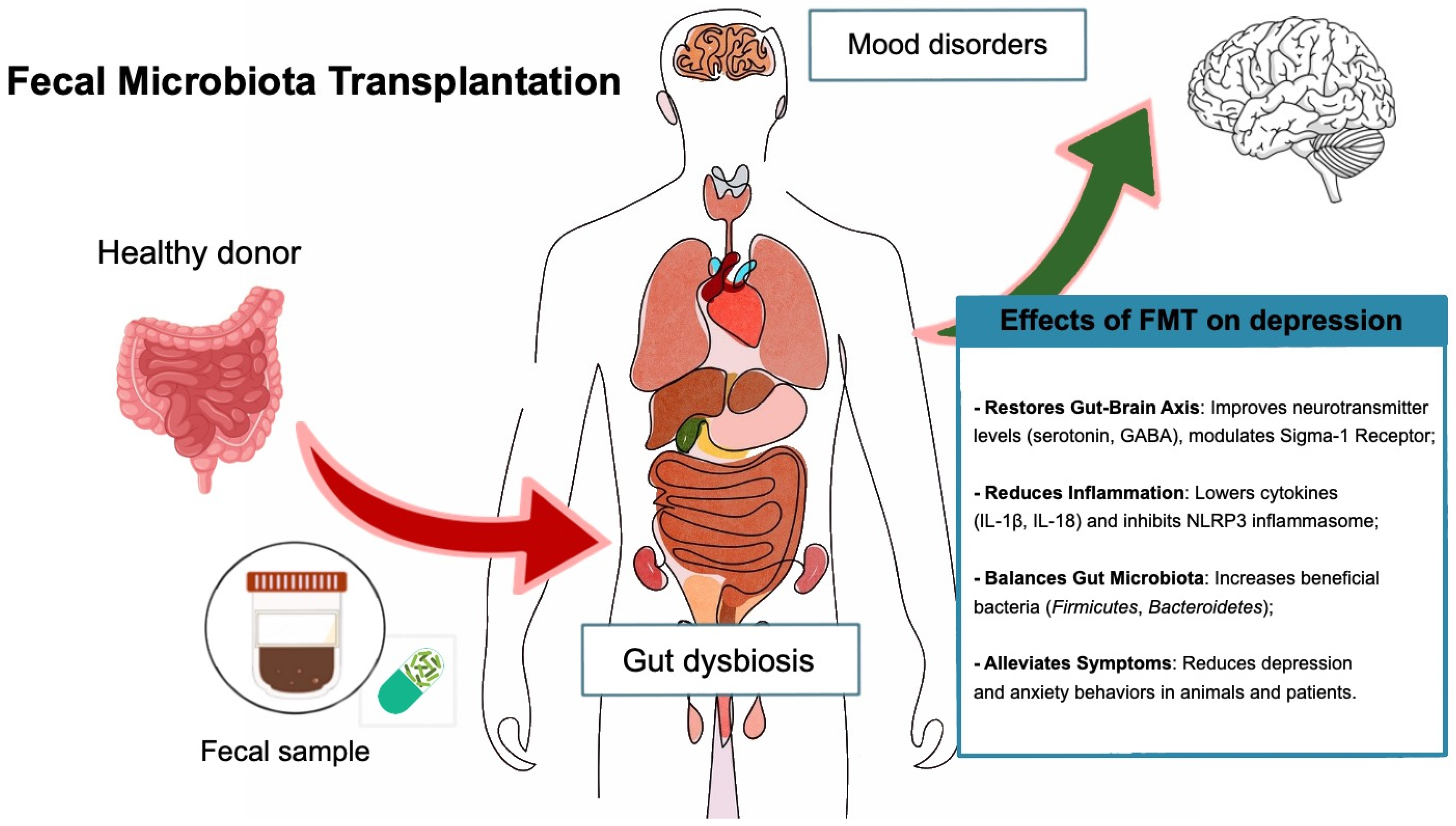
5.3. Fecal Microbiota Transplantation
5.4. New Strategies for Modulating the Gut Microbiota in Mental Health
6. Conclusions, Limitations and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Thursby, E.; Juge, N. Introduction to the human gut microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef] [PubMed]
- Cresci, G.A.; Bawden, E. Gut Microbiome. Nutr. Clin. Pract. 2015, 30, 734–746. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Zhao, Z.; Wang, W.; Liu, X. Bifidobacterium Longum: Protection against Inflammatory Bowel Disease. J. Immunol. Res. 2021, 2021, 8030297. [Google Scholar] [CrossRef] [PubMed]
- Fernández, J.; Saettone, P.; Franchini, M.C.; Villar, C.J.; Lombó, F. Antitumor bioactivity and gut microbiota modulation of polyhydroxybutyrate (PHB) in a rat animal model for colorectal cancer. Int. J. Biol. Macromol. 2022, 203, 638–649. [Google Scholar] [CrossRef] [PubMed]
- Toader, C.; Dobrin, N.; Costea, D.; Glavan, L.A.; Covache-Busuioc, R.A.; Dumitrascu, D.I.; Bratu, B.G.; Costin, H.P.; Ciurea, A.V. Mind, Mood and Microbiota-Gut-Brain Axis in Psychiatric Disorders. Int. J. Mol. Sci. 2024, 25, 3340. [Google Scholar] [CrossRef]
- Chen, M.; Ruan, G.; Chen, L.; Ying, S.; Li, G.; Xu, F.; Xiao, Z.; Tian, Y.; Lv, L.; Ping, Y.; et al. Neurotransmitter and Intestinal Interactions: Focus on the Microbiota-Gut-Brain Axis in Irritable Bowel Syndrome. Front. Endocrinol. 2022, 13, 817100. [Google Scholar] [CrossRef]
- Vuong, H.E.; Hsiao, E.Y. Emerging Roles for the Gut Microbiome in Autism Spectrum Disorder. Biol. Psychiatry 2017, 81, 411–423. [Google Scholar] [CrossRef]
- James, S.L. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef]
- Cryan, J.F.; Dinan, T.G. Mind-altering microorganisms: The impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 2012, 13, 701–712. [Google Scholar] [CrossRef]
- Evans, S.J.; Bassis, C.M.; Hein, R.; Assari, S.; Flowers, S.A.; Kelly, M.B.; Young, V.B.; Ellingrod, V.E.; McInnis, M.G. The gut microbiome composition associates with bipolar disorder and illness severity. J. Psychiatr. Res. 2017, 87, 23–29. [Google Scholar] [CrossRef]
- Zheng, P.; Yang, J.; Li, Y.; Wu, J.; Liang, W.; Yin, B.; Tan, X.; Huang, Y.; Chai, T.; Zhang, H.; et al. Gut Microbial Signatures Can Discriminate Unipolar from Bipolar Depression. Adv. Sci. 2020, 7, 1902862. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Huang, J.; Wang, S.; Zhang, K. Bipolar disorder and the gut microbiota: A bibliometric analysis. Front. Neurosci. 2024, 18, 1290826. [Google Scholar] [CrossRef]
- Li, Z.; Lai, J.; Zhang, P.; Ding, J.; Jiang, J.; Liu, C.; Huang, H.; Zhen, H.; Xi, C.; Sun, Y.; et al. Multi-omics analyses of serum metabolome, gut microbiome and brain function reveal dysregulated microbiota-gut-brain axis in bipolar depression. Mol. Psychiatry 2022, 27, 4123–4135. [Google Scholar] [CrossRef] [PubMed]
- Valle, C.G.-D.; Fernández, J.; Solá, E.; Montoya-Castilla, I.; Morillas, C.; Bañuls, C. Association between gut microbiota and psychiatric disorders: A systematic review. Front. Pshychology 2023, 14, 1215674. [Google Scholar] [CrossRef]
- Clapp, M.; Aurora, N.; Herrera, L.; Bhatia, M.; Wilen, E.; Wakefield, S. Gut Microbiota’s Effect on Mental Health: The Gut-Brain Axis. Clin. Pract. 2017, 7, 987. [Google Scholar] [CrossRef] [PubMed]
- Merlo, G.; Bachtel, G.; Sugden, S.G. Gut microbiota, nutrition, and mental health. Front. Nutr. 2024, 11, 1337889. [Google Scholar] [CrossRef]
- Xiong, R.G.; Li, J.; Cheng, J.; Zhou, D.D.; Wu, S.X.; Huang, S.Y.; Saimaiti, A.; Yang, Z.J.; Gan, R.Y.; Li, H.B. The Role of Gut Microbiota in Anxiety, Depression, and Other Mental Disorders as Well as the Protective Effects of Dietary Components. Nutrients 2023, 15, 3258. [Google Scholar] [CrossRef] [PubMed]
- Gomaa, E.Z. Human gut microbiota/microbiome in health and diseases: A review. Antonie van Leeuwenhoek 2020, 113, 2019–2040. [Google Scholar] [CrossRef]
- García-Montero, C.; Fraile-Martínez, O.; Gómez-Lahoz, A.M.; Pekarek, L.; Castellanos, A.J.; Noguerales-Fraguas, F.; Coca, S.; Guijarro, L.G.; García-Honduvilla, N.; Asúnsolo, A.; et al. Nutritional components in western diet versus mediterranean diet at the gut microbiota-immune system interplay. implications for health and disease. Nutrients 2021, 13, 699. [Google Scholar] [CrossRef]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef]
- Van Hul, M.; Cani, P.D.; Petitfils, C.; De Vos, W.M.; Tilg, H.; El-Omar, E.M. What defines a healthy gut microbiome? Gut 2024, 73, 1893–1908. [Google Scholar] [CrossRef] [PubMed]
- Hou, K.; Wu, Z.X.; Chen, X.Y. Microbiota in health and diseases. Signal Transduct. Target Ther. 2022, 7, 135. [Google Scholar] [CrossRef] [PubMed]
- Berding, K.; Vlckova, K.; Marx, W.; Schellekens, H.; Stanton, C.; Clarke, G.; Jacka, F.; Dinan, T.G.; Cryan, J.F. Diet and the Microbiota-Gut-Brain Axis: Sowing the Seeds of Good Mental Health. Adv. Nutr. 2021, 12, 1239–1285. [Google Scholar] [CrossRef] [PubMed]
- Finotello, F.; Mastrorilli, E.; Di Camillo, B. Measuring the diversity of the human microbiota with targeted next-generation sequencing. Brief. Bioinform. 2018, 19, 679–692. [Google Scholar] [CrossRef]
- Marano, G.; Mazza, M.; Lisci, F.M.; Ciliberto, M.; Traversi, G.; Kotzalidis, G.D.; De Berardis, D.; Laterza, L.; Sani, G.; Gasbarrini, A.; et al. The Microbiota–Gut–Brain Axis: Psychoneuroimmunological Insights. Nutrients 2023, 15, 1496. [Google Scholar] [CrossRef]
- Adak, A.; Khan, M.R. An insight into gut microbiota and its functionalities. Cell. Mol. Life Sci. 2019, 76, 473–493. [Google Scholar] [CrossRef] [PubMed]
- Meerveld, B.G.-V.; Johnson, A.C.; Grundy, D. Gastrointestinal Physiology and Function. In Gastrointestinal Pharmacology; Springer: Cham, Switzerland, 2017; pp. 1–16. [Google Scholar] [CrossRef]
- Koga, Y. Microbiota in the stomach and application of probiotics to gastroduodenal diseases. World J. Gastroenterol. 2022, 28, 6702–6715. [Google Scholar] [CrossRef]
- Ohno, H.; Satoh-Takayama, N. Stomach microbiota, Helicobacter pylori, and group 2 innate lymphoid cells. Exp. Mol. Med. 2020, 52, 1377–1382. [Google Scholar] [CrossRef]
- Procházková, N.; Falony, G.; Dragsted, L.O.; Licht, T.R.; Raes, J.; Roager, H.M. Advancing human gut microbiota research by considering gut transit time. Gut 2023, 72, 180–191. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, S.; Nie, Q.; He, H.; Tan, H.; Geng, F.; Ji, H.; Hu, J.; Nie, S. Gut firmicutes: Relationship with dietary fiber and role in host homeostasis. Crit. Rev. Food Sci. Nutr. 2023, 63, 12073–12088. [Google Scholar] [CrossRef]
- Wang, L.Y.; He, L.H.; Xu, L.J.; Li, S.B. Short-chain fatty acids: Bridges between diet, gut microbiota, and health. J. Gastroenterol. Hepatol. 2024, 39, 1728–1736. [Google Scholar] [CrossRef] [PubMed]
- Cataldi, S.; Poli, L.; Şahin, F.N.; Patti, A.; Santacroce, L.; Bianco, A.; Greco, G.; Ghinassi, B.; Di Baldassarre, A.; Fischetti, F. The Effects of Physical Activity on the Gut Microbiota and the Gut–Brain Axis in Preclinical and Human Models: A Narrative Review. Nutrients 2022, 14, 3293. [Google Scholar] [CrossRef]
- Vandenplas, Y.; Carnielli, V.P.; Ksiazyk, J.; Luna, M.S.; Migacheva, N.; Mosselmans, J.M.; Picaud, J.C.; Possner, M.; Singhal, A.; Wabitsch, M. Factors affecting early-life intestinal microbiota development. Nutrition 2020, 78, 110812. [Google Scholar] [CrossRef]
- Zhang, C.; Li, L.; Jin, B.; Xu, X.; Zuo, X.; Li, Y.; Li, Z. The Effects of Delivery Mode on the Gut Microbiota and Health: State of Art. Front. Microbiol. 2021, 12, 724449. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Martínez, C.; Santaella-Pascual, M.; Yagüe-Guirao, G.; Martínez-Graciá, C. Infant gut microbiota colonization: Influence of prenatal and postnatal factors, focusing on diet. Front. Microbiol. 2023, 14, 1236254. [Google Scholar] [CrossRef] [PubMed]
- Chong, H.-Y.; Tan, L.T.; Law, J.W.; Hong, K.W.; Ratnasingam, V.; Ab Mutalib, N.S.; Lee, L.H.; Letchumanan, V. Exploring the Potential of Human Milk and Formula Milk on Infants’ Gut and Health. Nutrients 2022, 14, 3554. [Google Scholar] [CrossRef]
- Yaron, S.; Shachar, D.; Abramas, L.; Riskin, A.; Bader, D.; Litmanovitz, I.; Bar-Yoseph, F.; Cohen, T.; Levi, L.; Lifshitz, Y.; et al. Effect of High β-Palmitate Content in Infant Formula on the Intestinal Microbiota of Term Infants. J. Pediatr. Gastroenterol. Nutr. 2013, 56, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Van den Elsen, L.W.J.; Garssen, J.; Burcelin, R.; Verhasselt, V. Shaping the Gut Microbiota by Breastfeeding: The Gateway to Allergy Prevention? Front. Pediatr. 2019, 7, 47. [Google Scholar] [CrossRef]
- Mangiola, F.; Nicoletti, A.; Gasbarrini, A.; Ponziani, F.R. Gut microbiota and aging. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 7404–7413. [Google Scholar]
- Du, Y.; Gao, X.-R.; Peng, L.; Ge, J.-F. Crosstalk between the microbiota-gut-brain axis and depression. Heliyon 2020, 6, e04097. [Google Scholar] [CrossRef]
- Sekirov, I.; Russell, S.L.; Antunes, L.C.M.; Finlay, B.B. Gut Microbiota in Health and Disease. Physiol. Rev. 2010, 90, 859–904. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.A.; Neufeld, K.A.M. Gut-brain axis: How the microbiome influences anxiety and depression. Trends Neurosci. 2013, 36, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, F.H.; Ussery, D.W.; Nielsen, J.; Nookaew, I. A Closer Look at Bacteroides: Phylogenetic Relationship and Genomic Implications of a Life in the Human Gut. Microb. Ecol. 2011, 61, 473–485. [Google Scholar] [CrossRef] [PubMed]
- Severance, E.; Tveiten, D.; Lindström, L.; Yolken, R.; Reichelt, K. The Gut Microbiota and the Emergence of Autoimmunity: Relevance to Major Psychiatric Disorders. Curr. Pharm. Des. 2016, 22, 6076–6086. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.S.; Hooper, L.V. Epithelial Cells and Their Neighbors. IV. Bacterial contributions to intestinal epithelial barrier integrity. Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 289, G779–G784. [Google Scholar] [CrossRef]
- Yatsunenko, T.; Rey, F.E.; Manary, M.J.; Trehan, I.; Dominguez-Bello, M.G.; Contreras, M.; Magris, M.; Hidalgo, G.; Baldassano, R.N.; Anokhin, A.P.; et al. Human gut microbiome viewed across age and geography. Nature 2012, 486, 222–227. [Google Scholar] [CrossRef]
- Davey, K.J.; Cotter, P.D.; O’Sullivan, O.; Crispie, F.; Dinan, T.G.; Cryan, J.F.; O’Mahony, S.M. Antipsychotics and the gut microbiome: Olanzapine-induced metabolic dysfunction is attenuated by antibiotic administration in the rat. Transl. Psychiatry 2013, 3, e309. [Google Scholar] [CrossRef]
- Dominguez-Bello, M.G.; Costello, E.K.; Contreras, M.; Magris, M.; Hidalgo, G.; Fierer, N.; Knight, R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl. Acad. Sci. USA 2010, 107, 11971–11975. [Google Scholar] [CrossRef] [PubMed]
- Fallani, M.; Young, D.; Scott, J.; Norin, E.; Amarri, S.; Adam, R.; Aguilera, M.; Khanna, S.; Gil, A.; Edwards, C.A.; et al. Intestinal microbiota of 6-week-old infants across Europe: Geographic influence beyond delivery mode, breast-feeding, and antibiotics. J. Pediatr. Gastroenterol. Nutr. 2010, 51, 77–84. [Google Scholar] [CrossRef]
- Patterson, E.; Ryan, P.M.; Wiley, N.; Carafa, I.; Sherwin, E.; Moloney, G.; Franciosi, E.; Mandal, R.; Wishart, D.S.; Tuohy, K.; et al. Gamma-aminobutyric acid-producing lactobacilli positively affect metabolism and depressive-like behaviour in a mouse model of metabolic syndrome. Sci. Rep. 2019, 9, 16323. [Google Scholar] [CrossRef]
- Busnelli, M.; Manzini, S.; Chiesa, G. The gut microbiota affects host pathophysiology as an endocrine organ: A focus on cardiovascular disease. Nutrients 2020, 12, 79. [Google Scholar] [CrossRef]
- Fülling, C.; Dinan, T.G.; Cryan, J.F. Gut Microbe to Brain Signaling: What Happens in Vagus. Neuron 2019, 101, 998–1002. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhan, G.; Cai, Z.; Jiao, B.; Zhao, Y.; Li, S.; Luo, A. Vagus nerve stimulation in brain diseases: Therapeutic applications and biological mechanisms. Neurosci. Biobehav. Rev. 2021, 127, 37–53. [Google Scholar] [CrossRef]
- Bonaz, B.; Bazin, T.; Pellissier, S. The Vagus Nerve at the Interface of the Microbiota-Gut-Brain Axis. Front. Neurosci. 2018, 12, 336468. [Google Scholar] [CrossRef]
- Wang, G.-J. Food addiction A common neurobiological mechanism with drug abuse. Front. Biosci. 2018, 23, 4618. [Google Scholar] [CrossRef]
- Wang, Y.; Duan, C.; Du, X.; Zhu, Y.; Wang, L.; Hu, J.; Sun, Y. Vagus Nerve and Gut-Brain Communication. Neuroscientist 2024, 10738584241259702. [Google Scholar] [CrossRef]
- Scaldaferri, F.; Gerardi, V.; Lopetuso, L.R.; Del Zompo, F.; Mangiola, F.; Boškoski, I.; Bruno, G.; Petito, V.; Laterza, L.; Cammarota, G.; et al. Gut Microbial Flora, Prebiotics, and Probiotics in IBD: Their Current Usage and Utility. Biomed. Res. Int. 2013, 2013, 435268. [Google Scholar] [CrossRef]
- Bolon, B. Cellular and Molecular Mechanisms of Autoimmune Disease. Toxicol. Pathol. 2012, 40, 216–229. [Google Scholar] [CrossRef]
- Dinan, T.G.; Cryan, J.F. The impact of gut microbiota on brain and behaviour. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 552–558. [Google Scholar] [CrossRef]
- Round, J.L.; O’Connell, R.M.; Mazmanian, S.K. Coordination of tolerogenic immune responses by the commensal microbiota. J. Autoimmun. 2010, 34, J220–J225. [Google Scholar] [CrossRef]
- Misiak, B.; Łoniewski, I.; Marlicz, W.; Frydecka, D.; Szulc, A.; Rudzki, L.; Samochowiec, J. The HPA axis dysregulation in severe mental illness: Can we shift the blame to gut microbiota? Prog. Neuropsychopharmacol. Biol. Psychiatry 2020, 102, 109951. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Xing, C.; Long, W.; Wang, H.Y.; Liu, Q.; Wang, R.-F. Impact of microbiota on central nervous system and neurological diseases: The gut-brain axis. J. Neuroinflammation 2019, 16, 53. [Google Scholar] [CrossRef]
- Doroszkiewicz, J.; Groblewska, M.; Mroczko, B. The Role of Gut Microbiota and Gut–Brain Interplay in Selected Diseases of the Central Nervous System. Int. J. Mol. Sci. 2021, 22, 10028. [Google Scholar] [CrossRef] [PubMed]
- Clarke, G.; Stone, T.W.; Schwarcz, R. The kynurenine pathway: Towards metabolic equilibrium. Neuropharmacology 2017, 112, 235–236. [Google Scholar] [CrossRef][Green Version]
- Schwarcz, R.; Stone, T.W. The kynurenine pathway and the brain: Challenges, controversies and promises. Neuropharmacology 2017, 112, 237–247. [Google Scholar] [CrossRef]
- Kadriu, B.; Farmer, C.A.; Yuan, P.; Park, L.T.; Deng, Z.D.; Moaddel, R.; Henter, I.D.; Shovestul, B.; Ballard, E.D.; Kraus, C.; et al. The kynurenine pathway and bipolar disorder: Intersection of the monoaminergic and glutamatergic systems and immune response. Mol. Psychiatry 2021, 26, 4085–4095. [Google Scholar] [CrossRef] [PubMed]
- Potter, M.C.; Elmer, G.I.; Bergeron, R.; Albuquerque, E.X.; Guidetti, P.; Wu, H.Q.; Schwarcz, R. Reduction of Endogenous Kynurenic Acid Formation Enhances Extracellular Glutamate, Hippocampal Plasticity, and Cognitive Behavior. Neuropsychopharmacology 2010, 35, 1734–1742. [Google Scholar] [CrossRef]
- Lin, P.; Li, D.; Shi, Y.; Li, Q.; Guo, X.; Dong, K.; Chen, Q.; Lou, X.; Li, Z.; Li, P. Dysbiosis of the Gut Microbiota and Kynurenine (Kyn) Pathway Activity as Potential Biomarkers in Patients with Major Depressive Disorder. Nutrients 2023, 15, 1752. [Google Scholar] [CrossRef]
- Inam, M.E.; Inam, M.E.; Enduru, N.; Quevedo, J.; Zhao, Z. The kynurenine pathway in major depressive disorder, bipolar disorder, and schizophrenia: A systematic review and meta-analysis of cerebrospinal fluid studies. Braz. J. Psychiatry 2023, 45, 343–355. [Google Scholar] [CrossRef]
- Nettis, M.A.; Lombardo, G.; Hastings, C.; Zajkowska, Z.; Mariani, N.; Nikkheslat, N.; Sforzini, L.; Worrell, C.; Begum, A.; Brown, M.; et al. The interaction between kynurenine pathway, suicidal ideation and augmentation therapy with minocycline in patients with treatment-resistant depression. J. Psychopharmacol. 2023, 37, 531–538. [Google Scholar] [CrossRef]
- Dash, S.; Clarke, G.; Berk, M.; Jacka, F.N. The gut microbiome and diet in psychiatry. Curr. Opin. Psychiatry 2015, 28, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Lach, G.; Schellekens, H.; Dinan, T.G.; Cryan, J.F. Anxiety, Depression, and the Microbiome: A Role for Gut Peptides. Neurotherapeutics 2018, 15, 36–59. [Google Scholar] [CrossRef] [PubMed]
- Lucidi, L.; Pettorruso, M.; Vellante, F.; Di Carlo, F.; Ceci, F.; Santovito, M.C.; Di Muzio, I.; Fornaro, M.; Ventriglio, A.; Tomasetti, C.; et al. Gut Microbiota and Bipolar Disorder: An Overview on a Novel Biomarker for Diagnosis and Treatment. Int. J. Mol. Sci. 2021, 22, 3723. [Google Scholar] [CrossRef] [PubMed]
- Painold, A.; Mörkl, S.; Kashofer, K.; Halwachs, B.; Dalkner, N.; Bengesser, S.; Birner, A.; Fellendorf, F.; Platzer, M.; Queissner, R.; et al. A step ahead: Exploring the gut microbiota in inpatients with bipolar disorder during a depressive episode. Bipolar Disord. 2019, 21, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Ling, Z.; Zhang, Y.; Mao, H.; Ma, Z.; Yin, Y.; Wang, W.; Tang, W.; Tan, Z.; Shi, J.; et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav. Immun. 2015, 48, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Marano, G.; Traversi, G.; Gaetani, E.; Gasbarrini, A.; Mazza, M. Gut microbiota in women: The secret of psychological and physical well-being. World J. Gastroenterol. 2023, 29, 5945–5952. [Google Scholar] [CrossRef]
- Naseribafrouei, A.; Hestad, K.; Avershina, E.; Sekelja, M.; Linløkken, A.; Wilson, R.; Rudi, K. Correlation between the human fecal microbiota and depression. Neurogastroenterol. Motil. 2014, 26, 1155–1162. [Google Scholar] [CrossRef]
- Huang, T.-T.; Lai, J.-B.; Du, Y.-L.; Xu, Y.; Ruan, L.-M.; Hu, S.-H. Current Understanding of Gut Microbiota in Mood Disorders: An Update of Human Studies. Front. Genet. 2019, 10, 98. [Google Scholar] [CrossRef]
- Huang, Y.; Shi, X.; Li, Z.; Shen, Y.; Shi, X.; Wang, L.; Li, G.; Yuan, Y.; Wang, J.; Zhang, Y.; et al. Possible association of Firmicutes in the gut microbiota of patients with major depressive disorder. Neuropsychiatr. Dis. Treat. 2018, 14, 3329–3337. [Google Scholar] [CrossRef]
- Gondalia, S.; Parkinson, L.; Stough, C.; Scholey, A. Gut microbiota and bipolar disorder: A review of mechanisms and potential targets for adjunctive therapy. Psychopharmacology 2019, 236, 1433–1443. [Google Scholar] [CrossRef]
- Borodovitsyna, O.; Flamini, M.; Chandler, D. Noradrenergic Modulation of Cognition in Health and Disease. Neural. Plast. 2017, 2017, 6031478. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.O.; Battagello, D.S.; Cardoso, A.R.; Hauser, D.N.; Bittencourt, J.C.; Correa, R.G. Dopamine: Functions, Signaling, and Association with Neurological Diseases. Cell. Mol. Neurobiol. 2019, 39, 31–59. [Google Scholar] [CrossRef] [PubMed]
- Kleinridders, A.; Pothos, E.N. Impact of Brain Insulin Signaling on Dopamine Function, Food Intake, Reward, and Emotional Behavior. Curr. Nutr. Rep. 2019, 8, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Grice, E.A.; Segre, J.A. The Human Microbiome: Our Second Genome. Annu. Rev. Genomics Hum. Genet. 2012, 13, 151–170. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Kong, L.; Huang, H.; Pan, Y.; Zhang, D.; Jiang, J.; Shen, Y.; Xi, C.; Lai, J.; Ng, C.H.; et al. Gut Microbiota—A Potential Contributor in the Pathogenesis of Bipolar Disorder. Front. Neurosci. 2022, 16, 830748. [Google Scholar] [CrossRef]
- Rieder, R.; Wisniewski, P.J.; Alderman, B.L.; Campbell, S.C. Microbes and mental health: A review. Brain Behav. Immun. 2017, 66, 9–17. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef]
- Caso, J.; Balanzá-Martínez, V.; Palomo, T.; García-Bueno, B. The Microbiota and Gut-Brain Axis: Contributions to the Immunopathogenesis of Schizophrenia. Curr. Pharm. Des. 2016, 22, 6122–6133. [Google Scholar] [CrossRef]
- Addolorato, G.; De Lorenzi, G.; Abenavoli, L.; Leggio, L.; Capristo, E.; Gasbarrini, G. Psychological support counselling improves gluten-free diet compliance in coeliac patients with affective disorders. Aliment. Pharmacol. Ther. 2004, 20, 777–782. [Google Scholar] [CrossRef]
- Fond, G.; Loundou, A.; Hamdani, N.; Boukouaci, W.; Dargel, A.; Oliveira, J.; Roger, M.; Tamouza, R.; Leboyer, M.; Boyer, L. Anxiety and depression comorbidities in irritable bowel syndrome (IBS): A systematic review and meta-analysis. Eur. Arch. Psychiatry Clin. Neurosci. 2014, 264, 651–660. [Google Scholar] [CrossRef]
- Gao, J. Correlation between anxiety-depression status and cytokines in diarrhea-predominant irritable bowel syndrome. Exp. Ther. Med. 2013, 6, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.-M.; Su, T.P.; Tsai, S.J.; Wen-Fei, C.; Li, C.T.; Pei-Chi, T.; Mu-Hong, C. Comparison of inflammatory cytokine levels among type I/type II and manic/hypomanic/euthymic/depressive states of bipolar disorder. J. Affect. Disord. 2014, 166, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.; Maes, M. Bipolar Disorder: Role of Immune-Inflammatory Cytokines, Oxidative and Nitrosative Stress and Tryptophan Catabolites. Curr. Psychiatry Rep. 2015, 17, 8. [Google Scholar] [CrossRef] [PubMed]
- Flowers, S.A.; Ward, K.M.; Clark, C.T. The gut microbiome in bipolar disorder and pharmacotherapy management. Neuropsychobiology 2020, 79, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Marano, G.; Traversi, G.; Gaetani, E.; Pola, R.; Claro, A.E.; Mazza, M. Alcohol use disorder and liver injury related to the COVID-19 pandemic. World J. Hepatol. 2022, 14, 1875–1883. [Google Scholar] [CrossRef] [PubMed]
- Neuman, M.G.; French, S.W.; Zakhari, S.; Malnick, S.; Seitz, H.K.; Cohen, L.B.; Salaspuro, M.; Voinea-Griffin, A.; Barasch, A.; Kirpich, I.A.; et al. Alcohol, microbiome, life style influence alcohol and non-alcoholic organ damage. Exp. Mol. Pathol. 2017, 102, 162–180. [Google Scholar] [CrossRef]
- Legendre, T.; Boudebesse, C.; Henry, C.; Etain, B. Antibiomania: Penser au syndrome maniaque secondaire à une antibiothérapie. Encephale 2017, 43, 183–186. [Google Scholar] [CrossRef]
- Kelly, J.R.; Kennedy, P.J.; Cryan, J.F.; Dinan, T.G.; Clarke, G.; Hyland, N.P. Breaking down the barriers: The gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front. Cell. Neurosci. 2015, 9, 392. [Google Scholar] [CrossRef]
- Reininghaus, E.Z.; Wetzlmair, L.C.; Fellendorf, F.T.; Platzer, M.; Queissner, R.; Birner, A.; Pilz, R.; Hamm, C.; Maget, A.; Koidl, C.; et al. The Impact of Probiotic Supplements on Cognitive Parameters in Euthymic Individuals with Bipolar Disorder: A Pilot Study. Neuropsychobiology 2020, 79, 63–70. [Google Scholar] [CrossRef]
- Winter, G.; Hart, R.A.; Charlesworth, R.P.G.; Sharpley, C.F. Gut microbiome and depression: What we know and what we need to know. Rev. Neurosci. 2018, 29, 629–643. [Google Scholar] [CrossRef]
- Li, N.; Wang, Q.; Wang, Y.; Sun, A.; Lin, Y.; Jin, Y.; Li, X. Fecal microbiota transplantation from chronic unpredictable mild stress mice donors affects anxiety-like and depression-like behavior in recipient mice via the gut microbiota-inflammation-brain axis. Stress 2019, 22, 592–602. [Google Scholar] [CrossRef] [PubMed]
- Simpson, C.A.; Diaz-Arteche, C.; Eliby, D.; Schwartz, O.S.; Simmons, J.G.; Cowan, C.S.M. The gut microbiota in anxiety and depression—A systematic review. Clin. Psychol. Rev. 2021, 83, 101943. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, L.; Wang, X.; Wang, Z.; Zhang, J.; Jiang, R.; Wang, X.; Wang, K.; Liu, Z.; Xia, Z.; et al. Similar Fecal Microbiota Signatures in Patients with Diarrhea-Predominant Irritable Bowel Syndrome and Patients with Depression. Clin. Gastroenterol. Hepatol. 2016, 14, 1602–1611.e5. [Google Scholar] [CrossRef]
- Aizawa, E.; Tsuji, H.; Asahara, T.; Takahashi, T.; Teraishi, T.; Yoshida, S.; Ota, M.; Koga, N.; Hattori, K.; Kunugi, H. Possible association of Bifidobacterium and Lactobacillus in the gut microbiota of patients with major depressive disorder. J. Affect. Disord. 2016, 202, 254–257. [Google Scholar] [CrossRef] [PubMed]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef]
- He, Y.; He, Y.; Wu, W.; Zheng, H.M.; Li, P.; McDonald, D.; Sheng, H.F.; Chen, M.X.; Chen, Z.H.; Ji, G.Y.; et al. Regional variation limits applications of healthy gut microbiome reference ranges and disease models. Nat. Med. 2018, 24, 1532–1535. [Google Scholar] [CrossRef]
- Bonder, M.J.; Kurilshikov, A.; Tigchelaar, E.F.; Mujagic, Z.; Imhann, F.; Vila, A.V.; Deelen, P.; Vatanen, T.; Schirmer, M.; Smeekens, S.P.; et al. The effect of host genetics on the gut microbiome. Nat. Genet. 2016, 48, 1407–1412. [Google Scholar] [CrossRef]
- O’Toole, P.W.; Jeffery, I.B. Gut microbiota and aging. Science 2015, 350, 1214–1215. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zheng, S.; Su, G.; Lu, X.; Yang, J.; Xiong, Z.; Wu, C. In vivo study on the neurotransmitters and their metabolites change in depressive disorder rat plasma by ultra high performance liquid chromatography coupled to tandem mass spectrometry. J. Chromatogr. B 2015, 988, 59–65. [Google Scholar] [CrossRef]
- Yadid, G.; Friedman, A. Dynamics of the dopaminergic system as a key component to the understanding of depression. In Serotonin–Dopamine Interaction: Experimental Evidence and Therapeutic Relevance; Elsevier: Amsterdam, The Netherlands, 2008; pp. 265–286. [Google Scholar] [CrossRef]
- Willner, P.; Hale, A.S.; Argyropoulos, S. Dopaminergic mechanism of antidepressant action in depressed patients. J. Affect. Disord. 2005, 86, 37–45. [Google Scholar] [CrossRef]
- Fidalgo, T.M.; Morales-Quezada, J.L.; Muzy, G.S.; Chiavetta, N.M.; Mendonca, M.E.; Santana, M.V.; Goncalves, O.F.; Brunoni, A.R.; Fregni, F. Biological Markers in Noninvasive Brain Stimulation Trials in Major Depressive Disorder. J. ECT 2014, 30, 47–61. [Google Scholar] [CrossRef] [PubMed]
- Sudo, N.; Chida, Y.; Aiba, Y.; Sonoda, J.; Oyama, N.; Yu, X.N.; Kubo, C.; Koga, Y. Postnatal microbial colonization programs the hypothalamic–pituitary–adrenal system for stress response in mice. J. Physiol. 2004, 558, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Rogers, G.B.; Keating, D.J.; Young, R.L.; Wong, M.-L.; Licinio, J.; Wesselingh, S. From gut dysbiosis to altered brain function and mental illness: Mechanisms and pathways. Mol. Psychiatry 2016, 21, 738–748. [Google Scholar] [CrossRef] [PubMed]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef]
- Han, W.; Tellez, L.A.; Perkins, M.H.; Perez, I.O.; Qu, T.; Ferreira, J.; Ferreira, T.L.; Quinn, D.; Liu, Z.W.; Gao, X.B.; et al. A Neural Circuit for Gut-Induced Reward. Cell 2018, 175, 665–678.e23. [Google Scholar] [CrossRef]
- Bansal, T.; Englert, D.; Lee, J.; Hegde, M.; Wood, T.K.; Jayaraman, A. Differential Effects of Epinephrine, Norepinephrine, and Indole on Escherichia coli O157:H7 Chemotaxis, Colonization, and Gene Expression. Infect. Immun. 2007, 75, 4597–4607. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, P.M.; Aviles, H.; Lyte, M.; Sonnenfeld, G. Enhancement of In Vitro Growth of Pathogenic Bacteria by Norepinephrine: Importance of Inoculum Density and Role of Transferrin. Appl. Environ. Microbiol. 2006, 72, 5097–5099. [Google Scholar] [CrossRef]
- Hoban, A.E.; Moloney, R.D.; Golubeva, A.V.; McVey Neufeld, K.A.; O’Sullivan, O.; Patterson, E.; Stanton, C.; Dinan, T.G.; Clarke, G.; Cryan, J.F. Behavioural and neurochemical consequences of chronic gut microbiota depletion during adulthood in the rat. Neuroscience 2016, 339, 463–477. [Google Scholar] [CrossRef]
- Liao, J.F.; Hsu, C.C.; Chou, G.T.; Hsu, J.S.; Liong, M.T.; Tsai, Y.C. Lactobacillus paracasei PS23 reduced early-life stress abnormalities in maternal separation mouse model. Benef. Microbes 2019, 10, 425–436. [Google Scholar] [CrossRef]
- Wei, C.-L.; Wang, S.; Yen, J.T.; Cheng, Y.F.; Liao, C.L.; Hsu, C.C.; Wu, C.C.; Tsai, Y.C. Antidepressant-like activities of live and heat-killed Lactobacillus paracasei PS23 in chronic corticosterone-treated mice and possible mechanisms. Brain Res. 2019, 1711, 202–213. [Google Scholar] [CrossRef]
- Koronyo-Hamaoui, M.; Ko, M.K.; Koronyo, Y.; Azoulay, D.; Seksenyan, A.; Kunis, G.; Pham, K.; Bakhsheshian, J.; Rogeri, P.; Black, K.L.; et al. Attenuation of AD-like neuropathology by harnessing peripheral immune cells: Local elevation of IL-10 and MMP-9. J. Neurochem. 2009, 111, 1409–1424. [Google Scholar] [CrossRef] [PubMed]
- Dinan, T.G.; Stilling, R.M.; Stanton, C.; Cryan, J.F. Collective unconscious: How gut microbes shape human behavior. J. Psychiatr. Res. 2015, 63, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Marx, W.; Lane, M.; Hockey, M.; Aslam, H.; Berk, M.; Walder, K.; Borsini, A.; Firth, J.; Pariante, C.M.; Berding, K.; et al. Diet and depression: Exploring the biological mechanisms of action. Mol. Psychiatry 2021, 26, 134–150. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, G.; Ferri, A.; Clarke, G.; Cryan, J.F. Diet and the microbiota-gut-brain-axis: A primer for clinical nutrition. Curr. Opin. Clin. Nutr. Metab. Care 2022, 25, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Swainson, J.; Reeson, M.; Malik, U.; Stefanuk, I.; Cummins, M.; Sivapalan, S. Diet and depression: A systematic review of whole dietary interventions as treatment in patients with depression. J. Affect. Disord. 2023, 327, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Choi, J.; Lee, H.-J. Flavonoid-Rich Orange Juice Intake and Altered Gut Microbiome in Young Adults with Depressive Symptom: A Randomized Controlled Study. Nutrients 2020, 12, 1815. [Google Scholar] [CrossRef]
- Thompson, R.S.; Bowers, S.J.; Vargas, F.; Hopkins, S.; Kelley, T.; Gonzalez, A.; Lowry, C.A.; Dorrestein, P.C.; Vitaterna, M.H.; Turek, F.W.; et al. A Prebiotic Diet Containing Galactooligosaccharides and Polydextrose Produces Dynamic and Reproducible Changes in the Gut Microbial Ecosystem in Male Rats. Nutrients 2024, 16, 1790. [Google Scholar] [CrossRef]
- Tarutani, S.; Omori, M.; Ido, Y.; Yano, M.; Komatsu, T.; Okamura, T. Effects of 4G-beta-D-Galactosylsucrose in patients with depression: A randomized, double-blinded, placebo-controlled, parallel-group comparative study. J. Psychiatr. Res. 2022, 148, 110–120. [Google Scholar] [CrossRef]
- Vaghef-Mehrabani, E.; Harouni, R.; Behrooz, M.; Ranjbar, F.; Asghari-Jafarabadi, M.; Ebrahimi-Mameghani, M. Effects of inulin supplementation on inflammatory biomarkers and clinical symptoms of women with obesity and depression on a calorie-restricted diet: A randomised controlled clinical trial. Br. J. Nutr. 2023, 129, 1897–1907. [Google Scholar] [CrossRef]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, Types, Sources, Mechanisms, and Clinical Applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef]
- Yadav, M.K.; Kumari, I.; Singh, B.; Sharma, K.K.; Tiwari, S.K. Probiotics, prebiotics and synbiotics: Safe options for next-generation therapeutics. Appl. Microbiol. Biotechnol. 2022, 106, 505–521. [Google Scholar] [CrossRef] [PubMed]
- Vandeputte, D.; Falony, G.; Vieira-Silva, S.; Wang, J.; Sailer, M.; Theis, S.; Verbeke, K.; Raes, J. Prebiotic inulin-type fructans induce specific changes in the human gut microbiota. Gut 2017, 66, 1968–1974. [Google Scholar] [CrossRef] [PubMed]
- Ribera, C.; Sánchez-Ortí, J.V.; Clarke, G.; Marx, W.; Mörkl, S.; Balanzá-Martínez, V. Probiotic, prebiotic, synbiotic and fermented food supplementation in psychiatric disorders: A systematic review of clinical trials. Neurosci. Biobehav. Rev. 2024, 158, 105561. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Binda, S.; Tremblay, A.; Iqbal, U.H.; Kassem, O.; Le Barz, M.; Thomas, V.; Bronner, S.; Perrot, T.; Ismail, N.; Parker, J.A. Psychobiotics and the Microbiota–Gut–Brain Axis: Where Do We Go from Here? Microorganisms 2024, 12, 634. [Google Scholar] [CrossRef]
- Kim, S.-K.; Guevarra, R.B.; Kim, Y.T.; Kwon, J.; Kim, H.; Cho, J.H.; Kim, H.B.; Lee, J.H. Role of Probiotics in Human Gut Microbiome-Associated Diseases. J. Microbiol. Biotechnol. 2019, 29, 1335–1340. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, B.; Delgado, S.; Blanco-Míguez, A.; Lourenço, A.; Gueimonde, M.; Margolles, A. Probiotics, gut microbiota, and their influence on host health and disease. Mol. Nutr. Food Res. 2017, 61, 1600240. [Google Scholar] [CrossRef]
- Gasbarrini, G.; Bonvicini, F.; Gramenzi, A. Probiotics History. J. Clin. Gastroenterol. 2016, 50 (Suppl. S2), S116–S119. [Google Scholar] [CrossRef] [PubMed]
- Dinan, T.G.; Stanton, C.; Cryan, J.F. Psychobiotics: A novel class of psychotropic. Biol. Psychiatry 2013, 74, 720–726. [Google Scholar] [CrossRef]
- Sharma, R.; Gupta, D.; Mehrotra, R.; Mago, P. Psychobiotics: The Next-Generation Probiotics for the Brain. Curr. Microbiol. 2021, 78, 449–463. [Google Scholar] [CrossRef] [PubMed]
- Ait-Belgnaoui, A.; Durand, H.; Cartier, C.; Chaumaz, G.; Eutamene, H.; Ferrier, L.; Houdeau, E.; Fioramonti, J.; Bueno, L.; Theodorou, V. Prevention of gut leakiness by a probiotic treatment leads to attenuated HPA response to an acute psychological stress in rats. Psychoneuroendocrinology 2012, 37, 1885–1895. [Google Scholar] [CrossRef]
- Ng, Q.X.; Peters, C.; Ho, C.Y.X.; Lim, D.Y.; Yeo, W.-S. A meta-analysis of the use of probiotics to alleviate depressive symptoms. J. Affect. Disord. 2018, 228, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Tian, P.; Chen, Y.; Zhu, H.; Wang, L.; Qian, X.; Zou, R.; Zhao, J.; Zhang, H.; Qian, L.; Wang, Q.; et al. Bifidobacterium breve CCFM1025 attenuates major depression disorder via regulating gut microbiome and tryptophan metabolism: A randomized clinical trial. Brain Behav. Immun. 2022, 100, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Schneider, E.; Doll, J.P.K.; Schweinfurth, N.; Kettelhack, C.; Schaub, A.C.; Yamanbaeva, G.; Varghese, N.; Mählmann, L.; Brand, S.; Eckert, A. Effect of short-term, high-dose probiotic supplementation on cognition, related brain functions and BDNF in patients with depression: A secondary analysis of a randomized controlled trial. J. Psychiatry Neurosci. 2023, 48, E23–E33. [Google Scholar] [CrossRef]
- Yamanbaeva, G.; Schaub, A.C.; Schneider, E.; Schweinfurth, N.; Kettelhack, C.; Doll, J.P.K.; Mählmann, L.; Brand, S.; Beglinger, C.; Borgwardt, S.; et al. Effects of a probiotic add-on treatment on fronto-limbic brain structure, function, and perfusion in depression: Secondary neuroimaging findings of a randomized controlled trial. J. Affect. Disord. 2023, 324, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, S.; Zhang, M.; Ren, F.; Ren, Y.; Li, Y.; Liu, N.; Zhang, Y.; Zhang, Q.; Wang, R. Effects of Fermented Milk Containing Lacticaseibacillus paracasei Strain Shirota on Constipation in Patients with Depression: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2021, 13, 2238. [Google Scholar] [CrossRef]
- Casertano, M.; Dekker, M.; Valentino, V.; De Filippis, F.; Fogliano, V.; Ercolini, D. Gaba-producing lactobacilli boost cognitive reactivity to negative mood without improving cognitive performance: A human Double-Blind Placebo-Controlled Cross-Over study. Brain Behav. Immun. 2024, 122, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Chahwan, B.; Kwan, S.; Isik, A.; van Hemert, S.; Burke, C.; Roberts, L. Gut feelings: A randomised, triple-blind, placebo-controlled trial of probiotics for depressive symptoms. J. Affect. Disord. 2019, 253, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Rudzki, L.; Ostrowska, L.; Pawlak, D.; Małus, A.; Pawlak, K.; Waszkiewicz, N.; Szulc, A. Probiotic Lactobacillus Plantarum 299v decreases kynurenine concentration and improves cognitive functions in patients with major depression: A double-blind, randomized, placebo controlled study. Psychoneuroendocrinology 2019, 100, 213–222. [Google Scholar] [CrossRef]
- Borkent, J.; Ioannou, M.; Neijzen, D.; Haarman, B.C.M.; Sommer, I.E.C. Probiotic Formulation for Patients With Bipolar or Schizophrenia Spectrum Disorder: A Double-Blind, Randomized Placebo-Controlled Trial. Schizophr. Bull. 2024, sbae188. [Google Scholar] [CrossRef]
- Godzien, J.; Godzien, J.; Kalaska, B.; Rudzki, L.; Barbas-Bernardos, C.; Swieton, J.; Lopez-Gonzalvez, A.; Ostrowska, L.; Szulc, A.; Waszkiewicz, N.; et al. Probiotic Lactobacillus plantarum 299v supplementation in patients with major depression in a double-blind, randomized, placebo-controlled trial: A metabolomics study. J. Affect. Disord. 2025, 368, 80–190. [Google Scholar] [CrossRef] [PubMed]
- Reininghaus, E.Z.; Wetzlmair, L.C.; Fellendorf, F.T.; Platzer, M.; Queissner, R.; Birner, A.; Pilz, R.; Hamm, C.; Maget, A.; Rieger, A. Probiotic Treatment in Individuals with Euthymic Bipolar Disorder: A Pilot-Study on Clinical Changes and Compliance. Neuropsychobiology 2020, 79, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Antushevich, H. Fecal microbiota transplantation in disease therapy. Clin. Chim. Acta 2020, 503, 90–98. [Google Scholar] [CrossRef]
- Wang, J.-W.; Kuo, C.H.; Kuo, F.C.; Wang, Y.K.; Hsu, W.H.; Yu, F.J.; Hu, H.M.; Hsu, P.I.; Wang, J.Y.; Wu, D.C. Fecal microbiota transplantation: Review and update. J. Formos. Med. Assoc. 2019, 118, S23–S31. [Google Scholar] [CrossRef]
- Xu, H.M.; Huang, H.L.; Zhou, Y.L.; Zhao, H.L.; Xu, J.; Shou, D.W.; Liu, Y.D.; Zhou, Y.J.; Nie, Y.Q. Fecal Microbiota Transplantation: A New Therapeutic Attempt from the Gut to the Brain. Gastroenterol. Res. Pract. 2021, 2021, 6699268. [Google Scholar] [CrossRef]
- Zhang, Q.; Bi, Y.; Zhang, B.; Jiang, Q.; Mou, C.K.; Lei, L.; Deng, Y.; Li, Y.; Yu, J.; Liu, W.; et al. Current landscape of fecal microbiota transplantation in treating depression. Front. Immunol. 2024, 15, 1416961. [Google Scholar] [CrossRef]
- Rao, J.; Qiao, Y.; Xie, R.; Lin, L.; Jiang, J.; Wang, C.; Li, G. Fecal microbiota transplantation ameliorates stress-induced depression-like behaviors associated with the inhibition of glial and NLRP3 inflammasome in rat brain. J. Psychiatr. Res. 2021, 137, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Cai, T.; Zheng, S.P.; Shi, X.; Yuan, L.Z.; Hu, H.; Zhou, B.; Xiao, S.L.; Wang, F. Therapeutic effect of fecal microbiota transplantation on chronic unpredictable mild stress-induced depression. Front. Cell. Infect. Microbiol. 2022, 12, 900652. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.; Zeng, B.; Zhou, C.; Liu, M.; Fang, Z.; Xu, X.; Zeng, L.; Chen, J.; Fan, S.; Du, X.; et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol. Psychiatry 2016, 21, 786–796. [Google Scholar] [CrossRef]
- Settanni, C.R.; Ianiro, G.; Bibbò, S.; Cammarota, G.; Gasbarrini, A. Gut microbiota alteration and modulation in psychiatric disorders: Current evidence on fecal microbiota transplantation. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 109, 110258. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Lin, H.; Chen, P.; Tan, S.; Wen, Z.; Lin, L.; He, J.; Wen, J.; Lu, S. Dynamic changes of intestinal flora in patients with irritable bowel syndrome combined with anxiety and depression after oral administration of enterobacteria capsules. Bioengineered 2021, 12, 11885–11897. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, S.; Borody, T.J.; Zhang, F. Encyclopedia of fecal microbiota transplantation: A review of effectiveness in the treatment of 85 diseases. Chin. Med. J. 2022, 135, 1927–1939. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.W.; Adams, J.B.; Coleman, D.M.; Pollard, E.L.; Maldonado, J.; McDonough-Means, S.; Caporaso, J.G.; Krajmalnik-Brown, R. Long-term benefit of Microbiota Transfer Therapy on autism symptoms and gut microbiota. Sci. Rep. 2019, 9, 5821. [Google Scholar] [CrossRef]
- Kwak, M.; Adams, J.B.; Coleman, D.M.; Pollard, E.L.; Maldonado, J.; McDonough-Means, S.; Caporaso, J.G.; Krajmalnik-Brown, R. Psychobiotics and fecal microbial transplantation for autism and attention-deficit/hyperactivity disorder: Microbiome modulation and therapeutic mechanisms. Front. Cell. Infect. Microbiol. 2023, 13, 1238005. [Google Scholar] [CrossRef]
- Green, J.E.; McGuinness, A.J.; Berk, M.; Castle, D.; Athan, E.; Hair, C.; Strandwitz, P.; Loughman, A.; Nierenberg, A.A.; Cryan, J.F.; et al. Safety and feasibility of faecal microbiota transplant for major depressive disorder: Study protocol for a pilot randomised controlled trial. Pilot. Feasibility Stud. 2023, 9, 5. [Google Scholar] [CrossRef] [PubMed]
- Pu, Y.; Pu, Y.; Tan, Y.; Qu, Y.; Chang, L.; Wang, S.; Wei, Y.; Wang, X.; Hashimoto, K. A role of the subdiaphragmatic vagus nerve in depression-like phenotypes in mice after fecal microbiota transplantation from Chrna7 knock-out mice with depression-like phenotypes. Brain Behav. Immun. 2021, 94, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Angelova, I.Y.; Kovtun, A.S.; Averina, O.V.; Koshenko, T.A.; Danilenko, V.N. Unveiling the Connection between Microbiota and Depressive Disorder through Machine Learning. Int. J. Mol. Sci. 2023, 24, 16459. [Google Scholar] [CrossRef]
- Jiang, Y.; Qu, Y.; Shi, L. The role of gut microbiota and metabolomic pathways in modulating the efficacy of SSRIs for major depressive disorder. Transl. Psychiatry 2024, 14, 493. [Google Scholar] [CrossRef] [PubMed]
- Snigdha, S.; Ha, K.; Tsai, P.; Dinan, T.G.; Bartos, J.D.; Shahid, M. Probiotics: Potential novel therapeutics for microbiota-gut-brain axis dysfunction across gender and lifespan. Pharmacol. Ther. 2022, 231, 107978. [Google Scholar] [CrossRef]
- Sun, Y.; Gao, S.; Ye, C.; Zhao, W. Gut microbiota dysbiosis in polycystic ovary syndrome: Mechanisms of progression and clinical applications. Front. Cell. Infect. Microbiol. 2023, 24, 1142041. [Google Scholar]
- Li, Y.; Zhang, H.; Zheng, P.; Yang, J.; Wu, J.; Huang, Y.; Hu, X.; Tan, X.; Duan, J.; Chai, T.; et al. Perturbed gut microbiota is gender-segregated in unipolar and bipolar depression. J. Affect. Disord. 2022, 317, 166–175. [Google Scholar] [CrossRef] [PubMed]
| Author (Year) | Changes in Microbiota |
|---|---|
| Painold et al., 2019 [75] | Decreased Abundance: Faecalibacterium (correlated with severe symptoms such as sleep disturbances and psychotic episodes). |
| Grice & Segre, 2012 [85]; Lucidi et al., 2021 [74]; Gondalia et al., 2019 [81] | Increased Abundance: Actinobacteria (particularly Coriobacteria), Prevotella, Enterobacter species (Gram-negative). |
| Lucidi et al., 2021 [74]; Gondalia et al., 2019 [81] | Increased Abundance (Gram-positive): Atopobium Cluster, Clostridium, Flavinofractor. |
| Lucidi et al., 2021 [74] | Subtype Differences: Prevotella more prevalent in BD type 1; Collinsella more abundant in BD type 2. |
| Author (Year) | Microbiota Changes |
|---|---|
| Liu et al., 2016 [104] | Reduced Diversity: Decreased microbial alpha and beta diversity. |
| Decreased Abundance: Firmicutes, Bacteroides, Proteobacteria, Bifidobacterium, Lactobacillus, Faecalibacterium, Ruminococcus. | |
| Increased Abundance: Actinobacteria, Fusobacteria, Prevotellaceae, Lachnospiraceae, Flavonifractor. | |
| Aizawa et al., 2016 [105] | Depletion: Coprococcus, Dialister; associations noted with Alistipes, Faecalibacterium, and Ruminococcus. |
| Therapeutic Approach | Main Effect on Gut Microbiota | Type of Article | Author (Year) |
|---|---|---|---|
| Dietary Modulation (e.g., Mediterranean Diet) | Promotes gut microbial diversity and increases beneficial bacteria, such as those producing SCFAs. Reduces inflammation and improves brain function. Associated with better mental outcome. | Review | Ribeiro et al., 2022 [126] |
| Systematic review | Swainson et al., 2023 [127] | ||
| Flavonoid-Rich Foods (e.g., Orange Juice) | Increases Lachnospiraceae and Bifidobacterium abundance, correlated with serum BDNF levels; reduced Clostridium, potentially improving depressive symptoms. | Randomized Controlled Trial | Park et al., 2020 [128] |
| Prebiotic Supplementation (e.g., fructans, GOS) | Enhances beneficial bacteria (e.g., Bacteroides, Parabacteroides); may support mental health indirectly by improving gut microbiota composition. | Clinical study | Thompson et al., 2024 [129] |
| 24-week 4G-beta-D-Galactosylsucrose (LS) supplementation | Increases microbial diversity and Bifidobacterium abundance. Limited direct improvement in depressive symptoms. | Randomized Controlled Trial | Tarutani et al., 2022 [130] |
| 8-week inulin supplementation | No significant impact on gut microbiota composition or function. | Randomized Controlled Trial | Vaghef-Mehrabani et al., 2023 [131] |
| Author (Year) | Study Design | Study Sample | Duration (Weeks) | Probiotic Characteristics | Results |
|---|---|---|---|---|---|
| Xiang Ng et al. (2018) [144] | Meta-analysis | 1349 Participants (1147 healthy individuals; 40 MDD; 44 with IBS and mild to moderate anxiety and/or depression; 39 with CFS; 79 with at least moderate scores on self-report mood measures) | - | Various probiotic strains, doses, and durations | No significant impact on mood overall, but significant benefit for mild to moderate depression (SMD = −0.684, p = 0.029). Adjunctive therapy potential. |
| Tian et al. (2022) [145] | Placebo-controlled, double-blind RCT | 45 MDD patients | 4 | Bifidobacterium breve CCFM1025 (1010 CFU daily) | Significant reductions in depressive symptoms (HDRS-24 p = 0.036; MADRS p = 0.037), changes in tryptophan metabolism and serotonin turnover; mild changes in gut microbial composition. |
| Schneider et al. (2022) [146] | Placebo-controlled, double-blind RCT | 60 MDD patients | 4 | Multi-strain probiotic (900 billion CFU daily, including Bifidobacterium spp., Lactobacillus spp., S. thermophilus) | Improved immediate recall on VLMT, reduced hippocampal activation, no significant changes in BDNF levels or other cognitive measures. |
| Yamanbaeva et al. (2022) [147] | Placebo-controlled, double-blind RCT | 32 MDD patients | 4 | Multi-strain probiotic (e.g., Bifidobacterium spp., Lactobacillus spp., S. thermophilus) | Stabilized uncinate fasciculus diffusivity, improved resting-state connectivity, reduced hippocampal activation, cognitive and emotional improvements. |
| Zhang et al. (2022) [148] | Placebo-controlled, double-blind RCT | 82 depressed patients with constipation | 9 | Lacticaseibacillus paracasei strain Shirota (LcS, 10^8 CFU/mL) | Improved specific constipation symptoms, beneficial gut microbiota changes, reduced IL-6. No significant differences in depressive symptoms vs. placebo. |
| Casertano et al. (2024) [149] | Randomized, double-blind, placebo-controlled, cross-over study | 77 healthy adults, with mild/moderate stress score in the DASS-42 questionnaire | 12 | Levilactobacillus brevis P30021, Lactiplantibacillus plantarum P30025 | Reduced rumination (LEIDS-r), no significant effects on cognitive performance (DASS-42), increased probiotic genera abundance. |
| Chahwan et al. (2019) [150] | Placebo-controlled, triple-blind, RCT. | 71 depressed patients | 8 | Multi-strain probiotic (e.g., Bifidobacterium spp., Lactobacillus spp., Lactococcus lactis) | Reduced cognitive reactivity, shifted participants from clinical to no depression diagnosis, no significant gut microbiota changes. |
| Rudzki et al. (2024) [151] | Placebo-controlled, double-blind RCT | 79 MDD patients on SSRI | 8 | Lactiplantibacillus plantarum 299 v (10 × 109 CFU per capsule) | Improved cognitive function, decreased kynurenine levels, increased the 3-HK/KA ratio. Cognitive performance and mood regulation improvements. |
| Reininghaus et al. (2018) [100] | Pilot study | 20 euthymic BD patients | 12 | Multi-strain probiotic (e.g., Bifidobacterium spp., Lactobacillus spp., Lactococcus lactis) | Improved attention, psychomotor speed, and executive function. Highlights cognitive benefits for BD. |
| Borkent et al. (2024) [152] | Double-blind, placebo-controlled RCT | BD and SSD patients | 12 | Multi-strain probiotic (e.g., Bifidobacterium spp., Lactobacillus spp., Lactococcus lactis) | Borderline improvement in verbal memory, positive effects on intestinal permeability, inflammation markers reduced (zonulin, alpha-1 antitrypsin). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marano, G.; Rossi, S.; Sfratta, G.; Traversi, G.; Lisci, F.M.; Anesini, M.B.; Pola, R.; Gasbarrini, A.; Gaetani, E.; Mazza, M. Gut Microbiota: A New Challenge in Mood Disorder Research. Life 2025, 15, 593. https://doi.org/10.3390/life15040593
Marano G, Rossi S, Sfratta G, Traversi G, Lisci FM, Anesini MB, Pola R, Gasbarrini A, Gaetani E, Mazza M. Gut Microbiota: A New Challenge in Mood Disorder Research. Life. 2025; 15(4):593. https://doi.org/10.3390/life15040593
Chicago/Turabian StyleMarano, Giuseppe, Sara Rossi, Greta Sfratta, Gianandrea Traversi, Francesco Maria Lisci, Maria Benedetta Anesini, Roberto Pola, Antonio Gasbarrini, Eleonora Gaetani, and Marianna Mazza. 2025. "Gut Microbiota: A New Challenge in Mood Disorder Research" Life 15, no. 4: 593. https://doi.org/10.3390/life15040593
APA StyleMarano, G., Rossi, S., Sfratta, G., Traversi, G., Lisci, F. M., Anesini, M. B., Pola, R., Gasbarrini, A., Gaetani, E., & Mazza, M. (2025). Gut Microbiota: A New Challenge in Mood Disorder Research. Life, 15(4), 593. https://doi.org/10.3390/life15040593











