Prevention of Osteoporosis in SAMP6 Mice by Rikkunshi-To: Japanese Kampo Medicine
Abstract
1. Introduction
2. Material and Methods
2.1. Animals
2.2. General Procedure
2.3. Effects of RKT on Osteoporosis and Osteopenia in SAMP6 Mice
2.4. Rikkunshi-To
2.5. Analysis of HU of the Fourth Lumbar Spine of Mice
2.6. Statistical Analysis
3. Results
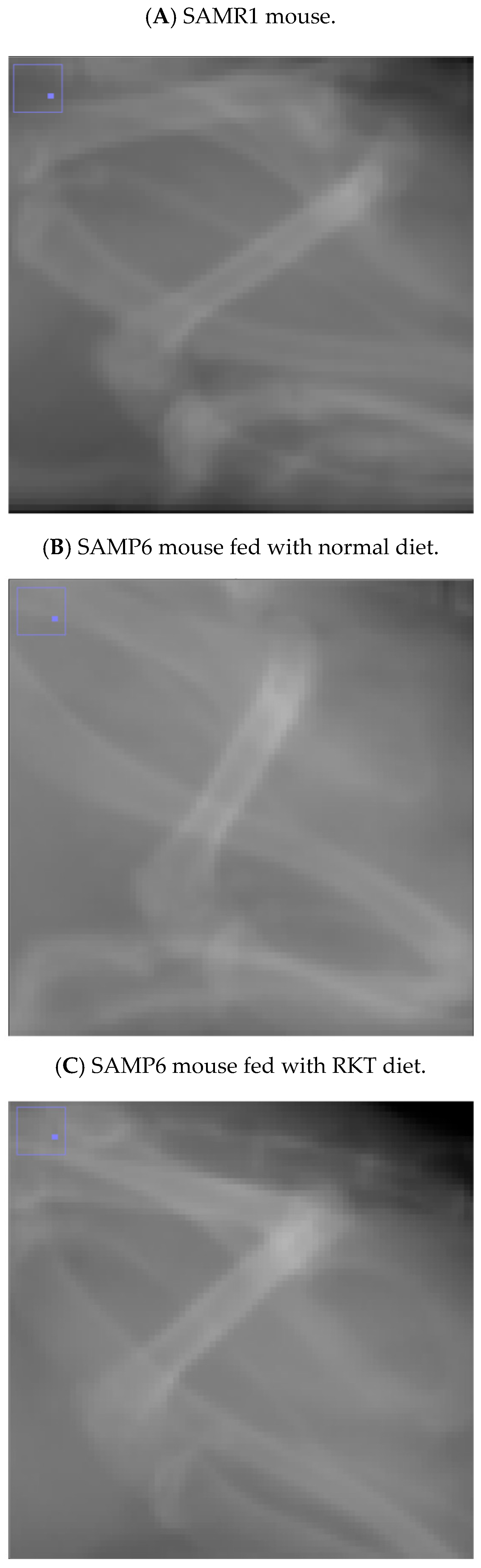
3.1. C/F Index of SAMR1 Mice
3.2. C/F Index of SAMP6 Mice Fed Normal Diet
3.3. C/F Index of SAMP6 Mice Fed RKT Diet
3.4. HUL4 Index of SAMR1 and SAMP6 Mice Fed Diet With or Without RKT
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ANOVA | analysis of variance |
| BMU | basic multicellular unit |
| BMD | bone mineral density |
| C/F index | ratio of cortical thickness of femur |
| CT | computed tomography |
| DXA | dual-energy X-ray absorptiometry |
| GERD | gastroesophageal reflux disease |
| GI | gastrointestinal |
| HU | Hounsfield units |
| IL | interleukin |
| OVX | ovariectomized |
| RKT | Rikkunshi-To |
| ROI | region of interest |
| SAM | male senescence-accelerated mouse strain |
| S.D. | standard deviation |
References
- Bolamperti, S.; Villa, I.; Rubinacci, A. Bone remodeling: An operational process ensuring survival and bone mechanical competence. Bone Res. 2022, 10, 48. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xiao, P.L.; Cui, A.Y.; Hsu, C.J.; Peng, R.; Jiang, N.; Xu, X.H.; Ma, Y.G.; Liu, D.; Lu, H.D. Global, regional prevalence, and risk factors of osteoporosis according to the World Health Organization diagnostic criteria: A systematic review and meta-analysis. Osteoporos Int. 2022, 33, 2137–2153. [Google Scholar] [CrossRef] [PubMed]
- Tella, S.H.; Gallagher, J.C. Prevention and treatment of postmenopausal osteoporosis. J. Steroid Biochem. Mol. Biol. 2014, 142, 155–170. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jarrell, L. Osteoporosis management in primary care. Nurse Pract. 2023, 48, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Gennari, L.; Bilezikian, J.P. New and developing pharmacotherapy for osteoporosis in men. Expert Opin. Pharmacother. 2018, 19, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, K.; Kato, M.; Takeda, H.; Shimoyama, Y.; Umegaki, E.; Iwakiri, R.; Furuta, K.; Sakurai, K.; Odaka, T.; Kusunoki, H.; et al. A randomized, placebo-controlled, double-blind clinical trial of rikkunshito for patients with non-erosive reflux disease refractory to proton-pump inhibitor: The G-PRIDE study. J. Gastroenterol. 2014, 49, 1392–1405. [Google Scholar] [CrossRef] [PubMed]
- Salehi, M.; Karegar-Borzi, H.; Karimi, M.; Rahimi, R. Medicinal Plants for Management of Gastroesophageal Reflux Disease: A Review of Animal and Human Studies. J. Altern. Complement. Med. 2017, 23, 82–95. [Google Scholar] [CrossRef] [PubMed]
- Chiba, H.; Kim, H.; Matsumoto, A.; Akiyama, S.; Ishimi, Y.; Suzuki, K.; Uehara, M. Hesperidin prevents androgen deficiency-induced bone loss in male mice. Phytother. Res. 2014, 28, 289–295. [Google Scholar] [CrossRef] [PubMed]
- King, A.B.; Fiorentino, D.M. Medicare payment cuts for osteoporosis testing reduced use despite tests’ benefit in reducing fractures. Health Aff. 2011, 30, 2362–2370. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, M.; Yamamoto, K.; Matsuzawa, H.; Yamamoto, Y.; Ishida, T. Osteoporosis Screening Using Chest Radiographs. Med. Imaging Inf. Sci. 2018, 35, 30–34. [Google Scholar]
- Saeki, S.; Yamamoto, K.; Tomizawa, R.; Meszaros, S.; Horvath, C.; Zoldi, L.; Szabo, H.; Tarnoki, A.D.; Tarnoki, D.L.; Ishida, T.; et al. Utilizing Graphical Analysis of Chest Radiographs for Primary Screening of Osteoporosis. Medicina 2022, 30, 1765. [Google Scholar] [CrossRef]
- Takeda, T.; Hosokawa, M.; Takeshita, S.; Irino, M.; Higuchi, K.; Matsushita, T.; Tomita, Y.; Yasuhira, K.; Hamamoto, H.; Shimizu, K.; et al. A new murine model of accelerated senescence. Mech. Ageing Dev. 1981, 17, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhou, X.; Emura, S.; Shoumura, S. Site-specific bone loss in senescence-accelerated mouse (SAMP6): A murine model for senile osteoporosis. Exp. Gerontol. 2009, 44, 792–798. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Shoumura, S.; Emura, S. Ultrastructural changes in bones of the senescence-accelerated mouse (SAMP6): A murine model for senile osteoporosis. Histol. Histopathol. 2004, 19, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Takeda, T. Senescence-accelerated mouse (SAM): A biogerontological resource in aging research. Neurobiol. Aging 1999, 20, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Sato, Y.; Hagihara, K.; Kirikihira, K.; Jotaki, A.; Michihara, A.; Miyake, Y. Effects of Rikkunshi-To, a Japanese kampo medicine, on donepezil-induced gastrointestinal side effects in mice. J. Pharmacol. Sci. 2022, 150, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Isogai, Y.; Ishida, T.; Hagihara, K. Enhancement of ghrelin-signaling system by Rikkunshi-To attenuates teriparatide-induced pica in rats. J. Pharmacol. Sci. 2018, 137, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Martel, D.; Monga, A.; Chang, G. Osteoporosis Imaging. Radiol. Clin. N. Am. 2022, 60, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Gu, C.; Zhang, W.; Zhu, J.; He, J.; Huang, Z.; Zhu, J.; Xu, Z. A few-shot learning framework for the diagnosis of osteopenia and osteoporosis using knee X-ray images. J. Int. Med. Res. 2024, 52, 3000605241274576. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kumar, D.A.; Anburajan, M. The role of hip and chest radiographs in osteoporotic evaluation among south Indian women population: A comparative scenario with DXA. J. Endocrinol. Investig. 2014, 37, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.D.; Rothenberg, S.A. AI and Chest Radiographs: A Dawning Era in Osteoporosis Screening. Radiology 2024, 311, e241339. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tsai, D.J.; Lin, C.; Lin, C.S.; Lee, C.C.; Wang, C.H.; Fang, W.H. Artificial Intelligence-enabled Chest X-ray Classifies Osteoporosis and Identifies Mortality Risk. J. Med. Syst. 2024, 48, 12. [Google Scholar] [CrossRef] [PubMed]
- Kruse, C.; Eiken, P.; Vestergaard, P. Machine Learning Principles Can Improve Hip Fracture Prediction. Calcif. Tissue Int. 2017, 100, 348–360. [Google Scholar] [CrossRef] [PubMed]
- Kasai, S.; Shimizu, M.; Matsumura, T.; Okudaira, S.; Matsushita, M.; Tsuboyama, T.; Nakamura, T.; Hosokawa, M. Consistency of low bone density across bone sites in SAMP6 laboratory mice. J. Bone Miner. Metab. 2004, 22, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.J.; Brodt, M.D.; Uthgenannt, B.A. Morphological and mechanical properties of caudal vertebrae in the SAMP6 mouse model of senile osteoporosis. Bone 2004, 35, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Bouxsein, M.L.; Myers, K.S.; Shultz, K.L.; Donahue, L.R.; Rosen, C.J.; Beamer, W.G. Ovariectomy-induced bone loss varies among inbred strains of mice. J. Bone Miner. Res. 2005, 20, 1085–1092. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.M.; Bab, I.; Fish, S.; Müller, R.; Uchiyama, T.; Gronowicz, G.; Nahounou, M.; Zhao, Q.; White, D.W.; Chorev, M.; et al. Human parathyroid hormone 1-34 reverses bone loss in ovariectomized mice. J. Bone Miner. Res. 2001, 16, 1665–1673. [Google Scholar] [CrossRef] [PubMed]
- Bisazza, K.T.; Nelson, B.B.; Sikes, K.J.; Nakamura, L.; Easley, J.T. Computed Tomography Provides Improved Quantification of Trabecular Lumbar Spine Bone Loss Compared to Dual-Energy X-Ray Absorptiometry in Ovariectomized Sheep. JBMR Plus 2023, 7, e10807. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC10731101/pdf/JBM4-7-e10807.pdf (accessed on 23 March 2025). [CrossRef] [PubMed] [PubMed Central]
- Shi, J.; Lee, S.; Uyeda, M.; Tanjaya, J.; Kim, J.K.; Pan, H.C.; Reese, P.; Stodieck, L.; Lin, A.; Ting, K.; et al. Guidelines for Dual Energy X-Ray Absorptiometry Analysis of Trabecular Bone-Rich Regions in Mice: Improved Precision, Accuracy, and Sensitivity for Assessing Longitudinal Bone Changes. Tissue Eng. C Methods 2016, 22, 451–463. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Takeda, H.; Sadakane, C.; Hattori, T.; Katsurada, T.; Ohkawara, T.; Nagai, K.; Asaka, M. Rikkunshito, an herbal medicine, suppresses cisplatin-induced anorexia in rats via 5-HT2 receptor antagonism. Gastroenterology 2008, 134, 2004–2013. [Google Scholar] [CrossRef] [PubMed]
- Takeda, H.; Muto, S.; Nakagawa, K.; Ohnishi, S.; Asaka, M. Rikkunshito and ghrelin secretion. Curr. Pharm. Des. 2012, 8, 4827–4838. [Google Scholar] [CrossRef] [PubMed]
- Horcajada, M.N.; Habauzit, V.; Trzeciakiewicz, A.; Morand, C.; Gil-Izquierdo, A.; Mardon, J.; Lebecque, P.; Davicco, M.J.; Chee, W.S.; Coxam, V.; et al. Hesperidin inhibits ovariectomized-induced osteopenia and shows differential effects on bone mass and strength in young and adult intact rats. J. Appl. Physiol. 2008, 104, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Song, X.; Chen, X.; Jiang, R.; Peng, K.; Tang, X.; Liu, Z. Antiosteoporotic effect of hesperidin against ovariectomy-induced osteoporosis in rats via reduction of oxidative stress and inflammation. J. Biochem. Mol. Toxicol. 2021, 35, e22832. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, M.H.; Siddiqi, M.Z.; Ahn, S.; Kang, S.; Kim, Y.J.; Sathishkumar, N.; Yang, D.U.; Yang, D.C. Ginseng saponins and the treatment of osteoporosis: Mini literature review. J. Ginseng Res. 2013, 37, 261–268. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hwang, Y.H.; Jang, S.A.; Lee, A.; Kim, T.; Ha, H. Poria cocos Ameliorates Bone Loss in Ovariectomized Mice and Inhibits Osteoclastogenesis In Vitro. Nutrients 2020, 12, 1383. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Galanis, D.; Soultanis, K.; Lelovas, P.; Zervas, A.; Papadopoulos, P.; Galanos, A.; Argyropoulou, K.; Makropoulou, M.; Patsaki, A.; Passali, C.; et al. Protective effect of Glycyrrhiza glabra roots extract on bone mineral density of ovariectomized rats. Biomedicine 2019, 9, 8. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC6533940/pdf/bmdcn-9-8.pdf (accessed on 23 March 2025). [CrossRef] [PubMed] [PubMed Central]
- Ahmed, B.; Bio Rashwan, A.K.; Karim, N.; Rezaul Islam Shishir, M.; Bao, T.; Lu, Y.; Chen, W. Jujube fruit: A potential nutritious fruit for the development of functional food products. J. Funct. Food 2020, 75, 104205. [Google Scholar]
- He, J.; Li, X.; Wang, Z.; Bennett, S.; Chen, K.; Xiao, Z.; Zhan, J.; Chen, S.; Hou, Y.; Chen, J.; et al. Therapeutic Anabolic and Anticatabolic Benefits of Natural Chinese Medicines for the Treatment of Osteoporosis. Front. Pharmacol. 2019, 10, 1344. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tarui, I.; Okada, E.; Okada, C.; Saito, A.; Takimoto, H. Trends in BMI among elderly Japanese population: Findings from 1973 to 2016 Japan National Health and Nutrition Survey. Public Health Nutr. 2020, 23, 1907–1915. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamamoto, K.; Yamamoto, K. Prevention of Osteoporosis in SAMP6 Mice by Rikkunshi-To: Japanese Kampo Medicine. Life 2025, 15, 557. https://doi.org/10.3390/life15040557
Yamamoto K, Yamamoto K. Prevention of Osteoporosis in SAMP6 Mice by Rikkunshi-To: Japanese Kampo Medicine. Life. 2025; 15(4):557. https://doi.org/10.3390/life15040557
Chicago/Turabian StyleYamamoto, Kouichi, and Keiko Yamamoto. 2025. "Prevention of Osteoporosis in SAMP6 Mice by Rikkunshi-To: Japanese Kampo Medicine" Life 15, no. 4: 557. https://doi.org/10.3390/life15040557
APA StyleYamamoto, K., & Yamamoto, K. (2025). Prevention of Osteoporosis in SAMP6 Mice by Rikkunshi-To: Japanese Kampo Medicine. Life, 15(4), 557. https://doi.org/10.3390/life15040557





