Therapeutic Potential of Ishige okamurae Yendo as a Multi-Target Inhibitor Against Dementia Symptoms
Abstract
1. Introduction
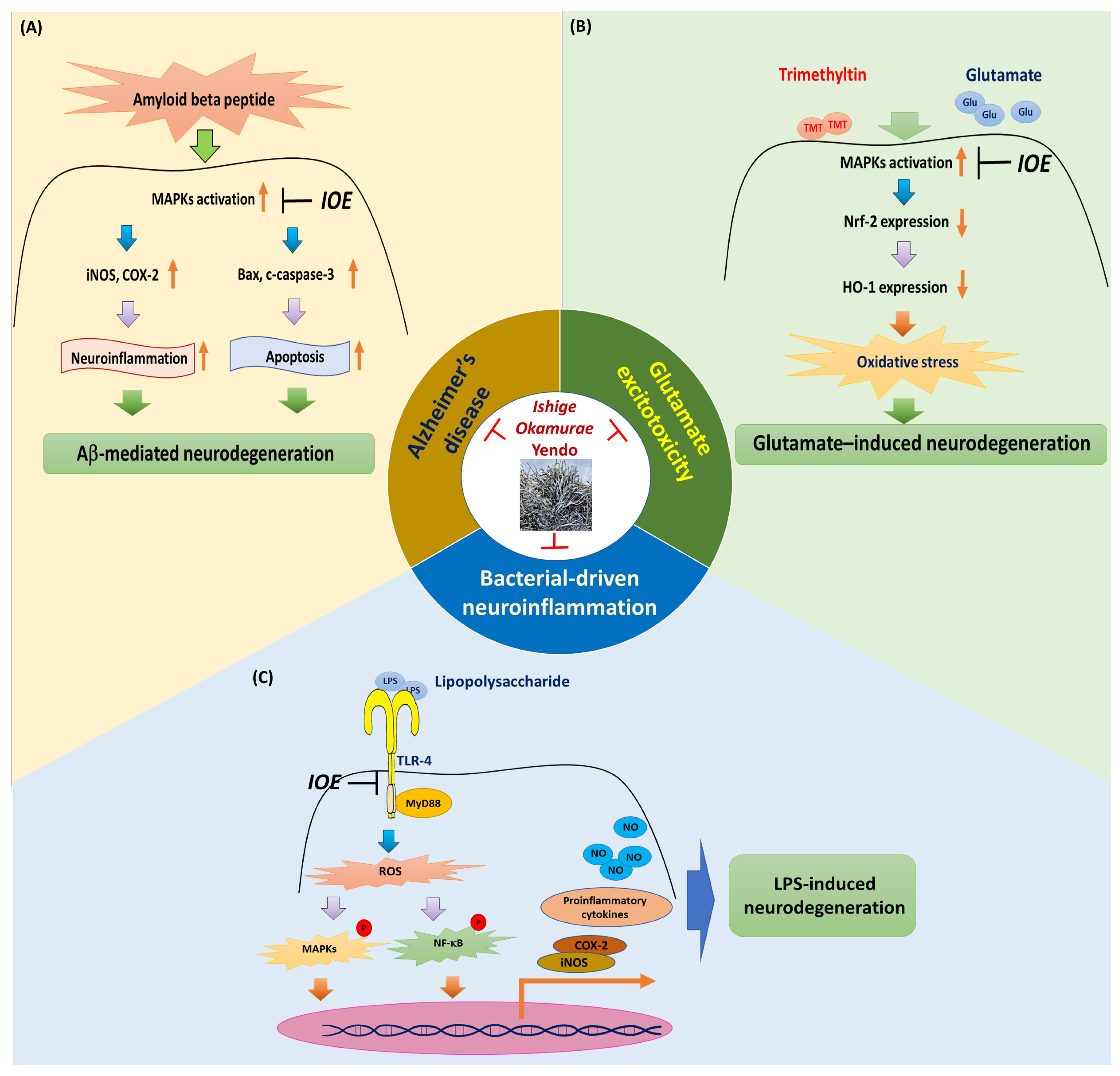
2. Ameliorating I. okamurae Activity in Amyloid Beta-Mediated AD
3. Ameliorating I. okamurae Activity Against Glutamate Excitotoxicity
4. Bacterial-Driven Neuroinflammation Attenuation by I. okamurae by Targeting the TLR-4/MyD88 Pathway
5. Marine Algae-Derived Anti-Dementia Agents
6. Limitations
7. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gale, S.A.; Acar, D.; Daffner, K.R. Dementia. Am. J. Med. 2018, 131, 1161–1169. [Google Scholar] [CrossRef] [PubMed]
- Livingston, G.; Huntley, J.; Liu, K.Y.; Costafreda, S.G.; Selbaek, G.; Alladi, S.; Ames, D.; Banerjee, S.; Burns, A.; Brayne, C.; et al. Dementia prevention, intervention, and care: 2024 report of the Lancet standing Commission. Lancet 2024, 404, 572–628. [Google Scholar] [CrossRef] [PubMed]
- Masrori, P.; Van Damme, P. Amyotrophic lateral sclerosis: A clinical review. Eur. J. Neurol. 2020, 27, 1918–1929. [Google Scholar] [CrossRef]
- Orad, R.I.; Shiner, T. Differentiating dementia with Lewy bodies from Alzheimer’s disease and Parkinson’s disease dementia: An update on imaging modalities. J. Neurol. 2022, 269, 639–653. [Google Scholar] [CrossRef]
- Brett, B.L.; Gardner, R.C.; Godbout, J.; Dams-O’Connor, K.; Keene, C.D. Traumatic Brain Injury and Risk of Neurodegenerative Disorder. Biol. Psychiatry 2022, 91, 498–507. [Google Scholar] [CrossRef]
- Mizutani, S.; Mizutani, T. Malignant Lymphoma of the Brain, and Dementia. Brain Nerve 2016, 68, 383–390. [Google Scholar] [PubMed]
- Raz, L.; Knoefel, J.; Bhaskar, K. The neuropathology and cerebrovascular mechanisms of dementia. J. Cereb. Blood Flow Metab. 2016, 36, 172–186. [Google Scholar] [CrossRef]
- GBD 2019 Dementia Forecasting Collaborators. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: An analysis for the Global Burden of Disease Study 2019. Lancet Public Health 2022, 7, e105–e125. [Google Scholar] [CrossRef]
- Lythgoe, M.P.; Jenei, K.; Prasad, V. Regulatory decisions diverge over aducanumab for Alzheimer’s disease. BMJ 2022, 376, e069780. [Google Scholar] [CrossRef]
- Malik, R.; Kalra, S.; Bhatia, S.; Harrasi, A.A.; Singh, G.; Mohan, S.; Makeen, H.A.; Albratty, M.; Meraya, A.; Bahar, B.; et al. Overview of therapeutic targets in management of dementia. Biomed. Pharmacother. 2022, 152, 113168. [Google Scholar] [CrossRef]
- Fedele, E. Anti-Amyloid Therapies for Alzheimer’s Disease and the Amyloid Cascade Hypothesis. Int. J. Mol. Sci. 2023, 24, 14499. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.; Wang, J.; Xia, Y.; Zhang, J.; Chen, L. Recent advances in Alzheimer’s disease: Mechanisms, clinical trials and new drug development strategies. Signal Transduct. Target Ther. 2024, 9, 211. [Google Scholar] [CrossRef]
- Miculas, D.C.; Negru, P.A.; Bungau, S.G.; Behl, T.; Hassan, S.S.U.; Tit, D.M. Pharmacotherapy Evolution in Alzheimer’s Disease: Current Framework and Relevant Directions. Cells 2022, 12, 131. [Google Scholar] [CrossRef]
- Ray, W.J.; Buggia-Prevot, V. Novel Targets for Alzheimer’s Disease: A View Beyond Amyloid. Annu. Rev. Med. 2021, 72, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Geoffroy, C.; Paoletti, P.; Mony, L. Positive allosteric modulation of NMDA receptors: Mechanisms, physiological impact and therapeutic potential. J. Physiol. 2022, 600, 233–259. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yang, J.W.; Lin, L.T.; Huang, J.; Wang, X.R.; Su, X.T.; Cao, Y.; Fisher, M.; Liu, C.Z. Acupuncture Attenuates Inflammation in Microglia of Vascular Dementia Rats by Inhibiting miR-93-Mediated TLR4/MyD88/NF-κB Signaling Pathway. Oxid. Med. Cell. Longev. 2020, 2020, 8253904. [Google Scholar] [PubMed]
- Cao, Z.; Kong, F.; Ding, J.; Chen, C.; He, F.; Deng, W. Promoting Alzheimer’s disease research and therapy with stem cell technology. Stem Cell Res. Ther. 2024, 15, 136. [Google Scholar] [CrossRef]
- Isakovic, J.; Serer, K.; Barisic, B.; Mitrecic, D. Mesenchymal stem cell therapy for neurological disorders: The light or the dark side of the force? Front. Bioeng. Biotechnol. 2023, 11, 1139359. [Google Scholar] [CrossRef]
- Volarevic, V.; Markovic, B.S.; Gazdic, M.; Volarevic, A.; Jovicic, N.; Arsenijevic, N.; Armstrong, L.; Djonov, V.; Lako, M.; Stojkovic, M. Ethical and Safety Issues of Stem Cell-Based Therapy. Int. J. Med. Sci. 2018, 15, 36–45. [Google Scholar] [CrossRef]
- El Menyiy, N.; El Allam, A.; Aboulaghras, S.; Jaouadi, I.; Bakrim, S.; El Omari, N.; Shariati, M.A.; Miftakhutdinov, A.; Wilairatana, P.; Mubarak, M.S.; et al. Inflammatory auto-immune diseases of the intestine and their management by natural bioactive compounds. Biomed. Pharmacother. 2022, 151, 113158. [Google Scholar] [CrossRef]
- Mushtaq, S.; Abbasi, B.H.; Uzair, B.; Abbasi, R. Natural products as reservoirs of novel therapeutic agents. EXCLI J. 2018, 17, 420–451. [Google Scholar]
- Zou, Y.; Qian, Z.J.; Li, Y.; Kim, M.M.; Lee, S.H.; Kim, S.K. Antioxidant effects of phlorotannins isolated from Ishige okamurae in free radical mediated oxidative systems. J. Agric. Food Chem. 2008, 56, 7001–7009. [Google Scholar] [CrossRef] [PubMed]
- Park, E.; Yu, H.; Lim, J.H.; Hee Choi, J.; Park, K.J.; Lee, J. Seaweed metabolomics: A review on its nutrients, bioactive compounds and changes in climate change. Food Res. Int. 2023, 163, 112221. [Google Scholar] [CrossRef]
- Hamid, S.S.; Wakayama, M.; Ichihara, K.; Sakurai, K.; Ashino, Y.; Kadowaki, R.; Soga, T.; Tomita, M. Metabolome profiling of various seaweed species discriminates between brown, red, and green algae. Planta 2019, 249, 1921–1947. [Google Scholar] [CrossRef]
- Leal, M.C.; Munro, M.H.; Blunt, J.W.; Puga, J.; Jesus, B.; Calado, R.; Rosa, R.; Madeira, C. Biogeography and biodiscovery hotspots of macroalgal marine natural products. Nat. Prod. Rep. 2013, 30, 1380–1390. [Google Scholar] [CrossRef]
- Kim, M.; Cho, C.; Lee, C.; Ryu, B.; Kim, S.; Hur, J.; Lee, S.H. Ishige okamurae Ameliorates Methylglyoxal-Induced Nephrotoxicity via Reducing Oxidative Stress, RAGE Protein Expression, and Modulating MAPK, Nrf2/ARE Signaling Pathway in Mouse Glomerular Mesangial Cells. Foods 2021, 10, 2000. [Google Scholar] [CrossRef]
- Kim, M.M.; Rajapakse, N.; Kim, S.K. Anti-inflammatory effect of Ishige okamurae ethanolic extract via inhibition of NF-κB transcription factor in RAW 264.7 cells. Phytother. Res. 2009, 23, 628–634. [Google Scholar] [CrossRef]
- Min, K.H.; Kim, H.J.; Jeon, Y.J.; Han, J.S. Ishige okamurae ameliorates hyperglycemia and insulin resistance in C57BL/KsJ-db/db mice. Diabetes Res. Clin. Pract. 2011, 93, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Kwon, O.Y.; Lee, S.H. Ameliorating Activity of Ishige okamurae on the Amyloid Beta-Induced Cognitive Deficits and Neurotoxicity through Regulating ERK, p38 MAPK, and JNK Signaling in Alzheimer’s Disease-Like Mice Model. Mol. Nutr. Food Res. 2020, 64, e1901220. [Google Scholar] [CrossRef] [PubMed]
- Kwon, O.Y.; Lee, S.H. Ishige okamurae Suppresses Trimethyltin-Induced Neurodegeneration and Glutamate-Mediated Excitotoxicity by Regulating MAPKs/Nrf2/HO-1 Antioxidant Pathways. Antioxidants 2021, 10, 440. [Google Scholar] [CrossRef]
- Kwon, O.Y.; Lee, S.H. Ishige okamurae Attenuates Neuroinflammation and Cognitive Deficits in Mice Intracerebroventricularly Injected with LPS via Regulating TLR-4/MyD88-Dependent Pathways. Antioxidants 2022, 12, 78. [Google Scholar] [CrossRef] [PubMed]
- Breijyeh, Z.; Karaman, R. Comprehensive Review on Alzheimer’s Disease: Causes and Treatment. Molecules 2020, 25, 5789. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, H.; Li, R.; Sterling, K.; Song, W. Amyloid beta-based therapy for Alzheimer’s disease: Challenges, successes and future. Signal Transduct. Target Ther. 2023, 8, 248. [Google Scholar] [CrossRef]
- Araki, W. Abeta Oligomer Toxicity-Reducing Therapy for the Prevention of Alzheimer’s Disease: Importance of the Nrf2 and PPARgamma Pathways. Cells 2023, 12, 1386. [Google Scholar] [CrossRef]
- Benilova, I.; Karran, E.; De Strooper, B. The toxic Abeta oligomer and Alzheimer’s disease: An emperor in need of clothes. Nat. Neurosci. 2012, 15, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Madhu, P.; Mukhopadhyay, S. Distinct types of amyloid-beta oligomers displaying diverse neurotoxicity mechanisms in Alzheimer’s disease. J. Cell. Biochem. 2021, 122, 1594–1608. [Google Scholar] [CrossRef]
- Hu, Y.; Sun, C.; Chen, Y.; Liu, Y.D.; Fan, J.G. Pipeline of New Drug Treatment for Non-alcoholic Fatty Liver Disease/Metabolic Dysfunction-associated Steatotic Liver Disease. J. Clin. Transl. Hepatol. 2024, 12, 802–814. [Google Scholar] [CrossRef]
- Couzin-Frankel, J. Side effects loom over Alzheimer’s drugs. Science 2023, 381, 466–467. [Google Scholar] [CrossRef]
- Morris, R.G. NMDA receptors and memory encoding. Neuropharmacology 2013, 74, 32–40. [Google Scholar] [CrossRef]
- Vyklicky, V.; Korinek, M.; Smejkalova, T.; Balik, A.; Krausova, B.; Kaniakova, M.; Lichnerova, K.; Cerny, J.; Krusek, J.; Dittert, I.; et al. Structure, function, and pharmacology of NMDA receptor channels. Physiol. Res. 2014, 63, S191–S203. [Google Scholar] [CrossRef]
- Verma, M.; Lizama, B.N.; Chu, C.T. Excitotoxicity, calcium and mitochondria: A triad in synaptic neurodegeneration. Transl. Neurodegener. 2022, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Iovino, L.; Tremblay, M.E.; Civiero, L. Glutamate-induced excitotoxicity in Parkinson’s disease: The role of glial cells. J. Pharmacol. Sci. 2020, 144, 151–164. [Google Scholar] [CrossRef]
- Wang, R.; Reddy, P.H. Role of Glutamate and NMDA Receptors in Alzheimer’s Disease. J. Alzheimers Dis. 2017, 57, 1041–1048. [Google Scholar] [CrossRef]
- Ferreira, I.L.; Ferreiro, E.; Schmidt, J.; Cardoso, J.M.; Pereira, C.M.; Carvalho, A.L.; Oliveira, C.R.; Rego, A.C. Abeta and NMDAR activation cause mitochondrial dysfunction involving ER calcium release. Neurobiol. Aging 2015, 36, 680–692. [Google Scholar] [CrossRef]
- Pardo-Moreno, T.; Gonzalez-Acedo, A.; Rivas-Dominguez, A.; Garcia-Morales, V.; Garcia-Cozar, F.J.; Ramos-Rodriguez, J.J.; Melguizo-Rodriguez, L. Therapeutic Approach to Alzheimer’s Disease: Current Treatments and New Perspectives. Pharmaceutics 2022, 14, 1117. [Google Scholar] [CrossRef]
- Sies, H.; Belousov, V.V.; Chandel, N.S.; Davies, M.J.; Jones, D.P.; Mann, G.E.; Murphy, M.P.; Yamamoto, M.; Winterbourn, C. Defining roles of specific reactive oxygen species (ROS) in cell biology and physiology. Nat. Rev. Mol. Cell Biol. 2022, 23, 499–515. [Google Scholar] [CrossRef]
- Choi, Y.K.; Kim, Y.M. Beneficial and Detrimental Roles of Heme Oxygenase-1 in the Neurovascular System. Int. J. Mol. Sci. 2022, 23, 7041. [Google Scholar] [CrossRef] [PubMed]
- DiSabato, D.J.; Quan, N.; Godbout, J.P. Neuroinflammation: The devil is in the details. J. Neurochem. 2016, 139 (Suppl. S2), 136–153. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xiao, D.; Mao, Q.; Xia, H. Role of neuroinflammation in neurodegeneration development. Signal Transduct. Target Ther. 2023, 8, 267. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, X.; Zhang, J.; Xia, X.; Li, H.; Qiu, C.; Liao, Y.; Chen, H.; He, Z.; Song, Z.; et al. Activated STAT3 signaling pathway by ligature-induced periodontitis could contribute to neuroinflammation and cognitive impairment in rats. J. Neuroinflamm. 2021, 18, 80. [Google Scholar] [CrossRef]
- Qin, Z.; Zhou, C.; Xiao, X.; Guo, C. Metformin attenuates sepsis-induced neuronal injury and cognitive impairment. BMC Neurosci. 2021, 22, 78. [Google Scholar] [CrossRef] [PubMed]
- Tran, V.T.A.; Lee, L.P.; Cho, H. Neuroinflammation in neurodegeneration via microbial infections. Front. Immunol. 2022, 13, 907804. [Google Scholar] [CrossRef] [PubMed]
- Emery, D.C.; Shoemark, D.K.; Batstone, T.E.; Waterfall, C.M.; Coghill, J.A.; Cerajewska, T.L.; Davies, M.; West, N.X.; Allen, S.J. 16S rRNA Next Generation Sequencing Analysis Shows Bacteria in Alzheimer’s Post-Mortem Brain. Front. Aging Neurosci. 2017, 9, 195. [Google Scholar] [CrossRef]
- Fulop, T.; Witkowski, J.M.; Bourgade, K.; Khalil, A.; Zerif, E.; Larbi, A.; Hirokawa, K.; Pawelec, G.; Bocti, C.; Lacombe, G.; et al. Can an Infection Hypothesis Explain the Beta Amyloid Hypothesis of Alzheimer’s Disease? Front. Aging Neurosci. 2018, 10, 224. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.; Li, Y.; Liu, F.; Fang, Y.; He, J.; Ma, J.; Xu, T.; Wang, L.; Lei, P.; Dong, H.; et al. Microbiota-Gut-Brain Axis Dysregulation in Alzheimer’s Disease: Multi-Pathway Effects and Therapeutic Potential. Aging Dis. 2024, 15, 1108–1131. [Google Scholar] [CrossRef]
- Brown, G.C. The endotoxin hypothesis of neurodegeneration. J. Neuroinflamm. 2019, 16, 180. [Google Scholar] [CrossRef]
- Gorina, R.; Font-Nieves, M.; Marquez-Kisinousky, L.; Santalucia, T.; Planas, A.M. Astrocyte TLR4 activation induces a proinflammatory environment through the interplay between MyD88-dependent NFκB signaling, MAPK, and Jak1/Stat1 pathways. Glia 2011, 59, 242–255. [Google Scholar] [CrossRef]
- Kwon, H.S.; Koh, S.H. Neuroinflammation in neurodegenerative disorders: The roles of microglia and astrocytes. Transl. Neurodegener. 2020, 9, 42. [Google Scholar] [CrossRef]
- Malko, P.; Syed Mortadza, S.A.; McWilliam, J.; Jiang, L.H. TRPM2 Channel in Microglia as a New Player in Neuroinflammation Associated With a Spectrum of Central Nervous System Pathologies. Front. Pharmacol. 2019, 10, 239. [Google Scholar] [CrossRef]
- Kou, J.J.; Shi, J.Z.; He, Y.Y.; Hao, J.J.; Zhang, H.Y.; Luo, D.M.; Song, J.K.; Yan, Y.; Xie, X.M.; Du, G.H.; et al. Luteolin alleviates cognitive impairment in Alzheimer’s disease mouse model via inhibiting endoplasmic reticulum stress-dependent neuroinflammation. Acta Pharmacol. Sin. 2022, 43, 840–849. [Google Scholar] [CrossRef]
- Stojanovic, T.D.; Rakic, M.R.; Cosic, M.V.; Oalde Pavlovic, M.M.; Sabovljevic, A.D.; Sabovljevic, M.S.; Bozic, B.D.; Bozic Nedeljkovic, B.D.; Vujicic, M.M.; Lunic, T.M. Moss Extracts as Natural Neuroprotective Agents: Mitigating LPS-Induced Neuroinflammation and Microglial Activation. Cells 2025, 14, 780. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yin, H.; Zhao, M.; Lu, Q. TLR2 and TLR4 in autoimmune diseases: A comprehensive review. Clin. Rev. Allergy Immunol. 2014, 47, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Sun, C.; Ma, Y.; Wang, S.; Wang, X.; Zhang, Y. Inhibition of TLR4 Induces M2 Microglial Polarization and Provides Neuroprotection via the NLRP3 Inflammasome in Alzheimer’s Disease. Front. Neurosci. 2020, 14, 444. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, Y.; Xu, C.; Zhang, H.; Lin, C. TLR4 Targeting as a Promising Therapeutic Strategy for Alzheimer Disease Treatment. Front. Neurosci. 2020, 14, 602508. [Google Scholar] [CrossRef]
- Lee, H.L.; Go, M.J.; Lee, H.S.; Heo, H.J. Ecklonia cava Ameliorates Cognitive Impairment on Amyloid beta-Induced Neurotoxicity by Modulating Oxidative Stress and Synaptic Function in Institute of Cancer Research (ICR) Mice. Antioxidants 2024, 13, 951. [Google Scholar] [CrossRef]
- Nho, J.A.; Shin, Y.S.; Jeong, H.R.; Cho, S.; Heo, H.J.; Kim, G.H.; Kim, D.O. Neuroprotective Effects of Phlorotannin-Rich Extract from Brown Seaweed Ecklonia cava on Neuronal PC-12 and SH-SY5Y Cells with Oxidative Stress. J. Microbiol. Biotechnol. 2020, 30, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.L.; Yang, H.; Jeong, K.J.; Lee, H.W.; Hong, E.J. Neuroprotective Effects of Ecklonia cava in a Chronic Neuroinflammatory Disease Model. Nutrients 2023, 15, 2007. [Google Scholar] [CrossRef]
- Bogie, J.; Hoeks, C.; Schepers, M.; Tiane, A.; Cuypers, A.; Leijten, F.; Chintapakorn, Y.; Suttiyut, T.; Pornpakakul, S.; Struik, D.; et al. Dietary Sargassum fusiforme improves memory and reduces amyloid plaque load in an Alzheimer’s disease mouse model. Sci. Rep. 2019, 9, 4908. [Google Scholar] [CrossRef]
- Ko, W.; Lee, H.; Kim, N.; Jo, H.G.; Woo, E.R.; Lee, K.; Han, Y.S.; Park, S.R.; Ahn, G.; Cheong, S.H.; et al. The Anti-Oxidative and Anti-Neuroinflammatory Effects of Sargassum horneri by Heme Oxygenase-1 Induction in BV2 and HT22 Cells. Antioxidants 2021, 10, 859. [Google Scholar] [CrossRef]
- Cho, J.H.; Kim, D.H.; Lee, J.S.; Seo, M.S.; Kim, M.E.; Lee, J.S. Sargassum horneri (Turner) C. Agardh Extract Regulates Neuroinflammation In Vitro and In Vivo. Curr. Issues Mol. Biol. 2022, 44, 5416–5426. [Google Scholar] [CrossRef]
- Heo, S.J.; Cha, S.H.; Kim, K.N.; Lee, S.H.; Ahn, G.; Kang, D.H.; Oh, C.; Choi, Y.U.; Affan, A.; Kim, D.; et al. Neuroprotective effect of phlorotannin isolated from Ishige okamurae against H2O2-induced oxidative stress in murine hippocampal neuronal cells, HT22. Appl. Biochem. Biotechnol. 2012, 166, 1520–1532. [Google Scholar] [CrossRef] [PubMed]
| Disease Models | Extract | Animal/Cell | Constructs | IOE Treatments | Effects | Ref. | |
|---|---|---|---|---|---|---|---|
| Alzheimer’s disease | In vivo | 70% EtOH | C57BL/6 male mice (4-weeks-old) | Aβ25–35 ICV-injection (410 μmol/5 μL) | Oral gavage for 4 weeks (7.5, 12.5 mg/kg bw/day) | - Memory deficits [↓], - Aβ plaque [↓] - Neuronal loss [↓], - Apoptosis [↓], - Neuroinflammation [↓] | [29] |
| In vitro | Rat pheochromocytoma cells (PC12) | Aβ25–35 (70 µM) for 14 h | Pretreated for 2 h (0.075, 0.125 mg/mL) | - Apoptosis [↓], - Neurotoxicity [↓] - Neuroinflammation [↓] - Oxidative stress [↓] | |||
| Glutamate excitotoxicity | In vivo | 70% EtOH | C57BL/6 male mice (4-weeks-old) | TMT IP-injection (2.5 mg/kg/bw) | Oral gavage for 3 weeks (20 mg/kg bw/day) | - Memory deficits [↓] - Neuronal loss [↓], -Apoptosis [↓] - Oxidative stress defense system [↑] | [30] |
| In vitro | Mouse hippocampalneuronal cells (HT22) | Glutamate (5 mM) for 16 h | Pretreated for 2 h (0.05, 0.1 mg/mL) | - Apoptosis [↓], -Neurotoxicity [↓] - Oxidative stress defense system [↑] | |||
| Bacterial-driven neuroinflam-mation | In vivo | 70% EtOH | C57BL/6 male mice (4-weeks-old) | LPS ICV-Injection (4 μg/μL, 3 μL) | Oral gavage for 4 weeks (20 mg/kg bw/day) | - Memory deficits [↓], -Aβ plaque [↓] - Neuronal loss [↓], - Neuroinflammation [↓] - Activation of TLR-4 signaling [↓] | [31] |
| In vitro | Rat glioblastoma multiforme cells (C6 glioma) | LPS (1 µg/mL) for 15 h | Pretreated for 2 h (0.1, 0.2 mg/mL) | - Activation of TLR-4 signaling [↓] - Neuroinflammation [↓] - Oxidative stress [↓] | |||
| Species | Extracts | Neurotoxic Agents | Experimental Models | Study Type | Effects | Refs. |
|---|---|---|---|---|---|---|
| Ecklonia cava | 70% EtOH | Aβ1–42 | ICR mice (410 μmol/10 μL, ICV injection) | In vivo | - Attenuation of cognitive deficits - Reduction in oxidative stress - Improvement of mitochondrial dysfunction - Enhancing the synapse function | [65] |
| 50% EtOH | H2O2 | PC12 cells, SH-SY5Y cells | In vitro | - Reduction in oxidative stress | [66] | |
| Water | LPS | ICR mice (750 μg/kg/bw, 1.5 mg/kg/bw) | In vivo | - Inhibition of neuroinflammation - Attenuation of neuronal apoptosis | [67] | |
| Sargassum fusiforme | Chloroform and methanol (2:1 (v/v)) | - | APPswePS1ΔE9 mice | In vivo | - Improvement of memory function - Attenuation of Aβ plaque formation | [68] |
| Sargassum horneri | CH2Cl2 -soluble fraction of 70% EtOH extracts | LPS and glutamate | BV2 cells HT22 cells | In vitro | - Inhibition of neuroinflammation - Attenuation of cell damage - Reduction in oxidative stress | [69] |
| 70% EtOH | LPS | BV2 cells C57/BL6 mice (1 mg/kg, IP injection) | In vitro and In vivo | - Attenuation of inflammation - Inhibition of microglial activation - Modulation of p38MAPK/NF-κB pathway | [70] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, O.Y.; Lee, S.H. Therapeutic Potential of Ishige okamurae Yendo as a Multi-Target Inhibitor Against Dementia Symptoms. Life 2025, 15, 1699. https://doi.org/10.3390/life15111699
Kwon OY, Lee SH. Therapeutic Potential of Ishige okamurae Yendo as a Multi-Target Inhibitor Against Dementia Symptoms. Life. 2025; 15(11):1699. https://doi.org/10.3390/life15111699
Chicago/Turabian StyleKwon, Oh Yun, and Seung Ho Lee. 2025. "Therapeutic Potential of Ishige okamurae Yendo as a Multi-Target Inhibitor Against Dementia Symptoms" Life 15, no. 11: 1699. https://doi.org/10.3390/life15111699
APA StyleKwon, O. Y., & Lee, S. H. (2025). Therapeutic Potential of Ishige okamurae Yendo as a Multi-Target Inhibitor Against Dementia Symptoms. Life, 15(11), 1699. https://doi.org/10.3390/life15111699








