Synchronous Ileal Metastasis from Pancreatic Ductal Adenocarcinoma: Case Report and Narrative Review with Practical Diagnostic and Management Points
Abstract
1. Introduction
2. Case Presentation
2.1. Patient Information
2.2. Clinical Findings
2.3. Timeline
2.4. Diagnostic Assessment
2.5. Therapeutic Intervention
2.6. Follow-Up and Outcomes
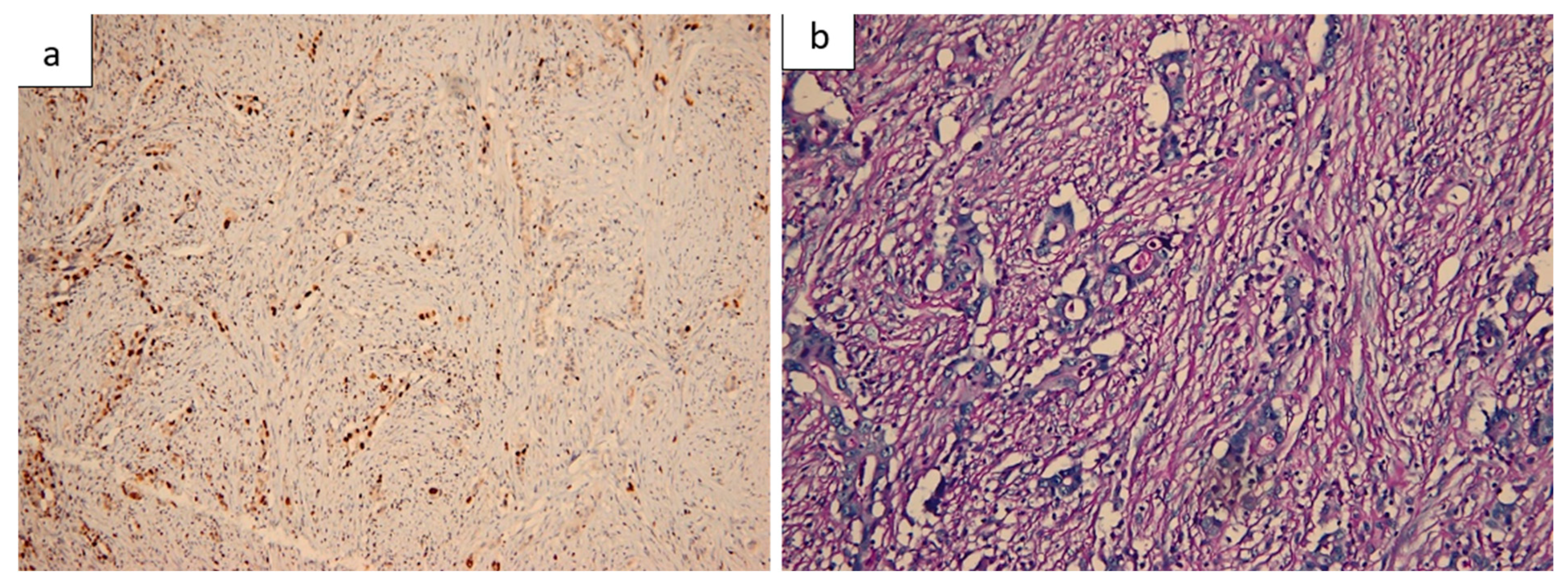
2.7. Histopathological Findings
3. Materials and Methods
3.1. Literature Search and Selection
3.2. Limitations
4. Discussion
4.1. Epidemiology and Mechanisms of Spread
4.2. Correlation with Reported Cases
4.3. Clinical Presentation and Diagnostic Work-Up
4.4. Histopathological and Immunohistochemical Evidence
4.5. Molecular and Therapeutic Considerations
4.6. Practical Management Algorithm for Intestinal Metastasis from PDAC
4.7. Prognosis and Clinical Lessons
4.8. Reported Cases in the Literature
4.9. Key Messages
- Intestinal metastases from PDAC are exceptional events, often presenting with acute surgical emergencies.
- Surgical resection provides palliative benefit and may enable systemic therapy in selected patients.
- The role of systemic chemotherapy is crucial, but evidence remains anecdotal.
- Multidisciplinary evaluation is essential, as no standardized guidelines exist.
4.10. Review Synthesis and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pavlidis, E.T.; Galanis, I.N.; Pavlidis, T.E. Updates in the diagnosis and management of ductal adenocarcinoma of the pancreas. World J. Clin. Oncol. 2025, 16, 105601. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Liu, S.; Yang, M. Clinical diagnosis and management of pancreatic cancer: Markers, molecular mechanisms, and treatment options. World J. Gastroenterol. 2022, 28, 6827–6845. [Google Scholar] [CrossRef]
- Cannistrà, M.; Ruggiero, M.; Zullo, A.; Serafini, S.; Grande, R.; Nardo, B. Metastases of pancreatic adenocarcinoma: A systematic review of literature and a new functional concept. Int. J. Surg. 2015, 21 (Suppl. 1), S15–S21. [Google Scholar] [CrossRef] [PubMed]
- Conroy, T.; Pfeiffer, P.; Vilgrain, V.; Lamarca, A.; Seufferlein, T.; O’Reilly, E.M.; Hackert, T.; Golan, T.; Prager, G.; Haustermans, K.; et al. Pancreatic cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2023, 34, 987–1002. [Google Scholar] [CrossRef]
- Springfeld, C.; Bailey, P.; Büchler, M.W.; Neoptolemos, J.P. ESMO 2023 pancreatic cancer guidelines signal stepwise progress. Hepatobiliary Surg. Nutr. 2024, 13, 362–365. [Google Scholar] [CrossRef]
- Sohal, D.P.S.; Kennedy, E.B.; Cinar, P.; Conroy, T.; Copur, M.S.; Crane, C.H.; Garrido-Laguna, I.; Lau, M.W.; Johnson, T.; Krishnamurthi, S.; et al. Metastatic Pancreatic Cancer: ASCO Guideline Update. J. Clin. Oncol. 2020, 38, 3217–3230. [Google Scholar] [CrossRef]
- Hayat, U.; Croce, P.S.; Saadeh, A.; Desai, K.; Appiah, J.; Khan, S.; Khan, Y.I.; Kumar, K.; Hanif, A. Current and Emerging Treatment Options for Pancreatic Cancer: A Comprehensive Review. J. Clin. Med. 2025, 14, 1129. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.-W.; Tseng, I.-T.; Wu, C.-C. Rare Case of Metachronous Small-Bowel Metastasis from Pancreatic Cancer Presenting as Anemia Approximately 5 Years Postpancreatoduodenectomy. J. Gastroenterol. Dig. Syst. 2024, 8, 1–6. [Google Scholar] [CrossRef]
- Miyasaka, M.; Noji, T.; Tanaka, K.; Nakanishi, Y.; Asano, T.; Ebihara, Y.; Kurashima, Y.; Nakamura, T.; Murakami, S.; Tsuchikawa, T.; et al. Oncological emergency surgery for metachronous large and small bowel metastases after pancreaticoduodenectomy for pancreatic cancer: A case report. Surg. Case Rep. 2018, 4, 99. [Google Scholar] [CrossRef]
- Fasano, M.; Della Corte, C.M.; Vicidomini, G.; Scotti, V.; Rambaldi, P.F.; Fiorelli, A.; Accardo, M.; De Vita, F.; Santini, M.; Ciardiello, F.; et al. Small bowel metastasis from pancreatic cancer in a long-term survival patient with synchronous advanced malignant pleural mesothelioma: A case report and literature review. Oncol. Lett. 2016, 12, 4505–4509. [Google Scholar] [CrossRef]
- Meng, N.; Han, P.; Liu, L.; Liu, J.; Liu, J. Colon Metastasis from Pancreatic Cancer: A Case Report. OncoTargets Ther. 2023, 16, 739–744. [Google Scholar] [CrossRef] [PubMed]
- Barbu, L.A.; Vasile, L.; Cercelaru, L.; Șurlin, V.; Mogoantă, S.-S.; Mogoș, G.F.R.; Țenea Cojan, T.S.; Mărgăritescu, N.-D.; Buliman, A. Non-Variceal Upper Gastrointestinal Bleeding: A Retrospective Cohort of 364 Cases, Historical Comparison, and Updated Management Algorithm. Life 2025, 15, 1320. [Google Scholar] [CrossRef]
- Vasile, L.; Barbu, L.A.; Mogoş, G.F.R.; Şurlin, V.; Vîlcea, I.D.; Cercelaru, L.; Mogoantă, S.Ş.; Mărgăritescu, N.D.; Nimigean, V.; Nimigean, V.R.; et al. Neuroendocrine tumors of the appendix: A comprehensive review of the literature and case presentation. Rom. J. Morphol. Embryol. 2025, 66, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Barbu, L.A.; Cercelaru, L.; Vîlcea, I.-D.; Șurlin, V.; Mogoantă, S.-S.; Țenea Cojan, T.S.; Mărgăritescu, N.-D.; Popescu, M.; Mogoș, G.F.R.; Vasile, L. Coexistence of Acute Appendicitis and Mesenteric Cystic Lymphatic Malformation in an Adult: A Case Report and Narrative Review of Intraoperative Management Strategies. Life 2025, 15, 1390. [Google Scholar] [CrossRef]
- Barbu, L.A.; Vasile, L.; Cercelaru, L.; Vîlcea, I.-D.; Șurlin, V.; Mogoantă, S.-S.; Mogoș, G.F.R.; Țenea Cojan, T.S.; Mărgăritescu, N.-D. Fused Ischiorectal Phlegmon with Pre- and Retroperitoneal Extension: Case Report and Narrative Literature Review. J. Clin. Med. 2025, 14, 4959. [Google Scholar] [CrossRef] [PubMed]
- Calomino, N.; Fusario, D.; Cencini, E.; Lazzi, S. Two secondary localisation of non-Hodgkin’s lymphomas in the upper gastrointestinal tract. BMJ Case Rep. 2022, 15, e247607. [Google Scholar] [CrossRef]
- Waters, A.M.; Der, C.J. KRAS: The Critical Driver and Therapeutic Target for Pancreatic Cancer. Cold Spring Harb. Perspect. Med. 2018, 8, a031435. [Google Scholar] [CrossRef]
- Ardalan, B.; Azqueta, J.; Sleeman, D. Cobimetinib Plus Gemcitabine: An Active Combination in KRAS G12R-Mutated Pancreatic Ductal Adenocarcinoma Patients in Previously Treated and Failed Multiple Chemotherapies. J. Pancreat. Cancer 2021, 7, 65–70. [Google Scholar] [CrossRef]
- Mehdi, M.; Thalji, S.Z.; Shreenivas, A.; Chakrabarti, S.; Thomas, J.P.; Christians, K.K.; Evans, D.B.; Hall, W.A.; Erickson, B.; Thapa, B.; et al. MEK-Inhibitor (inh) and Hydroxychloroquine (HCQ) in KRAS-Mutated Advanced Pancreatic Ductal Adenocarcinoma (PDAC). J. Clin. Oncol. 2022, 40, e16260. [Google Scholar] [CrossRef]
- Golan, T.; Hammel, P.; Reni, M.; Van Cutsem, E.; Macarulla, T.; Hall, M.J.; Park, J.O.; Hochhauser, D.; Arnold, D.; Oh, D.Y.; et al. Maintenance Olaparib for Germline BRCA-Mutated Metastatic Pancreatic Cancer. N. Engl. J. Med. 2019, 381, 317–327. [Google Scholar] [CrossRef]
- Ghidini, M.; Lampis, A.; Mirchev, M.B.; Okuducu, A.F.; Ratti, M.; Valeri, N.; Hahne, J.C. Immune-Based Therapies and the Role of Microsatellite Instability in Pancreatic Cancer. Genes 2020, 12, 33. [Google Scholar] [CrossRef]
- Mosele, F.; Remon, J.; Mateo, J.; Westphalen, C.B.; Barlesi, F.; Lolkema, M.P.; Normanno, N.; Scarpa, A.; Robson, M.; Meric-Bernstam, F.; et al. Recommendations for the use of next-generation sequencing (NGS) for patients with metastatic cancers: A report from the ESMO Precision Medicine Working Group. Ann. Oncol. 2020, 31, 1491–1505. [Google Scholar] [CrossRef]
- Tempero, M.A.; Malafa, M.P.; Al-Hawary, M.; Behrman, S.W.; Benson, A.B.; Cardin, D.B.; Chiorean, E.G.; Chung, V.; Czito, B.; Del Chiaro, M.; et al. Pancreatic Adenocarcinoma, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 439–457. [Google Scholar] [CrossRef]
- O’Sullivan, B.; Burton, T.; Van Dalen, R.; Welsh, F.; Pandita, A.; Fischer, J. Beware the pancreatic incidentaloma in colorectal tumours: Pancreatic adenocarcinoma with metastases to the colon and rectum. J. Surg. Case Rep. 2022, 2022, rjab629. [Google Scholar] [CrossRef]
- Daamen, L.A.; Molenaar, I.Q.; Groot, V.P. Recent Advances and Future Challenges in Pancreatic Cancer Care: Early Detection, Liquid Biopsies, Precision Medicine and Artificial Intelligence. J. Clin. Med. 2023, 12, 7485. [Google Scholar] [CrossRef] [PubMed]
- Bugazia, D.; Al-Najjar, E.; Esmail, A.; Abdelrahim, S.; Abboud, K.; Abdelrahim, A.; Umoru, G.; Rayyan, H.A.; Abudayyeh, A.; Al Moustafa, A.E.; et al. Pancreatic ductal adenocarcinoma: The latest on diagnosis, molecular profiling, and systemic treatments. Front. Oncol. 2024, 14, 1386699. [Google Scholar] [CrossRef] [PubMed]
- Nakaya, T.; Oshiro, H.; Saito, T.; Sakuma, Y.; Horie, H.; Sata, N.; Tanaka, A. Metastasis of pancreatic cancer within primary colon cancer by overtaking the stromal microenvironment. Int. J. Clin. Exp. Pathol. 2018, 11, 3141–3146. [Google Scholar] [PubMed]
- Park, D.Y.; Krishnamurthi, S.; Chahal, P.; Downs-Kelly, E.; Morris-Stiff, G. Pancreatic metastases to the colon: An unusual cause of colonic obstruction. BMJ Case Rep. 2019, 12, e228578. [Google Scholar] [CrossRef]
- Kahl, R.; George, K.; Patel, K.; Stawick, L. Pancreatic Adenocarcinoma with Rare Sigmoid Colon Metastasis. ACG Case Rep. J. 2019, 6, e00132. [Google Scholar] [CrossRef]
- Ardalan, B.; Azqueta, J.; England, J.; Hartmann, R. Pancreatic cancer presenting as bowel obstruction and role of next generation sequencing: A case report. Int. J. Surg. Case Rep. 2022, 90, 106654. [Google Scholar] [CrossRef]
- Pacheco, F.; Luciano, E.; Hebert, D.; Marar, O. Metastatic pancreatic adenocarcinoma presenting as large bowel obstruction: A case report. Int. J. Surg. Case Rep. 2023, 102, 107801. [Google Scholar] [CrossRef]
- Casolino, R.; Biankin, A.V. Treatment of pancreatic cancer in 2022. Camb. Prism. Precis. Med. 2023, 1, e14. [Google Scholar] [CrossRef]
- Netto, D.; Frizziero, M.; Foy, V.; McNamara, M.G.; Backen, A.; Hubner, R.A. Systemic Therapy for Metastatic Pancreatic Cancer—Current Landscape and Future Directions. Curr. Oncol. 2024, 31, 5206–5223. [Google Scholar] [CrossRef]
- Stoop, T.F.; Javed, A.A.; Oba, A.; Koerkamp, B.G.; Seufferlein, T.; Wilmink, J.W.; Besselink, M.G. Pancreatic cancer. Lancet 2025, 405, 1182–1202. [Google Scholar] [CrossRef] [PubMed]
- Wainberg, Z.A.; Melisi, D.; Macarulla, T.; Pazo Cid, R.; Chandana, S.R.; De La Fouchardière, C.; Dean, A.; Kiss, I.; Lee, W.J.; Goetze, T.O.; et al. NALIRIFOX versus nab-paclitaxel and gemcitabine in treatment-naive patients with metastatic pancreatic ductal adenocarcinoma (NAPOLI 3): A randomised, open-label, phase 3 trial. Lancet 2023, 402, 1272–1281. [Google Scholar] [CrossRef]
- Miyabayashi, K.; Nakagawa, H.; Koike, K. Molecular and Phenotypic Profiling for Precision Medicine in Pancreatic Cancer: Current Advances and Future Perspectives. Front. Oncol. 2021, 11, 682872. [Google Scholar] [CrossRef] [PubMed]
- Ratner, E.S.; Sartorelli, A.C.; Lin, Z.P. Poly (ADP-ribose) polymerase inhibitors: On the horizon of tailored and personalized therapies for epithelial ovarian cancer. Curr. Opin. Oncol. 2012, 24, 564–571. [Google Scholar] [CrossRef]
- Wang, H.; Li, Z.W.; Ou, Q.; Wu, X.; Nagasaka, M.; Shao, Y.; Ou, S.I.; Yang, Y. NTRK fusion positive colorectal cancer is a unique subset of CRC with high TMB and microsatellite instability. Cancer Med. 2022, 11, 2541–2549. [Google Scholar] [CrossRef] [PubMed]
- Joshi, D.C.; Sharma, A.; Prasad, S.; Singh, K.; Kumar, M.; Sherawat, K.; Tuli, H.S.; Gupta, M. Novel therapeutic agents in clinical trials: Emerging approaches in cancer therapy. Discov. Oncol. 2024, 15, 342. [Google Scholar] [CrossRef]
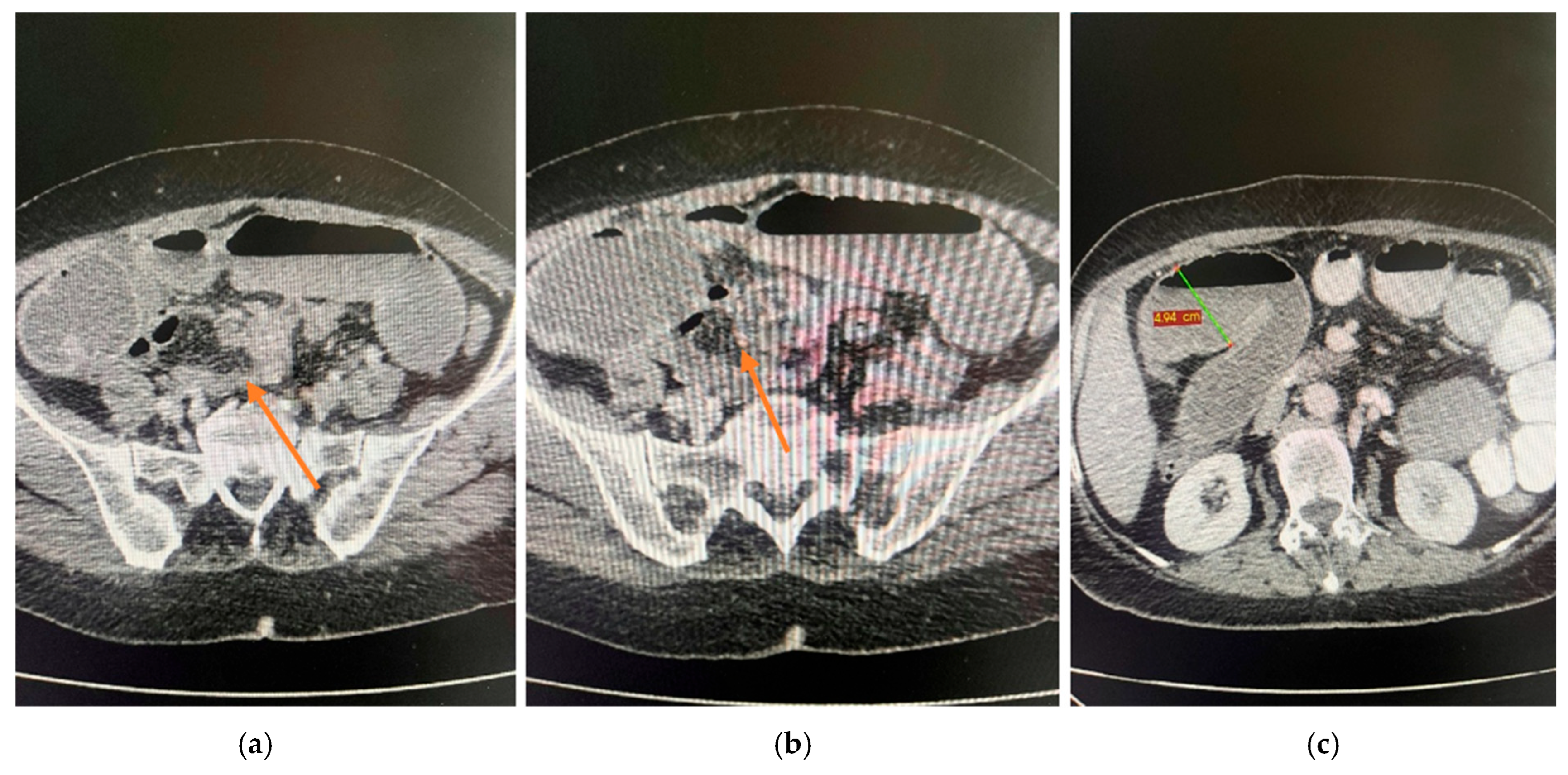
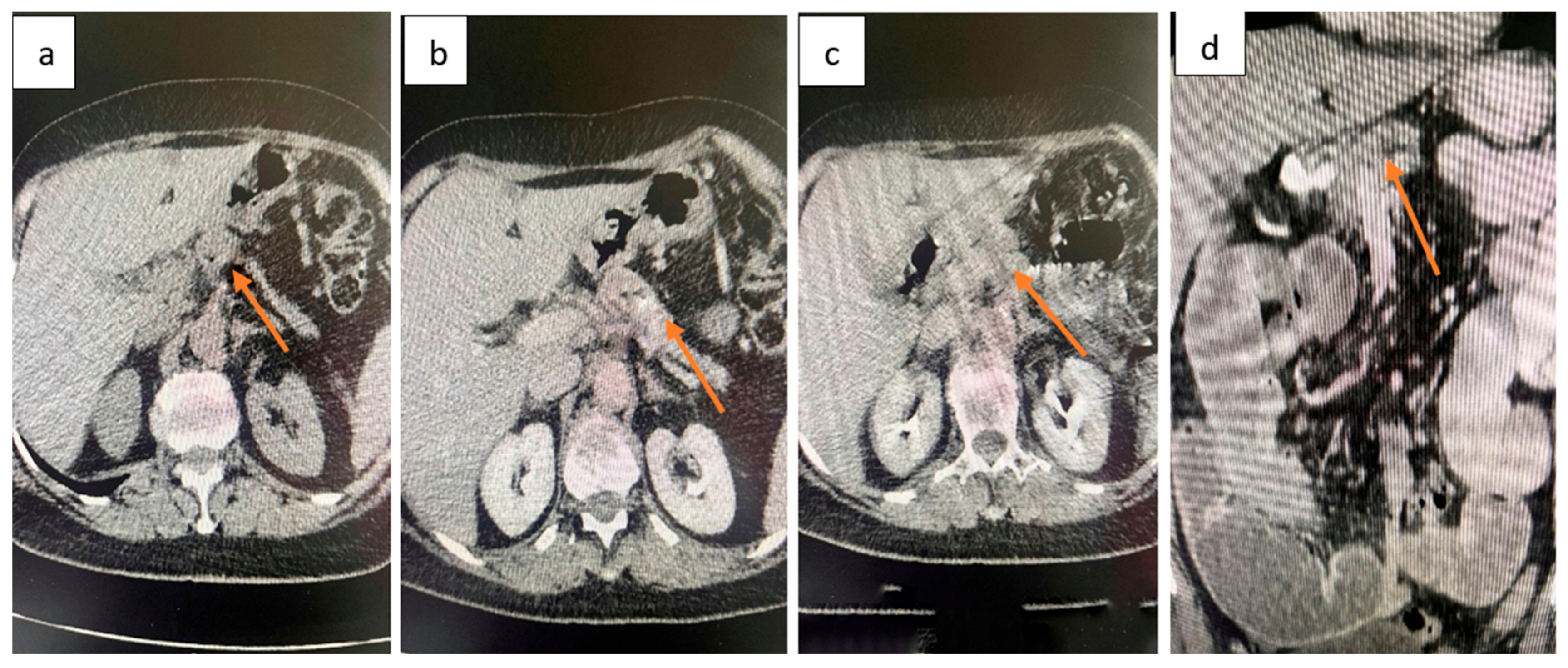



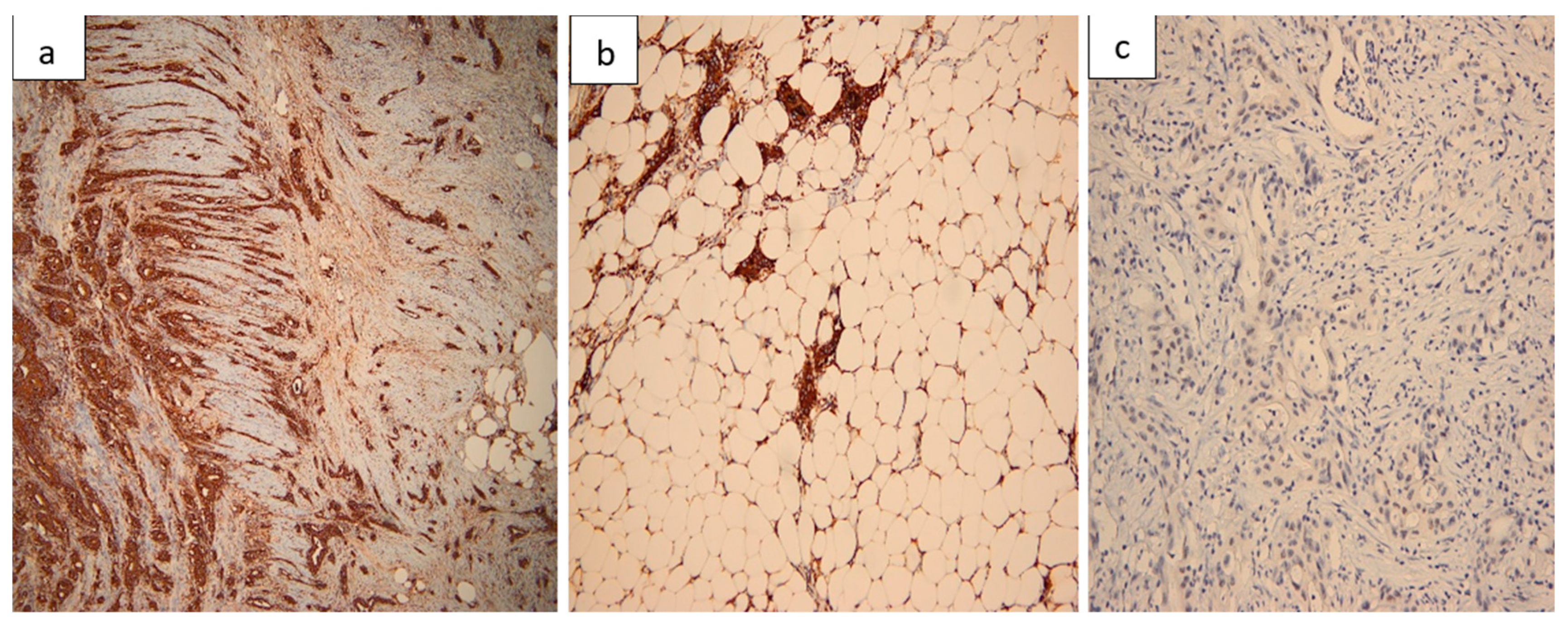
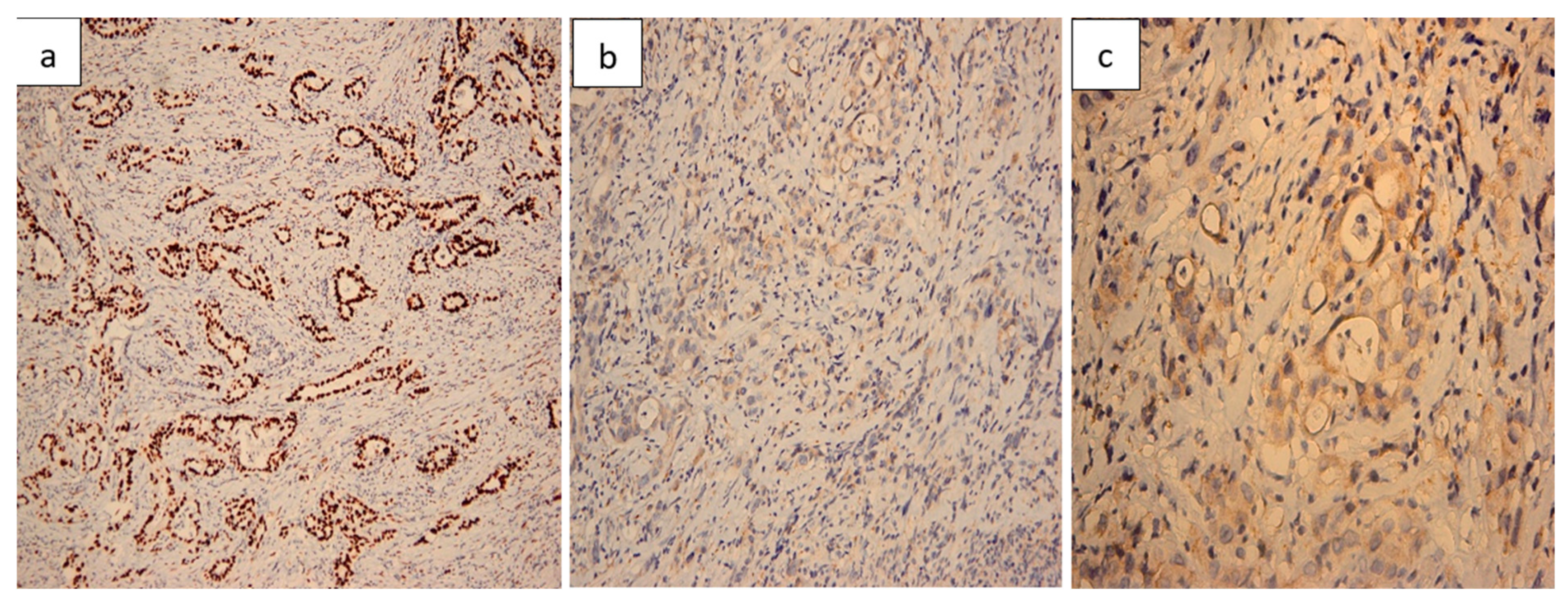

| Day | Clinical Event |
|---|---|
| Day 0 | Onset of diffuse abdominal pain, fecaloid vomiting, and absence of intestinal transit. |
| Day 2 | Admission to hospital; clinical and imaging workup performed. |
| Day 2 (evening) | Decision for surgical intervention; exploratory laparotomy performed. |
| Postoperative Days 1–3 | Gradual clinical improvement; ileostomy functional; oral intake initiated. |
| Postoperative Days 4–9 | Continued recovery without complications. |
| Postoperative Day 10 | Discharged home in good general condition. |
| Parameter | Result | Normal Range |
|---|---|---|
| White blood cell count | 13.40 × 103/μL | 4–10 × 103/μL |
| Neutrophil proportion | 77.9% | 40–70% |
| Hemoglobin | 13.4 g/dL | 12–16 g/dL |
| Platelet count | 304 × 103/μL | 150–450 × 103/μL |
| Creatinine | 0.7 mg/dL | 0.6–1.3 mg/dL |
| Urea | 28.37 mg/dL | 15–45 mg/dL |
| INR | 1.21 | 0.8–1.2 |
| Prothrombin time | 14.3 s | 11–15 s |
| Fibrinogen | 1115 mg/dL | 200–400 mg/dL |
| C-reactive protein | — | <0.5 mg/dL |
| ESR | 55 mm/h | <20 mm/h |
| CEA | 1.23 ng/mL | <5 ng/mL |
| CA 19-9 | 53.11 U/mL | <37 U/mL |
| Author/Year | Patient (Age/Sex) | Interval from Primary PDAC | Site of Metastasis | Presentation | Management | Outcome |
|---|---|---|---|---|---|---|
| Fasano [10] | Long-term survivor (M) | Synchronous | Small bowel | Acute abdomen | Emergency small bowel resection | First described intestinal metastasis in long-term PDAC survivor with mesothelioma |
| Miyasaka [9] | 63/M | 3 months post-pancreaticoduodenectomy | Jejunum + colon | Abdominal pain, fever, diarrhea | Emergency resection (jejunum + colon) | Death at 7 months due to relapse |
| Nakaya [27] | 72/M | Synchronous | Colon (within preexisting carcinoma) | Bowel obstruction | Colectomy | Rare phenomenon of PDAC metastasis colonizing colon carcinoma |
| Park [28] | 73/F | Synchronous | Transverse colon | Large bowel obstruction | Segmental colectomy | Palliative survival, <1 year |
| Kahl [29] | 91/F | Synchronous | Sigmoid colon | Large bowel obstruction | Sigmoid colectomy | Died shortly after surgery |
| Meng [11] | 65/M | Concomitant | Sigmoid colon | Colon lesion detected on work-up | Diagnosis confirmed by IHC; chemotherapy | Extremely rare metastatic route |
| Ardalan [30] | 66/F | Synchronous | Sigmoid colon | Acute obstruction | Emergency left hemicolectomy + IHC; FOLFIRINOX | Poor tolerance, palliative course |
| O’Sullivan [24] | Cases (2) | Synchronous | Rectum/colon | Obstruction, GI symptoms | Surgical resection/supportive | Reported as very rare synchronous cases |
| Tseng [8] | 56/F | ~5 years post-pancreaticoduodenectomy | Jejunum | Anemia (Hb 8–9 g/dL), occult bleeding | Segmental jejunal resection | Uneventful recovery; continued systemic therapy |
| Pacheco [31] | 68/M | Metachronous (interval not specified) | Sigmoid colon | Symptomatic metastasis, bowel obstruction | Surgical resection | Reported as the third symptomatic colonic metastasis from PDAC |
| Present case (2025) | F | Concomitant | Ileum | Acute intestinal obstruction | Emergency ileal resection + anastomosis | Favorable recovery; referred to oncology. |
| Practice Point | Rationale |
|---|---|
| When technically feasible, target submucosa | Superficial mucosal biopsies may mimic a colorectal phenotype; submucosal sampling can improve diagnostic accuracy, but should not be considered mandatory |
| Always run CK7/CK20/CDX2/SATB2 ± MUC1/SMAD4 | Distinguishes PDAC metastasis (CK7+/CDX2−/SATB2−) from primary intestinal adenocarcinoma. |
| Re-discuss at MDT before major surgery | Avoids inappropriate CRC-style resections and aligns care with systemic PDAC therapy. Avoiding inappropriate surgery in such rare scenarios is not based on a standardized algorithm, but rather on individualized, case-by-case multidisciplinary decision-making, aimed at aligning management with the underlying pancreatic primary. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Țenea Cojan, T.S.; Șurlin, V.; Mogoantă, S.-S.; Mărgăritescu, N.-D.; Caragea, D.-C.; Țenea Cojan, I.-A.; Căluianu, V.; Marinaș, M.C.; Mogoș, G.F.R.; Vasile, L.; et al. Synchronous Ileal Metastasis from Pancreatic Ductal Adenocarcinoma: Case Report and Narrative Review with Practical Diagnostic and Management Points. Life 2025, 15, 1684. https://doi.org/10.3390/life15111684
Țenea Cojan TS, Șurlin V, Mogoantă S-S, Mărgăritescu N-D, Caragea D-C, Țenea Cojan I-A, Căluianu V, Marinaș MC, Mogoș GFR, Vasile L, et al. Synchronous Ileal Metastasis from Pancreatic Ductal Adenocarcinoma: Case Report and Narrative Review with Practical Diagnostic and Management Points. Life. 2025; 15(11):1684. https://doi.org/10.3390/life15111684
Chicago/Turabian StyleȚenea Cojan, Tiberiu Stefăniță, Valeriu Șurlin, Stelian-Stefaniță Mogoantă, Nicolae-Dragoș Mărgăritescu, Daniel-Cosmin Caragea, Ioana-Alexia Țenea Cojan, Valentina Căluianu, Marius Cristian Marinaș, Gabriel Florin Răzvan Mogoș, Liviu Vasile, and et al. 2025. "Synchronous Ileal Metastasis from Pancreatic Ductal Adenocarcinoma: Case Report and Narrative Review with Practical Diagnostic and Management Points" Life 15, no. 11: 1684. https://doi.org/10.3390/life15111684
APA StyleȚenea Cojan, T. S., Șurlin, V., Mogoantă, S.-S., Mărgăritescu, N.-D., Caragea, D.-C., Țenea Cojan, I.-A., Căluianu, V., Marinaș, M. C., Mogoș, G. F. R., Vasile, L., & Barbu, L. A. (2025). Synchronous Ileal Metastasis from Pancreatic Ductal Adenocarcinoma: Case Report and Narrative Review with Practical Diagnostic and Management Points. Life, 15(11), 1684. https://doi.org/10.3390/life15111684






