In this retrospective cohort of 98 patients admitted for variceal upper gastrointestinal bleeding between 2009 and 2014, we observed outcomes that are consistent with the historical burden of this condition. Despite advances in diagnosis and endoscopic therapy, VUGIB continues to carry substantial short-term mortality, in line with the reported 6-week mortality of 15–20% in international cohorts [
1]. Our findings underscore the importance of early recognition, timely admission, and the adoption of evidence-based therapeutic strategies.
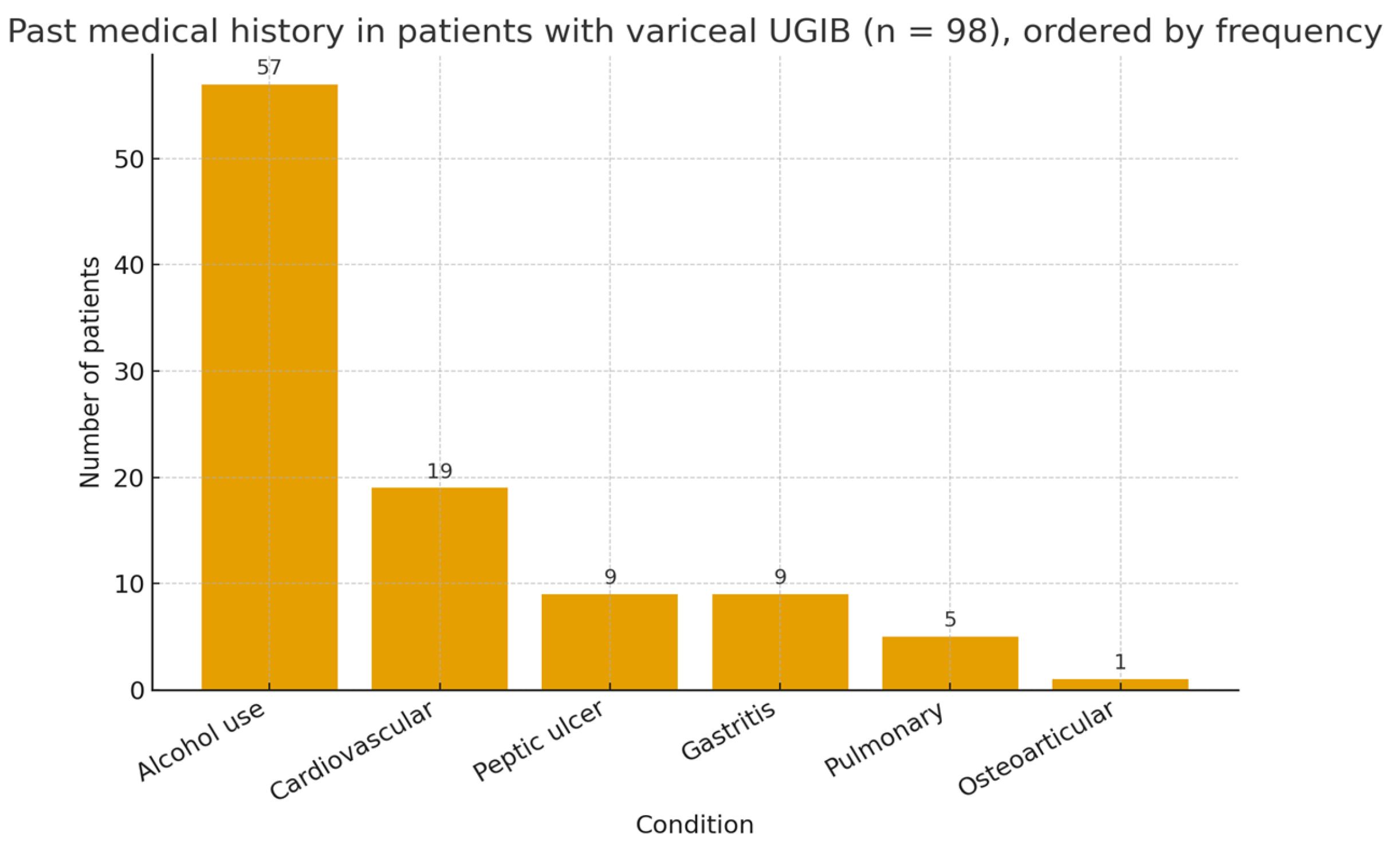
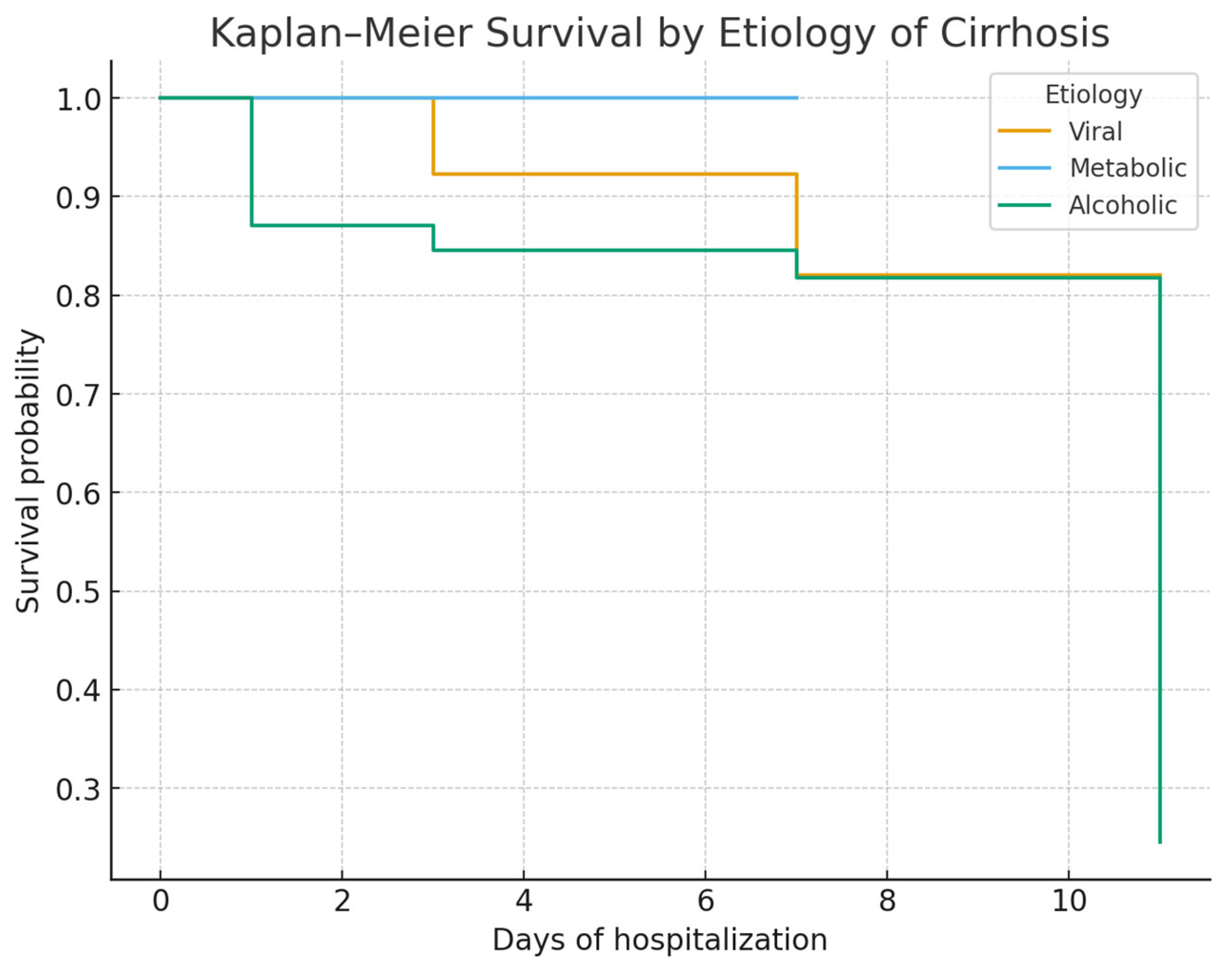
Our study confirms that mortality in VUGIB is driven primarily by the severity of hemorrhage at admission and by delays in hospital presentation, rather than by the underlying etiology of cirrhosis. In our cohort, alcoholic cirrhosis was predominant, in accordance with regional epidemiology and prior Eastern European studies, while viral and metabolic etiologies were less frequent. The predominance of alcoholic cirrhosis may reflect the regional patterns of alcohol consumption and the associated liver disease burden. These findings highlight the need for rapid intervention and appropriate risk stratification regardless of cirrhosis etiology.
4.1. Prognostic Factors and Comparison with Literature
Our study confirms that mortality in variceal upper gastrointestinal bleeding (UGIB) is determined mainly by hemorrhage severity at admission and delays in hospital presentation, rather than by cirrhosis etiology. Alcoholic cirrhosis predominated in our cohort (73%), consistent with Romanian and international data [
8,
9], while esophageal varices were the leading bleeding source [
10]. The overall mortality of 17.3% aligns with European reports (15–22%) [
11].
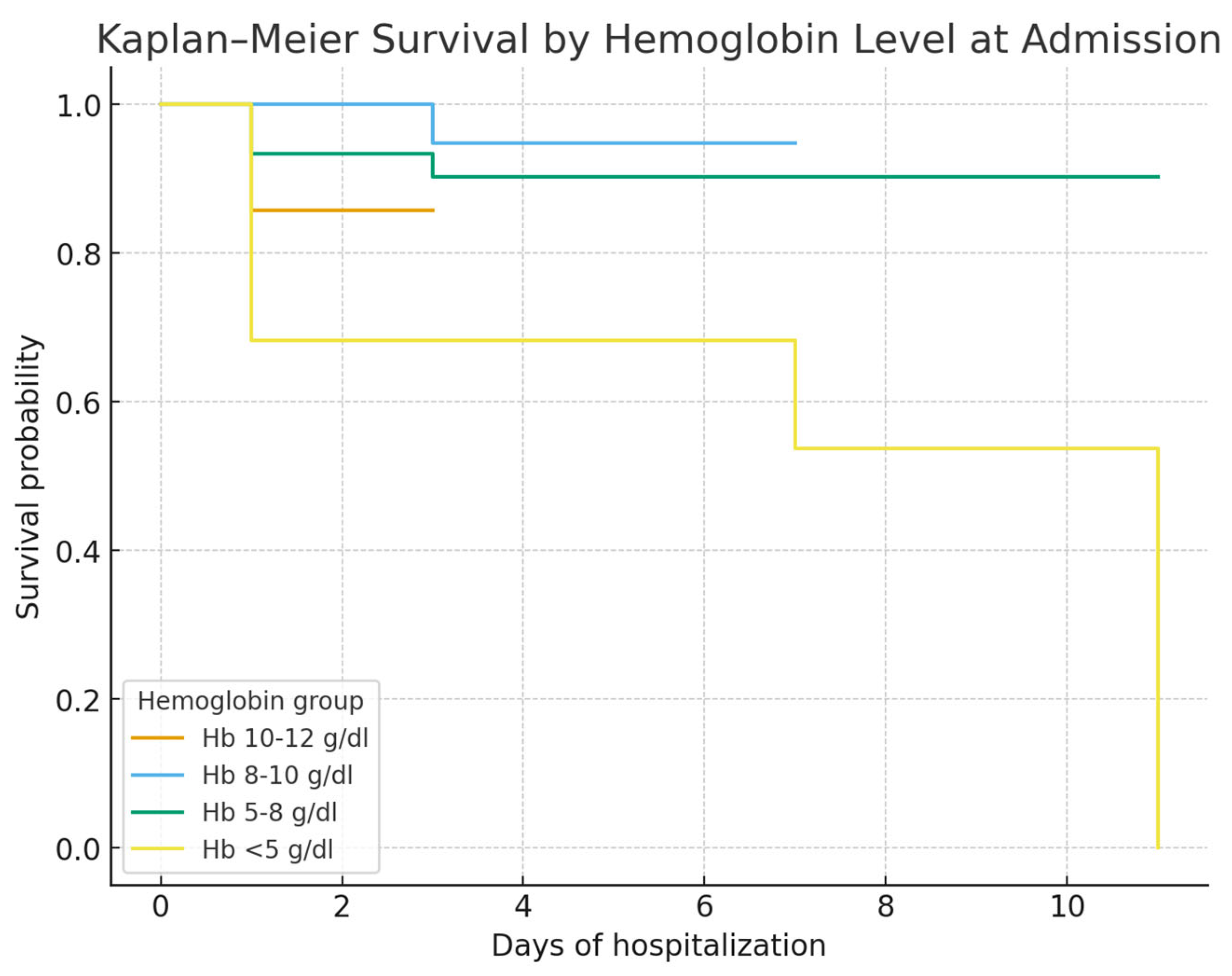
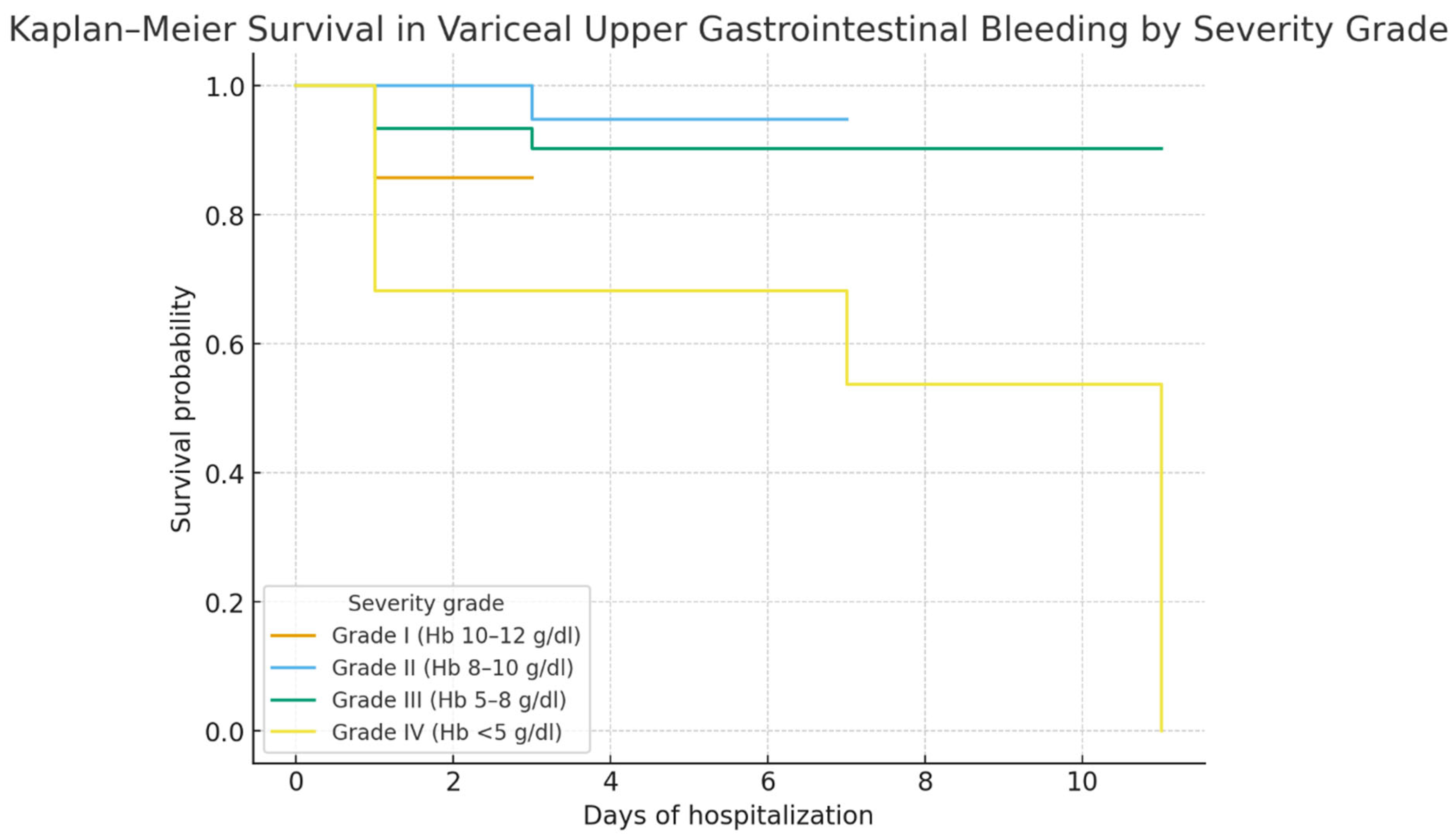
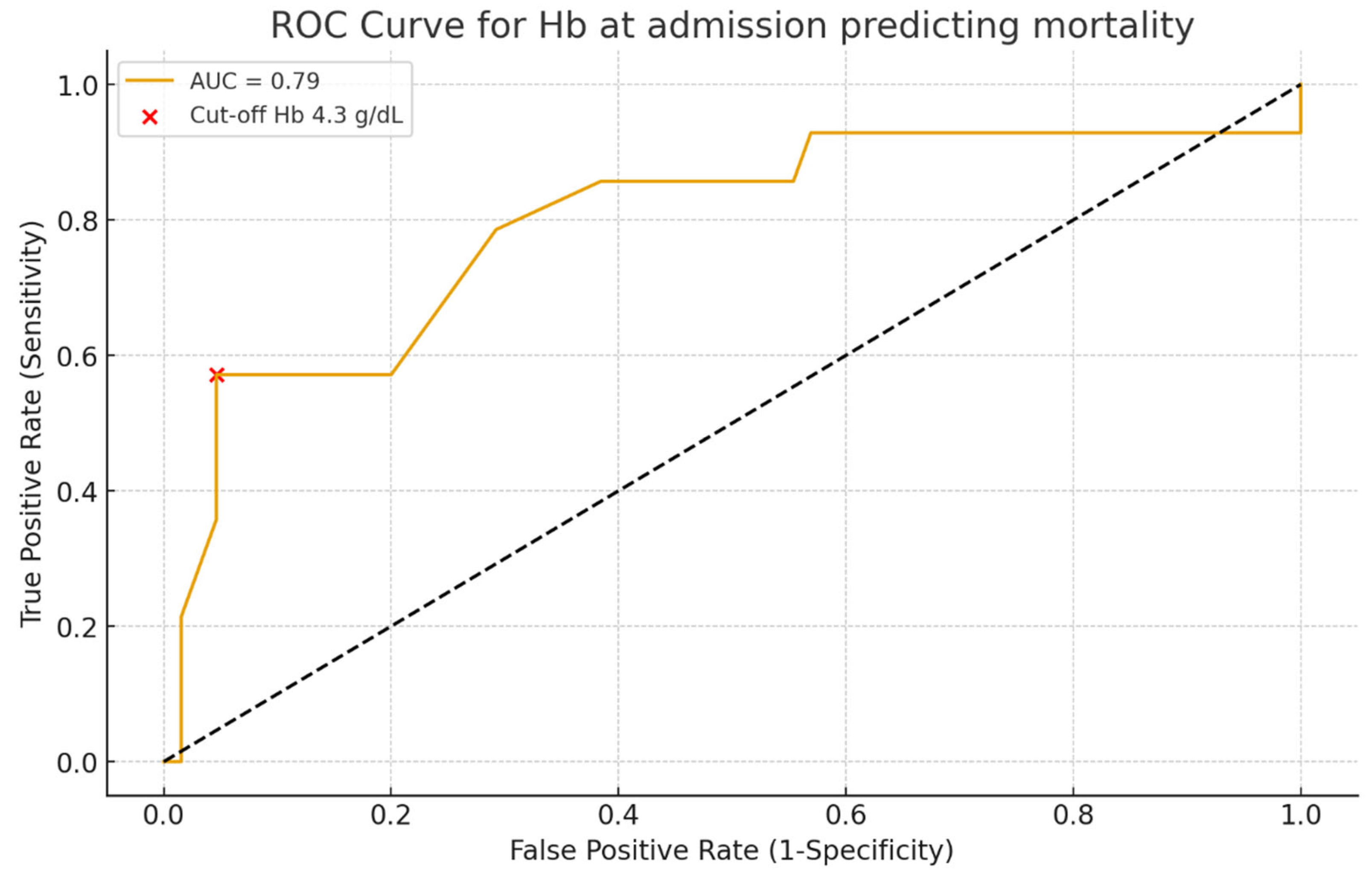
Hemoglobin on admission was a strong prognostic marker: patients with Hb < 5 g/dL had the poorest survival, consistent with the concept that profound anemia reflects uncontrolled bleeding and limited reserve. ROC curve analysis confirmed its discriminative value (AUC 0.79), though interpretation should consider other clinical factors [
12,
13]. Admission delay was also critical: both late presentation (>48–72 h) and very early admission (<6 h) were linked to higher mortality, reflecting the combined risks of uncontrolled bleeding and delayed intervention [
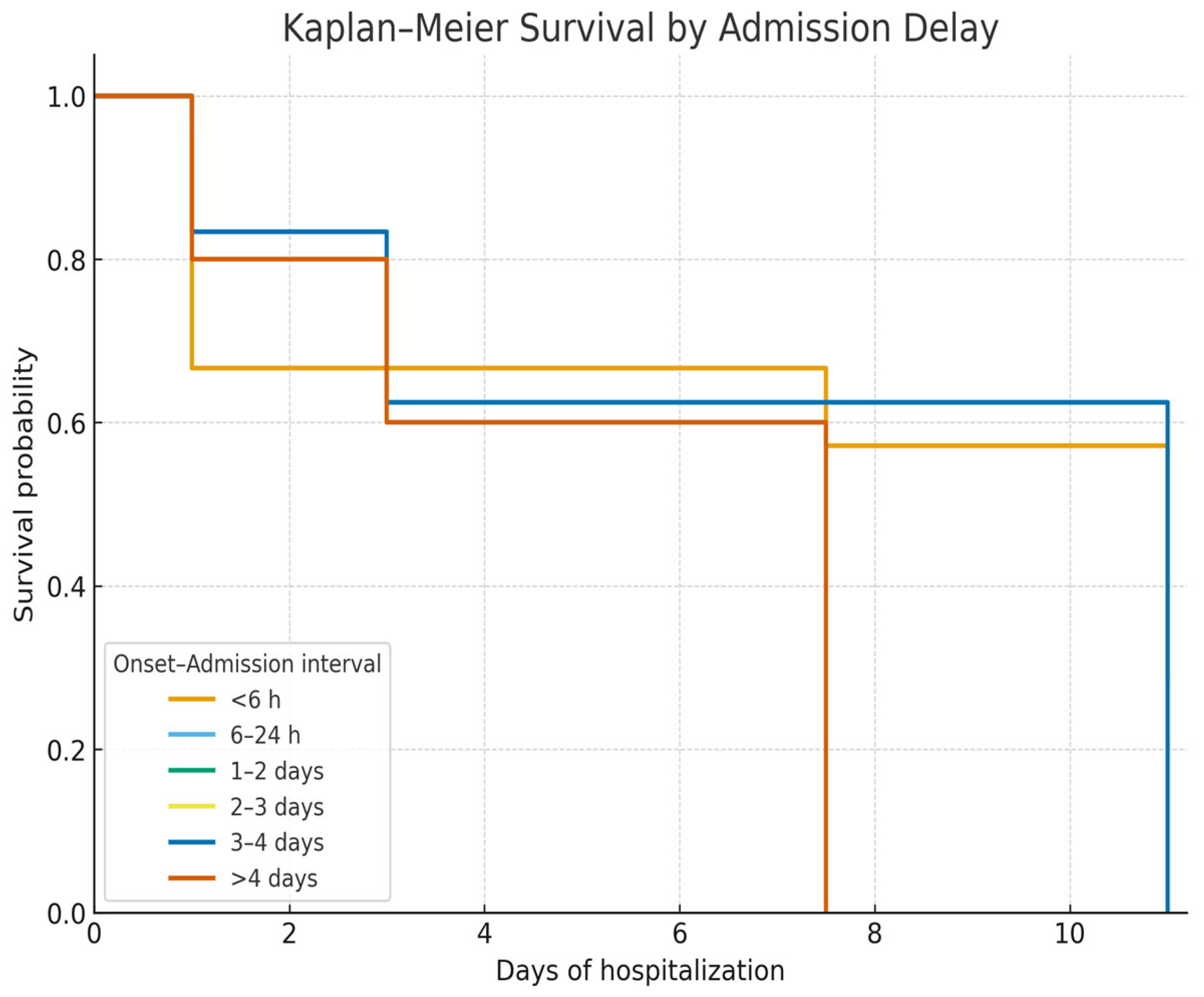
14].
The underuse of pre-emptive TIPS in our historical cohort may have contributed to higher mortality. Prospective trials, notably García-Pagán et al., demonstrated survival benefit from early TIPS in Child–Pugh B/C patients [
13], supporting current Baveno VII consensus, which emphasizes immediate vasoactive therapy, prophylactic antibiotics, and endoscopy within 12 h [
15].
Taken together, our findings highlight hemoglobin levels and admission delay as key prognostic factors, reinforcing the importance of rapid referral, early endoscopy, and optimized transfusion strategies. These elements should be integrated into risk stratification and management algorithms to improve outcomes in variceal UGIB [
13,
14,
15,
16,
17,
18,
19].
4.2. Etiology and Risk Stratification
In our cohort, patients admitted more than 24 h after bleeding onset had significantly worse outcomes, underscoring the importance of rapid referral and early specialized care. Previous studies consistently show that postponing admission or delaying therapeutic endoscopy increases mortality due to uncontrolled bleeding and early rebleeding [
20,
21,
22]. Current guidelines, including ESGE and Baveno VII, recommend endoscopy within 12 h of stabilization, and our results support this timeframe [
15,
23].
Bleeding severity was another strong predictor: profound anemia (Hb < 8 g/dL, especially <5 g/dL) was closely linked to mortality, consistent with evidence that hemoglobin reflects both blood loss and prognosis [
24,
25,
26,
27]. Restrictive transfusion strategies (Hb 7–9 g/dL) have been shown to reduce rebleeding and death, with the pivotal trial by Villanueva et al. confirming improved survival and bleeding control [
12,
28,
29].
Anemia severity and admission delay often coexisted, with late presenters showing greater hemodynamic instability, deeper anemia, and organ dysfunction, all contributing to poor outcomes [
20,
21,
27]. These findings emphasize the need for efficient prehospital triage, early vasoactive therapy, restrictive but timely transfusion, and urgent endoscopic intervention.
In summary, our findings identify delayed presentation and severe anemia at admission as critical predictors of mortality in variceal bleeding, reinforcing the importance of rapid referral systems and adherence to guideline-based transfusion and endoscopic strategies [
15,
23,
28].
4.3. Impact of Admission Delay and Initial Severity
Admission delay beyond 24 h was strongly associated with reduced survival, emphasizing the importance of timely referral and early intervention in variceal upper gastrointestinal bleeding (VUGIB). Prior studies have shown that postponing hospital admission or endoscopic therapy significantly increases mortality through uncontrolled hemorrhage and early rebleeding [
20,
21,
22]. Current ESGE and Baveno VII recommendations advise endoscopy within 12 h of stabilization, and our findings support this threshold [
15,
23].
Bleeding severity was another major prognostic factor. Severe anemia (Hb < 8 g/dL), particularly <5 g/dL, was independently linked to mortality, consistent with evidence that low hemoglobin reflects both bleeding severity and poor prognosis [
24,
25,
27]. Randomized trials and meta-analyses confirm that a restrictive transfusion strategy (Hb 7–9 g/dL) lowers rebleeding and mortality compared with liberal thresholds [
28,
29], as demonstrated in the pivotal trial by Villanueva et al. [
12].
Notably, admission delay and anemia severity are interrelated, with late presenters more likely to exhibit hemodynamic instability, profound anemia, and multi-organ dysfunction, thereby amplifying risk [
20,
21,
27]. This interplay underscores the need for coordinated prehospital triage, early vasoactive therapy, restrictive transfusion, and urgent endoscopy.
Overall, our findings confirm that delayed admission and severe anemia are key predictors of mortality in VUGIB, consistent with international guidelines [
15,
23,
28], and highlight opportunities to improve survival through optimized referral systems and adherence to restrictive transfusion policies.
4.4. Therapeutic Strategies and Historical Context
During the study period (2009–2014), endoscopic therapy—mainly band ligation and, less frequently, sclerotherapy—was the cornerstone of treatment for variceal upper gastrointestinal bleeding in our center. Pharmacological therapy with vasoactive agents was inconsistently administered, and pre-emptive TIPS was rarely available. These limitations, reflecting both therapeutic standards of the time and resource constraints typical of many Eastern European centers, likely contributed to the suboptimal survival compared with contemporary series from Western Europe and North America [
30,
31].
Since then, therapeutic strategies have evolved substantially. Current consensus emphasizes a combined approach: immediate initiation of vasoactive therapy, routine administration of broad-spectrum antibiotics, and endoscopic band ligation within 12 h of admission [
32]. Endoscopic sclerotherapy, once common, has been largely abandoned in favor of band ligation due to its superior safety profile [
32].
A major advance has been the use of pre-emptive TIPS in selected high-risk patients (Child–Pugh C < 14 or Child–Pugh B > 7 with active bleeding). Randomized trials demonstrated that early TIPS reduces treatment failure and improves survival [
33], although access remains limited in many low- and middle-income countries [
34].
Our findings underscore the gap between available therapies during the study period and current evidence-based standards. Patients were treated mainly with conventional endoscopic procedures, while interventions now considered essential—such as early vasoactive therapy, prophylactic antibiotics, and pre-emptive TIPS—were not systematically applied. This therapeutic gap likely contributed to the higher mortality observed, reinforcing that adherence to guideline-based protocols can significantly improve prognosis in acute variceal bleeding [
32,
33]. It should be emphasized that our cohort reflects a historical period (2009–2014), when current standards of care such as early vasoactive therapy, routine prophylactic antibiotics, restrictive transfusion, and pre-emptive TIPS were not consistently applied. Therefore, the outcomes observed in our series should not be interpreted as representative of present-day management but rather as a benchmark of real-world practice in Eastern Europe at that time. This historical perspective also highlights the progress achieved over the last decade and underscores the survival gains expected with contemporary evidence-based strategies.
4.9. Coagulation Management and Adjunctive Therapies
Traditionally, cirrhosis was viewed as a hypo-coagulable state, with variceal hemorrhage attributed to impaired clotting factor synthesis and thrombocytopenia. More recent data support the concept of “rebalanced hemostasis,” in which decreased procoagulant activity is offset by reduced anticoagulant function and endothelial dysfunction, placing patients at risk for both bleeding and thrombosis, particularly portal vein thrombosis [
30,
32,
42].
These insights have reshaped transfusion strategies. Fresh frozen plasma and platelet concentrates were previously given prophylactically for elevated INR or low platelet counts, but evidence shows that INR is not a reliable predictor of bleeding and that indiscriminate transfusion may increase portal pressure and rebleeding risk [
30]. Current guidelines therefore recommend restricting transfusion to cases of severe thrombocytopenia (<50,000/µL) or uncontrolled hemorrhage [
33,
49].
Viscoelastic assays such as thrombo-elastography (TEG) and rotational thrombo-elastometry (ROTEM) provide real-time assessment of clot dynamics, offering a more accurate measure of hemostasis than conventional assays. Their use can reduce unnecessary transfusions without increasing bleeding complications in cirrhotic patients undergoing invasive procedures [
34].
Adjunctive pharmacologic measures remain crucial. Prophylactic antibiotics are essential given the adverse impact of bacterial infections on outcomes [
50], while proton pump inhibitors are often used peri-endoscopically to minimize post-procedural ulcer risk. Antifibrinolytics have been explored but lack sufficient evidence for routine use.
In summary, coagulation management in cirrhosis should balance bleeding and thrombosis risk through restrictive transfusion policies, integration of viscoelastic testing, and targeted adjunctive therapies as part of evidence-based variceal bleeding care.
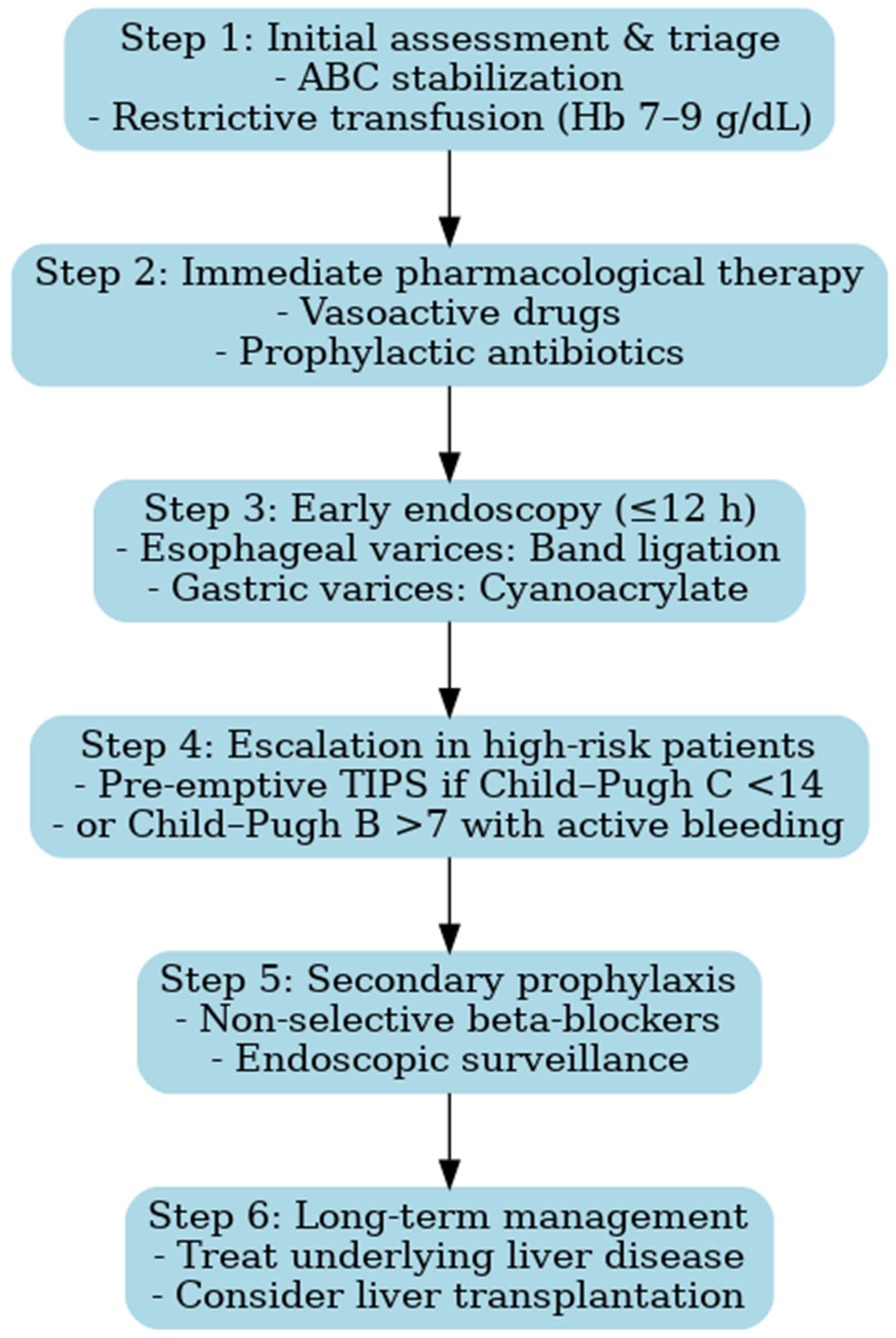
4.10. Clinical Implications and Proposal for an Updated Algorithm
The findings of our study have important clinical implications. Despite therapeutic advances, outcomes in variceal upper gastrointestinal bleeding (VUGIB) remain largely determined by admission delay and bleeding severity. This underscores the need for streamlined pathways that enable early recognition, rapid referral, and strict adherence to evidence-based protocols.
Recent consensus statements, including ESGE (2022), Baveno VII (2022), and AEEH (2025), provide standardized frameworks for acute variceal bleeding management. Integrating these recommendations with our observations, we propose a revised diagnostic and therapeutic algorithm that reflects contemporary standards while addressing gaps identified in routine practice [
15,
51]:
Early triage and resuscitation with restrictive transfusion—Patients should be rapidly identified at first contact and transferred to specialized centers whenever possible. Hemodynamic stabilization must be prioritized, with a restrictive transfusion strategy (target Hb 7–9 g/dL), as liberal transfusion has been associated with increased portal pressure and higher risk of rebleeding [
15,
23].
Prompt initiation of vasoactive drugs and antibiotics—Vasoactive agents (terlipressin, somatostatin, or octreotide) should be started immediately upon suspicion of variceal bleeding, before endoscopic confirmation, and continued for 2–5 days. In parallel, broad-spectrum antibiotics are mandatory to reduce the incidence of bacterial infections, which can precipitate rebleeding and worsen survival [
42,
51].
Early endoscopy within 12 h—Endoscopic band ligation is the first-line treatment for esophageal varices and should be performed within 12 h of admission, once the patient is stabilized. Sclerotherapy may be considered only if band ligation is not feasible. For gastric varices, cyanoacrylate injection is recommended [
42,
51,
52,
53].
Pre-emptive TIPS in high-risk patients—In patients with advanced liver disease (Child–Pugh C < 14 points or Child–Pugh B > 7 with active bleeding), early TIPS placement within 72 h has been shown to reduce treatment failure and improve survival [
42,
53]. The adoption of this strategy represents a paradigm shift compared to the period covered by our study, when TIPS was rarely available.
Secondary prophylaxis with non-selective beta-blockers (NSBBs) plus endoscopic surveillance—To prevent recurrent bleeding, combination therapy with NSBBs (e.g., propranolol or carvedilol) and repeated band ligation is recommended. This approach reduces both rebleeding rates and overall mortality [
12,
42].
The implementation of such an algorithm is expected to improve both short- and long-term outcomes, particularly in healthcare settings where gaps between guideline recommendations and real-world practice remain (
Figure 7). The diagnostic and therapeutic algorithm proposed here does not aim to introduce novel concepts beyond established international guidelines. Its main value lies in contextualizing these recommendations for Eastern European healthcare systems, where resources may be limited and access to advanced interventions such as pre-emptive TIPS remains restricted. By integrating evidence-based strategies into a practical framework adapted to local realities, the algorithm provides clinicians with a structured approach to patient management in similar settings. Moreover, it illustrates the progress achieved since the 2009–2014 study period and highlights the contrast between historical practices and current standards of care.
4.11. Strengths and Limitations
This study has notable strengths, including its status as one of the largest single-center retrospective cohorts of variceal upper gastrointestinal bleeding (VUGIB) from Eastern Europe, comprising 98 consecutive cases over a six-year period. The comprehensive collection of clinical, laboratory, and survival data enabled robust subgroup analyses, including risk stratification by etiology, hemoglobin levels at admission, and admission delay. Additionally, survival analysis using Kaplan–Meier curves provide valuable prognostic insights and establishes a historical benchmark for evaluating practice improvements. A further strength is the comparison of our findings with contemporary international guidelines, allowing for the development of an updated diagnostic and therapeutic algorithm applicable to both high-resource and resource-limited settings.
However, several limitations must be acknowledged. The retrospective design introduces potential biases related to data completeness and accuracy. As a retrospective study, it is susceptible to selection bias, reporting bias, and missing data, which can affect the reliability of the findings. Furthermore, the single-center nature of the study limits the generalizability of the findings to other healthcare systems, particularly in countries with different clinical settings and resources. As a tertiary surgical referral center, our hospital may also have received disproportionately severe cases, potentially inflating mortality rates compared with community hospitals. The single-center design, modest sample size, and retrospective methodology inherently constrain the external validity and generalizability of our findings. These limitations should be considered when interpreting the results.
Although relatively large for a single-center study in Eastern Europe, the sample size remains modest compared with multinational cohorts, limiting the statistical power of subgroup and regression analyses. The lack of standardized hemodynamic assessment (e.g., hepatic venous pressure gradient [HVPG]) precludes a precise characterization of portal hypertension severity, an increasingly important prognostic factor in cirrhosis. Additionally, systematic information on concomitant medications, including nonsteroidal anti-inflammatory drugs (NSAIDs), antiplatelet agents, beta-blockers, anticoagulants, and antiviral therapies, was not available, limiting our ability to assess their influence on bleeding risk and survival. Although comorbidities such as cardiovascular disease, diabetes, and renal dysfunction were documented, their independent prognostic impact was not evaluated. Moreover, data on alcohol abstinence and viral suppression were lacking, which limits the ability to contextualize outcomes based on etiology.
The incomplete information regarding the dose and duration of therapies, including vasoactive agents, antibiotics, and transfusions, reduces comparability with more contemporary cohorts. Importantly, during the study period (2009–2014), all patients were admitted and managed within the Surgical Department, in line with institutional protocols of that time. This organizational factor partly explains the limited use of vasoactive drugs, prophylactic antibiotics, and pre-emptive TIPS compared with current standards of care. An important limitation of our study is therefore the outdated timeframe (2009–2014). The cohort predates key advances in variceal bleeding management, including systematic use of vasoactive drugs at presentation, prophylactic antibiotics, restrictive transfusion strategies, and early pre-emptive TIPS in high-risk patients. Consequently, the direct clinical applicability of our findings to current practice is limited. However, this limitation also provides value: the data serve as a historical benchmark for Eastern Europe, allowing meaningful comparison with contemporary outcomes and illustrating the improvements achieved by guideline-based care.
Finally, the lack of long-term follow-up beyond hospitalization and six-week survival data prevented the evaluation of recurrent bleeding and long-term prognosis, which remains a critical aspect of VUGIB management. Taken together, the strengths and limitations of this study provide context for our findings and highlight the need for future multicenter prospective studies with standardized data collection, including detailed medication history, comorbidity assessment, and therapy protocols. Such studies should integrate validated risk scores (e.g., Child–Pugh, MELD), evaluate modern therapies such as early TIPS, and bridge the gap between guideline recommendations and real-world practice.