Age-Related Alterations of Cerebral Autoregulation
Abstract
1. Introduction
2. Mechanisms of Cerebral Autoregulation
3. Age-Related Changes in Cerebral Autoregulation
3.1. Structural Changes Contributing to Impaired Autoregulation
3.2. Functional Impairments
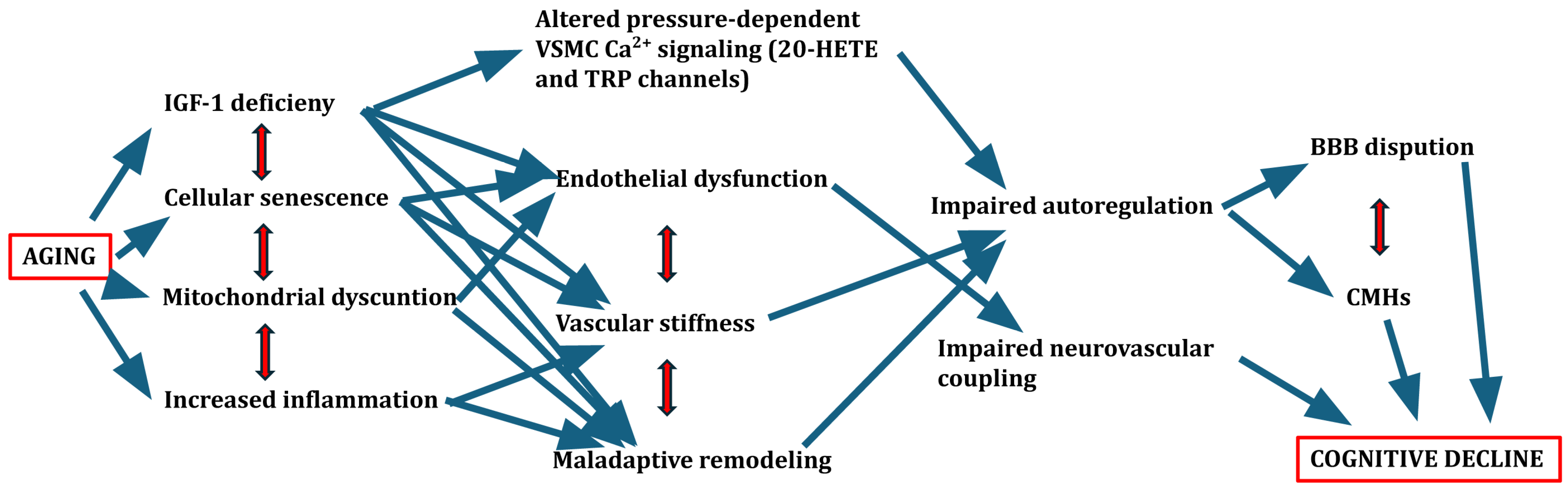
3.3. Emerging Role of Cellular and Molecular Aging Mechanisms
4. Consequences of Impaired Autoregulation in Aging
5. Methodological Considerations
6. Potential Interventions and Future Directions
7. Limitations
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lassen, N.A. Cerebral blood flow and oxygen consumption in man. Physiol. Rev. 1959, 39, 183–238. [Google Scholar] [CrossRef]
- Armstead, W.M. Cerebral Blood Flow Autoregulation and Dysautoregulation. Anesthesiol. Clin. 2016, 34, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Claassen, J.; Thijssen, D.H.J.; Panerai, R.B.; Faraci, F.M. Regulation of cerebral blood flow in humans: Physiology and clinical implications of autoregulation. Physiol. Rev. 2021, 101, 1487–1559. [Google Scholar] [CrossRef]
- Knox, E.G.; Aburto, M.R.; Clarke, G.; Cryan, J.F.; O’Driscoll, C.M. The blood-brain barrier in aging and neurodegeneration. Mol. Psychiatry 2022, 27, 2659–2673. [Google Scholar] [CrossRef]
- Kalaria, R.N. Vascular basis for brain degeneration: Faltering controls and risk factors for dementia. Nutr. Rev. 2010, 68 (Suppl. 2), S74–S87. [Google Scholar] [CrossRef]
- Weijs, R.W.; Oudegeest-Sander, M.H.; Hopman, M.T.; Thijssen, D.H.; Claassen, J.A. Cerebrovascular CO(2) reactivity and dynamic cerebral autoregulation through the eighth decade of life and their implications for cognitive decline. J. Cereb. Blood Flow. Metab. 2024, 44, 712–725. [Google Scholar] [CrossRef]
- Markus, H.S. Cerebral perfusion and stroke. J. Neurol. Neurosurg. Psychiatry 2004, 75, 353–361. [Google Scholar] [CrossRef]
- Wang, Y.; Payne, S.J. Static autoregulation in humans. J. Cereb. Blood Flow. Metab. 2024, 44, 1191–1207. [Google Scholar] [CrossRef] [PubMed]
- Abadjiev, D.S.; Toschi-Dias, E.; Salinet, A.S.; Gaykova, N.N.; Lo, M.T.; Nogueira, R.C.; Hu, K. Daily rhythm of dynamic cerebral autoregulation in patients after stroke. J. Cereb. Blood Flow. Metab. 2023, 43, 989–998. [Google Scholar] [CrossRef] [PubMed]
- Panerai, R.B.; Barnes, S.C.; Batterham, A.P.; Robinson, T.G.; Haunton, V.J. Directional sensitivity of dynamic cerebral autoregulation during spontaneous fluctuations in arterial blood pressure at rest. J. Cereb. Blood Flow. Metab. 2023, 43, 552–564. [Google Scholar] [CrossRef]
- Heutz, R.; Claassen, J.; Feiner, S.; Davies, A.; Gurung, D.; Panerai, R.B.; Heus, R.; Beishon, L.C. Dynamic cerebral autoregulation in Alzheimer’s disease and mild cognitive impairment: A systematic review. J. Cereb. Blood Flow. Metab. 2023, 43, 1223–1236. [Google Scholar] [CrossRef]
- Toth, P.; Tarantini, S.; Csiszar, A.; Ungvari, Z. Functional vascular contributions to cognitive impairment and dementia: Mechanisms and consequences of cerebral autoregulatory dysfunction, endothelial impairment, and neurovascular uncoupling in aging. Am. J. Physiol. Heart Circ. Physiol. 2017, 312, H1–H20. [Google Scholar] [CrossRef]
- Tan, C.O.; Hamner, J.W.; Taylor, J.A. The role of myogenic mechanisms in human cerebrovascular regulation. J. Physiol. 2013, 591, 5095–5105. [Google Scholar] [CrossRef] [PubMed]
- Newell, D.W.; Aaslid, R.; Lam, A.; Mayberg, T.S.; Winn, H.R. Comparison of flow and velocity during dynamic autoregulation testing in humans. Stroke 1994, 25, 793–797. [Google Scholar] [CrossRef]
- Ghosh, D.; Syed, A.U.; Prada, M.P.; Nystoriak, M.A.; Santana, L.F.; Nieves-Cintrón, M.; Navedo, M.F. Calcium Channels in Vascular Smooth Muscle. Adv. Pharmacol. 2017, 78, 49–87. [Google Scholar] [CrossRef]
- Davis, M.J.; Hill, M.A. Signaling mechanisms underlying the vascular myogenic response. Physiol. Rev. 1999, 79, 387–423. [Google Scholar] [CrossRef] [PubMed]
- Sommerville, L.E.; Hartshorne, D.J. Intracellular calcium and smooth muscle contraction. Cell Calcium 1986, 7, 353–364. [Google Scholar] [CrossRef]
- Brayden, J.E.; Earley, S.; Nelson, M.T.; Reading, S. Transient receptor potential (TRP) channels, vascular tone and autoregulation of cerebral blood flow. Clin. Exp. Pharmacol. Physiol. 2008, 35, 1116–1120. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Brayden, J.E. Rho kinase activity governs arteriolar myogenic depolarization. J. Cereb. Blood Flow. Metab. 2017, 37, 140–152. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, J.; Tong, X. Cross-Talk between Mechanosensitive Ion Channels and Calcium Regulatory Proteins in Cardiovascular Health and Disease. Int. J. Mol. Sci. 2021, 22, 8782. [Google Scholar] [CrossRef]
- Ainslie, P.N.; Brassard, P. Why is the neural control of cerebral autoregulation so controversial? F1000Prime Rep. 2014, 6, 14. [Google Scholar] [CrossRef] [PubMed]
- Murkin, J.M. Cerebral autoregulation: The role of CO2 in metabolic homeostasis. Semin. Cardiothorac. Vasc. Anesth. 2007, 11, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Winn, H.R.; Morii, S.; Berne, R.M. The role of adenosine in autoregulation of cerebral blood flow. Ann. Biomed. Eng. 1985, 13, 321–328. [Google Scholar] [CrossRef]
- Mascia, L.; Piper, I.R.; Andrews, P.J.; Souter, M.J.; Webb, D.J. The role of endothelin-1 in pressure autoregulation of cerebral blood flow in rats. Intensive Care Med. 1999, 25, 1282–1286. [Google Scholar] [CrossRef]
- Buchanan, J.E.; Phillis, J.W. The role of nitric oxide in the regulation of cerebral blood flow. Brain Res. 1993, 610, 248–255. [Google Scholar] [CrossRef]
- Leffler, C.W.; Parfenova, H. Cerebral arteriolar dilation to hypoxia: Role of prostanoids. Am. J. Physiol. 1997, 272, H418–H424. [Google Scholar] [CrossRef] [PubMed]
- Iadecola, C.; Davisson, R.L. Hypertension and cerebrovascular dysfunction. Cell Metab. 2008, 7, 476–484. [Google Scholar] [CrossRef]
- van Hespen, K.M.; Mackaaij, C.; Waas, I.S.E.; de Bree, M.P.; Zwanenburg, J.J.M.; Kuijf, H.J.; Daemen, M.; Hendrikse, J.; Hermkens, D.M.A. Arterial Remodeling of the Intracranial Arteries in Patients With Hypertension and Controls: A Postmortem Study. Hypertension 2021, 77, 135–146. [Google Scholar] [CrossRef]
- Toth, P.; Csiszar, A.; Tucsek, Z.; Sosnowska, D.; Gautam, T.; Koller, A.; Schwartzman, M.L.; Sonntag, W.E.; Ungvari, Z. Role of 20-HETE, TRPC channels, and BKCa in dysregulation of pressure-induced Ca2+ signaling and myogenic constriction of cerebral arteries in aged hypertensive mice. Am. J. Physiol. Heart Circ. Physiol. 2013, 305, H1698–H1708. [Google Scholar] [CrossRef]
- Bareiro, F.A.Q.; Carnicero, J.A.; Acha, A.A.; Artalejo, C.R.; Jimenez, M.C.G.; Manas, L.R.; Garcia Garcia, F.J. How cognitive performance changes according to the ankle-brachial index score in an elderly cohort? Results from the Toledo Study of Healthy Ageing. Geroscience 2024, 46, 609–620. [Google Scholar] [CrossRef]
- Zhang, H.; Leng, S.; Gao, F.; Kovalik, J.P.; Tan, R.S.; Wee, H.N.; Chua, K.V.; Ching, J.; Zhao, X.; Allen, J.; et al. Longitudinal aortic strain, ventriculo-arterial coupling and fatty acid oxidation: Novel insights into human cardiovascular aging. Geroscience 2024, 46, 5459–5471. [Google Scholar] [CrossRef]
- Bareiro, F.A.Q.; Carnicero, J.A.; Acha, A.A.; Artalejo, C.R.; Jimenez, M.C.G.; Manas, L.R.; Garcia Garcia, F.J. Carotid-femoral pulse wave velocity score, an estimator of cognitive performance in the elderly: Results from the Toledo Study for Healthy Aging. Geroscience 2024, 46, 5711–5723. [Google Scholar] [CrossRef] [PubMed]
- Reeve, E.H.; Barnes, J.N.; Moir, M.E.; Walker, A.E. Impact of arterial stiffness on cerebrovascular function: A review of evidence from humans and preclincal models. Am. J. Physiol. Heart Circ. Physiol. 2024, 326, H689–H704. [Google Scholar] [CrossRef]
- Yam, A.T.; Lang, E.W.; Lagopoulos, J.; Yip, K.; Griffith, J.; Mudaliar, Y.; Dorsch, N.W. Cerebral autoregulation and ageing. J. Clin. Neurosci. 2005, 12, 643–646. [Google Scholar] [CrossRef]
- van Beek, A.H.; Claassen, J.A.; Rikkert, M.G.; Jansen, R.W. Cerebral autoregulation: An overview of current concepts and methodology with special focus on the elderly. J. Cereb. Blood Flow. Metab. 2008, 28, 1071–1085. [Google Scholar] [CrossRef]
- Tarumi, T.; Zhang, R. Cerebral blood flow in normal aging adults: Cardiovascular determinants, clinical implications, and aerobic fitness. J. Neurochem. 2018, 144, 595–608. [Google Scholar] [CrossRef] [PubMed]
- Tomoto, T.; Lu, M.; Khan, A.M.; Liu, J.; Pasha, E.P.; Tarumi, T.; Zhang, R. Cerebral blood flow and cerebrovascular resistance across the adult lifespan: A multimodality approach. J. Cereb. Blood Flow. Metab. 2023, 43, 962–976. [Google Scholar] [CrossRef]
- Hoffman, W.E.; Albrecht, R.F.; Miletich, D.J. The influence of aging and hypertension on cerebral autoregulation. Brain Res. 1981, 214, 196–199. [Google Scholar] [CrossRef]
- Armstrong, M.K.; Jain, S.; Nuckols, V.; Pewowaruk, R.; Zhang, X.; DuBose, L.; Sodoma, M.; Madero, B.; Voss, M.W.; Pierce, G.L. The association of structural versus load-dependent large artery stiffness mechanisms with cerebrovascular damage and cortical atrophy in humans. Geroscience 2024, 46, 5587–5597. [Google Scholar] [CrossRef]
- van Dijk, S.E.; Drenth, N.; Hafkemeijer, A.; Labadie, G.; Witjes-Ane, M.W.; Blauw, G.J.; Rombouts, S.A.; van der Grond, J.; van Rooden, S. Neurovascular coupling in early stage dementia - A case-control study. J. Cereb. Blood Flow. Metab. 2024, 44, 1013–1023. [Google Scholar] [CrossRef] [PubMed]
- Ball, J.D.; Hills, E.; Altaf, A.; Ramesh, P.; Green, M.; Surti, F.B.; Minhas, J.S.; Robinson, T.G.; Bond, B.; Lester, A.; et al. Neurovascular coupling methods in healthy individuals using transcranial doppler ultrasonography: A systematic review and consensus agreement. J. Cereb. Blood Flow. Metab. 2024, 44, 1409–1429. [Google Scholar] [CrossRef]
- Lourenço, C.F.; Ledo, A.; Caetano, M.; Barbosa, R.M.; Laranjinha, J. Age-Dependent Impairment of Neurovascular and Neurometabolic Coupling in the Hippocampus. Front. Physiol. 2018, 9, 913. [Google Scholar] [CrossRef] [PubMed]
- Santisteban, M.M.; Iadecola, C.; Carnevale, D. Hypertension, Neurovascular Dysfunction, and Cognitive Impairment. Hypertension 2023, 80, 22–34. [Google Scholar] [CrossRef]
- Schaum, N.; Lehallier, B.; Hahn, O.; Palovics, R.; Hosseinzadeh, S.; Lee, S.E.; Sit, R.; Lee, D.P.; Losada, P.M.; Zardeneta, M.E.; et al. Ageing hallmarks exhibit organ-specific temporal signatures. Nature 2020, 583, 596–602. [Google Scholar] [CrossRef]
- Lopez-Otin, C.; Kroemer, G. Hallmarks of health. Cell 2021, 184, 1929–1939. [Google Scholar] [CrossRef]
- Lopez-Otin, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of aging: An expanding universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef]
- Tartiere, A.G.; Freije, J.M.P.; Lopez-Otin, C. The hallmarks of aging as a conceptual framework for health and longevity research. Front. Aging 2024, 5, 1334261. [Google Scholar] [CrossRef]
- Pospiech, E.; Bar, A.; Pisarek-Pacek, A.; Karas, A.; Branicki, W.; Chlopicki, S. Epigenetic clock in the aorta and age-related endothelial dysfunction in mice. Geroscience 2024, 46, 3993–4002. [Google Scholar] [CrossRef] [PubMed]
- Karas, A.; Bar, A.; Pandian, K.; Jasztal, A.; Kurylowicz, Z.; Kutryb-Zajac, B.; Buczek, E.; Rocchetti, S.; Mohaissen, T.; Jedrzejewska, A.; et al. Functional deterioration of vascular mitochondrial and glycolytic capacity in the aortic rings of aged mice. Geroscience 2024, 46, 3831–3844. [Google Scholar] [CrossRef]
- Bencivenga, L.; Strumia, M.; Rolland, Y.; Martinez, L.; Cestac, P.; Guyonnet, S.; Andrieu, S.; Parini, A.; Lucas, A.; Vellas, B.; et al. Biomarkers of mitochondrial dysfunction and inflammaging in older adults and blood pressure variability. Geroscience 2023, 45, 797–809. [Google Scholar] [CrossRef] [PubMed]
- Ting, K.K.; Coleman, P.; Kim, H.J.; Zhao, Y.; Mulangala, J.; Cheng, N.C.; Li, W.; Gunatilake, D.; Johnstone, D.M.; Loo, L.; et al. Vascular senescence and leak are features of the early breakdown of the blood-brain barrier in Alzheimer’s disease models. Geroscience 2023, 45, 3307–3331. [Google Scholar] [CrossRef] [PubMed]
- Summer, S.; Borrell-Pages, M.; Bruno, R.M.; Climie, R.E.; Dipla, K.; Dogan, A.; Eruslanova, K.; Fraenkel, E.; Mattace-Raso, F.; Pugh, C.J.A.; et al. Centenarians-the way to healthy vascular ageing and longevity: A review from VascAgeNet. Geroscience 2024, 47, 685–702. [Google Scholar] [CrossRef]
- Csiszar, A.; Ungvari, A.; Patai, R.; Gulej, R.; Yabluchanskiy, A.; Benyo, Z.; Kovacs, I.; Sotonyi, P.; Kirkpartrick, A.C.; Prodan, C.I.; et al. Atherosclerotic burden and cerebral small vessel disease: Exploring the link through microvascular aging and cerebral microhemorrhages. Geroscience 2024, 46, 5103–5132. [Google Scholar] [CrossRef]
- Lambert, M.; Miquel, G.; Villeneuve, L.; Thorin-Trescases, N.; Thorin, E. The senolytic ABT-263 improves cognitive functions in middle-aged male, but not female, atherosclerotic LDLr(-/-);hApoB(100)(+/+) mice. Geroscience 2025, 47, 4577–4600. [Google Scholar] [CrossRef]
- Cummings, S.R.; Lui, L.Y.; Zaira, A.; Mau, T.; Fielding, R.A.; Atkinson, E.J.; Patel, S.; LeBrasseur, N. Biomarkers of cellular senescence and major health outcomes in older adults. Geroscience 2024, 47, 3407–3415. [Google Scholar] [CrossRef]
- Faakye, J.; Nyul-Toth, A.; Muranyi, M.; Gulej, R.; Csik, B.; Shanmugarama, S.; Tarantini, S.; Negri, S.; Prodan, C.; Mukli, P.; et al. Preventing spontaneous cerebral microhemorrhages in aging mice: A novel approach targeting cellular senescence with ABT263/navitoclax. Geroscience 2023, 46, 21–37. [Google Scholar] [CrossRef]
- Csik, B.; Nyul-Toth, A.; Gulej, R.; Patai, R.; Kiss, T.; Delfavero, J.; Nagaraja, R.Y.; Balasubramanian, P.; Shanmugarama, S.; Ungvari, A.; et al. Senescent Endothelial Cells in Cerebral Microcirculation Are Key Drivers of Age-Related Blood-Brain Barrier Disruption, Microvascular Rarefaction, and Neurovascular Coupling Impairment in Mice. Aging Cell 2025, 24, e70048. [Google Scholar] [CrossRef]
- Kiss, T.; Nyul-Toth, A.; Balasubramanian, P.; Tarantini, S.; Ahire, C.; Yabluchanskiy, A.; Csipo, T.; Farkas, E.; Wren, J.D.; Garman, L.; et al. Nicotinamide mononucleotide (NMN) supplementation promotes neurovascular rejuvenation in aged mice: Transcriptional footprint of SIRT1 activation, mitochondrial protection, anti-inflammatory, and anti-apoptotic effects. Geroscience 2020, 42, 527–546. [Google Scholar] [CrossRef] [PubMed]
- Bartman, S.; Coppotelli, G.; Ross, J.M. Mitochondrial Dysfunction: A Key Player in Brain Aging and Diseases. Curr. Issues Mol. Biol. 2024, 46, 1987–2026. [Google Scholar] [CrossRef] [PubMed]
- Zinovkin, R.A.; Romaschenko, V.P.; Galkin, I.I.; Zakharova, V.V.; Pletjushkina, O.Y.; Chernyak, B.V.; Popova, E.N. Role of mitochondrial reactive oxygen species in age-related inflammatory activation of endothelium. Aging (Albany NY) 2014, 6, 661–674. [Google Scholar] [CrossRef]
- Salvagno, M.; Sterchele, E.D.; Zaccarelli, M.; Mrakic-Sposta, S.; Welsby, I.J.; Balestra, C.; Taccone, F.S. Oxidative Stress and Cerebral Vascular Tone: The Role of Reactive Oxygen and Nitrogen Species. Int. J. Mol. Sci. 2024, 25, 3007. [Google Scholar] [CrossRef]
- Grossini, E.; Venkatesan, S.; Ola Pour, M.M. Mitochondrial Dysfunction in Endothelial Cells: A Key Driver of Organ Disorders and Aging. Antioxidants 2025, 14, 372. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, J.; Wang, J.; He, L.; Lai, H.; Zhang, T.; Wang, X.; Li, W. Mitochondrial oxidative stress in brain microvascular endothelial cells: Triggering blood-brain barrier disruption. Mitochondrion 2023, 69, 71–82. [Google Scholar] [CrossRef]
- Mukli, P.; Pinto, C.B.; Owens, C.D.; Csipo, T.; Lipecz, A.; Szarvas, Z.; Peterfi, A.; Langley, A.; Hoffmeister, J.; Racz, F.S.; et al. Impaired Neurovascular Coupling and Increased Functional Connectivity in the Frontal Cortex Predict Age-Related Cognitive Dysfunction. Adv. Sci. 2024, 11, e2303516. [Google Scholar] [CrossRef]
- Patai, R.; Patel, K.; Csik, B.; Gulej, R.; Nagaraja, R.Y.; Nagy, D.; Chandragiri, S.S.; Shanmugarama, S.; Kordestan, K.V.; Nagykaldi, M.; et al. Aging, mitochondrial dysfunction, and cerebral microhemorrhages: A preclinical evaluation of SS-31 (elamipretide) and development of a high-throughput machine learning-driven imaging pipeline for cerebromicrovascular protection therapeutic screening. Geroscience 2025, 47, 4871–4887. [Google Scholar] [CrossRef]
- Szarka, N.; Pabbidi, M.R.; Amrein, K.; Czeiter, E.; Berta, G.; Pohoczky, K.; Helyes, Z.; Ungvari, Z.; Koller, A.; Buki, A.; et al. Traumatic Brain Injury Impairs Myogenic Constriction of Cerebral Arteries: Role of Mitochondria-Derived H2O2 and TRPV4-Dependent Activation of BKca Channels. J. Neurotrauma 2018, 35, 930–939. [Google Scholar] [CrossRef]
- Li, T.; Huang, Y.; Cai, W.; Chen, X.; Men, X.; Lu, T.; Wu, A.; Lu, Z. Age-related cerebral small vessel disease and inflammaging. Cell Death Dis. 2020, 11, 932. [Google Scholar] [CrossRef]
- Del Cuore, A.; Pacinella, G.; Riolo, R.; Tuttolomondo, A. The Role of Immunosenescence in Cerebral Small Vessel Disease: A Review. Int. J. Mol. Sci. 2022, 23, 7136. [Google Scholar] [CrossRef] [PubMed]
- Bickel, M.A.; Csik, B.; Gulej, R.; Ungvari, A.; Nyul-Toth, A.; Conley, S.M. Cell non-autonomous regulation of cerebrovascular aging processes by the somatotropic axis. Front. Endocrinol. 2023, 14, 1087053. [Google Scholar] [CrossRef] [PubMed]
- Tarantini, S.; Valcarcel-Ares, N.M.; Yabluchanskiy, A.; Springo, Z.; Fulop, G.A.; Ashpole, N.; Gautam, T.; Giles, C.B.; Wren, J.D.; Sonntag, W.E.; et al. Insulin-like growth factor 1 deficiency exacerbates hypertension-induced cerebral microhemorrhages in mice, mimicking the aging phenotype. Aging Cell 2017, 16, 469–479. [Google Scholar] [CrossRef]
- Toth, P.; Tucsek, Z.; Tarantini, S.; Sosnowska, D.; Gautam, T.; Mitschelen, M.; Koller, A.; Sonntag, W.E.; Csiszar, A.; Ungvari, Z. IGF-1 deficiency impairs cerebral myogenic autoregulation in hypertensive mice. J. Cereb. Blood Flow. Metab. 2014, 34, 1887–1897. [Google Scholar] [CrossRef]
- Sonntag, W.E.; Deak, F.; Ashpole, N.; Toth, P.; Csiszar, A.; Freeman, W.; Ungvari, Z. Insulin-like growth factor-1 in CNS and cerebrovascular aging. Front. Aging Neurosci. 2013, 5, 27. [Google Scholar] [CrossRef]
- Gulej, R.; Csik, B.; Faakye, J.; Tarantini, S.; Shanmugarama, S.; Chandragiri, S.S.; Mukli, P.; Conley, S.; Csiszar, A.; Ungvari, Z.; et al. Endothelial deficiency of insulin-like growth factor-1 receptor leads to blood-brain barrier disruption and accelerated endothelial senescence in mice, mimicking aspects of the brain aging phenotype. Microcirculation 2024, 31, e12840. [Google Scholar] [CrossRef] [PubMed]
- Stankovics, L.; Ungvari, A.; Fekete, M.; Nyul-Toth, A.; Mukli, P.; Patai, R.; Csik, B.; Gulej, R.; Conley, S.; Csiszar, A.; et al. The vasoprotective role of IGF-1 signaling in the cerebral microcirculation: Prevention of cerebral microhemorrhages in aging. Geroscience 2024, 47, 445–455. [Google Scholar] [CrossRef]
- Miller, L.R.; Bickel, M.A.; Vance, M.L.; Vaden, H.; Nagykaldi, D.; Nyul-Toth, A.; Bullen, E.C.; Gautam, T.; Tarantini, S.; Yabluchanskiy, A.; et al. Vascular smooth muscle cell-specific Igf1r deficiency exacerbates the development of hypertension-induced cerebral microhemorrhages and gait defects. Geroscience 2024, 46, 3481–3501. [Google Scholar] [CrossRef]
- Gulej, R.; Nyul-Toth, A.; Csik, B.; Patai, R.; Petersen, B.; Negri, S.; Chandragiri, S.S.; Shanmugarama, S.; Mukli, P.; Yabluchanskiy, A.; et al. Young blood-mediated cerebromicrovascular rejuvenation through heterochronic parabiosis: Enhancing blood-brain barrier integrity and capillarization in the aged mouse brain. Geroscience 2024, 46, 4415–4442. [Google Scholar] [CrossRef]
- Toth, L.; Czigler, A.; Hegedus, E.; Komaromy, H.; Amrein, K.; Czeiter, E.; Yabluchanskiy, A.; Koller, A.; Orsi, G.; Perlaki, G.; et al. Age-related decline in circulating IGF-1 associates with impaired neurovascular coupling responses in older adults. Geroscience 2022, 44, 2771–2783. [Google Scholar] [CrossRef]
- Nyul-Toth, A.; Patai, R.; Csiszar, A.; Ungvari, A.; Gulej, R.; Mukli, P.; Yabluchanskiy, A.; Benyo, Z.; Sotonyi, P.; Prodan, C.I.; et al. Linking peripheral atherosclerosis to blood-brain barrier disruption: Elucidating its role as a manifestation of cerebral small vessel disease in vascular cognitive impairment. Geroscience 2024, 46, 6511–6536. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Sohn, E.H.; Oh, E.; Lee, A.Y. Characteristics of Cerebral Microbleeds. Dement. Neurocogn Disord. 2018, 17, 73–82. [Google Scholar] [CrossRef]
- Caunca, M.R.; Del Brutto, V.; Gardener, H.; Shah, N.; Dequatre-Ponchelle, N.; Cheung, Y.K.; Elkind, M.S.; Brown, T.R.; Cordonnier, C.; Sacco, R.L.; et al. Cerebral Microbleeds, Vascular Risk Factors, and Magnetic Resonance Imaging Markers: The Northern Manhattan Study. J. Am. Heart Assoc. 2016, 5, e003477. [Google Scholar] [CrossRef]
- Caunca, M.R.; De Leon-Benedetti, A.; Latour, L.; Leigh, R.; Wright, C.B. Neuroimaging of Cerebral Small Vessel Disease and Age-Related Cognitive Changes. Front. Aging Neurosci. 2019, 11, 145. [Google Scholar] [CrossRef]
- Werring, D.J.; Gregoire, S.M.; Cipolotti, L. Cerebral microbleeds and vascular cognitive impairment. J. Neurol. Sci. 2010, 299, 131–135. [Google Scholar] [CrossRef] [PubMed]
- de Laat, K.F.; van den Berg, H.A.; van Norden, A.G.; Gons, R.A.; Olde Rikkert, M.G.; de Leeuw, F.E. Microbleeds are independently related to gait disturbances in elderly individuals with cerebral small vessel disease. Stroke 2011, 42, 494–497. [Google Scholar] [CrossRef]
- Charidimou, A.; Karayiannis, C.; Song, T.J.; Orken, D.N.; Thijs, V.; Lemmens, R.; Kim, J.; Goh, S.M.; Phan, T.G.; Soufan, C.; et al. Brain microbleeds, anticoagulation, and hemorrhage risk: Meta-analysis in stroke patients with AF. Neurology 2017, 89, 2317–2326. [Google Scholar] [CrossRef] [PubMed]
- Csipo, T.; Lipecz, A.; Mukli, P.; Peterfi, A.; Szarvas, Z.; Ungvari, A.; Alaoui, L.E.; Sandor, M.; Kallai, A.; Fekete, M.; et al. Advancing prediction of age-related vascular cognitive impairment based on peripheral and retinal vascular health in a pilot study: A novel comprehensive assessment developed for a prospective workplace-based cohort (The Semmelweis Study). Geroscience 2024, 47, 1329–1344. [Google Scholar] [CrossRef]
- Ungvari, Z.; Tarantini, S.; Kirkpatrick, A.C.; Csiszar, A.; Prodan, C.I. Cerebral microhemorrhages: Mechanisms, consequences, and prevention. Am. J. Physiol. Heart Circ. Physiol. 2017, 312, H1128–H1143. [Google Scholar] [CrossRef]
- Ungvari, Z.; Muranyi, M.; Gulej, R.; Negri, S.; Nyul-Toth, A.; Csik, B.; Patai, R.; Conley, S.; Milan, M.; Bagwell, J.; et al. Longitudinal detection of gait alterations associated with hypertension-induced cerebral microhemorrhages in mice: Predictive role of stride length and stride time asymmetry and increased gait entropy. Geroscience 2024, 46, 4743–4760. [Google Scholar] [CrossRef] [PubMed]
- Tipirneni, S.; Stanwell, P.; Weissert, R.; Bhaskar, S.M.M. Prevalence and Impact of Cerebral Microbleeds on Clinical and Safety Outcomes in Acute Ischaemic Stroke Patients Receiving Reperfusion Therapy: A Systematic Review and Meta-Analysis. Biomedicines 2023, 11, 2865. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, J.; Fang, X.; Yu, Y.; Xu, M.; Li, T.; Yan, J. Association Between Cerebral Microbleeds and Neurological Outcomes in Patients Who Underwent Extracorporeal Membrane Oxygenation. J. Am. Heart Assoc. 2024, 13, e037029. [Google Scholar] [CrossRef]
- Fang, X.; Crumpler, R.F.; Thomas, K.N.; Mazique, J.N.; Roman, R.J.; Fan, F. Contribution of cerebral microvascular mechanisms to age-related cognitive impairment and dementia. Physiol. Int. 2022, 109, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Klop, M.; de Heus, R.A.A.; Maier, A.B.; van Alphen, A.; Floor-Westerdijk, M.J.; Bronkhorst, M.; Melis, R.J.F.; Meskers, C.G.M.; Claassen, J.; van Wezel, R.J.A. Capturing postural blood pressure dynamics with near-infrared spectroscopy-measured cerebral oxygenation. Geroscience 2023, 45, 2643–2657. [Google Scholar] [CrossRef]
- Magkas, N.; Tsioufis, C.; Thomopoulos, C.; Dilaveris, P.; Georgiopoulos, G.; Sanidas, E.; Papademetriou, V.; Tousoulis, D. Orthostatic hypotension: From pathophysiology to clinical applications and therapeutic considerations. J. Clin. Hypertens 2019, 21, 546–554. [Google Scholar] [CrossRef]
- Wang, L.; Pronk, A.C.; van Poelgeest, E.P.; Briggs, R.; Claassen, J.; Jansen, S.; Klop, M.; de Lange, F.J.; Meskers, C.; Odekerken, V.J.J.; et al. Applying systems thinking to unravel the mechanisms underlying orthostatic hypotension related fall risk. Geroscience 2023, 45, 2743–2755. [Google Scholar] [CrossRef]
- Kern, K.C.; Wright, C.B.; Bergfield, K.L.; Fitzhugh, M.C.; Chen, K.; Moeller, J.R.; Nabizadeh, N.; Elkind, M.S.V.; Sacco, R.L.; Stern, Y.; et al. Blood Pressure Control in Aging Predicts Cerebral Atrophy Related to Small-Vessel White Matter Lesions. Front. Aging Neurosci. 2017, 9, 132. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Li, Y.; Hu, J.; Wu, J.; Huang, Y. A Study on the Pathogenesis of Vascular Cognitive Impairment and Dementia: The Chronic Cerebral Hypoperfusion Hypothesis. J. Clin. Med. 2022, 11, 4742. [Google Scholar] [CrossRef] [PubMed]
- Rajeev, V.; Chai, Y.L.; Poh, L.; Selvaraji, S.; Fann, D.Y.; Jo, D.G.; De Silva, T.M.; Drummond, G.R.; Sobey, C.G.; Arumugam, T.V.; et al. Chronic cerebral hypoperfusion: A critical feature in unravelling the etiology of vascular cognitive impairment. Acta Neuropathol. Commun. 2023, 11, 93. [Google Scholar] [CrossRef]
- Iadecola, C. The overlap between neurodegenerative and vascular factors in the pathogenesis of dementia. Acta Neuropathol. 2010, 120, 287–296. [Google Scholar] [CrossRef]
- Qi, X.; Tang, H.; Luo, Q.; Ding, B.; Chen, J.; Cui, P.; Chen, S.; Ling, H.; Ma, J. White Matter Hyperintensities Predict Cognitive Decline: A Community-Based Study. Can. J. Neurol. Sci. 2019, 46, 383–388. [Google Scholar] [CrossRef]
- Makin, S.D.; Turpin, S.; Dennis, M.S.; Wardlaw, J.M. Cognitive impairment after lacunar stroke: Systematic review and meta-analysis of incidence, prevalence and comparison with other stroke subtypes. J. Neurol. Neurosurg. Psychiatry 2013, 84, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Heinen, R.; Vlegels, N.; de Bresser, J.; Leemans, A.; Biessels, G.J.; Reijmer, Y.D. The cumulative effect of small vessel disease lesions is reflected in structural brain networks of memory clinic patients. Neuroimage Clin. 2018, 19, 963–969. [Google Scholar] [CrossRef]
- Lorenzini, L.; Maranzano, A.; Ingala, S.; Collij, L.E.; Tranfa, M.; Blennow, K.; Di Perri, C.; Foley, C.; Fox, N.C.; Frisoni, G.B.; et al. Association of Vascular Risk Factors and Cerebrovascular Pathology With Alzheimer Disease Pathologic Changes in Individuals Without Dementia. Neurology 2024, 103, e209801. [Google Scholar] [CrossRef]
- Govindpani, K.; McNamara, L.G.; Smith, N.R.; Vinnakota, C.; Waldvogel, H.J.; Faull, R.L.; Kwakowsky, A. Vascular Dysfunction in Alzheimer’s Disease: A Prelude to the Pathological Process or a Consequence of It? J. Clin. Med. 2019, 8, 651. [Google Scholar] [CrossRef]
- Deng, W.; Guo, S.; van Veluw, S.J.; Yu, Z.; Chan, S.J.; Takase, H.; Arai, K.; Ning, M.; Greenberg, S.M.; Lo, E.H.; et al. Effects of cerebral amyloid angiopathy on the brain vasculome. Aging Cell 2022, 21, e13503. [Google Scholar] [CrossRef]
- Noto, N.M.; Speth, R.C.; Robison, L.S. Cerebral amyloid angiopathy: A narrative review. Front. Aging Neurosci. 2025, 17, 1632252. [Google Scholar] [CrossRef]
- Bateman, G.A.; Bateman, A.R. A lumped parameter modelling study of cerebral autoregulation in normal pressure hydrocephalus suggests the brain chooses to be ischemic. Sci. Rep. 2024, 14, 24373. [Google Scholar] [CrossRef]
- Bateman, G.A. Vascular compliance in normal pressure hydrocephalus. AJNR Am. J. Neuroradiol. 2000, 21, 1574–1585. [Google Scholar] [PubMed]
- Zlokovic, B.V. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat. Rev. Neurosci. 2011, 12, 723–738. [Google Scholar] [CrossRef] [PubMed]
- Eisenmenger, L.B.; Peret, A.; Famakin, B.M.; Spahic, A.; Roberts, G.S.; Bockholt, J.H.; Johnson, K.M.; Paulsen, J.S. Vascular contributions to Alzheimer’s disease. Transl. Res. 2023, 254, 41–53. [Google Scholar] [CrossRef]
- Segura-Hernández, A.; Gómez-Amarillo, D.F.; Mejía-Michelsen, I.; Vargas-Osorio, M.P.; González, M.; Domínguez, M.T.; Mattar, S.M.; Volcinschi, D.; Ordóñez-Rubiano, E.G.; Ramos-Márquez, A.; et al. Reversal of cerebral pseudoatrophy in normal pressure hydrocephalus after ventriculoatrial shunt placement. Sci. Rep. 2025, 15, 24817. [Google Scholar] [CrossRef]
- Momjian, S.; Owler, B.K.; Czosnyka, Z.; Czosnyka, M.; Pena, A.; Pickard, J.D. Pattern of white matter regional cerebral blood flow and autoregulation in normal pressure hydrocephalus. Brain 2004, 127, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.; Tozer, D.J.; Chen, Y.; Brown, R.B.; Low, A.; Markus, H.S. Perivascular space dysfunction in cerebral small vessel disease is related to neuroinflammation. Brain 2025, 148, 1540–1550. [Google Scholar] [CrossRef]
- Zhong, J.; Lin, W.; Chen, J.; Gao, Q. Higher critical closing pressure is independently associated with enlarged basal ganglia perivascular spaces. Front. Neurol. 2023, 14, 1165469. [Google Scholar] [CrossRef]
- Nogueira, R.C.; Beishon, L.; Bor-Seng-Shu, E.; Panerai, R.B.; Robinson, T.G. Cerebral Autoregulation in Ischemic Stroke: From Pathophysiology to Clinical Concepts. Brain Sci. 2021, 11, 511. [Google Scholar] [CrossRef]
- Kovács, K.B.; Bencs, V.; Hudák, L.; Oláh, L.; Csiba, L. Hemorrhagic Transformation of Ischemic Strokes. Int. J. Mol. Sci. 2023, 24, 14067. [Google Scholar] [CrossRef] [PubMed]
- Sheriff, F.; Castro, P.; Kozberg, M.; LaRose, S.; Monk, A.; Azevedo, E.; Li, K.; Jafari, S.; Rao, S.; Otite, F.O.; et al. Dynamic Cerebral Autoregulation Post Endovascular Thrombectomy in Acute Ischemic Stroke. Brain Sci. 2020, 10, 641. [Google Scholar] [CrossRef]
- Nishijima, Y.; Akamatsu, Y.; Weinstein, P.R.; Liu, J. Collaterals: Implications in cerebral ischemic diseases and therapeutic interventions. Brain Res. 2015, 1623, 18–29. [Google Scholar] [CrossRef]
- Farooq, M.U.; Goshgarian, C.; Min, J.; Gorelick, P.B. Pathophysiology and management of reperfusion injury and hyperperfusion syndrome after carotid endarterectomy and carotid artery stenting. Exp. Transl. Stroke Med. 2016, 8, 7. [Google Scholar] [CrossRef] [PubMed]
- Kibrik, P.; Stonko, D.P.; Alsheekh, A.; Holscher, C.; Zarkowsky, D.; Abularrage, C.J.; Hicks, C.W. Association of carotid revascularization approach with perioperative outcomes based on symptom status and degree of stenosis among octogenarians. J. Vasc. Surg. 2022, 76, 769–777.e762. [Google Scholar] [CrossRef]
- Jones, J.D.; Castanho, P.; Bazira, P.; Sanders, K. Anatomical variations of the circle of Willis and their prevalence, with a focus on the posterior communicating artery: A literature review and meta-analysis. Clin. Anat. 2021, 34, 978–990. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.A.; Carpenter, K.L.; Hutchinson, P.J.; Smielewski, P.; Helmy, A. Candidate neuroinflammatory markers of cerebral autoregulation dysfunction in human acute brain injury. J. Cereb. Blood Flow. Metab. 2023, 43, 1237–1253. [Google Scholar] [CrossRef]
- Czosnyka, M.; Balestreri, M.; Steiner, L.; Smielewski, P.; Hutchinson, P.J.; Matta, B.; Pickard, J.D. Age, intracranial pressure, autoregulation, and outcome after brain trauma. J. Neurosurg. 2005, 102, 450–454. [Google Scholar] [CrossRef]
- Gardner, R.C.; Dams-O’Connor, K.; Morrissey, M.R.; Manley, G.T. Geriatric Traumatic Brain Injury: Epidemiology, Outcomes, Knowledge Gaps, and Future Directions. J. Neurotrauma 2018, 35, 889–906. [Google Scholar] [CrossRef]
- Toth, P.; Szarka, N.; Farkas, E.; Ezer, E.; Czeiter, E.; Amrein, K.; Ungvari, Z.; Hartings, J.A.; Buki, A.; Koller, A. Traumatic brain injury-induced autoregulatory dysfunction and spreading depression-related neurovascular uncoupling: Pathomechanisms, perspectives, and therapeutic implications. Am. J. Physiol. Heart Circ. Physiol. 2016, 311, H1118–H1131. [Google Scholar] [CrossRef] [PubMed]
- Zeiler, F.A.; Aries, M.; Czosnyka, M.; Smielewski, P. Cerebral Autoregulation Monitoring in Traumatic Brain Injury: An Overview of Recent Advances in Personalized Medicine. J. Neurotrauma 2022, 39, 1477–1494. [Google Scholar] [CrossRef]
- Toth, L.; Czigler, A.; Horvath, P.; Kornyei, B.; Szarka, N.; Schwarcz, A.; Ungvari, Z.; Buki, A.; Toth, P. Traumatic brain injury-induced cerebral microbleeds in the elderly. Geroscience 2021, 43, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Ikonomovic, M.D.; Mi, Z.; Abrahamson, E.E. Disordered APP metabolism and neurovasculature in trauma and aging: Combined risks for chronic neurodegenerative disorders. Ageing Res. Rev. 2017, 34, 51–63. [Google Scholar] [CrossRef]
- Svedung Wettervik, T.; Beqiri, E.; Hånell, A.; Bögli, S.Y.; Olakorede, I.; Chen, X.; Helmy, A.; Lavinio, A.; Hutchinson, P.J.; Smielewski, P. Autoregulatory-guided management in traumatic brain injury: Does age matter? Acta Neurochir 2025, 167, 55. [Google Scholar] [CrossRef]
- Weijs, R.W.J.; Oudegeest-Sander, M.H.; Vloet, J.I.A.; Hopman, M.T.E.; Claassen, J.; Thijssen, D.H.J. A decade of aging in healthy older adults: Longitudinal findings on cerebrovascular and cognitive health. Geroscience 2023, 45, 2629–2641. [Google Scholar] [CrossRef]
- Burma, J.S.; Roy, M.A.; Kennedy, C.M.; Labrecque, L.; Brassard, P.; Smirl, J.D. A systematic review, meta-analysis and meta-regression amalgamating the driven approaches used to quantify dynamic cerebral autoregulation. J. Cereb. Blood Flow. Metab. 2024, 44, 1271–1297. [Google Scholar] [CrossRef]
- Bird, J.D.; MacLeod, D.B.; Griesdale, D.E.; Sekhon, M.S.; Hoiland, R.L. Shining a light on cerebral autoregulation: Are we anywhere near the truth? J. Cereb. Blood Flow. Metab. 2024, 44, 1057–1060. [Google Scholar] [CrossRef] [PubMed]
- Tarumi, T.; Zhang, R. Point-Counterpoint: Transfer function analysis of dynamic cerebral autoregulation: To band or not to band? J. Cereb. Blood Flow. Metab. 2023, 43, 1625–1627. [Google Scholar] [CrossRef] [PubMed]
- Panerai, R.B.; Brassard, P.; Burma, J.S.; Castro, P.; Claassen, J.A.; van Lieshout, J.J.; Liu, J.; Lucas, S.J.; Minhas, J.S.; Mitsis, G.D.; et al. Transfer function analysis of dynamic cerebral autoregulation: A CARNet white paper 2022 update. J. Cereb. Blood Flow Metab. 2023, 43, 3–25. [Google Scholar] [CrossRef]
- Favilla, C.G.; Mullen, M.T.; Kahn, F.; Rasheed, I.D.; Messe, S.R.; Parthasarathy, A.B.; Yodh, A.G. Dynamic cerebral autoregulation measured by diffuse correlation spectroscopy. J. Cereb. Blood Flow. Metab. 2023, 43, 1317–1327. [Google Scholar] [CrossRef]
- Larsen, F.S.; Olsen, K.S.; Hansen, B.A.; Paulson, O.B.; Knudsen, G.M. Transcranial Doppler is valid for determination of the lower limit of cerebral blood flow autoregulation. Stroke 1994, 25, 1985–1988. [Google Scholar] [CrossRef]
- Sarkar, S.; Ghosh, S.; Ghosh, S.K.; Collier, A. Role of transcranial Doppler ultrasonography in stroke. Postgrad. Med. J. 2007, 83, 683–689. [Google Scholar] [CrossRef]
- Rivera-Lara, L.; Zeiler, F.A. Continuous Cerebral Autoregulation Monitoring Using TCD. In Neurovascular Sonography; Springer: Berlin/Heidelberg, Germany, 2022; pp. 241–247. [Google Scholar]
- Grade, M.; Hernandez Tamames, J.A.; Pizzini, F.B.; Achten, E.; Golay, X.; Smits, M. A neuroradiologist’s guide to arterial spin labeling MRI in clinical practice. Neuroradiology 2015, 57, 1181–1202. [Google Scholar] [CrossRef]
- Sainbhi, A.S.; Gomez, A.; Froese, L.; Slack, T.; Batson, C.; Stein, K.Y.; Cordingley, D.M.; Alizadeh, A.; Zeiler, F.A. Non-Invasive and Minimally-Invasive Cerebral Autoregulation Assessment: A Narrative Review of Techniques and Implications for Clinical Research. Front. Neurol. 2022, 13, 872731. [Google Scholar] [CrossRef] [PubMed]
- de Boorder, M.J.; Hendrikse, J.; van der Grond, J. Phase-contrast magnetic resonance imaging measurements of cerebral autoregulation with a breath-hold challenge: A feasibility study. Stroke 2004, 35, 1350–1354. [Google Scholar] [CrossRef]
- Pyne, J.D.; Morales, C.D.; Kraal, A.Z.; Alshikho, M.J.; Lao, P.J.; Turney, I.C.; Amarante, E.; Lippert, R.V.; Chang, J.F.; Gutierrez, J.; et al. Phase contrast-derived cerebral blood flow is associated with neurodegeneration and cerebrovascular injury in older adults. Front. Neurosci. 2025, 19, 1538956. [Google Scholar] [CrossRef]
- Konig, S.; Jayarajan, V.; Wray, S.; Kamm, R.; Moeendarbary, E. Mechanobiology of the blood-brain barrier during development, disease and ageing. Nat. Commun. 2025, 16, 7233. [Google Scholar] [CrossRef]
- Ling, Y.H.; Chi, N.F.; Pan, L.H.; Wang, Y.F.; Wu, C.H.; Lirng, J.F.; Fuh, J.L.; Wang, S.J.; Chen, S.P. Association between impaired dynamic cerebral autoregulation and BBB disruption in reversible cerebral vasoconstriction syndrome. J. Headache Pain. 2023, 24, 170. [Google Scholar] [CrossRef]
- Oghabian, M.A.; Fatemidokht, A.; Haririchian, M.H. Quantification of Blood-Brain-Barrier Permeability Dysregulation and Inflammatory Activity in MS Lesions by Dynamic-Contrast Enhanced MR Imaging. Basic. Clin. Neurosci. 2022, 13, 117–128. [Google Scholar] [CrossRef]
- Li, Y.; Sadiq, A.; Wang, Z. Arterial Spin Labelling-Based Blood-Brain Barrier Assessment and Its Applications. Investig. Magn. Reson. Imaging 2022, 26, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.J.; Abdelhafez, Y.G.; Spencer, B.A.; Jones, T.; Tran, Q.; Nardo, L.; Chen, M.S., Jr.; Sarkar, S.; Medici, V.; Lyo, V.; et al. Quantitative PET imaging and modeling of molecular blood-brain barrier permeability. medRxiv 2024. [Google Scholar] [CrossRef]
- Pieniazek, W.; Dimitrow, P.P. Autoregulation of cerebral circulation: Adaptation to hypertension and re-adaptation in response to antihypertensive treatment. Przegl Lek. 2006, 63, 688–690. [Google Scholar]
- Tzeng, Y.C.; Ainslie, P.N. Blood pressure regulation IX: Cerebral autoregulation under blood pressure challenges. Eur. J. Appl. Physiol. 2014, 114, 545–559. [Google Scholar] [CrossRef] [PubMed]
- Chau, A.C.M.; Cheung, E.Y.W.; Chan, K.H.; Chow, W.S.; Shea, Y.F.; Chiu, P.K.C.; Mak, H.K.F. Impaired cerebral blood flow in type 2 diabetes mellitus - A comparative study with subjective cognitive decline, vascular dementia and Alzheimer’s disease subjects. Neuroimage Clin. 2020, 27, 102302. [Google Scholar] [CrossRef] [PubMed]
- Mankovsky, B.N.; Piolot, R.; Mankovsky, O.L.; Ziegler, D. Impairment of cerebral autoregulation in diabetic patients with cardiovascular autonomic neuropathy and orthostatic hypotension. Diabet. Med. 2003, 20, 119–126. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, R.J.; Resnick, S.M.; Zonderman, A.B.; Ferrucci, L.; Crain, B.J.; Pletnikova, O.; Rudow, G.; Iacono, D.; Riudavets, M.A.; Driscoll, I.; et al. Neuropathologic studies of the Baltimore Longitudinal Study of Aging (BLSA). J. Alzheimers Dis. 2009, 18, 665–675. [Google Scholar] [CrossRef]
- Beishon, L.; Clough, R.H.; Kadicheeni, M.; Chithiramohan, T.; Panerai, R.B.; Haunton, V.J.; Minhas, J.S.; Robinson, T.G. Vascular and haemodynamic issues of brain ageing. Pflugers Arch. 2021, 473, 735–751. [Google Scholar] [CrossRef]
- M, A.S.; O’Connor, J.D.; Boyle, R.; Newman, L.; Knight, S.P.; Hernandez, B.; Whelan, R.; Meaney, J.F.; Kenny, R.A. Slower speed of blood pressure recovery after standing is associated with accelerated brain aging: Evidence from The Irish Longitudinal Study on Ageing (TILDA). Cereb. Circ. Cogn. Behav. 2024, 6, 100212. [Google Scholar] [CrossRef]
- Sorond, F.; Purkayastha, S.; Lipsitz, L.; Leveille, S. Impaired Cerebral Autoregualtion is Associated with a Greater 7-year Decline in Gait Speed in an Elderly Population: The MOBILIZE Boston Study (S58. 007). Neurology 2014, 82, S58–007. [Google Scholar] [CrossRef]
- Bakker, S.L.; de Leeuw, F.E.; den Heijer, T.; Koudstaal, P.J.; Hofman, A.; Breteler, M.M. Cerebral haemodynamics in the elderly: The rotterdam study. Neuroepidemiology 2004, 23, 178–184. [Google Scholar] [CrossRef]
- Shannon, O.M.; Mendes, I.; Köchl, C.; Mazidi, M.; Ashor, A.W.; Rubele, S.; Minihane, A.M.; Mathers, J.C.; Siervo, M. Mediterranean Diet Increases Endothelial Function in Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Nutr. 2020, 150, 1151–1159. [Google Scholar] [CrossRef] [PubMed]
- Koep, J.L.; Bond, B.; Barker, A.R.; Ruediger, S.L.; Pizzey, F.K.; Coombes, J.S.; Bailey, T.G. Sex modifies the relationship between age and neurovascular coupling in healthy adults. J. Cereb. Blood Flow. Metab. 2023, 43, 1254–1266. [Google Scholar] [CrossRef]
- DuBose, L.E.; Babcock, M.C.; Kohrt, W.M.; Stauffer, B.L.; Hildreth, K.L.; Walker, J.; Armstrong, M.K.; Moreau, K.L. Gonadal status modulates large elastic artery stiffness in healthy middle-aged and older men. Geroscience 2024, 47, 3277–3289. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Medina, D.; Stockwell, R.; McFadden, S.; Quinn, K.; Peck, M.R.; Bartke, A.; Hascup, K.N.; Hascup, E.R. Sexual dimorphic metabolic and cognitive responses of C57BL/6 mice to Fisetin or Dasatinib and quercetin cocktail oral treatment. Geroscience 2023, 45, 2835–2850. [Google Scholar] [CrossRef] [PubMed]
- Ungvari, A.; Gulej, R.; Patai, R.; Papp, Z.; Toth, A.; Szabo, A.A.; Podesser, B.K.; Sotonyi, P.; Benyo, Z.; Yabluchanskiy, A.; et al. Sex-specific mechanisms in vascular aging: Exploring cellular and molecular pathways in the pathogenesis of age-related cardiovascular and cerebrovascular diseases. Geroscience 2025, 47, 301–337. [Google Scholar] [CrossRef]
- Nist, K.M.; Bard, H.; McBride, B.; Capriglione, A.L.; Moreira, J.D.; Farb, D.H.; Wainford, R.D. Losartan attenuates sex-dependent hypertension, neuroinflammation, and cognitive impairment in the aging male sprague-dawley rat. Geroscience 2024, 47, 3007–3026. [Google Scholar] [CrossRef]
- Fernandez, A.; Cuesta, P.; Marcos, A.; Montenegro-Pena, M.; Yus, M.; Rodriguez-Rojo, I.C.; Bruna, R.; Maestu, F.; Lopez, M.E. Sex differences in the progression to Alzheimer’s disease: A combination of functional and structural markers. Geroscience 2024, 46, 2619–2640. [Google Scholar] [CrossRef]
- Pandics, T.; Major, D.; Fazekas-Pongor, V.; Szarvas, Z.; Peterfi, A.; Mukli, P.; Gulej, R.; Ungvari, A.; Fekete, M.; Tompa, A.; et al. Exposome and unhealthy aging: Environmental drivers from air pollution to occupational exposures. Geroscience 2023, 45, 3381–3408. [Google Scholar] [CrossRef]
- Ungvari, Z.; Tabak, A.G.; Adany, R.; Purebl, G.; Kaposvari, C.; Fazekas-Pongor, V.; Csipo, T.; Szarvas, Z.; Horvath, K.; Mukli, P.; et al. The Semmelweis Study: A longitudinal occupational cohort study within the framework of the Semmelweis Caring University Model Program for supporting healthy aging. Geroscience 2024, 46, 191–218. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Border, J.J.; Zhang, H.; Gregory, A.; Bai, S.; Fang, X.; Liu, Y.; Wang, S.; Hwang, S.H.; Gao, W.; et al. Inhibition of soluble epoxide hydrolase ameliorates cerebral blood flow autoregulation and cognition in alzheimer’s disease and diabetes-related dementia rat models. Geroscience 2025, 47, 4429–4449. [Google Scholar] [CrossRef]
- Orr, M.E.; Kotkowski, E.; Ramirez, P.; Bair-Kelps, D.; Liu, Q.; Brenner, C.; Schmidt, M.S.; Fox, P.T.; Larbi, A.; Tan, C.; et al. A randomized placebo-controlled trial of nicotinamide riboside in older adults with mild cognitive impairment. Geroscience 2024, 46, 665–682. [Google Scholar] [CrossRef]
- Jiang, Z.; He, Q.; Wezeman, J.; Darvas, M.; Ladiges, W. A cocktail of rapamycin, acarbose, and phenylbutyrate prevents age-related cognitive decline in mice by targeting multiple aging pathways. Geroscience 2024, 46, 4855–4868. [Google Scholar] [CrossRef]
- Seman, A.; Chandra, P.K.; Byrum, S.D.; Mackintosh, S.G.; Gies, A.J.; Busija, D.W.; Rutkai, I. Targeting mitochondria in the aged cerebral vasculature with SS-31, a proteomic study of brain microvessels. Geroscience 2023, 45, 2951–2965. [Google Scholar] [CrossRef]
- Van Skike, C.E.; DeRosa, N.; Galvan, V.; Hussong, S.A. Rapamycin restores peripheral blood flow in aged mice and in mouse models of atherosclerosis and Alzheimer’s disease. Geroscience 2023, 45, 1987–1996. [Google Scholar] [CrossRef] [PubMed]
- Fielding, R.A.; Atkinson, E.J.; Aversa, Z.; White, T.A.; Heeren, A.A.; Achenbach, S.J.; Mielke, M.M.; Cummings, S.R.; Pahor, M.; Leeuwenburgh, C.; et al. Associations between biomarkers of cellular senescence and physical function in humans: Observations from the lifestyle interventions for elders (LIFE) study. Geroscience 2022, 44, 2757–2770. [Google Scholar] [CrossRef] [PubMed]
| Modality | Metric | Strengths | Limitations | Best Application |
|---|---|---|---|---|
| TCD | cerebral blood flow velocity (cm/s) in major intracranial arteries and derived autoregulatory indices | bedside applicability high temporal resolution | poor spatial resolution interobserver variability requires bone window | continuous bedside monitoring of dynamic autoregulation in an ICU setting |
| NIRS | TOI (tissue oxygenation index) TOx (moving correlation coefficient of TOI and MAP, with positive values indicating impaired, and negative values reflecting maintained autoregulation) | bedside applicability high temporal resolution real-time regional autoregulatory information | limited penetration depth mainly cortical measurements | continuous, bedside autoregulatory monitoring with excellent temporal and acceptable spatial resolution |
| ASL | regional perfusion (mL/100g/min) | non-contrast perfusion maps high spatial resolution | poor temporal resolution, not applicable for dCA sensitive to motion artifacts | monitoring of functional hyperemia (neurovascular coupling) |
| PC-MRI | vessel-specific flow (mL/min) and pulsatility quantification | quantitative blood flow measurement in specific major arteries | limited temporal resolution, not applicable for dCA susceptible to motion artifacts | vessel-specific metrics of flow and pulsatility |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ungvari, A.; Kállai, A.; Stankovics, L.; Lendvai-Emmert, D.; Gulej, R.; Pal, E.; Patai, R.; Csik, B.; Fekete, M.; Lipecz, Á.; et al. Age-Related Alterations of Cerebral Autoregulation. Life 2025, 15, 1669. https://doi.org/10.3390/life15111669
Ungvari A, Kállai A, Stankovics L, Lendvai-Emmert D, Gulej R, Pal E, Patai R, Csik B, Fekete M, Lipecz Á, et al. Age-Related Alterations of Cerebral Autoregulation. Life. 2025; 15(11):1669. https://doi.org/10.3390/life15111669
Chicago/Turabian StyleUngvari, Anna, Attila Kállai, Levente Stankovics, Dominika Lendvai-Emmert, Rafal Gulej, Eva Pal, Roland Patai, Boglarka Csik, Mónika Fekete, Ágnes Lipecz, and et al. 2025. "Age-Related Alterations of Cerebral Autoregulation" Life 15, no. 11: 1669. https://doi.org/10.3390/life15111669
APA StyleUngvari, A., Kállai, A., Stankovics, L., Lendvai-Emmert, D., Gulej, R., Pal, E., Patai, R., Csik, B., Fekete, M., Lipecz, Á., Csípő, T., Benyó, Z., Csiszar, A., & Toth, P. (2025). Age-Related Alterations of Cerebral Autoregulation. Life, 15(11), 1669. https://doi.org/10.3390/life15111669








