Abstract
Intact regulation of cerebral blood flow (CBF) is essential for preserving cognitive function and reducing the risk of cerebrovascular events, particularly in the aging population. Autoregulation of CBF is one of the fundamental mechanisms that ensure constant supply for brain tissue by maintaining relatively stable perfusion despite fluctuations in systemic blood pressure. It also acts as a critical protective mechanism, shielding the fragile cerebral microcirculation from potentially harmful pressure fluctuations and hence excessive pulsatility. The loss or attenuation of this protective mechanism with aging or disease increases the vulnerability of the microvasculature to structural damage, blood–brain barrier (BBB) disruption, and the development of cerebral small vessel disease. This mini-review summarizes current understanding of how aging affects cerebral autoregulation, highlighting underlying mechanisms, clinical consequences, and potential strategies to preserve cerebrovascular health in older adults.
1. Introduction
The human brain is critically dependent on a constant supply of oxygen and nutrients, which is ensured by precise regulation of cerebral blood flow (CBF). Cerebral autoregulation refers to the intrinsic ability of regulation of CBF to maintain relatively stable perfusion of cerebral tissue despite fluctuations in systemic blood pressure [1]. Importantly, cerebral autoregulation also serves as a critical protective mechanism, shielding the fragile cerebral microcirculation from potentially harmful pressure fluctuations and hence excessive pulsatility [2]. By preventing the direct transmission of pulsatile or excessive pressure waves into the microvascular bed, autoregulation protects capillaries, arterioles, and the blood–brain barrier (BBB) from mechanical stress and injury [3]. The loss or attenuation of this protective mechanism with aging or disease increases the vulnerability of the microvasculature to structural damage, BBB disruption, and the development of cerebral small vessel disease [4]. Thus, intact autoregulation is fundamental not only for maintaining adequate perfusion but also for preserving the structural and functional integrity of the cerebral microcirculation [2].
Intact regulation of CBF is essential for preserving cognitive function and reducing the risk of cerebrovascular events, particularly in the aging population, where both vascular and neural systems undergo profound structural and functional changes [5]. The process of aging is associated with increased vulnerability to cerebrovascular insults, yet the role of impaired cerebral autoregulation in this context is often underrecognized [6,7].
This mini-review summarizes current understanding of how aging affects cerebral autoregulation, highlighting underlying mechanisms, clinical consequences, and potential strategies to preserve cerebrovascular health in older adults.
2. Mechanisms of Cerebral Autoregulation
Under physiological conditions, autoregulation operates effectively within a mean arterial pressure (MAP) range of approximately 50 to 150 mmHg [3]. Within these limits, the cerebrovascular system dynamically adjusts vascular resistance to counterbalance changes in perfusion pressure, ensuring stable delivery of oxygen and nutrients to the brain and protecting the fragile microcirculation from pressure-induced damage [4].
Importantly, a distinction is made between static autoregulation [8], which reflects the steady-state relationship between MAP and CBF, and dynamic autoregulation [9,10,11], which describes the rapid adjustments of cerebrovascular resistance to transient blood pressure fluctuations. Dynamic autoregulation is often more sensitive to early vascular dysfunction and is therefore particularly relevant when considering age-related impairments [12].
This complex regulatory process is governed by several interacting mechanisms. One of the most immediate contributors is the myogenic response, whereby vascular smooth muscle cells contract or relax in direct response to changes in transmural pressure [13]. This rapid adjustment stabilizes blood flow on a beat-to-beat basis and protects the delicate cerebral microvasculature from excessive pressure fluctuations [14]. The rapid myogenic adjustment of vascular tone is mainly controlled by cellular mechanisms in vascular smooth muscle cells, which convert mechanical stimuli into contractile responses. A key aspect of this process is the activation of mechanosensitive ion channels, especially voltage-gated calcium channels (VGCCs), which are essential for triggering vasoconstriction [15].
When transmural pressure increases, the resulting stretch of the vascular wall activates mechanosensors embedded in the smooth muscle cell membrane. This mechanical stimulus triggers the opening of L-type voltage-gated calcium channels (Cav1.2) [16], allowing extracellular calcium to enter the cytoplasm. An increase in intracellular calcium concentration acts as the key signal to start smooth muscle contraction. It activates the calcium–calmodulin complex and stimulates myosin light chain kinase (MLCK), which phosphorylates myosin light chains and enables actin–myosin cross-bridge cycling [17].
In addition to VGCCs, transient receptor potential (TRP) channels, particularly those of the TRPC and TRPV subfamilies, contribute to myogenic constriction. These non-selective cation channels are sensitive to mechanical stretch and facilitate calcium influx or membrane depolarization, which in turn enhances the activation of VGCCs and amplifies the contractile response [18].
The myogenic response is further modulated by a network of intracellular signaling pathways, including the activation of Rho-associated kinase (ROCK) and protein kinase C (PKC) [19]. These kinases promote calcium sensitization, a process whereby vascular smooth muscle contraction is maintained or enhanced independently of further increases in intracellular calcium levels. Specifically, ROCK inhibits myosin light chain phosphatase (MLCP), thereby sustaining myosin light chain phosphorylation and augmenting vasoconstriction [16].
Together, these coordinated molecular events—activation of mechanosensitive ion channels, calcium influx through VGCCs and TRP channels, and kinase-mediated calcium sensitization—allow vascular smooth muscle cells to adjust vascular tone rapidly and effectively. This adjustment of tone changes vascular resistance in response to pressure fluctuations, helping to maintain stable cerebral perfusion [20].
In addition to intrinsic vascular responses, neurogenic control also plays a role, particularly in larger cerebral vessels. Sympathetic and parasympathetic innervation modulates vascular tone, providing an additional layer of regulation that complements local mechanisms [21].
The brain’s ability to match blood flow to metabolic demands is further supported by metabolic regulation. Changes in local concentrations of carbon dioxide, pH, and metabolites such as adenosine lead to adjustments in arteriolar diameter, ensuring that neuronal activity is matched by an adequate supply of oxygen and nutrients in a negative feedback manner [22,23].
Endothelial function represents another crucial component of cerebral autoregulation. The vascular endothelium fine-tunes vascular tone by releasing vasoactive substances, including nitric oxide (NO), prostanoids, and endothelin-1 [24,25,26]. These mediators orchestrate the delicate balance between vasodilation and vasoconstriction, contributing to the dynamic stability of CBF.
In the setting of chronic hypertension, the cerebrovascular system undergoes structural and functional adaptations designed to preserve autoregulatory capacity [27]. These include remodeling of resistance vessels, alterations in prostanoid signaling pathways, and the involvement of TRP channels and calcium-dependent signaling cascades [26,28,29]. While such adaptations may initially help maintain CBF stability, they can also render the system more susceptible to dysregulation, a vulnerability that becomes particularly pronounced with advancing age [29].
3. Age-Related Changes in Cerebral Autoregulation
3.1. Structural Changes Contributing to Impaired Autoregulation
Aging is associated with progressive stiffening of large arteries [30,31,32], degradation of elastin fibers, and increased transmission of pulsatile pressure to the cerebral microcirculation. These changes diminish vascular compliance, reducing the ability of vessels to buffer pressure fluctuations and compromising the autoregulatory reserve [33].
In the context of hypertension, aging alters the normal adaptive remodeling of cerebral vessels. Studies suggest that the ability to remodel appropriately is diminished in older mammals, with dysregulated prostanoid signaling, TRP channel function, and impaired calcium signaling contributing to reduced vascular adaptability [29].
3.2. Functional Impairments
Functional assessments of cerebral autoregulation consistently demonstrate age-related attenuation, particularly in dynamic autoregulatory responses, which are essential for stabilizing CBF during rapid or transient blood pressure changes [34,35]. Both older individuals and aged laboratory animals exhibit weakened myogenic responses of cerebral resistance vessels, limiting the vasculature’s ability to constrict or dilate in response to perfusion pressure fluctuations. This impairment in cerebrovascular autoregulation contributes to diminished cerebral blood flow regulation with aging, increasing vulnerability to cerebral hypoperfusion during hemodynamic stress. While detailed quantitative measures such as transfer-function gain, phase, and autoregulatory index values vary across studies and methodologies, this decline in myogenic responsiveness is a consistent feature of cerebral vascular aging [36]. This diminished responsiveness compromises the brain’s capacity to buffer hemodynamic stress, increasing vulnerability to both hypoperfusion and hyperperfusion [2].
Moreover, age-related endothelial dysfunction, characterized by reduced NO bioavailability, increased endothelin-1 production, and heightened oxidative stress, further impairs the fine-tuning of cerebrovascular tone [37]. The endothelium’s ability to dynamically modulate vasodilation and vasoconstriction is essential for effective autoregulation, and its deterioration with aging undermines this regulatory capacity.
In addition to these baseline functional impairments, the adaptive response of the cerebrovasculature to chronic hypertension is also compromised in aging [29]. Under normal conditions, cerebral vessels undergo structural and functional remodeling in response to sustained hypertension, shifting the autoregulatory curve to maintain stable CBF at higher perfusion pressures [38]. However, aging impairs this protective adaptation. Older individuals show inadequate vascular remodeling and altered signaling pathways—including dysregulated prostanoid production, impaired TRP channel function, and disrupted calcium handling—that limit the ability of the cerebral vasculature to effectively compensate for chronic hypertension [29]. As a result, hypertensive older adults are at increased risk for cerebrovascular injury [32,39], as the protective autoregulatory range narrows and the brain becomes more susceptible to both hypo- and hyperperfusion-related damage.
Additionally, neurovascular coupling [40,41], the tightly regulated increase in CBF in response to neuronal activity, is often disrupted with aging. Impaired signaling between neurons, astrocytes, and endothelial cells diminishes the ability of the cerebrovasculature to meet local metabolic demands, further contributing to functional cerebrovascular insufficiency and cognitive decline [42].
Collectively, these functional impairments—including diminished dynamic autoregulation, endothelial dysfunction, inadequate adaptation to hypertension, and disrupted neurovascular coupling—converge to compromise cerebrovascular resilience in older adults, increasing the risk of cognitive impairment and cerebrovascular disease [43].
3.3. Emerging Role of Cellular and Molecular Aging Mechanisms
By definition, age-related functional decline must involve the fundamental biological mechanisms of aging. The conceptual framework provided by the hallmarks of aging offers a unifying perspective to understand the molecular drivers of age-associated cerebrovascular dysfunction [44,45,46,47]. These hallmarks include genomic instability, telomere attrition, epigenetic alterations [48], loss of proteostasis, deregulated nutrient sensing, mitochondrial dysfunction [49,50], cellular senescence [51], stem cell exhaustion, altered intercellular communication, heightened state of inflammation [50] and increased oxidative stress [52,53].
In the context of cerebral autoregulation and dysregulation of CBF, cellular senescence plays a central role, particularly within the cerebrovascular endothelium. Senescent endothelial cells lose their proliferative capacity and exhibit profound alterations in function, including increased oxidative stress, diminished NO bioavailability, and the secretion of pro-inflammatory and matrix-degrading factors—a profile collectively referred to as the senescence-associated secretory phenotype (SASP) [54,55]. These changes disrupt vascular homeostasis and blunt the dynamic regulation of vascular tone, undermining the brain’s ability to maintain stable perfusion. Moreover, accumulating evidence indicates that increased vascular senescence contributes to several pathological features associated with cerebrovascular aging. Specifically, endothelial senescence has been linked to the development of CMHs, subtle yet clinically significant markers of microvascular fragility and impaired hemodynamic control [56]. Senescence is also implicated in BBB disruption [57], a hallmark of cerebrovascular dysfunction that further compromises brain homeostasis and increases susceptibility to neurodegeneration. In addition, neurovascular coupling (NVC) impairment [58], which reflects the inability of cerebral blood vessels to adjust perfusion in response to neuronal activity, has been associated with vascular aging and senescence-driven endothelial dysfunction [57]. These interconnected processes highlight the vulnerability of the aging cerebrovascular system and underscore the importance of studying cerebral autoregulation and vascular smooth muscle cell (VSMC) function as critical determinants of brain health. Understanding how senescence disrupts both endothelial and smooth muscle components of the vascular wall is essential for identifying therapeutic strategies aimed at preserving autoregulatory capacity and reducing the risk of cerebrovascular pathology in aging.
Another key hallmark implicated in age-related cerebrovascular impairment is mitochondrial dysfunction [59]. Age-associated decline in mitochondrial function leads to excessive production of reactive oxygen species (ROS), which exacerbates oxidative stress, impairs endothelial signaling, and promotes vasoconstriction [60]. This oxidative environment compromises NO signaling, a critical determinant of vascular tone regulation, and amplifies vascular inflammation, thereby destabilizing cerebrovascular homeostasis [61]. Emerging evidence further links mitochondrial dysfunction to endothelial dysfunction, which represents an early and central feature of cerebrovascular aging. Mitochondrial impairment within endothelial cells disrupts energy production, increases oxidative burden, and compromises endothelial barrier integrity [62]. Consequently, mitochondrial dysfunction has been implicated in BBB disruption, a key pathological feature contributing to increased vascular permeability and susceptibility to neuroinflammation in the aging brain [63]. In addition, mitochondrial dysfunction is increasingly recognized as a contributor to NVC impairment [62,63]. This deficit impairs the brain’s ability to match metabolic demand with adequate perfusion, contributing to cognitive decline and functional brain deficits in older adults [64]. While mitochondrial dysfunction has been strongly linked to endothelial dysfunction, BBB breakdown, and impaired NVC, its direct role in the pathogenesis of CMHs remains less clearly defined [65]. Further research is warranted to elucidate whether mitochondrial decline contributes to autoregulatory dysfunction. For example, traumatic brain injury-induced excessive production of mitochondria derived H2O2 was shown to impair myogenic constriction of cerebral vessels through TRPV4-dependent activation of BK Ca channels [66].
Chronic low-grade inflammation, or “inflammaging”, represents a systemic manifestation of altered intercellular communication, another hallmark of aging. In the cerebral vasculature, this persistent inflammatory state further destabilizes autoregulatory mechanisms by promoting endothelial activation, vascular stiffness, and maladaptive remodeling [67,68]. In addition to the cell-intrinsic mechanisms outlined above, cell non-autonomous processes play a fundamental role in the age-related decline of cerebrovascular function. One of the most extensively studied systemic factors contributing to vascular aging is the progressive decline in circulating levels of insulin-like growth factor-1 (IGF-1) [69]. IGF-1 is a key mediator of vascular maintenance and repair, exerting pleiotropic protective effects on endothelial and smooth muscle cells [69]. The age-associated reduction in IGF-1 production, primarily due to diminished growth hormone (GH) signaling and hepatic output, represents a hallmark of disrupted intercellular communication—a core pillar of the aging process. Experimental studies in mouse models have provided compelling evidence for the critical role of IGF-1 in maintaining cerebrovascular health [69,70,71]. Mice with reduced circulating IGF-1 levels exhibit impaired cerebral autoregulation, characterized by diminished myogenic responses and blunted vascular reactivity to pressure fluctuations. Specifically, the autoregulatory range in IGF-1 deficient mice was narrower (80–140 mmHg) compared to controls (80–170 mmHg), indicating reduced capacity to maintain stable cerebral blood flow across a range of pressures. Although transfer-function gain and phase values were not directly reported, pressure-diameter relationships showed significantly impaired myogenic tone development in IGF-1 deficient hypertensive mice, reflecting marked autoregulatory dysfunction [71]. This impairment compromises the brain’s ability to buffer systemic blood pressure changes, increasing susceptibility to hypoperfusion and microvascular damage. Importantly, the adaptive response to chronic hypertension is also disrupted in the setting of IGF-1 deficiency, as vascular remodeling and functional adjustments required to maintain CBF at elevated perfusion pressures are impaired [71]. The inability to mount an effective hypertensive adaptation exacerbates cerebrovascular vulnerability in aged individuals with comorbid hypertension, a common clinical scenario. IGF-1 deficiency also contributes to endothelial dysfunction, heightened oxidative stress, and increased vascular inflammation [72]. These alterations mirror the endothelial changes observed in aged individuals and synergize with other hallmarks of aging, such as mitochondrial dysfunction and cellular senescence [56,65], to destabilize cerebrovascular homeostasis. In line with these mechanisms, animal studies have shown that IGF-1 deficiency exacerbates BBB disruption [73], a key pathological feature contributing to increased vascular permeability, neuroinflammation, and neuronal injury. Moreover, reduced IGF-1 signaling has been linked to a higher burden of CMHs, further highlighting the role of systemic growth factor deficiency in promoting microvascular fragility and structural damage in aging [74,75]. Findings obtained in heterochronic parabiosis models further emphasize that age-related cerebrovascular dysregulation cannot be fully understood without considering the contribution of cell non-autonomous mechanisms [76], particularly the decline in circulating IGF-1 [77]. Restoring systemic trophic support represents a promising avenue for preserving CBF regulation, protecting microvascular integrity, and mitigating the progression of cerebrovascular pathology in the aging brain.
Together, these interconnected aging hallmarks provide a mechanistic basis for the decline in cerebral autoregulation with advancing age. Importantly, they also identify potential targets for therapeutic intervention aimed at vascular rejuvenation. Strategies that reverse endothelial senescence [56], mitigate mitochondrial dysfunction, or suppress chronic inflammation may hold promise for restoring autoregulatory capacity and protecting cerebrovascular health in older adults. Understanding these processes through the lens of fundamental aging biology is essential for the development of effective interventions to preserve brain function across the lifespan.
4. Consequences of Impaired Autoregulation in Aging
Impaired cerebral autoregulation increases the brain’s vulnerability to both hypoperfusion and hyperperfusion, creating conditions that promote microvascular damage. In older adults with compromised autoregulatory capacity, even modest fluctuations in systemic blood pressure can translate into significant alterations in CBF, exceeding the adaptive capacity of the microcirculation [2,3,4]. During hypotensive episodes, this vulnerability manifests as cerebral hypoperfusion, which reduces oxygen and nutrient delivery to the brain and increases the risk of ischemic injury, particularly in wat00ershed regions [2].
Conversely, loss of effective autoregulatory buffering exposes the fragile cerebral microvasculature to damaging surges in blood pressure during hypertensive events [2]. These exaggerated CBF fluctuations impose significant mechanical stress on the capillary and arteriolar networks, which can disrupt the BBB and increase vascular permeability [78]. Over time, this cumulative microvascular injury contributes to the development of CMHs—small, often asymptomatic foci of hemosiderin deposition detectable by susceptibility-weighted imaging (SWI) on MRI [79].
CMHs are increasingly recognized as radiological markers of microvascular fragility and have been strongly linked to aging, hypertension, and cerebral small vessel disease [80]. They predominantly localize to deep brain regions, cortical-subcortical junctions, and lobar territories, depending on the underlying vascular pathology [79,81]. Importantly, CMHs are not merely incidental findings but are associated with cognitive impairment, gait disturbances, and an elevated risk of intracerebral hemorrhage, particularly in patients with mixed cerebrovascular pathology [82,83,84]. Beyond their role as imaging biomarkers, CMHs represent focal sites of brain tissue damage, where the leakage of blood products into the surrounding parenchyma triggers neuroinflammation, oxidative stress, and neuronal injury. Repeated or cumulative CMHs can disrupt neural networks, impair brain connectivity, and contribute to progressive functional decline, reinforcing their significance as both markers and mediators of brain damage in aging and cerebrovascular disease [82,83].
The pathogenesis of CMHs in the context of impaired autoregulation is thought to involve several converging mechanisms [85]. Chronic exposure to hemodynamic stress weakens the structural integrity of small vessels, promoting microaneurysm formation, perivascular inflammation, and degeneration of vascular smooth muscle cells [86]. Age-related endothelial dysfunction and increased oxidative stress further exacerbate vessel wall fragility, impair repair mechanisms, and compromise BBB integrity [53]. In this vulnerable state, transient hypertensive spikes or orthostatic blood pressure variability—common in older adults—can precipitate microvascular rupture, resulting in CMH formation [87]. Given the strong association between CMHs and adverse neurological outcomes [88,89], maintaining effective cerebral autoregulation is critical for protecting microvascular integrity in the aging brain. Understanding how age-related decline in autoregulatory function contributes to CMH development not only provides insight into the mechanisms of vascular cognitive impairment but also highlights potential therapeutic targets aimed at preserving cerebrovascular resilience and reducing the burden of microvascular injury [90].
In addition to these well-established consequences, orthostatic hypotension (OH) represents a clinically relevant manifestation of impaired cerebral autoregulatory capacity in older adults [91]. OH, defined as a significant drop in blood pressure upon standing, is prevalent in aging populations and is often exacerbated by coexisting hypertension, autonomic dysfunction, or polypharmacy [92]. In healthy individuals, intact cerebral autoregulation rapidly compensates for transient reductions in perfusion pressure, maintaining stable CBF during postural changes [2]. However, with advancing age and autoregulatory decline, this compensatory response is reduced, increasing the likelihood of cerebral hypoperfusion during episodes of OH [2,3]. Importantly, recurrent or prolonged cerebral hypoperfusion associated with OH has been linked to cognitive impairment, falls [93], and an elevated risk of ischemic events, particularly in individuals with existing cerebrovascular disease or compromised microvascular integrity. In the context of aging and small vessel disease, the brain’s diminished ability to buffer blood pressure fluctuations may amplify the adverse effects of OH, contributing to progressive white matter damage, microinfarcts, and functional decline [94]. Given the high prevalence of OH in older adults and its potential to exacerbate cerebrovascular injury [92], assessment and management of OH should be considered an integral component of cerebrovascular risk reduction strategies, particularly in individuals with known impaired autoregulation or evidence of microvascular disease.
The deterioration of cerebral autoregulation has been implicated in the pathogenesis of vascular cognitive impairment and dementia (VCID), where chronic hypoperfusion and microvascular dysfunction play central roles [95,96]. Moreover, impaired autoregulation may interact with neurodegenerative processes in mixed dementia, where both VCID and Alzheimer’s disease (AD) pathology coexist [97]. In the development of VCID white matter lesions (WMLs) and lacunar infarcts most likely play critical role. Probably not directly related to autoregulatory dysfunction, white matter hyperintensities, the most common neuroimaging manifestation of cerebral small vessel disease, consistently predict cognitive decline and dementia across diverse populations [98]. These lesions particularly impair executive function and processing speed, reflecting disruption of frontal-subcortical circuits that are essential for complex cognitive tasks. Lacunar infarcts, small subcortical strokes affecting deep brain structures, contribute to cognitive decline despite their modest size, with studies showing that approximately 37% of patients develop mild cognitive impairment or dementia following lacunar stroke [99]. The cognitive impact appears disproportionate to lesion size because lacunar infarcts often occur in the context of widespread cerebral small vessel disease, creating a cumulative burden of microvascular damage. Both WMLs and lacunar infarcts disrupt white matter microstructural integrity, as demonstrated by diffusion tensor imaging studies showing that the count of lacunar infarcts and diffusivity changes in normal-appearing white matter are independent predictors of executive dysfunction [100]. The underlying pathophysiology involves chronic hypoperfusion, blood–brain barrier disruption, and inflammatory responses that lead to demyelination and axonal damage in vulnerable deep white matter regions, ultimately compromising the neural networks essential for cognitive function. As mentioned above, the bidirectional interaction between vascular factors and AD represents a complex, synergistic relationship where cerebrovascular dysfunction both contributes to and results from AD pathology [101]. Vascular dysfunction appears early in AD pathogenesis, often preceding cognitive symptoms and traditional biomarkers, with chronic cerebral hypoperfusion and blood–brain barrier disruption creating conditions that promote amyloid-β (Aβ) accumulation in brain tissue [102]. Conversely, Aβ deposits exert direct neurotoxic effects on cerebral blood vessels, particularly through cerebral amyloid angiopathy (CAA), where Aβ40 and Aβ42 accumulate in vessel walls, causing smooth muscle cell degeneration, vessel wall weakening, and impaired cerebrovascular autoregulation [103,104]. This creates a “two-hit” vascular hypothesis, where initial microvascular insults (hit 1) lead to Aβ-independent neuronal dysfunction and BBB disruption, followed by Aβ-dependent vascular toxicity (hit 2) that creates a positive feedback loop of progressive cerebrovascular damage. Recent evidence demonstrates that vascular risk factors and cerebral small vessel disease severity are directly linked to amyloid deposition and downstream tau pathology, suggesting that cardiovascular health interventions may represent important targets for AD prevention. The clinical significance is substantial, as studies show that up to 50% of dementia cases, including AD, may have a vascular component, emphasizing that effective AD treatment strategies must address both neurodegenerative and cerebrovascular pathways simultaneously.
Interestingly, autoregulatory dysfunction may contribute to the development of age-related disorders of cerebrospinal fluid circulation. Accordingly, normal pressure hydrocephalus (NPH) involves complex vascular mechanisms that extend far beyond simple mechanical compression of brain tissue. The pathophysiology centers on impaired cerebrovascular autoregulation, where studies consistently demonstrate reduced cerebral blood flow (approximately 20–28% below normal) coupled with preserved but unused autoregulatory reserve, creating an apparent paradox where the ischemic brain fails to utilize available compensatory mechanisms [105]. Vascular compliance abnormalities play a central role, with NPH patients showing significantly reduced compliance in the superior sagittal sinus territory compared to healthy controls, leading to altered arteriovenous delay patterns and restricted cerebrospinal fluid outflow [106]. The “two-hit” vascular hypothesis suggests that vascular risk factors (hypertension, diabetes, white matter lesions) predispose to NPH development, with population-based studies demonstrating strong associations between these cardiovascular comorbidities and both clinical and imaging features of NPH [107,108]. Furthermore, recent modeling studies indicate that NPH involves balanced increases in both arterial and venous resistance, with the cerebral blood flow being actively limited by arteriolar constriction rather than passive compression, and this resistance pattern can be partially reversed following shunt surgery [109]. The vascular dysfunction creates a periventricular watershed region with maximal blood flow reduction occurring approximately 9 mm from the ventricular wall, explaining the characteristic pattern of white matter damage and the preferential vulnerability of deep brain structures in NPH [110].
Autoregulatory dysfunction and enlarged perivascular spaces (EPVS) are also interrelated phenomena. Impaired autoregulation can increase the transmission of blood pressure fluctuations to small vessels, promoting endothelial dysfunction and diminished perivascular clearance, factors that further contribute to EPVS formation [111]. Studies have demonstrated that patients with severe basal ganglia EPVS have higher critical closing pressure—a dynamic parameter that also reflects autoregulatory reserve—compared to those with fewer EPVS [112].
Finally, in the context of acute cerebrovascular events such as stroke, impaired autoregulation increases susceptibility to poor outcomes, including larger infarct size and higher risk of hemorrhagic transformation following reperfusion [113,114,115].
An often-overlooked consequence of impaired cerebral autoregulation is its detrimental impact on interhemispheric blood flow compensation following unilateral carotid artery occlusion. Under physiological conditions, intact autoregulatory mechanisms and a functional circle of Willis enable redistribution of blood flow from the contralateral hemisphere to maintain perfusion in territories affected by carotid stenosis or occlusion. However, with advancing age and associated vascular dysfunction, these compensatory pathways become increasingly compromised [33]. Impaired autoregulation blunts the ability of cerebral vessels in the contralateral hemisphere to appropriately dilate in response to increased demand, limiting effective collateral recruitment [116].
This dysfunctional compensatory response is of particular relevance during vascular interventions such as carotid artery stenting (CAS) or carotid endarterectomy (CEA), where temporary or prolonged alterations in perfusion dynamics can occur [117]. In older adults with pre-existing cerebrovascular aging and impaired autoregulation, these procedures carry a higher risk of inadequate interhemispheric flow redistribution, leaving vulnerable brain regions susceptible to hypoperfusion, ischemia, and subsequent neurological complications [118]. Moreover, in the setting of incomplete or hypoplastic components of the circle of Willis—a condition prevalent in a substantial portion of the population [119]—the reliance on intact autoregulatory capacity for adequate collateral flow becomes even more critical. Therefore, understanding the interplay between aging, autoregulatory decline, and interhemispheric perfusion is essential for optimizing perioperative management and minimizing the risk of ischemic injury during carotid revascularization procedures. Preoperative assessment of autoregulatory function and collateral capacity may help identify high-risk individuals and guide tailored intervention strategies to mitigate these risks.
In addition to these vascular and surgical contexts, traumatic brain injury (TBI) represents another clinical scenario where impaired cerebral autoregulation has critical consequences [120], particularly in the aging brain [121]. Older adults are at increased risk of TBI due to higher rates of falls and greater cerebral vulnerability associated with vascular aging, brain atrophy, and comorbidities [122]. Following TBI, cerebral autoregulation is frequently disrupted, impairing the brain’s ability to maintain stable CBF in response to fluctuations in systemic blood pressure [123]. This loss of autoregulatory control exacerbates the risk of both secondary ischemic injury and hyperperfusion-related damage, contributing to poor neurological outcomes [124]. Importantly, age-related deterioration in baseline autoregulatory capacity further amplifies this vulnerability in older TBI patients [122]. Studies have shown that impaired autoregulation after TBI is associated with larger lesion volumes, greater BBB disruption, and an increased burden of cerebral microhemorrhages—pathological features that are already prevalent in the aging brain [121,125]. Moreover, the combination of TBI-induced and age-related cerebrovascular dysfunction may synergistically promote cognitive decline and neurodegeneration [126]. Given these observations, early assessment of autoregulatory function and careful management of blood pressure are essential components of TBI care, particularly in older adults, to mitigate secondary brain injury and improve clinical outcomes [127]. The mechanism described is summarized in Figure 1, which schematically illustrates the age-related changes affecting cerebral blood flow regulation and their potential consequences.
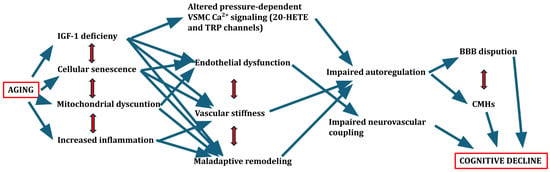
Figure 1.
Schematic figure of age-related changes affecting the regulation of cerebral blood flow and possible consequences.
5. Methodological Considerations
Noninvasively assessing cerebral autoregulation in older adults presents methodological challenges [128,129,130,131,132,133]. Techniques such as transcranial Doppler (TCD) ultrasound, arterial spin labeling (ASL) MRI, and near-infrared spectroscopy (NIRS) provide non-invasive estimates of CBF and autoregulatory function but differ in applicability across populations. Selecting the optimal modality requires balancing temporal resolution, spatial coverage, invasiveness, logistical demands, and susceptibility to technical pitfalls. Accordingly, TCD excels at real-time assessment of cerebral haemodynamic responses, which makes it ideal to assess dynamic cerebral autoregulation in neurointensive units [133,134]. NIRS provides continuous, non-invasive and operator-independent measurements, being ideal to assess, for example, delayed ischemic changes by the bedside [135,136]. ASL offers quantitative, global perfusion maps, having the highest spatial resolution among the non-invasive methods [137,138]. Phase-contrast MRI quantitatively measures vessel-specific flow and pulsatility, enabling for regional assessment of autoregulation [139,140]. In conclusion, no single modality is universally superior, each reflects answers a different aspect of the autoregulation puzzle. Integrating their complementary strengths—rather than substituting one for another—yields the richest, most reliable haemodynamic assessment of the injured or at-risk brain. It has to be noted that cost-effectiveness should be considered when planning monitoring of autoregulatory function in older individuals. The below comparative table (Table 1) describes these aspects in detail.

Table 1.
Comparison of non-invasive methods used to assess different aspects of cerebral autoregulation.
Blood–brain barrier disruption and increased permeability are important consequences of autoregulatory impairment [141,142]. Different modalities provide data on distinct aspects of BBB function and molecular transport. Dynamic contrast-enhanced (DCE) MRI measures the rate of movement of contrast from plasma to extravascular extracellular space, its volume fraction and the rate of contrast reflux back to plasma, providing parallel, high-resolution data on perfusion and permeability [143]. ASL MRI techniques can be adjusted to assess perfusion and permeability alike, by using magnetically labelled water molecules as tracers and quantifying their exchange between compartments, obviating the need for intravenous contrast administration [144]. PET tracers may only cross into the parenchyma by specific transport mechanisms, capturing the integrity of the BBB at the molecular level [145]. These methodologies should be applied complementarily to unveil the pathobiology of permeability changes in detail.
The presence of common age-related comorbidities, including hypertension, diabetes, and atherosclerosis, further complicates the interpretation of autoregulation measurements, highlighting the need for careful study design and analysis. For example, chronic hypertension thickens arteriolar wall, shifting autoregulatory breakpoints [146,147]. Type-2 diabetes induces endothelial dysfunction and impairs nitric-oxide signaling, narrowing the plateau, and increasing inter-beat flow variability [148,149]. Accordingly, cerebral-autoregulation research therefore demands comorbidity-aware recruitment and analytic models that incorporate vascular phenotype as an interactive covariate.
Longitudinal, population-based studies tracking the decline of autoregulatory function with aging are scarce and contradictory [6,150,151,152,153]. For example, Weijs et al. showed that in a relatively small group of patients that a decade of aging did not lead to deterioration in CBF or autoregulation, while reductions in CBF and increases in cerebrovascular resistance were associated with early subjective cognitive decline [128]. Contrary, the MOBILIZE Boston Study showed that in 96 participants impaired autoregulation was obtained at baseline (indicated by higher transfer function gain), which was associated with greater decline in gait function during a 7 years follow up [153]. The longitudinal findings reported by Weijs et al. and the MOBILIZE Boston Study present seemingly conflicting conclusions regarding age-related changes in cerebral autoregulation and their clinical implications. Weijs et al. observed, in a relatively small cohort, that a decade of aging did not result in significant deterioration of cerebral blood flow or autoregulatory function. Instead, their data indicated that reductions in cerebral blood flow and increases in cerebrovascular resistance were more closely associated with early subjective cognitive decline. In contrast, the MOBILIZE Boston Study, encompassing a larger sample of 96 participants, documented impaired cerebral autoregulation at baseline, as evidenced by elevated transfer function gain, which correlated with greater decline in gait function over a seven-year follow-up period.
These divergent results may be reconciled by careful consideration of methodological and population differences between the two studies. First, the sample sizes differ markedly, with the MOBILIZE Boston Study having greater statistical power to detect subtle autoregulatory impairments and their functional consequences. Second, the study designs differ; Weijs et al. employed a longitudinal approach with a small sample concentrated on cerebrovascular parameters and cognitive outcomes, whereas MOBILIZE incorporated broader functional assessments including gait, suggesting differential sensitivity to cerebrovascular changes. Third, participant comorbidities vary; the MOBILIZE Boston cohort likely included greater heterogeneity in vascular risk factors and baseline clinical status, which may augment autoregulatory dysfunction and its downstream effects. Lastly, the metrics employed differ: Weijs et al. focused primarily on cerebral blood flow and cerebrovascular resistance measures, while the MOBILIZE Boston Study utilized transfer function analysis of cerebral autoregulation and linked these measurements to motor function decline.
Together, these distinctions underscore the complexity inherent in measuring cerebral autoregulatory function longitudinally, particularly in aging populations with varying baseline risks. They highlight the necessity for large-scale, multimodal, prospective studies incorporating comprehensive assessments of cerebrovascular physiology, comorbid burden, and clinically relevant functional outcomes to clarify the trajectory and clinical significance of age-related changes in cerebral autoregulation [128,153].
Although, cross-sectional studies reported increased arterial stiffness and reduced vasomotor activity associated with hypertension and atherosclerosis, study design limits the ability to distinguish age effects from disease-related changes [154]. These results highlight the need for further multimodal prospective research to assess age-related changes in autoregulation and its consequences on brain function. Especially, that animal models of cerebrovascular aging, while informative, often fail to fully recapitulate the complexity of human vascular aging, underscoring the translational gap in the field.
6. Potential Interventions and Future Directions
Lifestyle interventions that preserve vascular health, such as regular aerobic exercise and adherence to dietary patterns like the Mediterranean diet, hold promise for mitigating age-related cerebrovascular dysfunction. Evidence suggests that such strategies can improve endothelial function and promote cerebrovascular resilience [155]. The role of Mediterranean diet in preventing age-related alterations in the regulation of CBF should be tested in the future. It is increasingly recognized that sex-specific mechanisms play a critical role in vascular aging [156] and may influence both the trajectory of cerebrovascular decline and the response to preventive interventions. Differences in sex hormones [157,158], genetic factors, and vascular biology contribute to distinct patterns of endothelial dysfunction, vascular stiffness, and CBF regulation between men and women as they age [159]. These differences underscore the need for sex-specific considerations [160,161] when designing and evaluating lifestyle interventions aimed at preserving cerebrovascular health. Moreover, epidemiological studies aimed at understanding the determinants of cerebrovascular health across the lifespan [162] are essential for identifying modifiable risk factors and potential targets for intervention [163]. Such studies can provide critical insights into how lifestyle, metabolic, and environmental factors interact with biological aging processes to shape cerebrovascular outcomes. Future studies are warranted to test how complex lifestyle interventions impact autoregulation of CBF in older adults.
Pharmacological interventions targeting the molecular hallmarks of vascular aging represent an exciting, yet largely exploratory, avenue [164,165]. Various anti-aging treatment regimens [166], including antioxidants [167], mTOR inhibitors [168], and senolytics [158,169] that selectively eliminate senescent cells are under investigation for their potential to rejuvenate vascular function, including cerebral autoregulatory capacity.
Future research should prioritize interventional studies to determine whether improving vascular health translates into restored autoregulation and cognitive protection in older adults. Integration of vascular aging biomarkers into personalized risk stratification frameworks could further guide prevention and intervention strategies.
7. Limitations
There are several factors that may influence age-related alterations in cerebrovascular autoregulation, including sex differences, ethnic and racial disparities in cerebrovascular aging, sleep and sleep disorders, COVID-19 and its long-term vascular and endothelial sequelae, computational modeling of autoregulation, and medication effects (such as antihypertensives, statins, vasoactive drugs, and dementia subtypes). However, quantifying the impact of these factors is beyond the scope of the present manuscript but could provide valuable directions for future research.
The role of IGF-1 deficiency in pathophysiological changes in the regulation of cerebral blood flow has been suggested by animal studies. Although we recently demonstrated that IGF-1 deficiency is associated with impaired neurovascular coupling responses in older individuals, further clinical studies are needed to examine the role of deficient IGF-1 related signaling in age-related alterations of regulation of CBF.
8. Conclusions
Aging impairs cerebral autoregulation through a complex interplay of structural, functional, and molecular mechanisms. The resulting instability in CBF contributes to increased vulnerability to ischemic injury, cognitive decline, and poor outcomes following cerebrovascular insults. Preserving cerebral autoregulatory function is a critical but underappreciated target for promoting healthy brain aging. A concerted research effort is needed to elucidate the mechanistic underpinnings of autoregulatory decline and to develop targeted interventions capable of maintaining or restoring cerebrovascular resilience in older adults. Based on this, a clear and easy to follow clinical algorithm should be developed for risk stratification, methodological modality, interpretation and management.
Author Contributions
Conceptualization: P.T. and A.C.; methodology: A.K., M.F. and Á.L.; investigation, A.U., R.G., E.P. and T.C.; writing—original draft preparation, A.U., A.K. and L.S.; writing—review and editing, L.S. and D.L.-E.; supervision, P.T. and A.C.; project administration, D.L.-E., R.P., B.C. and Z.B.; funding acquisition, P.T., A.C. and Z.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from the American Heart Association (ST: AHA CDA941290), the Oklahoma Center for the Advancement of Science and Technology, the National Institute on Aging (RF1AG072295, R01AG055395, R01AG068295; R01AG070915, K01AG073614, K01AG073613, R03AG070479), the National Institute of Neurological Disorders and Stroke (R01NS100782), the National Cancer Institute (R01CA255840), the Presbyterian Health Foundation, the Oklahoma Nathan Shock Center (P30AG050911), by funding through Project no. TKP2021-NKTA-47, implemented with the support provided by the Ministry of Innovation and Technology of Hungary from the National Research, Development and Innovation Fund, financed under the TKP2021-NKTA funding scheme; by funding through the National Cardiovascular Laboratory Program (RRF-2.3.1-21-2022-00003) provided by the Ministry of Innovation and Technology of Hungary from the National Research, Development and Innovation Fund; Project no. 135784 implemented with the support provided from the National Research, Development and Innovation Fund of Hungary, financed under the K_20 funding scheme. Further financial support was received from the National Research, Development and Innovation Office (OTKA K-134555, OTKA FK-146334 to PT), the National Clinical Neuroscience Laboratory (RRF-2.3.1-21-2022-00011), and the Thematic Excellence Program 2021 Health sub-program of the Ministry for Innovation and Technology in Hungary, within the framework of the EGA-16 project of the University of Pecs (to PT). ZB was supported by ADVANCED_24 research grant provided by the National Research, Development and Innovation Fund. This work was also supported by the EKÖP-2024-9 New National Excellence Program of the Ministry for Culture and Innovation from the source of the National Research, Development, and Innovation Fund. The funding sources had no role in the writing of the manuscript; and in the decision to submit the article for publication.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable.
Acknowledgments
A GPT-based AI grammar check was used to improve the English of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lassen, N.A. Cerebral blood flow and oxygen consumption in man. Physiol. Rev. 1959, 39, 183–238. [Google Scholar] [CrossRef]
- Armstead, W.M. Cerebral Blood Flow Autoregulation and Dysautoregulation. Anesthesiol. Clin. 2016, 34, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Claassen, J.; Thijssen, D.H.J.; Panerai, R.B.; Faraci, F.M. Regulation of cerebral blood flow in humans: Physiology and clinical implications of autoregulation. Physiol. Rev. 2021, 101, 1487–1559. [Google Scholar] [CrossRef]
- Knox, E.G.; Aburto, M.R.; Clarke, G.; Cryan, J.F.; O’Driscoll, C.M. The blood-brain barrier in aging and neurodegeneration. Mol. Psychiatry 2022, 27, 2659–2673. [Google Scholar] [CrossRef]
- Kalaria, R.N. Vascular basis for brain degeneration: Faltering controls and risk factors for dementia. Nutr. Rev. 2010, 68 (Suppl. 2), S74–S87. [Google Scholar] [CrossRef]
- Weijs, R.W.; Oudegeest-Sander, M.H.; Hopman, M.T.; Thijssen, D.H.; Claassen, J.A. Cerebrovascular CO(2) reactivity and dynamic cerebral autoregulation through the eighth decade of life and their implications for cognitive decline. J. Cereb. Blood Flow. Metab. 2024, 44, 712–725. [Google Scholar] [CrossRef]
- Markus, H.S. Cerebral perfusion and stroke. J. Neurol. Neurosurg. Psychiatry 2004, 75, 353–361. [Google Scholar] [CrossRef]
- Wang, Y.; Payne, S.J. Static autoregulation in humans. J. Cereb. Blood Flow. Metab. 2024, 44, 1191–1207. [Google Scholar] [CrossRef] [PubMed]
- Abadjiev, D.S.; Toschi-Dias, E.; Salinet, A.S.; Gaykova, N.N.; Lo, M.T.; Nogueira, R.C.; Hu, K. Daily rhythm of dynamic cerebral autoregulation in patients after stroke. J. Cereb. Blood Flow. Metab. 2023, 43, 989–998. [Google Scholar] [CrossRef] [PubMed]
- Panerai, R.B.; Barnes, S.C.; Batterham, A.P.; Robinson, T.G.; Haunton, V.J. Directional sensitivity of dynamic cerebral autoregulation during spontaneous fluctuations in arterial blood pressure at rest. J. Cereb. Blood Flow. Metab. 2023, 43, 552–564. [Google Scholar] [CrossRef]
- Heutz, R.; Claassen, J.; Feiner, S.; Davies, A.; Gurung, D.; Panerai, R.B.; Heus, R.; Beishon, L.C. Dynamic cerebral autoregulation in Alzheimer’s disease and mild cognitive impairment: A systematic review. J. Cereb. Blood Flow. Metab. 2023, 43, 1223–1236. [Google Scholar] [CrossRef]
- Toth, P.; Tarantini, S.; Csiszar, A.; Ungvari, Z. Functional vascular contributions to cognitive impairment and dementia: Mechanisms and consequences of cerebral autoregulatory dysfunction, endothelial impairment, and neurovascular uncoupling in aging. Am. J. Physiol. Heart Circ. Physiol. 2017, 312, H1–H20. [Google Scholar] [CrossRef]
- Tan, C.O.; Hamner, J.W.; Taylor, J.A. The role of myogenic mechanisms in human cerebrovascular regulation. J. Physiol. 2013, 591, 5095–5105. [Google Scholar] [CrossRef] [PubMed]
- Newell, D.W.; Aaslid, R.; Lam, A.; Mayberg, T.S.; Winn, H.R. Comparison of flow and velocity during dynamic autoregulation testing in humans. Stroke 1994, 25, 793–797. [Google Scholar] [CrossRef]
- Ghosh, D.; Syed, A.U.; Prada, M.P.; Nystoriak, M.A.; Santana, L.F.; Nieves-Cintrón, M.; Navedo, M.F. Calcium Channels in Vascular Smooth Muscle. Adv. Pharmacol. 2017, 78, 49–87. [Google Scholar] [CrossRef]
- Davis, M.J.; Hill, M.A. Signaling mechanisms underlying the vascular myogenic response. Physiol. Rev. 1999, 79, 387–423. [Google Scholar] [CrossRef] [PubMed]
- Sommerville, L.E.; Hartshorne, D.J. Intracellular calcium and smooth muscle contraction. Cell Calcium 1986, 7, 353–364. [Google Scholar] [CrossRef]
- Brayden, J.E.; Earley, S.; Nelson, M.T.; Reading, S. Transient receptor potential (TRP) channels, vascular tone and autoregulation of cerebral blood flow. Clin. Exp. Pharmacol. Physiol. 2008, 35, 1116–1120. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Brayden, J.E. Rho kinase activity governs arteriolar myogenic depolarization. J. Cereb. Blood Flow. Metab. 2017, 37, 140–152. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, J.; Tong, X. Cross-Talk between Mechanosensitive Ion Channels and Calcium Regulatory Proteins in Cardiovascular Health and Disease. Int. J. Mol. Sci. 2021, 22, 8782. [Google Scholar] [CrossRef]
- Ainslie, P.N.; Brassard, P. Why is the neural control of cerebral autoregulation so controversial? F1000Prime Rep. 2014, 6, 14. [Google Scholar] [CrossRef] [PubMed]
- Murkin, J.M. Cerebral autoregulation: The role of CO2 in metabolic homeostasis. Semin. Cardiothorac. Vasc. Anesth. 2007, 11, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Winn, H.R.; Morii, S.; Berne, R.M. The role of adenosine in autoregulation of cerebral blood flow. Ann. Biomed. Eng. 1985, 13, 321–328. [Google Scholar] [CrossRef]
- Mascia, L.; Piper, I.R.; Andrews, P.J.; Souter, M.J.; Webb, D.J. The role of endothelin-1 in pressure autoregulation of cerebral blood flow in rats. Intensive Care Med. 1999, 25, 1282–1286. [Google Scholar] [CrossRef]
- Buchanan, J.E.; Phillis, J.W. The role of nitric oxide in the regulation of cerebral blood flow. Brain Res. 1993, 610, 248–255. [Google Scholar] [CrossRef]
- Leffler, C.W.; Parfenova, H. Cerebral arteriolar dilation to hypoxia: Role of prostanoids. Am. J. Physiol. 1997, 272, H418–H424. [Google Scholar] [CrossRef] [PubMed]
- Iadecola, C.; Davisson, R.L. Hypertension and cerebrovascular dysfunction. Cell Metab. 2008, 7, 476–484. [Google Scholar] [CrossRef]
- van Hespen, K.M.; Mackaaij, C.; Waas, I.S.E.; de Bree, M.P.; Zwanenburg, J.J.M.; Kuijf, H.J.; Daemen, M.; Hendrikse, J.; Hermkens, D.M.A. Arterial Remodeling of the Intracranial Arteries in Patients With Hypertension and Controls: A Postmortem Study. Hypertension 2021, 77, 135–146. [Google Scholar] [CrossRef]
- Toth, P.; Csiszar, A.; Tucsek, Z.; Sosnowska, D.; Gautam, T.; Koller, A.; Schwartzman, M.L.; Sonntag, W.E.; Ungvari, Z. Role of 20-HETE, TRPC channels, and BKCa in dysregulation of pressure-induced Ca2+ signaling and myogenic constriction of cerebral arteries in aged hypertensive mice. Am. J. Physiol. Heart Circ. Physiol. 2013, 305, H1698–H1708. [Google Scholar] [CrossRef]
- Bareiro, F.A.Q.; Carnicero, J.A.; Acha, A.A.; Artalejo, C.R.; Jimenez, M.C.G.; Manas, L.R.; Garcia Garcia, F.J. How cognitive performance changes according to the ankle-brachial index score in an elderly cohort? Results from the Toledo Study of Healthy Ageing. Geroscience 2024, 46, 609–620. [Google Scholar] [CrossRef]
- Zhang, H.; Leng, S.; Gao, F.; Kovalik, J.P.; Tan, R.S.; Wee, H.N.; Chua, K.V.; Ching, J.; Zhao, X.; Allen, J.; et al. Longitudinal aortic strain, ventriculo-arterial coupling and fatty acid oxidation: Novel insights into human cardiovascular aging. Geroscience 2024, 46, 5459–5471. [Google Scholar] [CrossRef]
- Bareiro, F.A.Q.; Carnicero, J.A.; Acha, A.A.; Artalejo, C.R.; Jimenez, M.C.G.; Manas, L.R.; Garcia Garcia, F.J. Carotid-femoral pulse wave velocity score, an estimator of cognitive performance in the elderly: Results from the Toledo Study for Healthy Aging. Geroscience 2024, 46, 5711–5723. [Google Scholar] [CrossRef] [PubMed]
- Reeve, E.H.; Barnes, J.N.; Moir, M.E.; Walker, A.E. Impact of arterial stiffness on cerebrovascular function: A review of evidence from humans and preclincal models. Am. J. Physiol. Heart Circ. Physiol. 2024, 326, H689–H704. [Google Scholar] [CrossRef]
- Yam, A.T.; Lang, E.W.; Lagopoulos, J.; Yip, K.; Griffith, J.; Mudaliar, Y.; Dorsch, N.W. Cerebral autoregulation and ageing. J. Clin. Neurosci. 2005, 12, 643–646. [Google Scholar] [CrossRef]
- van Beek, A.H.; Claassen, J.A.; Rikkert, M.G.; Jansen, R.W. Cerebral autoregulation: An overview of current concepts and methodology with special focus on the elderly. J. Cereb. Blood Flow. Metab. 2008, 28, 1071–1085. [Google Scholar] [CrossRef]
- Tarumi, T.; Zhang, R. Cerebral blood flow in normal aging adults: Cardiovascular determinants, clinical implications, and aerobic fitness. J. Neurochem. 2018, 144, 595–608. [Google Scholar] [CrossRef] [PubMed]
- Tomoto, T.; Lu, M.; Khan, A.M.; Liu, J.; Pasha, E.P.; Tarumi, T.; Zhang, R. Cerebral blood flow and cerebrovascular resistance across the adult lifespan: A multimodality approach. J. Cereb. Blood Flow. Metab. 2023, 43, 962–976. [Google Scholar] [CrossRef]
- Hoffman, W.E.; Albrecht, R.F.; Miletich, D.J. The influence of aging and hypertension on cerebral autoregulation. Brain Res. 1981, 214, 196–199. [Google Scholar] [CrossRef]
- Armstrong, M.K.; Jain, S.; Nuckols, V.; Pewowaruk, R.; Zhang, X.; DuBose, L.; Sodoma, M.; Madero, B.; Voss, M.W.; Pierce, G.L. The association of structural versus load-dependent large artery stiffness mechanisms with cerebrovascular damage and cortical atrophy in humans. Geroscience 2024, 46, 5587–5597. [Google Scholar] [CrossRef]
- van Dijk, S.E.; Drenth, N.; Hafkemeijer, A.; Labadie, G.; Witjes-Ane, M.W.; Blauw, G.J.; Rombouts, S.A.; van der Grond, J.; van Rooden, S. Neurovascular coupling in early stage dementia - A case-control study. J. Cereb. Blood Flow. Metab. 2024, 44, 1013–1023. [Google Scholar] [CrossRef] [PubMed]
- Ball, J.D.; Hills, E.; Altaf, A.; Ramesh, P.; Green, M.; Surti, F.B.; Minhas, J.S.; Robinson, T.G.; Bond, B.; Lester, A.; et al. Neurovascular coupling methods in healthy individuals using transcranial doppler ultrasonography: A systematic review and consensus agreement. J. Cereb. Blood Flow. Metab. 2024, 44, 1409–1429. [Google Scholar] [CrossRef]
- Lourenço, C.F.; Ledo, A.; Caetano, M.; Barbosa, R.M.; Laranjinha, J. Age-Dependent Impairment of Neurovascular and Neurometabolic Coupling in the Hippocampus. Front. Physiol. 2018, 9, 913. [Google Scholar] [CrossRef] [PubMed]
- Santisteban, M.M.; Iadecola, C.; Carnevale, D. Hypertension, Neurovascular Dysfunction, and Cognitive Impairment. Hypertension 2023, 80, 22–34. [Google Scholar] [CrossRef]
- Schaum, N.; Lehallier, B.; Hahn, O.; Palovics, R.; Hosseinzadeh, S.; Lee, S.E.; Sit, R.; Lee, D.P.; Losada, P.M.; Zardeneta, M.E.; et al. Ageing hallmarks exhibit organ-specific temporal signatures. Nature 2020, 583, 596–602. [Google Scholar] [CrossRef]
- Lopez-Otin, C.; Kroemer, G. Hallmarks of health. Cell 2021, 184, 1929–1939. [Google Scholar] [CrossRef]
- Lopez-Otin, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of aging: An expanding universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef]
- Tartiere, A.G.; Freije, J.M.P.; Lopez-Otin, C. The hallmarks of aging as a conceptual framework for health and longevity research. Front. Aging 2024, 5, 1334261. [Google Scholar] [CrossRef]
- Pospiech, E.; Bar, A.; Pisarek-Pacek, A.; Karas, A.; Branicki, W.; Chlopicki, S. Epigenetic clock in the aorta and age-related endothelial dysfunction in mice. Geroscience 2024, 46, 3993–4002. [Google Scholar] [CrossRef] [PubMed]
- Karas, A.; Bar, A.; Pandian, K.; Jasztal, A.; Kurylowicz, Z.; Kutryb-Zajac, B.; Buczek, E.; Rocchetti, S.; Mohaissen, T.; Jedrzejewska, A.; et al. Functional deterioration of vascular mitochondrial and glycolytic capacity in the aortic rings of aged mice. Geroscience 2024, 46, 3831–3844. [Google Scholar] [CrossRef]
- Bencivenga, L.; Strumia, M.; Rolland, Y.; Martinez, L.; Cestac, P.; Guyonnet, S.; Andrieu, S.; Parini, A.; Lucas, A.; Vellas, B.; et al. Biomarkers of mitochondrial dysfunction and inflammaging in older adults and blood pressure variability. Geroscience 2023, 45, 797–809. [Google Scholar] [CrossRef] [PubMed]
- Ting, K.K.; Coleman, P.; Kim, H.J.; Zhao, Y.; Mulangala, J.; Cheng, N.C.; Li, W.; Gunatilake, D.; Johnstone, D.M.; Loo, L.; et al. Vascular senescence and leak are features of the early breakdown of the blood-brain barrier in Alzheimer’s disease models. Geroscience 2023, 45, 3307–3331. [Google Scholar] [CrossRef] [PubMed]
- Summer, S.; Borrell-Pages, M.; Bruno, R.M.; Climie, R.E.; Dipla, K.; Dogan, A.; Eruslanova, K.; Fraenkel, E.; Mattace-Raso, F.; Pugh, C.J.A.; et al. Centenarians-the way to healthy vascular ageing and longevity: A review from VascAgeNet. Geroscience 2024, 47, 685–702. [Google Scholar] [CrossRef]
- Csiszar, A.; Ungvari, A.; Patai, R.; Gulej, R.; Yabluchanskiy, A.; Benyo, Z.; Kovacs, I.; Sotonyi, P.; Kirkpartrick, A.C.; Prodan, C.I.; et al. Atherosclerotic burden and cerebral small vessel disease: Exploring the link through microvascular aging and cerebral microhemorrhages. Geroscience 2024, 46, 5103–5132. [Google Scholar] [CrossRef]
- Lambert, M.; Miquel, G.; Villeneuve, L.; Thorin-Trescases, N.; Thorin, E. The senolytic ABT-263 improves cognitive functions in middle-aged male, but not female, atherosclerotic LDLr(-/-);hApoB(100)(+/+) mice. Geroscience 2025, 47, 4577–4600. [Google Scholar] [CrossRef]
- Cummings, S.R.; Lui, L.Y.; Zaira, A.; Mau, T.; Fielding, R.A.; Atkinson, E.J.; Patel, S.; LeBrasseur, N. Biomarkers of cellular senescence and major health outcomes in older adults. Geroscience 2024, 47, 3407–3415. [Google Scholar] [CrossRef]
- Faakye, J.; Nyul-Toth, A.; Muranyi, M.; Gulej, R.; Csik, B.; Shanmugarama, S.; Tarantini, S.; Negri, S.; Prodan, C.; Mukli, P.; et al. Preventing spontaneous cerebral microhemorrhages in aging mice: A novel approach targeting cellular senescence with ABT263/navitoclax. Geroscience 2023, 46, 21–37. [Google Scholar] [CrossRef]
- Csik, B.; Nyul-Toth, A.; Gulej, R.; Patai, R.; Kiss, T.; Delfavero, J.; Nagaraja, R.Y.; Balasubramanian, P.; Shanmugarama, S.; Ungvari, A.; et al. Senescent Endothelial Cells in Cerebral Microcirculation Are Key Drivers of Age-Related Blood-Brain Barrier Disruption, Microvascular Rarefaction, and Neurovascular Coupling Impairment in Mice. Aging Cell 2025, 24, e70048. [Google Scholar] [CrossRef]
- Kiss, T.; Nyul-Toth, A.; Balasubramanian, P.; Tarantini, S.; Ahire, C.; Yabluchanskiy, A.; Csipo, T.; Farkas, E.; Wren, J.D.; Garman, L.; et al. Nicotinamide mononucleotide (NMN) supplementation promotes neurovascular rejuvenation in aged mice: Transcriptional footprint of SIRT1 activation, mitochondrial protection, anti-inflammatory, and anti-apoptotic effects. Geroscience 2020, 42, 527–546. [Google Scholar] [CrossRef] [PubMed]
- Bartman, S.; Coppotelli, G.; Ross, J.M. Mitochondrial Dysfunction: A Key Player in Brain Aging and Diseases. Curr. Issues Mol. Biol. 2024, 46, 1987–2026. [Google Scholar] [CrossRef] [PubMed]
- Zinovkin, R.A.; Romaschenko, V.P.; Galkin, I.I.; Zakharova, V.V.; Pletjushkina, O.Y.; Chernyak, B.V.; Popova, E.N. Role of mitochondrial reactive oxygen species in age-related inflammatory activation of endothelium. Aging (Albany NY) 2014, 6, 661–674. [Google Scholar] [CrossRef]
- Salvagno, M.; Sterchele, E.D.; Zaccarelli, M.; Mrakic-Sposta, S.; Welsby, I.J.; Balestra, C.; Taccone, F.S. Oxidative Stress and Cerebral Vascular Tone: The Role of Reactive Oxygen and Nitrogen Species. Int. J. Mol. Sci. 2024, 25, 3007. [Google Scholar] [CrossRef]
- Grossini, E.; Venkatesan, S.; Ola Pour, M.M. Mitochondrial Dysfunction in Endothelial Cells: A Key Driver of Organ Disorders and Aging. Antioxidants 2025, 14, 372. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, J.; Wang, J.; He, L.; Lai, H.; Zhang, T.; Wang, X.; Li, W. Mitochondrial oxidative stress in brain microvascular endothelial cells: Triggering blood-brain barrier disruption. Mitochondrion 2023, 69, 71–82. [Google Scholar] [CrossRef]
- Mukli, P.; Pinto, C.B.; Owens, C.D.; Csipo, T.; Lipecz, A.; Szarvas, Z.; Peterfi, A.; Langley, A.; Hoffmeister, J.; Racz, F.S.; et al. Impaired Neurovascular Coupling and Increased Functional Connectivity in the Frontal Cortex Predict Age-Related Cognitive Dysfunction. Adv. Sci. 2024, 11, e2303516. [Google Scholar] [CrossRef]
- Patai, R.; Patel, K.; Csik, B.; Gulej, R.; Nagaraja, R.Y.; Nagy, D.; Chandragiri, S.S.; Shanmugarama, S.; Kordestan, K.V.; Nagykaldi, M.; et al. Aging, mitochondrial dysfunction, and cerebral microhemorrhages: A preclinical evaluation of SS-31 (elamipretide) and development of a high-throughput machine learning-driven imaging pipeline for cerebromicrovascular protection therapeutic screening. Geroscience 2025, 47, 4871–4887. [Google Scholar] [CrossRef]
- Szarka, N.; Pabbidi, M.R.; Amrein, K.; Czeiter, E.; Berta, G.; Pohoczky, K.; Helyes, Z.; Ungvari, Z.; Koller, A.; Buki, A.; et al. Traumatic Brain Injury Impairs Myogenic Constriction of Cerebral Arteries: Role of Mitochondria-Derived H2O2 and TRPV4-Dependent Activation of BKca Channels. J. Neurotrauma 2018, 35, 930–939. [Google Scholar] [CrossRef]
- Li, T.; Huang, Y.; Cai, W.; Chen, X.; Men, X.; Lu, T.; Wu, A.; Lu, Z. Age-related cerebral small vessel disease and inflammaging. Cell Death Dis. 2020, 11, 932. [Google Scholar] [CrossRef]
- Del Cuore, A.; Pacinella, G.; Riolo, R.; Tuttolomondo, A. The Role of Immunosenescence in Cerebral Small Vessel Disease: A Review. Int. J. Mol. Sci. 2022, 23, 7136. [Google Scholar] [CrossRef] [PubMed]
- Bickel, M.A.; Csik, B.; Gulej, R.; Ungvari, A.; Nyul-Toth, A.; Conley, S.M. Cell non-autonomous regulation of cerebrovascular aging processes by the somatotropic axis. Front. Endocrinol. 2023, 14, 1087053. [Google Scholar] [CrossRef] [PubMed]
- Tarantini, S.; Valcarcel-Ares, N.M.; Yabluchanskiy, A.; Springo, Z.; Fulop, G.A.; Ashpole, N.; Gautam, T.; Giles, C.B.; Wren, J.D.; Sonntag, W.E.; et al. Insulin-like growth factor 1 deficiency exacerbates hypertension-induced cerebral microhemorrhages in mice, mimicking the aging phenotype. Aging Cell 2017, 16, 469–479. [Google Scholar] [CrossRef]
- Toth, P.; Tucsek, Z.; Tarantini, S.; Sosnowska, D.; Gautam, T.; Mitschelen, M.; Koller, A.; Sonntag, W.E.; Csiszar, A.; Ungvari, Z. IGF-1 deficiency impairs cerebral myogenic autoregulation in hypertensive mice. J. Cereb. Blood Flow. Metab. 2014, 34, 1887–1897. [Google Scholar] [CrossRef]
- Sonntag, W.E.; Deak, F.; Ashpole, N.; Toth, P.; Csiszar, A.; Freeman, W.; Ungvari, Z. Insulin-like growth factor-1 in CNS and cerebrovascular aging. Front. Aging Neurosci. 2013, 5, 27. [Google Scholar] [CrossRef]
- Gulej, R.; Csik, B.; Faakye, J.; Tarantini, S.; Shanmugarama, S.; Chandragiri, S.S.; Mukli, P.; Conley, S.; Csiszar, A.; Ungvari, Z.; et al. Endothelial deficiency of insulin-like growth factor-1 receptor leads to blood-brain barrier disruption and accelerated endothelial senescence in mice, mimicking aspects of the brain aging phenotype. Microcirculation 2024, 31, e12840. [Google Scholar] [CrossRef] [PubMed]
- Stankovics, L.; Ungvari, A.; Fekete, M.; Nyul-Toth, A.; Mukli, P.; Patai, R.; Csik, B.; Gulej, R.; Conley, S.; Csiszar, A.; et al. The vasoprotective role of IGF-1 signaling in the cerebral microcirculation: Prevention of cerebral microhemorrhages in aging. Geroscience 2024, 47, 445–455. [Google Scholar] [CrossRef]
- Miller, L.R.; Bickel, M.A.; Vance, M.L.; Vaden, H.; Nagykaldi, D.; Nyul-Toth, A.; Bullen, E.C.; Gautam, T.; Tarantini, S.; Yabluchanskiy, A.; et al. Vascular smooth muscle cell-specific Igf1r deficiency exacerbates the development of hypertension-induced cerebral microhemorrhages and gait defects. Geroscience 2024, 46, 3481–3501. [Google Scholar] [CrossRef]
- Gulej, R.; Nyul-Toth, A.; Csik, B.; Patai, R.; Petersen, B.; Negri, S.; Chandragiri, S.S.; Shanmugarama, S.; Mukli, P.; Yabluchanskiy, A.; et al. Young blood-mediated cerebromicrovascular rejuvenation through heterochronic parabiosis: Enhancing blood-brain barrier integrity and capillarization in the aged mouse brain. Geroscience 2024, 46, 4415–4442. [Google Scholar] [CrossRef]
- Toth, L.; Czigler, A.; Hegedus, E.; Komaromy, H.; Amrein, K.; Czeiter, E.; Yabluchanskiy, A.; Koller, A.; Orsi, G.; Perlaki, G.; et al. Age-related decline in circulating IGF-1 associates with impaired neurovascular coupling responses in older adults. Geroscience 2022, 44, 2771–2783. [Google Scholar] [CrossRef]
- Nyul-Toth, A.; Patai, R.; Csiszar, A.; Ungvari, A.; Gulej, R.; Mukli, P.; Yabluchanskiy, A.; Benyo, Z.; Sotonyi, P.; Prodan, C.I.; et al. Linking peripheral atherosclerosis to blood-brain barrier disruption: Elucidating its role as a manifestation of cerebral small vessel disease in vascular cognitive impairment. Geroscience 2024, 46, 6511–6536. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Sohn, E.H.; Oh, E.; Lee, A.Y. Characteristics of Cerebral Microbleeds. Dement. Neurocogn Disord. 2018, 17, 73–82. [Google Scholar] [CrossRef]
- Caunca, M.R.; Del Brutto, V.; Gardener, H.; Shah, N.; Dequatre-Ponchelle, N.; Cheung, Y.K.; Elkind, M.S.; Brown, T.R.; Cordonnier, C.; Sacco, R.L.; et al. Cerebral Microbleeds, Vascular Risk Factors, and Magnetic Resonance Imaging Markers: The Northern Manhattan Study. J. Am. Heart Assoc. 2016, 5, e003477. [Google Scholar] [CrossRef]
- Caunca, M.R.; De Leon-Benedetti, A.; Latour, L.; Leigh, R.; Wright, C.B. Neuroimaging of Cerebral Small Vessel Disease and Age-Related Cognitive Changes. Front. Aging Neurosci. 2019, 11, 145. [Google Scholar] [CrossRef]
- Werring, D.J.; Gregoire, S.M.; Cipolotti, L. Cerebral microbleeds and vascular cognitive impairment. J. Neurol. Sci. 2010, 299, 131–135. [Google Scholar] [CrossRef] [PubMed]
- de Laat, K.F.; van den Berg, H.A.; van Norden, A.G.; Gons, R.A.; Olde Rikkert, M.G.; de Leeuw, F.E. Microbleeds are independently related to gait disturbances in elderly individuals with cerebral small vessel disease. Stroke 2011, 42, 494–497. [Google Scholar] [CrossRef]
- Charidimou, A.; Karayiannis, C.; Song, T.J.; Orken, D.N.; Thijs, V.; Lemmens, R.; Kim, J.; Goh, S.M.; Phan, T.G.; Soufan, C.; et al. Brain microbleeds, anticoagulation, and hemorrhage risk: Meta-analysis in stroke patients with AF. Neurology 2017, 89, 2317–2326. [Google Scholar] [CrossRef] [PubMed]
- Csipo, T.; Lipecz, A.; Mukli, P.; Peterfi, A.; Szarvas, Z.; Ungvari, A.; Alaoui, L.E.; Sandor, M.; Kallai, A.; Fekete, M.; et al. Advancing prediction of age-related vascular cognitive impairment based on peripheral and retinal vascular health in a pilot study: A novel comprehensive assessment developed for a prospective workplace-based cohort (The Semmelweis Study). Geroscience 2024, 47, 1329–1344. [Google Scholar] [CrossRef]
- Ungvari, Z.; Tarantini, S.; Kirkpatrick, A.C.; Csiszar, A.; Prodan, C.I. Cerebral microhemorrhages: Mechanisms, consequences, and prevention. Am. J. Physiol. Heart Circ. Physiol. 2017, 312, H1128–H1143. [Google Scholar] [CrossRef]
- Ungvari, Z.; Muranyi, M.; Gulej, R.; Negri, S.; Nyul-Toth, A.; Csik, B.; Patai, R.; Conley, S.; Milan, M.; Bagwell, J.; et al. Longitudinal detection of gait alterations associated with hypertension-induced cerebral microhemorrhages in mice: Predictive role of stride length and stride time asymmetry and increased gait entropy. Geroscience 2024, 46, 4743–4760. [Google Scholar] [CrossRef] [PubMed]
- Tipirneni, S.; Stanwell, P.; Weissert, R.; Bhaskar, S.M.M. Prevalence and Impact of Cerebral Microbleeds on Clinical and Safety Outcomes in Acute Ischaemic Stroke Patients Receiving Reperfusion Therapy: A Systematic Review and Meta-Analysis. Biomedicines 2023, 11, 2865. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, J.; Fang, X.; Yu, Y.; Xu, M.; Li, T.; Yan, J. Association Between Cerebral Microbleeds and Neurological Outcomes in Patients Who Underwent Extracorporeal Membrane Oxygenation. J. Am. Heart Assoc. 2024, 13, e037029. [Google Scholar] [CrossRef]
- Fang, X.; Crumpler, R.F.; Thomas, K.N.; Mazique, J.N.; Roman, R.J.; Fan, F. Contribution of cerebral microvascular mechanisms to age-related cognitive impairment and dementia. Physiol. Int. 2022, 109, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Klop, M.; de Heus, R.A.A.; Maier, A.B.; van Alphen, A.; Floor-Westerdijk, M.J.; Bronkhorst, M.; Melis, R.J.F.; Meskers, C.G.M.; Claassen, J.; van Wezel, R.J.A. Capturing postural blood pressure dynamics with near-infrared spectroscopy-measured cerebral oxygenation. Geroscience 2023, 45, 2643–2657. [Google Scholar] [CrossRef]
- Magkas, N.; Tsioufis, C.; Thomopoulos, C.; Dilaveris, P.; Georgiopoulos, G.; Sanidas, E.; Papademetriou, V.; Tousoulis, D. Orthostatic hypotension: From pathophysiology to clinical applications and therapeutic considerations. J. Clin. Hypertens 2019, 21, 546–554. [Google Scholar] [CrossRef]
- Wang, L.; Pronk, A.C.; van Poelgeest, E.P.; Briggs, R.; Claassen, J.; Jansen, S.; Klop, M.; de Lange, F.J.; Meskers, C.; Odekerken, V.J.J.; et al. Applying systems thinking to unravel the mechanisms underlying orthostatic hypotension related fall risk. Geroscience 2023, 45, 2743–2755. [Google Scholar] [CrossRef]
- Kern, K.C.; Wright, C.B.; Bergfield, K.L.; Fitzhugh, M.C.; Chen, K.; Moeller, J.R.; Nabizadeh, N.; Elkind, M.S.V.; Sacco, R.L.; Stern, Y.; et al. Blood Pressure Control in Aging Predicts Cerebral Atrophy Related to Small-Vessel White Matter Lesions. Front. Aging Neurosci. 2017, 9, 132. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Li, Y.; Hu, J.; Wu, J.; Huang, Y. A Study on the Pathogenesis of Vascular Cognitive Impairment and Dementia: The Chronic Cerebral Hypoperfusion Hypothesis. J. Clin. Med. 2022, 11, 4742. [Google Scholar] [CrossRef] [PubMed]
- Rajeev, V.; Chai, Y.L.; Poh, L.; Selvaraji, S.; Fann, D.Y.; Jo, D.G.; De Silva, T.M.; Drummond, G.R.; Sobey, C.G.; Arumugam, T.V.; et al. Chronic cerebral hypoperfusion: A critical feature in unravelling the etiology of vascular cognitive impairment. Acta Neuropathol. Commun. 2023, 11, 93. [Google Scholar] [CrossRef]
- Iadecola, C. The overlap between neurodegenerative and vascular factors in the pathogenesis of dementia. Acta Neuropathol. 2010, 120, 287–296. [Google Scholar] [CrossRef]
- Qi, X.; Tang, H.; Luo, Q.; Ding, B.; Chen, J.; Cui, P.; Chen, S.; Ling, H.; Ma, J. White Matter Hyperintensities Predict Cognitive Decline: A Community-Based Study. Can. J. Neurol. Sci. 2019, 46, 383–388. [Google Scholar] [CrossRef]
- Makin, S.D.; Turpin, S.; Dennis, M.S.; Wardlaw, J.M. Cognitive impairment after lacunar stroke: Systematic review and meta-analysis of incidence, prevalence and comparison with other stroke subtypes. J. Neurol. Neurosurg. Psychiatry 2013, 84, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Heinen, R.; Vlegels, N.; de Bresser, J.; Leemans, A.; Biessels, G.J.; Reijmer, Y.D. The cumulative effect of small vessel disease lesions is reflected in structural brain networks of memory clinic patients. Neuroimage Clin. 2018, 19, 963–969. [Google Scholar] [CrossRef]
- Lorenzini, L.; Maranzano, A.; Ingala, S.; Collij, L.E.; Tranfa, M.; Blennow, K.; Di Perri, C.; Foley, C.; Fox, N.C.; Frisoni, G.B.; et al. Association of Vascular Risk Factors and Cerebrovascular Pathology With Alzheimer Disease Pathologic Changes in Individuals Without Dementia. Neurology 2024, 103, e209801. [Google Scholar] [CrossRef]
- Govindpani, K.; McNamara, L.G.; Smith, N.R.; Vinnakota, C.; Waldvogel, H.J.; Faull, R.L.; Kwakowsky, A. Vascular Dysfunction in Alzheimer’s Disease: A Prelude to the Pathological Process or a Consequence of It? J. Clin. Med. 2019, 8, 651. [Google Scholar] [CrossRef]
- Deng, W.; Guo, S.; van Veluw, S.J.; Yu, Z.; Chan, S.J.; Takase, H.; Arai, K.; Ning, M.; Greenberg, S.M.; Lo, E.H.; et al. Effects of cerebral amyloid angiopathy on the brain vasculome. Aging Cell 2022, 21, e13503. [Google Scholar] [CrossRef]
- Noto, N.M.; Speth, R.C.; Robison, L.S. Cerebral amyloid angiopathy: A narrative review. Front. Aging Neurosci. 2025, 17, 1632252. [Google Scholar] [CrossRef]
- Bateman, G.A.; Bateman, A.R. A lumped parameter modelling study of cerebral autoregulation in normal pressure hydrocephalus suggests the brain chooses to be ischemic. Sci. Rep. 2024, 14, 24373. [Google Scholar] [CrossRef]
- Bateman, G.A. Vascular compliance in normal pressure hydrocephalus. AJNR Am. J. Neuroradiol. 2000, 21, 1574–1585. [Google Scholar] [PubMed]
- Zlokovic, B.V. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat. Rev. Neurosci. 2011, 12, 723–738. [Google Scholar] [CrossRef] [PubMed]
- Eisenmenger, L.B.; Peret, A.; Famakin, B.M.; Spahic, A.; Roberts, G.S.; Bockholt, J.H.; Johnson, K.M.; Paulsen, J.S. Vascular contributions to Alzheimer’s disease. Transl. Res. 2023, 254, 41–53. [Google Scholar] [CrossRef]
- Segura-Hernández, A.; Gómez-Amarillo, D.F.; Mejía-Michelsen, I.; Vargas-Osorio, M.P.; González, M.; Domínguez, M.T.; Mattar, S.M.; Volcinschi, D.; Ordóñez-Rubiano, E.G.; Ramos-Márquez, A.; et al. Reversal of cerebral pseudoatrophy in normal pressure hydrocephalus after ventriculoatrial shunt placement. Sci. Rep. 2025, 15, 24817. [Google Scholar] [CrossRef]
- Momjian, S.; Owler, B.K.; Czosnyka, Z.; Czosnyka, M.; Pena, A.; Pickard, J.D. Pattern of white matter regional cerebral blood flow and autoregulation in normal pressure hydrocephalus. Brain 2004, 127, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.; Tozer, D.J.; Chen, Y.; Brown, R.B.; Low, A.; Markus, H.S. Perivascular space dysfunction in cerebral small vessel disease is related to neuroinflammation. Brain 2025, 148, 1540–1550. [Google Scholar] [CrossRef]
- Zhong, J.; Lin, W.; Chen, J.; Gao, Q. Higher critical closing pressure is independently associated with enlarged basal ganglia perivascular spaces. Front. Neurol. 2023, 14, 1165469. [Google Scholar] [CrossRef]
- Nogueira, R.C.; Beishon, L.; Bor-Seng-Shu, E.; Panerai, R.B.; Robinson, T.G. Cerebral Autoregulation in Ischemic Stroke: From Pathophysiology to Clinical Concepts. Brain Sci. 2021, 11, 511. [Google Scholar] [CrossRef]
- Kovács, K.B.; Bencs, V.; Hudák, L.; Oláh, L.; Csiba, L. Hemorrhagic Transformation of Ischemic Strokes. Int. J. Mol. Sci. 2023, 24, 14067. [Google Scholar] [CrossRef] [PubMed]
- Sheriff, F.; Castro, P.; Kozberg, M.; LaRose, S.; Monk, A.; Azevedo, E.; Li, K.; Jafari, S.; Rao, S.; Otite, F.O.; et al. Dynamic Cerebral Autoregulation Post Endovascular Thrombectomy in Acute Ischemic Stroke. Brain Sci. 2020, 10, 641. [Google Scholar] [CrossRef]
- Nishijima, Y.; Akamatsu, Y.; Weinstein, P.R.; Liu, J. Collaterals: Implications in cerebral ischemic diseases and therapeutic interventions. Brain Res. 2015, 1623, 18–29. [Google Scholar] [CrossRef]
- Farooq, M.U.; Goshgarian, C.; Min, J.; Gorelick, P.B. Pathophysiology and management of reperfusion injury and hyperperfusion syndrome after carotid endarterectomy and carotid artery stenting. Exp. Transl. Stroke Med. 2016, 8, 7. [Google Scholar] [CrossRef] [PubMed]
- Kibrik, P.; Stonko, D.P.; Alsheekh, A.; Holscher, C.; Zarkowsky, D.; Abularrage, C.J.; Hicks, C.W. Association of carotid revascularization approach with perioperative outcomes based on symptom status and degree of stenosis among octogenarians. J. Vasc. Surg. 2022, 76, 769–777.e762. [Google Scholar] [CrossRef]
- Jones, J.D.; Castanho, P.; Bazira, P.; Sanders, K. Anatomical variations of the circle of Willis and their prevalence, with a focus on the posterior communicating artery: A literature review and meta-analysis. Clin. Anat. 2021, 34, 978–990. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.A.; Carpenter, K.L.; Hutchinson, P.J.; Smielewski, P.; Helmy, A. Candidate neuroinflammatory markers of cerebral autoregulation dysfunction in human acute brain injury. J. Cereb. Blood Flow. Metab. 2023, 43, 1237–1253. [Google Scholar] [CrossRef]
- Czosnyka, M.; Balestreri, M.; Steiner, L.; Smielewski, P.; Hutchinson, P.J.; Matta, B.; Pickard, J.D. Age, intracranial pressure, autoregulation, and outcome after brain trauma. J. Neurosurg. 2005, 102, 450–454. [Google Scholar] [CrossRef]
- Gardner, R.C.; Dams-O’Connor, K.; Morrissey, M.R.; Manley, G.T. Geriatric Traumatic Brain Injury: Epidemiology, Outcomes, Knowledge Gaps, and Future Directions. J. Neurotrauma 2018, 35, 889–906. [Google Scholar] [CrossRef]
- Toth, P.; Szarka, N.; Farkas, E.; Ezer, E.; Czeiter, E.; Amrein, K.; Ungvari, Z.; Hartings, J.A.; Buki, A.; Koller, A. Traumatic brain injury-induced autoregulatory dysfunction and spreading depression-related neurovascular uncoupling: Pathomechanisms, perspectives, and therapeutic implications. Am. J. Physiol. Heart Circ. Physiol. 2016, 311, H1118–H1131. [Google Scholar] [CrossRef] [PubMed]
- Zeiler, F.A.; Aries, M.; Czosnyka, M.; Smielewski, P. Cerebral Autoregulation Monitoring in Traumatic Brain Injury: An Overview of Recent Advances in Personalized Medicine. J. Neurotrauma 2022, 39, 1477–1494. [Google Scholar] [CrossRef]
- Toth, L.; Czigler, A.; Horvath, P.; Kornyei, B.; Szarka, N.; Schwarcz, A.; Ungvari, Z.; Buki, A.; Toth, P. Traumatic brain injury-induced cerebral microbleeds in the elderly. Geroscience 2021, 43, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Ikonomovic, M.D.; Mi, Z.; Abrahamson, E.E. Disordered APP metabolism and neurovasculature in trauma and aging: Combined risks for chronic neurodegenerative disorders. Ageing Res. Rev. 2017, 34, 51–63. [Google Scholar] [CrossRef]
- Svedung Wettervik, T.; Beqiri, E.; Hånell, A.; Bögli, S.Y.; Olakorede, I.; Chen, X.; Helmy, A.; Lavinio, A.; Hutchinson, P.J.; Smielewski, P. Autoregulatory-guided management in traumatic brain injury: Does age matter? Acta Neurochir 2025, 167, 55. [Google Scholar] [CrossRef]
- Weijs, R.W.J.; Oudegeest-Sander, M.H.; Vloet, J.I.A.; Hopman, M.T.E.; Claassen, J.; Thijssen, D.H.J. A decade of aging in healthy older adults: Longitudinal findings on cerebrovascular and cognitive health. Geroscience 2023, 45, 2629–2641. [Google Scholar] [CrossRef]
- Burma, J.S.; Roy, M.A.; Kennedy, C.M.; Labrecque, L.; Brassard, P.; Smirl, J.D. A systematic review, meta-analysis and meta-regression amalgamating the driven approaches used to quantify dynamic cerebral autoregulation. J. Cereb. Blood Flow. Metab. 2024, 44, 1271–1297. [Google Scholar] [CrossRef]
- Bird, J.D.; MacLeod, D.B.; Griesdale, D.E.; Sekhon, M.S.; Hoiland, R.L. Shining a light on cerebral autoregulation: Are we anywhere near the truth? J. Cereb. Blood Flow. Metab. 2024, 44, 1057–1060. [Google Scholar] [CrossRef] [PubMed]
- Tarumi, T.; Zhang, R. Point-Counterpoint: Transfer function analysis of dynamic cerebral autoregulation: To band or not to band? J. Cereb. Blood Flow. Metab. 2023, 43, 1625–1627. [Google Scholar] [CrossRef] [PubMed]
- Panerai, R.B.; Brassard, P.; Burma, J.S.; Castro, P.; Claassen, J.A.; van Lieshout, J.J.; Liu, J.; Lucas, S.J.; Minhas, J.S.; Mitsis, G.D.; et al. Transfer function analysis of dynamic cerebral autoregulation: A CARNet white paper 2022 update. J. Cereb. Blood Flow Metab. 2023, 43, 3–25. [Google Scholar] [CrossRef]
- Favilla, C.G.; Mullen, M.T.; Kahn, F.; Rasheed, I.D.; Messe, S.R.; Parthasarathy, A.B.; Yodh, A.G. Dynamic cerebral autoregulation measured by diffuse correlation spectroscopy. J. Cereb. Blood Flow. Metab. 2023, 43, 1317–1327. [Google Scholar] [CrossRef]
- Larsen, F.S.; Olsen, K.S.; Hansen, B.A.; Paulson, O.B.; Knudsen, G.M. Transcranial Doppler is valid for determination of the lower limit of cerebral blood flow autoregulation. Stroke 1994, 25, 1985–1988. [Google Scholar] [CrossRef]
- Sarkar, S.; Ghosh, S.; Ghosh, S.K.; Collier, A. Role of transcranial Doppler ultrasonography in stroke. Postgrad. Med. J. 2007, 83, 683–689. [Google Scholar] [CrossRef]
- Rivera-Lara, L.; Zeiler, F.A. Continuous Cerebral Autoregulation Monitoring Using TCD. In Neurovascular Sonography; Springer: Berlin/Heidelberg, Germany, 2022; pp. 241–247. [Google Scholar]
- Grade, M.; Hernandez Tamames, J.A.; Pizzini, F.B.; Achten, E.; Golay, X.; Smits, M. A neuroradiologist’s guide to arterial spin labeling MRI in clinical practice. Neuroradiology 2015, 57, 1181–1202. [Google Scholar] [CrossRef]
- Sainbhi, A.S.; Gomez, A.; Froese, L.; Slack, T.; Batson, C.; Stein, K.Y.; Cordingley, D.M.; Alizadeh, A.; Zeiler, F.A. Non-Invasive and Minimally-Invasive Cerebral Autoregulation Assessment: A Narrative Review of Techniques and Implications for Clinical Research. Front. Neurol. 2022, 13, 872731. [Google Scholar] [CrossRef] [PubMed]
- de Boorder, M.J.; Hendrikse, J.; van der Grond, J. Phase-contrast magnetic resonance imaging measurements of cerebral autoregulation with a breath-hold challenge: A feasibility study. Stroke 2004, 35, 1350–1354. [Google Scholar] [CrossRef]
- Pyne, J.D.; Morales, C.D.; Kraal, A.Z.; Alshikho, M.J.; Lao, P.J.; Turney, I.C.; Amarante, E.; Lippert, R.V.; Chang, J.F.; Gutierrez, J.; et al. Phase contrast-derived cerebral blood flow is associated with neurodegeneration and cerebrovascular injury in older adults. Front. Neurosci. 2025, 19, 1538956. [Google Scholar] [CrossRef]
- Konig, S.; Jayarajan, V.; Wray, S.; Kamm, R.; Moeendarbary, E. Mechanobiology of the blood-brain barrier during development, disease and ageing. Nat. Commun. 2025, 16, 7233. [Google Scholar] [CrossRef]
- Ling, Y.H.; Chi, N.F.; Pan, L.H.; Wang, Y.F.; Wu, C.H.; Lirng, J.F.; Fuh, J.L.; Wang, S.J.; Chen, S.P. Association between impaired dynamic cerebral autoregulation and BBB disruption in reversible cerebral vasoconstriction syndrome. J. Headache Pain. 2023, 24, 170. [Google Scholar] [CrossRef]
- Oghabian, M.A.; Fatemidokht, A.; Haririchian, M.H. Quantification of Blood-Brain-Barrier Permeability Dysregulation and Inflammatory Activity in MS Lesions by Dynamic-Contrast Enhanced MR Imaging. Basic. Clin. Neurosci. 2022, 13, 117–128. [Google Scholar] [CrossRef]
- Li, Y.; Sadiq, A.; Wang, Z. Arterial Spin Labelling-Based Blood-Brain Barrier Assessment and Its Applications. Investig. Magn. Reson. Imaging 2022, 26, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.J.; Abdelhafez, Y.G.; Spencer, B.A.; Jones, T.; Tran, Q.; Nardo, L.; Chen, M.S., Jr.; Sarkar, S.; Medici, V.; Lyo, V.; et al. Quantitative PET imaging and modeling of molecular blood-brain barrier permeability. medRxiv 2024. [Google Scholar] [CrossRef]
- Pieniazek, W.; Dimitrow, P.P. Autoregulation of cerebral circulation: Adaptation to hypertension and re-adaptation in response to antihypertensive treatment. Przegl Lek. 2006, 63, 688–690. [Google Scholar]
- Tzeng, Y.C.; Ainslie, P.N. Blood pressure regulation IX: Cerebral autoregulation under blood pressure challenges. Eur. J. Appl. Physiol. 2014, 114, 545–559. [Google Scholar] [CrossRef] [PubMed]
- Chau, A.C.M.; Cheung, E.Y.W.; Chan, K.H.; Chow, W.S.; Shea, Y.F.; Chiu, P.K.C.; Mak, H.K.F. Impaired cerebral blood flow in type 2 diabetes mellitus - A comparative study with subjective cognitive decline, vascular dementia and Alzheimer’s disease subjects. Neuroimage Clin. 2020, 27, 102302. [Google Scholar] [CrossRef] [PubMed]
- Mankovsky, B.N.; Piolot, R.; Mankovsky, O.L.; Ziegler, D. Impairment of cerebral autoregulation in diabetic patients with cardiovascular autonomic neuropathy and orthostatic hypotension. Diabet. Med. 2003, 20, 119–126. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, R.J.; Resnick, S.M.; Zonderman, A.B.; Ferrucci, L.; Crain, B.J.; Pletnikova, O.; Rudow, G.; Iacono, D.; Riudavets, M.A.; Driscoll, I.; et al. Neuropathologic studies of the Baltimore Longitudinal Study of Aging (BLSA). J. Alzheimers Dis. 2009, 18, 665–675. [Google Scholar] [CrossRef]
- Beishon, L.; Clough, R.H.; Kadicheeni, M.; Chithiramohan, T.; Panerai, R.B.; Haunton, V.J.; Minhas, J.S.; Robinson, T.G. Vascular and haemodynamic issues of brain ageing. Pflugers Arch. 2021, 473, 735–751. [Google Scholar] [CrossRef]
- M, A.S.; O’Connor, J.D.; Boyle, R.; Newman, L.; Knight, S.P.; Hernandez, B.; Whelan, R.; Meaney, J.F.; Kenny, R.A. Slower speed of blood pressure recovery after standing is associated with accelerated brain aging: Evidence from The Irish Longitudinal Study on Ageing (TILDA). Cereb. Circ. Cogn. Behav. 2024, 6, 100212. [Google Scholar] [CrossRef]
- Sorond, F.; Purkayastha, S.; Lipsitz, L.; Leveille, S. Impaired Cerebral Autoregualtion is Associated with a Greater 7-year Decline in Gait Speed in an Elderly Population: The MOBILIZE Boston Study (S58. 007). Neurology 2014, 82, S58–007. [Google Scholar] [CrossRef]
- Bakker, S.L.; de Leeuw, F.E.; den Heijer, T.; Koudstaal, P.J.; Hofman, A.; Breteler, M.M. Cerebral haemodynamics in the elderly: The rotterdam study. Neuroepidemiology 2004, 23, 178–184. [Google Scholar] [CrossRef]
- Shannon, O.M.; Mendes, I.; Köchl, C.; Mazidi, M.; Ashor, A.W.; Rubele, S.; Minihane, A.M.; Mathers, J.C.; Siervo, M. Mediterranean Diet Increases Endothelial Function in Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Nutr. 2020, 150, 1151–1159. [Google Scholar] [CrossRef] [PubMed]
- Koep, J.L.; Bond, B.; Barker, A.R.; Ruediger, S.L.; Pizzey, F.K.; Coombes, J.S.; Bailey, T.G. Sex modifies the relationship between age and neurovascular coupling in healthy adults. J. Cereb. Blood Flow. Metab. 2023, 43, 1254–1266. [Google Scholar] [CrossRef]
- DuBose, L.E.; Babcock, M.C.; Kohrt, W.M.; Stauffer, B.L.; Hildreth, K.L.; Walker, J.; Armstrong, M.K.; Moreau, K.L. Gonadal status modulates large elastic artery stiffness in healthy middle-aged and older men. Geroscience 2024, 47, 3277–3289. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Medina, D.; Stockwell, R.; McFadden, S.; Quinn, K.; Peck, M.R.; Bartke, A.; Hascup, K.N.; Hascup, E.R. Sexual dimorphic metabolic and cognitive responses of C57BL/6 mice to Fisetin or Dasatinib and quercetin cocktail oral treatment. Geroscience 2023, 45, 2835–2850. [Google Scholar] [CrossRef] [PubMed]
- Ungvari, A.; Gulej, R.; Patai, R.; Papp, Z.; Toth, A.; Szabo, A.A.; Podesser, B.K.; Sotonyi, P.; Benyo, Z.; Yabluchanskiy, A.; et al. Sex-specific mechanisms in vascular aging: Exploring cellular and molecular pathways in the pathogenesis of age-related cardiovascular and cerebrovascular diseases. Geroscience 2025, 47, 301–337. [Google Scholar] [CrossRef]
- Nist, K.M.; Bard, H.; McBride, B.; Capriglione, A.L.; Moreira, J.D.; Farb, D.H.; Wainford, R.D. Losartan attenuates sex-dependent hypertension, neuroinflammation, and cognitive impairment in the aging male sprague-dawley rat. Geroscience 2024, 47, 3007–3026. [Google Scholar] [CrossRef]
- Fernandez, A.; Cuesta, P.; Marcos, A.; Montenegro-Pena, M.; Yus, M.; Rodriguez-Rojo, I.C.; Bruna, R.; Maestu, F.; Lopez, M.E. Sex differences in the progression to Alzheimer’s disease: A combination of functional and structural markers. Geroscience 2024, 46, 2619–2640. [Google Scholar] [CrossRef]
- Pandics, T.; Major, D.; Fazekas-Pongor, V.; Szarvas, Z.; Peterfi, A.; Mukli, P.; Gulej, R.; Ungvari, A.; Fekete, M.; Tompa, A.; et al. Exposome and unhealthy aging: Environmental drivers from air pollution to occupational exposures. Geroscience 2023, 45, 3381–3408. [Google Scholar] [CrossRef]
- Ungvari, Z.; Tabak, A.G.; Adany, R.; Purebl, G.; Kaposvari, C.; Fazekas-Pongor, V.; Csipo, T.; Szarvas, Z.; Horvath, K.; Mukli, P.; et al. The Semmelweis Study: A longitudinal occupational cohort study within the framework of the Semmelweis Caring University Model Program for supporting healthy aging. Geroscience 2024, 46, 191–218. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Border, J.J.; Zhang, H.; Gregory, A.; Bai, S.; Fang, X.; Liu, Y.; Wang, S.; Hwang, S.H.; Gao, W.; et al. Inhibition of soluble epoxide hydrolase ameliorates cerebral blood flow autoregulation and cognition in alzheimer’s disease and diabetes-related dementia rat models. Geroscience 2025, 47, 4429–4449. [Google Scholar] [CrossRef]
- Orr, M.E.; Kotkowski, E.; Ramirez, P.; Bair-Kelps, D.; Liu, Q.; Brenner, C.; Schmidt, M.S.; Fox, P.T.; Larbi, A.; Tan, C.; et al. A randomized placebo-controlled trial of nicotinamide riboside in older adults with mild cognitive impairment. Geroscience 2024, 46, 665–682. [Google Scholar] [CrossRef]
- Jiang, Z.; He, Q.; Wezeman, J.; Darvas, M.; Ladiges, W. A cocktail of rapamycin, acarbose, and phenylbutyrate prevents age-related cognitive decline in mice by targeting multiple aging pathways. Geroscience 2024, 46, 4855–4868. [Google Scholar] [CrossRef]
- Seman, A.; Chandra, P.K.; Byrum, S.D.; Mackintosh, S.G.; Gies, A.J.; Busija, D.W.; Rutkai, I. Targeting mitochondria in the aged cerebral vasculature with SS-31, a proteomic study of brain microvessels. Geroscience 2023, 45, 2951–2965. [Google Scholar] [CrossRef]
- Van Skike, C.E.; DeRosa, N.; Galvan, V.; Hussong, S.A. Rapamycin restores peripheral blood flow in aged mice and in mouse models of atherosclerosis and Alzheimer’s disease. Geroscience 2023, 45, 1987–1996. [Google Scholar] [CrossRef] [PubMed]
- Fielding, R.A.; Atkinson, E.J.; Aversa, Z.; White, T.A.; Heeren, A.A.; Achenbach, S.J.; Mielke, M.M.; Cummings, S.R.; Pahor, M.; Leeuwenburgh, C.; et al. Associations between biomarkers of cellular senescence and physical function in humans: Observations from the lifestyle interventions for elders (LIFE) study. Geroscience 2022, 44, 2757–2770. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).