Effects of Asparagus Powder Supplementation on Glycemic Control, Lipid Profile, and Oxidative Stress in Overweight and Obese Adults: An Exploratory Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
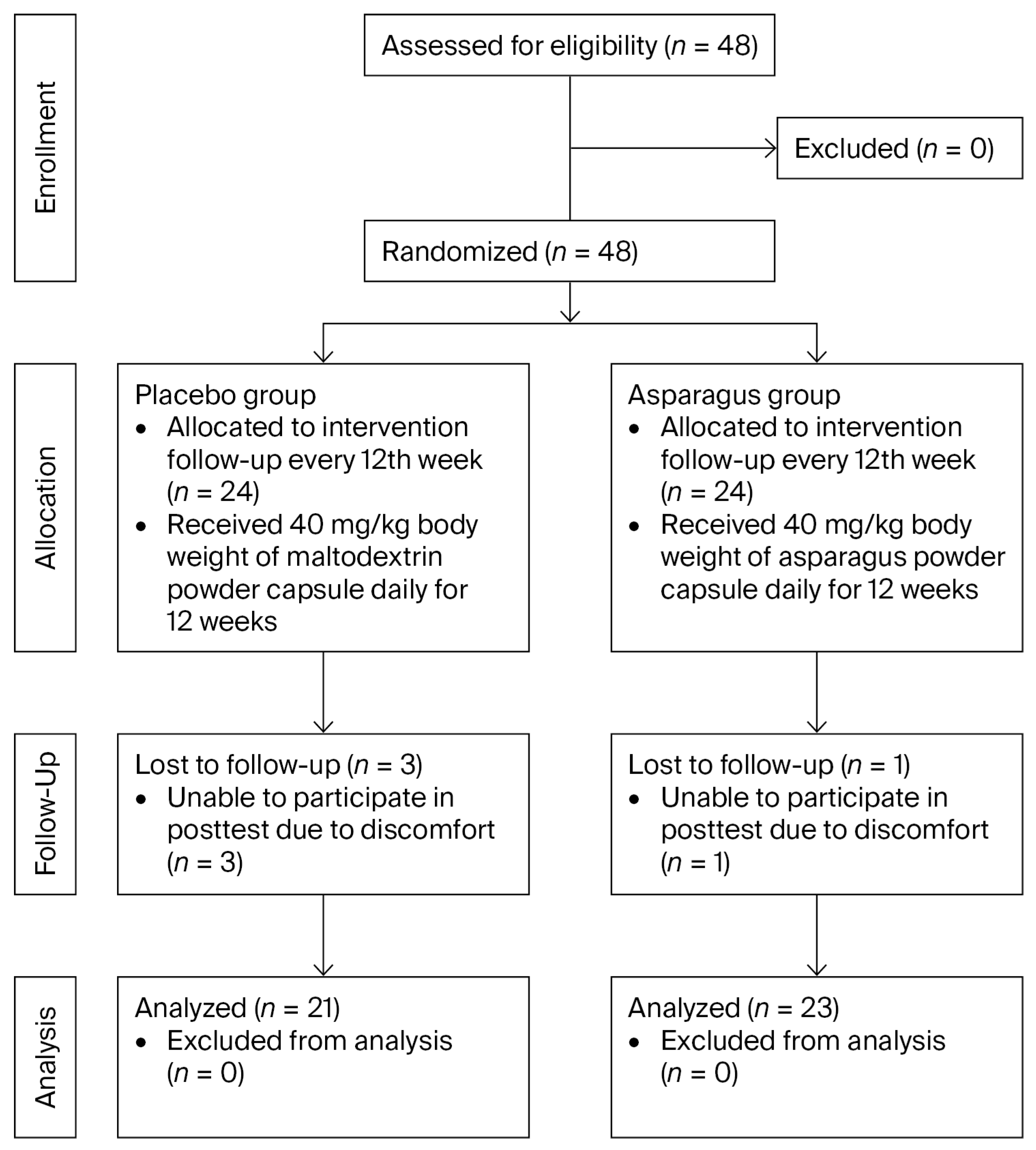
2.1. Participants and Sample Size
2.2. Ethical Consideration
2.3. Screening of Participants
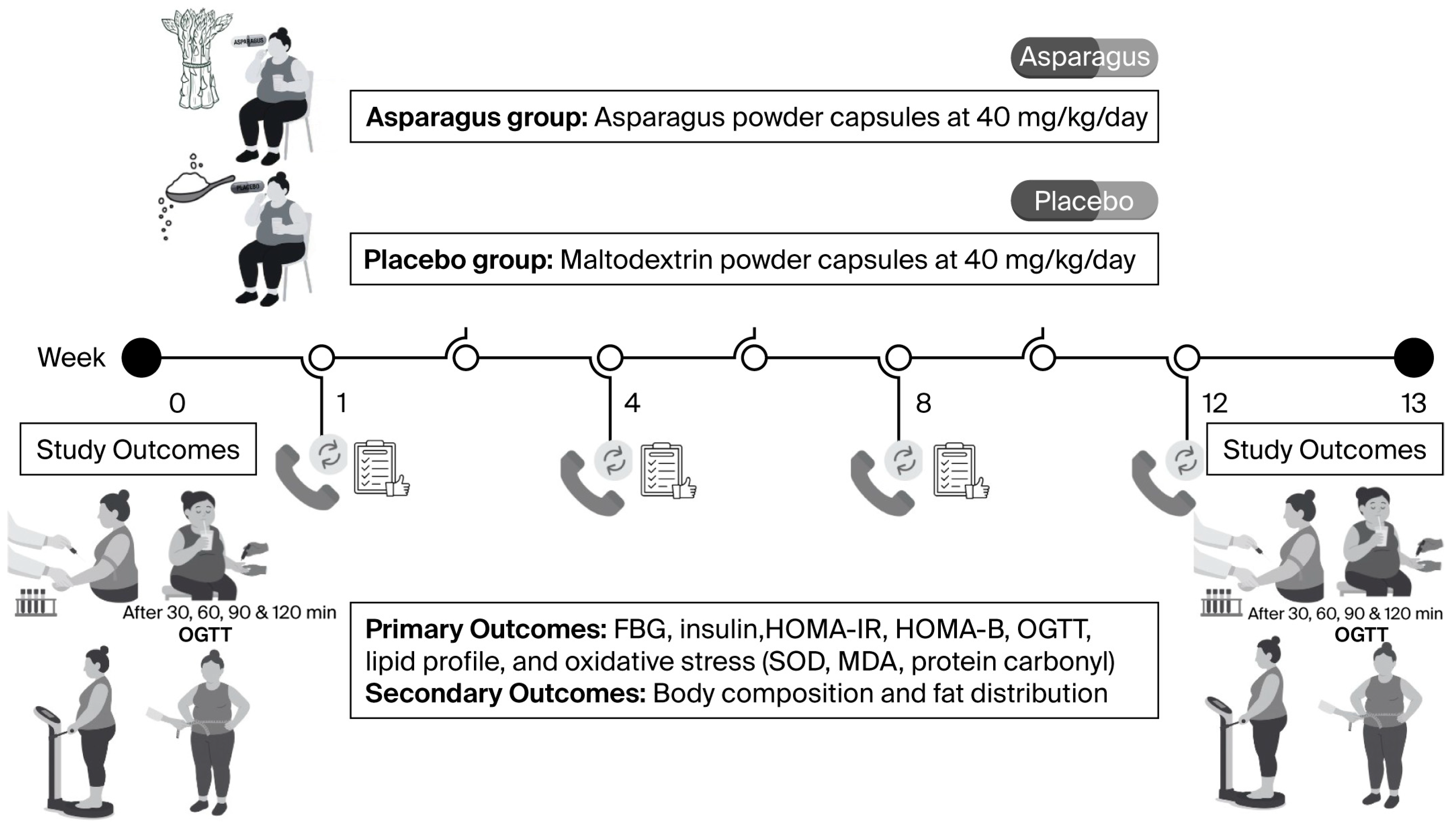
2.4. Experimental Protocols
2.4.1. Baseline Measurements
2.4.2. Randomization and Supplementation
2.4.3. Post-Supplementation Measurements
2.5. Preparation of Asparagus Powder and Placebo
2.6. Analysis of Asparagus Powder Constituents
2.7. Biochemical Assays
2.8. Oxidative Stress Assays
2.9. Body Composition and Fat Distribution Measurements
2.10. Data Analyses
3. Results
3.1. Baseline Participant Characteristics
3.2. Body Composition and Fat Distribution
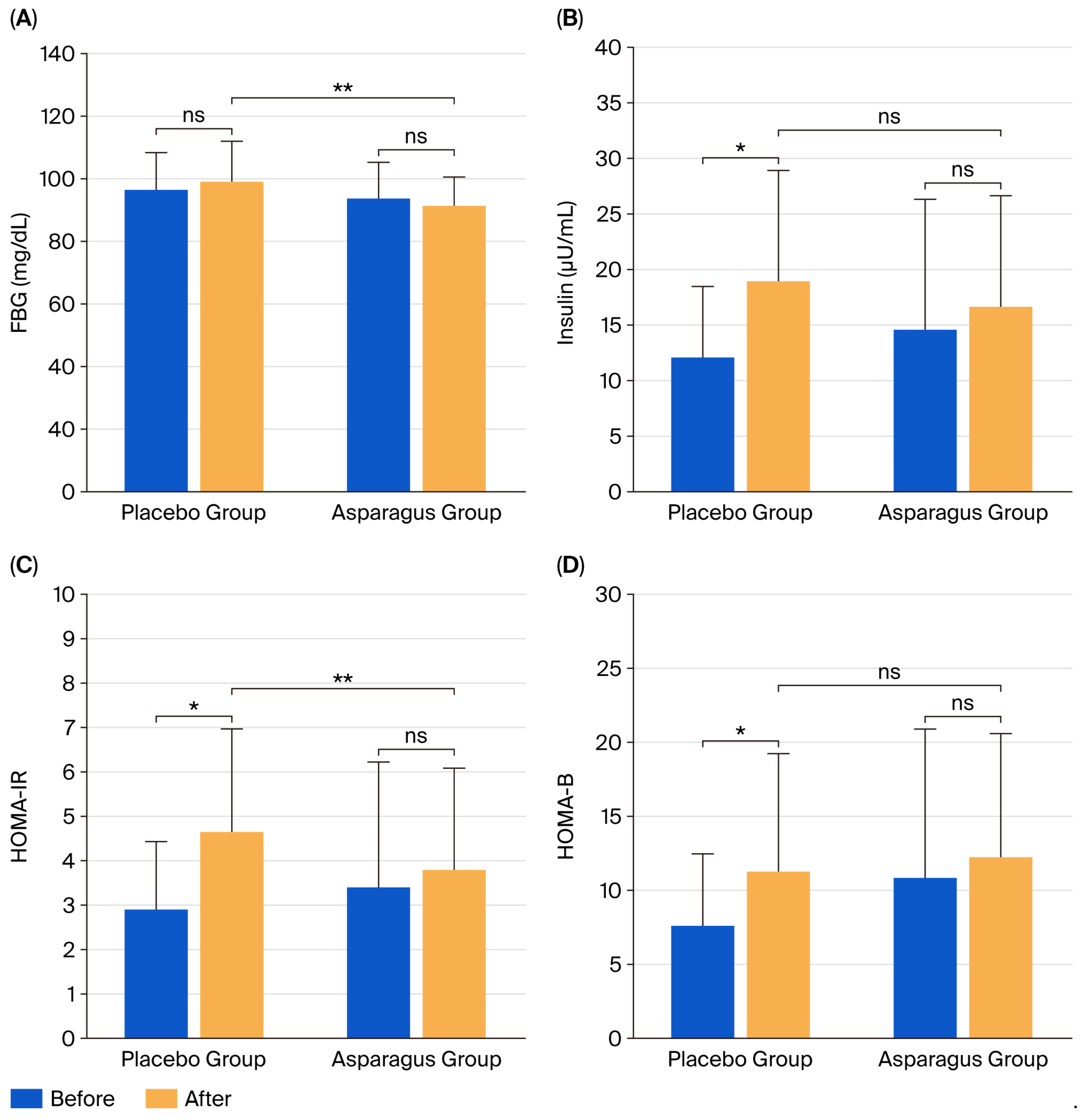
3.3. FBG and Insulin Concentrations, HOMA-IR, and HOMA-B
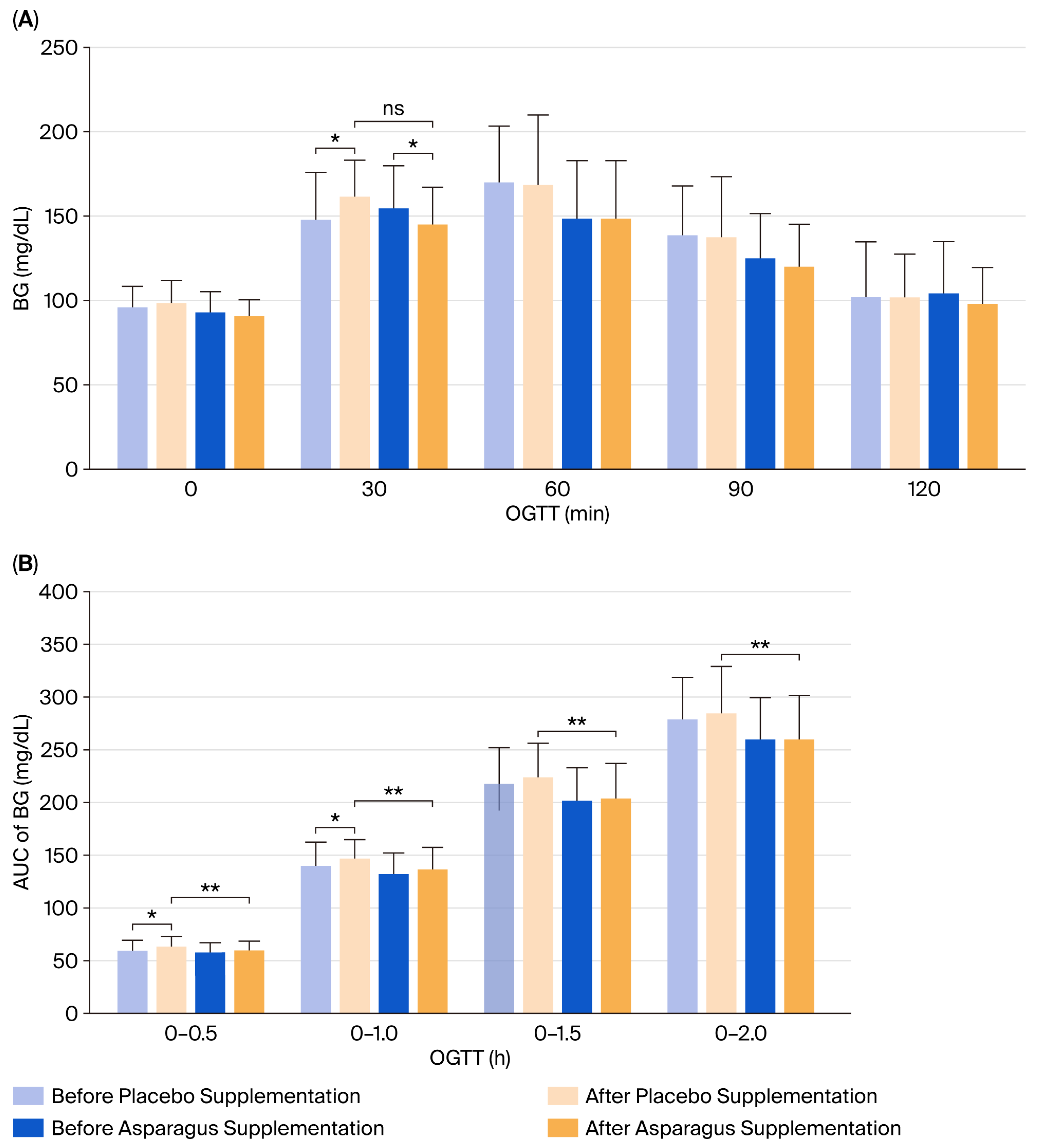
3.4. Blood Glucose Concentration in Response to OGTT
3.5. Lipid Profile
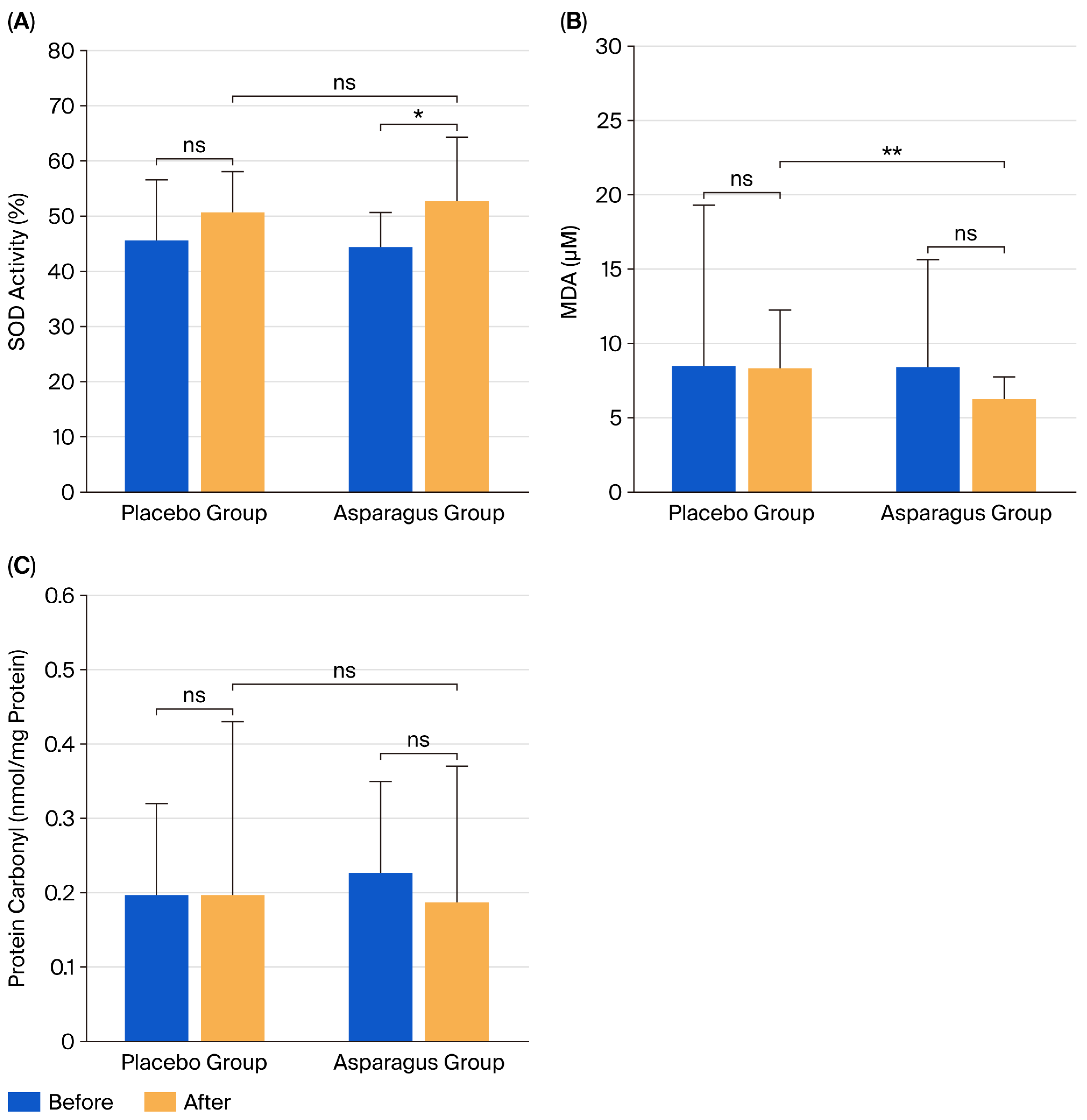
3.6. Oxidative Stress
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| AUC | Area under the curve |
| BG | Blood glucose |
| BM | Body mass |
| BMI | Body mass index |
| BP | Blood pressure |
| DBP | Diastolic blood pressure |
| FBG | Fasting blood glucose |
| GC-MS | Gas chromatography–mass spectrometry |
| GSH | Glutathione |
| HDL-C | High-density lipoprotein cholesterol |
| HOMA-B | Homeostasis model assessment of β-cell function |
| HOMA-IR | Homeostasis model assessment of insulin resistance |
| IL | Interleukin |
| LDL-C | Low-density lipoprotein cholesterol |
| MDA | Malondialdehyde |
| OGTT | Oral glucose tolerance test |
| ROS | Reactive oxygen species |
| SBP | Systolic blood pressure |
| SOD | Superoxide dismutase |
| TC | Total cholesterol |
| TG | Triglyceride |
| TNF-α | Tumor necrosis factor-alpha |
| W/H | Waist-to-hip |
References
- GBD 2021 Adult BMI Collaborators. Global, regional, and national prevalence of adult overweight and obesity, 1990-2021, with forecasts to 2050: A forecasting study for the Global Burden of Disease Study 2021. Lancet 2025, 405, 813–838. [Google Scholar] [CrossRef]
- Ansari, S.; Haboubi, H.; Haboubi, N. Adult obesity complications: Challenges and clinical impact. Ther. Adv. Endocrinol. Metab. 2020, 11, 2042018820934955. [Google Scholar] [CrossRef] [PubMed]
- Murphy, E.A.; Velazquez, K.T.; Herbert, K.M. Influence of high-fat diet on gut microbiota: A driving force for chronic disease risk. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Krajmalnik-Brown, R.; Ilhan, Z.E.; Kang, D.W.; DiBaise, J.K. Effects of gut microbes on nutrient absorption and energy regulation. Nutr. Clin. Pract. 2012, 27, 201–214. [Google Scholar] [CrossRef] [PubMed]
- Boulangé, C.L.; Neves, A.L.; Chilloux, J.; Nicholson, J.K.; Dumas, M.E. Impact of the gut microbiota on inflammation, obesity, and metabolic disease. Genome Med. 2016, 8, 42. [Google Scholar] [CrossRef]
- Wondmkun, Y.T. Obesity, insulin resistance, and type 2 diabetes: Associations and therapeutic implications. Diabetes Metab. Syndr. Obes. 2020, 13, 3611–3616. [Google Scholar] [CrossRef]
- Adnan, M.T.; Amin, M.N.; Uddin, M.G.; Hussain, M.S.; Sarwar, M.S.; Hossain, M.K.; Uddin, S.M.N.; Islam, M.S. Increased concentration of serum MDA, decreased antioxidants and altered trace elements and macro-minerals are linked to obesity among Bangladeshi population. Diabetes Metab. Syndr. 2019, 13, 933–938. [Google Scholar] [CrossRef]
- Frohnert, B.I.; Sinaiko, A.R.; Serrot, F.J.; Foncea, R.E.; Moran, A.; Ikramuddin, S.; Choudry, U.; Bernlohr, D.A. Increased adipose protein carbonylation in human obesity. Obesity 2011, 19, 1735–1741. [Google Scholar] [CrossRef]
- Agrawal, N.; Singh, S.K. Obesity: An independent risk factor for oxidative stress. Int. J. Adv. Med. 2017, 4, 718–721. [Google Scholar] [CrossRef]
- Derosa, G.; D’Angelo, A.; Angelini, F.; Belli, L.; Cicero, A.F.G.; Da Ros, R.; De Pergola, G.; Gaudio, G.V.; Lupi, A.; Sartore, G.; et al. Nutraceuticals and supplements in management of prediabetes and diabetes. Nutrients 2024, 17, 14. [Google Scholar] [CrossRef]
- Redondo-Cuenca, A.; García-Alonso, A.; Rodríguez-Arcos, R.; Castro, I.; Alba, C.; Miguel Rodríguez, J.; Goñi, I. Nutritional composition of green asparagus (Asparagus officinalis L.), edible part and by-products, and assessment of their effect on the growth of human gut-associated bacteria. Food Res. Int. 2023, 163, 112284. [Google Scholar] [CrossRef]
- Chitrakar, B.; Zhang, M.; Adhikari, B. Asparagus (Asparagus officinalis): Processing effect on nutritional and phytochemical composition of spear and hard-stem byproducts. Trends Food. Sci. Technol. 2019, 93, 1–11. [Google Scholar] [CrossRef]
- Goñi, I.; García-Alonso, A.; Alba, C.; Rodríguez, J.M.; Sánchez-Mata, M.C.; Guillén-Bejarano, R.; Redondo-Cuenca, A. Composition and functional properties of the edible spear and by-products from Asparagus officinalis L. and their potential prebiotic effect. Foods 2024, 13, 1154. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Hamayun, M.; Siraj, M.; Khan, S.A.; Kim, H.Y.; Lee, B. Recent advances in prebiotics: Classification, mechanisms, and health applications. Future Foods 2025, 12, 100680. [Google Scholar] [CrossRef]
- Nishimura, M.; Ohkawara, T.; Kagami-Katsuyama, H.; Sato, H.; Nishihira, J. Improvement of blood pressure, glucose metabolism, and lipid profile by the intake of powdered asparagus (Lu Sun) bottom-stems and cladophylls. J. Tradit. Complement. Med. 2013, 3, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.P.; Hussain, A.; Siddiqui, H.H.; Wahab, S.; Adak, M. Effect of methanolic extract of Asparagus racemosus Willd. on lipopolysaccharide induced-oxidative stress in rats. Pak. J. Pharm. Sci. 2015, 28, 509–513. [Google Scholar]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [PubMed]
- Good Manufacturing Practice (GMP) 420; Methods of Production, Production Tools and Equipment, and Food Storage. Ministry of Public Health: Nonthaburi, Thailand, 2020.
- Song, Y.; Manson, J.E.; Tinker, L.; Howard, B.V.; Kuller, L.H.; Nathan, L.; Rifai, N.; Liu, S. Insulin sensitivity and insulin secretion determined by homeostasis model assessment and risk of diabetes in a multiethnic cohort of women: The Women’s Health Initiative Observational Study. Diabetes Care 2007, 30, 1747–1752. [Google Scholar] [CrossRef]
- Yoon, H.; Jeon, D.J.; Park, C.E.; You, H.S.; Moon, A.E. Relationship between homeostasis model assessment of insulin resistance and beta cell function and serum 25-hydroxyvitamin D in non-diabetic Korean adults. J. Clin. Biochem. Nutr. 2016, 59, 139–144. [Google Scholar] [CrossRef]
- Nielsen, F.; Mikkelsen, B.B.; Nielsen, J.B.; Andersen, H.R.; Grandjean, P. Plasma malondialdehyde as biomarker for oxidative stress: Reference interval and effects of life-style factors. Clin. Chem. 1997, 43, 1209–1214. [Google Scholar] [CrossRef]
- Levine, R.L.; Garland, D.; Oliver, C.N.; Amici, A.; Climent, I.; Lenz, A.G.; Ahn, B.W.; Shaltiel, S.; Stadtman, E.R. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990, 186, 464–478. [Google Scholar] [CrossRef]
- Prasertsri, P.; Phoemsapthawee, J.; Kuamsub, S.; Poolpol, K.; Boonla, O. Effects of long-term regular continuous and intermittent walking on oxidative stress, metabolic profile, heart rate variability, and blood pressure in older adults with hypertension. J. Environ. Public Health 2022, 2022, 5942947. [Google Scholar] [CrossRef]
- Boonla, O.; Booranasuksakul, U.; Padkao, T.; Phoemsapthawee, J.; Tangwattanachuleeporn, M.; Koowattanatianchai, S.; Prasertsri, P. Effects of 4-week Eri silkworm cornflakes supplementation on oxidative stress and antioxidant status in male university athletes: A preliminary crossover study. Nutr. Health 2024, 2024, 2601060241302387. [Google Scholar] [CrossRef] [PubMed]
- Conn, C.A.; Vaughan, R.A.; Garver, W.S. Nutritional genetics and energy metabolism in human obesity. Curr. Nutr. Rep. 2013, 2, 142–150. [Google Scholar] [CrossRef]
- Ahmed, B.; Sultana, R.; Greene, M.W. Adipose tissue and insulin resistance in obese. Biomed. Pharmacother. 2021, 137, 111315. [Google Scholar] [CrossRef]
- Li, Y.; Ding, L.; Hassan, W.; Abdelkader, D.; Shang, J. Adipokines and hepatic insulin resistance. J. Diabetes Res. 2013, 2013, 170532. [Google Scholar] [CrossRef]
- Zhang, C.; Klett, E.L.; Coleman, R.A. Lipid signals and insulin resistance. Clin. Lipidol. 2013, 8, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, E.J.; Watson, A.; Theis, S.; Holz, A.; Harper, L.D.; Russell, M. A comparison of isomaltulose versus maltodextrin ingestion during soccer-specific exercise. Eur. J. Appl. Physiol. 2017, 117, 2321–2333. [Google Scholar] [CrossRef]
- Nickerson, K.P.; McDonald, C. Crohn’s disease-associated adherent-invasive Escherichia coli adhesion is enhanced by exposure to the ubiquitous dietary polysaccharide maltodextrin. PLoS ONE 2012, 7, e52132. [Google Scholar] [CrossRef]
- Jackson, P.P.; Wijeyesekera, A.; Williams, C.M.; Theis, S.; van Harsselaar, J.; Rastall, R.A. Inulin-type fructans and 2′fucosyllactose alter both microbial composition and appear to alleviate stress-induced mood state in a working population compared to placebo (maltodextrin): The EFFICAD Trial, a randomized, controlled trial. Am. J. Clin. Nutr. 2023, 118, 938–955. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, W.; Zhu, X.; Zhao, D.; Wang, K.; Wang, R.; Qu, W. The aqueous extract of Asparagus officinalis L. by-product exerts hypoglycaemic activity in streptozotocin-induced diabetic rats. J. Sci. Food. Agric. 2011, 91, 2095–2099. [Google Scholar] [CrossRef]
- Hafizur, R.M.; Kabir, N.; Chishti, S. Asparagus officinalis extract controls blood glucose by improving insulin secretion and β-cell function in streptozotocin-induced type 2 diabetic rats. Br. J. Nutr. 2012, 108, 1586–1595. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zhang, W.; Pang, X.; Wang, J.; Zhao, J.; Qu, W. Hypolipidemic effect of n-butanol extract from Asparagus officinalis L. in mice fed a high-fat diet. Phytother. Res. 2011, 25, 1119–1124. [Google Scholar] [CrossRef]
- García, M.D.; De la Puerta, R.; Sáenz, M.T.; Marquez-Martín, A.; Fernández-Arche, M.A. Hypocholesterolemic and hepatoprotective effects of “triguero” asparagus from andalusia in rats fed a high cholesterol diet. Evid.-Based Complement. Altern. Med. 2012, 2012, 814752. [Google Scholar] [CrossRef] [PubMed]
- Sanae, M.; Yasuo, A. Green asparagus (Asparagus officinalis) prevented hypertension by an inhibitory effect on angiotensin-converting enzyme activity in the kidney of spontaneously hypertensive rats. J. Agric. Food. Chem. 2013, 61, 5520–5525. [Google Scholar] [CrossRef] [PubMed]
- Matsuzawa, Y. Is diastolic blood pressure key to detecting risk and preventing heart failure with preserved ejection fraction? Hypertens. Res. 2023, 46, 534–536. [Google Scholar] [CrossRef]
- Al-Lahham, S.H.; Peppelenbosch, M.P.; Roelofsen, H.; Vonk, R.J.; Venema, K. Biological effects of propionic acid in humans; metabolism, potential applications and underlying mechanisms. Biochim. Biophys. Acta 2010, 1801, 1175–1183. [Google Scholar] [CrossRef]
- Martínez-Martínez, E.; Cachofeiro, V. Oxidative stress in obesity. Antioxidants 2022, 11, 639. [Google Scholar] [CrossRef]
- Ho, K.T.; Homma, K.; Takanari, J.; Bai, H.; Kawahara, M.; Nguyen, K.T.K.; Takahashi, M. A standardized extract of Asparagus officinalis stem improves HSP70-mediated redox balance and cell functions in bovine cumulus-granulosa cells. Sci. Rep. 2021, 11, 18175. [Google Scholar] [CrossRef]
- Alyami, N.M.; Almeer, R.; Alyami, H.M. Protective effects of Asparagus officinalis (asparagus) against lead toxicity in mice. Environ. Sci. Pollut. Res. Int. 2023, 30, 18718–18730. [Google Scholar] [CrossRef]
- Poormoosavi, S.M.; Najafzadehvarzi, H.; Behmanesh, M.A.; Amirgholami, R. Protective effects of Asparagus officinalis extract against Bisphenol A- induced toxicity in Wistar rats. Toxicol. Rep. 2018, 5, 427–433. [Google Scholar] [CrossRef]
- Ming, D.; Xu, X.; Jiang, X.; Li, Y.; Sun, W.; Xiang, J.; Huang, M.; Pi, Y.; Li, X. Indole-3-propionic acid enhances growth performance and reduces diarrhea via modulating redox status and intestinal inflammation in weaned piglets. Anim. Nutr. 2024, 19, 240–247. [Google Scholar] [CrossRef]
- Salehi, M.; Bagheri, D.; Sotoudeh, E.; Ghasemi, A.; Mozanzadeh, M.T. The combined effects of propionic acid and a mixture of Bacillus spp. probiotic in a plant protein-rich diet on growth, digestive enzyme activities, antioxidant capacity, and immune-related genes mRNA transcript abundance in Lates calcarifer fry. Probiotics Antimicrob. Proteins 2023, 15, 655–667. [Google Scholar] [CrossRef]
- Asmaey, M.A. Unravelling the secrets of α-pyrones from Aspergillus fungi: A comprehensive review of their natural sources, biosynthesis, and biological activities. Chem. Biodivers. 2023, 20, e202301185. [Google Scholar] [CrossRef]
- Purushothaman, R.; Vishnuram, G.; Ramanathan, T. Antiinflammatory efficacy of n-Hexadecanoic acid from a mangrove plant Excoecaria agallocha L. through in silico, in vitro and in vivo. Pharmacol. Res. Nat. Prod. 2025, 7, 100203. [Google Scholar] [CrossRef]

| Parameters | Placebo Group (n = 21) | Asparagus Group (n = 23) | p-Value (After vs. After) | ||||
|---|---|---|---|---|---|---|---|
| Before | After | Δ Change (%) | Before | After | Δ Change (%) | ||
| Age (years) | 24.52 ± 5.98 | - | - | 21.78 ± 4.08 | - | - | 0.081 |
| Sex (male/female) (n, %) | 2/19 (10/90) | - | - | 4/19 (17/83) | - | - | 0.459 |
| Height (cm) | 161.62 ± 5.63 | - | - | 160.91 ± 7.53 | - | - | 0.729 |
| Body mass (kg) | 74.81 ± 9.93 | 75.85 ± 9.17 * | 1.03 (1.38) | 71.53 ± 9.55 | 72.47 ± 9.86 | 0.93 (1.30) | 0.353 |
| Body mass index (kg/m2) | 28.68 ± 4.01 | 29.09 ± 3.66 * | 0.41 (1.42) | 27.74 ± 4.34 | 28.06 ± 4.07 | 0.32 (1.16) | 0.517 |
| Body fat (%) | 40.77 ± 5.37 | 41.47 ± 5.53 | 0.70 (1.71) | 37.75 ± 7.51 | 38.92 ± 7.39 | 1.17 (3.10) | 0.200 |
| Muscle mass (kg) | 24.47 ± 3.49 | 24.41 ± 3.48 | −0.06 (−0.23) | 24.24 ± 4.18 | 24.18 ± 4.33 | −0.06 (−0.25) | 0.937 |
| Fat-free mass (kg) | 31.28 ± 6.59 | 31.72 ± 6.69 | 0.44 (1.42) | 27.71 ± 7.76 | 28.37 ± 7.44 | 0.66 (2.38) | 0.155 |
| Water mass (kg) | 32.53 ± 4.14 | 32.51 ± 4.18 | −0.02 (−0.07) | 32.65 ± 5.06 | 32.29 ± 5.23 | −0.36 (−1.09) | 0.919 |
| Protein mass (kg) | 8.77 ± 1.14 | 8.75 ± 1.16 | −0.02 (−0.22) | 8.71 ± 1.38 | 8.68 ± 1.42 | −0.03 (−0.30) | 0.920 |
| Mineral mass (kg) | 3.13 ± 0.33 | 3.13 ± 0.32 | 0.00 (−0.02) | 3.12 ± 0.47 | 3.13 ± 0.52 | 0.01 (0.21) | 0.911 |
| Waist circumference (cm) | 94.17 ± 10.57 | 94.62 ± 10.53 | 0.45 (0.48) | 89.89 ± 11.18 | 89.76 ± 11.37 | −0.13 (−0.15) | 0.216 |
| Hip circumference (cm) | 107.31 ± 6.89 | 108.19 ± 6.79 | 0.88 (0.82) | 104.20 ± 13.65 | 103.76 ± 13.51 | −0.43 (−0.42) | 0.305 |
| Waist/hip ratio | 0.88 ± 0.09 | 0.88 ± 0.10 | 0.00 (−0.24) | 0.87 ± 0.10 | 0.86 ± 0.10 * | −0.01 (−0.51) | 0.271 |
| Visceral fat level | 14.19 ± 4.55 | 14.95 ± 3.28 | 0.76 (5.37) | 12.70 ± 4.28 | 13.13 ± 4.16 | 0.43 (3.42) | 0.150 |
| Basal metabolic rate (kcal) | 1329.52 ± 121.05 | 1328.48 ± 122.37 | −1.05 (−0.08) | 1322.70 ± 146.55 | 1322.35 ± 154.41 | −0.35 (−0.03) | 0.921 |
| Fitness score | 62.19 ± 5.60 | 61.57 ± 5.87 | −0.62 (−1.00) | 64.78 ± 6.95 | 63.96 ± 6.69 | −0.83 (−1.28) | 0.194 |
| Systolic blood pressure (mmHg) | 107.21 ± 12.55 | 108.62 ± 12.01 | 1.41 (1.31) | 109.57 ± 10.30 | 108.98 ± 10.04 | −0.58 (−0.53) | 0.407 |
| Diastolic blood pressure (mmHg) | 72.90 ± 6.38 | 71.06 ± 5.99 | −1.84 (−2.53) | 73.90 ± 9.25 | 69.73 ± 6.44 * | −4.17 (−5.64) | 0.871 |
| Pulse rate (/min) | 81.30 ± 11.02 | 82.98 ± 12.76 | 1.68 (2.07) | 75.78 ± 8.77 | 77.18 ± 10.87 | 1.40 (1.85) | 0.215 |
| Respiratory rate (/min) | 18.29 ± 2.35 | 18.86 ± 1.49 | 0.57 (3.13) | 18.52 ± 2.59 | 18.96 ± 1.33 | 0.43 (2.35) | 0.929 |
| Body temperature (°C) | 36.36 ± 0.36 | 36.40 ± 0.23 | 0.05 (0.13) | 36.07 ± 0.55 | 36.45 ± 0.26 * | 0.39 (1.07) | 0.683 |
| Hemoglobin saturation (%) | 97.71 ± 0.85 | 97.90 ± 0.83 | 0.19 (0.19) | 97.78 ± 1.00 | 98.26 ± 0.81 * | 0.48 (0.49) | 0.416 |
| Physical activity (kcal/day) | 1408.82 ± 157.89 | 1394.15 ± 150.43 | −14.67 (−1.04) | 1406.26 ± 77.21 | 1402.50 ± 71.03 | −3.76 (−0.27) | 0.659 |
| Dietary intake (kcal/day) | 1425.37 ± 54.36 | 1426.11 ± 40.63 | 0.74 (0.05) | 1407.31 ± 82.77 | 1400.06 ± 72.03 | −7.25 (−0.52) | 0.107 |
| Parameters | Placebo Group (n = 21) | Asparagus Group (n = 23) | p-Value (After vs. After) | ||||
|---|---|---|---|---|---|---|---|
| Before | After | Δ Change (%) | Before | After | Δ Change (%) | ||
| FBG (mg/dL) | 97.38 ± 11.04 | 99.90 ± 12.17 | 2.52 (2.59) | 94.52 ± 10.83 | 92.27 ± 8.32 ** | −2.25 (−2.38) | 0.045 |
| Insulin (µU/mL) | 12.31 ± 6.18 | 19.17 ± 9.76 * | 6.86 (55.74) | 14.82 ± 11.50 | 16.89 ± 9.75 | 2.07 (13.94) | 0.271 |
| HOMA-IR | 2.96 ± 1.48 | 4.71 ± 2.26 * | 1.75 (59.17) | 3.46 ± 2.77 | 3.85 ± 2.24 | 0.40 (11.45) | 0.143 |
| HOMA-B | 7.80 ± 4.68 | 11.46 ± 7.79 * | 3.65 (46.80) | 11.04 ± 9.87 | 12.44 ± 8.17 | 1.40 (12.65) | 0.950 |
| TC (mg/dL) | 203.00 ± 34.13 | 206.29 ± 23.47 | 3.29 (1.62) | 200.61 ± 44.21 | 202.86 ± 41.16 | 2.25 (1.12) | 0.612 |
| TG (mg/dL) | 138.76 ± 85.25 | 181.33 ± 128.58 * | 42.57 (30.68) | 116.26 ± 72.98 | 113.23 ± 54.69 ** | −3.03 (−2.61) | 0.043 |
| LDL-C (mg/dL) | 136.91 ± 47.36 | 129.64 ± 44.12 | −7.28 (−5.31) | 141.48 ± 37.87 | 129.52 ± 28.35 * | −11.95 (−8.45) | 0.891 |
| HDL-C (mg/dL) | 50.10 ± 9.71 | 49.10 ± 12.44 | −1.00 (−2.00) | 53.13 ± 11.53 | 53.68 ± 15.48 | 0.55 (1.04) | 0.538 |
| TC/HDL-C ratio | 4.22 ± 1.20 | 4.46 ± 1.22 * | 0.24 (5.76) | 3.99 ± 1.38 | 4.07 ± 1.41 | 0.08 (2.01) | 0.499 |
| TG/HDL-C ratio | 3.02 ± 2.19 | 4.26 ± 3.63 * | 1.23 (40.81) | 2.42 ± 1.71 | 2.42 ± 1.55 ** | 0.01 (0.22) | 0.032 |
| LDL-C/HDL-C ratio | 2.96 ± 1.15 | 2.85 ± 1.07 | −0.11 (−3.81) | 2.78 ± 1.32 | 2.68 ± 1.32 | −0.10 (−3.59) | 0.746 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mongraykang, J.; Padkao, T.; Boonla, O.; Teethaisong, Y.; Roengrit, T.; Koowattanatianchai, S.; Prasertsri, P. Effects of Asparagus Powder Supplementation on Glycemic Control, Lipid Profile, and Oxidative Stress in Overweight and Obese Adults: An Exploratory Randomized Controlled Trial. Life 2025, 15, 1584. https://doi.org/10.3390/life15101584
Mongraykang J, Padkao T, Boonla O, Teethaisong Y, Roengrit T, Koowattanatianchai S, Prasertsri P. Effects of Asparagus Powder Supplementation on Glycemic Control, Lipid Profile, and Oxidative Stress in Overweight and Obese Adults: An Exploratory Randomized Controlled Trial. Life. 2025; 15(10):1584. https://doi.org/10.3390/life15101584
Chicago/Turabian StyleMongraykang, Jittima, Tadsawiya Padkao, Orachorn Boonla, Yothin Teethaisong, Thapanee Roengrit, Sukrisd Koowattanatianchai, and Piyapong Prasertsri. 2025. "Effects of Asparagus Powder Supplementation on Glycemic Control, Lipid Profile, and Oxidative Stress in Overweight and Obese Adults: An Exploratory Randomized Controlled Trial" Life 15, no. 10: 1584. https://doi.org/10.3390/life15101584
APA StyleMongraykang, J., Padkao, T., Boonla, O., Teethaisong, Y., Roengrit, T., Koowattanatianchai, S., & Prasertsri, P. (2025). Effects of Asparagus Powder Supplementation on Glycemic Control, Lipid Profile, and Oxidative Stress in Overweight and Obese Adults: An Exploratory Randomized Controlled Trial. Life, 15(10), 1584. https://doi.org/10.3390/life15101584






