Gut Microbiota and Obsessive–Compulsive Disorder: A Systematic Review of Mechanistic Links, Evidence from Human and Preclinical Studies, and Therapeutic Prospects
Abstract
1. Introduction
2. Materials and Methods
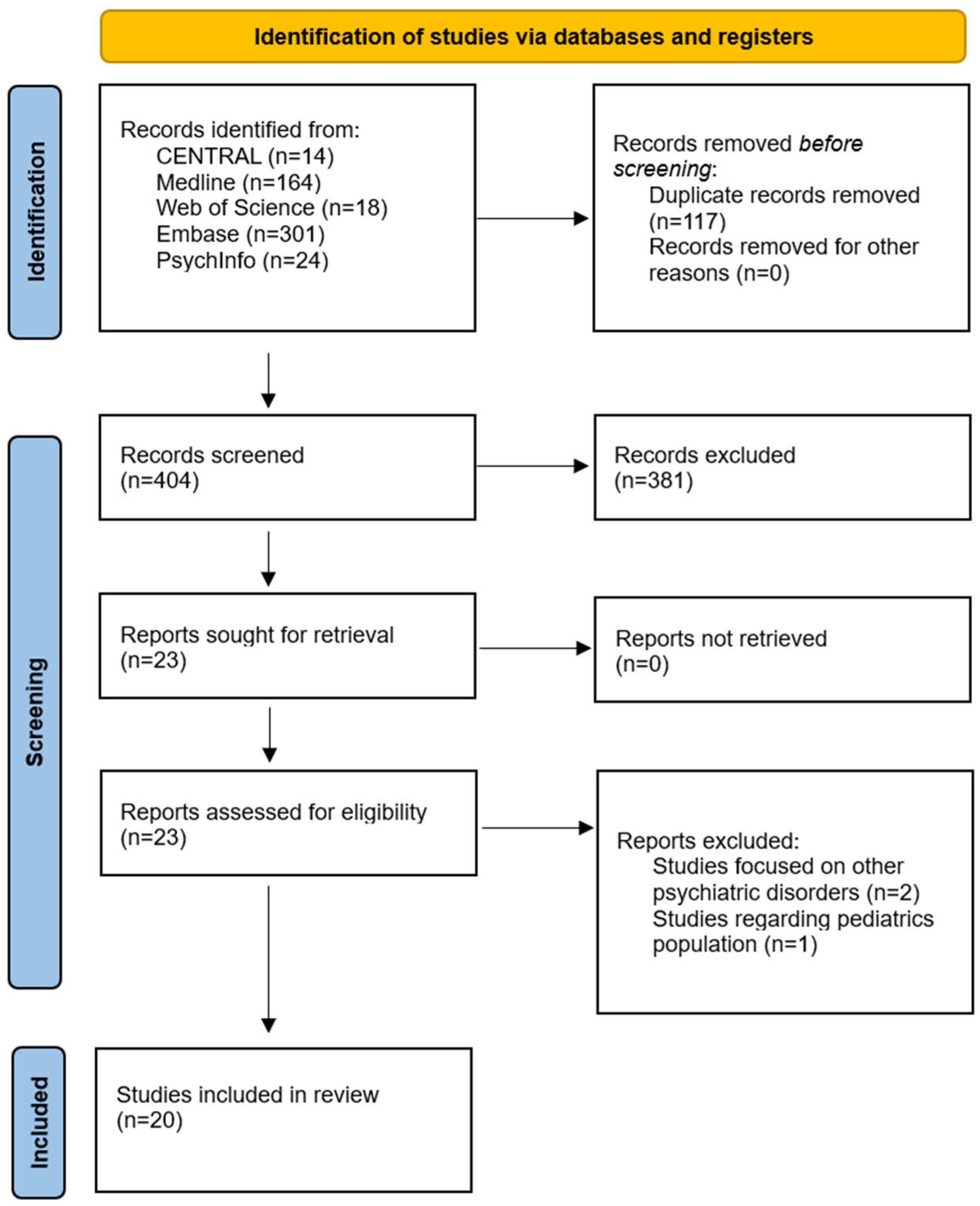
2.1. Protocol and Registration
2.2. Eligibility Criteria
- Immune and inflammatory markers (such as cytokines, acute-phase reactants, immune cell phenotypes);
- Metabolic and mitochondrial parameters (such as SCFAs, organic acids, oxidative phosphorylation activity);
- Neuroendocrine measures (such as cortisol, ACTH);
- Epigenetic modifications (DNA methylation, histone modifications, non-coding RNAs);
- Neurotransmitter systems (glutamate, GABA, serotonin, dopamine);
- Barrier function markers (such as zonulin, occludin);
- OCD symptom severity or compulsive-like behavior in animal models.
2.3. Information Sources
2.4. Study Selection
2.5. Data Extraction
2.6. Risk of Bias Assessment
2.7. Evidence Synthesis
3. Results
3.1. Step 1: Biological and Molecular Alterations in OCD Pathophysiology
3.1.1. Overview of Included Studies
3.1.2. Glutamatergic and GABAergic Dysfunction in OCD
3.1.3. Monoaminergic System Alterations in OCD Pathophysiology
3.1.4. Immune and Inflammatory Activation in OCD
3.1.5. Oxidative Stress and Mitochondrial Dysfunction in OCD
3.1.6. HPA Axis Dysregulation and Neuroendocrine Changes in OCD
3.1.7. Epigenetic Modifications and Gene Expression Changes in OCD
3.1.8. Neurotrophic Factor Alterations and Neuroplasticity Changes in OCD
3.1.9. Peripheral Metabolic Alterations and Tryptophan-Kynurenine Pathway Changes in OCD
3.2. Step 2: Gut Microbiome Alterations and Interventions in OCD
3.2.1. Study Selection and Overview
3.2.2. Human Gut Microbiome Studies in OCD Patients
3.2.3. Preclinical Models: Microbiome Manipulation in OCD-like Behaviors
3.3. Bridge to Step 1 Biomarker Axes
3.4. Mechanistic Causal Triangulation Across Microbiome-OCD Axes
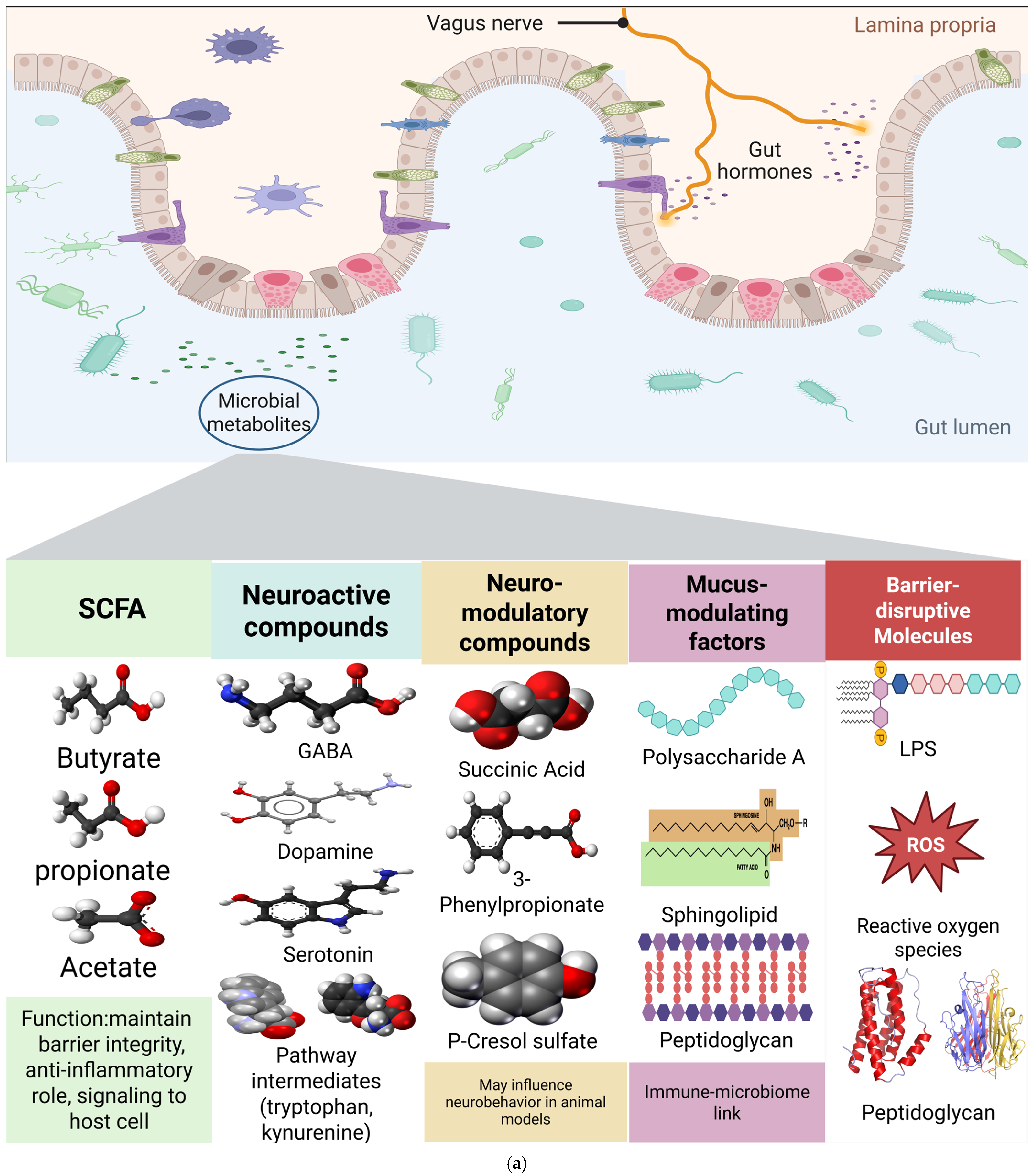
3.4.1. SCFAs/Microbial Metabolites
3.4.2. Endotoxin/Barrier Integrity
3.4.3. Monoaminergic Signaling (5-HT/DA)
4. Discussion
4.1. Integrating OCD Biology with Gut–Brain Mechanisms
4.2. Convergent Pathways: From Microbiome Dysfunction to OCD Symptoms
4.2.1. SCFA Deficiency and Intestinal Barrier Dysfunction in OCD Pathogenesis
4.2.2. Endotoxin-Mediated Barrier Disruption and Neuroinflammation in OCD
4.2.3. Microbiome-Mediated Monoaminergic Dysfunction in OCD: Tryptophan-Kynurenine and Dopamine-Serotonin Pathways
4.3. Cross-Species Bridge: Metabolic Signatures
4.4. What Is Probably True Now
4.5. Critical Confounding Factors in Microbiome-OCD Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABX | Antibiotic treatment |
| ACTH | Adrenocorticotropic hormone |
| BBB | Blood–brain barrier |
| BDNF | Brain-derived neurotrophic factor |
| CFU | Colony-forming unit |
| CRP | C-reactive protein |
| DA | Dopamine |
| DNA | Deoxyribonucleic acid |
| DSM | Diagnostic and Statistical Manual of Mental Disorders |
| ERP | Exposure and response prevention |
| FDR | False discovery rate |
| FMT | Fecal microbiota transplantation |
| GABA | Gamma-aminobutyric acid |
| GWAS | Genome-wide association study |
| HIP | Hippurate |
| HPA axis | Hypothalamic–pituitary–adrenal axis |
| HTR2A | 5-hydroxytryptamine receptor type 2A |
| IL | Interleukin |
| JBI | Joanna Briggs Institute |
| LPS | Lipopolysaccharide |
| MAOA | Monoamine oxidase A |
| mGluR5 | Metabotropic glutamate receptor 5 |
| mPFC | Medial prefrontal cortex |
| MR | Mendelian randomization |
| NGF | Nerve growth factor |
| OCD | Obsessive–compulsive disorder |
| OCI-R | Obsessive–Compulsive Inventory–Revised |
| OXTR | Oxytocin receptor |
| PERMANOVA | Permutational multivariate analysis of variance |
| pCS | p-Cresol sulfate |
| SERT | Serotonin transporter |
| SCFA | Short-chain fatty acid |
| SLC6A4 | Solute carrier family 6 member 4 (serotonin transporter gene) |
| SNP | Single nucleotide polymorphism |
| SSRI | Selective serotonin reuptake inhibitor |
| SYRCLE | Systematic Review Centre for Laboratory Animal Experimentation |
| TNF-α | Tumor necrosis factor-alpha |
| Trp | Tryptophan |
| WGS | Whole-genome sequencing |
| Y-BOCS | Yale–Brown Obsessive Compulsive Scale |
References
- Shavitt, R.G.; de Mathis, M.A.; Oki, F.; Ferrao, Y.A.; Fontenelle, L.F.; Torres, A.R.; Diniz, J.B.; Costa, D.L.; do Rosário, M.C.; Hoexter, M.Q. Phenomenology of OCD: Lessons from a large multicenter study and implications for ICD-11. J. Psychiatr. Res. 2014, 57, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Alvarenga, P.G.; Do Rosario, M.C.; Cesar, R.C.; Manfro, G.G.; Moriyama, T.S.; Bloch, M.H.; Shavitt, R.G.; Hoexter, M.Q.; Coughlin, C.G.; Leckman, J.F. Obsessive–compulsive symptoms are associated with psychiatric comorbidities, behavioral and clinical problems: A population-based study of Brazilian school children. Eur. Child Adolesc. Psychiatry 2016, 25, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Eghdami, S.; Eissazade, N.; Heidari Mokarar, M.; Boroon, M.; Orsolini, L.; Shalbafan, M. The safety and efficacy of N-acetylcysteine as an augmentation in the treatment of obsessive-compulsive disorder in adults: A systematic review and meta-analysis of randomized clinical trials. Front. Psychiatry 2024, 15, 1421150. [Google Scholar] [CrossRef]
- Shalbafan, M.; Malekpour, F.; Tadayon Najafabadi, B.; Ghamari, K.; Dastgheib, S.-A.; Mowla, A.; Shirazi, E.; Eftekhar Ardebili, M.; Ghazizadeh-Hashemi, M.; Akhondzadeh, S. Fluvoxamine combination therapy with tropisetron for obsessive-compulsive disorder patients: A placebo-controlled, randomized clinical trial. J. Psychopharmacol. 2019, 33, 1407–1414. [Google Scholar] [CrossRef]
- Westwell-Roper, C.; Williams, K.A.; Samuels, J.; Bienvenu, O.J.; Cullen, B.; Goes, F.S.; Grados, M.A.; Geller, D.; Greenberg, B.D.; Knowles, J.A. Immune-related comorbidities in childhood-onset obsessive compulsive disorder: Lifetime prevalence in the obsessive compulsive disorder collaborative genetics association study. J. Child Adolesc. Psychopharmacol. 2019, 29, 615–624. [Google Scholar] [CrossRef]
- Askari, S.; Mokhtari, S.; Shariat, S.V.; Shariati, B.; Yarahmadi, M.; Shalbafan, M. Memantine augmentation of sertraline in the treatment of symptoms and executive function among patients with obsessive-compulsive disorder: A double-blind placebo-controlled, randomized clinical trial. BMC Psychiatry 2022, 22, 34. [Google Scholar] [CrossRef] [PubMed]
- Hadi, F.; Kashefinejad, S.; Kamalzadeh, L.; Hoobehfekr, S.; Shalbafan, M. Glutamatergic medications as adjunctive therapy for moderate to severe obsessive-compulsive disorder in adults: A systematic review and meta-analysis. BMC Pharmacol. Toxicol. 2021, 22, 69. [Google Scholar] [CrossRef]
- Mahjani, B.; Bey, K.; Boberg, J.; Burton, C. Genetics of obsessive-compulsive disorder. Psychol. Med. 2021, 51, 2247–2259. [Google Scholar] [CrossRef]
- Tavasoli, A.; Kachuei, M.; Talebi, S.; Eghdami, S. Complex mitochondrial disease caused by the mutation of COX10 in a toddler: A case-report study. Ann. Med. Surg. 2024, 86, 3753–3756. [Google Scholar] [CrossRef]
- Jang, S.-H.; Woo, Y.S.; Lee, S.-Y.; Bahk, W.-M. The brain–gut–microbiome axis in psychiatry. Int. J. Mol. Sci. 2020, 21, 7122. [Google Scholar] [CrossRef] [PubMed]
- Domènech, L.; Willis, J.; Alemany-Navarro, M.; Morell, M.; Real, E.; Escaramís, G.; Bertolín, S.; Sánchez Chinchilla, D.; Balcells, S.; Segalàs, C. Changes in the stool and oropharyngeal microbiome in obsessive-compulsive disorder. Sci. Rep. 2022, 12, 1448. [Google Scholar] [CrossRef]
- Karimzadeh, P.; Kachuei, M.; Eghdami, S.; Beglar, M.B.; Azizi, M. Outcomes of Ketogenic Diet in Pediatric Intractable Epilepsy and Neurometabolic Disorders: Insights from the First Iranian Registry. Pediatr. Neurol. 2025, 169, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.V.-A. Gut microbiome composition and diversity are related to human personality traits. Hum. Microbiome J. 2020, 15, 100069. [Google Scholar] [CrossRef] [PubMed]
- Park, E.; Yun, K.E.; Kim, M.-H.; Kim, J.; Chang, Y.; Ryu, S.; Kim, H.-L.; Kim, H.-N.; Jung, S.-C. Correlation between gut microbiota and six facets of neuroticism in Korean adults. J. Pers. Med. 2021, 11, 1246. [Google Scholar] [CrossRef] [PubMed]
- Yunes, R.; Poluektova, E.; Dyachkova, M.; Klimina, K.; Kovtun, A.; Averina, O.; Orlova, V.; Danilenko, V. GABA production and structure of gadB/gadC genes in Lactobacillus and Bifidobacterium strains from human microbiota. Anaerobe 2016, 42, 197–204. [Google Scholar] [CrossRef]
- Villageliú, D.; Lyte, M. Dopamine production in Enterococcus faecium: A microbial endocrinology-based mechanism for the selection of probiotics based on neurochemical-producing potential. PLoS ONE 2018, 13, e0207038. [Google Scholar] [CrossRef]
- Gargus, M.; Ben-Azu, B.; Landwehr, A.; Dunn, J.; Errico, J.P.; Tremblay, M.-È. Mechanisms of vagus nerve stimulation for the treatment of neurodevelopmental disorders: A focus on microglia and neuroinflammation. Front. Neurosci. 2025, 18, 1527842. [Google Scholar] [CrossRef] [PubMed]
- Burns, A.M.; Farinelli-Scharly, M.; Hugues-Ascery, S.; Sanchez-Mut, J.V.; Santoni, G.; Gräff, J. The HDAC inhibitor CI-994 acts as a molecular memory aid by facilitating synaptic and intracellular communication after learning. Proc. Natl. Acad. Sci. USA 2022, 119, e2116797119. [Google Scholar] [CrossRef]
- Zhang, X.; Zou, Q.; Zhao, B.; Zhang, J.; Zhao, W.; Li, Y.; Liu, R.; Liu, X.; Liu, Z. Effects of alternate-day fasting, time-restricted fasting and intermittent energy restriction DSS-induced on colitis and behavioral disorders. Redox Biol. 2020, 32, 101535. [Google Scholar]
- Ahmari, S.E.; Spellman, T.; Douglass, N.L.; Kheirbek, M.A.; Simpson, H.B.; Deisseroth, K.; Gordon, J.A.; Hen, R. Repeated cortico-striatal stimulation generates persistent OCD-like behavior. Science 2013, 340, 1234–1239. [Google Scholar] [CrossRef]
- Bendriss, G.; MacDonald, R.; McVeigh, C. Microbial reprogramming in obsessive–compulsive disorders: A review of gut–brain communication and emerging evidence. Int. J. Mol. Sci. 2023, 24, 11978. [Google Scholar]
- Chinna Meyyappan, A.; Forth, E.; Wallace, C.J.; Milev, R. Effect of fecal microbiota transplant on symptoms of psychiatric disorders: A systematic review. BMC Psychiatry 2020, 20, 299. [Google Scholar] [CrossRef]
- Huang, Y.; Shi, X.; Li, Z.; Shen, Y.; Shi, X.; Wang, L.; Li, G.; Yuan, Y.; Wang, J.; Zhang, Y. Possible association of Firmicutes in the gut microbiota of patients with major depressive disorder. Neuropsychiatr. Dis. Treat. 2018, 14, 3329–3337. [Google Scholar] [CrossRef]
- Hamamah, S.; Aghazarian, A.; Nazaryan, A.; Hajnal, A.; Covasa, M. Role of microbiota-gut-brain axis in regulating dopaminergic signaling. Biomedicines 2022, 10, 436. [Google Scholar] [CrossRef]
- Gao, K.; Mu, C.-L.; Farzi, A.; Zhu, W.-Y. Tryptophan metabolism: A link between the gut microbiota and brain. Adv. Nutr. 2020, 11, 709–723. [Google Scholar] [CrossRef]
- Wilson, C.; Gattuso, J.J.; Kuznetsova, M.; Li, S.; Connell, S.; Choo, J.M.; Rogers, G.B.; Gubert, C.; Hannan, A.J.; Renoir, T. Experience-dependent grooming microstructure alterations and gastrointestinal dysfunction in the SAPAP3 knockout mouse model of compulsive behaviour. J. Affect. Disord. 2024, 363, 520–531. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.; Sandhu, K.V.; Bastiaanssen, T.F.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V. The microbiota-gut-brain axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Cheng, Y.; Liu, X.; Xu, X.; Ding, W.; Ling, Z.; Liu, J.; Cai, G. The Microbiota-Gut-Brain Axis in Depression: Unraveling the Relationships and Therapeutic Opportunities. Front. Immunol. 2025, 16, 1644160. [Google Scholar]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The role of short-chain fatty acids from gut microbiota in gut-brain communication. Front. Endocrinol. 2020, 11, 508738. [Google Scholar] [CrossRef] [PubMed]
- Dehhaghi, M.; Kazemi Shariat Panahi, H.; Guillemin, G.J. Microorganisms, tryptophan metabolism, and kynurenine pathway: A complex interconnected loop influencing human health status. Int. J. Tryptophan Res. 2019, 12, 1178646919852996. [Google Scholar] [CrossRef] [PubMed]
- Strandwitz, P.; Kim, K.H.; Terekhova, D.; Liu, J.K.; Sharma, A.; Levering, J.; McDonald, D.; Dietrich, D.; Ramadhar, T.R.; Lekbua, A. GABA-modulating bacteria of the human gut microbiota. Nat. Microbiol. 2019, 4, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Madison, A.; Kiecolt-Glaser, J.K. Stress, depression, diet, and the gut microbiota: Human–bacteria interactions at the core of psychoneuroimmunology and nutrition. Curr. Opin. Behav. Sci. 2019, 28, 105–110. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Abramowitz, J.S.; Jacoby, R.J. Obsessive-compulsive disorder in the DSM-5. Clin. Psychol. Sci. Pract. 2014, 21, 221. [Google Scholar] [CrossRef]
- Stein, D.J.; Kogan, C.S.; Atmaca, M.; Fineberg, N.A.; Fontenelle, L.F.; Grant, J.E.; Matsunaga, H.; Reddy, Y.C.J.; Simpson, H.B.; Thomsen, P. The classification of obsessive–compulsive and related disorders in the ICD-11. J. Affect. Disord. 2016, 190, 663–674. [Google Scholar] [CrossRef] [PubMed]
- Mueen Ahmed, K.; Dhubaib, B.E.A. Zotero: A bibliographic assistant to researcher. J. Pharmacol. Pharmacother. 2011, 2, 304–305. [Google Scholar] [CrossRef]
- Grammatopoulos, T.; Hunter, J.W.; Munn, Z.; Stone, J.C.; Barker, T.H. Reporting quality and risk of bias in JBI systematic reviews evaluating the effectiveness of interventions: A methodological review protocol. JBI Evid. Synth. 2023, 21, 584–591. [Google Scholar] [CrossRef]
- Hooijmans, C.R.; Rovers, M.M.; De Vries, R.B.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef]
- Akkus, F.; Terbeck, S.; Ametamey, S.M.; Rufer, M.; Treyer, V.; Burger, C.; Johayem, A.; Mancilla, B.G.; Sovago, J.; Buck, A. Metabotropic glutamate receptor 5 binding in patients with obsessive-compulsive disorder. Int. J. Neuropsychopharmacol. 2014, 17, 1915–1922. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; Khanna, S.; Chakrabarty, K.; Mahadevan, A.; Christopher, R.; Shankar, S. Anti-brain autoantibodies and altered excitatory neurotransmitters in obsessive–compulsive disorder. Neuropsychopharmacology 2009, 34, 2489–2496. [Google Scholar] [PubMed]
- Lee, S.W.; Kim, S.; Chang, Y.; Cha, H.; Noeske, R.; Choi, C.; Lee, S.J. Quantification of glutathione and its associated spontaneous neuronal activity in major depressive disorder and obsessive-compulsive disorder. Biol. Psychiatry 2025, 97, 279–289. [Google Scholar] [CrossRef]
- Naaijen, J.; Zwiers, M.P.; Amiri, H.; Williams, S.C.R.; Durston, S.; Oranje, B.; Brandeis, D.; Boecker-Schlier, R.; Ruf, M.; Wolf, I.; et al. Erratum: Fronto-Striatal Glutamate in Autism Spectrum Disorder and Obsessive Compulsive Disorder. Neuropsychopharmacology 2017, 42, 2466–2467. [Google Scholar] [CrossRef]
- Rodriguez, C.I.; Kegeles, L.S.; Levinson, A.; Ogden, R.T.; Mao, X.; Milak, M.S.; Vermes, D.; Xie, S.; Hunter, L.; Flood, P. In vivo effects of ketamine on glutamate-glutamine and gamma-aminobutyric acid in obsessive-compulsive disorder: Proof of concept. Psychiatry Res. Neuroimaging 2015, 233, 141–147. [Google Scholar] [CrossRef]
- Adams, K.H.; Hansen, E.S.; Pinborg, L.H.; Hasselbalch, S.G.; Svarer, C.; Holm, S.; Bolwig, T.G.; Knudsen, G.M. Patients with obsessive–compulsive disorder have increased 5-HT2A receptor binding in the caudate nuclei. Int. J. Neuropsychopharmacol. 2005, 8, 391–401. [Google Scholar] [PubMed]
- Matsumoto, R.; Ichise, M.; Ito, H.; Ando, T.; Takahashi, H.; Ikoma, Y.; Kosaka, J.; Arakawa, R.; Fujimura, Y.; Ota, M. Reduced serotonin transporter binding in the insular cortex in patients with obsessive–compulsive disorder: A [11C] DASB PET study. Neuroimage 2010, 49, 121–126. [Google Scholar] [PubMed]
- Zitterl, W.; Aigner, M.; Stompe, T.; Zitterl-Eglseer, K.; Gutierrez-Lobos, K.; Wenzel, T.; Zettinig, G.; Hornik, K.; Pirker, W.; Thau, K. Changes in thalamus–hypothalamus serotonin transporter availability during clomipramine administration in patients with obsessive–compulsive disorder. Neuropsychopharmacology 2008, 33, 3126–3134. [Google Scholar]
- Denys, D.; Van Nieuwerburgh, F.; Deforce, D.; Westenberg, H.G. Association between serotonergic candidate genes and specific phenotypes of obsessive compulsive disorder. J. Affect. Disord. 2006, 91, 39–44. [Google Scholar] [CrossRef]
- Olver, J.S.; O’Keefe, G.; Jones, G.R.; Burrows, G.D.; Tochon-Danguy, H.J.; Ackermann, U.; Scott, A.; Norman, T.R. Dopamine D1 receptor binding in the striatum of patients with obsessive–compulsive disorder. J. Affect. Disord. 2009, 114, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Tao, Q.; Dang, J.; Guo, H.; Zhang, M.; Niu, X.; Kang, Y.; Sun, J.; Ma, L.; Wei, Y.; Wang, W. Abnormalities in static and dynamic intrinsic neural activity and neurotransmitters in first-episode OCD. J. Affect. Disord. 2024, 363, 609–618. [Google Scholar] [CrossRef]
- Rao, N.P.; Venkatasubramanian, G.; Ravi, V.; Kalmady, S.; Cherian, A.; Yc, J.R. Plasma cytokine abnormalities in drug-naïve, comorbidity-free obsessive–compulsive disorder. Psychiatry Res. 2015, 229, 949–952. [Google Scholar]
- Sarker, R.; Qusar, M.S.; Islam, S.M.A.; Bhuiyan, M.A.; Islam, M.R. Association of granulocyte macrophage colony-stimulating factor and interleukin-17 levels with obsessive–compulsive disorder: A case–control study findings. Sci. Rep. 2023, 13, 18976. [Google Scholar]
- Sarmin, N.; Roknuzzaman, A.; Sarker, R.; Rashid, M.-O.; Hasan, A.; Qusar, M.S.; Kabir, E.R.; Islam, M.R.; Mahmud, Z.A. Exploring the role of interleukin-1β and interleukin-6 in the pathophysiology of obsessive-compulsive disorder. PLoS ONE 2024, 19, e0306125. [Google Scholar] [CrossRef]
- Turna, J.; Grosman Kaplan, K.; Anglin, R.; Patterson, B.; Soreni, N.; Bercik, P.; Surette, M.; Van Ameringen, M. The gut microbiome and inflammation in obsessive-compulsive disorder patients compared to age- and sex-matched controls: A pilot study. Acta Psychiatr. Scand. 2020, 142, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Alici, D.; Bulbul, F.; Virit, O.; Unal, A.; Altindag, A.; Alpak, G.; Alici, H.; Ermis, B.; Orkmez, M.; Taysi, S. Evaluation of oxidative metabolism and oxidative DNA damage in patients with obsessive–compulsive disorder. Psychiatry Clin. Neurosci. 2016, 70, 109–115. [Google Scholar] [CrossRef]
- Kang, J.I.; Park, C.I.; Lin, J.; Kim, S.T.; Kim, H.W.; Kim, S.J. Alterations of cellular aging markers in obsessive–compulsive disorder: Mitochondrial DNA copy number and telomere length. J. Psychiatry Neurosci. 2021, 46, E451–E458. [Google Scholar] [CrossRef]
- Orhan, N.; Kucukali, C.I.; Cakir, U.; Seker, N.; Aydin, M. Genetic variants in nuclear-encoded mitochondrial proteins are associated with oxidative stress in obsessive compulsive disorders. J. Psychiatr. Res. 2012, 46, 212–218. [Google Scholar] [CrossRef]
- Shrivastava, A.; Kar, S.K.; Sharma, E.; Mahdi, A.A.; Dalal, P.K. A study of oxidative stress biomarkers in obsessive compulsive disorder. J. Obs. -Compuls. Relat. Disord. 2017, 15, 52–56. [Google Scholar]
- Erbay, L.G.; Kavuran, N.A.; Taşkapan, Ç.; Lara Utku, İ.; Yoloğlu, S.; Temelli, H.G.; Ünal, S. Serum IL-1, IL-2, IL-4, IL-6, IL-10, TNF-α, and IFN-γ levels in drug-free, comorbidity-free obsessive-compulsive disorder patients. Anadolu Psikiyatr. Derg. 2018, 19, 157–162. [Google Scholar] [CrossRef]
- Kluge, M.; Schüssler, P.; Künzel, H.E.; Dresler, M.; Yassouridis, A.; Steiger, A. Increased nocturnal secretion of ACTH and cortisol in obsessive compulsive disorder. J. Psychiatr. Res. 2007, 41, 928–933. [Google Scholar] [CrossRef]
- Malisiova, E.K.; Mourikis, I.; Chalimourdas, T.; Nianiakas, N.; Michou, M.; Mantzou, A.; Darviri, C.; Vaidakis, N.; Zervas, I.M.; Chrousos, G.P. Low hair cortisol concentrations in obsessive compulsive disorder: A cross-sectional study. J. Psychiatr. Res. 2020, 131, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Gruenblatt, E.; Marinova, Z.; Roth, A.; Gardini, E.; Ball, J.; Geissler, J.; Wojdacz, T.K.; Romanos, M.; Walitza, S. Combining genetic and epigenetic parameters of the serotonin transporter gene in obsessive-compulsive disorder. J. Psychiatr. Res. 2018, 96, 209–217. [Google Scholar] [CrossRef]
- McGregor, N.; Hemmings, S.; Erdman, L.; Calmarza-Font, I.; Stein, D.; Lochner, C. Modification of the association between early adversity and obsessive-compulsive disorder by polymorphisms in the MAOA, MAOB and COMT genes. Psychiatry Res. 2016, 246, 527–532. [Google Scholar] [CrossRef]
- Park, C.I.; Kim, H.W.; Jeon, S.; Kang, J.I.; Kim, S.J. Reduced DNA methylation of the oxytocin receptor gene is associated with obsessive-compulsive disorder. Clin. Epigenetics 2020, 12, 101. [Google Scholar] [CrossRef]
- D’Addario, C.; Bellia, F.; Benatti, B.; Grancini, B.; Vismara, M.; Pucci, M.; De Carlo, V.; Viganò, C.; Galimberti, D.; Fenoglio, C. Exploring the role of BDNF DNA methylation and hydroxymethylation in patients with obsessive compulsive disorder. J. Psychiatr. Res. 2019, 114, 17–23. [Google Scholar] [CrossRef]
- Da Rocha, F.; Malloy--Diniz, L.; Lage, N.; Correa, H. The relationship between the Met allele of the BDNF Val66Met polymorphism and impairments in decision making under ambiguity in patients with obsessive–compulsive disorder. Genes Brain Behav. 2011, 10, 523–529. [Google Scholar] [CrossRef]
- Fontenelle, L.F.; Barbosa, I.G.; Luna, J.V.; Rocha, N.P.; Miranda, A.S.; Teixeira, A.L. Neurotrophic factors in obsessive-compulsive disorder. Psychiatry Res. 2012, 199, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Gomes, C.K.F.; Vieira-Fonseca, T.; Melo-Felippe, F.B.; de Salles Andrade, J.B.; Fontenelle, L.F.; Kohlrausch, F.B. Corrigendum to “Association analysis of SLC6A4 and HTR2A genes with obsessive-compulsive disorder: Influence of the STin2 polymorphism” [Compr. Psychiatry 82 (2018) 1–6]. Compr. Psychiatry 2018, 86, 144. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Chen, X.; Wei, C.; Zhang, H.; Zhang, L.; Han, L.; Sun, K.; Li, B.; Wen, S. BDNF alleviates microglial inhibition and stereotypic behaviors in a mouse model of obsessive-compulsive disorder. Front. Mol. Neurosci. 2022, 15, 926572. [Google Scholar] [CrossRef] [PubMed]
- Alp, H.H.; Kurhan, F.; Akbay, H.İ. Predictive value of kynurenine pathway metabolites in the severity of patients with obsessive-compulsive disorder. Psychiatry Clin. Neurosci. 2025, 79, 378–388. [Google Scholar] [CrossRef]
- Ari, M.; Ozturk, O.H.; Bez, Y.; Arica, S.; Can, Y.; Erduran, D. Serum adiponectin and resistin levels in patients with obsessive compulsive disorder. J. Affect. Disord. 2012, 136, 979–982. [Google Scholar] [CrossRef]
- Delen, E.; Kucukali, C.I.; Karaaslan, Z.; Yuceer, H.; Punar, S.; Hakan, M.T.; Yaylim, I.; Ozkok, E. Investigation of the effects of oxidative stress, inflammation on the pathway of tryptophan/kynurenine in OCD. Acta Neuropsychiatr. 2025, 37, e21. [Google Scholar] [CrossRef]
- Emül, H.M.; Serteser, M.; Kurt, E.; Ozbulut, O.; Guler, O.; Gecici, O. Ghrelin and leptin levels in patients with obsessive–compulsive disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2007, 31, 1270–1274. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-D.; Shi, D.-D.; Liao, B.-B.; Li, Y.; Zhang, S.; Gao, J.; Lin, L.-J.; Wang, Z. Human microbiota from drug-naive patients with obsessive-compulsive disorder drives behavioral symptoms and neuroinflammation via succinic acid in mice. Mol. Psychiatry 2024, 29, 1782–1797. [Google Scholar] [CrossRef]
- Chen, L.L.; Abbaspour, A.; Aspvall, K.; Rück, C.; Bulik, C.M.; Pascal, D. Longitudinal study of gut microbiome in obsessive–compulsive disorder. Brain Behav. 2023, 13, e3115. [Google Scholar] [CrossRef] [PubMed]
- Zengil, S.; Laloğlu, E. Evaluation of serum zonulin and occludin levels in obsessive-compulsive disorder and the effect of major depressive disorder comorbidity. Front. Psychiatry 2024, 15, 1395235. [Google Scholar] [CrossRef]
- He, M.; Zhang, H.; Luo, Z.; Duan, X.; Zhao, F.; Su, P.; Zeng, Z.; Zhou, L.; Chen, C.; Qiu, J. Causal link between gut microbiota and obsessive-compulsive disorder: A two-sample Mendelian randomization analysis. J. Affect. Disord. 2025, 379, 852–860. [Google Scholar] [CrossRef]
- Cox, L.M.; Tatematsu, B.K.; Guo, L.; LeServe, D.S.; Mayrink, J.; Oliveira, M.G.; Donnelly, D.; Fonseca, R.C.; Lemos, L.; Lanser, T.B. Gamma-delta T cells suppress microbial metabolites that activate striatal neurons and induce repetitive/compulsive behavior in mice. Brain Behav. Immun. 2024, 117, 242–254. [Google Scholar] [CrossRef]
- Bruce-Keller, A.J.; Fernandez-Kim, S.-O.; Townsend, R.L.; Kruger, C.; Carmouche, R.; Newman, S.; Salbaum, J.M.; Berthoud, H.-R. Maternal obese-type gut microbiota differentially impact cognition, anxiety and compulsive behavior in male and female offspring in mice. PLoS ONE 2017, 12, e0175577. [Google Scholar] [CrossRef]
- Miranda-Ribera, A.; Serena, G.; Cetinbas, M.; Pearce, O.; Fiorentino, M.R. Microbiota-Dependent Behavioral Abnormalities in the Zonulin Transgenic Mouse Characterized by Increased Gut Permeability. Gastroenterology 2019, 156, S-2. [Google Scholar] [CrossRef]
- Ghuge, S.; Rahman, Z.; Bhale, N.A.; Dikundwar, A.G.; Dandekar, M.P. Multistrain probiotic rescinds quinpirole-induced obsessive-compulsive disorder phenotypes by reshaping of microbiota gut-brain axis in rats. Pharmacol. Biochem. Behav. 2023, 232, 173652. [Google Scholar] [CrossRef] [PubMed]
- Sanikhani, N.S.; Modarressi, M.H.; Jafari, P.; Vousooghi, N.; Shafei, S.; Akbariqomi, M.; Heidari, R.; Lavasani, P.S.; Yazarlou, F.; Motevaseli, E. The effect of Lactobacillus casei consumption in improvement of obsessive–compulsive disorder: An animal study. Probiotics Antimicrob. Proteins 2020, 12, 1409–1419. [Google Scholar] [CrossRef]
- Kantak, P.A.; Bobrow, D.N.; Nyby, J.G. Obsessive–compulsive-like behaviors in house mice are attenuated by a probiotic (Lactobacillus rhamnosus GG). Behav. Pharmacol. 2014, 25, 71–79. [Google Scholar] [CrossRef]
- Fortunato, M.C.; Neves, J.; Pais, M.L.; Fonseca, C.; Silva, D.; Martins, J.; Castelo-Branco, M.; Fortuna, A.; Gonçalves, J. Maternal Tryptophan Supplementation Alters Offspring Gut-Brain Axis and Behavior in a Sex-Specific Manner. J. Neurochem. 2025, 169, e70161. [Google Scholar] [CrossRef]
- Deng, W.; Ke, H.; Wang, S.; Li, Z.; Li, S.; Lv, P.; Li, F.; Chen, Y. Metformin Alleviates Autistic-Like Behaviors Elicited by High-Fat Diet Consumption and Modulates the Crosstalk Between Serotonin and Gut Microbiota in Mice. Behav. Neurol. 2022, 2022, 6711160. [Google Scholar] [CrossRef]
- Scheepers, I.M.; Cryan, J.F.; Bastiaanssen, T.F.; Rea, K.; Clarke, G.; Jaspan, H.B.; Harvey, B.H.; Hemmings, S.M.; Santana, L.; van der Sluis, R. Natural compulsive-like behaviour in the deer mouse (Peromyscus maniculatus bairdii) is associated with altered gut microbiota composition. Eur. J. Neurosci. 2020, 51, 1419–1427. [Google Scholar] [CrossRef]
- Jung, T.D.; Jung, P.S.; Raveendran, L.; Farbod, Y.; Dvorkin-Gheva, A.; Sakic, B.; Surette, M.G.; Szechtman, H. Changes in gut microbiota during development of compulsive checking and locomotor sensitization induced by chronic treatment with the dopamine agonist quinpirole. Behav. Pharmacol. 2018, 29, 211–224. [Google Scholar] [CrossRef] [PubMed]
- Merchán, A.; Pérez-Fernández, C.; López, M.J.; Moreno, J.; Moreno, M.; Sánchez-Santed, F.; Flores, P. Dietary tryptophan depletion alters the faecal bacterial community structure of compulsive drinker rats in schedule-induced polydipsia. Physiol. Behav. 2021, 233, 113356. [Google Scholar] [CrossRef]
- Kim, E.; Paik, D.; Ramirez, R.N.; Biggs, D.G.; Park, Y.; Kwon, H.-K.; Choi, G.B.; Huh, J.R. Maternal gut bacteria drive intestinal inflammation in offspring with neurodevelopmental disorders by altering the chromatin landscape of CD4+ T cells. Immunity 2022, 55, 145–158.e147. [Google Scholar] [CrossRef] [PubMed]
- Lange, O.; Proczko-Stepaniak, M.; Mika, A. Short-Chain Fatty Acids-A Product of the Microbiome and Its Participation in Two-Way Communication on the Microbiome-Host Mammal Line. Curr. Obes. Rep. 2023, 12, 108–126. [Google Scholar] [CrossRef] [PubMed]
- Ahmari, S.E. Using mice to model Obsessive Compulsive Disorder: From genes to circuits. Neuroscience 2016, 321, 121–137. [Google Scholar] [CrossRef]
- Nguyen, T.P.; Cribb, L.; Ng, C.H.; Byrne, G.J.; Castle, D.; Brakoulias, V.; Blair-West, S.; Oliver, G.; Ee, C.; Dean, O.M.; et al. Dietary quality and nutrient intake in adults with obsessive–compulsive disorder. BJPsych Open 2021, 7, e218. [Google Scholar] [CrossRef]
- Lukić, I.; Getselter, D.; Ziv, O.; Oron, O.; Reuveni, E.; Koren, O.; Elliott, E. Antidepressants affect gut microbiota and Ruminococcus flavefaciens is able to abolish their effects on depressive-like behavior. Transl. Psychiatry 2019, 9, 133. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.J.; Vangay, P.; Al-Ghalith, G.A.; Hillmann, B.M.; Ward, T.L.; Shields-Cutler, R.R.; Kim, A.D.; Shmagel, A.K.; Syed, A.N.; Walter, J. Daily sampling reveals personalized diet-microbiome associations in humans. Cell Host Microbe 2019, 25, 789–802.e785. [Google Scholar] [CrossRef]
- Yang, J.; Zheng, P.; Li, Y.; Wu, J.; Tan, X.; Zhou, J.; Sun, Z.; Chen, X.; Zhang, G.; Zhang, H. Landscapes of bacterial and metabolic signatures and their interaction in major depressive disorders. Sci. Adv. 2020, 6, eaba8555. [Google Scholar] [CrossRef]
- Browne, H.P.; Forster, S.C.; Anonye, B.O.; Kumar, N.; Neville, B.A.; Stares, M.D.; Goulding, D.; Lawley, T.D. Culturing of ‘unculturable’ human microbiota reveals novel taxa and extensive sporulation. Nature 2016, 533, 543–546. [Google Scholar] [CrossRef]
- Voigt, R.M.; Forsyth, C.B.; Green, S.J.; Mutlu, E.; Engen, P.; Vitaterna, M.H.; Turek, F.W.; Keshavarzian, A. Circadian disorganization alters intestinal microbiota. PLoS ONE 2014, 9, e97500. [Google Scholar] [CrossRef]
- Minichino, A.; Preston, T.; Fanshawe, J.B.; Fusar-Poli, P.; McGuire, P.; Burnet, P.W.J.; Lennox, B.R. Psycho-Pharmacomicrobiomics: A Systematic Review and Meta-Analysis. Biol. Psychiatry 2024, 95, 611–628. [Google Scholar] [CrossRef]
- Pollock, J.; Glendinning, L.; Wisedchanwet, T.; Watson, M. The madness of microbiome: Attempting to find consensus “best practice” for 16S microbiome studies. Appl. Environ. Microbiol. 2018, 84, e02627-17. [Google Scholar] [CrossRef]
- Bastiaanssen, T.F.; Cussotto, S.; Claesson, M.J.; Clarke, G.; Dinan, T.G.; Cryan, J.F. Gutted! Unraveling the role of the microbiome in major depressive disorder. Harv. Rev. Psychiatry 2020, 28, 26–39. [Google Scholar] [CrossRef]
- Long-Smith, C.; O’Riordan, K.J.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Microbiota-gut-brain axis: New therapeutic opportunities. Annu. Rev. Pharmacol. Toxicol. 2020, 60, 477–502. [Google Scholar] [CrossRef] [PubMed]


| Study | Design/Cohort | n (OCD/HC) | Specimen | Method (16S/Shotgun/Other) | α Diversity (per Index) | β Diversity (Metric; PERMANOVA) | FDR-Sig Taxa (Direction) | Gut-Axis Biomarkers (Assay) | Clinical Link | Notes |
|---|---|---|---|---|---|---|---|---|---|---|
| He 2025 [76] | Two-sample Mendelian randomization (exposure: gut taxa GWAS; outcome: OCD GWAS) | MiBioGen exposure: 18,340 across 211 taxa; OCD outcome: FinnGen Europeans N = 199,169 | Summary GWAS | MR | — | — | Positive causal signals reported for Bacillales, Eubacterium ruminantium group, Lachnospiraceae UCG001; protective for Ruminococcaceae and Bilophila (IVW-anchored, sensitivity tested) | — | — | Data sources and significance handling described; authors note potential weakness using p < 1 × 10−5 instruments in microbiome GWAS |
| Zhang 2024 [73] | Human → mouse FMT mechanistic study; donors drug-naïve | FMT donors: 4/4; separate serum cohort: 32/32 | Mouse stool (post-FMT); human serum | 16S full-length (mouse); targeted metabolomics (human serum) | — | Mouse FMT groups show compositional separation (methods/results narrative) | — | Succinic acid (SA) increased in OCD patients’ serum (p = 0.0016; N = 32/32); SA increased in OCD-colonized mice serum and mPFC; SA correlates with OCI-R in humans (R2 = 0.1278; p = 0.0037) | SA-OCI-R positive correlation; SA associated with higher Gammaproteobacteria and Clostridia, lower Bacilli (joint analysis) | Methods list full-length 16S for mice; human serum targeted LC-MS described; donor feces used for FMT |
| Zengil 2024 [75] | Case–control; adults | 60/30 | Serum | Other (barrier proteins) | — | — | — | Zonulin and occludin markedly increased in OCD; both correlate with Y-BOCS and duration (positive) | Positive correlations (Y-BOCS/HDRS) | No microbiota profiling performed. |
| Chen 2023 [74] | Longitudinal case–control with ERP follow-up | 32/32 | Stool | Shotgun WGS | Richness/Shannon/Faith’s PD: no differences at baseline; also, ns post-ERP (exact W and p in text) | Bray–Curtis, unweighted and weighted UniFrac: ns (F ≈ 0.02; p ≥ 0.50–0.97) | None | Fiber intake lower in OCD (mean 24.22 g vs. 34.90 g; q = 0.04) | Marked symptom improvement after ERP (Y-BOCS and OCI-R reductions reported) | Sequencing noted as WGS in Methods; diet assessed via FFQ linked to Swedish DB |
| D’Addario 2022 [64] | Case–control (adults) | 64/51 | PBMCs; saliva | Other (OXTR mRNA and DNA methylation in PBMCs; OXTR methylation in saliva; saliva phyla by rRNA gene PCR) | — | — | (Saliva phyla abundances quantified; not a 16S survey) | PBMC OXTR mRNA/methylation measured; saliva OXTR methylation; saliva phyla (Actinobacteria, Firmicutes, Fusobacteria, Bacteroidetes, Proteobacteria) | No correlation with Y-BOCS found in saliva methylation analysis; meds stable ≥1 month in many OCD participants; some confounders not recorded | — |
| Domènech 2022 [11] | Case–control + within-OCD pre/post (paired for a subset) | 38/33 (controls) | Stool and oropharyngeal | 16S rRNA amplicon | Indices computed: Shannon, Chao1, Observed, Faith’s PD, Evenness-methods | PERMANOVA with multiple distance metrics (999 perms-methods) | — | — | — | Cohort make-up: baseline 54 stool and 62 oropharyngeal samples; 28 OCD had paired T0/T3; +7 single-timepoint OCD; 33 HCs |
| Turna 2020 [53] | Cross-sectional case–control (adults) | 21/22 | Stool | 16S rRNA amplicon | Inverse Simpson decreased in OCD | No between-group separation in Jaccard/Bray–Curtis/weighted and unweighted UniFrac | Lower Oscillospira (OTU13), Odoribacter (OTU92), Anaerostipes (OTU137); W-stats shown; indicates FDR p < 0.02 | CRP increased in OCD vs. HC. | CRP correlated with Y-BOCS. | — |
| Human Case–Control Studies | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study (Year) | I1. Case Definition Adequate | I2. Control Definition Appropriate | I3. Case Selection Appropriate | I4. Control Selection Appropriate | I5. Matching/Comparability | I6. Exposure Measured Validly | I7. Same Exposure Measurement for Groups | I8. Confounders Identified | I9. Strategies for Confounding | I10. Appropriate Statistics | Overall RoB | |||||||
| Zengil 2024 [75] | Unclear | Unclear | Unclear | Unclear | Unclear | Yes | Yes | Unclear | Unclear | Yes | Moderate–High | |||||||
| Zhang 2024 [73] | Yes | Unclear | Yes | Unclear | Yes | Yes | Yes | Unclear | Unclear | Unclear | High | |||||||
| Chen 2023 [74] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low | |||||||
| Domènech 2022 [11] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | Yes | Moderate | |||||||
| D’Addario 2022 [64] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Partially | No | Yes | Moderate | |||||||
| Turna 2020 [53] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low | |||||||
| Human Mendelian randomizationstudy | ||||||||||||||||||
| Study | Assessment Tool | Instrument Relevance | Independence from Confounders | Exclusion Restriction/Pleiotropy | Heterogeneity (Cochran’s Q) | Population Stratification/Sample Overlap | Multiple Testing Across Taxa | Sensitivity Analyses | Overall Risk of Bias | |||||||||
| He 2025 [76] | MR-specific | Low | Low | Low | Low | Low | High | Low | High | |||||||||
| Study | Model/Strain/Sex | Gut Manipulation | Microbiome Profiling | Behavior (Direction) | Gut-Axis Biomarkers | α/β Diversity (If Reported) | Notes |
|---|---|---|---|---|---|---|---|
| Fortunato 2025 [83] | Maternal tryptophan (Trp)-enriched diet (1.5% vs. 0.7% control) altered offspring gut microbiome and produced sex-specific behaviors: ↑ repetitive behavior (marble burying) in males; ↑ anxiety-like behavior in females; associative gut–brain axis evidence (no FMT/ABX). | None | 16S rRNA amplicon | — | LEfSe families (sex-specific): Female TRP+ ↑ Tannerellaceae (0.049 ± 0.008 vs. 0.010 ± 0.003; p < 0.0001), ↑ Muribaculaceae (0.154 ± 0.030 vs. 0.092 ± 0.031; p = 0.0243), ↑ Eubacterium (0.009 ± 0.004 vs. 1.85 × 10−4 ± 1.61 × 10−4; p = 0.0075); ↓ Marinifilaceae (4.93 × 10−4 ± 0.001 vs. 0.024 ± 0.006; p = 0.0071), ↓ Saccharimonadaceae (0.002 ± 4.52 × 10−4 vs. 0.041 ± 0.028; p = 0.039), ↓ Oscillospiraceae (0.025 ± 0.007 vs. 0.063 ± 0.002; p = 0.0014). TRP+ sex-dimorphism: Bacteroidaceae higher in males than females (0.144 ± 0.050 vs. 0.062 ± 0.026; p = 0.0409); Monoglobaceae higher in females (p = 0.0170). | β: Females: Shannon CTR 4.84 ± 0.12 vs. TRP+ 4.31 ± 0.06 (p = 0.0358); richness (Chao) ns. Sex effect on Shannon in CTR lost in TRP+. | — |
| Cox 2024 [77] | γδ T-cell–deficient (TCRδ−/−) mice display microbiota-dependent repetitive/compulsive (marble-burying) behavior; microbiota transfer induces/abolishes phenotype; direct gut microbiome ↔ compulsive-like link. | FMT; Antibiotic | 16S rRNA amplicon | — | Cecum: 3-phenylpropionate (3-PP) ↑ (~50×) in γδ−/−; 3-(4-hydroxyphenyl) propionate ↑ (~30×) in WT; Serum/CSF: HIP and p-cresol sulfate (pCS) ↑ in γδ−/−; HIP ↑ ~8× in CSF; pCS ↑ ~4× (Welch’s t-tests, p < 0.05). | α: Alpha: no differences between groups after colonization. | β: Beta: unweighted UniFrac; ADONIS significant by microbiota treatment, not genotype. | — |
| Wilson 2024 [26] | SAPAP3 knockout (validated compulsive/OCD-like model); gut function assessed plus baseline stool microbiome (16S) in SH mice; WT vs. KO showed no dysbiosis (species richness, alpha and beta diversity equivalent). | None (environmental housing; not microbiome-targeted) | 16S rRNA amplicon (baseline SH subset) | Fecal water content ↑ in KO vs. WT (estimate 9.67, SE 3.13, t = 3.09, p = 0.005); fecal output ↑ in KO vs. WT (p = 0.045); gut permeability (FITC-dextran) trend ↑ in KO (p = 0.065); gut transit time slower in EE vs. SH (coef −0.66, exp(coef) = 0.52, p = 0.033). | Alpha diversity: no KO vs. WT difference. Beta diversity: no KO vs. WT difference. | — | |
| Zhang 2024 [73] | Human stool from drug-naïve OCD vs. healthy; FMT into germ-free mice altered compulsive-like grooming/locomotion | FMT | Shotgun | Mouse FMT (OCD donor): ↑ grooming time (p < 0.01), ↑ locomotor activity (p < 0.05) vs. HC FMT; partial rescue with SCFA supplementation | Pathways (HUMAnN3, FDR < 0.05): ↓ butyrate biosynthesis, ↓ tryptophan metabolism, ↑ LPS biosynthesis; SCFA (stool acetate, propionate, butyrate) ↓ in OCD (p < 0.05, GC–MS) | α: Shannon: ↓ in OCD vs. HC (p = 0.029); Simpson: ns; Chao1: ↓ (p = 0.022) | β: Bray–Curtis: PERMANOVA R2 = 0.034, p = 0.001; Weighted UniFrac: PERMANOVA R2 = 0.027, p = 0.001 | Metagenomics + FMT causality; extensive taxa, pathway, SCFA and behavior data |
| D’Addario 2022 [64] | Human: DSM-5 OCD vs. HC with OXTR epigenetics and oral (saliva) phyla qPCR (not gut); Animal: social isolation rats with fecal phyla qPCR + SCFAs and stereotyped behaviors | None | Other | Animal behavior (ISO vs. CTRL): Open field center time ↓ (t = 4.31, p < 0.001); wall rearing ↑ (t = 9.56, p < 0.001); hole-board head dippings ↑ (t = 4.69, p < 0.001). Rat PFC Oxtr mRNA ↓ (0.55 ± 0.10 vs. 1.10 ± 0.20; p = 0.007) | SCFAs (rat feces, LC–MS): Total SCFAs ↓ at T1 (CTRL 38.92 ± 7.42 vs. ISO 20.83 ± 3.59; p = 0.049); Butyrate ↓ at T1 (11.65 ± 1.54 vs. 6.69 ± 1.35; p = 0.049). Human: OXTR mRNA (PBMC) ↓ (0.35 ± 0.05 vs. 1.10 ± 0.10; p < 0.0001); OXTR exon 3 methylation ↑ (PBMC avg CpGs 5.21 ± 0.32 vs. 3.74 ± 0.15; p = 0.006; saliva avg 4.43 ± 0.31 vs. 2.95 ± 0.27; p = 0.021) | — | Human microbiome = oral (excluded for human gut analyses). Animal part qualifies (associative; no ABX/FMT). Microbiome measured by phylum-qPCR, not 16S/shotgun; no α/β stats. Correlations: PFC Oxtr vs. behavior (e.g., center time r = 0.554, p = 0.035) |
| Deng 2022 [84] | HFD induces ↑ repetitive behavior (marble burying, self-grooming) vs. ND; metformin reverses; gut 16S profiles and gut 5-HT pathway (Trp, 5-HT, 5-HIAA; TPH1/SERT) measured; associative gut–brain axis mechanism proposed (no FMT/ABX). | None | 16S rRNA amplicon | — | HFD vs. ND: ↑ Lactococcus, Trichococcus, Romboutsia, Faecalibaculum; HFD + Met: ↑ Intestinimonas, Lactobacillus reuteri (LDA ≥ 4); HFD/HFD + Met: Melainabacteria ↓; HFD + Met vs. HFD: Tenericutes ↑; F/B ratio ↑ in HFD, metformin ↑ Bacteroidetes. | β: Richness (Observed_OTUs) ↓ in HFD vs. ND (significant); Simpson ns. | — |
| Merchán 2021 [87] | Schedule-induced polydipsia (SIP) model of compulsivity; High-Drinkers (HD) vs. Low-Drinkers (LD) ± chronic tryptophan-free diet; fecal microbiota profiled; compulsive licking increased only in HD under TRP-depletion. | None (dietary TRP manipulation) | 16S (PCR-DGGE fingerprinting) | Plasma 5-HT ↓with TRP depletion (treatment effect F1,24 = 10.754, p < 0.01); BDNF: no group/treatment effects; 5-HT ↔ BDNF: positive correlation r = +0.514 (p < 0.01; N = 27). | Bray–Curtis cluster analysis: HD T- animals form a distinct cluster; LD T+/LD T- cluster together; HD T+ separate sub-cluster. PL functional organization: Fo ~66% (HD T-, 62% (HD T+ and LD T+), 56% (LD T-). | — | Behavior (compulsive licking): TRP depletion increased total licks (only in HD) (group × treatment × session F5,120 = 2.529, p < 0.05); HD T negative more than HD T positive from session three (p < 0.01). |
| Sanikhani 2020 [81] | Quinpirole-induced OCD-like behaviors in Wistar rats; probiotic L. casei Shirota (1 × 109 CFU/g, daily ×4 wks) post-induction; open-field compulsive-checking metrics reported | Probiotic | Other (no microbiome profiling) | Open-field: exploratory behavior improved with L. casei (p = 0.018) and L. casei + fluoxetine (p = 0.004); group difference in key-zone time profiles p = 0.03; direction = decrease in OCD-like preference for corner “home base” zones. | — | — | No gut microbiome sequencing; probiotic is gut targeted. Quinpirole 0.5 mg/kg i.p. twice weekly ×5 wks; five groups × 6 rats. |
| Scheepers 2020 [85] | Natural compulsive-like large nest building (LNB) vs. normal nest building (NNB) in deer mice; gut microbiome profiled | None | 16S | — | — | β: Aitchison (genus-level CLR): PERMANOVA p < 0.05; PC1 = 13.77%; PC2 = 10.91% | Duplicate with: Single timepoint fecal sampling during first hour of dark cycle; 86 genera detected; minimum read count ≥10,000; median read depth 34,686 reads/sample; n per group includes three males and eight females |
| Zhang 2020 [19] | DSS-induced colitis in male C57BL/6 mice; EPM and obsessive–compulsive-like (marble burying) behaviors measured | None | 16S | EPM: % open arm entries ↑ vs. DSS (TRF, IER; p < 0.01); MBT: marbles buried ↓ vs. DSS (TRF, IER; p < 0.05) | LPS (serum) ↓ (TRF, IER; p < 0.01; ELISA Xinle Biotech); TNF-α (colon) ↓ (TRF, IER; p < 0.01; ELISA Xinle Biotech); IL-1β (colon) ↓ (TRF, IER; p < 0.01; ELISA Xinle Biotech); MDA (colon) ↓ (TRF p < 0.01, IER p < 0.05; Nanjing Jiancheng kit); TNF-α, IL-1β, IL-6 (brain) ↓ (TRF, IER; p < 0.01; RT-PCR); MDA, GSSG ↓, GSH ↑ (brain; p < 0.01; commercial kits); SCFAs (acetate, butyrate, isobutyrate) ↑ (TRF, IER; p < 0.05; GC Shimadzu) | — | Colitis model with OCD-like component; ADF arm worsened survival/colitis and excluded from behavioral/molecular follow-up; only TRF and IER analyzed for microbiome and behavior outcomes |
| Miranda-Ribera 2019 [79] | Zonulin transgenic mice (Ztm) with increased gut permeability; females show pronounced repetitive behavior (marble burying); males show increased anxiety-like behavior; gut microbiota depletion with antibiotics rescues behavioral phenotype; direct microbiota–behavior link (animal). | Antibiotic | Not stated (stool microbiota composition assessed) | Antibiotic depletion: decreases zonulin and inflammatory markers in brain, normalizes BBB tight junctions, and rescues behavioral phenotype (no statistics provided). | Small-intestinal permeability ↑; BBB tight-junction genes dysregulated; brain inflammatory markers ↑ (qualitative per abstract). | — | Ztm show dysbiosis; behavioral tests included marble burying (repetitive/compulsive-like) and elevated zero maze (anxiety-like). Outcomes and microbiome details are qualitative because this is a conference abstract. |
| Jung 2018 [86] | Quinpirole-induced compulsive checking rat model; serial fecal 16S at injections 1, 5, 9 | None | 16S | At injection 9: all four compulsive-checking criteria differed QNP vs. saline (t-tests p ≤ 0.003); “time to next checking bout” shorter in QNP (t = 2.028, p = 0.037, 1-tail). Locomotion: distance ↑, 2SDE ↓, path stereotypy ↑ (all p < 0.001 at injection 9). | — | — | Preprint; Raw reads rarefied to 43,345/sample; feces collected ~55 min post-injection; no α/β diversity stats or PERMANOVA reported. |
| Bruce-Keller 2017 [78] | Maternal gut microbiota manipulated (antibiotic depletion → FMT from HFD vs. CD donors); male offspring from HFD-microbiota dams show ↑ stereotypical/compulsive marble burying; direct gut microbiome ↔ compulsive-like link (animal). | FMT; Antibiotic | 16S rRNA amplicon | — | Pre-pregnancy dams: PERMANOVA (weighted UniFrac) F = 5.2, p = 0.009; Offspring females: PERMANOVA F = 2.51, p = 0.003; Offspring males: PERMANOVA F = 2.05, p = 0.034. | — | Male offspring from HFD-microbiota dams: marble burying increased (p < 0.01); Open field inner-zone time/entries ↓ (p < 0.01–0.001); sucrose preference ↑ (ad lib); fear-conditioning freezing ↓ (tone test)—females largely unaffected. |
| Kantak 2014 [82] | RU 24969–induced OCD-like behaviors; probiotic pretreatment tested | Probiotic | — | L. rhamnosus GG (ATCC 53103) 1 × 109 CFU/day oral gavage for 2 or 4 wks; male BALB/cJ mice; RU 24969 10 mg/kg i.p.; behaviors scored 60–90 min post-injection; no microbiome data | — | — | — |
| Study | Sequence Randomization | Baseline Characteristics | Allocation Concealment | Random Housing | Blinding of Investigators | Blinding of Outcome Assessment | Incomplete Outcome Data | Selective Outcome Reporting | Other Sources of Bias | Overall Risk of Bias |
|---|---|---|---|---|---|---|---|---|---|---|
| Furtunato 2025 [83] | Unclear | Unclear | Low risk | Low risk | Low risk | High risk | Low risk | Low risk | High risk | High risk |
| Wilson 2024 [26] | Unclear | Low risk | Unclear | Unclear | Low risk | High risk | Low risk | Low risk | Low risk | High |
| Cox 2024 [77] | Low risk | Low risk | Unclear | Unclear | Unclear | Low risk | Low risk | Low risk | Low risk | Moderate risk |
| Ghuge 2023 [80] | Unclear | Low risk | Unclear | Unclear | Unclear | High risk | Low risk | Low risk | Low risk | High risk |
| Deng 2022 [84] | Unclear | Low risk | Unclear | Unclear | High risk | Low risk | Low risk | Low risk | Low risk | High risk |
| D’Addario 2022 [64] | Unclear | Low risk | Unclear | Low risk | Low risk | High risk | Low risk | Low risk | Low risk | High |
| Merchán 2021 [87] | Unclear | Low risk | Unclear | Low risk | Low risk | High risk | Low risk | Low risk | Unclear | High |
| Sanikhani 2020 [81] | Unclear | Low risk | Unclear | Unclear | Unclear | High risk | Low risk | Low risk | Low risk | High |
| Scheepers 2020 [85] | Unclear | Unclear | Unclear | Low risk | Low risk | High risk | Low risk | Low risk | High risk | High |
| Zhang 2020 [19] | Unclear | Low risk | Unclear | Low risk | Low risk | High risk | Low risk | Low risk | Low risk | High |
| Miranda-Ribera 2019 [79] | Unclear | Low risk | Unclear | Unclear | Low risk | Low risk | High risk | Low risk | Low risk | High |
| Jung 2018 [86] | Unclear | Low risk | Unclear | Unclear | Low risk | Low risk | Low risk | Low risk | Low risk | Moderate risk |
| Bruce-Keller 2017 [78] | Low risk | Unclear | Unclear | Low risk | Unclear | High risk | Unclear | Low risk | Low risk | High risk |
| Kantak 2014 [82] | Unclear | Low risk | Unclear | Low risk | Unclear | High risk | Low risk | Low risk | Low risk | High |
| Axis | Human Observation | Transfer (Human → Mouse FMT) | Depletion/Transfer (ABX/FMT in Models) | Rescue (Metabolite/Probiotic/Diet) | Behavioral Direction |
|---|---|---|---|---|---|
| SCFAs | ↓ stool SCFAs; ↓ butyrate-producer species; ↓ butyrate pathways [73] | OCD-FMT ↑ grooming/locomotion [73] | — | SCFAs partially rescue; TRF/IER ↑ SCFAs [19,73] | Toward control with SCFA restoration |
| Endotoxin/Barrier | ↑ serum zonulin/occludin (severity-linked); ↑ LPS-biosynthesis functions [19,73,75] | — | ABX rescue in zonulin-Tg [79] | TRF/IER ↓ serum LPS; improved histology; probiotic histology gains [19,81] | Reduced repetitive and anxiety-like behavior |
| Monoaminergic | ↓ tryptophan-metabolism pathways [73] | — | ABX/FMT causality; metabolites (HIP, 3-PP) induce behavior via D1R [77] | Metformin improves 5-HT-pathway markers and behavior; maternal Trp diet shifts biomarkers and behavior; probiotic with gene-expression changes [81,83,84] | Behavior modified with pathway-targeted interventions |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eghdami, S.; Saeidi, M.; Gunturu, S.; Boroon, M.; Shalbafan, M. Gut Microbiota and Obsessive–Compulsive Disorder: A Systematic Review of Mechanistic Links, Evidence from Human and Preclinical Studies, and Therapeutic Prospects. Life 2025, 15, 1585. https://doi.org/10.3390/life15101585
Eghdami S, Saeidi M, Gunturu S, Boroon M, Shalbafan M. Gut Microbiota and Obsessive–Compulsive Disorder: A Systematic Review of Mechanistic Links, Evidence from Human and Preclinical Studies, and Therapeutic Prospects. Life. 2025; 15(10):1585. https://doi.org/10.3390/life15101585
Chicago/Turabian StyleEghdami, Shayan, Mahdieh Saeidi, Sasidhar Gunturu, Mahsa Boroon, and Mohammadreza Shalbafan. 2025. "Gut Microbiota and Obsessive–Compulsive Disorder: A Systematic Review of Mechanistic Links, Evidence from Human and Preclinical Studies, and Therapeutic Prospects" Life 15, no. 10: 1585. https://doi.org/10.3390/life15101585
APA StyleEghdami, S., Saeidi, M., Gunturu, S., Boroon, M., & Shalbafan, M. (2025). Gut Microbiota and Obsessive–Compulsive Disorder: A Systematic Review of Mechanistic Links, Evidence from Human and Preclinical Studies, and Therapeutic Prospects. Life, 15(10), 1585. https://doi.org/10.3390/life15101585








