Shared Immunopathogenic Mechanisms in Chronic Spontaneous Urticaria, Vitiligo, and Hashimoto’s Thyroiditis: The Role of Oxidative Stress and Vitamin D
Abstract
1. Introduction
2. Materials and Methods
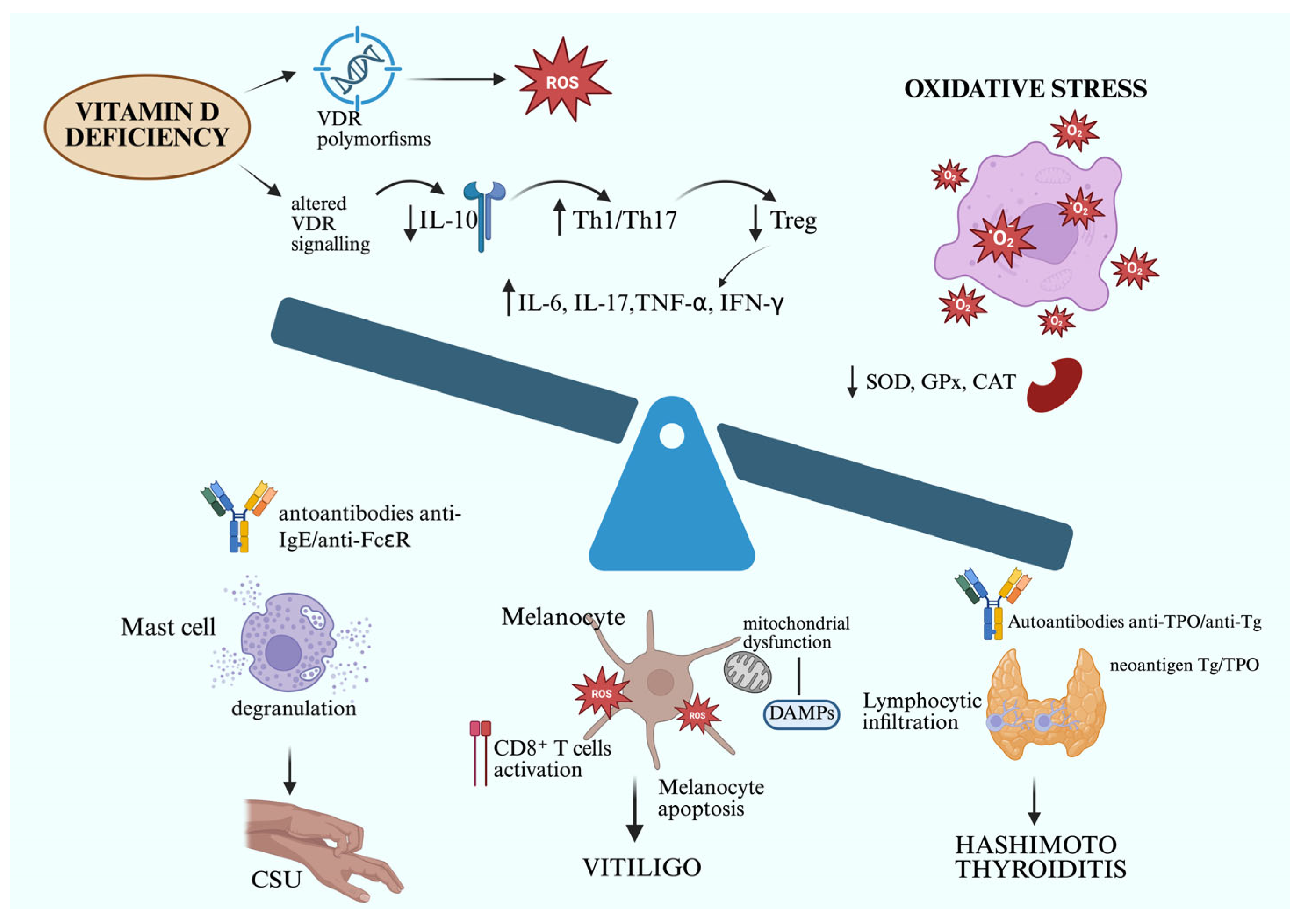
3. Discussion
3.1. Low Level of Vitamin D and Its Role in the Pathogenesis of Chronic Urticaria, Vitiligo, and Autoimmune Thyroiditis
3.1.1. Correlation Between CSU and Vitamin D Serum Levels
3.1.2. Correlation Between Vitiligo and Vitamin D Serum Levels
3.1.3. Correlation Between Autoimmune Thyroiditis and Vitamin D Serum Levels
3.1.4. Bidirectional Relationship Between Autoimmune Thyroiditis and the Main Autoimmune Skin Diseases
3.2. Role of Oxidative Stress in Chronic Spontaneous Urticaria, Hashimoto’s Thyroiditis, and Vitiligo
3.2.1. Correlation Between Chronic Spontaneous Urticaria and Oxidative/Nitrosative Stress
3.2.2. Hashimoto’s Thyroiditis and Oxidative Stress
3.2.3. Correlation Between Vitiligo and Oxidative Stress
Highlights
- -
- Vitiligo, HT, and CSU share common pathogenic mechanisms, including redox imbalance, autoimmunity, and genetic susceptibility.
- -
- OS and NS play a central role across all three conditions, contributing to tissue damage, autoantibody production, and immune system overactivation.
- -
- Vitamin D deficiency is highly prevalent in patients with vitiligo, HT, and CSU, and is associated with increased disease activity and immune dysregulation.
- -
- Vitamin D supplementation improves immune balance, reducing proinflammatory cytokines and enhancing Treg function, supporting its therapeutic role.
- -
- Cross-screening for autoimmune comorbidities among patients with vitiligo, HT, or CSU is recommended to enable earlier diagnosis and patient-tailored management.
3.3. Study Limitations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bergqvist, C.; Ezzedine, K. Vitiligo: A Review. Dermatology 2020, 236, 571–592. [Google Scholar] [CrossRef] [PubMed]
- McLeod, D.S.; Cooper, D.S. The incidence and prevalence of thyroid autoimmunity. Endocrine 2012, 42, 252–265. [Google Scholar] [CrossRef] [PubMed]
- Colucci, R.; Lotti, F.; Dragoni, F.; Arunachalam, M.; Lotti, T.; Benvenga, S.; Moretti, S. High prevalence of circulating autoantibodies against thyroid hormones in vitiligo and correlation with clinical and historical parameters of patients. Br. J. Dermatol. 2014, 171, 786–798. [Google Scholar] [CrossRef] [PubMed]
- Colucci, R.; Lotti, F.; Arunachalam, M.; Lotti, T.; Dragoni, F.; Benvenga, S.; Moretti, S. Correlation of Serum Thyroid Hormones Autoantibodies with Self-Reported Exposure to Thyroid Disruptors in a Group of Nonsegmental Vitiligo Patients. Arch. Environ. Contam. Toxicol. 2015, 69, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Najafipour, M.; Zareizadeh, M.; Najafipour, F. Relationship between Chronic urticaria and autoimmune thyroid disease. J. Adv. Pharm. Technol. Res. 2018, 9, 158–161. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ralli, M.; Angeletti, D.; Fiore, M.; D’Aguanno, V.; Lambiase, A.; Artico, M.; de Vincentiis, M.; Greco, A. Hashimoto’s thyroiditis: An update on pathogenic mechanisms, diagnostic protocols, therapeutic strategies, and potential malignant transformation. Autoimmun. Rev. 2020, 19, 102649. [Google Scholar] [CrossRef] [PubMed]
- Kolkhir, P.; Borzova, E.; Grattan, C.; Asero, R.; Pogorelov, D.; Maurer, M. Autoimmune comorbidity in chronic spontaneous urticaria: A systematic review. Autoimmun. Rev. 2017, 16, 1196–1208. [Google Scholar] [CrossRef] [PubMed]
- Czarnecka-Operacz, M.; Sadowska-Przytocka, A.; Jenerowicz, D.; Szeliga, A.; Adamski, Z.; Łącka, K. Thyroid function and thyroid autoantibodies in patients with chronic spontaneous urticaria. Postep. Dermatol. Alergol. 2017, 34, 566–572. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Białczyk, A.; Wełniak, A.; Kamińska, B.; Czajkowski, R. Oxidative Stress and Potential Antioxidant Therapies in Vitiligo: A Narrative Review. Mol. Diagn. Ther. 2023, 27, 723–739. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ruggeri, R.M.; CampennÌ, A.; Giuffrida, G.; Casciaro, M.; Barbalace, M.C.; Hrelia, S.; Trimarchi, F.; CannavÒ, S.; Gangemi, S. Oxidative stress as a key feature of autoimmune thyroiditis: An update. Minerva Endocrinol. 2020, 45, 326–344. [Google Scholar] [CrossRef] [PubMed]
- Nettis, E.; Distaso, M.; Saitta, S.; Casciaro, M.; Cristani, M.; Saija, A.; Vacca, A.; Gangemi, S.; Minciullo, P.L. Involvement of new oxidative stress markers in chronic spontaneous urticaria. Postep. Dermatol. Alergol. 2017, 34, 448–452. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, M.; Murphy, E.; Amerson, E.H. Rethinking screening for thyroid autoimmunity in vitiligo. J. Am. Acad. Dermatol. 2016, 75, 1278–1280. [Google Scholar] [CrossRef] [PubMed]
- Quirk, S.K.; Rainwater, E.; Shure, A.K.; Agrawal, D.K. Vitamin D in atopic dermatitis, chronic urticaria and allergic contact dermatitis. Expert Rev. Clin. Immunol. 2016, 12, 839–847. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Murdaca, G.; Tonacci, A.; Negrini, S.; Greco, M.; Borro, M.; Puppo, F.; Gangemi, S. Emerging role of vitamin D in autoimmune diseases: An update on evidence and therapeutic implications. Autoimmun. Rev. 2019, 18, 102350. [Google Scholar] [CrossRef] [PubMed]
- Tuchinda, P.; Kulthanan, K.; Chularojanamontri, L.; Arunkajohnsak, S.; Sriussadaporn, S. Relationship between vitamin D and chronic spontaneous urticaria: A systematic review. Clin. Transl. Allergy 2018, 4, 51. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cheng, H.M.; Kim, S.; Park, G.-H.; Chang, S.E.; Bang, S.; Won, C.H.; Lee, M.W.; Choi, J.H.; Moon, K.C. Low vitamin D levels are associated with atopic dermatitis, but not allergic rhinitis, asthma, or IgE sensitization, in the adult Korean population. J. Allergy Clin. Immunol. 2014, 133, 1048–1055. [Google Scholar] [CrossRef] [PubMed]
- Ariaee, N.; Zarei, S.; Mohamadi, M.; Jabbari, F. Amelioration of patients with chronic spontaneous urticaria in treatment with vitamin D supplement. Clin. Mol. Allergy 2017, 15, 22. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sîrbe, C.; Rednic, S.; Grama, A.; Pop, T.L. An Update on the Effects of Vitamin D on the Immune System and Autoimmune Diseases. Int. J. Mol. Sci. 2022, 23, 9784. [Google Scholar] [CrossRef]
- Daryabor, G.; Gholijani, N.; Kahmini, F.R. A review of the critical role of vitamin D axis on the immune system. Exp. Mol. Pathol. 2023, 132–133, 104866. [Google Scholar] [CrossRef] [PubMed]
- Heine, G.; Hoefer, N.; Franke, A.; Nothling, U.; Schumann, R.R.; Hamann, L.; Worm, M. Association of vitamin D receptor gene polymorphisms with severe atopic dermatitis in adults. Br. J. Dermatol. 2013, 168, 855–858. [Google Scholar] [CrossRef]
- Bergler-Czop, B.; Brzezinska-Wcislo, L. Serum vitamin D level-the effect on the clinical course of psoriasis. Postep. Dermatol. Alergol. 2016, 33, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.K.; Ha, J.M.; Lee, Y.H.; Lee, Y.; Seo, Y.J.; Kim, C.D.; Lee, J.-H.; Im, M. Comparison of vitamin D levels in patients with and without acne: A case-control study combined with a randomized controlled trial. PLoS ONE 2016, 11, 0161162. [Google Scholar] [CrossRef]
- Powe, C.E.; Evans, M.K.; Wenger, J.; Zonderman, A.B.; Berg, A.H.; Nalls, M.; Tamez, H.; Zhang, D.; Bhan, I.; Karumanchi, S.A.; et al. Vitamin D-binding protein and vitamin D status of black Americans and white Americans. N. Engl. J. Med. 2013, 369, 1991–2000. [Google Scholar] [CrossRef]
- Zhang, J.; Chalmers, M.J.; Stayrook, K.R.; Burris, L.L.; Wang, Y.; Busby, S.A.; Pascal, B.D.; Garcia-Ordonez, R.D.; Bruning, J.B.; A Istrate, M.; et al. DNA binding alters coactivator interaction surfaces of the intact VDR-RXR complex. Nat. Struct. Mol. Biol. 2011, 18, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Bizzaro, G.; Antico, A.; Fortunato, A.; Bizzaro, N. Vitamin D and autoimmune diseases: Is vitamin D receptor (VDR) polymorphism the culprit? Isr. Med. Assoc. J. 2017, 19, 438–443. [Google Scholar]
- Nasiri-Kalmarzi, R.; Abdi, M.; Hosseini, J.; Babaei, E.; Mokarizadeh, A.; Vahabzadeh, Z. Evaluation of 1,25-dihydroxyvitamin D3 pathway in patients with chronic urticaria. QJM 2018, 111, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Woo, Y.R.; Jung, K.E.; Koo, D.W.; Lee, J.S. Vitamin D as a marker for disease severity in chronic urticaria and its possible role in pathogenesis. Ann. Dermatol. 2015, 27, 423–430. [Google Scholar] [CrossRef]
- Chandrashekar, L.; Rajappa, M.; Munisamy, M.; Ananthanarayanan, P.H.; Thappa, D.M.; Arumugam, B. 25-Hydroxy vitamin D levels in chronic urticaria and its correlation with disease severity from a tertiary care centre in South India. Clin. Chem. Lab. Med. 2014, 52, e115–e118. [Google Scholar] [CrossRef]
- Goetz, D.W. Vitamin D treatment of idiopathic itch, rash, and urticaria/angioedema. Allergy Asthma Proc. 2010, 31, 158–160. [Google Scholar] [CrossRef] [PubMed]
- Sindher, S.B.; Jariwala, S.; Gilbert, J.; Rosenstreich, D. Resolution of chronic urticaria coincident with vitamin D supplementation. Ann. Allergy Asthma Immunol. 2012, 109, 359–360. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.H.; Kan, W.C.; Jhen, R.N.; Chang, Y.M.; Kao, J.L.; Lai, H.Y.; Liou, H.H.; Shiao, C.C. Secondary hyperparathyroidism in chronic kidney disease: A narrative review focus on therapeutic strategy. Clin. Med. 2024, 24, 100238. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, X.; Li, X.; Shen, Y.; Wang, X. The association between serum vitamin D levels and urticaria: A meta-analysis of observational studies. G. Ital. Dermatol. Venereol. 2018, 153, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Z.; Wang, M.; Ding, Y.; Gao, F.; Feng, Y.Y.; Yakeya, B.; Wang, P.; Wu, X.J.; Hu, F.X.; Xian, J.; et al. Vitamin D receptor gene polymorphism, serum 25-hydroxyvitamin D levels, and risk of vitiligo: A meta-analysis. Medicine 2018, 97, e11506. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, K.; Shi, Q.; Yang, L.; Li, X.; Liu, L.; Wang, L.; Li, Q.; Wang, G.; Li, C.Y.; Gao, T.W. The association of vitamin D receptor gene polymorphisms and serum 25-hydroxyvitamin D levels with generalized vitiligo. Br. J. Dermatol. 2012, 167, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Upala, S.; Sanguankeo, A. Low 25-hydroxyvitamin D levels are associated with vitiligo: A systematic review and meta-analysis. Photodermatol. Photoimmunol. Photomed. 2016, 32, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Karagüzel, G.; Sakarya, N.P.; Bahadır, S.; Yaman, S.; Ökten, A. Vitamin D status and the effects of oral vitamin D treatment in children with vitiligo: A prospective study. Clin. Nutr. ESPEN 2016, 15, 28–31. [Google Scholar] [CrossRef] [PubMed]
- Sobeih, S.; Mashaly, H.M.; Gawdat, H.; Amr, K.; Hamid, M.F.; Shaalan, E. Evaluation of the correlation between serum levels of vitamin D and vitamin D receptor gene polymorphisms in an Egyptian population. Int. J. Dermatol. 2016, 55, 1329–1335. [Google Scholar] [CrossRef] [PubMed]
- Saleh, H.M.; Abdel Fattah, N.S.; Hamza, H.T. Evaluation of serum 25-hydroxyvitamin D levels in vitiligo patients with and without autoimmune diseases. Photodermatol. Photoimmunol. Photomed. 2013, 29, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Ustun, I.; Seraslan, G.; Gokce, C.; Motor, S.; Can, Y.; Ugur Inan, M.; Yilmaz, N. Investigation of vitamin D levels in patients with vitiligo vulgaris. Acta Dermatovenerol. Croat. 2014, 22, 110–113. [Google Scholar] [PubMed]
- Mahmmod, Z.; Ismael, D.K. Vitamin D Deficiency in Patients with Vitiligo: A Cross-Sectional Study From Basrah, Iraq. Cureus 2021, 13, e20733. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, X.; Yao, Z.; Wang, Y.; Chai, L.; Zhou, X. Vitamin D analogs combined with different types of phototherapy in the treatment of vitiligo: A systematic review of randomized trials and within-patient studies. Int. Immunopharmacol. 2022, 109, 108789. [Google Scholar] [CrossRef] [PubMed]
- AlGhamdi, K.; Kumar, A.; Moussa, N. The role of vitamin Din melanogenesis with an emphasis on vitiligo. Indian J. Dermatol. Venereol. Leprol. 2013, 79, 750–758. [Google Scholar] [CrossRef] [PubMed]
- George, C.A.; Chhabra, N.; Patel, S. Vitamin D and Interleukin-17: Are These Serum Biomarkers Useful in Non-Segmental Vitiligo? A Case Control Study from Central India. Indian J. Dermatol. 2023, 68, 725. [Google Scholar] [CrossRef] [PubMed]
- Vieira, I.H.; Rodrigues, D.; Paiva, I. Vitamin D and Autoimmune Thyroid Disease-Cause, Consequence, or a Vicious Cycle? Nutrients 2020, 12, 2791. [Google Scholar] [CrossRef]
- Barchetta, I.; Baroni, M.G.; Leonetti, F.; De Bernardinis, M.; Bertoccini, L.; Fontana, M.; Mazzei, E.; Fraioli, A.; Cavallo, M.G. TSH levels are associated with vitamin D status and seasonality in an adult population of euthyroid adults. Clin. Exp. Med. 2015, 15, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Mackawy, A.M.; Al-Ayed, B.M.; Al-Rashidi, B.M. Vitamin d deficiency and its association with thyroid disease. Int. J. Health Sci. 2013, 7, 267–275. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rogiers, V.; Benfenati, E.; Bernauer, U.; Bodin, L.; Carmichael, P.; Chaudhry, Q.; Coenraads, P.J.; Cronin, M.T.D.; Dent, M.; Dusinska, M.; et al. The way forward for assessing the human health safety of cosmetics in the EU—Workshop proceedings. Toxicology 2020, 436, 152421. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Song, E.; Oh, H.-S.; Park, S.; Kwon, H.; Jeon, M.J.; Shong, Y.K.; Kim, T.-Y.; Kim, W.G.; Kim, W.B. Vitamin D deficiency affects thyroid autoimmunity and dysfunction in iodine-replete area: Korea national health and nutrition examination survey. Endocrine 2017, 58, 332–339. [Google Scholar] [CrossRef]
- Hamdaoui, L.; Oudadesse, H.; Lefeuvre, B.; Mahmoud, A.; Naifer, M.; Badraoui, R.; Ayadi, F.; Rebai, T. Sub-chronic exposure to Kalach 360 SL, Glyphosate-based Herbicide, induced bone rarefaction in female Wistar rats. Toxicology 2020, 436, 152412. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, N.C.; Karbek, B.; Ucan, B.; Sahin, M.; Cakal, E.; Ozbek, M.; Delibasi, T. The association between severity of vitamin D deficiency and Hashimoto’s thyroiditis. Endocr. Pract. 2013, 19, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Zhang, W.; Ma, C.; Zhao, Y.; Xiong, R.; Wang, H.; Chen, W.; Zheng, S.G. Immunomodulatory Function of Vitamin D and Its Role in Autoimmune Thyroid Disease. Front. Immunol. 2021, 12, 574967. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, Q.; He, X.; Chen, W.; Jiu, J.; Gao, C.; Gao, T. Vitamin D3 attenuates autoimmune thyroiditis by regulating Th17/Treg cell differentiation via YAP/JAK1/STAT1 axis. Immunol. Lett. 2024, 269, 106890. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Shan, S.; Li, F.; Yun, P. Effects of vitamin D supplementation on autoantibodies and thyroid function in patients with Hashimoto’s thyroiditis: A systematic review and meta-analysis. Medicine 2023, 102, e36759. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tywanek, E.; Michalak, A.; Świrska, J.; Zwolak, A. Autoimmunity, New Potential Biomarkers and the Thyroid Gland-The Perspective of Hashimoto’s Thyroiditis and Its Treatment. Int. J. Mol. Sci. 2024, 25, 4703. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Botelho, I.M.B.; Moura Neto, A.; Silva, C.A.; Tambascia, M.A.; Alegre, S.M.; Zantut-Wittmann, D.E. Vitamin D in Hashimoto’s thyroiditis and its relationship with thyroid function and inflammatory status. Endocr. J. 2018, 65, 1029–1037. [Google Scholar] [CrossRef] [PubMed]
- Nodehi, M.; Ajami, A.; Izad, M.; Asgarian Omran, H.; Chahardoli, R.; Amouzegar, A.; Yekaninejad, S.; Hemmatabadi, M.; Azizi, F.; Esfahanian, F.; et al. Effects of vitamin D supplements on frequency of CD4+ T-cell subsets in women with Hashimoto’s thyroiditis: A double-blind placebo-controlled study. Eur. J. Clin. Nutr. 2019, 73, 1236–1243. [Google Scholar] [CrossRef] [PubMed]
- Huwiler, V.V.; Maissen-Abgottspon, S.; Stanga, Z.; Mühlebach, S.; Trepp, R.; Bally, L.; Bano, A. Selenium Supplementation in Patients with Hashimoto Thyroiditis: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Thyroid 2024, 34, 295–313. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gärtner, R.; Gasnier, B.C.; Dietrich, J.W.; Krebs, B.; Angstwurm, M.W. Selenium supplementation in patients with autoimmune thyroiditis decreases thyroid peroxidase antibodies concentrations. J. Clin. Endocrinol. Metab. 2002, 87, 1687–1691. [Google Scholar] [CrossRef] [PubMed]
- Szklarz, M.; Gontarz-Nowak, K.; Matuszewski, W.; Bandurska-Stankiewicz, E. Iron: Not Just a Passive Bystander in AITD. Nutrients 2022, 14, 4682. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Collins, A.B.; Pawlak, R. Prevalence of vitamin B-12 deficiency among patients with thyroid dysfunction. Asia Pac. J. Clin. Nutr. 2016, 25, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Wei, H.; Zhang, W.; Li, Z.; Ding, L.; Yu, T.; Tan, L.; Liu, Y.; Liu, T.; Wang, H.; et al. Severely low serum magnesium is associated with increased risks of positive anti-thyroglobulin antibody and hypothyroidism: A cross-sectional study. Sci. Rep. 2018, 8, 9904. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ma, J.; Wu, D.; Li, C.; Fan, C.; Chao, N.; Liu, J.; Li, Y.; Wang, R.; Miao, W.; Guan, H.; et al. Lower serum 25-hydroxyvitamin D level is associated with 3 types of autoimmune thyroid diseases. Medicine 2015, 94, e1639. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liang, L.; Xie, Z. Low vitamin D status is associated with increased thyrotropin-receptor antibody titer in Graves disease. Endocr. Pract. 2015, 21, 258–263. [Google Scholar] [CrossRef]
- Ahn, H.Y.; Chung, Y.J.; Cho, B.Y. Serum 25-hydroxyvitamin D might be an independent prognostic factor for Graves disease recurrence. Medicine 2017, 96, e7700. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ke, W.; Sun, T.; Zhang, Y.; He, L.; Wu, Q.; Liu, J.; Zha, B. 25-Hydroxyvitamin D serum level in Hashimoto’s thyroiditis, but not Graves’ disease is relatively deficient. Endocr. J. 2017, 64, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.Y.; Chung, Y.J. Vitamin D supplementation does not prevent the recurrence of Graves’ disease. Sci. Rep. 2020, 10, 16. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Misharin, A.; Hewison, M.; Chen, C.R.; Lagishetty, V.; Aliesky, H.A.; Mizutori, Y.; Rapoport, B.; McLachlan, S.M. Vitamin D deficiency modulates Graves’ hyperthyroidism induced in BALB/c mice by thyrotropin receptor immunization. Endocrinology 2009, 150, 1051–1060. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, J.H.; Ju, H.J.; Seo, J.M.; Almurayshid, A.; Kim, G.M.; Ezzedine, K.; Bae, J.M. Comorbidities in Patients with Vitiligo: A Systematic Review and Meta-Analysis. J. Investig. Dermatol. 2023, 143, 777–789.e6. [Google Scholar] [CrossRef] [PubMed]
- Naik, P.P.; Farrukh, S.N. Association between alopecia areata and thyroid dysfunction. Postgrad. Med. 2021, 133, 895–898. [Google Scholar] [CrossRef] [PubMed]
- Sagdic, A.; Sener, O.; Bulucu, F.; Karadurmus, N.; Yamanel, L.; Tasci, C.; Naharci, I.; Ocal, R.; Aydin, A. Oxidative stress status in patients with chronic idiopathic urticaria. Allergol. Immunopathol. 2011, 39, 150–153. [Google Scholar] [CrossRef]
- Biedrzycki, G.; Wolszczak-Biedrzycka, B.; Dorf, J.; Maciejczyk, M. The antioxidant barrier, oxidative/nitrosative stress, and protein glycation in allergy: From basic research to clinical practice. Front Immunol. 2024, 15, 1440313. [Google Scholar] [CrossRef]
- Okayama, Y. Oxidative stress in allergic and inflammatory skin diseases. Curr. Drug Targets-Inflamm. Allergy 2005, 4, 517–519. [Google Scholar] [CrossRef]
- Dilek, F.; Ozceker, D.; Ozkaya, E.; Guler, E.; Yazici, M.; Tamay, Z.; Kocyigit, A.; Guler, N. Nitrosative stress in children with chronic spontaneous urticaria. Int. Arch. Allergy Immunol. 2017, 172, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Cassano, N.; D’Argento, V.; Filoni, A.; Vena, G.A. Platelet oxidative stress and antioxidant enzyme activity in patients with chronic idiopathic urticaria: Effects of desloratadine treatment. Int. J. Immunopathol. Pharmacol. 2005, 18, 729–734. [Google Scholar]
- Galiniak, S.; Mołoń, M.; Biesiadecki, M.; Bożek, A.; Rachel, M. The Role of Oxidative Stress in Atopic Dermatitis and Chronic Urticaria. Antioxidants 2022, 11, 1590. [Google Scholar] [CrossRef]
- Gunes Bilgili, S.; Karadag, A.S. The role of oxidative stress in skin diseases. Curr. Probl. Dermatol. 2017, 50, 99–106. [Google Scholar] [CrossRef]
- Karanikas, G.; Schuetz, M.; Wahl, K.; Paul, M.; Kontur, S.; Pietschmann, P.; Kletter, K.; Dudczak, R.; Willheim, M. Relation of anti-TPO autoantibody titre and T-lymphocyte cytokine production patterns in Hashimoto’s thyroiditis. Clin. Endocrinol. 2005, 63, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.L.; Chung, K.R. The NADPH oxidase-mediated production of hydrogen peroxide (H2O2) and resistance to oxidative stress in the necrotrophic pathogen Alternaria alternata of citrus. Mol. Plant Pathol. 2012, 13, 900–914. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ates, I.; Yilmaz, F.M.; Altay, M.; Yilmaz, N.; Berker, D.; Güler, S. The relationship between oxidative stress and autoimmunity in Hashimoto’s thyroiditis. Eur. J. Endocrinol. 2015, 173, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, R.M.; Barbalace, M.C.; Cristani, M.T.; Alibrandi, A.; Giovinazzo, S.; Giuffrida, G.; Trimarchi, F.; Cannavò, S.; Campennì, A. Serum levels of advanced glycation end products (AGEs) are increased and their soluble receptor (sRAGE) reduced in Hashimoto’s thyroiditis. J. Endocrinol. Investig. 2020, 43, 1337–1342. [Google Scholar] [CrossRef] [PubMed]
- Kochman, J.; Jakubczyk, K.; Bargiel, P.; Janda-Milczarek, K. The Influence of Oxidative Stress on Thyroid Diseases. Antioxidants 2021, 10, 1442. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gowen, B.H.; Reyes, M.V.; Joseph, L.C.; Morrow, J.P. Mechanisms of Chronic Metabolic Stress in Arrhythmias. Antioxidants 2020, 9, 1012. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ventura, M.; Melo, M.; Carrilho, F. Selenium and Thyroid Disease: From Pathophysiology to Treatment. Int. J. Endocrinol. 2017, 2017, 1297658. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Burek, C.L.; Talor, M.V. Environmental triggers of autoimmune thyroiditis. J. Autoimmun. 2009, 33, 183–189. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pirola, I.; Rotondi, M.; Cristiano, A.; Maffezzoni, F.; Pasquali, D.; Marini, F.; Coperchini, F.; Paganelli, M.; Apostoli, P.; Chiovato, L.; et al. Selenium supplementation in patients with subclinical hypothyroidism affected by autoimmune thyroiditis: Results of the SETI study. Endocrinol. Diabetes Nutr. 2020, 67, 28–35, (In English, Spanish). [Google Scholar] [CrossRef] [PubMed]
- Toulis, K.A.; Anastasilakis, A.D.; Tzellos, T.G.; Goulis, D.G.; Kouvelas, D. Selenium supplementation in the treatment of Hashimoto’s thyroiditis: A systematic review and a meta-analysis. Thyroid 2010, 20, 1163–1173. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Feng, W.; Chen, H.; Shi, H.; Jiang, L.; Zheng, X.; Liu, X.; Zhang, W.; Ge, Y.; Liu, Y.; et al. Effect of selenium on thyroid autoimmunity and regulatory T cells in patients with Hashimoto’s thyroiditis: A prospective randomized-controlled trial. Clin. Transl. Sci. 2021, 14, 1390–1402. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moncada, S.; Palmer, R.M.; Higgs, E.A. Nitric oxide: Physiology, pathophysiology, and pharmacology. Pharmacol. Rev. 1991, 43, 109–142. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.L.; Ko, C.H. The Role of Oxidative Stress in Vitiligo: An Update on Its Pathogenesis and Therapeutic Implications. Cells 2023, 12, 936. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Marchioro, H.Z.; de Castro, C.C.S.; Fava, V.M.; Sakiyama, P.H.; Dellatorre, G.; Miot, H.A. Update on the pathogenesis of vitiligo. An. Bras. Dermatol. 2022, 97, 478–490. [Google Scholar] [CrossRef]
- Ujiie, H. IgE autoantibodies in bullous pemphigoid: Supporting role, or leading player? J. Dermatol. Sci. 2015, 78, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Denat, L.; Kadekaro, A.L.; Marrot, L.; Leachman, S.A.; Abdel-Malek, Z.A. Melanocytes as instigators and victims of oxidative stress. J. Investig. Dermatol. 2014, 134, 1512–1518. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Touni, A.A.; Shivde, R.S.; Echuri, H.; Abdel-Aziz, R.T.A.; Abdel-Wahab, H.; Kundu, R.V.; Le Poole, I.C. Melanocyte-keratinocyte cross-talk in vitiligo. Front. Med. 2023, 10, 1176781. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tang, A.; Eller, M.S.; Hara, M.; Yaar, M.; Hirohashi, S.; Gilchrest, B.A. E-cadherin is the major mediator of human melanocyte adhesion to keratinocytes in vitro. J. Cell Sci. 1994, 107, 983–992. [Google Scholar] [CrossRef] [PubMed]
- Wagner, R.Y.; Luciani, F.; Cario-André, M.; Rubod, A.; Petit, V.; Benzekri, L.; Ezzedine, K.; Lepreux, S.; Steingrimsson, E.; Taieb, A.; et al. Altered E-Cadherin Levels and Distribution in Melanocytes Precede Clinical Manifestations of Vitiligo. J. Investig. Dermatol. 2015, 135, 1810–1819. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, Y.; Cario-Andre, M.; Lepreux, S.; Pain, C.; Taïeb, A. Melanocyte detachment after skin friction in non lesional skin of patients with generalized vitiligo. Br. J. Dermatol. 2003, 148, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Matarrese, P.; Puglisi, R.; Mattia, G.; Samela, T.; Abeni, D.; Malorni, W. An Overview of the Biological Complexity of Vitiligo. Oxid. Med. Cell. Longev. 2024, 2024, 3193670. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bergqvist, C.; Ezzedine, K. Vitiligo: A focus on pathogenesis and its therapeutic implications. J. Dermatol. 2021, 48, 252–270. [Google Scholar] [CrossRef] [PubMed]
- Peterman, C.M.; Vadeboncoeur, S.; Mulliken, J.B.; Fishman, S.J.; Liang, M.G. Wilms tumor screening in diffuse capillary malformation with overgrowth and macrocephaly-capillary malformation: A retrospective study. J. Am. Acad. Dermatol. 2017, 77, 874–878. [Google Scholar] [CrossRef] [PubMed]
- Peterle, L.; Sanfilippo, S.; Borgia, F.; Li Pomi, F.; Vadalà, R.; Costa, R.; Cicero, N.; Gangemi, S. The Role of Nutraceuticals and Functional Foods in Skin Cancer: Mechanisms and Therapeutic Potential. Foods 2023, 12, 2629. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Borgia, F.; Li Pomi, F.; Vaccaro, M.; Alessandrello, C.; Papa, V.; Gangemi, S. Oxidative Stress and Phototherapy in Atopic Dermatitis: Mechanisms, Role, and Future Perspectives. Biomolecules 2022, 12, 1904. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Allegra, A.; Pioggia, G.; Tonacci, A.; Musolino, C.; Gangemi, S. Oxidative Stress and Photodynamic Therapy of Skin Cancers: Mechanisms, Challenges and Promising Developments. Antioxidants 2020, 9, 448. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li Pomi, F.; Gammeri, L.; Borgia, F.; Di Gioacchino, M.; Gangemi, S. Oxidative Stress and Skin Diseases: The Role of Lipid Peroxidation. Antioxidants 2025, 14, 555. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casella, R.; Li Pomi, F.; Borgia, F.; Nettis, E.; Gangemi, S. Shared Immunopathogenic Mechanisms in Chronic Spontaneous Urticaria, Vitiligo, and Hashimoto’s Thyroiditis: The Role of Oxidative Stress and Vitamin D. Life 2025, 15, 1535. https://doi.org/10.3390/life15101535
Casella R, Li Pomi F, Borgia F, Nettis E, Gangemi S. Shared Immunopathogenic Mechanisms in Chronic Spontaneous Urticaria, Vitiligo, and Hashimoto’s Thyroiditis: The Role of Oxidative Stress and Vitamin D. Life. 2025; 15(10):1535. https://doi.org/10.3390/life15101535
Chicago/Turabian StyleCasella, Rossella, Federica Li Pomi, Francesco Borgia, Eustachio Nettis, and Sebastiano Gangemi. 2025. "Shared Immunopathogenic Mechanisms in Chronic Spontaneous Urticaria, Vitiligo, and Hashimoto’s Thyroiditis: The Role of Oxidative Stress and Vitamin D" Life 15, no. 10: 1535. https://doi.org/10.3390/life15101535
APA StyleCasella, R., Li Pomi, F., Borgia, F., Nettis, E., & Gangemi, S. (2025). Shared Immunopathogenic Mechanisms in Chronic Spontaneous Urticaria, Vitiligo, and Hashimoto’s Thyroiditis: The Role of Oxidative Stress and Vitamin D. Life, 15(10), 1535. https://doi.org/10.3390/life15101535








