Evaluating the Linkage Between Resistin and Viral Seropositivity in Psoriasis: Evidence from a Tertiary Centre
Abstract
1. Introduction
Research Aim
2. Materials and Methods
2.1. Research Design
2.2. Inclusion and Exclusion Criteria
2.3. Sample Collection and Preparation
Detection of CRP Levels
2.4. ELISA (Enzyme-Linked Immuno Sorbent Assay)
2.5. Data Analysis
3. Results
4. Discussion
- Practical Considerations in Patient Care
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rajguru, J.P.; Maya, D.; Kumar, D.; Suri, P.; Bhardwaj, S.; Patel, N.D. Update on psoriasis: A review. J. Fam. Med. Prim. Care Rev. 2020, 29, 20. [Google Scholar]
- Tokura, Y.; Phadungsaksawasdi, P.; Kurihara, K.; Fujiyama, T.; Honda, T. Pathophysiology of Skin Resident Memory T Cells. Front. Immunol. 2021, 11, 618897. [Google Scholar] [CrossRef] [PubMed]
- Boehncke, W.H.; Brembilla, N.C. Autoreactive T-Lymphocytes in Inflammatory Skin Diseases. Front. Immunol. 2019, 10, 1198. [Google Scholar] [CrossRef]
- Veale, D.J.; Fearon, U. The pathogenesis of psoriatic arthritis. Lancet 2018, 391, 2273–2284. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A.W.; Read, C. Pathophysiology, Clinical Presentation, and Treatment of Psoriasis: A Review. JAMA 2020, 323, 1945–1960. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.N.; Bowman, S.; Laszik, Z.G.; North, J.P. Clinicopathologic overlap of psoriasis, eczema, and psoriasiform dermatoses: A retrospective study of T helper type 2 and 17 subsets, interleukin 36, and β-defensin 2 in spongioticpsoriasiform dermatitis, sebopsoriasis, and tumor necrosis factor α inhibitor–associated dermatitis. J. Am. Acad. Derm. 2020, 82, 430–439. [Google Scholar]
- Ji, Y.-Z.; Liu, S.-R. Koebner phenomenon leading to the formation of new psoriatic lesions: Evidences and mechanisms. Biosci. Rep. 2019, 39, BSR20193266. [Google Scholar] [CrossRef]
- Parisi, R.; Iskandar, I.Y.K.; Kontopantelis, E.; Augustin, M.; Griffiths, C.E.M.; Ashcroft, D.M. National, regional, and worldwide epidemiology of psoriasis: Systematic analysis and modelling study. BMJ 2020, 369, m1590. [Google Scholar] [CrossRef]
- Farber, E.M.; Nall, L. Psoriasis in the tropics: Epidemiologic, genetic, clinical, and therapeutic aspects. Dermatol. Clin. 1994, 12, 805–816. [Google Scholar] [CrossRef]
- Lebwohl, M. Psoriasis. Lancet 2003, 361, 1197–1204. [Google Scholar] [CrossRef]
- Dogra, S.; Yadav, S. Psoriasis in India: Prevalence and pattern. Indian J. Dermatol. Venereol. Leprol. 2010, 76, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, Q.; Cai, D.; Guo, H.; Fang, J.; Cui, H.; Gou, L.; Deng, J.; Wang, Z.; Zuo, Z. Resistin, a novel host defense peptide of innate immunity. Front. Immunol. 2021, 12, 699807. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.D.; Rajala, M.W.; Rossetti, L.; Scherer, P.E.; Shapiro, L. Disulfide-Dependent Multimeric Assembly of Resistin Family Hormones. Science 2004, 304, 1154–1158. [Google Scholar] [CrossRef]
- Holcomb, I.N.; Kabakoff, R.C.; Chan, B.; Baker, T.W.; Gurney, A.; Henzel, W.; Nelson, C.; Lowman, H.B.; Wright, B.D.; Skelton, N.J.; et al. FIZZ1, a novel cysteine-rich secreted protein associated with pulmonary inflammation, defines a new gene family. EMBO J. 2000, 19, 4046–4055. [Google Scholar] [CrossRef]
- Harris, T.A.; Gattu, S.; Propheter, D.C.; Kuang, Z.; Bel, S.; Ruhn, K.A.; Chara, A.L.; Edwards, M.; Zhang, C.; Jo, J.H.; et al. Resistin-like molecule α provides vitamin-A-dependent antimicrobial protection in the skin. Cell Host Microbe 2019, 25, 777–788. [Google Scholar] [CrossRef]
- Ghosh, S.; Singh, A.K.; Aruna, B.; Mukhopadhyay, S.; Ehtesham, N.Z. The Genomic Organization of Mouse Resistin Reveals Major Differences from the Human Resistin: Functional Implications. Gene 2003, 305, 27–34. [Google Scholar] [CrossRef]
- Gerber, M.; Boettner, A.; Seidel, B.; Lammert, A.; Bar, J.; Schuster, E.; Thiery, J.; Kiess, W.; Kratzsch, J. Serum resistin levels of obese and lean children and adolescents: Biochemical analysis and clinical relevance. J. Clin. Endocrinol. Metab. 2005, 90, 4503–4509. [Google Scholar] [CrossRef]
- Aruna, B.; Islam, A.; Ghosh, S.; Singh, A.K.; Vijayalakshmi, M.; Ahmad, F.; Ehtesham, N.Z. Biophysical analyses of human resistin: Oligomer formation suggests novel biological function. Biochemistry 2008, 47, 12457–12466. [Google Scholar] [CrossRef] [PubMed]
- Silswal, N.; Singh, A.K.; Aruna, B.; Mukhopadhyay, S.; Ghosh, S.; Ehtesham, N.Z. Human Resistin Stimulates the Pro-Inflammatory Cytokines TNF-a and IL-12 in Macrophages by NF-κb-Dependent Pathway. Biochem. Biophys. Res. Commun. 2005, 334, 1092–1101. [Google Scholar] [CrossRef]
- Aruna, B.; Ghosh, S.; Singh, A.K.; Mande, S.C.; Srinivas, V.; Chauhan, R.; Ehtesham, N.Z. Human recombinant resistin protein displays a tendency to aggregate by forming intermolecular disulfide linkages. Biochemistry 2003, 42, 10554–10559. [Google Scholar] [CrossRef]
- Rodriguez-Pacheco, F.; Novelle, M.G.; Vazquez, M.J.; Garcia-Escobar, E.; Soriguer, F.; Rojo-Martinez, G.; García-Fuentes, E.; Malagon, M.M.; Diéguez, C. Resistin regulates pituitary lipid metabolism and inflammation in vivo and in vitro. Mediat. Inflamm. 2013, 2013, 479739. [Google Scholar] [CrossRef]
- Choe, J.Y.; Bae, J.; Jung, H.Y.; Park, S.H.; Lee, H.J.; Kim, S.K. Serum resistin level is associated with radiographic changes in hand osteoarthritis: Cross-sectional study. Jt. Bone Spine 2012, 79, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Shieh, W.; Chen, C.; Hsu, S.; Chen, H. Lipopolysaccharide Increases Resistin Gene Expression In Vivo and In Vitro. FEBS Lett. 2002, 530, 158–162. [Google Scholar] [CrossRef]
- Kaser, S.; Kaser, A.; Sandhofer, A.; Ebenbichler, C.F.; Tilg, H.; Patsch, J.R. Resistin Messenger-RNA Expression Is Increased by Proinflammatory Cytokines In Vitro. Biochem. Biophys. Res. Commun. 2003, 309, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Filková, M.; Haluzík, M.; Gay, S.; Šenolt, L. The role of resistin as a regulator of inflammation: Implications for various human pathologies. Clin. Immunol. 2009, 133, 157–170. [Google Scholar] [CrossRef]
- Johnston, A.; Arnadottir, S.; Gudjonsson, J.E.; Aphale, A.; Sigmarsdottir, A.A.; Gunnarsson, S.I.; Steinsson, J.T.; Elder, J.T.; Valdimarsson, H. Obesity in psoriasis: Leptin and resistin as mediators of cutaneous inflammation. Br. J. Dermatol. 2008, 159, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Słuczanowska-Głabowska, S.; Staniszewska, M.; Marchlewicz, M.; Duchnik, E.; Łuczkowska, K.; Safranow, K.; Machaliński, B.; Pawlik, A. Adiponectin, leptin and resistin in patients with psoriasis. J. Clin. Med. 2023, 12, 663. [Google Scholar] [CrossRef]
- Vachatova, S.; Andrys, C.; Krejsek, J.; Salavec, M.; Ettler, K.; Rehacek, V.; Cermakova, E.; Malkova, A.; Fiala, Z.; Borska, L. Metabolic Syndrome and Selective Inflammatory Markers in Psoriatic Patients. J. Immunol. Res. 2016, 2016, 5380792. [Google Scholar] [CrossRef]
- Kawashima, K.; Torii, K.; Furuhashi, T.; Saito, C.; Nishio, E.; Nishida, E.; Shintani, Y.; Morita, A. Phototherapy reduces serum resistin levels in psoriasis patients. Photodermatol. Photoimmunol. Photomed. 2011, 27, 152–155. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, X.Q.; Cheng, J.; Hui, R.S.; Gao, T.W. Increased Th17 cells are accompanied by FoxP3+ Treg cell accumulation and correlated with psoriasis disease severity. Clin. Immunol. 2010, 135, 108–117. [Google Scholar] [CrossRef]
- Nakajima, H.; Nakajima, K.; Tarutani, M.; Sano, S. Clear association between serum levels of adipokines and T-helper 17-related cytokines in patients with psoriasis. Clin. Exp. Dermatol. 2013, 38, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Menter, A.; Tyring, S.K.; Gordon, K.; Kimball, A.B.; Leonardi, C.L.; Langley, R.G.; Strober, B.E.; Kaul, M.; Gu, Y.; Okun, M.; et al. Adalimumab therapy for moderate to severe psoriasis: A randomized, controlled phase III trial. J. Am. Acad. Dermatol. 2008, 58, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.; Xie, W.; Tao, X.; Liu, N.; Yu, Y.; Huang, Y.; Xu, D.; Fan, Y. Infection-Provoked Psoriasis: Induced or Aggravated (Review). Exp. Ther. Med. 2021, 21, 567. [Google Scholar] [CrossRef]
- Megna, M.; Lauletta, G.; Tommasino, N.; Salsano, A.; Battista, T.; Ruggiero, A.; Martora, F.; Potestio, L. Management of Psoriasis Patients with Serious Infectious Diseases. Adv. Ther. 2024, 41, 2099–2111. [Google Scholar] [CrossRef]
- Campanati, A.; Marani, A.; Martina, E.; Diotallevi, F.; Radi, G.; Offidani, A. Psoriasis as an immune-mediated and inflammatory systemic disease: From pathophysiology to novel therapeutic approaches. Biomedicines 2021, 9, 1511. [Google Scholar] [CrossRef]
- Schett, G.; Rahman, P.; Ritchlin, C.; McInnes, I.B.; Elewaut, D.; Scher, J.U. Psoriatic arthritis from a mechanistic perspective. Nat. Rev. Rheumatol. 2022, 18, 311–325. [Google Scholar] [CrossRef]
- Srikanth, M.; Rasool, M. Resistin–A plausible therapeutic target in the pathogenesis of psoriasis. Immunol. Investig. 2024, 53, 115–159. [Google Scholar] [CrossRef] [PubMed]
- Kiełbowski, K.; Bakinowska, E.; Ostrowski, P.; Pala, B.; Gromowska, E.; Gurazda, K.; Dec, P.; Modrzejewski, A.; Pawlik, A. The role of adipokines in the pathogenesis of psoriasis. Int. J. Mol. Sci. 2023, 24, 6390. [Google Scholar] [CrossRef]
- Antonelli, A.; Ferri, C.; Galeazzi, M.; Giannitti, C.; Manno, D.; Mieli-Vergani, G.; Menegatti, E.; Olivieri, I.; Puoti, M.; Palazzi, C.; et al. HCV infection: Pathogenesis, clinical manifestations and therapy. Clin. Exp. Rheumatol. 2008, 26, S39. [Google Scholar]
- Noe, M.H.; Grewal, S.K.; Shin, D.B.; Ogdie, A.; Takeshita, J.; Gelfand, J.M. Increased prevalence of HCV and hepatic decompensation in adults with psoriasis: A population-based study in the United Kingdom. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 1674–1680. [Google Scholar] [CrossRef]
- Imafuku, S.; Naito, R.; Nakayama, J. Possible association of hepatitis C virus infection with late-onset psoriasis: A hospital-based observational study. J. Dermatol. 2013, 40, 813–818. [Google Scholar] [CrossRef] [PubMed]
- Alpalhão, M.; Borges-Costa, J.; Filipe, P. Psoriasis in HIV Infection: An Update. Int. J. STD AIDS 2019, 30, 596–604. [Google Scholar] [CrossRef] [PubMed]
- Morar, N.A.; Willis-Owen, S.A.; Maurer, T.; Bunker, C.B. HIV-Associated Psoriasis: Pathogenesis, Clinical Features, and Management. Lancet Infect. Dis. 2010, 10, 470–478. [Google Scholar] [CrossRef]
- Colombo, D.; Chimenti, S.; Grossi, P.; Marchesoni, A.; Di Nuzzo, S.; Griseta, V.; Gargiulo, A.; Parodi, A.; Simoni, L.; Bellia, G. Prevalence of past and reactivated viral infections and efficacy of cyclosporine A as monotherapy or in combination in patients with psoriatic arthritis—Synergy study: A longitudinal observational study. BioMed Res. Int. 2014, 2014, 941767. [Google Scholar] [CrossRef]
- Colombo, D.; Chimenti, S.; Grossi, P.A.; Marchesoni, A.; Bardazzi, F.; Ayala, F.; Simoni, L.; Vassellatti, D.; Bellia, G. Prevalence of acute and chronic viral seropositivity and characteristics of disease in patients with psoriatic arthritis treated with cyclosporine: A post hoc analysis from a sex point of view on the observational study of infectious events in psoriasis complicated by active psoriatic arthritis. Clin. Cosmet. Investig. Dermatol. 2015, 22, 1–7. [Google Scholar]
- Tsai, S.Y.; Chen, H.J.; Lio, C.F.; Ho, H.P.; Kuo, C.F.; Jia, X.; Chen, C.; Chen, Y.T.; Chou, Y.T.; Yang, T.Y.; et al. Increased risk of herpes zoster in patients with psoriasis: A population-based retrospective cohort study. PLoS ONE 2017, 12, e0179447. [Google Scholar] [CrossRef]
- El Hayderi, L.; Colson, F.; Dezfoulian, B.; Nikkels, A.F. Herpes zoster in psoriasis patients undergoing treatment with biological agents: Prevalence, impact, and management challenges. Psoriasis Targets Ther. 2016, 6, 145–151. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, P.; Lv, Y.; Deng, Y.; Yao, M.; Wang, L.; Pan, G. Advancements in the study of biologic agents in comorbidities of psoriasis: A literature review. Clin. Cosmet. Investig. Dermatol. 2023, 31, 3487–3495. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Lou, F.; Yin, Q.; Gao, Y.; Sun, Y.; Bai, J.; Xu, Z.; Liu, Z.; Cai, W.; Ke, F.; et al. RIG-I antiviral signaling drives interleukin-23 production and psoriasis-like skin disease. EMBO Mol. Med. 2017, 9, 589–604. [Google Scholar] [CrossRef]
- Fuchs, D.; Hausen, A.; Reibnegger, G.; Werner, E.R.; Dierich, M.P.; Wachter, H. Psoriasis, gamma-interferon, and the acquired immunodeficiency syndrome. Ann. Intern. Med. 1987, 106, 165. [Google Scholar] [CrossRef]
- Namazi, M.R. Paradoxical exacerbation of psoriasis in AIDS: Proposed explanations including the potential roles of substance P and gramnegative bacteria. Autoimmunity 2004, 37, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Hsu, D.Y.; Gordon, K.; Silverberg, J.I. Serious Infections in Hospitalized Patients with Psoriasis in the United States. J. Am. Acad. Dermatol. 2016, 75, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Edwards, R.J.; Lavia, L.O.; Edwards, J.; Boyce, G. Psoriasis in Patients Attending a Large HIV Clinic in Trinidad. Med. Sci. 2022, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Chun, K.; Afshar, M.; Audish, D.; Kabigting, F.; Paik, A.; Gallo, R.; Hata, T. Hepatitis C may enhance key amplifiers of psoriasis. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 672–678. [Google Scholar] [CrossRef]
- Burchill, M.A.; GoldenMason, L.; WindRotolo, M.; Rosen, H.R. Memory redifferentiation and reduced lymphocyte activation in chronic HCVinfected patients receiving directacting antivirals. J. Viral Hepat. 2015, 22, 983991. [Google Scholar] [CrossRef] [PubMed]
- Spaan, M.; van Oord, G.; Kreefft, K.; Hou, J.; Hansen, B.E.; Janssen, H.L.; de Knegt, R.J.; Boonstra, A. Immunological Analysis During InterferonFree Therapy for Chronic Hepatitis C Virus Infection Reveals Modulation of the Natural Killer Cell Compartment. J. Infect. Dis. 2016, 213, 216223. [Google Scholar] [CrossRef] [PubMed]
- Serti, E.; Park, H.; Keane, M.; O’Keefe, A.C.; Rivera, E.; Liang, T.J.; Ghany, M.; Rehermann, B. Rapid decrease in hepatitis C viremia by direct acting antivirals improves the natural killer cell response to IFNα. Gut 2017, 66, 724735. [Google Scholar] [CrossRef] [PubMed]
- Damiani, G.; Franchi, C.; Pigatto, P.; Altomare, A.; Pacifico, A.; Petrou, S.; Leone, S.; Pace, M.C.; Fiore, M. Outcomes assessment of hepatitis C virus-positive psoriatic patients treated using pegylated interferon in combination with ribavirin compared to new Direct-Acting Antiviral agents. World J. Hepatol. 2018, 10, 329–336. [Google Scholar] [CrossRef]
- Bu, J.; Ding, R.; Zhou, L.; Chen, X.; Shen, E. Epidemiology of psoriasis and comorbid diseases: A narrative review. Front. Immunol. 2022, 13, 880201. [Google Scholar] [CrossRef]
- Ting, S.W.; Ting, S.Y.; Lin, Y.S.; Lin, M.S.; Kuo, G. Risk of herpes zoster in psoriasis patients receiving systemic therapies: A nationwide population-based cohort study. Sci. Rep. 2021, 11, 11824. [Google Scholar] [CrossRef]
- Singer, D.; Thompson-Leduc, P.; Ma, S.; Gupta, D.; Cheng, W.Y.; Sendhil, S.R.; Sundar, M.; Hagopian, E.; Stempniewicz, N.; Duh, M.S.; et al. Burden of herpes zoster among patients with psoriasis in the United States. Dermatol. Ther. 2023, 13, 2649–2668. [Google Scholar] [CrossRef] [PubMed]
- Megna, M.; Napolitano, M.; Ayala, F.; Balato, N. The risk of herpes zoster in patients with psoriasis: A retrospective records-based observational study. Indian J. Dermatol. Venereol. Leprol. 2016, 82, 744. [Google Scholar] [CrossRef] [PubMed]
- Garg, G.; Thami, G.P. Psoriasis Herpeticum due to Varicella Zoster Virus: A Kaposi’s Varicelliform Eruption in Erythrodermic Psoriasis. Indian J. Dermatol. 2012, 57, 213–214. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ku, C.C.; Padilla, J.A.; Grose, C.; Butcher, E.C.; Arvin, A.M. Tropism of varicella-zoster virus for human tonsillar CD4(+) T lymphocytes that express activation, memory, and skin homing markers. J. Virol. 2002, 76, 11425–11433. [Google Scholar] [CrossRef] [PubMed Central]
- Zerboni, L.; Sen, N.; Oliver, S.L.; Arvin, A.M. Molecular mechanisms of varicella zoster virus pathogenesis. Nat. Rev. Microbiol. 2014, 12, 197–210. [Google Scholar] [CrossRef] [PubMed Central]
- Gunther, C.; Carballido-Perrig, N.; Kaesler, S.; Carballido, J.M.; Biedermann, T. CXCL16 and CXCR6 are upregulated in psoriasis and mediate cutaneous recruitment of human CD8+ T cells. J. Investig. Dermatol. 2012, 132 Pt 1, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Sigmundsdottir, H.; Gudjonsson, J.E.; Jonsdottir, I.; Ludviksson, B.R.; Valdimarsson, H. The frequency of CLA+ CD8+ T cells in the blood of psoriasis patients correlates closely with the severity of their disease. Clin. Exp. Immunol. 2001, 126, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, M.; Krammig, S.; Henze, M.; Docke, W.D.; Sterry, W.; Asadullah, K. Flow cytometric characterization of lesional T cells in psoriasis: Intracellular cytokine and surface antigen expression indicates an activated, memory/effector type 1 immunophenotype. Arch. Dermatol. Res. 2000, 292, 519–521. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.J.; Haraldsen, G.; Pan, J.; Rottman, J.; Qin, S.; Ponath, P.; Andrew, D.P.; Warnke, R.; Ruffing, N.; Kassam, N.; et al. The chemokine receptor CCR4 in vascular recognition by cutaneous but not intestinal memory T cells. Nature 1999, 400, 776–780. [Google Scholar] [CrossRef]
- Chung, M.; Hakimi, M.; Yeroushalmi, S.; Bartholomew, E.; Bhutani, T. Improvement of psoriasis after initiation of antiviral therapy for hepatitis B. Dermatol. Online J. 2023, 29, 17. [Google Scholar] [CrossRef] [PubMed]
- Suh, H.-Y.; Yoon, Y.-B.; Ahn, J.-Y.; Park, M.-Y.; Youn, J.-I. Association of Hepatitis B Virus Infection and Psoriasis. Ann. Dermatol. 2017, 29, 822–824. [Google Scholar] [CrossRef] [PubMed]
- Kanada, K.N.; Schupp, C.W.; Armstrong, A.W. Association between Psoriasis and Viral Infections in the United States: Focusing on Hepatitis B, Hepatitis C and Human Immunodeficiency Virus. J. Eur. Acad. Dermatol. 2013, 27, 1312–1316. [Google Scholar] [CrossRef] [PubMed]
- Fedorova, U.V.; Syzon, O.O.; Dashko, M.O.; Voznyak, I.Y. Complex treatment the patients with psoriasis and concomitant activated herpes virus infection, types 1, 2. Wiad Lek 2020, 73, 1968–1972. [Google Scholar] [CrossRef] [PubMed]
- Almario, L.; Antonyan, A.S.; Porto, D.A.; Gomez-Roberts, H.; Alhousseini, A.; Gonik, B. Management of psoriasis herpeticum in pregnancy: A clinical conundrum. Case Rep. Obstet. Gynecol. 2016, 2016, 5319425. [Google Scholar] [CrossRef]
- Boyd, A.S.; King, L.E., Jr. Herpes simplex virus-induced psoriatic flares in a patient previously treated with tamoxifen: A follow-up. J. Am. Acad. Dermatol. 2002, 46, 797–798. [Google Scholar] [CrossRef] [PubMed]
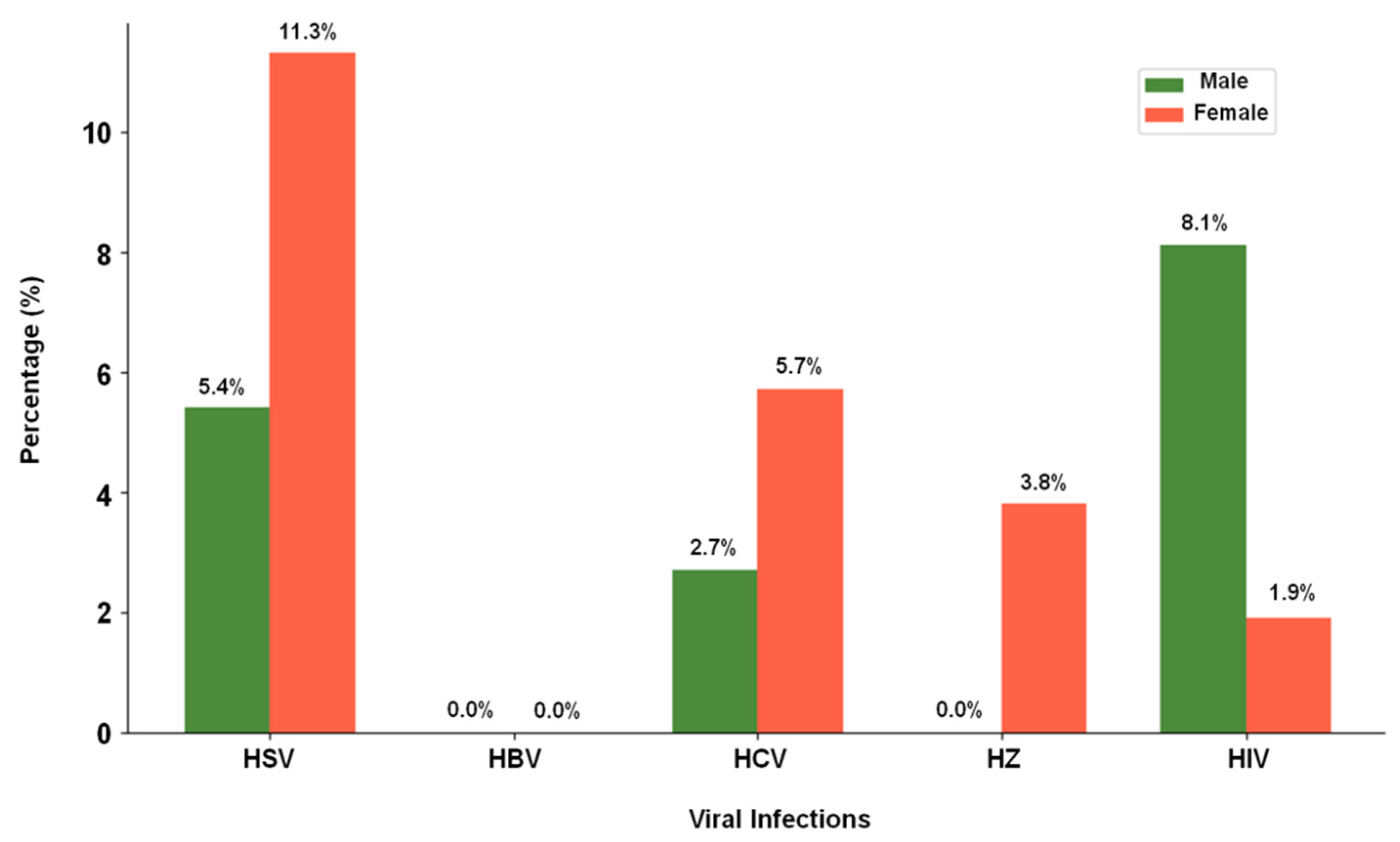
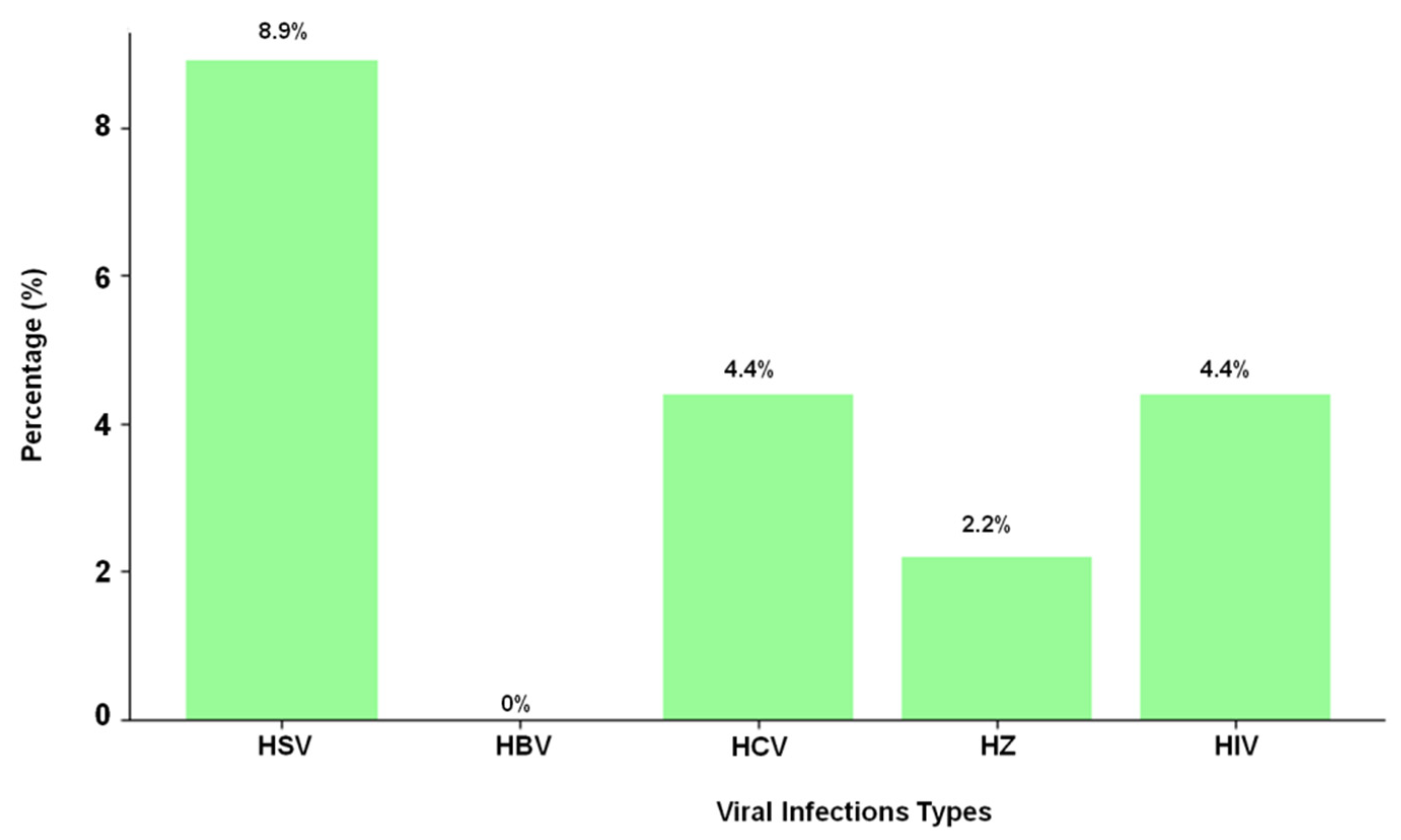
| Viral Infection | Male (n = 37) | Female (n = 53) |
|---|---|---|
| HSV | 2 (5.4%) | 6 (11.3%) |
| HBV | 0 (0%) | 0 (0%) |
| HCV | 1 (2.7%) | 3 (5.7%) |
| HZ | 0 (0%) | 2 (3.8%) |
| HIV | 3 (8.1%) | 1 (1.9%) |
| Viral Infection | Number of Cases (n = 90) | Percentage % |
|---|---|---|
| HSV | 8 | 8.9% |
| HBV | 0 | 0% |
| HCV | 4 | 4.4% |
| HZ | 2 | 2.2% |
| HIV | 4 | 4.4% |
| Viral Infection | Elevated Resistin (12–20 ng/mL) (n = 50) | Normal Resistin (4–12 ng/mL) (n = 30) | Low Resistin (<3–4 ng/mL) (n = 10) | p-Value (Trend Test) |
|---|---|---|---|---|
| HSV | 7 (14.0%) | 1 (3.3%) | 0 (0%) | 0.002 |
| HIV | 4 (8.0%) | 0 (0%) | 0 (0%) | 0.018 |
| HCV | 1 (2.0%) | 2 (6.7%) | 1 (10.0%) | 0.25 |
| HZ | 0 (0%) | 1 (3.3%) | 1 (10.0%) | 0.12 |
| HBV | 0 (0%) | 0 (0%) | 0 (0%) | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baig, H.A.; Sultana, W.; Soliman, M.; Alenizi, D.; Alenezy, A.; Mote, S.; Hegazy, A.M.S.; Alanazi, B.K.; Alanazi, M.S.; Albedaiwi, Y.; et al. Evaluating the Linkage Between Resistin and Viral Seropositivity in Psoriasis: Evidence from a Tertiary Centre. Life 2025, 15, 1054. https://doi.org/10.3390/life15071054
Baig HA, Sultana W, Soliman M, Alenizi D, Alenezy A, Mote S, Hegazy AMS, Alanazi BK, Alanazi MS, Albedaiwi Y, et al. Evaluating the Linkage Between Resistin and Viral Seropositivity in Psoriasis: Evidence from a Tertiary Centre. Life. 2025; 15(7):1054. https://doi.org/10.3390/life15071054
Chicago/Turabian StyleBaig, Habeeb Ali, Waseema Sultana, Mohamed Soliman, Dhaifallah Alenizi, Awwad Alenezy, Srinath Mote, Ahmed M. S. Hegazy, Bader Khalid Alanazi, Mansour Srhan Alanazi, Yousef Albedaiwi, and et al. 2025. "Evaluating the Linkage Between Resistin and Viral Seropositivity in Psoriasis: Evidence from a Tertiary Centre" Life 15, no. 7: 1054. https://doi.org/10.3390/life15071054
APA StyleBaig, H. A., Sultana, W., Soliman, M., Alenizi, D., Alenezy, A., Mote, S., Hegazy, A. M. S., Alanazi, B. K., Alanazi, M. S., Albedaiwi, Y., & Gouda, N. S. (2025). Evaluating the Linkage Between Resistin and Viral Seropositivity in Psoriasis: Evidence from a Tertiary Centre. Life, 15(7), 1054. https://doi.org/10.3390/life15071054







