Is a Bacteriophage Approach for Musculoskeletal Infection Management an Alternative to Conventional Therapy?
Abstract
1. Introduction
2. Background of Bacteriophages and Clinical Trials
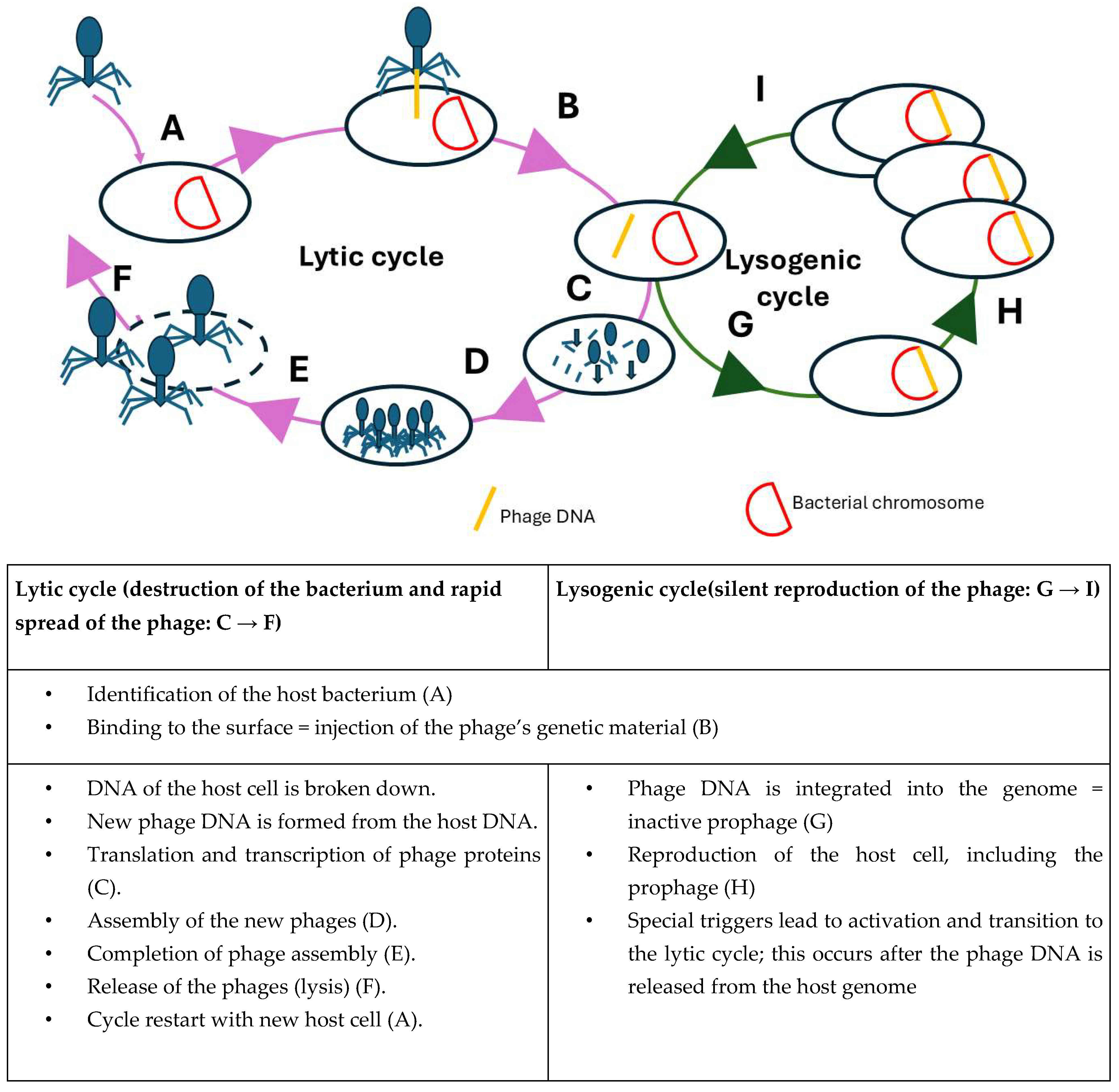
2.1. Bacteriophage Biology
2.2. Regulatory Framework and Aspects Regarding the Use of Phage Therapy
2.3. Clinical Trials
2.4. A Non-Standardized Global Framework
2.5. Challenges in Regulation
3. Therapy Strategies
3.1. Current Momentum and Emerging Solutions
3.2. Case Report
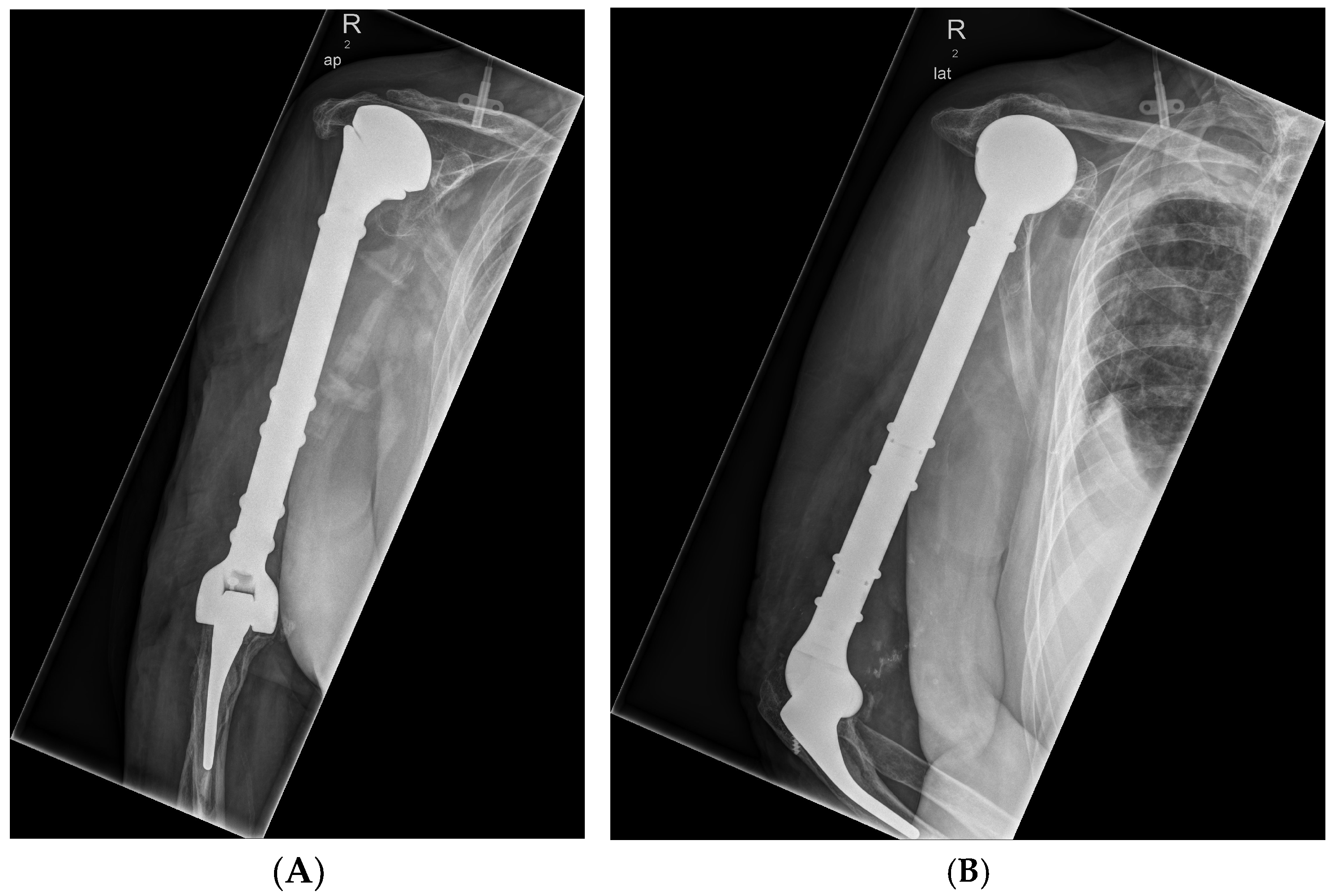
3.2.1. Case 1—Proximal Femur
- ▪
- Multi-fragmentary tibial shaft fracture on the left (AO 42 C3)
- ▪
- Implant-associated infection and soft tissue defect on the lateral thigh on the right following open reduction and internal osteosynthesis of a multi-fragmentary subtrochanteric femur fracture (AO 31A2) on the right with locking nail and two cerclages in September 2023 (Figure 2)
- ▪
- Subglottic stenosis after external tracheotomy and relocation of the tracheostoma ex domo
- ▪
- Multiple rib fractures on both sides, 4th to 8th ribs
- ▪
- Traumatic brain injury with ICB (intracranial bleeding)
- ▪
- Fracture of the transverse process of the thoracic vertebra body (TVB) 7
- ▪
- Clavicle fracture on the left
- ▪
- Fracture of the lateral mass of the sacrum on the left
- ▪
- Fracture of the anterior acetabular pillar on the left
- ▪
- Following sacral decubitus and decubitus of the heel on the right
3.2.2. Case 2—Humerus
- ▪ Chronic periprosthetic infection of the implanted alloplastic humerus and elbow joint replacement on the right side with fistula
- ▪ Infection-related loosening of a modular elbow joint endoprosthesis with osteitis of the proximal humerus on the right side
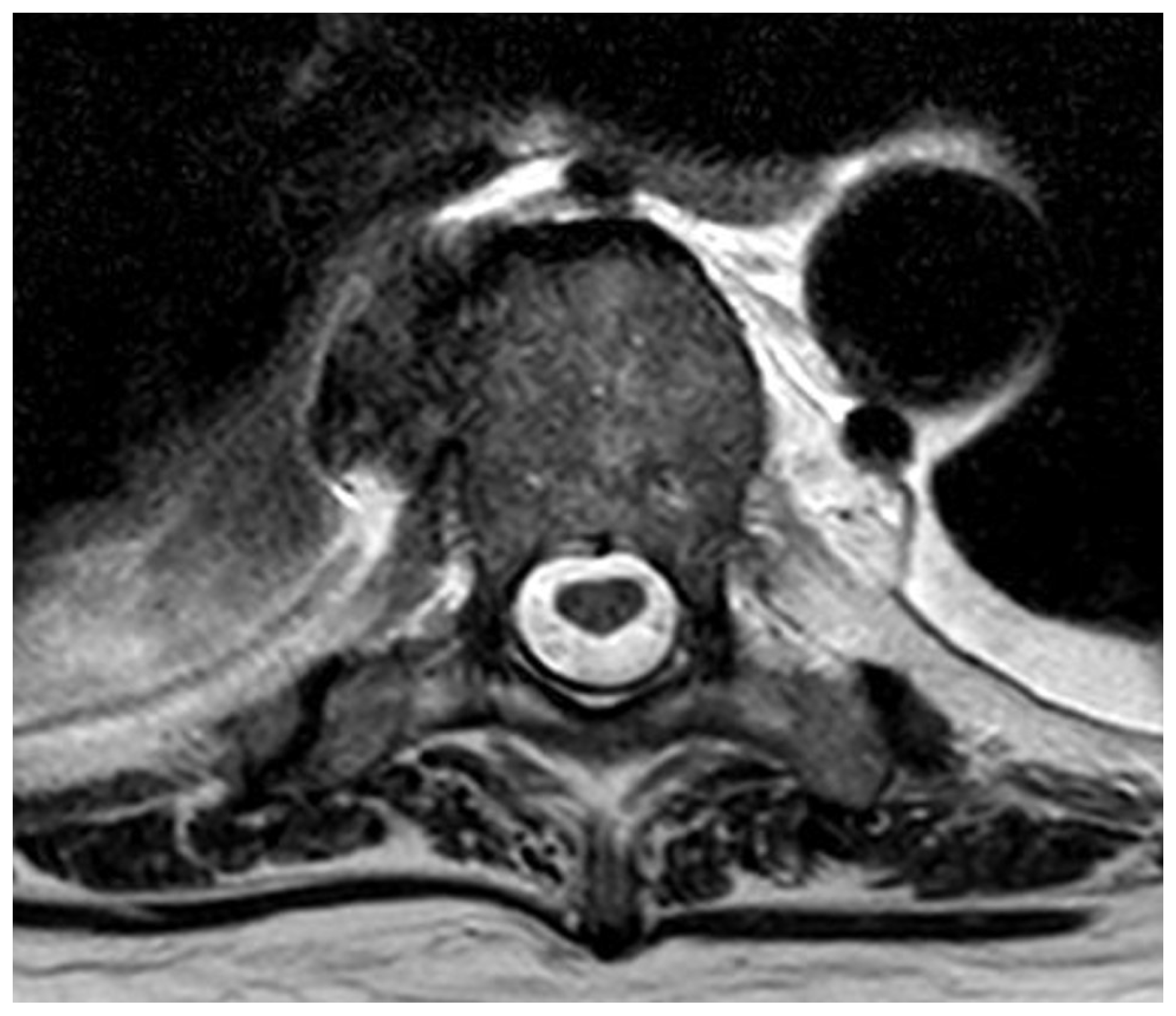
3.2.3. Case 3—Spine/Knee
- ▪ Multi-level spondylodiscitis in segments TVB 8/9, TVB 11/12, LVB (lumbal vertebra body) 2/3, and LVB 4-SVB (sacral vertebra body) 1 with inflammatory reaction and epidural abscess formation at the posterior edges of LVB 4 and LVB 5, as well as a long-distance, meningeal, inflammatory surrounding reaction at TVB 8–12 and LVB-2/SVB-1 with accompanying consecutive absolute spinal canal stenosis in the LVB 4–5 segment (Figure 4 and Figure 5)
- ▪ bilateral partially chambered psoas abscesses
- ▪ Epidural abscess at the posterior edges of LS (lumbal segment) 5 and LS6 with consecutive absolute spinal canal stenosis in the LS5/SS (sacral segment) 1 segment
- ▪ Shoulder joint empyema on the left
- ▪ Periprosthetic infection with an implanted revision total knee arthroplasty on the right
- ▪ Exclusion of periprosthetic infection with implanted total hip arthroplasty on the left
4. Discussion
4.1. Clinical Trials
4.2. Regulatory Affairs
4.3. Case Reports
4.4. Limitations of Phage Therapy
4.5. Outlook
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMR | antimicrobial resistance |
| AO | Arbeitsgemeinschaft Osteosysnthese |
| BP | Bacteriophage |
| CTS | Clinical Trials |
| DNA | Desoxyribonukleidacid |
| ICB | Intracranial bleeding |
| LVB | Lumbal vertebra body |
| LS | lumbal segment |
| MRP | multi-resistant pathogens |
| FDA | Food and Drug Administration |
| EMA | European Medicines Agency |
| RNA | Ribonukleidacid |
| SVB | Sacral vertebra body |
| SS | Sacral segment |
| TVB | Thoracic vertebra body |
| WHO | World Health Organization |
References
- Dedrick, R.M.; Guerrero-Bustamante, C.A.; Garlena, R.A.; Russell, D.A.; Ford, K.; Harris, K.; Gilmour, K.C.; Soothill, J.; Jacobs-Sera, D.; Schooley, R.T.; et al. Engineered bacteriophages for treatment of a patient with a disseminated drug-resistant Mycobacterium abscessus. Nat. Med. 2019, 25, 730–733. [Google Scholar] [CrossRef]
- RKI. Antimikrobielle Resistenzen: Krankheitslast in G7-Staaten und Weltweit EIN Dringender Aufruf Zum Handeln. Available online: https://www.rki.de/DE/Themen/Infektionskrankheiten/Antibiotikaresistenz/Grundwissen/Broschuere_IHME_RKI.pdf?__blob=publicationFile&v=1 (accessed on 25 February 2025).
- Zalewska-Piątek, B. Phage Therapy—Challenges, Opportunities and Future Prospects. Pharmaceuticals 2023, 16, 1638. [Google Scholar] [CrossRef]
- Deutscher Bundestag. Technikfolgenabschätzung (TA)—Bakteriophagen in Medizin, Land- und Lebensmittelwirtschaft—Anwendungsperspektiven, Innovations- und Regulierungsfragen; 2023. Available online: https://dserver.bundestag.de/btd/20/076/2007600.pdf (accessed on 23 September 2025).
- Willy, C.; Bröcker, F. Phagentherapie in Deutschland-auf dem Weg zur Wiedereinführung in die Militärmedizin. Wehrmed. Monatsschrift 2023, 2023, 237–244. [Google Scholar]
- Gastmeier, P.; Geffers, C.; Herrmann, M.; Lemmen, S.; Salzberger, B.; Seifert, H.; Kern, W.; Fätkenheuer, G. Nosokomiale Infektionen und Infektionen mit multiresistenten Erregern-Häufigkeit und Sterblichkeit. DMW-Dtsch. Med. Wochenschr. 2016, 141, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Walter, N.; Deng, L.; Brochhausen, C.; Alt, V.; Rupp, M. Behandlung von Knochen-und Protheseninfektionen mit Bakteriophagen: Ein systematisches Review. Der Orthop. 2022, 51, 138. [Google Scholar] [CrossRef]
- Olawade, D.B.; Fapohunda, O.; Egbon, E.; Ebiesuwa, O.A.; Usman, S.O.; Faronbi, A.O.; Fidelis, S.C. Phage therapy: A targeted approach to overcoming antibiotic resistance. Microb. Pathog. 2024, 197, 107088. [Google Scholar] [CrossRef]
- Ferry, T.; Kolenda, C.; Briot, T.; Souche, A.; Lustig, S.; Josse, J.; Batailler, C.; Pirot, F.; Medina, M.; Leboucher, G.; et al. Past and future of phage therapy and phage-derived proteins in patients with bone and joint infection. Viruses 2021, 13, 2414. [Google Scholar] [CrossRef] [PubMed]
- Clokie, M.R.J.; Kropinski, A.M.; Lavigne, R. Bacteriophages; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Onsea, J.; Wagemans, J.; Pirnay, J.P.; Di Lucas, M.; Gonzalez-Moreno, M.; Lavigne, R.; Trampuz, A.; Moriarty, T.F.; Metsemakers, W.J. Bacteriophage therapy as a treatment strategy for orthopaedic-device-related infections: Where do we stand? Eur. Cells Mater. 2020, 39, 193–210. [Google Scholar] [CrossRef]
- Onsea, J.; Post, V.; Buchholz, T.; Schwegler, H.; Zeiter, S.; Wagemans, J.; Pirnay, J.-P.; Merabishvili, M.; D’Este, M.; Rotman, S.G.; et al. Bacteriophage therapy for the prevention and treatment of fracture-related infection caused by Staphylococcus aureus: A preclinical study. Microbiol. Spectr. 2021, 9, e01736-21. [Google Scholar] [CrossRef]
- Vogt, D.; Sperling, S.; Tkhilaishvili, T.; Trampuz, A.; Pirnay, J.P.; Willy, C. “Beyond antibiotic therapy”-Zukünftige antiinfektiöse Strategien-Update 2017. Unfallchirurg 2017, 120, 573–584. [Google Scholar] [CrossRef]
- Abdelsattar, A.; Dawoud, A.; Makky, S.; Nofal, R.; Aziz, R.K.; El-Shibiny, A. Bacteriophages: From isolation to application. Curr. Pharm. Biotechnol. 2022, 23, 337–360. [Google Scholar] [CrossRef]
- Calendar, R. The Bacteriophages; Oxford University Press: Oxford, UK, 2006. [Google Scholar]
- Guerin, E.; Hill, C. Shining light on human gut bacteriophages. Front. Cell. Infect. Microbiol. 2020, 10, 481. [Google Scholar] [CrossRef]
- Jeyaraman, M.; Jain, V.K.; Iyengar, K.P. Bacteriophage therapy in infection after fracture fixation (IAFF) in orthopaedic surgery. J. Clin. Orthop. Trauma 2022, 35, 102067. [Google Scholar] [CrossRef]
- Yang, S.; Mukh, A.A.; Abdelatif, E.; Schmidt, A.; Batailler, C.; Ferry, T.; Lustig, S. Bacteriophage therapy as an innovative strategy for the treatment of Periprosthetic Joint Infection: A systematic review. Int. Orthop. 2024, 48, 2809–2825. [Google Scholar] [CrossRef]
- Hendrix, R.W. Bacteriophages: Evolution of the majority. Theor. Popul. Biol. 2002, 61, 471–480. [Google Scholar] [CrossRef]
- Doub, J.B. Bacteriophage Therapy for Clinical Biofilm Infections: Parameters That Influence Treatment Protocols and Current Treatment Approaches. Antibiotics 2020, 9, 799. [Google Scholar] [CrossRef] [PubMed]
- Sharp, R. Bacteriophages: Biology and history. J. Chem. Technol. Biotechnol. 2001, 76, 667–672. [Google Scholar] [CrossRef]
- Leitner, L.; McCallin, S.; Kessler, T.M. Bacteriophages: What role may they play in life after spinal cord injury? Spinal Cord 2021, 59, 967–970. [Google Scholar] [CrossRef] [PubMed]
- Śliwka, P.; Ochocka, M.; Skaradzińska, A. Applications of bacteriophages against intracellular bacteria. Crit. Rev. Microbiol. 2022, 48, 222–239. [Google Scholar] [CrossRef]
- Lin, J.; Du, F.; Long, M.; Li, P. Limitations of phage therapy and corresponding optimization strategies: A review. Molecules 2022, 27, 1857. [Google Scholar] [CrossRef]
- Pinto, A.M.; Cerqueira, M.A.; Bañobre-Lópes, M.; Pastrana, L.M.; Sillankorva, S. Bacteriophages for chronic wound treatment: From traditional to novel delivery systems. Viruses 2020, 12, 235. [Google Scholar] [CrossRef]
- Bröcker, F.; Willy, C. Potenziale der Bakteriophagentherapie in Deutschland: Evidenzlage und klinische Relevanz. Bundesgesundheitsblatt-Gesundheitsforschung-Gesundheitsschutz 2025, 68, 608–616. [Google Scholar]
- Sanz-Gaitero, M.; Seoane-Blanco, M.; van Raaij, M.J. Structure and function of bacteriophages. In Bacteriophages: Biology, Technology, Therapy; Springer International Publishing: Cham, Germany, 2021; pp. 19–91. [Google Scholar]
- Dublanchet, A.; Fruciano, E. Brève histoire de la phagothérapie. Med. Mal. Infect. 2008, 38, 415–420. [Google Scholar] [CrossRef]
- Trudil, D. Phage lytic enzymes: A history. Virol. Sin. 2015, 30, 26–32. [Google Scholar] [CrossRef] [PubMed]
- van Belleghem, J.D.; Manasherob, R.; Miȩdzybrodzki, R.; Rogóż, P.; Górski, A.; Suh, G.A.; Bollyky, P.L.; Amanatullah, D.F. The rationale for using bacteriophage to treat and prevent periprosthetic joint infections. Front. Microbiol. 2020, 11, 591021. [Google Scholar] [CrossRef] [PubMed]
- Genevière, J.; McCallin, S.; Huttner, A.; Pham, T.-T.; Suva, D. A systematic review of phage therapy applied to bone and joint infections: An analysis of success rates, treatment modalities and safety. EFORT Open Rev. 2021, 6, 1148–1156. [Google Scholar] [CrossRef] [PubMed]
- Strathdee, S.A.; Hatfull, G.F.; Mutalik, V.K.; Schooley, R.T. Phage therapy: From biological mechanisms to future directions. Cell 2023, 186, 17–31. [Google Scholar] [CrossRef]
- Lewis, J.M.; Williams, J.; Sagona, A.P. Making the leap from technique to treatment—Genetic engineering is paving the way for more efficient phage therapy. Biochem. Soc. Trans. 2024, 52, 1373–1384. [Google Scholar] [CrossRef]
- Wagner, A. Bakteriophagen im Einsatz Gegen Schwere Infektionen; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Zillmann, H. Medizinethische Herausforderungen der Phagentherapie—Informierte Einwilligung, Studiendesign und Heilversuch. Bundesgesundheitsblatt—Gesundheitsforschung—Gesundheitsschutz 2025, 68, 631–637. [Google Scholar] [CrossRef]
- Meinen, A.; Tomczyk, S.; Wiegand, F.N.; Abu Sin, M.; Eckmanns, T.; Sebastian, H. Antibiotikaresistenz in Deutschland und Europa-Ein systematischer Review zur zunehmenden Bedrohung, beschleunigt durch den Klimawandel. J. Health Monit. 2023, 8, 102–119. [Google Scholar] [CrossRef]
- Pirnay, J.-P.; Djebara, S.; Steurs, G.; Griselain, J.; Cochez, C.; de Soir, S.; Glonti, T.; Spiessens, A.; Vanden Berghe, E.; Green, S.; et al. Personalized bacteriophage therapy outcomes for 100 consecutive cases: A multicentre, multinational, retrospective observational study. Nat. Microbiol. 2024, 9, 1434–1453. [Google Scholar] [CrossRef]
- Merabishvili, M.; Pirnay, J.-P.; Verbeken, G.; Chanishvili, N.; Tediashvili, M.; Lashkhi, N.; Glonti, T.; Krylov, V.; Mast, J.; van Parys, L.; et al. Quality-controlled small-scale production of a well-defined bacteriophage cocktail for use in human clinical trials. PLoS ONE 2009, 4, e4944. [Google Scholar] [CrossRef] [PubMed]
- Gibb, B.; Hyman, P.; Schneider, C.L. The many applications of engineered bacteriophages—An overview. Pharmaceuticals 2021, 14, 634. [Google Scholar] [CrossRef]
- Gibb, B.P.; Hadjiargyrou, M. Bacteriophage therapy for bone and joint infections. Bone Jt. J. 2021, 103-B, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Chatterjee, S.; Datta, S.; Prasad, R.; Dubey, D.; Prasad, R.K.; Vairale, M.G. Bacteriophages and its applications: An overview. Folia Microbiol. 2017, 62, 17–55. [Google Scholar] [CrossRef] [PubMed]
- Sulakvelidze, A.; Morris, J.G. Bacteriophages as therapeutic agents. In Annals of Medicine; Taylor & Francis: Abingdon, UK, 2001; Volume 33. [Google Scholar]
- Zhang, X.; Lv, W.; Teng, Z.; Zhao, N.; Zhou, Y.; Ma, D.; Ma, L.; Cheng, Y.; Wei, J.; He, J.; et al. Molecular detection of Rickettsiales and a potential novel Ehrlichia species closely related to Ehrlichia chaffeensis in ticks (Acari: Ixodidae) from Shaanxi Province, China, in 2022 to 2023. Front. Microbiol. 2024, 14, 1331434. [Google Scholar] [CrossRef]
- Papp, M.; Tóth, A.G.; Valcz, G.; Makrai, L.; Nagy, S.Á.; Farkas, R.; Solymosi, N. Antimicrobial resistance gene lack in tick-borne pathogenic bacteria. Sci. Rep. 2023, 13, 8167. [Google Scholar] [CrossRef]
- Faruk, O.; Jewel, Z.A.; Bairagi, S.; Rasheduzzaman, M.; Bagchi, H.; Tuha, A.S.M.; Hossain, I.; Bala, A.; Ali, S. Phage treatment of multidrug-resistant bacterial infections in humans, animals, and plants: The current status and future prospects. Infect. Med. 2025, 4, 100168. [Google Scholar] [CrossRef]
- Zhao, M.; Tan, X.; Liu, Z.; Dou, L.; Liu, D.; Pan, Y.; Ma, Y.; Yu, J. Engineered phage with cell-penetrating peptides for intracellular bacterial infections. Msystems 2023, 8, e00646-23. [Google Scholar] [CrossRef]
- Górski, A.; Międzybrodzki, R.; Jończyk-Matysiak, E.; Kniotek, M.; Letkiewicz, S. Therapeutic phages as modulators of the immune response: Practical implications. Clin. Infect. Dis. 2023, 77, S433–S439. [Google Scholar] [CrossRef]
- Washizaki, A.; Sakiyama, A.; Ando, H. Phage-specific antibodies: Are they a hurdle for the success of phage therapy? Essays Biochem. 2024, 68, 633–644. [Google Scholar] [CrossRef]
- Podlacha, M.; Gaffke, L.; Grabowski, Ł.; Mantej, J.; Grabski, M.; Pierzchalska, M.; Pierzynowska, K.; Węgrzyn, G.; Węgrzyn, A. Bacteriophage DNA induces an interrupted immune response during phage therapy in a chicken model. Nat. Commun. 2024, 15, 2274. [Google Scholar] [CrossRef]
- Lusiak-Szelachowska, M.; Weber-Dąbrowska, B.; Żaczek, M.; Międzybrodzki, R.; Górski, A. The Appearance of Antiphage Antibodies in Sera of Patients Treated with Phages. Antibiotics 2025, 14, 87. [Google Scholar] [CrossRef] [PubMed]
- Lusiak-Szelachowska, M.; Międzybrodzki, R.; Rogóż, P.; Weber-Dąbrowska, B.; Żaczek, M.; Górski, A. Do anti-phage antibodies persist after phage therapy? A preliminary report. Antibiotics 2022, 11, 1358. [Google Scholar] [CrossRef]
- Bernabéu-Gimeno, M.; Pardo-Freire, M.; Chan, B.K.; Turner, P.E.; Gil-Brusola, A.; Pérez-Tarazona, S.; Carrasco-Hernández, L.; Quintana-Gallego, E.; Domingo-Calap, P. Neutralizing antibodies after nebulized phage therapy in cystic fibrosis patients. Med 2024, 5, 1096–1111. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Yuan, M.; Chai, J. Mini review advantages and limitations of lytic phages compared with chemical antibiotics to combat bacterial infections. Heliyon 2024, 10, e34849. [Google Scholar] [CrossRef] [PubMed]



| NCT No. | Study Title | Study Status | Conditions | Interventions | Study Type |
|---|---|---|---|---|---|
| NCT05314426 | Mayo Clinic Phage Program Biobank | ENROLLING_BY_INVITATION | Bacteriophage Therapy | OBSERVATIONAL | |
| NCT05973721 | Clinical Study of Phage Therapy for Chronic Constipation Efficacy and Safety | UNKNOWN | Pib Specific Phage|Intractable Constipation | BIOLOGICAL: phage | INTERVENTIONAL |
| NCT03140085 | Bacteriophages for Treating Urinary Tract Infections in Patients Undergoing Transurethral Resection of the Prostate | COMPLETED | Intravesical Bacteriophage Treatment for Urinary Tract Infections | BIOLOGICAL: PYO Phage|DRUG: Antibiotics|OTHER: Sterile bacteriology media | INTERVENTIONAL |
| NCT04682964 | Bacteriophage Therapy in Tonsillitis | ACTIVE_NOT_RECRUITING | Acute Tonsillitis | DRUG: Nebulizer inhalation irrigation of the mucous membranes of the tonsils with a bacteriophage. | INTERVENTIONAL |
| NCT06814756 | Bacteriophage Therapy for Morganella Morganii Prosthetic Joint Infection | ACTIVE_NOT_RECRUITING | Prosthetic Joint Infections of Hip | BIOLOGICAL: phage therapy | INTERVENTIONAL |
| NCT04287478 | Bacteriophage Therapy in Patients With Urinary Tract Infections | TERMINATED | Urinary Tract Infection Bacterial | BIOLOGICAL: Bacteriophage Therapy | INTERVENTIONAL |
| NCT05498363 | Bacteriophage Therapy of Difficult-to-treat Infections | COMPLETED | Bacterial Infections | BIOLOGICAL: Bacteriophage therapy | OBSERVATIONAL |
| NCT04787250 | Bacteriophage Therapy in Patients With Prosthetic Joint Infections | WITHDRAWN | Prosthetic Joint Infection | BIOLOGICAL: Phage Therapy|PROCEDURE: Two-Stage Exchange Arthroplasty | INTERVENTIONAL |
| NCT00945087 | Experimental Phage Therapy of Bacterial Infections | UNKNOWN | Bacterial Infections | OTHER: Bacteriophage preparation | INTERVENTIONAL |
| NCT05537519 | Phage Therapy for the Treatment of Urinary Tract Infection | ACTIVE_NOT_RECRUITING | Recurrent Urinary Tract Infection | BIOLOGICAL: Phage Therapy | INTERVENTIONAL |
| NCT06409819 | Phage Therapy for Recurrent UTIs in Kidney Transplant Recipients | RECRUITING | Urinary Tract Infection, Recurrent | DRUG: phage therapy|DRUG: control | INTERVENTIONAL |
| NCT04803708 | Bacteriophage Therapy TP-102 in Diabetic Foot Ulcers | COMPLETED | Diabetic Foot Ulcer|Pseudomonas Aeruginosa Infection|Staphylococcus Aureus Infection|Acinetobacter Infection | BIOLOGICAL: TP-102 | INTERVENTIONAL |
| NCT05177107 | Bacteriophage Therapy in Patients With Diabetic Foot Osteomyelitis | TERMINATED | Osteomyelitis|Diabetic Foot Osteomyelitis | BIOLOGICAL: Bacteriophage Therapy|OTHER: Placebo | INTERVENTIONAL |
| NCT06456424 | Bacteriophage Therapy for Methicillin-Sensitive Staphylococcus Aureus Prosthetic Joint Infection | ACTIVE_NOT_RECRUITING | Prosthetic Joint Infections of Hip|Staphylococcus Aureus Infection | BIOLOGICAL: Phage therapy | INTERVENTIONAL |
| NCT05269134 | Bacteriophage Therapy in Patients With Prosthetic Joint Infections (PJI) | WITHDRAWN | Prosthetic Joint Infection | DRUG: Bacteriophage|DRUG: Placebo | INTERVENTIONAL |
| NCT06559618 | Bacteriophage Therapy in Spinal Cord Injury Patients With Bacteriuria | RECRUITING | Bacteriuria|Spinal Cord Injuries|Asymptomatic Bacteriuria|Escherichia Coli | DRUG: Phage Therapy|OTHER: Placebo | INTERVENTIONAL |
| NCT05269121 | Bacteriophage Therapy in First Time Chronic Prosthetic Joint Infections | WITHDRAWN | Prosthetic Joint Infection|Bacterial Infections | BIOLOGICAL: Phage Therapy | INTERVENTIONAL |
| NCT06942624 | Phage Therapy for the Treatment of a Chronic Enterococcus Faecium Periprosthetic Joint Infection | NOT_YET_RECRUITING | Periprosthetic Joint Infection | BIOLOGICAL: Phage Therapy | INTERVENTIONAL |
| NCT06827041 | Use of Phage Therapy for Treatment of a Periprosthetic Joint Infection | ACTIVE_NOT_RECRUITING | Periprosthetic Joint Infection | BIOLOGICAL: Phage (Cytophage Technologies) | INTERVENTIONAL |
| NCT07048704 | Taking Advantage of Phage Technologies (TAPT) to Facilitate Phage Therapy While Reducing the Use of Antibiotics in the Management of Cystic Fibrosis (CF) | NOT_YET_RECRUITING | Cystic Fibrosis (CF)|Klebsiella Pneumoniae Infection|E Coli Infections|Staphylococcus Aureus Infection|Achromobacter|Stenotrophomonas Maltophilia Infection | DRUG: Intravenous Bacteriophage Cocktail plus Standard IV Antibiotics | INTERVENTIONAL |
| NCT04684641 | CYstic Fibrosis bacterioPHage Study at Yale (CYPHY) | COMPLETED | Cystic Fibrosis | DRUG: Standard Dose YPT-01|OTHER: Placebo | INTERVENTIONAL |
| NCT05948592 | Bacteriophage Therapy TP-102 in Patients With Diabetic Foot Infection | RECRUITING | Diabetic Foot Infection | BIOLOGICAL: TP-102|OTHER: Placebo | INTERVENTIONAL |
| NCT06368388 | Bacteriophage Therapy for Difficult-to-treat Infections: the Implementation of a Multidisciplinary Phage Task Force | RECRUITING | Musculoskeletal Infection|Chronic Rhinosinusitis (Diagnosis)|Sepsis|Pulmonary Infection|Hidradenitis Suppurativa | OTHER: Prospective data collection|OTHER: Prospective data collection | OBSERVATIONAL |
| NCT03395743 | Individual Patient Expanded Access for AB-PA01, an Investigational Anti-Pseudomonas Aeruginosa Bacteriophage Therapeutic | NO_LONGER_AVAILABLE | BIOLOGICAL: AB-PA01 | EXPANDED_ACCESS | |
| NCT03395769 | Individual Patient Expanded Access for AB-SA01, an Investigational Anti-Staphylococcus Aureus Bacteriophage Therapeutic | NO_LONGER_AVAILABLE | BIOLOGICAL: AB-SA01 | EXPANDED_ACCESS | |
| NCT07076238 | Biomarker Investigation of Response to Bacteriophage Treatment for Bacterial Infection | RECRUITING | Nontuberculous Mycobacterial Lung Disease | BIOLOGICAL: Bacteriophage Treatment | OBSERVATIONAL |
| NCT04815798 | Phage Therapy for the Prevention and Treatment of Pressure Ulcers. | UNKNOWN | Pressure Ulcer | COMBINATION_PRODUCT: Bacteriophage-loaded Microcapsule Spray|COMBINATION_PRODUCT: Placebo|PROCEDURE: Standard of Care | INTERVENTIONAL |
| NCT05369104 | Phage Therapy in Prosthetic Joint Infection Due to Staphylococcus Aureus Treated With DAIR. | UNKNOWN | Infection of Total Hip Joint Prosthesis|Infection of Total Knee Joint Prosthesis | BIOLOGICAL: Anti-Staphylococcus aureus Bacteriophages | INTERVENTIONAL |
| NCT06870409 | Bacteriophages in Addition to Antibiotics for the Treatment of Patients With Infective Endocarditis | RECRUITING | Endocarditis, Bacterial | DRUG: Bacteriophage | INTERVENTIONAL |
| NCT05010577 | Nebulized Bacteriophage Therapy in Cystic Fibrosis Patients With Chronic Pseudomonas Aeruginosa Pulmonary Infection | COMPLETED | Chronic Pseudomonas Aeruginosa Infection|Cystic Fibrosis | DRUG: BX004-A|DRUG: Placebo | INTERVENTIONAL |
| NCT04650607 | Phage Safety Cohort Study | RECRUITING | Prosthetic Joint Infection|Severe Infection | OTHER: Adverse event after injection of phages | OBSERVATIONAL |
| NCT06798168 | Bacteriophage Clinical Trial for Periprosthetic Joint Infection of Multidrug Resistant Pseudomonas Aeruginosa | AVAILABLE | Joint Infection | BIOLOGICAL: Combining bacteriophage therapy with antibiotics for a case with hip PJI | EXPANDED_ACCESS |
| NCT04323475 | Phage Therapy for the Prevention and Treatment of Wound Infections in Burned Patients | UNKNOWN | Wound Infection | BIOLOGICAL: Bacteriophage cocktail spray|DRUG: Xeroform | INTERVENTIONAL |
| NCT06185920 | PHAGEinLYON Clinic Cohort Study: a Descriptive Study of Severe Infections Treated With Phage Therapy at the HCL. | RECRUITING | Severe Infection | OTHER: Description of severe infection | OBSERVATIONAL |
| NCT02664740 | Standard Treatment Associated With Phage Therapy Versus Placebo for Diabetic Foot Ulcers Infected by S. Aureus | UNKNOWN | Diabetic Foot|Staphylococcal Infections | DRUG: Topical anti-Staphylococcus bacteriophage therapy|DRUG: Topical placebo corresponding to anti-Staphylococcus bacteriophage therapy | INTERVENTIONAL |
| NCT05967130 | Treatment Chronic UTI Post Kidney Transplant | TERMINATED | Urinary Tract Infections|Transplant-Related Disorder | BIOLOGICAL: Phage | INTERVENTIONAL |
| NCT05616221 | Study to Evaluate the Safety, Phage Kinetics, and Efficacy of Inhaled AP-PA02 in Subjects With Non-Cystic Fibrosis Bronchiectasis and Chronic Pulmonary Pseudomonas Aeruginosa Infection | COMPLETED | Non-cystic Fibrosis Bronchiectasis|Pseudomonas Aeruginosa|Lung Infection | BIOLOGICAL: AP-PA02|OTHER: Placebo | INTERVENTIONAL |
| NCT02116010 | Evaluation of Phage Therapy for the Treatment of Escherichia Coli and Pseudomonas Aeruginosa Wound Infections in Burned Patients | UNKNOWN | Wound Infection | DRUG: E. coli Phages cocktail|DRUG: Standard of care: Silver Sulfadiazine|DRUG: P. Aeruginosa, Phages cocktail | INTERVENTIONAL |
| NCT06605651 | Proof of Concept Study to Assess Safety and Efficacy of Phage Therapy in Hip or Knee Prosthetic Joint Infections Due to Staphylococcus Aureus Treated by DAIR. | NOT_YET_RECRUITING | Hip Prosthesis Infection|Knee Prosthesis Infection | BIOLOGICAL: Anti-Staphylococcus aureus Bacteriophages (PP1493 and PP1815) intra-articular injection with 0.9% NaCl solution|DRUG: 0.9% NaCl solution | INTERVENTIONAL |
| NCT06998043 | Study With Phage for CF Subjects With Pseudomonas Lung Infection | RECRUITING | Chronic Pseudomonas Aeruginosa Infection|Cystic Fibrosis (CF) | BIOLOGICAL: BX004|OTHER: Placebo | INTERVENTIONAL |
| NCT05453578 | A Phase 1b/2 Trial of the Safety and Microbiological Activity of Bacteriophage Therapy in Cystic Fibrosis Subjects Colonized With Pseudomonas Aeruginosa | COMPLETED | Bacterial Disease Carrier|Cystic Fibrosis | OTHER: Placebo|BIOLOGICAL: WRAIR-PAM-CF1 | INTERVENTIONAL |
| NCT04636554 | Personalized Phage Treatment in COVID-19 Patients With Bacterial Co-Infections Microbials for Pneumonia or Bacteremia/Septicemia | NO_LONGER_AVAILABLE | COVID-19|Bacteremia|Septicemia|Acinetobacter Baumannii Infection|Pseudomonas Aeruginosa Infection|Staph Aureus Infection | OTHER: Phage Therapy | EXPANDED_ACCESS |
| NCT00937274 | Antibacterial Treatment Against Diarrhea in Oral Rehydration Solution | TERMINATED | Diarrhea | OTHER: T4 phage cocktail test|OTHER: Commercial T4 phage cocktail|OTHER: standard oral rehydration solution (ORS) | INTERVENTIONAL |
| NCT06370598 | Phase 1/2a to Assess the Safety and Tolerability of TP-122A for the Treatment of Ventilator-Associated Pneumonia | NOT_YET_RECRUITING | Pneumonia, Ventilator-Associated | BIOLOGICAL: TP-122A | INTERVENTIONAL |
| NCT05488340 | A Study of LBP-EC01 in the Treatment of Acute Uncomplicated UTI Caused by Drug Resistant E. coli (ELIMINATE Trial) | RECRUITING | Urinary Tract Infections | DRUG: LBP-EC01 0.1 × IV dose|DRUG: LBP-EC01 0.01 × IV Dose|DRUG: LBP-EC01 IV Infusion Dose|DRUG: Placebo|DRUG: LBP-EC01|DRUG: TMP/SMX | INTERVENTIONAL |
| NCT04596319 | Ph 1/2 Study Evaluating Safety and Tolerability of Inhaled AP-PA02 in Subjects With Chronic Pseudomonas Aeruginosa Lung Infections and Cystic Fibrosis | COMPLETED | Cystic Fibrosis|Pseudomonas Aeruginosa|Pseudomonas|Lung Infection|Lung Infection Pseudomonal | BIOLOGICAL: AP-PA02|OTHER: Placebo | INTERVENTIONAL |
| NCT06262282 | Mycobacteriophage Treatment of Non-tuberculosis Mycobacteria | ENROLLING_BY_INVITATION | Cystic Fibrosis|Nontuberculous Mycobacterial Lung Disease|Nontuberculous Mycobacterium Infection|Mycobacterium Infections|Mycobacterium; Pulmonary | BIOLOGICAL: mycobacteriophage | OBSERVATIONAL |
| NCT05184764 | Study Evaluating Safety, Tolerability, and Efficacy of Intravenous AP-SA02 in Subjects With S. Aureus Bacteremia | COMPLETED | Bacteremia|Staphylococcus Aureus|Staphylococcus Aureus Bacteremia|Bacteremia Staph|Bacteremia Due to Staphylococcus Aureus | BIOLOGICAL: AP-SA02|OTHER: Placebo | INTERVENTIONAL |
| NCT06750588 | Safety and Tolerability of NTR-101 in Patients With Acute Alcohol-Associated Hepatitis | NOT_YET_RECRUITING | Alcohol-Associated Hepatitis | DRUG: bacteriophage preparation|DRUG: Bacteriophage preparation|DRUG: bacteriophage preparation|DRUG: bacteriophage preparation | INTERVENTIONAL |
| NCT06319235 | Clinical Trial to Demonstrate the Safety and Efficacy of DUOFAG® | RECRUITING | Surgical Site Infection|Staphylococcus Aureus Infection|Pseudomonas Aeruginosa Infection|Bacterial Infections|Surgical Wound Infection | DRUG: IMP|DRUG: Placebo | INTERVENTIONAL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eschweiler, J.; Fischer, C.; Migliorini, F.; Greven, J.; Mendel, T.; Kobbe, P.; Langwald, S. Is a Bacteriophage Approach for Musculoskeletal Infection Management an Alternative to Conventional Therapy? Life 2025, 15, 1534. https://doi.org/10.3390/life15101534
Eschweiler J, Fischer C, Migliorini F, Greven J, Mendel T, Kobbe P, Langwald S. Is a Bacteriophage Approach for Musculoskeletal Infection Management an Alternative to Conventional Therapy? Life. 2025; 15(10):1534. https://doi.org/10.3390/life15101534
Chicago/Turabian StyleEschweiler, Jörg, Christian Fischer, Filippo Migliorini, Johannes Greven, Thomas Mendel, Philipp Kobbe, and Steffen Langwald. 2025. "Is a Bacteriophage Approach for Musculoskeletal Infection Management an Alternative to Conventional Therapy?" Life 15, no. 10: 1534. https://doi.org/10.3390/life15101534
APA StyleEschweiler, J., Fischer, C., Migliorini, F., Greven, J., Mendel, T., Kobbe, P., & Langwald, S. (2025). Is a Bacteriophage Approach for Musculoskeletal Infection Management an Alternative to Conventional Therapy? Life, 15(10), 1534. https://doi.org/10.3390/life15101534








