The Role of Plant-Derived Natural Products in the Management of Inflammatory Bowel Disease—What Is the Clinical Evidence So Far?
Abstract
1. Introduction
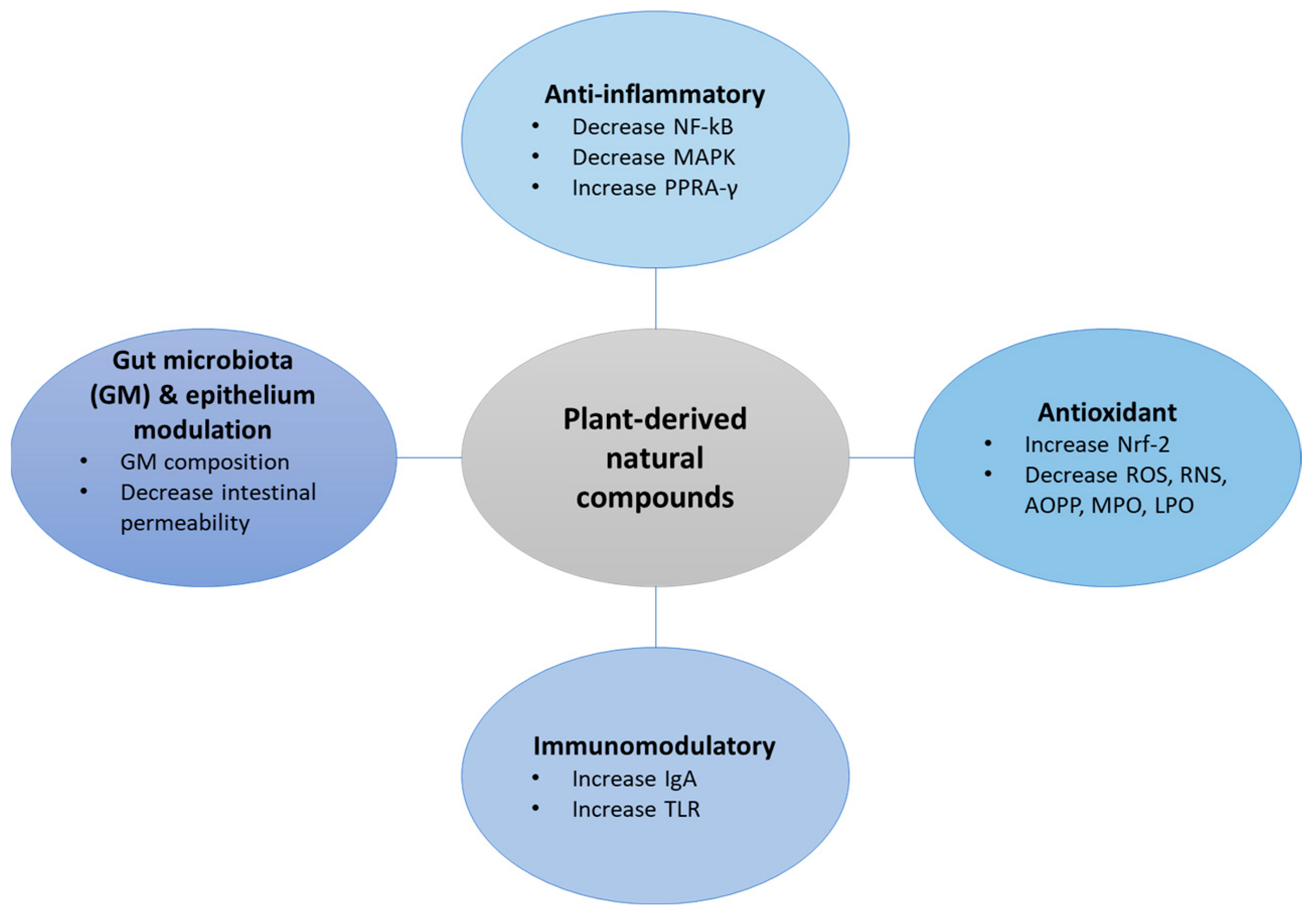
2. Plant-Derived Natural Products in IBD
3. Modulation of Gut Microbiota
4. Evidence from Human Studies
4.1. Curcumin
| Aspect Evaluated | Sample | Duration | Dose | MAIN Outcomes | Reference |
|---|---|---|---|---|---|
| Effect on IBD | 5 patients with UC | 2 months | 550 mg of curcumin twice daily for 1 month and then 3 times/d for another month for UC | Improved global score (number and quality of stools) (p < 0.02), serologic indexes, sedimentation rate, and CRP decrease within normal limits | [49] |
| 5 patients with CD | 3 months | 360 mg of curcumin 3 times/day for 1 month and then 360 mg 4 times/day for 2 months | Decrease in CDAI (mean reduction of 55 points); decrease in sedimentation rate (mean reduction of 10 mm/h); CRP was reduced by a mean of 0.1 mg/dL | ||
| Efficacy as a maintenance therapy in quiescent UC | 89 patients with quiescent UC | 6 months | Curcumin capsules 2 g/day plus sulfasalazine or mesalamine or placebo plus sulfasalazine or mesalamine | Improved clinical activity index (CAI) (p = 0.038) and endoscopic index (EI) (p = 0.0001) | [50] |
| Tolerability in children with IBD | 11 patients with IBD(6 with CD and 5 UC) | 9 weeks | 500 mg curcumin twice daily plus 1 g increase twice daily at week 3 plus 2 g twice daily at week 6 | Curcumin was well tolerated in doses up to 2 g twice per day | [51] |
| Efficacy and safety in distal UC | 45 patients with distal UC | 8 weeks | Curcumin enema plus oral 5-ASA or placebo enema plus 5-ASA | Higher remission in patients with curcumin treatment (p = 0.14); improvement in endoscopic parameters (p = 0.29) | [52] |
| Add-on therapy with optimised mesalamine in UC | 50 patients with active mild-to-moderate UC | 1 month | 3 g/day curcumin capsules with continued mesalamine or placebo capsules plus mesalamine | Higher clinical improvement and remission in patients on curcumin vs. placebo (p < 0.01) Higher endoscopic remission in the curcumin group vs. placebo (p = 0.043). | [53] |
| Effect on inducing remission in UC | 62 patients with active mild-to-moderate UC | 8 weeks | 450 mg/day curcumin plus mesalamine OR placebo plus mesalamine | No significant differences between groups | [59] |
| Effect of a nano formulation of curcuminoids in UC | 56 patients with mild-to-moderate UC | 4 weeks | 80 mg curcuminoid formula, 3 times/day orally plus mesalamine or placebo plus mesalamine | Score for urgency of defecation reduced; mean SCCAI score lower in curcuminoid group (p = 0.050) | [56] |
| Theracurmin efficacy and safety in CD. | 30 patients with active-to-moderate CD | 12 weeks | 360 mg/day Theracurmin or placebo | CDAI score significantly decreased (p = 0.005) in Theracurcumin group Reduction in endoscopic CD severity (p = 0.032) compared to baseline | [57] |
| Effect on improvement of disease activity in UC. | 70 patients with mild-to-moderate UC | 8 weeks | 1500 mg/day curcumin or placebo | Improvement in clinical outcomes Changes in SCCAI (p < 0.001) and IBDQ (p = 0.006) higher in curcumin group; serum hs-CRP and ESR decreased (p = 0.01 and p = 0.02) | [54] |
| Effect in preventing post-operative recurrence of CD | 62 patients with CD undergoing bowel resection | 6 months | Azathioprine (2.5 mg/kg) plus oral curcumin (3 g/day) or placebo | Curcumin not more effective than placebo to prevent CD recurrence. | [60] |
| Efficacy of a novel bio enhanced curcumin as add on therapy in UC. | 69 patients with mild-to-moderate UC | Up to 12 months (6-week, 3-month, 6-month, and 12-month follow-ups) | 50 mg of bio-enhanced curcumin (BEC) twice a day or placebo plus standard dose of mesalamine | Higher clinical and endoscopic remission compared to placebo (p < 0.01) at 6 weeks Maintenance of remission in 95% (6-mo) and 84% (12-mo) of BEC responders compared to none in the placebo. | [58] |
4.2. Mastiha
4.3. Boswellia serrata
4.4. Artemisia absinthium
5. Bioavailability and Safety Considerations
6. Future Implications and Conclusive Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bischoff, S.C.; Bernal, W.; Dasarathy, S.; Merli, M.; Plank, L.D.; Schütz, T.; Plauth, M. ESPEN practical guideline: Clinical nutrition in liver disease. Clin. Nutr. 2020, 39, 3533–3562. [Google Scholar] [CrossRef] [PubMed]
- Wędrychowicz, A.; Zając, A.; Tomasik, P. Advances in nutritional therapy in inflammatory bowel diseases: Review. World J. Gastroenterol. 2016, 22, 1045–1066. [Google Scholar] [CrossRef] [PubMed]
- Kayal, M.; Shah, S. Ulcerative Colitis: Current and Emerging Treatment Strategies. J. Clin. Med. 2019, 9, 94. [Google Scholar] [CrossRef]
- Roda, G.; Chien Ng, S.; Kotze, P.G.; Argollo, M.; Panaccione, R.; Spinelli, A.; Kaser, A.; Peyrin-Biroulet, L.; Danese, S. Crohn’s disease. Nat. Rev. Dis. Primers 2020, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- Mak, W.Y.; Zhao, M.; Ng, S.C.; Burisch, J. The epidemiology of inflammatory bowel disease: East meets west. J. Gastroenterol. Hepatol. 2020, 35, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Novak, E.A.; Mollen, K.P. Mitochondrial dysfunction in inflammatory bowel disease. Front. Cell Dev. Biol. 2015, 3, 62. [Google Scholar] [CrossRef] [PubMed]
- Sultan, S.; El-Mowafy, M.; Elgaml, A.; Ahmed, T.a.E.; Hassan, H.; Mottawea, W. Metabolic Influences of Gut Microbiota Dysbiosis on Inflammatory Bowel Disease. Front. Physiol. 2021, 12, 715506. [Google Scholar] [CrossRef]
- Ferrari, L.; Krane, M.K.; Fichera, A. Inflammatory bowel disease surgery in the biologic era. World J. Gastrointest. Surg. 2016, 8, 363–370. [Google Scholar] [CrossRef]
- De Conno, B.; Pesce, M.; Chiurazzi, M.; Andreozzi, M.; Rurgo, S.; Corpetti, C.; Seguella, L.; Del Re, A.; Palenca, I.; Esposito, G.; et al. Nutraceuticals and Diet Supplements in Crohn’s Disease: A General Overview of the Most Promising Approaches in the Clinic. Foods 2022, 11, 1044. [Google Scholar] [CrossRef]
- Zhang, Y.-J.; Gan, R.-Y.; Li, S.; Zhou, Y.; Li, A.-N.; Xu, D.-P.; Li, H.-B. Antioxidant Phytochemicals for the Prevention and Treatment of Chronic Diseases. Molecules 2015, 20, 21138–21156. [Google Scholar] [CrossRef]
- Thakur, M.; Singh, K.; Khedkar, R. 11—Phytochemicals: Extraction process, safety assessment, toxicological evaluations, and regulatory issues. In Functional and Preservative Properties of Phytochemicals; Prakash, B., Ed.; Academic Press: Cambridge, MA, USA, 2020. [Google Scholar]
- Campos-Vega, R.; Oomah, B.D. Chemistry and classification of phytochemicals. In Handbook of Plant Food Phytochemicals: Sources, Stability and Extraction; Tiwari, B.K., Brunton, N.P., Brennan, C., Eds.; Wiley-Blackwell: Chichester, UK, 2013; pp. 7–8. [Google Scholar]
- Larussa, T.; Imeneo, M.; Luzza, F. Potential role of nutraceutical compounds in inflammatory bowel disease. World J. Gastroenterol. 2017, 23, 2483–2492. [Google Scholar] [CrossRef] [PubMed]
- Kaulmann, A.; Bohn, T. Bioactivity of Polyphenols: Preventive and Adjuvant Strategies toward Reducing Inflammatory Bowel Diseases-Promises, Perspectives, and Pitfalls. Oxid. Med. Cell. Longev. 2016, 2016, 9346470. [Google Scholar] [CrossRef] [PubMed]
- Hossen, I.; Hua, W.; Ting, L.; Mehmood, A.; Jingyi, S.; Duoxia, X.; Yanping, C.; Hongqing, W.; Zhipeng, G.; Kaiqi, Z.; et al. Phytochemicals and inflammatory bowel disease: A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 1321–1345. [Google Scholar] [CrossRef] [PubMed]
- Holleran, G.; Lopetuso, L.; Petito, V.; Graziani, C.; Ianiro, G.; Mcnamara, D.; Gasbarrini, A.; Scaldaferri, F. The Innate and Adaptive Immune System as Targets for Biologic Therapies in Inflammatory Bowel Disease. Int. J. Mol. Sci. 2017, 18, 2020. [Google Scholar] [CrossRef]
- Cao, S.; Wang, C.; Yan, J.; Li, X.; Wen, J.; Hu, C. Curcumin ameliorates oxidative stress-induced intestinal barrier injury and mitochondrial damage by promoting Parkin dependent mitophagy through AMPK-TFEB signal pathway. Free Radic. Biol. Med. 2020, 147, 8–22. [Google Scholar] [CrossRef] [PubMed]
- Sodagari, H.; Aryan, Z.; Abdolghaffari, A.H.; Rezaei, N.; Sahebkar, A. Immunomodulatory and anti-inflammatory phytochemicals for the treatment of inflammatory bowel disease (IBD). J. Pharmacopunct. 2018, 21, 294–295. [Google Scholar] [CrossRef]
- Sameer, A.S.; Nissar, S. Toll-Like Receptors (TLRs): Structure, Functions, Signaling, and Role of Their Polymorphisms in Colorectal Cancer Susceptibility. BioMed. Res. Int. 2021, 2021, 1157023. [Google Scholar] [CrossRef]
- Gohda, J.; Matsumura, T.; Inoue, J.-I. Cutting Edge: TNFR-Associated Factor (TRAF) 6 Is Essential for MyD88-Dependent Pathway but Not Toll/IL-1 Receptor Domain-Containing Adaptor-Inducing IFN-β (TRIF)-Dependent Pathway in TLR Signaling. J. Immunol. 2004, 173, 2913–2917. [Google Scholar] [CrossRef]
- Amerikanou, C.; Papada, E.; Gioxari, A.; Smyrnioudis, I.; Kleftaki, S.A.; Valsamidou, E.; Bruns, V.; Banerjee, R.; Trivella, M.G.; Milic, N.; et al. Mastiha has efficacy in immune-mediated inflammatory diseases through a microRNA-155 Th17 dependent action. Pharmacol. Res. 2021, 171, 105753. [Google Scholar] [CrossRef]
- Valdes, A.M.; Walter, J.; Segal, E.; Spector, T.D. Role of the gut microbiota in nutrition and health. BMJ 2018, 361, k2179. [Google Scholar] [CrossRef]
- Sartor, R.B.; Mazmanian, S.K. Intestinal microbes in inflammatory bowel diseases. Am. J. Gastroenterol. Suppl. 2012, 1, 15. [Google Scholar] [CrossRef]
- Rechner, A.R.; Smith, M.A.; Kuhnle, G.; Gibson, G.R.; Debnam, E.S.; Srai, S.K.S.; Moore, K.P.; Rice-Evans, C.A. Colonic metabolism of dietary polyphenols: Influence of structure on microbial fermentation products. Free Rad. Biol. Med. 2004, 36, 212–225. [Google Scholar] [CrossRef]
- Santhiravel, S.; Bekhit, A.E.-D.A.; Mendis, E.; Jacobs, J.L.; Dunshea, F.R.; Rajapakse, N.; Ponnampalam, E.N. The Impact of Plant Phytochemicals on the Gut Microbiota of Humans for a Balanced Life. Int. J. Mol. Sci. 2022, 23, 8124. [Google Scholar] [CrossRef]
- Campbell, C.L.; Yu, R.; Li, F.; Zhou, Q.; Chen, D.; Qi, C.; Yin, Y.; Sun, J. Modulation of fat metabolism and gut microbiota by resveratrol on high-fat diet-induced obese mice. Diabetes Metab. Syndr. Obes. 2019, 12, 97–107. [Google Scholar] [CrossRef]
- Larrosa, M.; Yañéz-Gascón, M.J.; Selma, M.V.; González-Sarrías, A.; Toti, S.; Cerón, J.J.; Tomás-Barberán, F.; Dolara, P.; Espín, J.C. Effect of a low dose of dietary resveratrol on colon microbiota, inflammation and tissue damage in a DSS-induced colitis rat model. J. Agric. Food Chem. 2009, 57, 2211–2220. [Google Scholar] [CrossRef]
- Zhuang, Y.; Huang, H.; Liu, S.; Liu, F.; Tu, Q.; Yin, Y.; He, S. Resveratrol Improves Growth Performance, Intestinal Morphology, and Microbiota Composition and Metabolism in Mice. Front Microbiol. 2021, 12, 726878. [Google Scholar] [CrossRef]
- Wu, Z.; Huang, S.; Li, T.; Li, N.; Han, D.; Zhang, B.; Xu, Z.Z.; Zhang, S.; Pang, J.; Wang, S.; et al. Gut microbiota from green tea polyphenol-dosed mice improves intestinal epithelial homeostasis and ameliorates experimental colitis. Microbiome 2021, 9, 184. [Google Scholar] [CrossRef] [PubMed]
- Palm, N.W.; De Zoete, M.R.; Cullen, T.W.; Barry, N.A.; Stefanowski, J.; Hao, L.; Degnan, P.H.; Hu, J.; Peter, I.; Zhang, W.; et al. Immunoglobulin A coating identifies colitogenic bacteria in inflammatory bowel disease. Cell 2014, 158, 1000–1010. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Y.; Wu, L.; Wang, F.; Shen, X.; Lin, D. Carotenoid supplementation and retinoic acid in immunoglobulin A regulation of the gut microbiota dysbiosis. Exp. Biol. Med. 2018, 243, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Pozuelo, M.; Panda, S.; Santiago, A.; Mendez, S.; Accarino, A.; Santos, J.; Guarner, F.; Azpiroz, F.; Manichanh, C. Reduction of butyrate- and methane-producing microorganisms in patients with Irritable Bowel Syndrome. Sci. Rep. 2015, 5, 12693. [Google Scholar] [CrossRef] [PubMed]
- Peluso, I.; Romanelli, L.; Palmery, M. Interactions between prebiotics, probiotics, polyunsaturated fatty acids and polyphenols: Diet or supplementation for metabolic syndrome prevention? Int. J. Food. Sci. Nutr. 2014, 65, 259–267. [Google Scholar] [CrossRef]
- Lin, S.C.; Cheifetz, A.S. The use of complementary and alternative medicine in patients with Inflammatory Bowel Disease. Gastroenterol. Hepatol. 2018, 14, 415–425. [Google Scholar]
- Burge, K.; Gunasekaran, A.; Eckert, J.; Chaaban, H. Curcumin and Intestinal Inflammatory Diseases: Molecular Mechanisms of Protection. Int. J. Mol. Sci. 2019, 20, 1912. [Google Scholar] [CrossRef] [PubMed]
- Ukil, A.; Maity, S.; Karmakar, S.; Datta, N.; Vedasiromoni, J.R.; Das, P.K. Curcumin, the major component of food flavour turmeric, reduces mucosal injury in trinitrobenzene sulphonic acid-induced colitis. Br. J. Pharmacol. 2003, 139, 209–218. [Google Scholar] [CrossRef]
- Jian, Y.T.; Mai, G.F.; Wang, J.D.; Zhang, Y.L.; Luo, R.C.; Fang, Y.X. Preventive and therapeutic effects of NF-kappaB inhibitor curcumin in rats colitis induced by trinitrobenzene sulfonic acid. World J. Gastroenterol. 2005, 11, 1747–1752. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 969516, Curcumin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Curcumin (accessed on 31 July 2023).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 6654, alpha-PINENE. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/alpha-PINENE (accessed on 31 July 2023).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 14896, beta-Pinene. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/beta-Pinene (accessed on 31 July 2023).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 31253, Myrcene. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Myrcene (accessed on 31 July 2023).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 5951616, Masticadienonic acid. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Masticadienonic-acid (accessed on 31 July 2023).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 15559978, Isomasticadienonic acid. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Isomasticadienonic-acid (accessed on 31 July 2023).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 168928, beta-Boswellic acid. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/beta-Boswellic-acid (accessed on 31 July 2023).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 261491, Thujone. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Thujone (accessed on 31 July 2023).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 6616, Camphene. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Camphene (accessed on 31 July 2023).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 442138, Absinthin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Absinthin (accessed on 31 July 2023).
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem 2023 update. Nucleic. Acids. Res. 2023, 51, D1373–D1380. [Google Scholar] [CrossRef]
- Holt, P.R.; Katz, S.; Kirshoff, R. Curcumin therapy in inflammatory bowel disease: A pilot study. Dig. Dis. Sci. 2005, 50, 2191–2193. [Google Scholar] [CrossRef] [PubMed]
- Hanai, H.; Iida, T.; Takeuchi, K.; Watanabe, F.; Maruyama, Y.; Andoh, A.; Tsujikawa, T.; Fujiyama, Y.; Mitsuyama, K.; Sata, M.; et al. Curcumin maintenance therapy for ulcerative colitis: Randomized, multicenter, double-blind, placebo-controlled trial. Clin. Gastroenterol. Hepatol. 2006, 4, 1502–1506.e1. [Google Scholar] [CrossRef]
- Suskind, D.L.; Wahbeh, G.; Burpee, T.; Cohen, M.; Christie, D.; Weber, W. Tolerability of curcumin in pediatric inflammatory bowel disease: A forced-dose titration study. J. Pediatr. Gastroenterol. Nutr. 2013, 56, 277–279. [Google Scholar] [CrossRef]
- Singla, V.; Pratap Mouli, V.; Garg, S.K.; Rai, T.; Choudhury, B.N.; Verma, P.; Deb, R.; Tiwari, V.; Rohatgi, S.; Dhingra, R.; et al. Induction with NCB-02 (curcumin) enema for mild-to-moderate distal ulcerative colitis—A randomized, placebo-controlled, pilot study. J. Crohns. Colitis. 2014, 8, 208–214. [Google Scholar] [CrossRef]
- Lang, A.; Salomon, N.; Wu, J.C.; Kopylov, U.; Lahat, A.; Har-Noy, O.; Ching, J.Y.; Cheong, P.K.; Avidan, B.; Gamus, D.; et al. Curcumin in Combination with Mesalamine Induces Remission in Patients with Mild-to-Moderate Ulcerative Colitis in a Randomized Controlled Trial. Clin. Gastroenterol. Hepatol. 2015, 13, 1444–1449.e1. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, N.; Mansoori, A.; Shayesteh, A.; Hashemi, S.J. The effect of curcumin supplementation on clinical outcomes and inflammatory markers in patients with ulcerative colitis. Phytother. Res. 2020, 34, 1123–1133. [Google Scholar] [CrossRef] [PubMed]
- Lopresti, A.L. The problem of Curcumin and its bioavailability: Could its gastrointestinal influence contribute to its overall health-enhancing effects? Adv. Nutr. 2018, 9, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Masoodi, M.; Mahdiabadi, M.A.; Mokhtare, M.; Agah, S.; Kashani, A.H.F.; Rezadoost, A.M.; Sabzikarian, M.; Talebi, A.; Sahebkar, A. The efficacy of curcuminoids in improvement of ulcerative colitis symptoms and patients’ self-reported well-being: A randomized double-blind controlled trial. J. Cell. Biochem. 2018, 119, 9552–9559. [Google Scholar] [CrossRef]
- Sugimoto, K.; Ikeya, K.; Bamba, S.; Andoh, A.; Yamasaki, H.; Mitsuyama, K.; Nasuno, M.; Tanaka, H.; Matsuura, A.; Kato, M.; et al. Highly Bioavailable Curcumin Derivative Ameliorates Crohn’s Disease Symptoms: A Randomized, Double-Blind, Multicenter Study. J. Crohns. Colitis. 2020, 14, 1693–1701. [Google Scholar] [CrossRef]
- Banerjee, R.; Pal, P.; Penmetsa, A.; Kathi, P.; Girish, G.; Goren, I.; Reddy, D.N. Novel Bioenhanced Curcumin with Mesalamine for Induction of Clinical and Endoscopic Remission in Mild-to-Moderate Ulcerative Colitis: A Randomized Double-Blind Placebo-controlled Pilot Study. J. Clin. Gastroenterol. 2021, 55, 702–708. [Google Scholar] [CrossRef]
- Kedia, S.; Bhatia, V.; Thareja, S.; Garg, S.; Mouli, V.P.; Bopanna, S.; Tiwari, V.; Makharia, G.; Ahuja, V. Low dose oral curcumin is not effective in induction of remission in mild to moderate ulcerative colitis: Results from a randomized double blind placebo controlled trial. World J. Gastrointest. Pharmacol. Ther. 2017, 8, 147–154. [Google Scholar] [CrossRef]
- Bommelaer, G.; Laharie, D.; Nancey, S.; Hebuterne, X.; Roblin, X.; Nachury, M.; Peyrin-Biroulet, L.; Fumery, M.; Richard, D.; Pereira, B.; et al. Oral Curcumin No More Effective Than Placebo in Preventing Recurrence of Crohn’s Disease After Surgery in a Randomized Controlled Trial. Clin. Gastroenterol. Hepatol. 2020, 18, 1553–1560.e1. [Google Scholar] [CrossRef]
- Papada, E.; Kaliora, A.C. Antioxidant and Anti-Inflammatory Properties of Mastiha: A Review of Preclinical and Clinical Studies. Antioxidants 2019, 8, 208. [Google Scholar] [CrossRef]
- Soulaidopoulos, S.; Tsiogka, A.; Chrysohoou, C.; Lazarou, E.; Aznaouridis, K.; Doundoulakis, I.; Tyrovola, D.; Tousoulis, D.; Tsioufis, K.; Vlachopoulos, C.; et al. Overview of Chios Mastic Gum (Pistacia lentiscus) Effects on Human Health. Nutrients 2022, 14, 590. [Google Scholar] [CrossRef]
- European Medicines Agency. 2015. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Herbal__Herbal_monograph/2015/07/WC500190099.pdf (accessed on 1 July 2023).
- Kaliora, A.C.; Stathopoulou, M.G.; Triantafillidis, J.K.; Dedoussis, G.V.; Andrikopoulos, N.K. Chios mastic treatment of patients with active Crohn’s disease. World J. Gastroenterol. 2007, 13, 748–753. [Google Scholar] [CrossRef] [PubMed]
- Kaliora, A.C.; Stathopoulou, M.G.; Triantafillidis, J.K.; Dedoussis, G.V.; Andrikopoulos, N.K. Alterations in the function of circulating mononuclear cells derived from patients with Crohn’s disease treated with mastic. World J. Gastroenterol. 2007, 13, 6031–6036. [Google Scholar]
- Papada, E.; Forbes, A.; Amerikanou, C.; Torović, L.; Kalogeropoulos, N.; Tzavara, C.; Triantafillidis, J.K.; Kaliora, A.C. Antioxidative Efficacy of a Pistacia Lentiscus Supplement and Its Effect on the Plasma Amino Acid Profile in Inflammatory Bowel Disease: A Randomised, Double-Blind, Placebo-Controlled Trial. Nutrients 2018, 10, 1779. [Google Scholar] [CrossRef] [PubMed]
- Papada, E.; Gioxari, A.; Amerikanou, C.; Forbes, A.; Tzavara, C.; Smyrnioudis, I.; Kaliora, A.C. Regulation of faecal biomarkers in inflammatory bowel disease patients treated with oral mastiha (Pistacia lentiscus) supplement: A double-blind and placebo-controlled randomised trial. Phytother. Res. 2019, 33, 360–369. [Google Scholar] [CrossRef]
- Coulombe, G.; Langlois, A.; De Palma, G.; Langlois, M.-J.; Mccarville, J.L.; Gagné-Sanfaçon, J.; Perreault, N.; Feng, G.-S.; Bercik, P.; Boudreau, F.; et al. SHP-2 Phosphatase Prevents Colonic Inflammation by Controlling Secretory Cell Differentiation and Maintaining Host-Microbiota Homeostasis. J. Cell. Physiol. 2016, 231, 2529–2540. [Google Scholar] [CrossRef]
- Papada, E.; Amerikanou, C.; Torović, L.; Kalogeropoulos, N.; Tzavara, C.; Forbes, A.; Kaliora, A.C. Plasma free amino acid profile in quiescent Inflammatory Bowel Disease patients orally administered with Mastiha (Pistacia lentiscus); a randomised clinical trial. Phytomedicine 2019, 56, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Amerikanou, C.; Dimitropoulou, E.; Gioxari, A.; Papada, E.; Tanaini, A.; Fotakis, C.; Zoumpoulakis, P.; Kaliora, A.C. Linking the IL-17A immune response with NMR-based faecal metabolic profile in IBD patients treated with Mastiha. Biomed. Pharmacother. 2021, 138, 111535. [Google Scholar] [CrossRef]
- Varma, K.; Haponiuk, J.T.; Gopi, S. 7-Antiinflammatory activity of Boswellia. In Inflammation and Natural Products; Gopi, S., Amalraj, A., Kunnumakkara, A., Thomas, S., Eds.; Academic Press: London, UK, 2021; pp. 147–159. [Google Scholar]
- Gerhardt, H.; Seifert, F.; Buvari, P.; Vogelsang, H.; Repges, R. Therapy of active Crohn disease with Boswellia serrata extract H 15. Z. Gastroenterol. 2001, 39, 11–17. [Google Scholar] [CrossRef]
- Holtmeier, W.; Zeuzem, S.; Preiβ, J.; Kruis, W.; Böhm, S.; Maaser, C.; Raedler, A.; Schmidt, C.; Schnitker, J.; Schwarz, J.; et al. Randomized, placebo-controlled, double-blind trial of Boswellia serrata in maintaining remission of Crohn’s disease: Good safety profile but lack of efficacy. Inflamm. Bowel. Dis. 2011, 17, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, L.; Milano, E.; Franceschi, F.; Belcaro, G.; Gizzi, G.; Feragalli, B.; Dugall, M.; Luzzi, R.; Togni, S.; Eggenhoffner, R.; et al. Managing ulcerative colitis in remission phase: Usefulness of Casperome®, an innovative lecithin-based delivery system of Boswellia serrata extract. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 2695–2700. [Google Scholar]
- Szopa, A.; Pajor, J.; Klin, P.; Rzepiela, A.; Elansary, H.O.; Al-Mana, F.A.; Mattar, M.A.; Ekiert, H. Artemisia absinthium L.-Importance in the History of Medicine, the Latest Advances in Phytochemistry and Therapeutical, Cosmetological and Culinary Uses. Plants 2020, 9, 1063. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.C.; Choi, E.J.; Oh, H.M.; Lee, S.; Lee, J.K.; Lee, M.S.; Shin, Y.I.; Choi, S.J.; Chae, J.R.; Lee, K.M.; et al. DA-9601, a standardized extract of Artemisia asiatica, blocks TNF-alpha-induced IL-8 and CCL20 production by inhibiting p38 kinase and NF-kappaB pathways in human gastric epithelial cells. World J. Gastroenterol. 2006, 12, 4850–4858. [Google Scholar]
- Hatziieremia, S.; Gray, A.I.; Ferro, V.A.; Paul, A.; Plevin, R. The effects of cardamonin on lipopolysaccharide-induced inflammatory protein production and MAP kinase and NFkappaB signalling pathways in monocytes/macrophages. Br. J. Pharmacol. 2006, 149, 188–198. [Google Scholar] [CrossRef]
- Omer, B.; Krebs, S.; Omer, H.; Noor, T.O. Steroid-sparing effect of wormwood (Artemisia absinthium) in Crohn’s disease: A double-blind placebo-controlled study. Phytomedicine 2007, 14, 87–95. [Google Scholar] [CrossRef]
- Krebs, S.; Omer, T.N.; Omer, B. Wormwood (Artemisia absinthium) suppresses tumour necrosis factor alpha and accelerates healing in patients with Crohn’s disease—A controlled clinical trial. Phytomedicine 2010, 17, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Olivares-Morales, A.; Hatley, O.J.; Turner, D.; Galetin, A.; Aarons, L.; Rostami-Hodjegan, A. The use of ROC analysis for the qualitative prediction of human oral bioavailability from animal data. Pharm. Res. 2014, 31, 720–730. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- Siviero, A.; Gallo, E.; Maggini, V.; Gori, L.; Mugelli, A.; Firenzuoli, F.; Vannacci, A. Curcumin, a golden spice with a low bioavailability. J. Herb. Med. 2015, 5, 57–70. [Google Scholar] [CrossRef]
- Kesarwani, K.; Gupta, R.; Mukerjee, A. Bioavailability enhancers of herbal origin: An overview. Asian Pac. J. Trop Biomed. 2013, 3, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Johnson, I.T. Phytochemicals and health. In Handbook of Plant Food Phytochemicals: Sources, Stability and Extraction; Tiwari, B.K., Brunton, N.P., Brennan, C., Eds.; Wiley-Blackwell: Chichester, UK, 2013; p. 50. [Google Scholar]
- Domínguez-Perles, R.; Auñón, D.; Ferreres, F.; Gil-Izquierdo, A. Gender differences in plasma and urine metabolites from Sprague-Dawley rats after oral administration of normal and high doses of hydroxytyrosol, hydroxytyrosol acetate, and DOPAC. Eur. J. Nutr. 2017, 56, 215–224. [Google Scholar] [CrossRef]
- García-Villalba, R.; Larrosa, M.; Possemiers, S.; Tomás-Barberán, F.A.; Espín, J.C. Bioavailability of phenolics from an oleuropein-rich olive (Olea europaea) leaf extract and its acute effect on plasma antioxidant status: Comparison between pre- and postmenopausal women. Eur. J. Nutr. 2014, 53, 1015–1027. [Google Scholar] [CrossRef]
- Papada, E.; Gioxari, A.; Brieudes, V.; Amerikanou, C.; Halabalaki, M.; Skaltsounis, A.L.; Smyrnioudis, I.; Kaliora, A.C. Bioavailability of Terpenes and Postprandial Effect on Human Antioxidant Potential. An Open-Label Study in Healthy Subjects. Mol. Nutr. Food Res. 2018, 62, 1700751. [Google Scholar] [CrossRef] [PubMed]
- Shoba, G.; Joy, D.; Joseph, T.; Majeed, M.; Rajendran, R.; Srinivas, P.S. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998, 64, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Halegoua-Demarzio, D.; Navarro, V.; Ahmad, J.; Avula, B.; Barnhart, H.; Barritt, A.S.; Bonkovsky, H.L.; Fontana, R.J.; Ghabril, M.S.; Hoofnagle, J.H.; et al. Liver Injury Associated with Turmeric-A Growing Problem: Ten Cases from the Drug-Induced Liver Injury Network [DILIN]. Am. J. Med. 2023, 136, 200–206. [Google Scholar] [CrossRef] [PubMed]

| Aspect Evaluated | Sample | Duration | Dose | Main Outcomes | Reference |
|---|---|---|---|---|---|
| Effects on the clinical course of active CD | 10 patients with active CD and 8 healthy controls | 4 weeks | 6 Mastiha caps/day (2.2 g/day) | Significant reduction in CDAI (p = 0.05), CRP (p = 0.028), and plasma IL-6 (0.027). Total antioxidant potential (TAP) significantly increased in the Mastiha group (p = 0.036) | [64] |
| Effects on cytokine production of circulating mononuclear cells in active CD | Reduction in TNF-a secretion from PBMC in the Mastiha group (p = 0.028) and an increase in MIF (p = 0.026). No significant changes in IL-6, MCP-1, or GSH. | [65] | |||
| Effects on oxidative stress and plasma-free amino acid profile in active IBD | 60 patients with active IBD | 3 months | 2.8 g/day Mastiha (4 tabs 700 mg) plus conventional medical treatment or Placebo plus conventional medical treatment | Significant decrease in oxLDL (p = 0.031), oxLDL/HDL (p = 0.020) and oxLDL/LDL (p = 0.005) in the Mastiha group and amelioration of the decrease in plasma-free AAs in patients with UC. | [66] |
| Effects on QoL, inflammatory biomarkers, and clinical course in IBD | IBDQ significantly improved (p = 0.004); significant decrease in faecal lysozymes (p = 0.018) and fibrinogen (p = 0.006) in the Mastiha group. Significant increase in faecal lactoferrin (p = 0.001) and calprotectin (p = 0.029) in the placebo group | [67] | |||
| Effect on the clinical course and amino acid profile in inactive IBD | 68 patients with inactive IBD | 6 months | 2.8 g/day Mastiha plus conventional medical treatment or Placebo plus conventional medical treatment | Attenuation of the increase in free AA levels in the Mastiha group; significant decrease in oxidative stress biomarkers | [69] |
| Regulatory effect on IL-17A serum levels in IBD | 43 patients with UC and 86 patients with CD | 3 months in active and 6 months in inactive IBD | 2.8 g/day Mastiha plus conventional medical treatment or Placebo plus conventional medical treatment | Increase in serum IL-17A in the Mastiha group (p = 0.006) and significant difference between Mastiha and placebo in the mean change in inactive IBD. | [70] |
| Anti-inflammatory activity through regulation of miRNA in IBD | 60 patients with IBD (endoscopy-proven CD or UC) with usual medical treatment | 3–6 months | 2.8 g/day Mastiha or placebo | miR-155 increased in the placebo group in active UC (p = 0.054), while it was prevented by Mastiha. | [21] |
| Aspect Evaluated | Sample | Duration | Dose | Main Outcomes | Reference |
|---|---|---|---|---|---|
| Safety and efficacy of BS extract H15 on active CD | 102 participants | 8 weeks | 3.6 g/day BS extract H15 OR mesalamine | Reduction in CDAI score, but no significant superiority compared to mesalamine (p = 0.061) | [72] |
| Effect and safety of long-term therapy in CD | 108 patients with CD in remission | 52 weeks | Boswelan 3 × 2 capsules/day (400 mg each) OR placebo | Good tolerability and safety; no superiority versus placebo as maintenance therapy (p = 0.85) | [73] |
| Effect of BS extract (BSE) in UC | 43 participants with UC in remission for at least 1 year | 4 weeks | 250 mg/day BSE in a novel lecithin-based delivery form (Casperome®) OR no supplementation | Significant improvement in diffuse intestinal pain, blood in stools, bowel movements and cramps, and reduction in calprotectin levels (p < 0.05) | [74] |
| Aspect Evaluated | Sample | Duration | Dose | Main Outcomes | Reference |
|---|---|---|---|---|---|
| Steroid-sparing effect on CD | 40 participants with CD | 20 weeks | AA containing herbal blend (3 × 500 mg/day) (SedaCrohn®) plus steroids or placebo | Significantly higher clinical improvement using CDAI in SedaCrohn® group (p < 0.01) | [78] |
| TNF-α suppressing effect on CD | 20 participants with active CD | 6 weeks | 3 capsules SedaCrohn® 3 times/day (250 mg of powdered AA each one) plus conventional medical treatment or placebo | Significant reduction in CDAI and TNF-a levels compared with placebo | [79] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davila, M.M.; Papada, E. The Role of Plant-Derived Natural Products in the Management of Inflammatory Bowel Disease—What Is the Clinical Evidence So Far? Life 2023, 13, 1703. https://doi.org/10.3390/life13081703
Davila MM, Papada E. The Role of Plant-Derived Natural Products in the Management of Inflammatory Bowel Disease—What Is the Clinical Evidence So Far? Life. 2023; 13(8):1703. https://doi.org/10.3390/life13081703
Chicago/Turabian StyleDavila, Mariela Martinez, and Efstathia Papada. 2023. "The Role of Plant-Derived Natural Products in the Management of Inflammatory Bowel Disease—What Is the Clinical Evidence So Far?" Life 13, no. 8: 1703. https://doi.org/10.3390/life13081703
APA StyleDavila, M. M., & Papada, E. (2023). The Role of Plant-Derived Natural Products in the Management of Inflammatory Bowel Disease—What Is the Clinical Evidence So Far? Life, 13(8), 1703. https://doi.org/10.3390/life13081703







