Eicosapentaenoic Acid Induces the Inhibition of Adipogenesis by Reducing the Effect of PPARγ Activator and Mediating PKA Activation and Increased COX-2 Expression in 3T3-L1 Cells at the Differentiation Stage
Abstract
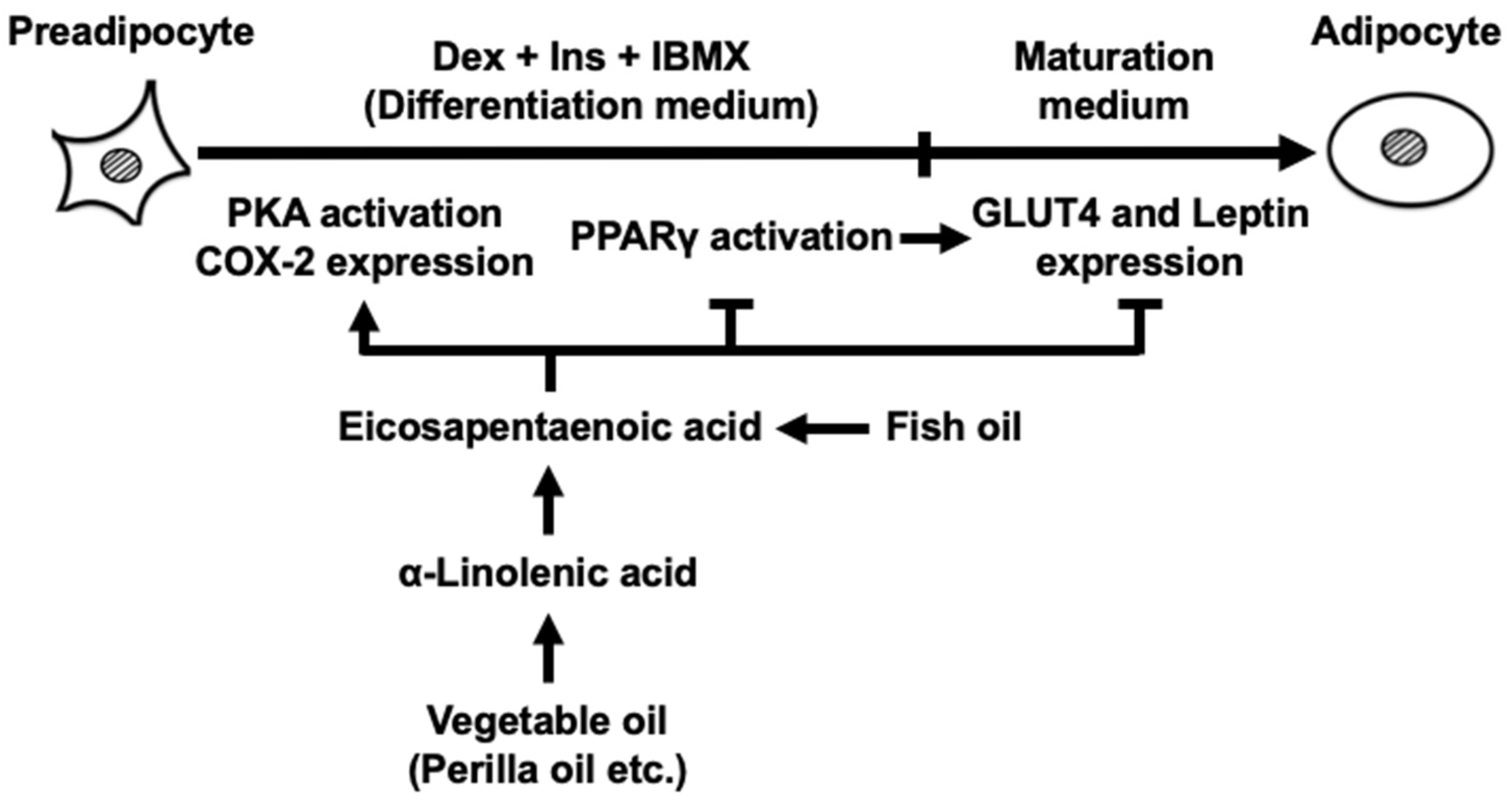
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Adipogenesis Induction by 3T3-L1 Cell Culture
2.3. Measurement of Intracellular Triacylglycerols and Proteins
2.4. Visualization of Intracellular Lipids by Oil Red O Staining
2.5. Measurement of Gene Expression Levels
2.6. Data Presentation and Statistical Analysis
3. Results
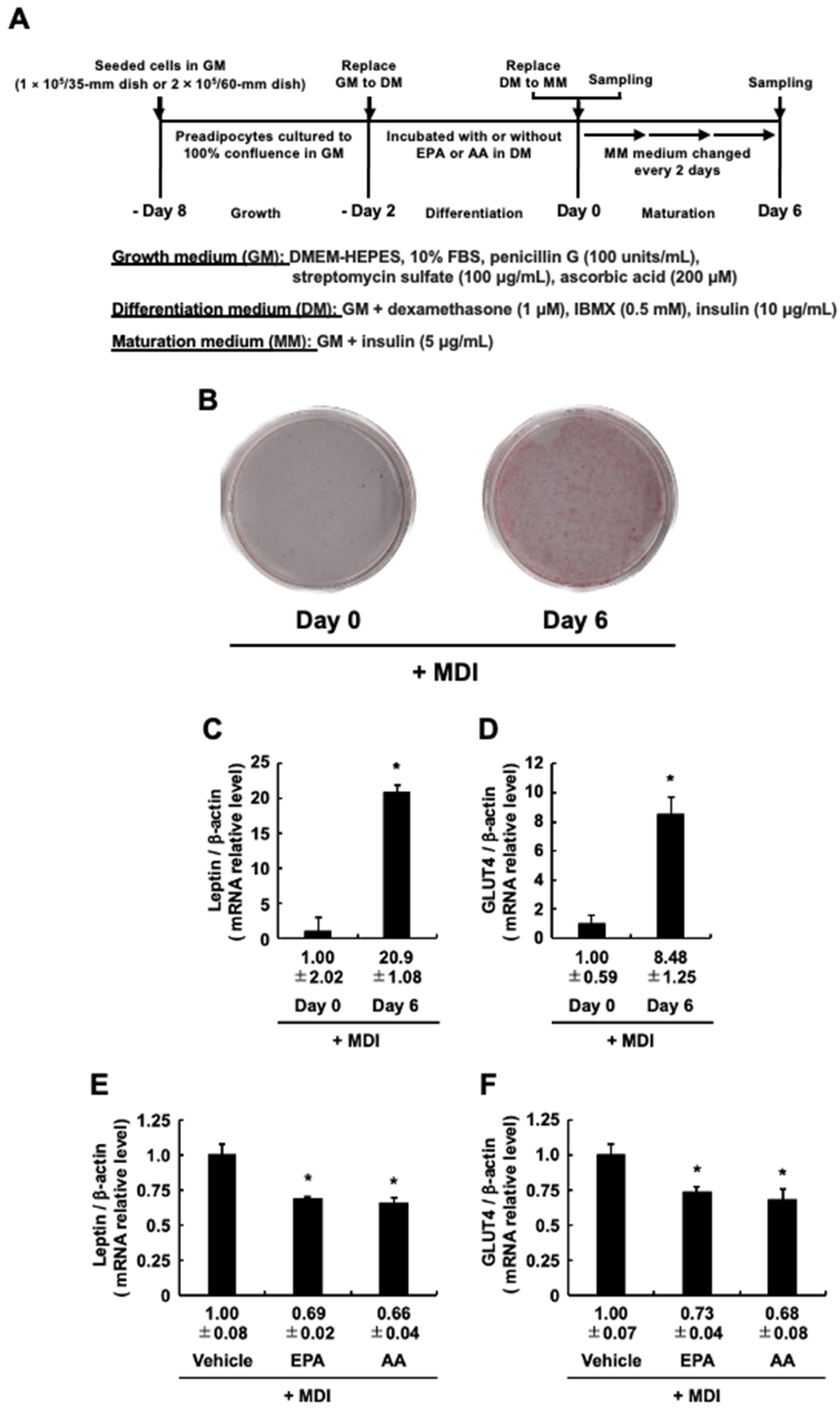
3.1. Addition of EPA during the Differentiation Stage Effectively Inhibited Adipogenesis Evoked by MDI
3.2. Adipocyte-Specific Marker Genes Expression during the Differentiation Stage When 3T3-L1 Cells Are Cultured with EPA or AA
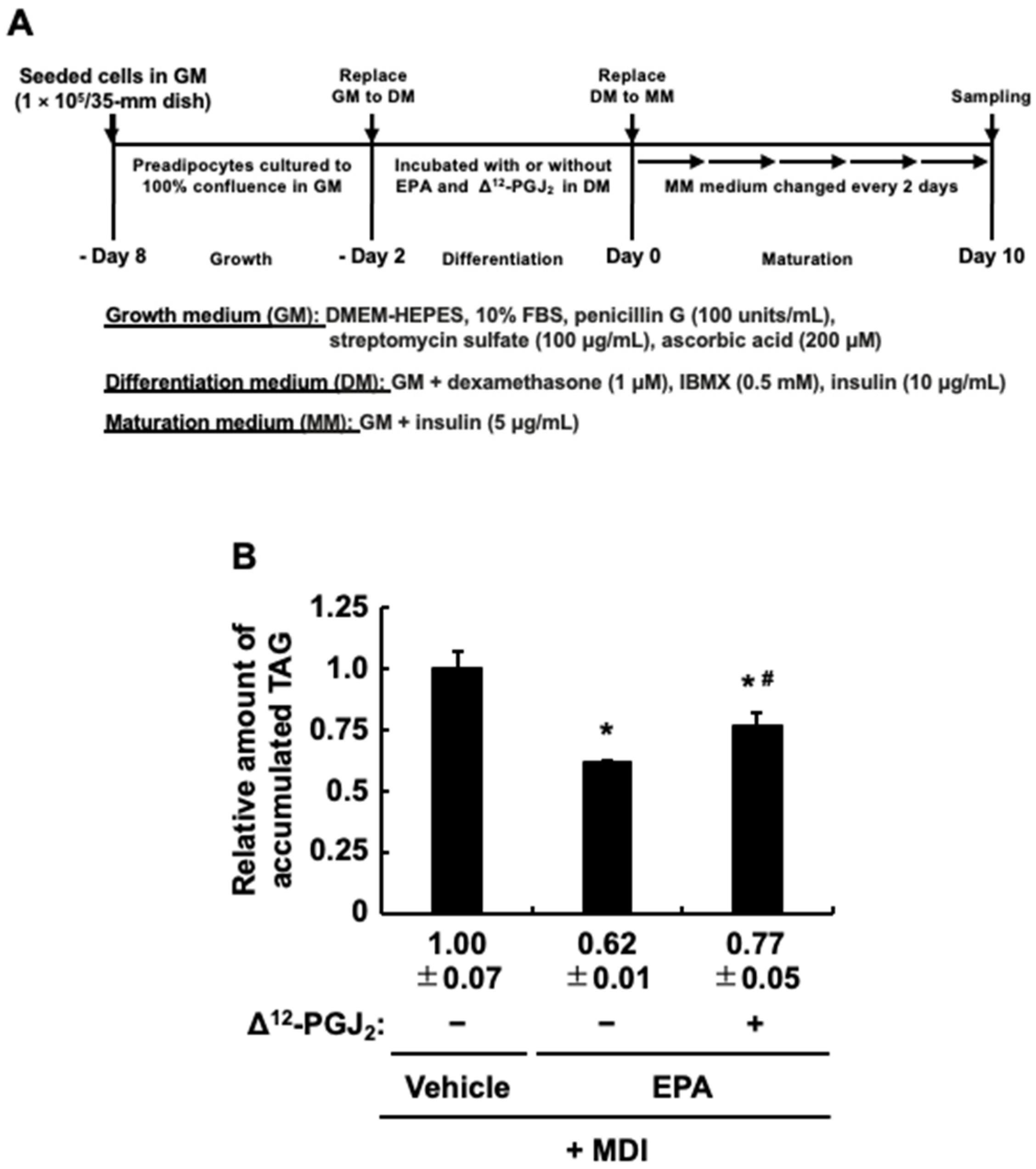
3.3. Δ12-PGJ2, a PPARγ Activator, Partially Attenuates the Anti-Adipogenic Effect of EPA
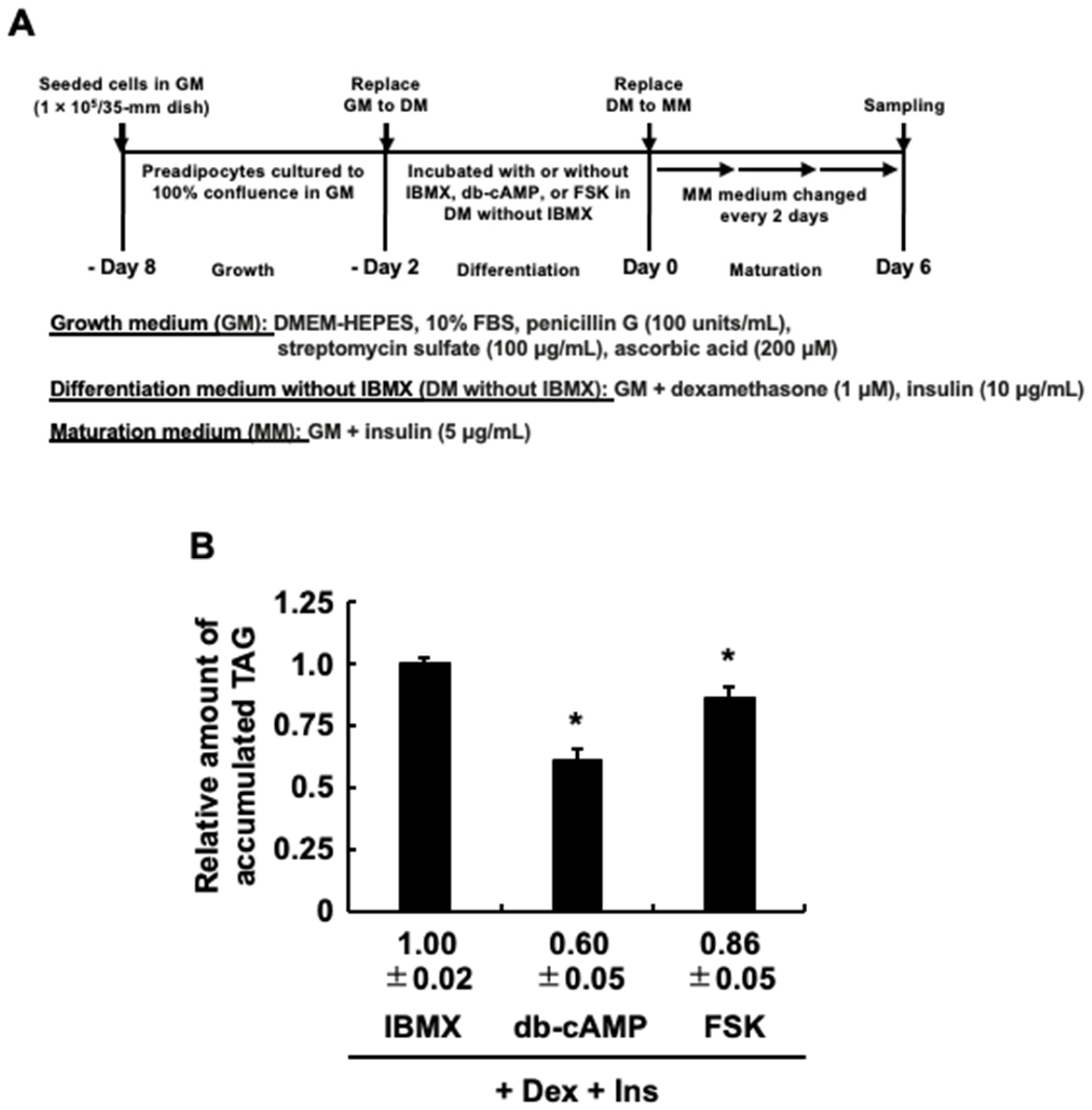
3.4. The Impact of Protein Kinase A (PKA) Activation on the Effect of EPA Added Exclusively during the Differentiation Stage of 3T3-L1 Cells on Adipogenesis Evoked by the MDI Cocktail
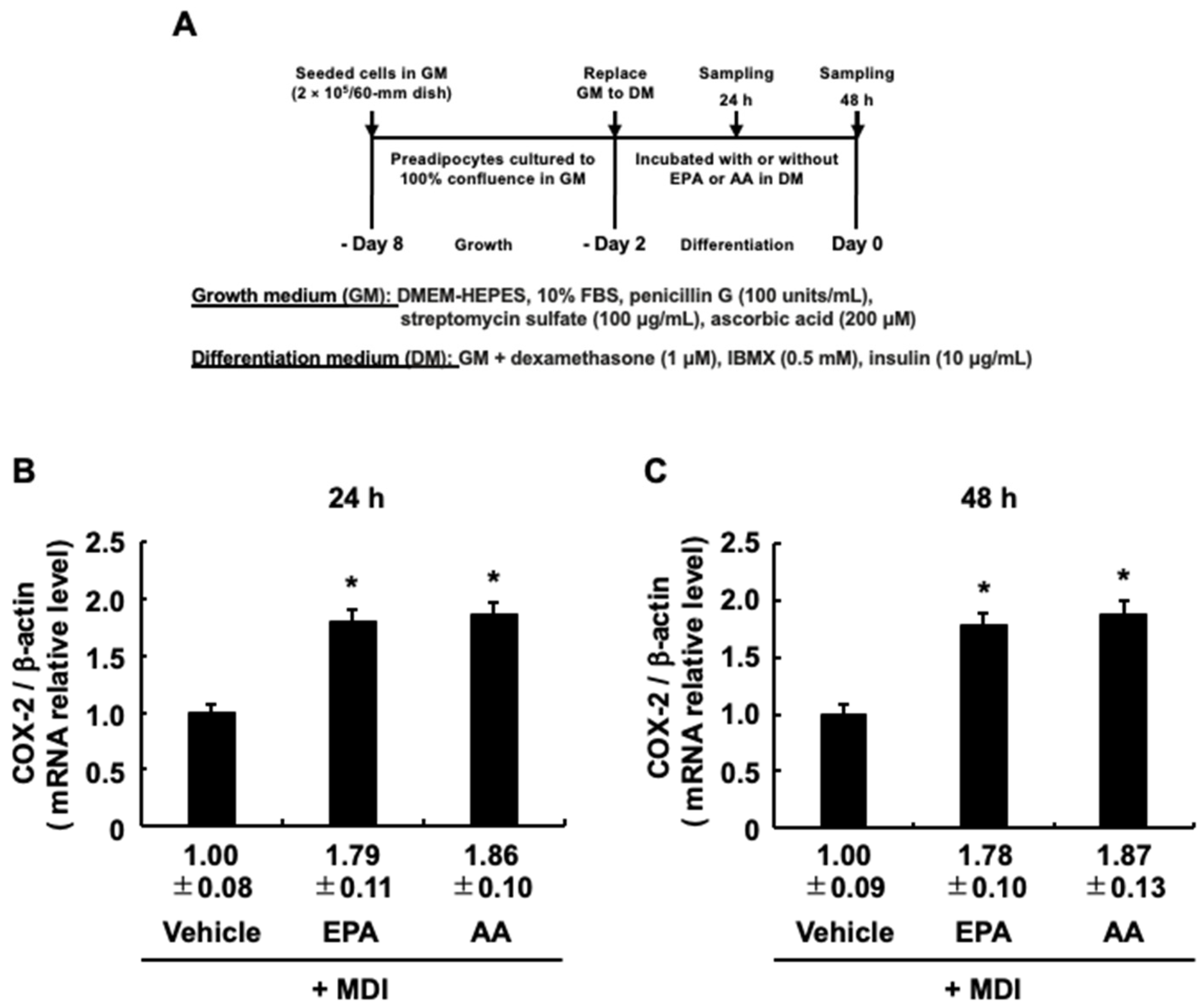
3.5. EPA-Induced COX-2 Expression during the Differentiation Stage
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seidell, J.C.; Halberstadt, J. The global burden of obesity and the challenges of prevention. Ann. Nutr. Metab. 2015, 66 (Suppl. S2), 7–12. [Google Scholar] [CrossRef] [PubMed]
- Green, H.; Kehinde, O. Sublines of mouse 3T3 cells that accumulate lipid. Cell 1974, 1, 113–116. [Google Scholar] [CrossRef]
- Green, H.; Kehinde, O. An established preadipose cell line and its differentiation in culture II. Factors affecting the adipose conversion. Cell 1975, 5, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Chu, X.; Nishimura, K.; Jisaka, M.; Nagaya, T.; Shono, F.; Yokota, K. Up-regulation of adipogenesis in adipocytes expressing stably cyclooxygenase-2 in the antisense direction. Prostaglandins Other Lipid Mediat. 2010, 91, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chu, X.; Xu, L.; Nishimura, K.; Jisaka, M.; Nagaya, T.; Shono, F.; Yokota, K. Suppression of adipogenesis program in cultured preadipocytes transfected stably with cyclooxygenase isoforms. Biochim. Biophys. Acta 2009, 1791, 273–280. [Google Scholar] [CrossRef]
- De Rooij, J.; Zwartkruis, F.J.; Verheijen, M.H.; Cool, R.H.; Nijman, S.M.; Wittinghofer, A.; Bos, J.L. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature 1998, 396, 474–477. [Google Scholar] [CrossRef]
- DiPilato, L.M.; Cheng, X.; Zhang, J. Fluorescent indicators of cAMP and Epac activation reveal differential dynamics of cAMP signaling within discrete subcellular compartments. Proc. Natl. Acad. Sci. USA 2004, 101, 16513–16518. [Google Scholar] [CrossRef]
- Osterrieder, W.; Brum, G.; Hescheler, J.; Trautwein, W.; Flockerzi, V.; Hofmann, F. Injection of subunits of cyclic AMP-dependent protein kinase into cardiac myocytes modulates Ca2+ current. Nature 1982, 298, 576–588. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.S. Prostacyclin: A major prostaglandin in the regulation of adipose tissue development. J. Cell. Physiol. 2019, 234, 3254–3262. [Google Scholar] [CrossRef]
- Sugimoto, Y.; Tsuboi, H.; Okuno, Y.; Tamba, S.; Tsuchiya, S.; Tsujimoto, G.; Ichikawa, A. Microarray evaluation of EP4 receptor-mediated prostaglandin E2 suppression of 3T3-L1 adipocyte differentiation. Biochem. Biophys. Res. Commun. 2004, 322, 911–917. [Google Scholar] [CrossRef]
- Tsuboi, H.; Sugimoto, Y.; Kainoh, T.; Ichikawa, A. Prostanoid EP4 receptor is involved in suppression of 3T3-L1 adipocyte differentiation. Biochem. Biophys. Res. Commun. 2004, 322, 1066–1072. [Google Scholar] [CrossRef]
- Inazumi, T.; Shirata, N.; Morimoto, K.; Takano, H.; Segi-Nishida, E.; Sugimoto, Y. Prostaglandin E2-EP4 signaling suppresses adipocyte differentiation in mouse embryonic fibroblasts via an autocrine mechanism. J. Lipid Res. 2011, 52, 1500–1508. [Google Scholar] [CrossRef]
- Miller, C.W.; Casimir, D.A.; Ntambi, J.M. The mechanism of inhibition of 3T3-L1 preadipocyte differentiation by prostaglandin F2α. Endocrinology 1996, 137, 5641–5650. [Google Scholar] [CrossRef]
- Fujimori, K.; Ueno, T.; Nagata, N.; Kashiwagi, K.; Aritake, K.; Amano, F.; Urade, Y. Suppression of Adipocyte Differentiation by Aldo-keto Reductase 1B3 Acting as Prostaglandin F2α Synthase. J. Biol. Chem. 2010, 285, 8880–8886. [Google Scholar] [CrossRef]
- Forman, B.M.; Tontonoz, P.; Chen, J.; Brun, R.P.; Spiegelman, B.M.; Evans, R.M. 15-Deoxy-Δ12,14-Prostaglandin J2 is a ligand for the adipocyte determination factor PPARγ. Cell 1995, 83, 803–812. [Google Scholar] [CrossRef] [PubMed]
- Kliewer, S.A.; Lenhard, J.M.; Willson, T.M.; Patel, I.; Morris, D.C.; Lehmann, J.M. A prostaglandin J2 metabolite binds peroxisome proliferator-activated receptor γ and promotes adipocyte differentiation. Cell 1995, 83, 813–819. [Google Scholar] [CrossRef]
- Wei, W.; Hu, M.; Huang, J.; Yu, S.; Li, X.; Li, Y.; Mao, L. Anti-obesity effects of DHA and EPA in high fat-induced insulin resistant mice. Food Funct. 2021, 12, 1614–1625. [Google Scholar] [CrossRef]
- Martínez-Fernández, L.; Laiglesia, L.M.; Huerta, A.E.; Martínez, J.A.; Moreno-Aliaga, M.J. Omega-3 fatty acids and adipose tissue function in obesity and metabolic syndrome. Prostaglandins Other Lipid Mediat. 2015, 121, 24–41. [Google Scholar] [CrossRef]
- Oh, D.Y.; Talukdar, S.; Bae, E.J.; Imamura, T.; Morinaga, H.; Fan, W.Q.; Li, P.; Lu, W.J.; Watkins, S.M.; Olefsky, J.M. GPR120 Is an Omega-3 Fatty Acid Receptor Mediating Potent Anti-inflammatory and Insulin-Sensitizing Effects. Cell 2010, 142, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Xie, W.; Lei, T.; Hamilton, J.A. Eicosapentaenoic acid, but not oleic acid, stimulates β-oxidation in adipocytes. Lipids 2005, 40, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Martins, F.F.; Aguila, M.B.; Mandarim-De-Lacerda, C.A. Eicosapentaenoic and docosapentaenoic acids lessen the expression of PPARγ/Cidec affecting adipogenesis in cultured 3T3-L1 adipocytes. Acta Histochem. 2020, 122, 151504. [Google Scholar] [CrossRef]
- Manickam, E.; Sinclair, A.J.; Cameron-Smith, D. Suppressive actions of eicosapentaenoic acid on lipid droplet formation in 3T3-L1 adipocytes. Lipids Health Dis. 2010, 9, 57. [Google Scholar] [CrossRef]
- Barber, E.; Sinclair, A.J.; Cameron-Smith, D. Comparative actions of omega-3 fatty acids on in-vitro lipid droplet formation. Prostaglandins Leukot. Essent. Fat. Acids 2013, 89, 359–366. [Google Scholar] [CrossRef]
- Nartey, M.N.N.; Jisaka, M.; Syeda, P.K.; Nishimura, K.; Shimizu, H.; Yokota, K. Arachidonic Acid Added during the Differentiation Phase of 3T3-L1 Cells Exerts Anti-Adipogenic Effect by Reducing the Effects of Pro-Adipogenic Prostaglandins. Life 2023, 13, 367. [Google Scholar] [CrossRef] [PubMed]
- Nartey, M.N.N.; Jisaka, M.; Syeda, P.K.; Nishimura, K.; Shimizu, H.; Yokota, K. Prostaglandin D2 Added during the Differentiation of 3T3-L1 Cells Suppresses Adipogenesis via Dysfunction of D-Prostanoid Receptor P1 and P2. Life 2023, 13, 370. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.S.; Khan, F.; Syeda, P.K.; Nishimura, K.; Jisaka, M.; Nagaya, T.; Shono, F.; Yokota, K. Endogenous synthesis of prostacyclin was positively regulated during the maturation phase of cultured adipocytes. Cytotechnology 2014, 66, 635–646. [Google Scholar] [CrossRef] [PubMed]
- Zając-Grabiec, A.; Bartusek, K.; Sroczyńska, K.; Librowski, T.; Gdula-Argasińska, J. Effect of Eicosapentaenoic Acid Supplementation on Murine Preadipocytes 3T3-L1 Cells Activated with Lipopolysaccharide and/or Tumor Necrosis Factor-α. Life 2021, 11, 977. [Google Scholar] [CrossRef]
- Krey, G.; Braissant, O.; L’horset, F.; Kalkhoven, E.; Perroud, M.; Parker, M.G.; Wahli, W. Fatty Acids, Eicosanoids, and Hypolipidemic Agents Identified as Ligands of Peroxisome Proliferator-Activated Receptors by Coactivator-Dependent Receptor Ligand Assay. Mol. Endocrinol. 1997, 11, 779–791. [Google Scholar] [CrossRef] [PubMed]
- Tishinsky, J.M.; Ma, D.W.; Robinson, L.E. Eicosapentaenoic Acid and Rosiglitazone Increase Adiponectin in an Additive and PPARγ-Dependent Manner in Human Adipocytes. Obesity 2011, 19, 262–268. [Google Scholar] [CrossRef]
- Petersen, R.K.; J⊘Rgensen, C.; Rustan, A.C.; Frøyland, L.; Muller-Decker, K.; Furstenberger, G.; Berge, R.K.; Kristiansen, K.; Madsen, L. Arachidonic acid-dependent inhibition of adipocyte differentiation requires PKA activity and is associated with sustained expression of cyclooxygenases. J. Lipid Res. 2003, 44, 2320–2330. [Google Scholar] [CrossRef]
- Madsen, L.; Pedersen, L.M.; Liaset, B.; Ma, T.; Petersen, R.K.; van den Berg, S.; Pan, J.; Müller-Decker, K.; Dülsner, E.D.; Kleemann, R.; et al. cAMP-dependent Signaling Regulates the Adipogenic Effect of n-6 Polyunsaturated Fatty Acids. J. Biol. Chem. 2008, 283, 7196–7205. [Google Scholar] [CrossRef]
- Longvah, T.; Deosthale, Y.G. Chemical and nutritional studies on hanshi (perilla frutescens), a traditional oilseed from northeast india. J. Am. Oil Chem. Soc. 1991, 68, 781–784. [Google Scholar] [CrossRef]
- Shin, H.-S.; Kim, S.-W. Lipid composition of perilla seed. J. Am. Oil Chem. Soc. 1994, 71, 619–622. [Google Scholar] [CrossRef]
- Yoshikiyo, K.; Yoshioka, Y.; Narumiya, Y.; Oe, S.; Kawahara, H.; Kurata, K.; Shimizu, H.; Yamamoto, T. Thermal stability and bioavailability of inclusion complexes of perilla oil with γ-cyclodextrin. Food Chem. 2019, 294, 56–59. [Google Scholar] [CrossRef] [PubMed]
- Yoshikiyo, K.; Takahashi, M.; Narumiya, Y.; Honda, M.; Iwasaki, K.; Ishigaki, M.; Nagato, E.G.; Noothalapati, H.; Shimizu, H.; Murota, K.; et al. Co-ingestion with γ-cyclodextrin improves bioavailability of α-linolenic acid in Perilla frutescens seed oil. Food Hydrocoll. Health 2023, 3, 100116. [Google Scholar] [CrossRef]
- Harris, R.B. Direct and indirect effects of leptin on adipocyte metabolism. Biochim. Biophys. Acta 2014, 1842, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.; Mei, F.C.; Cheng, X. Epac not PKA catalytic subunit is required for 3T3-L1 preadipocyte differentiation. Front. Biosci. 2010, E2, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Gabrielli, M.; Martini, C.N.; Brandani, J.N.; Iustman, L.J.; del Romero, D.G.; Vila, M.d.C. Exchange protein activated by cyclic AMP is involved in the regulation of adipogenic genes during 3T3-L1 fibroblasts differentiation. Dev. Growth Differ. 2014, 56, 143–151. [Google Scholar] [CrossRef]
- Li, F.; Wang, D.; Zhou, Y.; Zhou, B.; Yang, Y.; Chen, H.; Song, J. Protein kinase A suppresses the differentiation of 3T3-L1 preadipocytes. Cell Res. 2008, 18, 311–323. [Google Scholar] [CrossRef]
- Szentandrássy, N.; Pérez-Bido, M.R.; Alonzo, E.; Negretti, N.; O’Neill, S.C. Protein kinase A is activated by the n−3 polyunsaturated fatty acid eicosapentaenoic acid in rat ventricular muscle. J. Physiol. 2007, 582, 349–358. [Google Scholar] [CrossRef]
- Yamada, H.; Umemoto, T.; Kakei, M.; Momomura, S.-I.; Kawakami, M.; Ishikawa, S.-E.; Hara, K. Eicosapentaenoic acid shows anti-inflammatory effect via GPR120 in 3T3-L1 adipocytes and attenuates adipose tissue inflammation in diet-induced obese mice. Nutr. Metab. 2017, 14, 33. [Google Scholar] [CrossRef]
- Husted, A.S.; Ekberg, J.H.; Tripp, E.; Nissen, T.A.D.; Meijnikman, S.; O’Brien, S.L.; Ulven, T.; Acherman, Y.; Bruin, S.C.; Nieuwdorp, M.; et al. Autocrine negative feedback regulation of lipolysis through sensing of NEFAs by FFAR4/GPR120 in WAT. Mol. Metab. 2020, 42, 101103. [Google Scholar] [CrossRef]
- Nikolopoulou, E.; Papacleovoulou, G.; Jean-Alphonse, F.; Grimaldi, G.; Parker, M.G.; Hanyaloglu, A.C.; Christian, M. Arachidonic acid-dependent gene regulation during preadipocyte differentiation controls adipocyte potential. J. Lipid Res. 2014, 55, 2479–2490. [Google Scholar] [CrossRef]
- Sun, X.J.; Rothenberg, P.; Kahn, C.R.; Backer, J.M.; Araki, E.; Wilden, P.A.; Cahill, D.A.; Goldstein, B.J.; White, M.F. Structure of the insulin receptor substrate IRS-1 defines a unique signal transduction protein. Nature 1991, 352, 73–77. [Google Scholar] [CrossRef]
- White, M.F.; Kahn, C.R. The insulin signaling system. J. Biol. Chem. 1994, 269, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Schmitz-Peiffer, C.; Whitehead, J.P. IRS-1 Regulation in Health and Disease. IUBMB Life 2003, 55, 367–374. [Google Scholar] [CrossRef]
- Horike, N.; Takemori, H.; Katoh, Y.; Doi, J.; Min, L.; Asano, T.; Sun, X.J.; Yamamoto, H.; Kasayama, S.; Muraoka, M.; et al. Adipose-specific Expression, Phosphorylation of Ser794 in Insulin Receptor Substrate-1, and Activation in Diabetic Animals of Salt-inducible Kinase-2. J. Biol. Chem. 2003, 278, 18440–18447. [Google Scholar] [CrossRef] [PubMed]
- Katoh, Y.; Takemori, H.; Horike, N.; Doi, J.; Muraoka, M.; Min, L.; Okamoto, M. Salt-inducible kinase (SIK) isoforms: Their involvement in steroidogenesis and adipogenesis. Mol. Cell. Endocrinol. 2004, 217, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.F.; Herschkovitz, A.; Boura-Halfon, S.; Ronen, D.; Paz, K.; Leroith, D.; Zick, Y. Serine phosphorylation proximal to its phosphotyrosine binding domain inhibits insulin receptor substrate 1 function and promotes insulin resistance. Mol. Cell. Biol. 2004, 24, 9668–9681. [Google Scholar] [CrossRef]
- Smith, P.J.; Wise, L.S.; Berkowitz, R.; Wan, C.; Rubin, C.S. Insulin-like growth factor-I is an essential regulator of the differentiation of 3T3-L1 adipocytes. J. Biol. Chem. 1988, 263, 9402–9408. [Google Scholar] [CrossRef]
- Reginato, M.J.; Krakow, S.L.; Bailey, S.T.; Lazar, M.A. Prostaglandins Promote and Block Adipogenesis through Opposing Effects on Peroxisome Proliferator-activated Receptor γ. J. Biol. Chem. 1998, 273, 1855–1858. [Google Scholar] [CrossRef] [PubMed]
- Fujimori, K.; Yano, M.; Ueno, T. Synergistic Suppression of Early Phase of Adipogenesis by Microsomal PGE Synthase-1 (PTGES1)-Produced PGE2 and Aldo-Keto Reductase 1B3-Produced PGF2α. PLoS ONE 2012, 7, e44698. [Google Scholar] [CrossRef][Green Version]


| GenBank Accession No. | Target Genes | Primers (5→3′) | Length (bp) | Tm (°C) | Product Length (bp) |
|---|---|---|---|---|---|
| NM_008493 | Leptin | F: TTTCACACACGCAGTCGGTA | 20 | 59.90 | 149 |
| R: CACATTTTGGGAAGGCAGGC | 20 | 60.04 | |||
| AB008453 | Glut4 | F: GGATTCCATCCCACAAGGCA | 20 | 60.03 | 158 |
| R: CCAACACGGCCAAGACATTG | 20 | 60.04 | |||
| NM_011198.5 | COX-2 | F: GTTTGTTGAGTCATTCACCAG | 21 | 55.36 | 224 |
| R: TGTAGAGGGCTTTCAATTCTG | 21 | 55.59 | |||
| NM_007393.5 | β-Actin | F: GCGGGCGACGATGCT | 15 | 59.84 | 197 |
| R: TGCCAGATCTTCTCCATGTCG | 21 | 59.86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nartey, M.N.N.; Shimizu, H.; Sugiyama, H.; Higa, M.; Syeda, P.K.; Nishimura, K.; Jisaka, M.; Yokota, K. Eicosapentaenoic Acid Induces the Inhibition of Adipogenesis by Reducing the Effect of PPARγ Activator and Mediating PKA Activation and Increased COX-2 Expression in 3T3-L1 Cells at the Differentiation Stage. Life 2023, 13, 1704. https://doi.org/10.3390/life13081704
Nartey MNN, Shimizu H, Sugiyama H, Higa M, Syeda PK, Nishimura K, Jisaka M, Yokota K. Eicosapentaenoic Acid Induces the Inhibition of Adipogenesis by Reducing the Effect of PPARγ Activator and Mediating PKA Activation and Increased COX-2 Expression in 3T3-L1 Cells at the Differentiation Stage. Life. 2023; 13(8):1704. https://doi.org/10.3390/life13081704
Chicago/Turabian StyleNartey, Michael N. N., Hidehisa Shimizu, Hikaru Sugiyama, Manami Higa, Pinky Karim Syeda, Kohji Nishimura, Mitsuo Jisaka, and Kazushige Yokota. 2023. "Eicosapentaenoic Acid Induces the Inhibition of Adipogenesis by Reducing the Effect of PPARγ Activator and Mediating PKA Activation and Increased COX-2 Expression in 3T3-L1 Cells at the Differentiation Stage" Life 13, no. 8: 1704. https://doi.org/10.3390/life13081704
APA StyleNartey, M. N. N., Shimizu, H., Sugiyama, H., Higa, M., Syeda, P. K., Nishimura, K., Jisaka, M., & Yokota, K. (2023). Eicosapentaenoic Acid Induces the Inhibition of Adipogenesis by Reducing the Effect of PPARγ Activator and Mediating PKA Activation and Increased COX-2 Expression in 3T3-L1 Cells at the Differentiation Stage. Life, 13(8), 1704. https://doi.org/10.3390/life13081704







