Homoarginine Associates with Carotid Intima-Media Thickness and Atrial Fibrillation and Predicts Adverse Events after Stroke
Abstract
1. Introduction
2. Patients and Methods
2.1. Study Design, Ethical Approval, and Patient Consent
2.2. Clinical Assessment
2.3. Liquid Chromatography–Tandem Mass Spectrometric Measurement of Homoarginine
2.4. Statistical Analyses
3. Results
3.1. Homoarginine Levels and Stroke Risk Factors
3.2. Homoarginine Levels and Stroke Outcome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GBD 2019 Stroke Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021, 20, 795–820. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, D.H.; Polak, J.F.; Kronmal, R.A.; Manolio, T.A.; Burke, G.L.; Wolfson, S.K., Jr. Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N. Engl. J. Med. 1999, 340, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, M.W.; Markus, H.S.; Bots, M.L.; Rosvall, M.; Sitzer, M. Prediction of clinical cardiovascular events with carotid intima-media thickness: A systematic review and meta-analysis. Circulation 2007, 115, 459–467. [Google Scholar] [CrossRef]
- Choe, C.-U.; Atzler, D.; Wild, P.S.; Carter, A.M.; Böger, R.H.; Ojeda, F.; Simova, O.; Stockebrand, M.; Lackner, K.; Nabuurs, C.; et al. Homoarginine Levels Are Regulated by L-Arginine: Glycine Amidinotransferase and Affect Stroke Outcome. Circulation 2013, 128, 1451–1461. [Google Scholar] [CrossRef] [PubMed]
- Karetnikova, E.S.; Jarzebska, N.; Markov, A.G.; Weiss, N.; Lentz, S.R.; Rodionov, R.N. Is Homoarginine a Protective Cardiovascular Risk Factor? Arterioscler. Thromb. Vasc. Biol. 2019, 39, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Bell, E.A. The isolation of L-homoarginine from seeds of Lathyrus cicera. Biochem. J. 1962, 85, 91–93. [Google Scholar] [CrossRef]
- Rao, S.L. A look at the brighter facets of β-N-oxalyl-l-α,β-diaminopropionic acid, homoarginine and the grass pea. Food Chem. Toxicol. 2011, 49, 620–622. [Google Scholar] [CrossRef]
- Lambein, F.; Travella, S.; Kuo, Y.H.; Van Montagu, M.; Heijde, M. Grass pea (Lathyrus sativus L.): Orphan crop, nutraceutical or just plain food? Planta 2019, 250, 821–838. [Google Scholar] [CrossRef]
- Schmitz, M.; Hagemeister, H.; Erbersdobler, H.F. Homoarginine labeling is suitable for determination of protein absorption in miniature pigs. J. Nutr. 1991, 121, 1575–1580. [Google Scholar] [CrossRef]
- Bollenbach, A.; Cordts, K.; Hanff, E.; Atzler, D.; Choe, C.U.; Schwedhelm, E.; Tsikas, D. Evidence by GC-MS that lysine is an arginase-catalyzed metabolite of homoarginine in vitro and in vivo in humans. Anal. Biochem. 2019, 577, 59–66. [Google Scholar] [CrossRef]
- Papageorgiou, N.; Androulakis, E.; Papaioannou, S.; Antoniades, C.; Tousoulis, D. Homoarginine in the shadow of asymmetric dimethylarginine: From nitric oxide to cardiovascular disease. Amino Acids 2015, 47, 1741–1750. [Google Scholar] [CrossRef]
- Tsikas, D.; Bollenbach, A.; Hanff, E.; Kayacelebi, A.A. Asymmetric dimethylarginine (ADMA), symmetric dimethylarginine (SDMA) and homoarginine (hArg): The ADMA, SDMA and hArg paradoxes. Cardiovasc. Diabetol. 2018, 17, 1. [Google Scholar] [CrossRef]
- Ryu, W.S.; Lee, S.H.; Kim, C.K.; Kim, B.J.; Yoon, B.W. Increased serum alkaline phosphatase as a predictor of long-term mortality after stroke. Neurology 2010, 75, 1995–2002. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.; Che, D.; Qin, G.; Farouk, M.H.; Hailong, J.; Rui, H. Novel Biosynthesis, Metabolism and Physiological Functions of L-Homoarginine. Curr. Protein Pept. Sci. 2019, 20, 184–193. [Google Scholar] [CrossRef]
- Atzler, D.; Schwedhelm, E.; Nauck, M.; Ittermann, T.; Böger, R.H.; Friedrich, N. Serum reference intervals of homoarginine, ADMA, and SDMA in the study of health in Pomerania. Clin. Chem. Lab. Med. 2014, 52, 1835–1842. [Google Scholar] [CrossRef] [PubMed]
- Atzler, D.; Appelbaum, S.; Cordts, K.; Ojeda, F.M.; Wild, P.S.; Münzel, T.; Blankenberg, S.; Böger, R.H.; Blettner, M.; Beutel, M.E.; et al. Reference intervals of plasma homoarginine from the German Gutenberg Health Study. Clin. Chem. Lab. Med. 2016, 54, 1231–1237. [Google Scholar] [CrossRef]
- Valtonen, P.; Laitinen, T.; Lyyra-Laitinen, T.; Raitakari, O.T.; Juonala, M.; Viikari, J.S.; Heiskanen, N.; Vanninen, E.; Punnonen, K.; Heinonen, S. Serum L-homoarginine concentration is elevated during normal pregnancy and is related to flow-mediated vasodilatation. Circ. J. 2008, 72, 1879–1884. [Google Scholar] [CrossRef]
- Atzler, D.; Gore, M.O.; Ayers, C.R.; Choe, C.U.; Böger, R.H.; de Lemos, J.A.; McGuire, D.K.; Schwedhelm, E. Homoarginine and cardiovascular outcome in the population-based Dallas Heart Study. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2501–2507. [Google Scholar] [CrossRef] [PubMed]
- Haghikia, A.; Yanchev, G.R.; Kayacelebi, A.A.; Hanff, E.; Bledau, N.; Widera, C.; Sonnenschein, K.; Haghikia, A.; Weissenborn, K.; Bauersachs, J.; et al. The role of L-arginine/L-homoarginine/nitric oxide pathway for aortic distensibility and intima-media thickness in stroke patients. Amino Acids 2017, 49, 1111–1121. [Google Scholar] [CrossRef]
- Cordts, K.; Grzybowski, R.; Lezius, S.; Lüneburg, N.; Atzler, D.; Neu, A.; Hornig, S.; Böger, R.H.; Gerloff, C.; Magnus, T.; et al. Guanidino compound ratios are associated with stroke etiology, internal carotid artery stenosis and CHA(2)DS(2)-VASc score in three cross-sectional studies. J. Neurol. Sci. 2019, 397, 156–161. [Google Scholar] [CrossRef]
- Atzler, D.; Baum, C.; Ojeda, F.; Keller, T.; Cordts, K.; Schnabel, R.B.; Choe, C.U.; Lackner, K.J.; Münzel, T.; Böger, R.H.; et al. Low Homoarginine Levels in the Prognosis of Patients With Acute Chest Pain. J. Am. Heart Assoc. 2016, 5, e002565. [Google Scholar] [CrossRef] [PubMed]
- Büttner, P.; Bahls, M.; Böger, R.H.; Hindricks, G.; Thiele, H.; Schwedhelm, E.; Kornej, J. Arginine derivatives in atrial fibrillation progression phenotypes. J. Mol. Med. 2020, 98, 999–1008. [Google Scholar] [CrossRef] [PubMed]
- Schwedhelm, E.; Song, R.J.; Vasan, R.S.; van den Heuvel, E.R.; Hannemann, J.; Xanthakis, V.; Böger, R. Association of Lower Plasma Homoarginine Concentrations with Greater Risk of All-Cause Mortality in the Community: The Framingham Offspring Study. J. Clin. Med. 2020, 9, 2016. [Google Scholar] [CrossRef] [PubMed]
- Zinellu, A.; Paliogiannis, P.; Carru, C.; Mangoni, A. Homoarginine and all-cause mortality: A systematic review and meta-analysis. Eur. J. Clin. Investig. 2018, 48, e12960. [Google Scholar] [CrossRef] [PubMed]
- Schwedhelm, E.; Schwieren, L.; Tiedt, S.; Lucadou, M.V.; Gloyer, N.-O.; Böger, R.; Magnus, T.; Daum, G.; Thomalla, G.; Gerloff, C.; et al. Serum Sphingosine-1-Phosphate Levels Are Associated With Severity and Outcome in Patients With Cerebral Ischemia. Stroke 2021, 52, 3901–3907. [Google Scholar] [CrossRef]
- Atzler, D.; Mieth, M.; Maas, R.; Böger, R.H.; Schwedhelm, E. Stable isotope dilution assay for liquid chromatography–tandem mass spectrometric determination of l-homoarginine in human plasma. J. Chromatogr. B 2011, 879, 2294–2298. [Google Scholar] [CrossRef]
- Cordts, K.; Atzler, D.; Qaderi, V.; Sydow, K.; Böger, R.H.; Choe, C.U.; Schwedhelm, E. Measurement of homoarginine in human and mouse plasma by LC-MS/MS and ELISA: A comparison and a biological application. Amino Acids 2015, 47, 2015–2022. [Google Scholar] [CrossRef]
- Stockebrand, M.; Hornig, S.; Neu, A.; Atzler, D.; Cordts, K.; Böger, R.H.; Isbrandt, D.; Schwedhelm, E.; Choe, C.U. Homoarginine supplementation improves blood glucose in diet-induced obese mice. Amino Acids 2015, 47, 1921–1929. [Google Scholar] [CrossRef]
- Mokhaneli, M.C.; Fourie, C.M.T.; Botha-Le Roux, S.; Böger, R.H.; Schwedhelm, E.; Mels, C.M.C. Asymmetric dimethylarginine and L-homoarginine prospectively relate to carotid wall thickness in a South African cohort. Amino Acids 2020, 52, 965–973. [Google Scholar] [CrossRef]
- Jaźwińska-Kozuba, A.; Martens-Lobenhoffer, J.; Kruszelnicka, O.; Rycaj, J.; Chyrchel, B.; Surdacki, A.; Bode-Böger, S.M. Opposite Associations of Plasma Homoarginine and Ornithine with Arginine in Healthy Children and Adolescents. Int. J. Mol. Sci. 2013, 14, 21819–21832. [Google Scholar] [CrossRef]
- Nitz, K.; Lacy, M.; Bianchini, M.; Wichapong, K.; Kücükgöze, I.A.; Bonfiglio, C.A.; Migheli, R.; Wu, Y.; Burger, C.; Li, Y.; et al. The Amino Acid Homoarginine Inhibits Atherogenesis by Modulating T-Cell Function. Circ. Res. 2022, 131, 701–712. [Google Scholar] [CrossRef]
- Niekamp, C.; Atzler, D.; Ojeda, F.M.; Sinning, C.R.; Lackner, K.J.; Böger, R.H.; Munzel, T.; Beutel, M.E.; Schmidtmann, I.; Pfeiffer, N.; et al. Cross-Sectional Associations between Homoarginine, Intermediate Phenotypes, and Atrial Fibrillation in the Community-The Gutenberg Health Study. Biomolecules 2018, 8, 86. [Google Scholar] [CrossRef] [PubMed]
- Mokhaneli, M.C.; Botha-Le Roux, S.; Fourie, C.M.T.; Böger, R.; Schwedhelm, E.; Mels, C.M.C. L-homoarginine is associated with decreased cardiovascular- and all-cause mortality. Eur. J. Clin. Investig. 2021, 51, e13472. [Google Scholar] [CrossRef] [PubMed]
- Mokhaneli, M.C.; Botha-Le Roux, S.; Fourie, C.M.T.; Böger, R.; Schwedhelm, E.; Mels, C.M.C. Homoarginine and blood pressure: A 10-year prospective relationship in normotensives. J. Hum. Hypertens. 2022, 36, 135–143. [Google Scholar] [CrossRef]
- Seppälä, I.; Oksala, N.; Jula, A.; Kangas, A.J.; Soininen, P.; Hutri-Kähönen, N.; März, W.; Meinitzer, A.; Juonala, M.; Kähönen, M.; et al. The biomarker and causal roles of homoarginine in the development of cardiometabolic diseases: An observational and Mendelian randomization analysis. Sci. Rep. 2017, 7, 1130. [Google Scholar] [CrossRef]
- Atzler, D.; Schönhoff, M.; Cordts, K.; Ortland, I.; Hoppe, J.; Hummel, F.C.; Gerloff, C.; Jaehde, U.; Jagodzinski, A.; Böger, R.H.; et al. Oral supplementation with L-homoarginine in young volunteers. Br. J. Clin. Pharmacol. 2016, 82, 1477–1485. [Google Scholar] [CrossRef]
- Schönhoff, M.; Weineck, G.; Hoppe, J.; Hornig, S.; Cordts, K.; Atzler, D.; Gerloff, C.; Böger, R.; Neu, A.; Schwedhelm, E.; et al. Cognitive performance of 20 healthy humans supplemented with L-homoarginine for 4 weeks. J. Clin. Neurosci. 2018, 50, 237–241. [Google Scholar] [CrossRef]
- Kleist, C.J.; Choe, C.U.; Atzler, D.; Schönhoff, M.; Böger, R.; Schwedhelm, E.; Wicha, S.G. Population kinetics of homoarginine and optimized supplementation for cardiovascular risk reduction. Amino Acids 2022, 54, 889–896. [Google Scholar] [CrossRef]
| Characteristics | 1st Tertile (n = 125) <1.0 µmol/L | 2nd Tertile (n = 124) 1–1.5 µmol/L | 3rd Tertile (n = 125) >1.5 µmol/L | p-Value |
|---|---|---|---|---|
| Demographic parameters | ||||
| Age, years | 72.9 (11.6) | 67.4 (13.1) | 63.3 (12.6) | <0.001 *** |
| Male sex, % | 58 (46.4) | 91 (73.4) | 93 (74.4) | <0.001 *** |
| Smoking, % | 20 (16.0) | 42 (33.9) | 32 (25.6) | 0.004 ** |
| Hypertension, % | 91 (72.8) | 92 (74.2) | 87 (69.6) | 0.815 |
| Hyperlipidaemia, % | 34 (27.2) | 44 (35.5) | 43 (34.4) | 0.315 |
| Diabetes, % | 19 (15.2) | 27 (21.8) | 17 (13.6) | 0.259 |
| Atrial fibrillation, % | 37 (29.6) | 29 (23.4) | 15 (12.0) | 0.003 ** |
| Prior myocardial infarct, % | 15 (12.0) | 11 (8.9) | 15 (12.0) | 0.660 |
| Prior stroke, % | 15 (12.0) | 28 (22.6) | 19 (15.2) | 0.071 |
| BMI, kg/m2 | 26.7 (4.2) | 25.8 (4.3) | 26.5 (4.9) | 0.278 |
| Laboratory parameters | ||||
| HbA1c, % | 5.7 [5.4, 6.0] | 5.7 [5.4, 6.2] | 5.6 [5.4, 6.0] | 0.841 |
| GFR, mL/min | 72 [55, 89] | 77 [62, 92] | 84 [67, 97] | 0.001 ** |
| Triglycerides, mg/dL | 112 [87, 160] | 124 [93, 171] | 128 [97, 199] | 0.014 * |
| HDL, mg/dL | 49 [40, 60] | 49 [40, 61] | 46 [38, 56] | 0.319 |
| LDL, mg/dL | 105 [81, 134] | 108 [75, 137] | 101 [75, 132] | 0.710 |
| Homoarginine, µmol/L | 0.77 [0.59, 0.87] | 1.22 [1.11, 1.34] | 1.94 [1.66, 2.21] | <0.001 *** |
| Medication | ||||
| Blood-thinning, % | 105 (84.0) | 107 (86.3) | 109 (87.2) | 0.756 |
| Lipid-lowering, % | 81 (64.8) | 94 (75.8) | 95 (76.0) | 0.078 |
| Antihypertensive, % | 93 (74.4) | 90 (72.6) | 85 (68.0) | 0.512 |
| Neurological parameters | ||||
| NIHSS, points | 2 [0, 4] | 2 [0, 3] | 1 [0, 3] | 0.019 * |
| cIMT, mm | 1.4 [1.1, 1.8] | 1.2 [1.0, 1.6] | 1.2 [1.0, 1.4] | <0.001 * |
| Correlation Coefficient r | FDR-Corrected p–Value | |
|---|---|---|
| Age | −0.312 | <0.001 * |
| BMI | −0.042 | 0.431 |
| NIHSS at admission | −0.140 | 0.007 * |
| GFR | 0.225 | <0.001 * |
| Triglycerides | 0.159 | 0.002 * |
| cIMT | −0.232 | <0.001 * |
| Model | NIHSS | cIMT | AF | ||||
|---|---|---|---|---|---|---|---|
| Mean Factor [95% CI] | p-Value | Mean Factor [95% CI] | p-Value | Odds Ratio [95% CI] | p-Value | ||
| Homoarginine (per 2-fold increase) | 1 | −0.77 [−1.35, −0.19] | 0.010 * | −0.18 [−0.26, −0.10] | <0.001 *** | 0.41 [0.25, 0.67] | <0.001 *** |
| 2 | −0.65 [−1.27, −0.04] | 0.038 * | −0.13 [−0.22, −0.05] | 0.002 ** | 0.53 [0.32, 0.89] | 0.017 * | |
| 3 | −0.68 [−1.30, −0.07] | 0.030 * | −0.14 [−0.22, −0.05] | 0.002 ** | 0.57 [0.33, 0.96] | 0.036 * | |
| Model | 3rd Tertile Versus 1st Tertile | ||
|---|---|---|---|
| Hazard Ratio [95% CI] | p-Value | ||
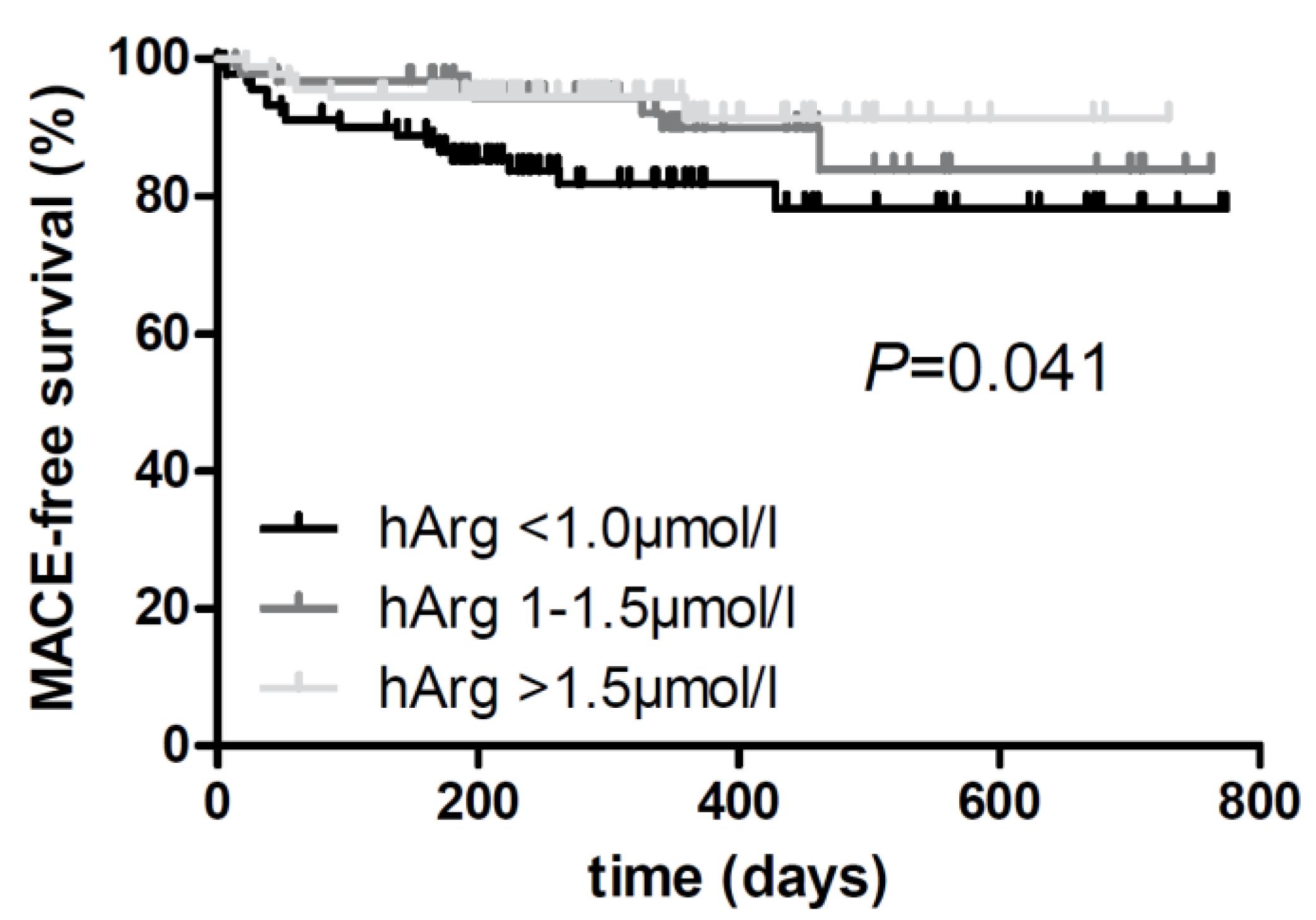
| Risk of major adverse events | 1 | 0.37 [0.14, 0.94] | 0.036 * |
| 2 | 0.24 [0.09, 0.66] | 0.006 ** | |
| 3 | 0.22 [0.08, 0.63] | 0.005 ** | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schwieren, L.; Jensen, M.; Schulz, R.; Lezius, S.; Laxy, E.; Milatz, M.; Thomalla, G.; Böger, R.; Gerloff, C.; Magnus, T.; et al. Homoarginine Associates with Carotid Intima-Media Thickness and Atrial Fibrillation and Predicts Adverse Events after Stroke. Life 2023, 13, 1590. https://doi.org/10.3390/life13071590
Schwieren L, Jensen M, Schulz R, Lezius S, Laxy E, Milatz M, Thomalla G, Böger R, Gerloff C, Magnus T, et al. Homoarginine Associates with Carotid Intima-Media Thickness and Atrial Fibrillation and Predicts Adverse Events after Stroke. Life. 2023; 13(7):1590. https://doi.org/10.3390/life13071590
Chicago/Turabian StyleSchwieren, Laura, Märit Jensen, Robert Schulz, Susanne Lezius, Elena Laxy, Magalie Milatz, Götz Thomalla, Rainer Böger, Christian Gerloff, Tim Magnus, and et al. 2023. "Homoarginine Associates with Carotid Intima-Media Thickness and Atrial Fibrillation and Predicts Adverse Events after Stroke" Life 13, no. 7: 1590. https://doi.org/10.3390/life13071590
APA StyleSchwieren, L., Jensen, M., Schulz, R., Lezius, S., Laxy, E., Milatz, M., Thomalla, G., Böger, R., Gerloff, C., Magnus, T., Schwedhelm, E., & Choe, C.-u. (2023). Homoarginine Associates with Carotid Intima-Media Thickness and Atrial Fibrillation and Predicts Adverse Events after Stroke. Life, 13(7), 1590. https://doi.org/10.3390/life13071590







