Abstract
The Latin word lupus, meaning wolf, was in the medical literature prior to the 1200s to describe skin lesions that devour flesh, and the resources available to physicians to help people were limited. The present text reviews the ethnobotanical and pharmacological aspects of medicinal plants and purified molecules from natural sources with efficacy against lupus conditions. Among these molecules are artemisinin and its derivatives, antroquinonol, baicalin, curcumin, emodin, mangiferin, salvianolic acid A, triptolide, the total glycosides of paeony (TGP), and other supplements such as fatty acids and vitamins. In addition, medicinal plants, herbal remedies, mushrooms, and fungi that have been investigated for their effects on different lupus conditions through clinical trials, in vivo, in vitro, or in silico studies are reviewed. A special emphasis was placed on clinical trials, active phytochemicals, and their mechanisms of action. This review can be helpful for researchers in designing new goal-oriented studies. It can also help practitioners gain insight into recent updates on supplements that might help patients suffering from lupus conditions.
1. Introduction
Systemic lupus erythematosus (SLE) is an autoimmune disease involving multiple organs and clinical manifestations. SLE is more common in young women. The incidence of SLE in males compared to females is 1:5~10 [1,2]. In SLE, autoantibodies and antibody-immune complexes are produced that eventually cause damage to body tissues and induce inflammation [3,4]. SLE patients experience relapsing and remission courses [5]. In SLE, various organs can be involved, including the skin, kidneys, joints, heart, lungs, liver, and blood vessels [6,7]. Since different organs are involved in SLE, a variety of indices can be used to assess the status of diseases, such as the Systemic Lupus Erythematosus Disease Activity Index (SLEDAI), Safety of Estrogens in Lupus Erythematosus National Assessment (SELENA), or British Isles Lupus Activity Group (BILAG) index [8].
The level of anti-double-stranded DNA (anti-dsDNA) antibodies is associated with disease activity, and anti-dsDNA plays an important role in the pathogenesis of SLE. In some cases, the goal of treatment is to bring the level of anti-dsDNA antibodies back to normal (5). Mechanisms involved in kidney damage due to lupus nephritis include dysregulation of T-regulatory cells due to overactivity of B and T lymphocytes, activation of inflammatory responses, improper production of autoantibodies, and deposition of immune complexes in kidney tissue [9,10].
The current medications used to treat SLE include glucocorticoids, immunosuppressive drugs, non-steroidal anti-inflammatory drugs, anti-malarial drugs, systemic lymph node irradiation therapy, and plasma treatment. Despite this, the morbidity and mortality ratios in SLE patients are still unacceptably high [6,11]. The mentioned medications lead patients to be exposed to side effects and also reduce the patient’s quality of life [12]. This encourages patients to try complementary and alternative medicines such as herbal remedies, medicinal plants, phytochemicals, vitamins and mineral supplements, acupuncture, moxibustion, and spiritual therapy such as yoga. Moreover, tremendous efforts have been made by researchers to develop safe and efficient drugs and supplements from natural molecules and their synthetic derivatives for the condition.
Some previous articles can be found on the treatment of lupus conditions that are not on natural products or supplements and mostly discussed orthodox medicine [13,14,15,16,17,18]. Some other review articles discussing natural products on a specific condition of lupus, such as cutaneous lupus [19] or lupus nephritis [20], can be found in the literature. Some discussed only a specific natural compound such as curcumin [21], omega-3 fatty acids [22], and triptolide [23]. On the other hand, some other review articles reflected a specific traditional medicine approach such as traditional Chinese medicine (TCM), traditional Iranian medicine (TIM) [24], or Ayurveda [25], which mostly focused on polyherbal formulations and plant extracts that are composed of a complex mixture of phytochemicals with a holistic approach. Some of these articles have neglected minerals, vitamins, and pure active phytoconstituents. Conversely, some reviews and meta-analyses specifically focused on fatty acids [22,26,27] or vitamins [28,29]. Moreover, some articles focused mostly on pharmacology and possible mechanisms of action of a group of natural components without scoping clinical trials or meta-analyses [30]. All the mentioned articles provided precious information for specific groups of researchers and highlighted specific aspects of treatment and drug development for lupus conditions. But a comprehensive systematic review that simultaneously gives detailed evidence-based information on the efficacy of natural products and supplements on different lupus conditions, which helps practitioners and clinicians to understand the mechanism of action and the level of evidence for any of these molecules, herbs, or supplements and researchers to design new goal-oriented experimental studies or clinical trials, seems to be lacking. The present article tries to cover the last-mentioned points in this paragraph and reviewed natural molecules, phytochemicals, medicinal plants, fungi, vitamins, minerals, and other supplements that have been reported to be beneficial for lupus conditions, with special emphasis on clinical trials and the molecules’ mechanism of actions, as well as their adverse effects and toxicity.
2. Materials and Methods
A comprehensive literature search was conducted in PubMed, Cochrane Library, Web of Science, Scopus, the National Library of Medicine (NLM) catalog, and Google Scholar, from January 1970 up until January 2023. The obtained records were assessed for eligibility in accordance with the PRISMA 2000 guidelines.
Inclusion criteria: Combinations of various keywords including systemic lupus erythematosus, SLE, lupus nephritis, or lupus AND natural molecule, natural medicine, phytochemicals, herbal medicine, medicinal plants, fungi, mushrooms, minerals, vitamins, fatty acids, supplements, nutrition, meta-analysis, and clinical trial, toxicity, and side effects have been considered in the search strategy. Moreover, the compound names and scientific names of the identified plants were searched again with keywords related to lupus. No restriction was set on the language. A special focus was set on reported purified molecules from natural sources including artemisinin and its derivatives, antroquinonol, baicalin, curcumin, emodin, mangiferin, salvianolic acid A, triptolide, the total glycosides of paeony (TGP), some fatty acids, and vitamins.
Exclusion criteria: (1) Duplicate article; (2) addressed a natural compound, but not related to lupus conditions; (3) article on lupus conditions, but not related to natural compounds; (4) did not address specific natural compounds or plant extracts; (5) studies involving entirely synthetic molecules or antibodies; (6) herbal remedies from traditional medicine that had not been scientifically evaluated for lupus conditions; and (7) polyherbal formulations from traditional medicine.
Extraction of data: The following entries were included: (1) study ID; (2) title; (3) aim or objective of the study; (4) study design; (5) possible conflicts of interests for study authors; (6) participants/population description (humans, animal, cell lines, etc.); (7) the total number of participants; (8) inclusion criteria; (9) exclusion criteria; (10) total number of experimental repeats; (11) the tested material (plants/compounds/extracts, fungi, mushroom, pure molecule, etc.); (12) the active ingredients or molecule were tested; (13) the used positive or negative controls and placebo; (14) primary outcome and findings on lupus conditions (lupus nephritis, cutaneous lupus, systemic lupus erythematosus (SLE), etc.)—was the intervention statically effective? (15) primary outcome—inflammation—was the intervention statically effective? (16) primary outcome—immunomodulation—was the intervention statically effective? (17) primary outcome—other organ systems—was the intervention statically efficacious? (18) the reported adverse effects for molecules or extracts; (19) the reported interaction (with drugs, food, or herbs) for the tested molecules or extracts.
3. Results
The search strategy yielded 14,300 studies. The titles or abstracts were reviewed to exclude duplicates or irrelevant ones. Excluded were 13,887 records that were identified as irrelevant, duplicate, or not reliable. As a result, 413 studies were included in the review and 74 were included in the synthesized tables.
3.1. Ethnobotany
The Latin word lupus, meaning wolf, was in the medical literature prior to the 1200s to describe skin lesions that devour flesh, and the resources available to physicians to help people were limited [31,32]. Traditional knowledge on how to deal with this condition involves the use of several medicinal plants or plant-based mixtures. Ethnobotanical and ethnopharmacological studies reveal that Cinchona spp. [33] and “Thanatka” made of Hesperethusa crenulata and Limonia acidissima bark [34] have dermatologic uses, specifically in the treatment of lupus erythematosus. Also, sieketroos Arctopus species [35], Juniperus species [36], Onopordum acanthium [37], and Centella asiatica [38] were documented to treat systemic lupus erythematosus. According to Iranian traditional medicine (traditional Persian medicine), infectious diseases and fever are the main reasons for nephritis, which is called “Varam-e-Kolye”. Several medicinal plants have been advised to control for lupus nephritis or “Varam-e-Kolye”, which are Anethum graveolens L., Carum carvi L., Coriandrum sativum L., Cucurbita pepo L., Cydonia oblonga Mill., Ficus carica L., Linum usitatissimum L., Melissa officinalis L., Prunus amygdalus, and Ziziphus jujuba Mill. Some recent research reported nephroprotective and anti-inflammatory properties of these plants [24,39,40].
As examples, Cuminum cyminum L. (in Persian كرويا or زيره سبز), Carum carvi L. (in Persian كمون كرماني or زيره سياه), Lagoecia cuminoides L. (in Persian زيره وحشي or قردمانا) [41,42], and Bunium persicum (Boiss.) Fedtch (in Persian Zire Kermani) [39] are other plants advised for Varam-e-Kolye and/or other kidney diseases such as “Riah-e-Gorde” [41,42]. Carvia (كرويا) is the Arabic version of the Latin word “craviya” or the Syriac word “Ceravi”; in Greek the word is “Azhamyon”, in Roman “Fadroni”, and in Arabic “Taghdeh”, “Taghrad”, and “Comone Roomi” [42]. B. persicum has shown antiglycation, antioxidant, anti-inflammatory, and nephroprotective (possibly due to antiglycation) effects [43,44,45].
According to the literature, several medicinal plants and fungi have been considered to be beneficial for conditions related to lupus. This review focused on those that are evidence-based with in vitro or in vivo studies or clinical trials. The ethnobotanical aspects of these plants are summarized in Table 1.

Table 1.
Main botanical aspects and pharmacological benefits of medicinal plants, mushrooms, and fungi advised for lupus conditions.
3.2. Purified Molecules from Natural Sources
Different herbal remedies, medicinal plants, and mushrooms have been utilized to cure a range of medical ailments in both developing and developed communities. Additionally, it is estimated that roughly 25% of currently marketed medicines were developed from the primary or secondary metabolites of natural medicines [90]. On the other hand, the absence of a well-organized regulatory and legal framework for herbal products has caused the World Health Organization (WHO) to express worry regarding the efficacy and safety of herbal treatments [91]. Due to varying growth circumstances and harvesting times, different primary and secondary metabolites have varying concentrations in medicinal plants [92]. These problems motivate researchers to find and purify the medicinal plant’s active components. Researchers have gained a greater understanding of the mechanisms of action by working with highly purified compounds. When compared to herbal extracts, pure natural molecules are more reliable at determining dosage and detecting unwanted effects or potential toxicities. Moreover, natural molecules can be considered lead compounds for developing new drugs. In the case of lupus, several natural products and their derivatives, in purified and structure-elucidated form, have been reported to exhibit considerable therapeutic potential. Although the mechanisms of action of some of these molecules have yet to be fully elucidated, more extensive research can generate new data that can be used in clinical trials. The reported data on these molecules is discussed in detail in the following (Table 2, Figure 1).

Table 2.
Purified molecules from natural sources and their derivatives, fatty acids, minerals, and vitamins with reported efficacy against lupus (↓: decrease in activity or level; ↑: increase in activity or level).
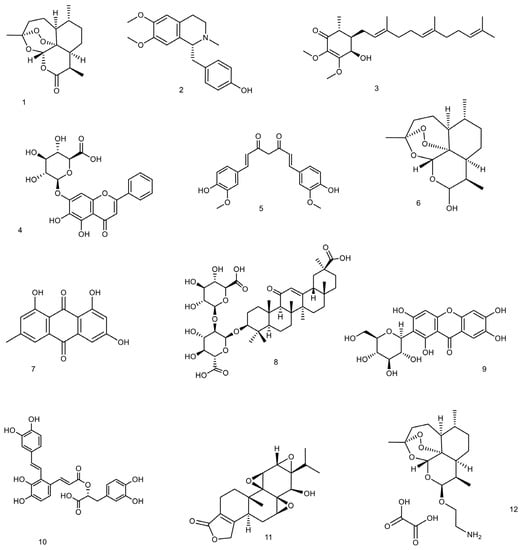
Figure 1.
The structures of small molecules from natural sources and their derivatives with reported efficacy against lupus conditions. The structures are 1: (+)—artemisinin, 2: (S)—armepavine, 3: antroquinonol, 4: baicalin, 5: curcumin, 6: dihydroartemisinin, 7: emodin, 8: glycyrrhizic acid, 9: mangiferin, 10: salvianolic acid A, 11: triptolide, and 12: β-aminoarteether maleate.
3.2.1. Artemisinin and Its Derivatives
Artemisinin is a sesquiterpene lactone with a peroxide bridge extracted from the plant Artemisia annua [138,139]. Several semi-synthetic derivatives of artemisinin with greater solubility or bioactivity, such as dihydroartemisinin, artemether, and arteether, artesunate have been developed and investigated in several research works [140,141].
Along with its anti-malarial effect, artemisinin and its derivatives have exhibited anti-inflammatory, immunoregulatory, and antioxidant properties [142]. Like some other conventional anti-malarial drugs, including chloroquine and hydroxychloroquine, artemisinin derivatives are assumed to have beneficial therapeutic effects on SLE [143,144]. But in contrast to chloroquine and hydroxychloroquine, which have serious side effects in some cases, no significant side effects have been associated with artemisinin except for mild side effects such as nausea and vomiting or diarrhea [145].
Studies that examined the effectiveness of artemisinin and its derivatives in patients with lupus have shown that long-term use can be effective in improving renal lesions and can prevent recurrence of lupus nephritis. They can relieve the symptoms of patients with SLE. They increased complement levels and also lowered creatinine and urinary protein levels and reduced erythrocyte sedimentation rates [144]. Artesunate increases CD3 and CD4 and increases the CD4/CD8 T lymphocytes ratio. It can regulate the immune function by increasing IL-2 activity and decreasing the level of soluble interleukin-2 receptor (sIL-2R) [144].
Artemisinin can be effective in improving kidney disorders by modulating immune-inflammatory responses. Anti-inflammatory effects of artemisinin are due to its ability to suppress nuclear factor-kB (NF-kB), phosphatidylinositol 3 kinase (PI3K)/protein kinase B (AKT) activity, signal transducer and activator of transcription (STAT), and toll-like receptors (TLRs) [142]. Following the use of artemisinin, the production of proinflammatory cytokines such as TNF-α, IL-6, IL-10, IL-17, and IL-21 is inhibited, but the production of anti-inflammatory cytokines such as IL-4 and IL-10 is increased [146].
Artesunate has suppressed the Jak2-Stat3 signaling pathway in MRL/lpr mice. It has also regulated T follicular helper cell differentiation; thus, it resulted in an increase in follicular regulatory T cells (Tfr) and a decrease in follicular T helper cells (Tfh). It has also reduced the levels of pathogenic cytokines such as IL-6, IFN-γ, and IL-21. It has reduced the level of anti-dsDNA antibodies deposited in the kidney. This means that it might be able to help lessen the symptoms of lupus nephritis [147].
Dihydroartemisinin has been shown to reduce the senescence of myeloid-derived suppressor cells (MDSCs) by regulating the Nrf2/HO-1 pathway. MDSCs are involved in exacerbating the pathogenesis of SLE [148]. Dihydroartemisinin can also restore balance in Treg/Th17 by inducing Foxp3 expression in T cells in mice model [106]. Therefore, dihydroartemisinin is assumed to be effective in improving the condition of SLE patients [148].
Toxicity and Side Effects
According to meta-analyses and large clinical studies on artemisinin and its derivatives, they did not demonstrate serious side effects. However, this group of compounds has a number of side effects that could be mentioned, such as neurotoxicity, genotoxicity, hematotoxicity, immunotoxicity, and cardiotoxicity. According to both animal and human studies, artemisinin toxicity is caused by long-term availability rather than by short-term peak concentrations. It is worth mentioning that taking artemisinin orally has a faster rate of elimination than administering it intramuscularly. Therefore, it provides a relatively safe route of administration. This explains why significant toxicities were discovered in the majority of animal research but not in those involving humans [149]. This topic is still open for further research [150].
3.2.2. Antroquinonol
Antroquinonol is a derivative of tetrahydro ubiquinone, which was found in the mycelium of Antrodia camphorata [151,152]. A. camphorata is a mushroom that grows in the inner cavity of the Cinnamomum kanehirai (Lauraceae) tree [153] and produces some antroquinonol drivatives, including antroquinonol, antroquinonol B, C, D, L, and M, and 4-acetyantroquinonol B [154]. Hocena is an antroquinonol capsule intended for the treatment of acute myeloid leukemia, hepatocellular carcinoma, and pancreatic cancer and has an orphan drug status from the US Food and Drug Administration [155]. Antroquinonol has been claimed to have the potential to prevent renal disorders and the worsening of lupus nephritis [156]. Inhibiting T cell activation and proliferation, lowering free radical and nitric oxide production, enhancing Nrf2 activation, and decreasing inflammation by inhibiting NF-kB function in the kidney are some of the proposed involved mechanisms [93,156].
In one study, the effect of antroquinonol on preventing the mild form of lupus nephritis from becoming severe was investigated. NZB/NZW F1 mice were used for this purpose and were treated orally with 15 mg/kg antroquinonol for 5 weeks. Eventually, A. camphorata reduced hematuria, proteinuria, and IL-18 production in the kidneys. T cell proliferation was also inhibited and Treg cell suppression was induced. Also, reactive oxygen species and nitric oxide production were inhibited, Nrf2 activation was increased, and NF-ĸB activation was inhibited. It was concluded that antroquinonol might be effective in preventing the progression of lupus nephritis [93]. In another study, antroquinonol reduced proteinuria and lowered creatinine and serum BUN levels. It also reduces the thickness of the kidney glomerular basement membrane and inhibits the production of TNF-α and IL-1β. Therefore, the use of A. camphorata in autoimmune diseases such as SLE can protect the kidneys [46].
Toxicity and Side Effects
In numerous research on animal toxicology, A. camphorata exhibited no obvious toxicity. Thus, no significant side effects or deaths were reported, and nausea, vomiting, and diarrhea were the most frequent side effects [157]. Although antroquinonol exhibits cytotoxic activities against cancer cell lines MCF-7, MDA-MB-231, Hep 3B, Hep G2, DU-145, and LNCaP with IC50 values ranging from 0.13 to 6.09 μM it is considered safe [158]. Antroquinonol dosages below 30 mg/kg/day do not appear to be associated with any adverse effects [159]. Overall, A. camphorata has revealed very little toxicity or side effects in clinical practice.
3.2.3. Baicalin
Baicalin is another compound that has a high potential to be considered as a bioactive molecule against SLE. It is a flavonoid isolated from the root of Scutellaria baicalensis and has anti-inflammatory and antioxidant effects [99]. Baicalin in MRL/lpr lupus-prone mice has been shown to reduce anti-ds-DNA antibody and urine protein levels. Baicalin has been able to inhibit mTOR activation and also reduce mTOR agonist-mediated Tfh cell expansion and increase Tfr cells. This molecule can inhibit IL-21 production, Tfh cell differentiation, and Foxp3+ regulatory T cell differentiation [98]. In a study on pristane-induced lupus in BALB/c mice, baicalin reduced the production of proinflammatory cytokines such as TNF-α, IL-6, IL-10, and IFN-γ. It also inhibited the overproduction of IL-6 and PGE2 and downregulated the aberrant activation of T cells. Thus, it was concluded that baicalin can reduce the severity of SLE and attenuate autoimmunity [99,160].
Toxicity and Side Effects
Scutellaria baicalensis has long been recognized as a safe and non-toxic herb. S. baicalensis oral preparation has no significant side effects; however, some patients may experience stomach discomfort, diarrhea, etc., and those with allergic constitutions may develop a blister-like medication eruption. When used in high doses of injectable preparations, S. baicalensis may also result in symptoms such as hypothermia, muscle discomfort, and leucopenia [161]. Some data about possible nephrotoxicity of high doses of baicalin are published, but as a whole, the safety and toxicity of this compound remain still insufficiently studied [162]. Various drug transporters and metabolic enzymes are involved in the disposition of baicalin, and they may be influenced or reciprocally influenced by co-administered medications. These factors can justify the wide herb-drug interactions between baicalin and chemical drugs. Baicalin can significantly alter the pharmacokinetics of medications that have a high protein binding affinity or share the same cytochrome P450 (CYP) enzymes. Phenacetin, theophylline, midazolam, dextromethorphan, nifedipine, and chlorzoxazone can be mentioned among drugs that can interfere with baicalin [163].
3.2.4. Curcumin
Curcumin is the major diarylheptanoid component of turmeric (Curcuma longa, Zingiberaceae) [21]. A variety of clinical trials assessing the curcumin effect on inflammation, skin, eye, CNS, respiratory, cardiovascular, gastrointestinal, urogenital, and metabolic disorders have been reported so far [164]. Since curcumin has shown immunomodulatory properties, it has been considered for the improvement of SLE patients. The recommended dosage for SLE ranges from 100–200 mg daily to 4.5 g/day [165]. Curcumin is found to have protective effects against aluminum toxicity and cisplatin-associated neurotoxicity and neuropathy [166,167]. Hypothetically, curcumin may help lupus induced peripheral neuropathy.
The immunomodulatory property of curcumin results from its interaction with various immune mediators, including B and T lymphocytes, macrophage and dendritic cells, cytokines, and various transcription factors such as nuclear factor kappa B (NF-κB), activator protein-1 (AP-1), and signal transducer and activator of transcription (STAT) [168,169,170,171,172,173]. It has been found that curcumin can inhibit the maturation and function of dendritic cells. This function of curcumin is achieved through reducing the expression of MHC-II and co-stimulatory molecules such as CD11c, CD40, CD54, CD80, CD83, CD86, CD252, and CD256. It can also be due to the reduction of proinflammatory cytokines such as IL-1, IL-6, IL-12, IL-12p40, IL-12p70, and TNF-α. In general, curcumin can keep dendritic cells in an immature state, and as a result, it suppresses dendritic cell-mediated stimulation of inflammatory T cells, which play a key role in the severity of symptoms observed in SLE [21].
In Vivo and In Vitro Studies
In a study that was conducted on six SLE patients and six healthy individuals, the balance between T helper 17 (Th17) and regulatory T cells (Treg) in SLE patients was investigated. The CD4+ cells of these people have been collected, stimulated by Th17 differentiating factors, and exposed to 0.1 and 1 µg/mL of curcumin. Finally, it was found that curcumin can decrease Th17 percentage, decrease IL-17a production, and can increase Treg percentage and increase TGF-β1 production on CD4+ T cells of SLE patients. In general, curcumin can modulate the Th17/Treg balance on CD4+ T cells of SLE patients without affecting healthy subjects [100].
A study conducted on lupus-prone female MRL/lpr mice has shown that curcumin has the potential to be considered for the treatment of lupus. In this study, mice were treated with 200 mg/kg of curcumin for 8 weeks. As a result, proteinuria, renal inflammation, and spleen size have decreased following the use of curcumin, and a decrease in NLRP3 inflammasome activation was also observed. Following in vitro studies, it has also been found that curcumin can inhibit anti-dsDNA serum induced expression of NLRP3 inflammasome in podocytes [101]. In another study, the ability of oral curcumin consumption to attenuate autoimmunity and renal injury during SLE was evaluated. In order to do this, the female NZBWF1 was given 500 mg/kg/day of curcumin through an oral gavage for 14 days. Finally, it was found that following the consumption of curcumin, weight and body composition were maintained and a decrease in spleen weight and renal injury (glomerulosclerosis) were observed compared to the control group. Ultimately, it has been determined that curcumin can modulate autoimmune activity and probably reduce renal injury in female mice with SLE [102].
In a study, the immune modulation effects of curcumin on pristane-induced lupus mice have been investigated. The female BALB/c mice received an intraperitoneal injection of 0.5 mL pristane for lupus induction. Afterwards, they were treated with 0, 12.5, 50, and 200 mg/kg of bw/day curcumin intragastrically for 16 weeks. As a result, the arthritis score and proteinuria level decreased. However, no significant alteration was observed in body weight. Following 200 mg/kg bw/day curcumin consumption, Th1, Th2, and Th17 percentages decreased, Treg percentages increased slightly, serum IL-6 and IFN-α levels decreased, and antinuclear antibody levels decreased significantly. Therefore, the results have shown that curcumin could be useful as a therapeutic intervention in SLE [103].
Toxicity and Side Effects
Long-standing safety data exist for curcumin. For instance, curcumin’s allowable daily intake (ADI) value is 0–3 mg/kg body weight, according to reports from the JECFA and EFSA organizations (the Joint United Nations and World Health Organization Expert Committee on Food Additives and the European Food Safety Authority, respectively) [174]. Despite its well-known safety, several unfavorable side effects have been documented. In a dose-response investigation, seven patients who received 500–12,000 mg and were monitored for 72 h reported symptoms including diarrhea, headache, rash, and yellow stools [175]. In a different study, some participants who received 0.45 to 3.6 g of curcumin per day for one to four months experienced diarrhea, nausea, and a rise in the levels of the enzymes lactate dehydrogenase and alkaline phosphatase in their serum [176].
3.2.5. Emodin
Emodin (1,3,8-trihydroxy-6-methylanthraquinone) is actually a natural anthraquinone that can be found in the barks and roots of many plants, lichens, and molds [177]. One of the main sources of emodin is Rheum palmatum (Polygonaceae) which is also known as Chinese rhubarb.
Emodin can reduce steroid resistance by inhibiting P-glycoprotein efflux function. Steroid therapy is part of the common treatment for SLE patients, and a decreased response to steroid therapy following overexpression of p-glycoprotein in peripheral lymphocytes has been observed in some patients [178].
An attempt was made to investigate the effect of emodin on nephritis in a study on BXSB lupus mice. Mice were treated with different doses of emodin for 30 days. As a result, it has been shown that following emodin consumption, the level of proteinuria is reduced and the expression of intercellular adhesion molecule-1 (ICAM 1) in the renal glomerulus is also reduced [107].
The effect of emodin on renal injury in lupus nephritis was investigated. Lupus-prone male BXSB mice were treated with 0, 5, 10, and 20 mg/kg/day emodin for 30 days. Finally, it was observed that following the administration of emodin, glomerular levels of TNF-α, ICAM-1, and fibronectin (FN) decreased, and the levels of urinary protein and serum anti-dsDNA antibody also decreased, and these decreases were dose-dependent. The mechanism of action of emodin is probably through inhibition of dsDNA antibody and decreased levels of TNF-α, ICAM-1, and FN in the glomeruli [108].
Toxicity and Side Effects
According to reports, emodin can reduce sperm motility in a dose-dependent manner in mice. Emodin has also been found to have dose- and time-dependent toxicity in kidney and liver cell lines. Intestinal discomfort and severe diarrhea brought on by an overdose of emodin due to its laxative properties lead to an electrolyte imbalance and dehydration [179]. Generally, it is also known to have kidney toxicity, hepatotoxicity, and reproductive toxicity, especially at high doses and long-term use [180] The extremely low bioavailability of emodin further limits its use in therapeutic applications [179].
3.2.6. Esculetin
Esculetin (also known as aesculetin, 6,7-dihydroxycoumarin, and cichorigenin) is a coumarin that has been isolated from a variety of medicinal and toxic plants such as Cichorium intybus (chicory) and in Hydrangea paniculate Siebold. In a study conducted on MRL/lpr mice, esculetin significantly attenuated renal impairment by reducing BUN, serum creatinine, and albuminuria. Esculetin could improve glomerular hypertrophy and tubular interstitial fibrosis and reduce mononuclear cell infiltration into the interstitium. It was suggested that this molecule could significantly down-regulate the complement cascade as well as the inflammation and fibrosis pathway. In addition, esculetin could up-regulate Nrf2-related antioxidation genes. The authors reported that esculetin could inhibit complement activation both in classical and alternative pathways. The molecule blocked the C3 convertase (C4b2a) to exert this inhibitory capability. Moreover, it was suggested that the antioxidation effect of esculetin was dependent on Nrf2 activation, which means that esculetin could inhibit NFκB nuclear translocation and TGFβ-smad3 profibrosis pathway [109]. Lupus nephritis is one of the important complications of lupus, and complement activation contributes to kidney injury; the inhibition of complement activation by herbal compounds might be beneficial for lupus. It was also reported that the coumarin derivates that are isolated from H. paniculata could improve renal injuries in cationized-BSA-induced membranous nephropathy. The suggested mechanism was the inhibition of complement activation and interleukin 10-mediated interstitial fibrosis [181].
Toxicity and Side Effects of Esculetin
Acute toxicity studies reported LD50 for intraperitoneal injection to mice as 1450 mg/kg and >2000 mg/kg by mouth. No reported adverse effects are known other than LD50 [182].
3.2.7. Mangiferin
The main source of mangiferin is Mangifera indica, although it is found in 96 species, 28 genera, and 19 families of angiospermic plants. Mangifera indica belongs to the family Anacardiaceae and is known as mango. Almost all parts of M. indica, such as fruits, twigs, leaves, and stem bark, contain mangiferin [183]. Mangiferin is a xanthonoid polyphenol with a variety of pharmacological effects such as anti-inflammatory, antioxidant, immunomodulatory, nephroprotective, hepatoprotective, anti-cancer, anti-diabetic, and anti-asthma [184]. According to certain research, its renal protective actions may be beneficial for those with lupus nephritis [166,167].
Mangiferin has been shown to improve lupus nephritis in lupus-prone B6/gld mice. In a study, the effect of mangiferin on lupus nephritis was investigated. Mice were treated orally with 20 or 40 mg/kg/day of mangiferin for 12 weeks. Finally, Mangiferin has been shown to be effective in treating lupus nephritis with its anti-inflammatory and immunomodulatory effects. Mangiferin was effective by suppressing mTOR signaling pathways, upregulating CD4+ FoxP3+ Tregs, and inhibiting T cell proliferation. Mangiferin improved renal immunopathology and reduced renal T cell infiltration. It also lowered serum creatinine and urinary protein levels and increased CD4+ FoxP3+ Treg frequencies in the spleens, lymph nodes, and kidneys [113].
Toxicity and Side Effects
Mangiferin is typically regarded as a non-toxic natural substance. Adults receiving 0.9 g of mangiferin orally demonstrated no toxicity. LD50 of the mangiferin was considered to be 400 mg/kg on mice [185]. Mangiferin was found to be safe and helpful in enhancing cellular function, according to numerous research works [186]. In a study that assessed the toxicity of mango leaf extract, which was given orally to rats for three months at a dose of 2 g/kg body weight per day, neither mortality nor toxic effects were observed [187]. The Mangifera indica leaf aqueous extract was not particularly mutagenic or genotoxic. Mangiferin has generally been shown to be safe in cell and animal research. In contrast, there are insufficient safety data from human research [186].
3.2.8. Salvianolic Acid A
Salvianolic acid A (or Dan phenolic acid A) is a phenolic compound extracted from Salvia miltiorrhiza (Lamiaceae family). The plant is also known as Chinese sage, Danshen, and red sage. Salvia species such as S. officinalis and S. miltiorrhiza have shown antioxidant, antibacterial, anti-cancer, and anti-diarrheal effects and have been used to treat lupus and autism, lower cholesterol, treat Alzheimer’s, reduce sweating, and reduce menopausal hot flashes [188].
In a study performed on BALB/c mice, the effect of salvianolic acid A isolated from the root of S. miltiorrhiza, on lupus nephritis was investigated. Mice were treated with 5 mg/kg/day of salvianolic acid A for 5 months. As a result, it was observed that following the consumption of salvianolic acid A, anti-Sm autoantibodies decreased, phosphorylation of IKK, IκB, and NFκB in kidney tissue was inhibited, and pathological effects were reduced [114].
Toxicity and Side Effects
In an acute toxicity study, the LD50 of salvianolic acid A was reported as 1161.2 mg/kg in mice. In dogs’ animal model, the minimum lethal dose and maximal non-lethal dose of salvianolic acid A were reported as 682 mg/kg and 455 mg/kg in dogs, respectively. Based on a 4-week repeated-dose, no observed adverse effect level was 20 mg/kg. It was suggested to examine liver and kidney function during the administration of salvianolic acid A in a clinic [189]. According to a system review of the drug’s safety, the clinical use of salvianolate injection did not result in the occurrence of any common or major side effects. Blood loss and allergic reactions are the most common adverse effects of salvianolic acid injections. In general, it has been determined that salvianolic acid is well tolerated in the general population. Rash, erythemas, pruritus, palpitations, headaches, dizziness, elevated blood bilirubin, elevated transaminases, elevated blood creatinine, positive fecal occult blood, and abnormal platelet count are among the most common adverse effects that were reported for salvianolic acids [190].
3.2.9. Triptolide
Triptolide is a diterpene triepoxide isolated from Tripterygium wilfordii Hook F (T. wilfordii). The molecule has immunosuppressive and anti-inflammatory effects and has been shown to have therapeutic effects on autoimmune and inflammatory diseases such as lupus nephritis, arthritis, neurodegenerative disorders, and asthma [23,139]. Despite the beneficial effects of Triptolide in the treatment of various inflammatory disorders, it should be noted that the use of T. wilfordii can cause severe toxicity and side effects. This may limit the clinical use of this plant [191]. Triptolide’s anti-inflammatory and immunosuppressive effects are due to its ability to inhibit the proliferation of immune cells and inflammation-related cells and reduce cytokines and proinflammatory mediators [192].
The effects of triptolide on SLE are assumed to be through induction of miR-125a-5p and an increase in the proportion of Treg [124]. Triptolide has been shown to reduce the expression of transforming growth factor-beta (TGF-β) and vascular cell adhesion molecule (VCAM-1) [193]. It can also reduce the expression of C3 and CD40, so it generally has immunosuppressive and anti-inflammatory effects and is useful in renal disorders [194]. Another way triptolide can be immunosuppressive and anti-inflammatory is through changing signaling pathways. Triptolide has been reported to inhibit nuclear factor-κB (NF-κB) signaling pathway [195], lower the IL-17 level, and suppress IL-6/signal transducer and transcription 3 (STAT3) signaling pathway [196].
(5R)-5-Hydroxytriptolide (LLDT-8) is a triptolide analogue. It has strong anti-inflammatory and immunosuppressive activity [196]. LLDT-8 improves anti-GBM glomerulonephritis because it can regulate Fcγ signaling pathway [197]. It can also improve lupus nephritis and reduce the infiltration of kidney immune cells because it inhibits the expression of renal chemokines [125].
Toxicity and Side Effects
The subject of triptolide‘s safety in clinical applications has been brought up because of its broad usage. Despite the valuable pharmacological effects of triptolide, its application requires particular caution because it is well known to have hepatotoxicity, nephrotoxicity, reproductive toxicity, etc. [198]. Hepatic cytochrome P450s are involved in the metabolism of triptolide, and triptolide toxicity and CPY3A also have a close relationship. Clinical case reports have shown through research that triptolide exposure can be involved in damaging a variety of organs, including the kidney, liver, heart, ovary, and testicles. Additionally, it has been shown that triptolide has a variety of harmful effects on cells, including damage to membranes, oxidative stress, endoplasmic reticulum stress, metabolism dysfunction, mitochondrial dysfunction, apoptosis, and autophagy [199].
3.2.10. Total Glycosides of Paeony (TGP)
Total glycosides of paeony (TGP) are extracted from the root of Paeonia lactiflora. TGP has long been used to treat autoimmune diseases [200]. The beneficial effects of TGP on lupus patients are dependent on its anti-inflammatory and immunosuppressive effects [201]. The effects of TGP on the production of proinflammatory cytokines, antibody production, apoptosis of lymphocytes, and lymphocyte proliferation are dual and dose-dependent [202]. TGP increased the mouse splenocytes’ proliferation at low doses (0.05~0.4 mg/L), while it decreased it at high doses (0.4~1.6 mg/L) [202,203,204]. The ratio of T helper cells to T suppressor cells (Th/Ts) increases at low doses of TGP (0.2 mg/L) and decreases at high doses of TGP (6.0 mg/L) [204]. IL-1 production increases at low doses of TGP (0.5~12.5 mg/L) and decreases at high doses of TGP (12.5~312.5 mg/L) [203]. IgM-antibody production increases at low doses of TGP (0.1~0.4 mg/L) and decreases at high doses of TGP (0.4~3.2 mg/L) [205,206]. Therefore, it is assumed that the immunomodulatory effects of TGP are dose-dependent, and the dose should be adjusted for best results.
The beneficial effect of TGP on SLE has been discussed in several studies. It was reported that the anti-inflammatory effect of TGP is due to its ability to inhibit the production of nitric oxide, leukotriene B4, and prostaglandin E2 [202]. TGP reduces the SLEDAI score in SLE patients and also reduces the average daily dose of prednisolone [115]. A decrease in renal pathology has been observed following the consumption of TGP by MRL/lpr mice. TGP has also reduced the levels of anti-dsDNA antibodies and antinuclear antibodies (ANA). It could also reduce urinary protein levels. Consequently, it was concluded that TGP consumption in patients with lupus nephritis can have therapeutic effects [119]. TGP causes down-regulated Foxp3 promoter methylation levels, thus increasing the expression of Foxp3 in lupus CD4+ T cells. TGP increased the number and percentage of Treg cells in lupus CD4+ T cells and increased IFN-γ and IL-2 expression [200]. TGP increases DNA methylation of ITGAL promoter in CD4+ T cells, thereby reducing CD11a gene expression [120].
Toxicity and Side Effects
In general, TGP is considered a safe and effective compound that is tolerable and does not cause any serious side effects. According to studies, the likelihood of developing diarrhea after consuming TGP may rise. TGP can accelerate the gastrointestinal tract’s peristalsis, which may be the cause of the diarrhea. The majority of patients only experience moderate and acceptable symptoms, and the gastrointestinal system is not organically harmed. Drowsiness, dry mouth, dizziness, and weakness are some additional side effects that have been reported [207].
3.3. Fatty Acids, Vitamins, and Minerals
Certain nutrients and vitamins as dietary supplements have been consumed to improve lupus [208]. The efficacy of some of them have been investigated and discussed in several studies (Table 2). Safety and side effects of fatty acids, vitamins, and minerals is well studied by numerous publications and are available even on the indications of these over-the-counter (OTC) medicines.
3.3.1. Fatty Acids
Unsaturated oils play an important role in the immune system regulation. In human nutrition and/or healthcare, seed oils have long been utilized as a daily supplement, a food ingredient, or a therapeutic cure. Long chain fatty acids (LCFAs) are fatty acids with more than 14 carbons and make up the majority of vegetable oils. They are necessary for the human body’s ongoing regular cell growth and development. Among these, polyunsaturated fatty acids (PUFA) like n-3 and n-6 fatty acids are crucial for the prevention and treatment of many chronic diseases, including diabetes, coronary artery disease, inflammatory and autoimmune disorders, and many other ailments. Some significant fatty acids, such as linoleic acid (an n-6 fatty acid) found in the majority of vegetable oils and plant seeds, are regarded as essential fatty acids (EFAs). Arachidonic acid, which can be further elongated and desaturated to form prostaglandins, thromboxanes, and leukotrienes, is one of these EFAs. A different class of EFA is the n-3 fatty acids, which include linolenic acid and are present in soy, linseed, and flaxseed oils. According to some evidence, n-3 fatty acids have protective effects on eicosanoid metabolism. Docosahexaenoic acid (DHA), a crucial component of cellular membranes and another significant n-3 fatty acid, has a favorable impact on coronary heart disease, inflammatory disease, atherosclerosis, and disorders of the nervous system [209]. Dietary lipids are also involved in autoimmune phenomena by affecting the balance between Th1 and Th2 cells [210,211].
Dysregulation of PUFAs induces a wide range of neurological and developmental disorders. Linoleic acid and linolenic acid are required as part of the immune cell membrane [212]. α-linolenic acid and γ-linolenic acid are among the omega-3 acids that have beneficial effects following the reduction of TNF-α and IL-2 in SLE patients. Omega-3 fatty acid supplementation has shown potential benefit on SLE disease activity as demonstrated by Systemic Lupus Activity Measure-Revised (SLAM-R), SLE Disease Activity Index (SLEDAI), and British Isles Lupus Assessment Group (BILAG) scores as well as plasma membrane arachidonic acid composition and urinary 8-isoprostane levels, with minimal adverse effects [213].
Finding the optimal ratio of ω-6/ω-3 PUFAs is essential in therapeutic interventions. As an example, linoleic/alpha-linolenic of 1:3 is the optimal ratio for enhancing both the proliferation and differentiation of cells such as neural stem cells [214]. Wei et al. concluded in a meta-analysis that low-ratio n-6/n-3 PUFA supplementation could significantly reduce serum TNF-α and IL-6 concentrations but not CRP concentrations [215].
In the NZB × NZW mice animal models, essential fatty acid deficient diets can reduce arachidonic acid levels, thus reducing proinflammatory prostaglandins and leukotrienes, and also reduce nephritis by inhibiting autoantibody production [212]. Studies have shown that the lifespan increased and autoantibody levels decreased in animal models of SLE following a diet rich in omega-3 fatty acids [22].
The presence of omega-3 PUFA in the diet of SLE patients can regulate blood pressure and proteinuria and also reduce anti-dsDNA levels, as well as TNF-α, IL-1α, IL-1β, and IL-2 [126,213].
A meta-analysis conducted in 2020 found that omega-3 fatty acids could reduce SLE activity. In this study, 136 patients in the comparison group and 138 in the treatment group were used, and the mean age of patients was 43 years. The follow-up time of the trial varied between 12 and 52 weeks. This study showed that the use of omega-3 fatty acids is more effective than placebo in reducing disease activity in SLE [22].
Eicosapentaenoic (EPA) and docosahexaenoic (DHA) are some of the unsaturated fatty acids that exert their anti-inflammatory effects by lowering the level of C reactive protein (CRP) and other inflammatory mediators [126,216,217]. The most widely available dietary source of EPA and DHA is cold-water oily fish, such as salmon, herring, mackerel, anchovies, and sardines.
EPA and DHA can affect the immune system through various mechanisms. They can inhibit the enzyme lipoxygenase and subsequently reduce the inflammatory factors derived from arachidonic acid. DHA can inhibit nuclear factor κB (NF-κB) and TNF-α [218].
DHA has increased the lifespan of and suppressed glomerulonephritis in NZB × NZW mice with systemic lupus erythematosus, possibly due to inhibition of IL-18 induction [126]. DHA has also reduced IL-18 levels, lowered serum levels of anti-dsDNA, and regulated IgG renal deposition in mice [126,219].
3.3.2. Vitamin A
Some studies have been conducted to investigate the effectiveness of vitamin A in lupus. Retinoic acid is a metabolite of vitamin A. Vitamin A deficiency in lupus patients has been shown to have a negative effect on the prognosis of the disease. Consumption of retinoic acid and vitamin A regulates the balance between Th17 and Treg. It was reported that following the intake of vitamin A by lupus patients, the level of Th17 decreased and the level of Treg increased [127,128].
3.3.3. Vitamin B
Vitamins B6, B12, and folate reduce homocysteine levels, so they can be helpful in improving atherosclerosis in SLE patients. They can also lower levels of inflammatory cytokines and C-reactive protein (CRP). Vitamin B6 can also reduce the risk of active disease by lowering homocysteine [129]. Following the use of niacin, a decrease in triglyceride and LDL-C levels was observed, with no significant effect on HDL-C levels [130]. In general, it was suggested that taking supplements of the vitamin B complex could be beneficial for people with SLE.
3.3.4. Vitamin C
Vitamin C has an antioxidant effect. It can release inflammatory mediators and modulate immune function. It also lowers anti-dsDNA levels and IgG. Vitamin C can prevent active SLE [131]. Concomitant use of 500 mg of vitamin C and 800 mg of vitamin E daily for 3 months has shown a slight decrease in lipid peroxidation. In SLE patients with high doses of vitamin C, ascorbate is found in the urine, so the maximum dose of vitamin C is 1000 mg/day [220].
3.3.5. Vitamin D
It has been shown that there is a link between vitamin D deficiency and the severity of SLE. Higher SLEDAI scores have been reported in patients with low levels of vitamin D. Supplementation with vitamin D in SLE patients inhibits dendritic cell activation and maturation [132]. Calcitriol is the active form of vitamin D and acts on autoimmune diseases such as SLE by regulating the response of T and B cells and boosting the innate immune response [133]. SLE patients are photosensitive and should use sunscreen when exposed to the sun. On the other hand, sunlight is needed to produce vitamin D, so it can be assumed that taking vitamin D supplements might be useful for SLE patients [221,222].
3.3.6. Vitamin E
Vitamin E has antioxidant and anti-inflammatory effects and, because of its anti-inflammatory effect, seeks to reduce IL-2, IL-4, and TNF-α, which can be effective in lupus [223]. Furthermore, vitamin E consumption by SLE patients reduces the generation of autoantibodies [134].
3.3.7. Calcium
In some SLE patients, a decrease in bone mineral density has been observed, which may or may not be dependent on corticosteroid use. On the other hand, these patients are mostly deficient in vitamin D and avoid exposure to sunlight. Therefore, adequate calcium intake is important for SLE patients [223].
3.3.8. Iron
There should be a balance of iron intake in SLE patients. Iron supplementation to MRL/MPJ-lpr/lpr mice resulted in cell damage, renal lesions, and worsened renal impairment in an in vivo study. Iron chelators have also been shown to be beneficial in autoimmune diseases. In contrast, iron deficiency increases the symptoms of the disease, so iron should be used in SLE patients who have anemia [135,224].
3.3.9. Selenium
Selenium supplementation has been considered in the treatment of lupus because of its antioxidant and anti-inflammatory effects. A study on NZB/NZW female mice found that survival increased following selenium supplementation, which may be due to increased levels of natural killer cell activity [136]. In an in vitro study performed on the B6.Sle1b mouse model of lupus, an attempt was made to find the mechanism of the effect of selenium on lupus. It has been shown that selenium can inhibit the activation, differentiation, and maturation of macrophages and B cells. Therefore, its use can be useful in patients with lupus [137]. Reduced selenium levels have been observed in patients with autoimmune diseases, which may be considered a risk factor for the onset of autoimmunity and inflammation. Due to the anti-inflammatory effect of selenium, it has been suggested that consuming a certain amount of selenium in patients with autoimmune diseases can lead to better management of disease complications [225].
3.3.10. Zinc
It was shown that a zinc-restricted diet can increase serum levels of corticosteroids and subsequently reduce the symptoms of autoimmune diseases such as SLE, so it can be useful in controlling SLE [226]. A study of NZB/NZW mice showed a decrease in autoantibody production. In MRL/lpr mice, the use of zinc-restricted diets decreased the level of anti-dsDNA, decreased lymphoproliferation, and recovered glomerulonephritis [210]. On the other hand, it should be noted that a study conducted on humans has shown that zinc deficiency causes immune dysfunction by acting on Th cells and can lead to neurosensory disorders and reduced body mass [227].
3.4. Herbal Medicines, Medicinal Plants, Mushrooms, and Fungi and Their Crude Extracts
A variety of medicinal plants and mushrooms have been reported to exhibit efficacy against lupus conditions (Table 3). In some traditional remedies, they have been used in the form of dried powdered plant material or fungi. In some others, a crude extract of the plant or fungus was prepared using solvents such as water or ethanol or a mixture of both (hydroalcoholic extract). Crude extracts are a complex mixture of molecules with the same range of polarity but different concentrations. Sometimes, several molecules with a variety of mechanisms work synergistically to produce a specific effect. Although the crude extracts of herbal medicine can reflect the efficacy of a special herb or fungi, due to the variation of compounds in the natural sources, special attention should be given to the standardization and authentication of them in order to have repeatable and reliable effects. Compared to purified bioactive molecules, crude extracts usually exhibit milder efficacy and side effects, and introducing supplements from herbal medicine to the market is much easier.

Table 3.
Medicinal plants, mushrooms, and fungi with reported efficacy against lupus.
The use of herbal medicines has long been used to treat various diseases, such as SLE. In this article, an attempt has been made to review effective herbs for improving lupus. The use of traditional medicine along with Western medicine can reduce the dose of Western medicine drugs, reduce their side effects, and ultimately improve the quality of life of SLE patients.
3.4.1. Tripterygium wilfordii Hook. F.
Tripterygium wilfordii Hook. F. (Celastraceae) has the common names of “thunder duke vine” and “thunder god vine” and is also known as “léi gōng téng” in Mandarin. The plant has long been used in traditional Chinese medicine (TCM) and it is widespread in southern and eastern China [88,253]. Its root extract is used, but its bark must be removed because of its toxicity. There are various compounds with a range of biological effects in the root extract, and the procedures and methods of extraction play a role in overcoming the plants’ toxicity [254,255]. In TCM, the T. wilfordii preparations have long been used for some health conditions, including sores and swelling, inflammations, ankylosing spondylitis, hepatitis, nephropathy, allergic skin diseases, inflammatory lesions of leprosy, and cancer [253,254,256,257,258,259]. The effect of T. wilfordii on autoimmune diseases such as SLE, rheumatoid arthritis (RA), BehCet’s disease, psoriasis, etc., has been investigated in several studies [253,260]. Its effect on kidney transplantation, nephrotic syndrome, and diabetic nephropathy has also been investigated [261,262,263].
Clinical Trials
A number of studies have examined the efficacy of T. wilfordii in lupus erythematosus. A clinical trial study compared the effect of T. wilfordii with prednisolone. In this study, there were 23 cases of lupus erythematosus, of which 15 had SLE and 8 had discoid lupus erythematosus (DLE). They had been given 45 mg/day of crude extract of T. wilfordii. The control group consisted of 19 cases of SLE treated with prednisolone. The rate of improvement was almost the same in the two groups, and no significant difference was observed. However, there are benefits to using T. wilfordii, including the recovery from erythematosus rash and arthralgia [249]. The immunomodulatory and anti-inflammatory effects of T. wilfordii have also been reported in a clinical study on 26 DLE cases [250].
Despite the benefits of this plant, side effects have also been reported. Headaches, gastrointestinal complications, nausea, diarrhea, infertility, etc., are among these adverse effects [249,250] One of the side effects reported due to long-term use of T. wilfordii in women is decreased bone mineral density [121]. Due to some toxic side effects of T. wilfordii such as kidney damage, its use is not recommended in SLE patients who also have nephropathy [249]. A systematic review has discussed cardiovascular, hematologic, and skin complications as well as infertility and gastrointestinal complications of this plant [122]). Side effects are more common with high concentrations. Consuming the right amounts and rational treatment will help control the side effects and obtain therapeutic effects [121].
Active Compounds and Possible Mechanisms of Efficacy
A variety of compounds have been isolated from T. wilfordii such as triptonide, tripodine, triptolide (TPT), tripdiolide (TPO), etc. [123]. Among the phytochemicals of T. wilfordii root extract, celastrol (tripterine) and triptolide (diterpenoid triepoxide) are the most investigated [124,258]. Triptolide’s anti-inflammatory and immunosuppressive effects are mediated by inhibition of T cells and inhibition of IL-17 and STAT3 transcription [125]. It has been observed that NF-κB activity is significantly reduced in patients with SLE after consuming T. wilfordii. It was suggested that T. wilfordii may exert an immunosuppressive effect on SLE patients by inhibiting NF-κB expression [123].
An in vitro study on dendritic cells (DCs) of SLE patients has shown that triptolide can inhibit the differentiation and maturation of DCs and reduce the immune function of DCs. In this study, doses of 0, 5, 10, and 30 μg/L of triptolide were used. Triptolide also reduced secretion of IFN-α, IL-6, and TNF-α [121]. In another study, to evaluate the effect of triptolide, BALB/c-un nude mice were used. Mice were treated orally with 5 mg/kg/d of triptolide, and their blood samples were collected before treatment and 1, 3, and 6 months after treatment. Finally, it was found that with the use of triptolide, a decrease in the percentage of CD8+, Tcl, Thl cells, CD4+/CD8+, Thl/Th2, and Tcl/Tc2 and an increase in the percentage of CD4+, Tc2, and Th2 cells was observed [122].
The effect of triptolide and tripdiolide on lupus nephritis in (NZB×NZW) F1 mice has been investigated. Mice were treated orally with 6 µg of triptolide or tripdiolide for 15 weeks. Finally, triptolide and tripdiolide have been shown to reduce BUN, proteinuria, and anti-dsDNA antibody levels, as well as the production of cytokines such as IL-6 and TNF and monocyte chemoattractant protein 1. Therefore, triptolide and tripdiolide have therapeutic effects in lupus nephritis [123].
In a study, the effect of triptolide on SLE was investigated in female MRL/lpr mice treated with 0.2 or 0.3 mg/kg/d of triptolide for 13 weeks. Compared to the control (vehicle) group, triptolide significantly reduced proteinuria, serum anti-dsDNA, and renal histopathologic assessment. The effect was comparable to that of cyclophosphamide (20 mg/kg/w). Triptolide also increased the proportion of Treg and induced expression of miR-125a-5p [124].
(5R)-5-Hydroxytriptolide (LLDT-8) is a triptolide derivative that has strong anti-inflammatory and immunosuppressive effects and low toxicity. In a study, the effect of LLDT-8 on lupus nephritis was investigated. Female MRL/lpr mice were treated with 0.125 mg/kg/2 days of LLDT-8 for 9 weeks. Finally, LLDT-8 has been shown to reduce proteinuria, serum creatinine, and glomerular IgG deposits, and it could also ameliorate histopathology and increase the lifespan of mice. LLDT-8 reduced the expression of inflammatory cytokines such as IFN-γ, IL-17, IL-6, and TNF-α and inhibited immune cell infiltration in the kidneys. It was suggested that LLDT-8 could have therapeutic effects on lupus nephritis [154].
Toxicity and Side Effects
Refer to the section on toxicity and side effects of triptolide in this text.
3.4.2. Ophiocordyceps sinensis (syn. Cordyceps sinensis)
Ophiocordyceps sinensis belongs to the Ophiocordycipitaceae family, is an entomogenous fungus used in TCM. It is also known as Yartsa gumba or caterpillar fungus [79,264].
Clinical Trials
Several clinical trials have evaluated the efficacy of the dry powder of O. sinensis mycelium (Bailing capsules) as a supplement in conjunction with prednisolone, cyclophosphamide, tacrolimus, or leflunomide on lupus nephritis. They have reported controversial results, which might be due to the small sample size or differences in control groups and study design [142]. A meta-analysis study was conducted on a total of 14 studies comprising 1301 participants, which were combined for analysis in the present study. In general, this study showed that consumption of O. sinensis mycelium (Bailing capsule) for lupus nephritis is more effective than not using it. Although there was no significant difference between the Bailing group and the control group in anti-ds-DNAIgM levels and complement C3 levels (which is associated with the existence of some immune diseases like SLE), other indicators of the disease, such as SLEDAI score, Alb, 24 h urinary protein, serum creatinine, and the number of effective treatments and complications, improved. It was concluded that O. sinensis could be beneficial in the treatment of lupus nephritis [142].
In a study of 61 lupus nephritis patients, the prevention of the recurrence of lupus nephritis by artemisinin and C. sinensis was evaluated. A total of 30 patients were in the control group, and 31 patients were treated with 2–4 g/d of C. sinensis and 0.6 g/d of artemisinin. C. sinensis was taken before three main meals and artemisinin after three main meals for three years. The control group took tripterygiitotorum and/or Baoshenkang. Finally, the creatinine clearance rate did not change between before and after treatment, and the complement C3 level stabilized in the normal range. It was concluded that the combination of C. sinensis and artemisinin could be effective in the prevention of recurrence of lupus nephritis [95].
Active Compounds and Possible Mechanisms of Efficacy
A variety of bioactive phytochemicals with biological activities have been reported from O. sinensis, such as cordycepin, cordycepic acid, polysaccharides, ergosterol, nucleosides, fatty acids, proteins, minerals, etc. The immunomodulatory effects of O. sinensis were mostly attributed to its polysaccharides, which affect both humoral and cellular immune responses in vivo and improve the serum levels of ovalbumin-specific IgG, IgG1, and IgG2b levels. This fungus’s mycelia polysaccharides have been shown to enhance the proliferation and phagocytosis of macrophages and stimulate macrophages [265]. Intracellular polysaccharides isolated from submerged cultures of O. sinensis have been reported to exhibit strong immunomodulatory effects on RAW264.7 macrophage cells via the MAPK and PI3K/Akt signaling pathways. It enhanced the phagocytic activity of RAW264.7 cells and increased cytokine production. This immunomodulatory response was mediated by the secretion of both proinflammatory cytokines (TNF-α, IL-6, and IL-1β) and anti-inflammatory substances (TGF-β1 and IL-10), producing NO and promoting the expression of iNOS [266].
Some of O. sinensis’ nucleotide contents have also been reported to exhibit immunomodulatory properties by lowering NO and increasing IL-lβ and TNF-α release from macrophages [265]. Deoxynucleic acids from O. sinensis activated mouse bone marrow-derived DCs via a toll-like receptor 9-dependent pathway [265]. Aside from the total extracts of O. sinensis, specific components, including 1-(5-Hydroxymethyl-2-furyl)-β-carboline, and cordymin (a purified peptide from O. sinensis), displayed significant anti-inflammatory properties [79,267,268,269].
Hypothetically, besides immunomodulatory and anti-inflammatory effects, the antioxidant [270], cardiovascular [271], and kidney protective [272] properties of O. sinensis may also have a role in the beneficial effects of this fungus on SLE.
A number of studies have been performed to investigate the effect of O. sinensis on lupus. A study was performed on lupus-prone (NZB/NZW) F1 hybrid mice. The mice were divided into four groups of different ages (three, six, and eight months) and were given 2.4 mg/g/day of cultured mycelia of C. sinensis orally. The fourth group was also used as a control. The results showed that in groups who started taking it at the ages of 3 and 6 months, survival increased, proteinuria decreased, and titers of anti-double-stranded DNA antibodies decreased. The percentage of CD4+ T cells in peripheral blood mononuclear cells (PBMC) decreased significantly, while the percentage of CD8+ T cells increased. Eventually, the results of this study showed that early administration of C. sinensis reduces the severity of lupus disease [242]. In another study on MRL lpr/lpr mice, a triterpenoid, component H1-A, was extracted from C. sinensis. Administration of 40 µg/kg/d of H1-A daily for 8 weeks to mice aged 12 weeks resulted in a reduction in the production of anti-dsDNA, lymphadenopathy, and proteinuria. Renal function has improved, and no significant changes have been observed in immune complex deposition. In general, H1-A intake has increased the survival of mice with lupus [96].
In another study, the effects of Chinese herbs on SLE were investigated in NZB/NZW F1 mice at one month of age. C. sinensis was found to inhibit anti-ds-DNA production and increase the lifespan of mice. Although A. sinensis does not inhibit anti-ds-DNA production, it has been able to increase the lifespan of mice [243].
Toxicity and Side Effects
In addition to the claimed therapeutic or positive effects for the chemicals derived from the Cordyceps fungi, cytotoxicity and/or neurological toxicity adverse effects have also been described for these compounds. After daily intake of Cordyceps fruiting bodies or associated products, reports of nausea, diarrhea, and even significant post-extraction bleeding have been documented. There have also been a few rare reports of dry mouth, nausea, and diarrhea [273].
3.4.3. Ganoderma Lucidum and Ganoderma Tsugae
Ganoderma lucidum belongs to the family Ganodermataceae or Polyporaceae and is also known as Lingzhi or Reishi. This fungus has been observed to have anti-inflammatory, antioxidant, and analgesic effects, and it has been used in TMC since the ancient era. This fungus is used for various diseases that, in most cases, have an inflammatory basis, such as arthritis, hepatitis, bronchitis, acute colitis, etc., as well as hypertension and malignancy [236].
G. lucidum has shown an immunomodulatory effect on PMNC (peripheral mononuclear cells). It has exhibited suppressive effects on tumor necrosis factor-α (TNF-α), IL-1β, IL-12, and IL-6, which are pathogenic cytokines associated with SLE [274,275].
Clinical Trials
The authors of the present study could not find a clinical trial on the efficacy of the G. lucidum on SLE, but there are several trials investigating the efficacy of this mushroom on some other conditions such as rheumatoid arthritis [276], fibromyalgia [111,277], neurasthenia [278], cancers [279], cardiovascular risk factors of metabolic syndrome [280], lower urinary tract symptoms (LUTS) [281], etc. Moreover, in a clinical trial, β-glucans of G. lucidum were reported as safe and well-tolerated immunomodulator supplements for children [282].
Active Compounds and Possible Mechanisms of Efficacy
A variety of natural polysaccharides have shown immunomodulatory, anti-inflammatory, and wound-healing properties [283,284]. G. lucidum polysaccharides (GLPs), such as ganoderan and β-glucans, have been extensively studied for their biological activities, which include antioxidant, antitumor, anti-inflammatory, anti-diabetes, and immunomodulatory properties [285]. GLPs can affect different immune effector cells, including lymphocytes and myeloid cells. They can modulate innate immunity, cellular immunity, and humoral immunity [286]. Also, some triterpenoids of G. lucidum (GLTs), such as ganosidone A and its derivatives [287], as well as ganoderic acid D [288], and 3-oxo-5α -lanosta-8, 24-dien-21-oic acid [289] have shown anti-inflammatory effects.
In a study, the combination of G. lucidum and San-Miao-San (SMS) has been used to evaluate the anti-inflammatory effect on SLE. SMS is a Chinese herbal medicine that consists of a combination of three herbs that include Phellodendri Cortex (Huangbai), Atractylodes Rhizome (Cangzhu), and Radix achyranthis bidentatae (Niuxi). The control group consisted of female Balb/c mice (at the age of 20–24 weeks). The study group included three groups of female MRL/lpr mice that had mild, moderate, and severe lupus. Initially, 500 mg/kg/day was administered orally for 7 days, and then 50 mg/kg/day was injected intraperitoneally for 7 days. Finally, a significant reduction in anti-ds-DNA in the study group with moderate and severe SLEs was observed. In the study group, the percentages of IL-10, CD4+, CD25+, Foxp3+ and Treg cells increased significantly, the concentrations of IL-2 and IL-12P70 increased significantly, and the concentrations of IL-21, IL-10, and IL-17A decreased significantly [236].
In a study, the effectiveness of Ganoderma tsugae on increasing the lifespan of NZB/NZW F1 mice was investigated. All groups of mice (two months of age) were given standard laboratory chow feeding. The first study group was given 0.1 cm3 of oral ganoderma extract, and the second study group was given 0.2 cm3 of oral ganoderma extract daily. The third study group was given 0.5 mg/kg/day of prednisolone. G. tsugae increased the life expectancy in mice with lupus and reduced anti-dsDNA autoantibody and proteinuria, as well as parenchyma and perivascular mononuclear cell infiltration [237].
Toxicity and Side Effects
Patients from various nations have reported developing human sensitivity to the G. lucidum antigen. Since G. lucidum has an anticoagulant effect and extending the prothrombin time has an additional effect on clotting factors, patients who were taking anticoagulants or antiplatelets should take caution. Hypoglycemic people should also take caution because it reduces blood sugar levels. G. lucidum is an anti-hypertensive agent, according to numerous research works. Before using it, persons with cardiac issues should visit a physician [290]. In a study, it has been determined that sub-chronic toxicity of the liver occurs when rats are given more than 1.2 mg/kg body weight of G. lucidum extract [291].
3.4.4. Urtica Dioica L.
Urtica dioica, also known as stinging nettle, grande ortie, or anonhasquara, belongs to the Urticaceae family. Different parts of the plant, such as roots, leaves, seeds, and aerial parts, have different therapeutic effects upon extraction by different methods. Following animal studies, various therapeutic effects without the appearance of serious side effects have been reported. This plant has shown anti-inflammatory, antioxidant, antimicrobial, antifungal, etc., effects. In traditional medicine and ethnomedicine, different parts of the plant have been used in diseases such as systemic lupus erythematosus and rheumatoid arthritis, diabetes, prostate cancer, breast cancer, atherosclerosis, cardiovascular diseases, etc. Non-aqueous extraction of the root of this plant has been found to be effective in SLE [292,293].
Clinical Trials
In a case report, one female lupus patient (24 years old) with a renal allograft status and elevated serum creatinine was featured. The patient was consuming immunosuppressants (prednisone, CellCept™ and Prograf™). After consuming an herbal remedy, consisting of a combination of Agropyron repens rhizome and U. dioica seed extracts (1:3, 5 mL, three times a day), the serum creatinine started to decline. After 46 days, U. dioica seed extract was used as a monotherapy for 3 months. Serum creatinine levels were normalized to acceptable levels [251].
Active Compounds and Possible Mechanisms of Efficacy
A variety of phytochemicals, including phenylpropanoids, flavonoids (such as chlorogenic acid, rutin and isoquercitrin, quercetin-3-O-rutinoside, kaempherol-3-O-rutinoside, and isorhamnetin3-O-glucoside), lignans (such as secoisolariciresinol), and coumarin (such as scopoletin), have been reported from nettle extracts. These extracts have shown anti-inflammatory and immunomodulatory effects with different selectivity toward the COX and LOX branches of the eicosanoid pathway [294,295]. Moreover, several plant sterols, such as sitosterol and its derivatives, have also been reported from the nettle root extract [296]. The root and leave extracts of U. dioica have shown immunomodulatory effects through different mechanisms, including lowering thromboxane production in human platelets and inhibiting the 12-LOX pathway. Different parts of the plant have shown antioxidant effects [294,297].
In a study, the effect of long-term injection of the Vβ8.3-specific superantigenic lectin U. dioica agglutinin (UDA) was investigated in MRL lpr/lpr mice (7 weeks of age). In contrast to the control group, injection of UDA (100 µg every two weeks for 4.5 months), inhibited the development of overt clinical signs of lupus and nephritis. Pathogenic T cell clones are thus found among the V8.3+ T cell population, which also contains an enlarged T cell clone. UDA affected autoantibody production in a sex-dependent manner [252].
Toxicity and Side Effects
Sweating and gastric discomfort are some of the side effects that have been reported to be associated with using stinging nettle. It should be noted that touching stinging nettle typically can cause skin irritation. Patients with renal conditions have been documented to experience hypersensitivity following consumption of this plant. Additionally, this plant has been shown to improve the effects of CNS depressive drugs. Consumption of stinging nettle concurrently with sedatives, such as lorazepam, phenobarbital, clonazepam, zolpidem, and others, may cause drowsiness and sleepiness [298].
3.4.5. Nelumbo nucifera Gaertn.
Nelumbo nucifera belongs to the Nelumbonaceae family and is also known as the sacred lotus and water lily. In addition to being used as a vegetable and food, this plant has had many therapeutic uses from the past to the present. All parts of the plants (fruits, leaves, flowers, seeds, roots, rhizomes, buds, stems, anthers, stalks, plumules, and stamens) have been used in traditional medicine. The plant has been comprehensively studied to investigate its medicinal benefits such as anti-obesity, anti-diabetic, antioxidant, anti-amnesic, anti-thrombotic, anticarcinogenic, anti-inflammatory, immunomodulatory activity, anti-neurodegenerative, antiproliferation, cardiovascular activity, etc.
The effectiveness of lotus on SLE is due to the presence of procyanadins, polyphenols, and polysaccharides in its seeds [299].
The effectiveness of N. nucifera on SLE has been investigated in a study. In this study, 12-week-old MRL/MpJ-lpr/lpr mice were used. N. nucifera seeds extracted with ethanol contain (S)-armepavine (C19H23O3N). One group was given corn oil orally as a control; another group was given 5 and 10 mg/kg/day of oral (S)-armepavine; and the other group was given 20 mg/kg/day of oral cyclosporine. Mice were treated for 6 weeks. Finally, it was observed that taking (S)-Armepavine increased the life expectancy of mice and inhibited splenocytes proliferation and prevented lymphadenopathy. It also inhibited the expression of IL-2, IL-4, IL-10, and IFN-γ genes and inhibited T cells proliferation. Consumption of (S)-armepavine reduced proteinuria and anti-dsDNA autoantibody [94].
Toxicity and Side Effects
Despite the N. nucifera’s long history of therapeutic use, research on its possible toxicity and safety is required. Numerous investigations up to this point have partially supported the safety of N. nucifera [300]. The toxicity and safety profile of N. nucifera and its components have been examined in several research. A number of in vitro studies have been conducted to evaluate the toxicity of N. nucifera using normal cell lines. N. nucifera has not been found to significantly affect cell viability or to have any toxic effects, according to the findings of a number of in vitro investigations. The safety profile of N. nucifera has also been examined in numerous in vivo studies. In general, side effects from in vivo investigations include an increase in lymphocytes, a decrease in basophils, and a drop in creatinine, cholesterol, and hematocrit [301].
3.4.6. Artemisia annua Pall.
Artemisia annua belongs to the Asteraceae family. A. annua has not only been used in TCM for various ailments but has also been identified as a medicinal plant in the United States, Europe, Australia, and Asia. This plant used to be found in western Asia and southeastern Europe. Today, it has spread all over the world and can be found in Australia, North and South America, and many parts of Asia and Europe. Since this plant is found in different parts of the world, it is known by many names, such as annual wormwood, sweet wormwood, Chinese wormwood, sweet sagewort, and sweet Annie. Antitumor, analgesic, anti-inflammatory, and antioxidant effects of A. annua have been discovered in studies on this species. In traditional medicine, this plant has been used in viral and bacterial diseases, jaundice, and autoimmune diseases such as rheumatoid arthritis and SLE, bacterial dysentery, hemorrhoids, and wound healing. It has also been used as an antipyretic in the treatment of tuberculosis and malaria. The 2015 Nobel Prize in Medicine was awarded for the discovery of sesquiterpene lactone artemisinin and its effects on the treatment of malaria [302]. Recently, the possibility of A. annua being useful in the treatment of COVID-19 has been investigated and satisfactory results have been reported [303,304,305].
Clinical Trial
In a clinical trial performed on 73 patients, including 36 patients with SLE and 37 patients with DLE, 60 or 80 mg of dihydroartemisinin was given orally for 9 weeks. Finally, dihydroartemisinin has been shown to be effective in most patients, and no serious side effects have been reported [306].
Phytochemicals and Possible Mechanisms of Action
The components identified from different parts of A. annua are classified as sesquiterpene lactones, coumarins, saponins, flavonoids, tannins, essential oils, polyalkenes, phenolic acids, fatty acids, proteins, and phytosterols [140,307,308]. Artemisinin, a sesquiterpene lactone in glandular hairs on the leaves and flowers of A. annua, is one of the important components of this plant, which is assumed to be one of the potential compounds against lupus. Apart from A. annua, other plants containing artemisinin, such as Artemisia apiacea, can also be effective in treating lupus [309].
Several semi-synthetic derivatives of artemisinin such as dihydroartemisinin, artemether, arteether, and artesunate have been investigated [140,302]. The effects of dihydroarteannuin (also known as dihydroqinghaosu, artenimol, or DHA) is one of the semi-synthetic derivatives of artemisinin. In a study, the effect of dihydroarteannuin and its mechanism on lupus in BXSB mice has been investigated. In this study, male BXSB mice were given dihydroarteannuin daily for ten days. One group was considered a control and the other three groups were given doses of 5 mg/kg, 25 mg/kg, or 125 mg/kg. As a result, dihydroarteannuin consumption was found to reduce TNF-alpha production, as well as nuclear factor-κB (NF-κB) activation and p65 subunit expression. Dihydroarteannuin inhibits NF-κB translocation to the nucleus and also inhibits IκB-α protein degradation [104]. In general, the level of TNF-alpha in the serum of SLE patients is higher than normal people. In addition, the expression of TNF-alpha receptors in peripheral blood lymphocytes of SLE patients is higher. NF-κB is a transcription factor located in the inactive cytoplasmic complex. It has two subunits, p50 and p65, and is attached to the IκB family, which are inhibitory proteins. Stimulation separates NF-κB from IκB and eventually translocates NF-κB to the nucleus, where it binds to DNA, inducing the expression of genes involved in the pathology of SLE progression [104].
In a study, the effect of A. annua was studied in female ICR mice, and it was found that, due to its suppressive effect on the immune system, it can be effective in diseases such as SLE and rheumatoid arthritis. Splenocytes of immunized mice were isolated and exposed to different concentrations, and finally, the number of specific antibodies was counted by indirect ELISA. The results indicated that the levels of a series of antibodies decreased following the consumption of ethanolic extract of A. annua. It was suggested that the plant might be useful in autoimmune diseases such as SLE and rheumatoid arthritis [228].
SM934 is another derivative of artemisinin that has more water solubility, bioavailability, and bioactivity but less toxicity. A study on MRL/lpr mice showed improvement in lupus syndrome. SM934 reduced the production of IFNγ and IL-17 by polyclonal CD4+ T cells activated by T cell receptor rearrangement in vitro, as well as the development of naive CD4+ T cells into Th1 and Th17 cells, but not Treg cells. In the in vivo study, administration of SM934 to mice for 4 weeks attenuated the renal lesion severity, proteinuria, and anti-dsDNA autoantibody. It also decreased the spleen size and the levels of serum IFN-Y and blood urea nitrogen. Following 8 weeks of consumption, the lifespan of mice increased. Ex vivo studies have shown an inhibition in the production of Th1 and Th17 while the level of Treg cells increased. In splenocytes, SM934 inhibited the complete activation of STAT-1, STAT-3, and STAT-5 proteins [97].
The effect of oral dihydroartemisinin (2 mg, daily, three days post-infection) on the immune system of BALB/C female mice infected with Toxoplasma gondii or Plasmodium berghei has been evaluated. Following the consumption of dihydroartemisinin, the number of B cells in the bloodstream and spleen cells decreased, and the ratio of T helper to CD8+ T cells increased. Dihydroartemisinin also decreased the proinflammatory cytokines. It is assumed that due to immunomodulating properties, dihydroartemisinin might be useful in autoimmune diseases such as SLE [105].
One of the factors associated with the progression of SLE is the imbalance between Treg/Th17. In a study, the effect of dihydroartemisinin alone or in combination with prednisolone on Treg/Th17 balance has been investigated. For this purpose, female BALB/c mice have been used. One group was considered the control, and one group was the SLE model group. The third group was given 100 mg/kg of dihydroartemisinin, the fourth group was given 5 mg/kg prednisolone, and the last group was given a combination of prednisolone and dihydroartemisinin with the same previous doses. Dihydroartemisinin and prednisolone were administered daily and orally for two months. As a result, the Treg/Th17 balance was restored, and the inflammation was inhibited. The effect of dihydroartemisinin has also been studied in vitro by isolating mouse spleen lymphocytes and exposing them to dihydroartemisinin or a combination of dihydroartemisinin and prednisolone. As a result, it was observed that the levels of TGF-β, IL-17, and Foxp3 increased, while transcription of RORγt decreased. Th17 cell differentiation is inhibited, but Treg cell differentiation is induced. Finally, dihydroartemisinin has been shown to have a synergistic effect on prednisolone [106]. There is also further evidence and studies to investigate the effect of A. annua on lupus [310,311,312].
Toxicity and Side Effects
Refer to the section on toxicity and side effects of artemisinin in the present text.
3.4.7. Linum usitatissimum L.
The plant belongs to the Linaceae family and is also known as flaxseed and linseed.
Clinical Trials
In a two-year nonplacebo-controlled crossover study, 23 patients suffering from lupus nephritis were divided into two groups. One group received ground flaxseed (30 g/day for one year), and the second group was considered the control. At the end of the one-year period, the two groups were switched after a 12-week period of washout. Fifteen volunteers remained until the end of the trial. Eventually, it was observed that the viscosity of serum and plasma lipids remained unchanged, but serum creatinine decreased. Although microalbumin decreased during both flaxseed consumption and control times, a further decrease was observed during flaxseed treatment. It was concluded that in lupus nephritis, flaxseed appears to be renoprotective, but this interpretation is hampered by underpowering due to poor adherence and possible Hawthorne effects [240].
In another study, the effect of flaxseed on lupus nephritis was investigated. Nine patients with lupus nephritis enrolled while eight of them accomplished the study. Patients consumed 15, 30, and 45 mg of flaxseed daily for four weeks, with a washout of five weeks between doses. Finally, it was observed that blood viscosity and LDL decreased significantly, and there was a further decrease following an increase of the dose. Inhibition of AF-induced platelet aggregation, a decrease in serum creatinine, a decrease in proteinuria, an increase in complement C3, and decreased expression of CD11b have been observed. It was concluded that a daily consumption of 30 mg of flaxseed is tolerable and useful for patients with lupus nephritis due to its anti-inflammatory effect and beneficial effects on the kidney and atherogenic mechanism [241].
Phytochemicals and Possible Mechanisms of Action
Flaxseed oil, or linseed oil, is rich in omega-3 fatty acids. It also contains linolenic acid, linoleic acid, secoisolariciresinol diglucoside (SDG), lignans, and cyclic peptides [313,314]. Flaxseed contains polysaccharides with immunomodulatory properties such as FP-1. In a study, this polysaccharide stimulated immune responses by inducing mRNA expression of TNF-α, NO, IL-6, and IL-12 in murine macrophages [315].
Polyphenols, polysaccharides, and lignans of flaxseed have been reported to have antioxidant and anti-angiogenic properties [316,317,318]. Flaxseed oil has been reported to possess anti-inflammatory and immunomodulatory properties [319,320]. Flaxseed oil is rich in phytosterols such as campesterol, brassicasterol, stigmasterol, β-sitosterol, and Δ5-avenasterol [321]. These phytosterols have been repeatedly reported as anti-inflammatory agents [322]. Adding flaxseed oil to the co-culture of 3T3-L1 adipocytes, RAW 264.7 macrophages, showed a dose-dependent shift in cytokines toward IL-4 but a decrease in TNF-α. In the in vivo model (C57bl/6 mice), oral flaxseed oil (4 weeks) increased IL-4 cytokine, serum anti-ova IgG1, and IgE levels. Anti-ova IgG2a, IgG2b, and IgG3 levels were also reduced [319]. Some polyunsaturated fatty acids (PUFA), such as α-linolenic acid, have a variety of biological activities including neural stem cell proliferative, anti-atherosclerotic, and anti-inflammatory effects [209,215,323]. They might exhibit cardiovascular protective properties and might be useful in preventing the progression of nephritis in patients with lupus [240]. The beneficial effect of flaxseed on the kidneys has been reported in a number of studies [324,325,326,327].
In general, flaxseed reduces inflammatory responses and lowers blood pressure and vascular disease due to its PUFA content, such as omega-3. Since blood pressure is a risk factor for chronic kidney disease and flaxseed has an anti-inflammatory effect on the kidneys, flaxseed improves kidney health [313].
Toxicity and Side Effects
The flaxseed contains certain chemicals that have been recognized as potentially harmful, including cyanogenic glycosides and linatine, even though no toxicity has been documented in clinical investigations with dietary supplementation of flaxseed. Intestinal β-glycosidase changes the glycoside into cyanohydrin, which subsequently breaks down into hydrogen cyanide. Acute cyanide poisoning from hydrogen cyanide could put the nervous and respiratory systems at risk. However, consuming 15–100 g of flaxseed has not been reported to cause any rise in plasma cyanide levels above the baseline. Theoretically, 1–2 tablespoons of flaxseed will result in the production of between 5 and 10 milligrams of hydrogen cyanide when consumed. Toxic effects are quite unlikely to result from this. It is crucial to note that the idea that dietary flaxseed is toxic due to any of these components has not been proven scientifically [328].
3.4.8. Rehmannia glutinosa (Gaertn.) DC.
Rehmanniae Radix (Di Huang) is the root of Rehmannia glutinosa (Gaertn.) DC. and belongs to the Orobanchaceae family. Rehmanniae Radix has long been used in TCM for medicinal purposes. Studies on Rehmanniae Radix have been shown to have antioxidant and anti-inflammatory effects and can lower blood sugar, activate the autonomic nervous system, and improve cognitive function. It has been used for treating dermatitis, cervical cancer, and nephrotic hypertension and improving liver damage. The effect of Rehmanniae Radix on lupus has also been investigated [302,329].
Clinical Trial
In a clinical trial in 72 patients with lupus, the combination of Radix Rehmanniae and Radix Astragali with glucocorticoid drugs was investigated. Patients were divided into control and treated groups. The control group was given prednisolone and cyclophosphamide, and the treated group was given a combination of Radix Rehmanniae and Radix Astragali on the basis of the control group. The duration of treatment in both groups was 6 months. The dose of prednisolone was reduced following the improvement in the patient’s condition. The dose reduction of prednisolone was greater in the treated group. In the treated group, there were fewer patients who had to increase the dose of prednisolone due to an aggravation of the disease. Infection, cardiovascular anomalies, hot flushes, insomnia, and Cushing’s syndrome were less common in the treated group. Although there was no difference in blood immunoglobulin G and blood complement 3 in the two groups, the protein in the 24 h urine was lower in the treated group. Therefore, following the use of Radix Rehmanniae and Radix Astragal with conventional Western medicine, fewer side effects were observed, and it was more convenient to withdraw corticosteroids [246]. A similar clinical trial was performed for patients with lupus nephritis, and it can be said that similar results were seen in general [330].
Phytochemicals and Possible Mechanisms of Action
In an in vitro study, the effect of fresh Rehmanniae radix Methanol extract has been investigated in adult female BALB/c mice. Therefore, mouse splenocytes were used. Finally, it has been observed that inflammatory cytokines such as IL-2, IFN-γ, IL-6 and IL-10 are reduced in the mouse splenocytes [247].
More than 100 chemical compounds have been reported from R. glutinosa that are mostly classified as iridoids, ionones, phenylethanoid glycosides, lignans, polysaccharides, and phenylpropanoids [331]. The polysaccharides of this plant have been reported to exhibit anti-inflammatory activity through suppression of IL-6 and TGFβ production on bacterial LPS-induced macrophages.
The anti-inflammatory effect of RG-B9, a polysaccharide isolated after processing of Rehmanniae Radix, involved AKT/ERK/JNK signaling pathway [332]. Two other polysaccharides, including SDH-WA and SDH-0.2A, have also been reported to have immunomodulatory properties. They increased lysozyme activity and TNF-α and IL-6 production by RAW264.7 cells. They did, however, reduce the secretion of lysozymes, TNF-α, IL-6, IL-1β, and nitric oxide by LPS-induced RAW264.7 cells [333]. In LPS-stimulated BV2 microglia, catalpol, an iridoid glucoside from R. glutinosa significantly suppressed LPS-induced secretion of proinflammatory mediators, NO and prostaglandin E2. Catalpol downregulated NO synthase and cyclooxygenase-2 expression. It also inhibited the TNF-α and IL-1β secretion. Moreover, catalpol downregulated the NF-κB signaling pathway, suppressed the expression of toll-like receptor 4 (TLR4), and lowered LPS-induced generation of ROS [334].
Toxicity and Side Effects
Although R. glutinosa is considered one of the safe plants, it can cause side effects such as dizziness, vertigo, headache, heart palpitations, nausea, diarrhea, allergies, and fatigue. It should be used with caution in patients with liver, digestive, and immune system problems [335].
3.4.9. Paeonia × suffruticosa Andrews
Paeonia suffruticosa belongs to the family Paeoniaceae and is also known as mǔdān and Moutan Cortex. Due to its anti-inflammatory, antibacterial, sedative, anti-diabetic, and analgesic effects, it is used for inflammatory diseases, menstrual problems, cardiovascular diseases, and atherosclerosis. Many benefits of this plant are due to the large number of monoterpenoids glucosides in it [336]. The root bark of this plant has been used in traditional Chinese medicine for lupus nephritis [337].
Clinical Trials
The effect of Moutan Cortex on improving the condition of SLE patients in a clinical trial has been investigated. A total of 84 patients in the study group were given Moutan Cortex extract, and 84 patients in the control group were given common drugs. Finally, it was observed that following the use of Moutan Cortex extract, the percentage of Th17 cells decreased and the percentage of Th1 cells increased. Decreases were also observed in the IL-6 level, ESR, and SLEDAI scores. Compared to the control group, fewer side effects were observed with the use of Moutan Cortex. Therefore, taking Moutan Cortex improves the condition of SLE patients [245].
Toxicity and Side Effects
Moutan Cortex can be considered a safe raw material, and no research has been done to support its toxic effects. Although, benzoic acid, a component of the extracts that are thought to be toxic, is present in small amounts in this species. It is important to consider how easily heavy metals from soil, pesticides, air dust, irrigation water, vehicle and industrial exhaust gases, and fertilizers can contaminate raw materials. Exogenous elements like heavy metals, pesticide residues, or an excessive amount of sulfur from sulfur fumigation can contaminate Moutan Cortex. To assure the high quality of the raw material, it is crucial to determine the trace elements present in Moutan Cortex [338].
3.4.10. Paeonia lactiflora Pall.
Paeonia lactiflora belongs to the family Ranunculaceae and the genus Paeonia and is also known as the Chinese peony, common garden peony, and shaoyao. P. lactiflora has a long history of use in TCM. Radix Paeoniae Alba and Radix Paeoniae Rubra are both derived from Paeonia roots, but in terms of the percentage of components and pharmacological actions, they are different. Radix Paeoniae Rubra, which is also known as chishao, RPR, and red peony root, is the dried root of P. lactiflora and has a cardiovascular and hepatoprotective effect. Radix Paeoniae Alba, which is known as baishao, RPA, and white peony root, is also the dried root of P. lactiflora, but there is also boiling and peeling in its production process. It affects the immune and nervous systems. Radix Paeoniae Alba and Radix Paeoniae Rubra both have antitumor and anti-inflammatory effects [339].
Clinical Trials
Total glycoside of paeony (TGP) in the hydroalcoholic extract of Radix Paeoniae Alba, which consists of more than 15 components. TGP has shown a direct anti-inflammatory effect by inhibiting the production of nitric oxide, leukotriene B4, and prostaglandin E2 [202].
A study investigated the effect of TGP in SLE patients. In this clinical study, one group of 29 cases received TGP for 5 years or more, the other group of 47 cases received TGP for one year or more (but less than 5 years), and the third group was the control. The results showed that following the daily intake of TGP, the required daily dose of prednisolone and cyclophosphamide decreased, and a decrease in SLEDAI score was observed, but no significant difference was observed in urinary protein. No side effects were observed following TGP use [115].
In another clinical trial, the effect of TGP on SLE patients was investigated on 70 SLE patients, who were divided into control and treatment groups. Both groups used conventional medicine, but the treatment group also received TGP for three months. Finally, it was found that the dose of glucocorticoids required by the patient decreased following the use of TGP. It was concluded that a combination of TGP and glucocorticoids might be beneficial for these patients. The side effects, including gastrointestinal effects, following the use of TGP were tolerable [116].
In a clinical trial, the effect of TGP on lupus nephritis was investigated. Forty patients were in the control group and were treated with prednisolone and cyclophosphamide. Forty patients were in the treatment group and were treated with TGP in addition to prednisolone and cyclophosphamide. As a result, following TGP intake, IL-18 and IL-6 levels decreased more than in the control group, and anti-dsDNA and serum creatinine levels decreased. Albumin and complement C3 levels increased following TGP intake. A meta-analysis on TGP, concluded that TGP is more efficient and safer when used in combination with conventional treatments. It could reduce the disease activity of SLE and the incidence of adverse reactions. Moreover, TGP could improve other outcomes related to SLE disease activity, including complement proteins (C3 and C4), immunoglobulins (IgA, IgM and, IgG), ESR, CRP, 24 h urine protein, and recurrence rate [117].
Phytochemicals and Possible Mechanisms of Action
P. lactiflora has therapeutic applications in lupus due to its anti-inflammatory and immunomodulatory effects. It has been many years since the decoction of the root of P. lactiflora has been used to treat SLE, rheumatoid arthritis, etc.
In a study, the effect of 12 weeks of treatment with Radix Paeoniae Rubra on lupus nephritis was investigated. MRL/lpr lupus mice were divided into three groups: control, prednisolone, and Radix Paeoniae Rubra. The results showed a decrease in renal pathological damage and urinary protein levels. Also, the expression of intercellular cell adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), and platelet endothelial cell adhesion molecule-1 (PECAM-1) decreased. Moreover, following the use of Radix Paeoniae Rubra, the required dose of prednisolone was reduced [244].
In another study on female MRL/lpr mice, the effect of oral administration of 50 mg/kg/d of TGP was investigated. As a result, ERα expression decreased, and DNA methyltransferases (DNMTs) expression increased. Besides a decrease in renal damage and the expression of IFN-γ, IL6, and IL12 cytokines, the serum dsDNA levels were inhibited [118]. In another study, following the consumption of TGP by MRL/lpr mice, the urinary protein content and the levels of anti-dsDNA antibodies and antinuclear antibodies (ANA) decreased [119].
In an in vitro study, the effect of TGP was investigated on the expression and DNA methylation status of the ITGAL gene (CD11a) in CD4+ T cells isolated from patients with SLE. TGP led to down-regulation of ITGAL mRNA and protein levels. In addition, DNA methylation of the ITGAL promoter was increased, which can result in the repression of the CD11a gene expression [120].
Among the components of TGP, paeoniflorin (Pae), hydroxyl-paeoniflorin, paeonin, albiflorin, and benzoylpaeoniflorin can be named. Paeoniflorin, accounting for about 40% of TGP, is the major active component of TGP. This compound has been shown to modify immune cell activities, reduce inflammatory medium formation, and correct aberrant signal pathways. Paeoniflorin has been found to regulate a variety of signaling pathways, including GPCR pathway, MAPKs/NF-κB pathway, PI3K/Akt/mTOR pathway, JAK2/STAT3 pathway, TGFβ/Smads, etc. [201].
Toxicity and Side Effects
Although there is not a lot of information about P. lactiflora’s toxicity, peonies are typically regarded as non-toxic in texts on traditional medicine. According to the research, the roots of paeony species are not toxic, but elevated doses of some of their components, such as pyrethrin I and phenol, can be harmful [340]. In the section of this article on purified molecules from natural sources, the side effects of this plant and TGP compound have been discussed.
3.4.11. Scutellaria baicalensis Georgi
Scutellaria baicalensis belongs to the family Lamiaceae and is also known as Baikal skullcap or Chinese skullcap. Wogonin, baicalin, and baicalein are some of the components of S. baicalensis. [248] Baicalin is a flavonoid isolated from the root of S. baicalensis and its anti-inflammatory, anti-cancer, and antioxidant effects have been studied [99].
Phytochemicals and Possible Mechanisms of Action
An in vitro study on pristane-induced lupus BALB/c mice found that the use of S. baicalensis downregulated the production of proinflammatory cytokines such as TNF-α, IL-6, IL-10, and IFN-γ. Also, the expression of CD69+ CD4+ T cells and CD4+ T cells decreased, but CD8+ did not decrease [248].
In another in vivo study, BALB/c mice were divided into three groups: healthy control mice, lupus control mice, and baicalin-treated mice. Mice were treated with 50 mg/kg of baicalin for 10 days. Finally, it was shown that following the use of baicalin, abnormal activation of T cells was downregulated and overproduction of IL-6 and PGE2 was inhibited [99,160].
To evaluate the effect of baicalin, a study was performed on lupus-prone MRL/lpr mice in which 200 mg/kg of baicalin was used peritoneally daily for 4 weeks. It was observed that following the use of baicalin, urine protein decreased, anti-dsDNA antibody titers were inhibited, and lupus nephritis was attenuated. Baicalin inhibits IL-21 production and Tfh cell differentiation and induces Foxp3+ regulatory T cell differentiation [98].
Toxicity and Side Effects
Refer to the section on toxicity and side effects of baicalin in this text.
3.4.12. Gentiana macrophylla Pall.
Gentiana macrophylla belongs to the Gentianaceae family, which is also known as Qinjiao. The Gentiana genus has protective effects on the cardiovascular, gastrointestinal, and reproductive systems. They have long been recognized for their immunomodulatory properties, as well as their beneficial effects on the skin, liver, and joints. Gentiana spp. have been used to control visceral pain [65,66,341]. Gentiana macrophylla root has been used in TCM to treat inflammation and pain and systemic lupus erythematosus [239].
Clinical Trial
In a clinical trial, the effect of G. macrophylla was compared with that of prednisolone. Sixty-two patients with SLE were treated with G. macrophylla complex tablets in the form of 10 tablets twice a day or 5 tablets three times a day with 10 to 30 mg of prednisolone daily. In the control group, 19 SLE patients were treated with prednisolone alone. As a result, it was observed that the recovery rate was significantly higher in the Gentiana group. Improvement of nephropathy, erythema, and arthralgia and restoration of ESR, LE cells, C3, and CH50 following G. macrophylla consumption was higher in than the control group, and no significant complication was observed [238].
Animal Studies
In a study, the cardiac protective effect of G. macrophylla on lupus mice was investigated. Thirty female NZB/W F1 mice were used, which were divided into three groups that included the control group, the cholesterol-consuming group, and the cholesterol- and G. macrophylla-consuming group. After 12 weeks, the mice’s heart tissue was used for further experiments. The results showed that following the use of G. macrophylla, cholesterol-aggravated apoptosis decreased, IGF-1 survival signal increased, and anti-apoptotic proteins increased. Therefore, G. macrophylla protects the heart against cholesterol-aggravated apoptosis in mice with lupus, so it can be useful in the treatment of CVD in patients with SLE [67].
In another study, the hepatoprotective effect of G. macrophylla on lupus mice was investigated. The female NZB/W F1 mice were divided into four groups and participated in the experiment for 8 weeks. The livers of mice were used for further experiments. Finally, they found that taking G. macrophylla could be effective in reducing liver inflammation in SLE patients [239].
Toxicity and Side Effects
In vivo studies on the Gentiana genus have not revealed any significant toxic effects when given elevated doses [65]. In Kunming mice treated with 500 mg/kg of G. macrophylla root extract, no abnormal performance or mortality was observed [342].
3.4.13. Glycyrrhiza glabra L.
Glycyrrhiza glabra belongs to the Fabaceae family and is also known as liquorice, sweet wood, and mulaithi [343]. G. glabra root contains glycyrrhizin and its derivatives, which are in the class of saponins [344]. G. glabra has long been used to treat a wide range of diseases. Studies have shown that it has anti-inflammatory, antioxidant, immunostimulatory, anticoagulant, hepatoprotective, and neuroprotective properties, as well as antibacterial, antiviral, antifungal, and anti-malarial effects [69,345,346].
Phytochemicals and Possible Mechanisms of Action
Licorice root is rich in triterpenoids and flavonoids, which are known for their antioxidant and anti-inflammatory properties, mostly by decreasing TNF, MMPs, PGE2, and free radicals [347].
G. glabra has been reported to be beneficial in improving SLE. High mobility group box 1 (HMGB1) has proinflammatory effects and an immune-stimulatory function and plays a role in the pathogenesis of inflammatory and autoimmune diseases such as SLE. Glycyrrhizin has the blocking effect of HMGB1 [110,348]. Elevated HMGB1 levels have been shown to be associated with exacerbation of SLE and elevated levels of proinflammatory cytokines such as IL-6 and TNF-α. The results of an animal study on female BALB/c mice that received glycyrrhizin (0.5 mg/day for two months) demonstrated that glycyrrhizin’s inhibition of HMGB1 function caused a sharp decline in serum HMGB1 levels, which in turn decreased the severity of SLE [110]. Glycyrrhizin directly binds to HMGB1, reducing both the extracellular release of HMGB1 and its cytokine actions [348].
In another study based on HMGB1, glycyrrhizin was used as an HMGB1 blocker. Glycyrrhizin (10 mg/kg) was injected every other three days into female BALB/c mice for 12 weeks. Finally, an improvement in lupus nephritis was achieved following the use of glycyrrhizin. Decreased levels of anti-dsDNA antibodies and decreased levels of inflammatory cytokines, as well as decreased glomerular IgG and C3 deposition and reduction of proteinuria, were observed [349].
In an in vitro study, glycyrrhizin was shown to inhibit the immunocomplex formation of 60S acidic ribosomal P proteins from porcine liver when combined with patient serum for SLE. It was concluded that a relatively high dose of glycyrrhizin could prevent the immunocomplex formation of 60S acidic ribosomal P proteins with their specific antibodies in the sera of SLE patients [112].
No clinical trial was found on the efficacy of licorice on SLE. Considering the results of preclinical studies and the application of licorice as an anti-inflammatory agent in a variety of diseases, further studies might lead researchers to find effective remedies.
Toxicity and Side Effects
The most significant adverse effects of glycyrrhizin and licorice are secondary diseases brought on by hypokalemia and hypertension. Additionally, it may result in fatal arrhythmias and cardiomyopathy. Hypokalemia, hypertension, anorexia nervosa, prolonged gastrointestinal, advanced age, and being of female sex all enhance the risk of adverse consequences from licorice. It should be mentioned that the positive effects of beta blockers and angiotensin-converting enzyme inhibitors (ACEIs) can be countered by the hypertensive impact of licorice [350,351].
3.4.14. Antrodia camphorata
Antrodia camphorata is a fungal parasite on Cinnamomum kanehirai. It is also known as “stout camphor fungus”. Antrodia camphorata has long been used in traditional medicine in China and Taiwan and is used to treat a wide range of diseases. Crude extracts of A. camphorata have been shown to have anti-cancer, antioxidant, anti-inflammatory, immunomodulatory, hepatoprotective, neuroprotective, anti-hypertensive, and vasorelaxant effects [153].
A study investigated the effect of A. camphorata on nephritis in SLE-prone NZB/W F1 mice. For this purpose, for 12 weeks, 100, 200, and 400 mg/kg of A. camphorata extract were administered orally on 5 consecutive days per week. As a result, the kidney glomerular basement membrane’s thickness was reduced and urine protein and serum BUN levels were markedly controlled by the extract of A. camphorate (400 mg/kg) [46]. Antroquinonol was reported to be the main active ingredient (refer to the antroquinonol section in this text).
Toxicity and Side Effects
Refer to the section on toxicity and side effects of antroquinonol in this text.
3.4.15. Astragalus propinquus Schischkin (syn. Astragalus membranaceus (Fisch.) Bunge)
Astragalus membranaceus belongs to the Fabaceae family and is also known as Mongolian milkvetch. It consists of the components astragaloside, astragalus flavonoids, and astragalus polysaccharide. It has an anti-inflammatory effect and reduces proteinuria and creatinine. It has been used to treat kidney disease [352]. A. membranaceus is used in lupus nephritis and its effects have been reviewed in articles. In a study, the effect of A. membranaceus and Tripterygium hypoglancum on SLE patients was investigated, and it was observed that NK activity decreased and therefore disease activity decreased [229]. A bioinformatics study has attempted to investigate the mechanism of the effect of A. membranaceus on lupus nephritis [352].
Toxicity and Side Effects
A. membranaceus has an LD50 of about 40 g/kg, which makes it safe and non-toxic. However, it has been found through in vivo research that a dose of 1 mg/kg of this plant in the form of AS-IV can have side effects such as fetal toxicity and reproductive toxicity. As a result, it should be used carefully throughout pregnancy and the postpartum period. However, astragalus extract is generally safe and has no significant side effects [353].
3.4.16. Bryophyllum pinnatum (Lam.) Oken
Bryophyllum pinnatum belongs to the Crassulaceae family and is also known as Kalanchoe pinnata, air plant, Zakham-e-hyat, life plant, and cathedral bells. The constituents of B. pinnatum include alkaloids, glycosides, triterpenes, cardienolides, flavonoids, steroids, lipids, and buffadienolides. It is used for a wide range of diseases and has been found to have antibacterial, antileishmaniasis, and antimutagenic effects, as well as hepatoprotective and nephroprotective effects. B. Pinnatum can be beneficial in the treatment of SLE due to its anti-inflammatory and immunosuppressive effects [354]. The effect of B. pinnatum on SLE has been investigated.
In a study on BALB/c mice, mice were treated with different doses of ethanolic extract of B. pinnatum leaves for 12 weeks. For this purpose, 4 groups including control and those with doses of 10.5, 21, and 42 mg/kg/day were used. As a result, following the consumption of B. pinnatum, a decrease in TNF-α, IL-17, IL-12, CRP, and matured B cells was observed. On the other hand, there was an increase in complement C3 and C4 and TGF-β. No specific side effects were reported with B. pinnatum [230,231].
In an in vitro study performed with the help of spleen cells of BALB/c mice, the effect of ethanolic extract of B. pinnatum leaves at doses of 0, 0.02, 0.1, or 0.5 µg/mL was investigated. As a result, B. pinnatum reduces B cell maturation and increases B cell apoptosis and decreases NF-ĸB p65 expression [232]. The effect of B. pinnatum on B cells has also been investigated in silico [355,356].
In vivo, the effect of aqueous extract of B. pinnatum leaf on lupus nephritis in female Balb/c mice was performed. Mice in the treatment group received 200, 400, or 600 mg/kg/day aqueous extract of B. pinnatum orally for 21 days. Eventually, it was found that proteinuria levels and glomerular inflammation were reduced. On the other hand, with the help of in silico studies, an attempt has been made to find the flavonoid composition that binds to the glucocorticoid receptor. It has been shown that bryophyllin A is probably the active compound of B. pinnatum that has an anti-inflammatory effect [233]. There is also further evidence and studies that investigate the effect of B. pinnatum on SLE and lupus nephritis [357,358,359,360].
Toxicity and Side Effects
Toxicological tests have been carried out mostly on leaf extracts to determine the safety of B. pinnatum. Even though the majority of research works have demonstrated low toxicity and acceptable safety, some have noted its cytotoxicity. For this plant, abnormalities in the animal’s testicles have also been observed. As a result, it’s seemed that B. pinnatum can be used safely in acute situations, but further research is required to determine its chronic toxicity [361].
3.4.17. Anemarrhenae aspheloidis
Anemarrhenae aspheloidis belongs to the family Asparagaceae and is also known as Zhi Mu. Anemarrhenae rhizoma has been used in TCM for many years to treat various ailments. One of the compounds of anemarrhenae rhizoma is mangiferin, which has antioxidant, anti-inflammatory, and immunomodulatory effects. The effect of mangiferin extracted from anemarrhenae rhizomaand Mangifera indica on lupus nephritis has been investigated (see the section on mangiferin in this text) [362].
Toxicity and Side Effects
Refer to the section on toxicity and side effects of mangiferin in this text.
3.4.18. Camellia sinensis (L.) Kuntze
Camellia sinensis belongs to the family Theaceae and is generally known as tea. The compounds in C. sinensis depend on various factors such as geographical environment, growing season, etc., but generally contain flavanols, flavonols, polyphenolic acids, and flavonol glycosides. C. sinensis has anti-inflammatory, antioxidant, and anti-arthritis effects [363]. The effect of C. sinensis on lupus has been studied. In an in silco study, the potency of green tea phytoconstituents as immunomodulators, anti-apoptosis agents, and anti-pyroptosis agents in SLE was investigated. The result of molecular docking can explain the mechanism of the active compound as anti-apoptosis and anti-pyroptosis. The docking results suggested theaflavin as one of the most active constituents [364].
Clinical Trial
The effect of C. sinensis on the improvement of SLE has been investigated due to its anti-inflammatory and immunomodulatory effects. In this clinical trial, 68 SLE patients were divided into control and study groups. The study group was treated with 1000 mg of C. sinensis extract daily for 12 weeks. Finally, it was observed that following the consumption of C. sinensis, the quality of life of patients and their general health increased and their disease activity decreased [234].
Toxicity and Side Effects
Although C. sinensis is one of the most commonly used and safe plants, a number of side effects have been reported for its excessive consumption, including diuresis, tremors, irritability during the day, heart irregularities, nervousness, anxiety, headache, and hypotension. In patients with anxiety, poor cardiovascular systems, renal disorders, and hyperthyroidism, it has been suggested that tea consumption be restricted [365].
3.4.19. Curcuma longa L.
Curcuma longa belongs to the Zingiberaceae family and is also known as turmeric. C. longa is widely used in Asian traditional medicine due to its medicinal properties. Since curcumin can interact with different molecular and cellular targets, it exhibits a wide range of pharmacological effects including anti-inflammatory, antioxidant, antimicrobial and chemotherapeutic activity [21]. It also has hepatoprotective effects and is useful in gastrointestinal disorders [366]. The effect of C. longa on lupus has been studied. One of the most active constituents is curcumin (see the section on curcumin in this text).
Clinical Trials
In a clinical trial, the effect of turmeric on the improvement of lupus nephritis has been investigated. For this purpose, 24 patients with lupus nephritis were divided into study and control groups. The study group was treated with 1500 mg of turmeric daily for 3 months. As a result, following turmeric consumption, proteinuria and hematuria decreased, and systolic blood pressure also decreased. No side effects were observed following short-term use of turmeric in this clinical trial [235].
Toxicity and Side Effects
Refer to the section on toxicity and side effects of curcumin in this text.
3.5. Other Plants with Lower Evidence for Lupus Conditions
In addition to the herbs listed so far, other herbs that have anti-inflammatory, antioxidant, or immunomodulatory effects can help improve SLE. By using these herbs with conventional treatment, the patient might experience fewer adverse effects, and a lower dosage of conventional medicine might be needed.
Cinchona officinalis L. belongs to the family Rubiaceae and is known as cinchona and Peruvian bark. Important components in C. officinalis include alkaloids such as Quinine, Quinidine, Chichonine, and Cinchonidine. It has anti-malarial, anti-inflammatory, antioxidant, antimicrobial, and anti-cancer effects and is used to treat lupus, malaria, etc. [367,368]. The plant and its phytoconstituents were applied to treat COVID-19 due to its anti-inflammatory and immunomodulatory properties [369,370].
Bupleurum falcatum L. (Apiaceae) is known as Chinese thoroughwax and sickle-leaf hare’s ear. Saikosaponins extracted from B. falcatum have anti-inflammatory, immunoregulatory, and anti-cancer effects. Investigating the potency of Saikosaponins to attenuate symptoms in autoimmune diseases such as lupus can be beneficial [371].
Centella asiatica(L.) Urb.(Apiceae family) is known as Hydrocotyle asiatica, Indischer Wassernabel, and Indian pennywort. [372] It contains triterpene saponins, madecassic acid, asiatic acid, asiaticoside, and madecassoside. It is mostly used to heal wounds, but it has also been used for skin conditions caused by lupus, leprosy, eczema, psoriasis, varicose ulcers, etc. [373].
Tinospora sinensis (Lour.) Merr. (syn. Tinospora cordifolia) belongs to the family Menispermaceae and is also known as guduchi, heart-leaved moonseed, and gurjo. This plant can be used in SLE due to its immunomodulatory effect. It has been reported for its anti-inflammatory, immunomodulatory, antioxidant, antibacterial, antifungal, and anti-diabetic effects. It has been used in some inflammatory and autoimmune diseases such as SLE, rheumatoid arthritis, psoriasis, etc. [374].
Acacia farnesiana (L.) Willd. (Fabaceae) is known as sweet acacia, needle bush, and huisache. A. farnesiana, due to its proteins, lectin, and α-amyrin, β-amyrin, and lupeol have anti-inflammatory and antioxidant effects and can down-regulate proinflammatory mediators [375,376,377].
Morinda citrifolia L. belongs to the family Rubiaceae and is also known as noni. In addition to its nutritional value, M. citrifolia has several biological activities, including, antibacterial, anti-fungal, anti-inflammatory, antioxidant, and antituberculosis effects, which have been reported for this plant and its phytochemicals [378]. M. citrifolia has also been claimed to be beneficial in treating lupus [379,380].
Cornus officinalis Siebold and Zucc. belongs to the Cornaceae family and is known as Asiatic Dogwood, Japanese Cornel Dogwood, and Shan Zhu Yu. Its ripe and dried fruit is called Corni Fructus. Corni Fructus has been used in traditional Chinese medicine (TCM) for a variety of conditions. It has exhibited antioxidant, anti-inflammatory, nephroprotective, hepatoprotective, neuroprotective, hypoglycemic, and anti-cancer effects [381]. The combination of Corni Fructus with other plants of traditional Chinese medicine has been used to treat lupus, although we could not find a study that has specifically examined the effect of Corni Fructus on SLE. It has been assumed that, owing to its anti-inflammatory and nephroprotective effects, it can be beneficial for patients with lupus. There are studies on the mechanism of its anti-inflammatory and kidney protective effect [382,383,384].
Allium sativum L. (Alliaceae) is known as garlic. Alliin and allicin are the most important sulfur components in A. sativum. It has exhibited a wide range of therapeutic properties, including anti-inflammatory, antioxidant, antimicrobial, antifungal, immunomodulatory, antiatherosclerotic, anti-hypertensive effects, etc. [385] A. sativum has also been suggested to be useful in improving lupus patients [386].
Wolfiporia extensa, or Poria cocos, is a fungus that belongs to the family Polyporaceae and grows on the roots of the pine tree. Poria cocos has long been used extensively in traditional Chinese medicine and is also known as Fuling, poria, and hoelen. Due to the presence of triterpenoid and ergosterol compounds, it has anti-inflammatory effects and also affects the immune system, so it is used in diseases such as rheumatoid arthritis and SLE. In addition to anti-inflammation and immunomodulation effects, this fungus also has antitumor, anti-diabetic, anti-aging, and antioxidant effects [387,388,389].
Evening primrose oil (EPO) can also be helpful in the healing process of lupus and is obtained from the seeds of Oenothera biennis L., which belongs to the Onagraceae family. The plant is also known as evening primrose, evening star, and sundrop. Evening primrose oil (EPO) contains a high concentration of γ-linolenic acid (GLA), which has anti-inflammatory, antioxidant, radical scavenging, and immunomodulatory properties [390,391]. Some clinical trials have shown that EPO can be beneficial in improving some inflammatory conditions such as atopic eczema and atopic dermatitis. There are studies on the effect of EPO on arthritis [391,392]. Due to the presence of γ-linolenic acid and prostaglandin E1, its use can be effective in SLE patients.
Andrographis paniculata (Burm.f.) Nees belongs to the family Acanthaceae and is also known as green chiretta and creat and Chuan Xin Lian. The aerial parts of this plant have been used in traditional Chinese medicine to treat inflammation, pain, and detoxification. A. paniculata contains flavonoids, polyphenols, and diterpenoids [393]. Immunomodulatory effects have been reported for the aqueous extract of A. paniculata leaves on rats [394].
Phyllanthus emblic G.L.Webster belongs to the family Euphorbiaceae. The genus Phyllanthus has long been used extensively in immune-related diseases such as SLE and has been shown to have an immunomodulatory effect [395].
Coriandrum sativum L. (Apiaceae): The essential oils in the seeds and leaves of this plant, such as linalool, are responsible for many of the plant’s benefits. C. sativum has anti-inflammatory, antioxidant, antibacterial, anti-cancer, immunostimulatory effects, etc. [396]. Consumption of this plant could be effective in lupus patients due to its anti-inflammatory and immunomodulatory effects [30].
Boswellia spp. (Burseraceae): The four main species of Boswellia, B. sacra, B. frereana, B. papyrifera, and B. serrata produce frankincense (also known as olibanum). Among the chemical compounds of frankincense 3-O-acetyl-11-keto-β boswellic acid, α- and β-boswellic acids, 11-keto-β-boswellic acid and other boswellic acids, lupeolic acids, incensole, cembrenes, triterpenediol, tirucallic acids, and olibanumols can be named. Frankincense exhibits anti-inflammatory effects through a variety of mechanisms including inhibition of leukotriene synthesis, cyclooxygenase 1/2 and 5-lipoxygenase, and oxidative stress and by regulation of immune cells from the innate and acquired immune systems. Additionally, it modifies signal transduction, which is in charge of cell cycle arrest as well as the suppression of proliferation, angiogenesis, invasion, and metastasis [397]. The major components of frankincense are boswellic acids, among which the most important and abundant is 3-O-acetyl-11-keto-β-boswellic acid (AKBA). This compound is a strong inhibitor of 5-lipoxygenase with anti-inflammatory and anti-arthritic properties [398]. Another suggested mechanism for the anti-inflammatory effects of boswellic acid is potently inhibiting cathepsin G [399].
In clinical trials, frankincense and its phytochemicals were found to be effective in treating a range of inflammatory disorders, such as osteoarthritis, multiple sclerosis, asthma, psoriasis and erythematous dermatitis, plaque-induced gingivitis, and pain [397,400,401]. We could not find a clinical trial investigating the efficacy of frankincense on SLE, but due to its anti-inflammatory and immunomodulatory effects, it might be poetically effective.
Dioscorea polystachya Turcz. (syn. Dioscorea batatas Decne.) belongs to the Dioscoreaceae family and is also known as Chinese yam or cinnamon-vine. In addition to being used as food in China, it has also been used in traditional Chinese medicine to treat various diseases such as asthma, diabetes, etc. It has anti-inflammatory and antioxidant effects [402,403] and has also been used to treat lupus [402,403,404].
Ocimum gratissimum L. belongs to the Lamiaceae family and is also known as Ram Tulshi, clove basil, and African basil. In addition to being used in food in some countries, this plant is also used in the treatment of some diseases. O. gratisimum has anti-malarial, anti-inflammatory, and antioxidant effects [405]. Owing to its immunomodulatory and anti-inflammatory effects, it can be effective in treating SLE patients [30,406].
Uncaria tomentosa (Willd. ex Schult.) DC. belongs to the Rubiaceae family and is also known as cat’s claw. The roots and bark of this plant have been used in traditional medicine to treat various diseases such as inflammation, viral infections, urinary tract infections, asthma, etc. U. tomentosa has anti-inflammatory, antioxidant, antimicrobial, and immunomodulatory effects and can be effective in treating lupus [407,408]. There is a case report that an SLE patient has ended up with acute renal failure following daily use of U. tomentosa [409].
Clerodendrum trichotomum Thunb. (Verbenaceae) is known as Chou Wu Tong and Kusagi. C. trichotomum has antioxidant, anti-inflammatory, analgesic, and sedative effects [410,411]. Due to the presence of taraxerol, friedelin, lupeol, and betulinic acid, it has an immunomodulatory effect [30].
Scrophularia ningpoensis Hemsl. belongs to the family Scrophulariaceae and is also known as Xuanshen. In traditional Chinese medicine, it has been used for many years to treat various diseases. Among the components of S. ningpoensis, phenylpropanoid glycosides and iridoid glycosides can be named to possess anti-inflammatory effects. The plant has been used to treat liver disease, cardiovascular disease, and diabetes and has antioxidant, anti-inflammatory, and anticarcinogenic effects [412]. Its effectiveness in lupus has also been considered [413].
4. Conclusions
Overall, a variety of natural molecules and their derivatives, in purified and structurally elucidated form, have been reported to exhibit beneficial effects in lupus conditions. Among these molecules, artemisinin and its derivatives, antroquinonol, baicalin, curcumin, emodin, mangiferin, salvianolic acid A, triptolide, and the total glycosides of paeony (TGP) have potential to be considered for further drug development studies. They showed their efficacy through interaction with various immune mediators, cytokines, and transcription factors such as nuclear factor kappa B (NF-κB), inhibition of anti-dsDNA, etc. Additionally, some omega-6 and omega-3 PUFAs, such as EPA, DHA, α-linolenic acid, and γ-linolenic acid, have been shown to be beneficial by lowering anti-dsDNA, TNF-α, IL-1, IL-1, IL-2, and/or CRP levels.
Some minerals (calcium, iron, selenium, and zinc) and vitamins (vitamins A, B, C, D, and E) have shown potential to exhibit degrees of beneficial effects. Considering the reported clinical trials on medicinal plants and fungi, T. wilfordii, O. sinensis, G. lucidum, A. annua, U. dioica, L. usitatissimum, R. glutinosa, P. × suffruticosa, P. lactiflora, G. macrophylla, and C. longa have exhibited efficacy against lupus conditions. Due to the small number of clinical trials and the small number of patents in each trial, it is not possible to do a meta-analysis on any of the phytochemicals or herbal medicines listed above. However, since many of these herbal medicines, minerals, or vitamins have a history of human consumption, until more studies are done, the use of these products in the form of complementary products can be beneficial for patients.
Author Contributions
Conceptualization, A.H., A.P., and B.H.; methodology, A.H., A.P., and B.H.; validation, A.H., A.P., and B.H.; investigation, A.H., A.P., A.T., E.K., and B.H.; writing—original draft preparation, A.H., A.P., A.T., E.K., and B.H.; writing—review and editing, A.H.; project administration, A.H. All authors have read and agreed to the published version of the manuscript.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article, except Ekaterina Kozuharova, who is grateful for the financial support for the research to European Union—NextGenerationEU—through the National Recovery and Resilience Plan of the Republic of Bulgaria, Project No. BG-RRP-2.004-0004-C01.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dörner, T.; Furie, R. Novel paradigms in systemic lupus erythematosus. Lancet 2019, 393, 2344–2358. [Google Scholar] [CrossRef] [PubMed]
- Veeranki, S.; Choubey, D. Systemic lupus erythematosus and increased risk to develop B cell malignancies: Role of the p200-family proteins. Immunol. Lett. 2010, 133, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Lightfoot, Y.L.; Blanco, L.P.; Kaplan, M.J. Metabolic abnormalities and oxidative stress in lupus. Curr. Opin. Rheumatol. 2017, 29, 442. [Google Scholar] [CrossRef] [PubMed]
- Bertsias, G.K.; Pamfil, C.; Fanouriakis, A.; Boumpas, D.T. Diagnostic criteria for systemic lupus erythematosus: Has the time come? Nat. Rev. Rheumatol. 2013, 9, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Bootsma, H.; Spronk, P.; de Boer, G.; Limburg, P.; Kallenberg, C.; Derksen, R.; Wolters-Dicke, J.; Gmelig-Meyling, F.; Kater, L.; Hermans, J. Prevention of relapses in systemic lupus erythematosus. Lancet 1995, 345, 1595–1599. [Google Scholar] [CrossRef]
- Loram, L.C.; Culp, M.E.; Connolly-Strong, E.C.; Sturgill-Koszycki, S. Melanocortin peptides: Potential targets in systemic lupus erythematosus. Inflammation 2015, 38, 260–271. [Google Scholar] [CrossRef]
- Fanouriakis, A.; Tziolos, N.; Bertsias, G.; Boumpas, D.T. Update οn the diagnosis and management of systemic lupus erythematosus. Ann. Rheum. Dis. 2021, 80, 14–25. [Google Scholar] [CrossRef]
- Mikdashi, J.; Nived, O. Measuring disease activity in adults with systemic lupus erythematosus: The challenges of administrative burden and responsiveness to patient concerns in clinical research. Arthritis Res. Ther. 2015, 17, 1–10. [Google Scholar] [CrossRef]
- Almaani, S.; Meara, A.; Rovin, B.H. Update on lupus nephritis. Clin. J. Am. Soc. Nephrol. 2017, 12, 825–835. [Google Scholar] [CrossRef]
- Hoover, P.J.; Costenbader, K.H. Insights into the epidemiology and management of lupus nephritis from the US rheumatologist’s perspective. Kidney Int. 2016, 90, 487–492. [Google Scholar] [CrossRef]
- Wallace, D.J. The evolution of drug discovery in systemic lupus erythematosus. Nat. Rev. Rheumatol. 2015, 11, 616–620. [Google Scholar] [CrossRef] [PubMed]
- Basta, F.; Fasola, F.; Triantafyllias, K.; Schwarting, A. Systemic lupus erythematosus (SLE) therapy: The old and the new. Rheumatol. Ther. 2020, 7, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, A.; Bonsmann, G.; Anders, H.-J.; Herzer, P.; Tenbrock, K.; Schneider, M. The diagnosis and treatment of systemic lupus erythematosus. Dtsch. Ärzteblatt Int. 2015, 112, 423. [Google Scholar] [CrossRef] [PubMed]
- Gurevitz, S.; Snyder, J.; Wessel, E.; Frey, J.; Williamson, B. Systemic lupus erythematosus: A review of the disease and treatment options. Consult. Pharm. 2013, 28, 110–121. [Google Scholar] [CrossRef]
- Tanaka, Y. State-of-the-art treatment of systemic lupus erythematosus. Int. J. Rheum. Dis. 2020, 23, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Liossis, S.N.; Staveri, C. What’s new in the treatment of systemic lupus erythematosus. Front. Med. 2021, 8, 655100. [Google Scholar] [CrossRef] [PubMed]
- Verdelli, A.; Corrà, A.; Mariotti, E.B.; Aimo, C.; Ruffo di Calabria, V.; Volpi, W.; Quintarelli, L.; Caproni, M. An update on the management of refractory cutaneous lupus erythematosus. Front. Med. 2022, 9, 941003. [Google Scholar] [CrossRef]
- Schwartz, N.; Stock, A.D.; Putterman, C. Neuropsychiatric lupus: New mechanistic insights and future treatment directions. Nat. Rev. Rheumatol. 2019, 15, 137–152. [Google Scholar] [CrossRef]
- Lubov, J.E.; Jamison, A.S.; Baltich Nelson, B.; Amudzi, A.A.; Haas, K.N.; Richmond, J.M. Medicinal plant extracts and natural compounds for the treatment of cutaneous lupus erythematosus: A systematic review. Front. Pharmacol. 2022, 13, 802624. [Google Scholar] [CrossRef]
- Cao, F.; Cheng, M.-H.; Hu, L.-Q.; Shen, H.-H.; Tao, J.-H.; Li, X.-M.; Pan, H.-F.; Gao, J. Natural products action on pathogenic cues in autoimmunity: Efficacy in systemic lupus erythematosus and rheumatoid arthritis as compared to classical treatments. Pharmacol. Res. 2020, 160, 105054. [Google Scholar] [CrossRef]
- Momtazi-Borojeni, A.A.; Haftcheshmeh, S.M.; Esmaeili, S.-A.; Johnston, T.P.; Abdollahi, E.; Sahebkar, A. Curcumin: A natural modulator of immune cells in systemic lupus erythematosus. Autoimmun. Rev. 2018, 17, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Duarte-Garcia, A.; Myasoedova, E.; Karmacharya, P.; Hocaoğlu, M.; Murad, M.H.; Warrington, K.J.; Crowson, C.S. Effect of omega-3 fatty acids on systemic lupus erythematosus disease activity: A systematic review and meta-analysis. Autoimmun. Rev. 2020, 19, 102688. [Google Scholar] [CrossRef] [PubMed]
- Yuan, K.; Li, X.; Lu, Q.; Zhu, Q.; Jiang, H.; Wang, T.; Huang, G.; Xu, A. Application and mechanisms of triptolide in the treatment of inflammatory diseases—A review. Front. Pharmacol. 2019, 10, 1469. [Google Scholar] [CrossRef] [PubMed]
- Vahedi-Mazdabadi, Y.; Saeedi, M. Treatment of Lupus Nephritis from Iranian Traditional Medicine and Modern Medicine Points of View: A Comparative Study. Evid.-Based Complement. Altern. Med. 2021, 2021, 6645319. [Google Scholar] [CrossRef]
- Gururaja, D.; Hegde, V. Ayurvedic management of systemic lupus erythematosus overlap vasculitis. J. Ayurveda Integr. Med. 2019, 10, 294–298. [Google Scholar] [CrossRef]
- Salek, M.; Hosseini Hooshiar, S.; Salek, M.; Poorebrahimi, M.; Jafarnejad, S. Omega-3 fatty acids: Current insights into mechanisms of action in systemic lupus erythematosus. Lupus 2023, 32, 7–22. [Google Scholar] [CrossRef]
- Huerta, M.D.R.; Trujillo-Martin, M.M.; Rúa-Figueroa, Í.; Cuellar-Pompa, L.; Quiros-Lopez, R.; Serrano-Aguilar, P.; Spanish, S. Healthy lifestyle habits for patients with systemic lupus erythematosus: A systemic review. Semin. Arthritis Rheum. 2016, 45, 463–470. [Google Scholar] [CrossRef]
- Zheng, R.; Gonzalez, A.; Yue, J.; Wu, X.; Qiu, M.; Gui, L.; Zhu, S.; Huang, L. Efficacy and safety of vitamin D supplementation in patients with systemic lupus erythematosus: A meta-analysis of randomized controlled trials. Am. J. Med. Sci. 2019, 358, 104–114. [Google Scholar] [CrossRef]
- Islam, M.A.; Khandker, S.S.; Alam, S.S.; Kotyla, P.; Hassan, R. Vitamin D status in patients with systemic lupus erythematosus (SLE): A systematic review and meta-analysis. Autoimmun. Rev. 2019, 18, 102392. [Google Scholar] [CrossRef]
- Balkrishna, A.; Thakur, P.; Singh, S.; Chandra Dev, S.N.; Varshney, A. Mechanistic paradigms of natural plant metabolites as remedial candidates for systemic lupus erythromatosus. Cells 2020, 9, 1049. [Google Scholar] [CrossRef]
- Thomas, D.E. The Lupus Encyclopedia: A Comprehensive Guide for Patients and Families; JHU Press: Baltimore, MD, USA, 2014. [Google Scholar]
- Fu, S.M.; Gaskin, F. History of systemic lupus erythematosus with an emphasis on certain recent major issues. In Systemic Lupus Erythematosus; Elsevier: Amsterdam, The Netherlands, 2021; pp. 3–8. [Google Scholar]
- Tene, V.; Malagon, O.; Finzi, P.V.; Vidari, G.; Armijos, C.; Zaragoza, T. An ethnobotanical survey of medicinal plants used in Loja and Zamora-Chinchipe, Ecuador. J. Ethnopharmacol. 2007, 111, 63–81. [Google Scholar] [CrossRef] [PubMed]
- Kyaw, M.S.; Aye, M.M.; Grinnell, M.; Rabach, M. Traditional and ethnobotanical dermatology practices in Myanmar. Clin. Dermatol. 2018, 36, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Magee, A.; Van Wyk, B.-E.; Van Vuuren, S. Ethnobotany and antimicrobial activity of sieketroos (Arctopus species). S. Afr. J. Bot. 2007, 73, 159–162. [Google Scholar] [CrossRef]
- Orhan, N.; Akkol, E.; Ergun, F. Evaluation of antiinflammatory and antinociceptive effects of some Juniperus species growing in Turkey. Turk. J. Biol. 2012, 36, 719–726. [Google Scholar] [CrossRef]
- Gilca, M.; Tiplica, G.S.; Salavastru, C.M. Traditional and ethnobotanical dermatology practices in Romania and other Eastern European countries. Clin. Dermatol. 2018, 36, 338–352. [Google Scholar] [CrossRef]
- Verma, R.; Singh, H.; Thakur, A.; Kohli, S. Ethnobotanical survey of medicinal and aromatic plants of Bhagalpur Region. Int. J. Appl. Sci. Biotechnol. 2020, 8, 216–222. [Google Scholar] [CrossRef]
- Sharififar, F.; Yassa, N.; Mozaffarian, V. Bioactivity of major components from the seeds of Bunium persicum (Boiss.) Fedtch. Pak. J. Pharm. Sci. 2010, 23, 300–304. [Google Scholar]
- Abou El-Soud, N.H.; El-Lithy, N.A.; El-Saeed, G.; Wahby, M.S.; Khalil, M.Y.; Morsy, F.; Shaffie, N. Renoprotective effects of caraway (Carum carvi L.) essential oil in streptozotocin induced diabetic rats. J. Appl. Pharm. Sci. 2014, 4, 027–033. [Google Scholar] [CrossRef]
- Aghili, M.H. Makhzan-al-Advia; Tehran University of Medical Sciences: Tehran, Iran, 2009; pp. 227–228. (In Persian) [Google Scholar]
- Ardakani, M.R.S.; Farjadmand, F.; Rahimi, R. Makhzan al adviyeh and pointing to the scientific names of medicinal plants for the first time in a persian book. Tradit. Integr. Med. 2018, 3, 186–195. [Google Scholar]
- Seri, A.; Khorsand, M.; Rezaei, Z.; Hamedi, A.; Takhshid, M.A. Inhibitory effect of bunium persicum hydroalcoholic extract on glucose-induced albumin glycation, oxidation, and aggregation in vitro. Iran. J. Med. Sci. 2017, 42, 369. [Google Scholar]
- Mehrabadi, M.M.; Zarshenas, M.M. A Concise Overview of Phytochemistry, Pharmacology and Clinical Aspects of Persian Cumin; Bunium persicum (Boiss.) B. Fedtsch. Curr. Drug Discov. Technol. 2021, 18, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Bansal, S.; Sharma, K.; Gautam, V.; Lone, A.A.; Malhotra, E.V.; Kumar, S.; Singh, R. A comprehensive review of Bunium persicum: A valuable medicinal spice. Food Rev. Int. 2021, 39, 1184–1202. [Google Scholar] [CrossRef]
- Chang, J.-M.; Lee, Y.-R.; Hung, L.-M.; Liu, S.-Y.; Kuo, M.-T.; Wen, W.-C.; Chen, P. An extract of Antrodia camphorata mycelia attenuates the progression of nephritis in systemic lupus erythematosus-prone NZB/W F1 mice. Evid.-Based Complement. Altern. Med. 2011, 2011, 465894. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Han, C.; Sheng, Y.; Wang, J.; Li, W.; Zhou, X.; Ruan, S. Antrodia camphorata polysaccharide improves inflammatory response in liver injury via the ROS/TLR4/NF-κB signal. J. Cell. Mol. Med. 2022, 26, 2706–2716. [Google Scholar] [CrossRef]
- Kushairi, N.; Tarmizi, N.A.K.A.; Phan, C.W.; Macreadie, I.; Sabaratnam, V.; Naidu, M.; David, P. Modulation of neuroinflammatory pathways by medicinal mushrooms, with particular relevance to Alzheimer’s disease. Trends Food Sci. Technol. 2020, 104, 153–162. [Google Scholar] [CrossRef]
- Septembre-Malaterre, A.; Lalarizo Rakoto, M.; Marodon, C.; Bedoui, Y.; Nakab, J.; Simon, E.; Hoarau, L.; Savriama, S.; Strasberg, D.; Guiraud, P.; et al. Artemisia annua, a Traditional Plant Brought to Light. Int. J. Mol. Sci. 2020, 21, 4986. [Google Scholar] [CrossRef]
- Alesaeidi, S.; Miraj, S. A Systematic Review of Anti-malarial Properties, Immunosuppressive Properties, Anti-inflammatory Properties, and Anti-cancer Properties of Artemisia Annua. Electron. Physician 2016, 8, 3150–3155. [Google Scholar] [CrossRef]
- Durazzo, A.; Nazhand, A.; Lucarini, M.; Silva, A.M.; Souto, S.B.; Guerra, F.; Severino, P.; Zaccardelli, M.; Souto, E.B.; Santini, A. Astragalus (Astragalus membranaceus Bunge): Botanical, geographical, and historical aspects to pharmaceutical components and beneficial role. Rend. Lincei. Sci. Fis. Nat. 2021, 32, 625–642. [Google Scholar] [CrossRef]
- Chibli, L.A.; Rodrigues, K.C.; Gasparetto, C.M.; Pinto, N.C.; Fabri, R.L.; Scio, E.; Alves, M.S.; Del-Vechio-Vieira, G.; Sousa, O.V. Anti-inflammatory effects of Bryophyllum pinnatum (Lam.) Oken ethanol extract in acute and chronic cutaneous inflammation. J. Ethnopharmacol. 2014, 154, 330–338. [Google Scholar] [CrossRef]
- Smith, G.F.; Figueiredo, E.; van Wyk, A.E. Chapter 12—Taxonomic Treatment. In Kalanchoe (Crassulaceae) in Southern Africa; Smith, G.F., Figueiredo, E., van Wyk, A.E., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 131–303. [Google Scholar]
- Mule, P.; Upadhye, M.; Taru, P.; Dhole, S. A Review on Bryophyllum pinnatum (Lam.) Oken. Res. J. Pharmacogn. Phytochem. 2020, 12, 111. [Google Scholar] [CrossRef]
- Sánchez, M.; González-Burgos, E.; Iglesias, I.; Lozano, R.; Gómez-Serranillos, M.P. The Pharmacological Activity of Camellia sinensis (L.) Kuntze on Metabolic and Endocrine Disorders: A Systematic Review. Biomolecules 2020, 10, 603. [Google Scholar] [CrossRef]
- Oliveira, A.; Guimarães, A.; Oliveira Junior, R.; Quintans, J.; Medeiros, F.; Barbosa Filho, J.; Quintans-Júnior, L.; Almeida, J.R. Camellia sinensis (L.) Kuntze: A Review of Chemical and Nutraceutical Properties. Nat. Prod. Res. Rev. 2016, 4, 21–62. [Google Scholar]
- Umesh, C.V. Chapter 13—Camellia sinensis. In Herbs, Spices and Their Roles in Nutraceuticals and Functional Foods; Amalraj, A., Kuttappan, S., Varma A.C, K., Matharu, A., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 219–231. [Google Scholar]
- Sahoo, J.P.; Behera, L.; Praveena, J.; Sawant, S.; Mishra, A.; Sharma, S.S.; Ghosh, L.; Mishra, A.P.; Sahoo, A.R.; Pradhan, P. The golden spice turmeric (Curcuma longa) and its feasible benefits in prospering human health—A review. Am. J. Plant Sci. 2021, 12, 455–475. [Google Scholar] [CrossRef]
- Varma, A.C.K.; Jude, S.; Varghese, B.A.; Kuttappan, S.; Amalraj, A. Chapter 2—Curcuma longa. In Herbs, Spices and Their Roles in Nutraceuticals and Functional Foods; Amalraj, A., Kuttappan, S., Varma A.C, K., Matharu, A., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 15–30. [Google Scholar]
- Jyotirmayee, B.; Mahalik, G. A review on selected pharmacological activities of Curcuma longa L. Int. J. Food Prop. 2022, 25, 1377–1398. [Google Scholar] [CrossRef]
- Wachtel-Galor, S.; Yuen, J.; Buswell, J.A.; Benzie, I.F.F. Ganoderma lucidum (Lingzhi or Reishi): A Medicinal Mushroom. In Herbal Medicine: Biomolecular and Clinical Aspects, 2nd ed.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2011. [Google Scholar]
- Sanodiya, B.S.; Thakur, G.S.; Baghel, R.K.; Prasad, G.B.; Bisen, P.S. Ganoderma lucidum: A potent pharmacological macrofungus. Curr. Pharm. Biotechnol. 2009, 10, 717–742. [Google Scholar] [CrossRef]
- Ahmad, R.; Riaz, M.; Khan, A.; Aljamea, A.; Algheryafi, M.; Sewaket, D.; Alqathama, A. Ganoderma lucidum (Reishi) an edible mushroom; a comprehensive and critical review of its nutritional, cosmeceutical, mycochemical, pharmacological, clinical, and toxicological properties. Phytother. Res. PTR 2021, 35, 6030–6062. [Google Scholar] [CrossRef]
- Yu, Y.H.; Kuo, H.P.; Hsieh, H.H.; Li, J.W.; Hsu, W.H.; Chen, S.J.; Su, M.H.; Liu, S.H.; Cheng, Y.C.; Chen, C.Y.; et al. Ganoderma tsugae Induces S Phase Arrest and Apoptosis in Doxorubicin-Resistant Lung Adenocarcinoma H23/0.3 Cells via Modulation of the PI3K/Akt Signaling Pathway. Evid.-Based Complement. Altern. Med. 2012, 2012, 371286. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Cui, B.-W.; Wu, Y.-L.; Nan, J.-X.; Lian, L.-H. Genus Gentiana: A review on phytochemistry, pharmacology and molecular mechanism. J. Ethnopharmacol. 2021, 264, 113391. [Google Scholar] [CrossRef]
- Zhang, X.; Zhan, G.; Jin, M.; Zhang, H.; Dang, J.; Zhang, Y.; Guo, Z.; Ito, Y. Botany, traditional use, phytochemistry, pharmacology, quality control, and authentication of Radix Gentianae Macrophyllae-A traditional medicine: A review. Phytomedicine 2018, 46, 142–163. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-Y.; Hsu, T.-C.; Kuo, W.-W.; Liou, Y.-F.; Lee, S.-D.; Ju, D.-T.; Kuo, C.-H.; Tzang, B.-S. The root extract of Gentiana macrophylla Pall. Alleviates cardiac apoptosis in lupus prone mice. PLoS ONE 2015, 10, e0127440. [Google Scholar] [CrossRef]
- Pasdaran, A.; Naychov, Z.; Batovska, D.; Kerr, P.; Favre, A.; Dimitrov, V.; Aneva, I.; Hamedi, A.; Kozuharova, E. Some European Gentiana Species Are Used Traditionally to Cure Wounds: Bioactivity and Conservation Issues. Diversity 2023, 15, 467. [Google Scholar] [CrossRef]
- Ravanfar, P.; Namazi, G.; Atigh, M.; Zafarmand, S.; Hamedi, A.; Salehi, A.; Izadi, S.; Borhani-Haghighi, A. Efficacy of whole extract of licorice in neurological improvement of patients after acute ischemic stroke. J. Herb. Med. 2016, 6, 12–17. [Google Scholar] [CrossRef]
- Petramfar, P.; Hajari, F.; Yousefi, G.; Azadi, S.; Hamedi, A. Efficacy of oral administration of licorice as an adjunct therapy on improving the symptoms of patients with Parkinson’s disease, A randomized double blinded clinical trial. J. Ethnopharmacol. 2020, 247, 112226. [Google Scholar] [CrossRef] [PubMed]
- Ayeka, P.A.; Bian, Y.; Githaiga, P.M.; Zhao, Y. The immunomodulatory activities of licorice polysaccharides (Glycyrrhiza uralensis Fisch.) in CT 26 tumor-bearing mice. BMC Complement. Altern. Med. 2017, 17, 536. [Google Scholar] [CrossRef]
- Jiang, M.; Zhao, S.; Yang, S.; Lin, X.; He, X.; Wei, X.; Song, Q.; Li, R.; Fu, C.; Zhang, J. An “essential herbal medicine”—Licorice: A review of phytochemicals and its effects in combination preparations. J. Ethnopharmacol. 2020, 249, 112439. [Google Scholar] [CrossRef]
- Ansari, R.; Zarshenas, M.M.; Dadbakhsh, A. A Review on Pharmacological and Clinical Aspects of Linum usitatissimum L. Curr. Drug Discov. Technol. 2018, 15, 148–158. [Google Scholar] [CrossRef]
- Ganguly, S.; Panjagari, N.R.; Raman, R.K. Flaxseed (Linum usitatissimum). In Oilseeds: Health Attributes and Food Applications; Springer: Berlin/Heidelberg, Germany, 2021; pp. 253–283. [Google Scholar]
- Heydarirad, G.; Tavakoli, A.; Cooley, K.; Pasalar, M. A review on medical plants advised for neuralgia from the perspective of “canon of medicine”. Adv. Integr. Med. 2021, 8, 230–235. [Google Scholar] [CrossRef]
- Mehta, N.; Patel, E.P.; Pragnesh, B.S.V.P.; Shah, B. Nelumbo nucifera (Lotus): A review on ethanobotany, phytochemistry and pharmacology. Indian J. Pharm. Biol. Res. 2013, 1, 152–167. [Google Scholar] [CrossRef]
- Sharma, B.R.; Gautam, L.N.S.; Adhikari, D.; Karki, R. A comprehensive review on chemical profiling of Nelumbo nucifera: Potential for drug development. Phytother. Res. 2017, 31, 3–26. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Yang, S.K.; Zhou, X.; Wang, M.; Tang, D.; Liu, F.Y.; Sun, L.; Xiao, L. Use of Ophiocordyceps sinensis (syn. Cordyceps sinensis) combined with angiotensin-converting enzyme inhibitors (ACEI)/angiotensin receptor blockers (ARB) versus ACEI/ARB alone in the treatment of diabetic kidney disease: A meta-analysis. Ren. Fail. 2015, 37, 614–634. [Google Scholar] [CrossRef]
- Lo, H.-C.; Hsieh, C.; Lin, F.-Y.; Hsu, T.-H. A systematic review of the mysterious caterpillar fungus Ophiocordyceps sinensis in DongChongXiaCao (冬蟲夏草 Dōng Chóng Xià Cǎo) and related bioactive ingredients. J. Tradit. Complement. Med. 2013, 3, 16–32. [Google Scholar] [CrossRef] [PubMed]
- He, D.Y.; Dai, S.M. Anti-inflammatory and immunomodulatory effects of paeonia lactiflora pall., a traditional chinese herbal medicine. Front. Pharmacol. 2011, 2, 10. [Google Scholar] [CrossRef]
- Wang, Z.; He, C.; Peng, Y.; Chen, F.; Xiao, P. Origins, Phytochemistry, Pharmacology, Analytical Methods and Safety of Cortex Moutan (Paeonia suffruticosa Andrew): A Systematic Review. Molecules 2017, 22, 946. [Google Scholar] [CrossRef]
- Kim, Y.G.; Komakech, R.; Jeong, D.H.; Park, Y.M.; Lee, T.K.; Kim, K.H.; Lee, A.Y.; Moon, B.C.; Kang, Y. Verification of the Field Productivity of Rehmannia glutinosa (Gaertn.) DC. Developed Through Optimized In Vitro Culture Method. Plants 2020, 9, 317. [Google Scholar] [CrossRef] [PubMed]
- Bian, Z.; Zhang, R.; Zhang, X.; Zhang, J.; Xu, L.; Zhu, L.; Ma, Y.; Liu, Y. Extraction, structure and bioactivities of polysaccharides from Rehmannia glutinosa: A review. J. Ethnopharmacol. 2023, 305, 116132. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Tang, H.; Xie, L.; Zheng, Y.; Ma, Z.; Sun, Q.; Li, X. Scutellaria baicalensis Georgi. (Lamiaceae): A review of its traditional uses, botany, phytochemistry, pharmacology and toxicology. J. Pharm. Pharmacol. 2019, 71, 1353–1369. [Google Scholar] [CrossRef]
- Song, J.-W.; Long, J.-Y.; Xie, L.; Zhang, L.-L.; Xie, Q.-X.; Chen, H.-J.; Deng, M.; Li, X.-F. Applications, phytochemistry, pharmacological effects, pharmacokinetics, toxicity of Scutellaria baicalensis Georgi. and its probably potential therapeutic effects on COVID-19: A review. Chin. Med. 2020, 15, 102. [Google Scholar] [CrossRef]
- Billah, M.M.; Khan, F.; Niaz, K. Chapter 3.38—Scutellaria baicalensis Georgi. In Nonvitamin and Nonmineral Nutritional Supplements; Nabavi, S.M., Silva, A.S., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 403–408. [Google Scholar]
- Song, C.Y.; Xu, Y.G.; Lu, Y.Q. Use of Tripterygium wilfordii Hook F for immune-mediated inflammatory diseases: Progress and future prospects. J. Zhejiang Univ. Sci. B 2020, 21, 280–290. [Google Scholar] [CrossRef]
- Brinker, A.M.; Ma, J.; Lipsky, P.E.; Raskin, I. Medicinal chemistry and pharmacology of genus Tripterygium (Celastraceae). Phytochemistry 2007, 68, 732–766. [Google Scholar] [CrossRef]
- Devkota, H.P.; Paudel, K.R.; Khanal, S.; Baral, A.; Panth, N.; Adhikari-Devkota, A.; Jha, N.K.; Das, N.; Singh, S.K.; Chellappan, D.K.; et al. Stinging Nettle (Urtica dioica L.): Nutritional Composition, Bioactive Compounds, and Food Functional Properties. Molecules 2022, 27, 5219. [Google Scholar] [CrossRef]
- Hamedi, A.; Sakhteman, A.; Moheimani, S.M. An in silico approach towards investigation of possible effects of essential oils constituents on receptors involved in cardiovascular diseases (CVD) and associated risk factors (Diabetes Mellitus and Hyperlipidemia). Cardiovasc. Hematol. Agents Med. Chem. (Former. Curr. Med. Chem.-Cardiovasc. Hematol. Agents) 2021, 19, 32–42. [Google Scholar] [CrossRef]
- Ebrahimi-Najafabadi, H.; Kazemeini, S.S.; Pasdaran, A.; Hamedi, A. A novel similarity search approach for high-performance thin-layer chromatography (HPTLC) fingerprinting of medicinal plants. Phytochem. Anal. 2019, 30, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Mojab, F.; Hamedi, A.; Nickavar, B.; Javidnia, K. Hydrodistilled volatile constituents of the leaves of Daucus carota L. subsp. sativus (Hoffman.) Arcang. (Apiaceae) from Iran. J. Essent. Oil Bear. Plants 2008, 11, 271–277. [Google Scholar] [CrossRef]
- Tsai, P.Y.; Ka, S.M.; Chang, J.M.; Lai, J.H.; Dai, M.S.; Jheng, H.L.; Kuo, M.T.; Chen, P.; Chen, A. Antroquinonol differentially modulates T cell activity and reduces interleukin-18 production, but enhances Nrf2 activation, in murine accelerated severe lupus nephritis. Arthritis Rheum. 2012, 64, 232–242. [Google Scholar] [CrossRef]
- Liu, C.-P.; Tsai, W.-J.; Shen, C.-C.; Lin, Y.-L.; Liao, J.-F.; Chen, C.-F.; Kuo, Y.-C. Inhibition of (S)-armepavine from Nelumbo nucifera on autoimmune disease of MRL/MpJ-lpr/lpr mice. Eur. J. Pharmacol. 2006, 531, 270–279. [Google Scholar] [CrossRef]
- Lan, L. Study on effect of Cordyceps sinensis and artemisinin in preventing recurrence of lupus nephritis. Chin. J. Integr. Tradit. West. Med. 2002, 8, 89. [Google Scholar] [CrossRef]
- Yang, L.-Y.; Chen, A.; Kuo, Y.-C.; Lin, C.-Y. Efficacy of a pure compound H1-A extracted from Cordyceps sinensis on autoimmune disease of MRL lpr/lpr mice. J. Lab. Clin. Med. 1999, 134, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.F.; He, S.J.; Li, X.; Yang, Y.; He, P.L.; Zhou, Y.; Zhu, F.H.; Yang, Y.F.; Li, Y.; Tang, W. Oral administration of artemisinin analog SM934 ameliorates lupus syndromes in MRL/lpr mice by inhibiting Th1 and Th17 cell responses. Arthritis Rheum. 2011, 63, 2445–2455. [Google Scholar] [CrossRef]
- Yang, J.; Yang, X.; Yang, J.; Li, M. Baicalin ameliorates lupus autoimmunity by inhibiting differentiation of Tfh cells and inducing expansion of Tfr cells. Cell Death Dis. 2019, 10, 140. [Google Scholar] [CrossRef]
- Chae, B.-S. Baicalin Ameliorates Dysimmunoregulation in Pristane-Induced Lupus Mice: Production of IL-6 and PGE2 and Activation of T cells. Nat. Prod. Sci. 2011, 17, 354–362. [Google Scholar]
- Handono, K.; Pratama, M.Z.; Endharti, A.T.; Kalim, H. Treatment of low doses curcumin could modulate Th17/Treg balance specifically on CD4+ T cell cultures of systemic lupus erythematosus patients. Cent. Eur. J. Immunol. 2015, 40, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wang, J.; Zhou, M.; Li, M.; Li, M.; Tan, H. Curcumin attenuates murine lupus via inhibiting NLRP3 inflammasome. Int. Immunopharmacol. 2019, 69, 213–216. [Google Scholar] [CrossRef] [PubMed]
- Dent, E.L.; Taylor, E.B.; Turbeville, H.R.; Ryan, M.J. Curcumin attenuates autoimmunity and renal injury in an experimental model of systemic lupus erythematosus. Physiol. Rep. 2020, 8, e14501. [Google Scholar] [CrossRef]
- Kalim, H.; Handono, K.; Khalasha, T.; Pratama, M.Z.; Dantara, T.W.I.; Wulandari, A.P.; Albinsaid, F.; Fitria, S.N.; Mahardika, M.V. Immune modulation effects of curcumin in pristane-induced lupus mice. Indian J. Rheumatol. 2017, 12, 86. [Google Scholar] [CrossRef]
- Li, W.-D.; Dong, Y.-J.; Tu, Y.-Y.; Lin, Z.-B. Dihydroarteannuin ameliorates lupus symptom of BXSB mice by inhibiting production of TNF-alpha and blocking the signaling pathway NF-kappa B translocation. Int. Immunopharmacol. 2006, 6, 1243–1250. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, Y.; Jiang, N.; Zhao, X.; Sang, X.; Yang, N.; Feng, Y.; Chen, R.; Chen, Q. Dihydroartemisinin regulates the immune system by promotion of CD8+ T lymphocytes and suppression of B cell responses. Sci. China Life Sci. 2020, 63, 737–749. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Tao, T.; Wang, W.; Yang, B.; Cha, X. Dihydroartemisinin attenuated the symptoms of mice model of systemic lupus erythematosus by restoring the Treg/Th17 balance. Clin. Exp. Pharmacol. Physiol. 2021, 48, 626–633. [Google Scholar] [CrossRef]
- Xia, Y.; Xu, S. Effect of emodin on nephritis of BXSB lupus mice and its pharmacological mechanism. Cent. China Med. J. 2003, 27, 63–64. [Google Scholar]
- Yuan, X.; Dai, B.; Yang, L.; Lin, B.; Lin, E.; Pan, Y. Emodin ameliorates renal injury in BXSB mice by modulating TNF-α/ICAM-1. Biosci. Rep. 2020, 40, BSR20202551. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Z.; Wu, H.; Wang, J.; Zhang, S. Esculetin alleviates murine lupus nephritis by inhibiting complement activation and enhancing Nrf2 signaling pathway. J. Ethnopharmacol. 2022, 288, 115004. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Yu, S.; Xu, W.; Gao, B.; Xiong, S. HMGB1 promotes systemic lupus erythematosus by enhancing macrophage inflammatory response. J. Immunol. Res. 2015, 2015, 946748. [Google Scholar] [CrossRef]
- Garcia-Gordillo, M.A.; Collado-Mateo, D.; Hernández-Mocholi, M.A.; Pazzi, F.; Gusi, N.; Dominguez-Munoz, F.J.; Adsuar, J.C. Cost-Utility Analysis of a Six-Weeks Ganoderma Lucidum-Based Treatment for Women with Fibromyalgia: A Randomized Double-Blind, Active Placebo-Controlled Trial. Myopain 2015, 23, 188–194. [Google Scholar] [CrossRef]
- Maekawa, T.; Kosuge, S.; Karino, A.; Okano, T.; Ito, J.; Munakata, H.; Ohtsuki, K. Biochemical characterization of 60S acidic ribosomal P proteins from porcine liver and the inhibition of their immunocomplex formation with sera from systemic lupus erythematosus (SLE) patients by glycyrrhizin in vitro. Biol. Pharm. Bull. 2000, 23, 27–32. [Google Scholar] [CrossRef]
- Liang, C.-L.; Lu, W.; Zhou, J.-Y.; Chen, Y.; Zhang, Q.; Liu, H.; Qiu, F.; Dai, Z. Mangiferin attenuates murine lupus nephritis by inducing CD4+ Foxp3+ regulatory T cells via suppression of mTOR signaling. Cell. Physiol. Biochem. 2018, 50, 1560–1573. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Yan, Y.; Zhang, H.; Chen, Y.; He, Y.; Wang, S.; Fang, L.; Lv, Y.; Du, G. Salvianolic acid A alleviates renal injury in systemic lupus erythematosus induced by pristane in BALB/c mice. Acta Pharm. Sin. B 2017, 7, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xiao, W.; Hou, P. Clinical study of total glucosides of paeony in patients with systemic lupus erythematosus. Zhongguo Zhong Xi Yi Jie He Za Zhi Zhongguo Zhongxiyi Jiehe Zazhi = Chin. J. Integr. Tradit. West. Med. 2011, 31, 476–479. [Google Scholar]
- Shuai, Z.-w.; Xu, J.-h.; Liu, S. Clinical study on effect of Total Glucosides of Peony in treating systemic lupus erythematosus as adjuvant treatment. Chin. J. Integr. Tradit. West. Med. 2003, 23, 188–191. [Google Scholar]
- Gong, X.; Li, H.; Guo, H.; Wu, S.; Lu, C.; Chen, Y.; Li, S. Efficacy and safety of total glucosides of paeony in the treatment of systemic lupus erythematosus: A systematic review and meta-analysis. Front. Pharmacol. 2022, 13, 932874. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Jiang, A. DNA methylation was involved in total glucosides of paeony regulating ERα for the treatment of female systemic lupus erythematosus mice. J. Pharmacol. Sci. 2019, 140, 187–192. [Google Scholar] [CrossRef]
- Ding, Z.-X.; Yang, S.-F.; Wu, Q.-F.; Lu, Y.; Chen, Y.-Y.; Nie, X.-L.; Jie, H.-Y.; Qi, J.-M.; Wang, F.-S. Therapeutic effect of total glucosides of paeony on lupus nephritis in MRL/lpr mice. J. South. Med. Univ. 2011, 31, 656–660. [Google Scholar]
- Zhao, M.; Liang, G.; Luo, S.; Lu, Q. Effect of total glucosides of peony on expression and DNA methylation status of ITGAL gene in CD4 (+) T cells of systemic lupus erythematosus. Zhong Nan Da Xue Xue Bao. Yi Xue Ban = J. Cent. South Univ. Med. Sci. 2012, 37, 463–468. [Google Scholar]
- Liu, L.; Jiao, W.; Zhang, X.; Zhang, Y.; Zhao, G.; Sun, Y.; Shuhua, L. Regulation on function and maturation of dendritic cells from systemic lupus erythematosus patients by triptolide. Chin. J. Microbiol. Immunol. 2011, 31, 824–829. [Google Scholar]
- Liu, Y.-F.; He, H.-Q.; Ding, Y.-L.; Wu, S.-Y.; Chen, D.-S.; CL, E. Effects of Triptolide on Tc and Th Cell Excursion in Peripheral Blood of Nude Mice with Systemic Lupus Erythematosus BALB/c-un. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2019, 27, 1691–1695. [Google Scholar] [PubMed]
- Tao, X.; Fan, F.; Hoffmann, V.; Gao, C.Y.; Longo, N.S.; Zerfas, P.; Lipsky, P.E. Effective therapy for nephritis in (NZB × NZW) F1 mice with triptolide and tripdiolide, the principal active components of the Chinese herbal remedy Tripterygium wilfordii Hook F. Arthritis Rheum. Off. J. Am. Coll. Rheumatol. 2008, 58, 1774–1783. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Tang, X.; Yan, Q.; Song, H.; Li, Z.; Wang, D.; Chen, H.; Sun, L. Triptolide ameliorates lupus via the induction of miR-125a-5p mediating Treg upregulation. Int. Immunopharmacol. 2019, 71, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-Y.; Li, H.; Wu, Y.-W.; Cheng, L.; Yan, Y.-X.; Yang, X.-Q.; Zhu, F.-H.; He, S.-J.; Tang, W.; Zuo, J.-P. (5R)-5-hydroxytriptolide ameliorates lupus nephritis in MRL/lpr mice by preventing infiltration of immune cells. Am. J. Physiol.-Ren. Physiol. 2017, 312, F769–F777. [Google Scholar] [CrossRef] [PubMed]
- Halade, G.V.; Rahman, M.M.; Bhattacharya, A.; Barnes, J.L.; Chandrasekar, B.; Fernandes, G. Docosahexaenoic acid-enriched fish oil attenuates kidney disease and prolongs median and maximal life span of autoimmune lupus-prone mice. J. Immunol. 2010, 184, 5280–5286. [Google Scholar] [CrossRef]
- Fettouh, D.S.; Saif, D.S.; El Gazzar, S.F.; Sonbol, A.A. Study the relationship between vitamin A deficiency, T helper 17, regulatory T cells, and disease activity in patients with systemic lupus erythematosus. Egypt. Rheumatol. Rehabil. 2019, 46, 244–250. [Google Scholar] [CrossRef]
- Handono, K.; Firdausi, S.N.; Pratama, M.Z.; Endharti, A.T.; Kalim, H. Vitamin A improve Th17 and Treg regulation in systemic lupus erythematosus. Clin. Rheumatol. 2016, 35, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Minami, Y.; Hirabayashi, Y.; Nagata, C.; Ishii, T.; Harigae, H.; Sasaki, T. Intakes of vitamin B6 and dietary fiber and clinical course of systemic lupus erythematosus: A prospective study of Japanese female patients. J. Epidemiol. 2011, 21, 246–254. [Google Scholar] [CrossRef]
- Shah, M.; Adams-Huet, B.; Kavanaugh, A.; Coyle, Y.; Lipsky, P. Nutrient intake and diet quality in patients with systemic lupus erythematosus on a culturally sensitive cholesterol lowering dietary program. J. Rheumatol. 2004, 31, 71–75. [Google Scholar]
- Minami, Y.; Sasaki, T.; Arai, Y.; Kurisu, Y.; Hisamichi, S. Diet and systemic lupus erythematosus: A 4 year prospective study of Japanese patients. J. Rheumatol. 2003, 30, 747–754. [Google Scholar] [PubMed]
- Ben-Zvi, I.; Aranow, C.; Mackay, M.; Stanevsky, A.; Kamen, D.L.; Marinescu, L.M.; Collins, C.E.; Gilkeson, G.S.; Diamond, B.; Hardin, J.A. The impact of vitamin D on dendritic cell function in patients with systemic lupus erythematosus. PLoS ONE 2010, 5, e9193. [Google Scholar] [CrossRef] [PubMed]
- Antico, A.; Tampoia, M.; Tozzoli, R.; Bizzaro, N. Can supplementation with vitamin D reduce the risk or modify the course of autoimmune diseases? A systematic review of the literature. Autoimmun. Rev. 2012, 12, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Maeshima, E.; Liang, X.-M.; Goda, M.; Otani, H.; Mune, M. The efficacy of vitamin E against oxidative damage and autoantibody production in systemic lupus erythematosus: A preliminary study. Clin. Rheumatol. 2007, 26, 401–404. [Google Scholar] [CrossRef]
- Leiter, L.M.; Reuhl, K.R.; Racis Jr, S.P.; Sherman, A.R. Iron status alters murine systemic lupus erythematosus. J. Nutr. 1995, 125, 474–484. [Google Scholar]
- O’Dell, J.R.; McGivern, J.P.; Kay, H.; Klassen, L.W. Improved survival in murine lupus as the result of selenium supplementation. Clin. Exp. Immunol. 1988, 73, 322. [Google Scholar]
- Soni, C.; Sinha, I.; Fasnacht, M.J.; Olsen, N.J.; Rahman, Z.S.; Sinha, R. Selenium supplementation suppresses immunological and serological features of lupus in B6. Sle1b mice. Autoimmunity 2019, 52, 57–68. [Google Scholar] [CrossRef]
- D’Cunha, N.M.; Peterson, G.; Baby, K.; Thomas, J. Impetigo: A need for new therapies in a world of increasing antimicrobial resistance. J. Clin. Pharm. Ther. 2017, 43, 150–153. [Google Scholar] [CrossRef]
- Corson, T.W.; Crews, C.M. Molecular understanding and modern application of traditional medicines: Triumphs and trials. Cell 2007, 130, 769–774. [Google Scholar] [CrossRef]
- Garcia, L.C. A Review of Artemisia annua L.: Its genetics, biochemical characteristics, and anti-malarial efficacy. Int. J. Sci. Technol. 2015, 5, 38–46. [Google Scholar]
- Krishna, S.; Bustamante, L.; Haynes, R.K.; Staines, H.M. Artemisinins: Their growing importance in medicine. Trends Pharmacol. Sci. 2008, 29, 520–527. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, T.; Qiu, X.; Tian, B.; Bi, C.; Yao, L. Effectiveness of Bailing capsules in the treatment of lupus nephritis: A meta-analysis. Mol. Med. Rep. 2020, 22, 2132–2140. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Fortin, P.; Li, Y.; Panaritis, T.; Gans, M.; Esdaile, J. Discontinuation of antimalarial drugs in systemic lupus erythematosus. J. Rheumatol. 1999, 26, 808–815. [Google Scholar] [PubMed]
- Mu, X.; Wang, C. Artemisinins—A promising new treatment for systemic lupus erythematosus: A descriptive review. Curr. Rheumatol. Rep. 2018, 20, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Golenser, J.; Waknine, J.H.; Krugliak, M.; Hunt, N.H.; Grau, G.E. Current perspectives on the mechanism of action of artemisinins. Int. J. Parasitol. 2006, 36, 1427–1441. [Google Scholar] [CrossRef] [PubMed]
- Efferth, T.; Oesch, F. The immunosuppressive activity of artemisinin-type drugs towards inflammatory and autoimmune diseases. Med. Res. Rev. 2021, 41, 3023–3061. [Google Scholar] [CrossRef] [PubMed]
- Dang, W.-Z.; Li, H.; Jiang, B.; Nandakumar, K.S.; Liu, K.-F.; Liu, L.-X.; Yu, X.-C.; Tan, H.-J.; Zhou, C. Therapeutic effects of artesunate on lupus-prone MRL/lpr mice are dependent on T follicular helper cell differentiation and activation of JAK2–STAT3 signaling pathway. Phytomedicine 2019, 62, 152965. [Google Scholar] [CrossRef]
- Li, D.; Qi, J.; Wang, J.; Pan, Y.; Li, J.; Xia, X.; Dou, H.; Hou, Y. Protective effect of dihydroartemisinin in inhibiting senescence of myeloid-derived suppressor cells from lupus mice via Nrf2/HO-1 pathway. Free Radic. Biol. Med. 2019, 143, 260–274. [Google Scholar] [CrossRef]
- Efferth, T.; Kaina, B. Toxicity of the antimalarial artemisinin and its dervatives. Crit. Rev. Toxicol. 2010, 40, 405–421. [Google Scholar] [CrossRef]
- Yin, J.; Wang, H.; Ding, R. Artemisinin and its derivatives: Progress in toxicology. Chin. J. Pharmacol. Toxicol. 2014, 6, 309–314. [Google Scholar]
- Lee, W.-T.; Lee, T.-H.; Cheng, C.-H.; Chen, K.-C.; Chen, Y.-C.; Lin, C.-W. Antroquinonol from Antrodia Camphorata suppresses breast tumor migration/invasion through inhibiting ERK-AP-1-and AKT-NF-κB-dependent MMP-9 and epithelial-mesenchymal transition expressions. Food Chem. Toxicol. 2015, 78, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Tsai, P.-Y.; Ka, S.-M.; Chao, T.-K.; Chang, J.-M.; Lin, S.-H.; Li, C.-Y.; Kuo, M.-T.; Chen, P.; Chen, A. Antroquinonol reduces oxidative stress by enhancing the Nrf2 signaling pathway and inhibits inflammation and sclerosis in focal segmental glomerulosclerosis mice. Free Radic. Biol. Med. 2011, 50, 1503–1516. [Google Scholar] [CrossRef] [PubMed]
- Geethangili, M.; Tzeng, Y.-M. Review of pharmacological effects of Antrodia camphorata and its bioactive compounds. Evid.-Based Complement. Altern. Med. 2011, 2011, 212641. [Google Scholar] [CrossRef]
- Zhang, B.-B.; Hu, P.-F.; Huang, J.; Hu, Y.-D.; Chen, L.; Xu, G.-R. Current advances on the structure, bioactivity, synthesis, and metabolic regulation of novel ubiquinone derivatives in the edible and medicinal mushroom Antrodia cinnamomea. J. Agric. Food Chem. 2017, 65, 10395–10405. [Google Scholar] [CrossRef]
- Villaume, M.T.; Sella, E.; Saul, G.; Borzilleri, R.M.; Fargnoli, J.; Johnston, K.A.; Zhang, H.; Fereshteh, M.P.; Dhar, T.M.; Baran, P.S. Antroquinonol a: Scalable synthesis and preclinical biology of a phase 2 drug candidate. ACS Cent. Sci. 2016, 2, 27–31. [Google Scholar] [CrossRef]
- Angamuthu, V.; Shanmugavadivu, M.; Nagarajan, G.; Velmurugan, B.K. Pharmacological activities of antroquinonol-Mini review. Chem.-Biol. Interact. 2019, 297, 8–15. [Google Scholar] [CrossRef]
- Kuang, Y.; Li, B.; Wang, Z.; Qiao, X.; Ye, M. Terpenoids from the medicinal mushroom Antrodia camphorata: Chemistry and medicinal potential. Nat. Prod. Rep. 2021, 38, 83–102. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Ju, D.-T.; Chang, C.-F.; Reddy, P.M.; Velmurugan, B.K. A review on the effects of current chemotherapy drugs and natural agents in treating non–small cell lung cancer. Biomedicine 2017, 7, 23. [Google Scholar] [CrossRef]
- Chang, W.-H.; Chen, M.C.; Cheng, I.H. Antroquinonol lowers brain amyloid-β levels and improves spatial learning and memory in a transgenic mouse model of Alzheimer’s disease. Sci. Rep. 2015, 5, 15067. [Google Scholar] [CrossRef]
- Chae, B.S. Effect of Baicalin on the Ex vivo Production of Cytokines in Pristane-Induced Lupus Mice. YAKHAK HOEJI 2016, 60, 21–28. [Google Scholar] [CrossRef]
- Delerue, T.; Barroso, M.F.; Dias-Teixeira, M.; Figueiredo-González, M.; Delerue-Matos, C.; Grosso, C. Interactions between Ginkgo biloba L. and Scutellaria baicalensis Georgi in multicomponent mixtures towards cholinesterase inhibition and ROS scavenging. Food Res. Int. 2021, 140, 109857. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Ma, W.; Xiao, Y.; Wu, B.; Li, X.; Liu, F.; Qiu, J.; Zhang, G. High doses of baicalin induces kidney injury and fibrosis through regulating TGF-β/Smad signaling pathway. Toxicol. Appl. Pharmacol. 2017, 333, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Liu, Y.; Zhang, C. Pharmacokinetics and bioavailability enhancement of baicalin: A review. Eur. J. Drug Metab. Pharmacokinet. 2019, 44, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Stojanović-Radić, Z.; Matejić, J.; Sharifi-Rad, M.; Anil Kumar, N.V.; Martins, N.; Sharifi-Rad, J. The therapeutic potential of curcumin: A review of clinical trials. Eur. J. Med. Chem. 2019, 163, 527–545. [Google Scholar] [CrossRef] [PubMed]
- Constantin, M.M.; Nita, I.E.; Olteanu, R.; Constantin, T.; Bucur, S.; Matei, C.; Raducan, A. Significance and impact of dietary factors on systemic lupus erythematosus pathogenesis. Exp. Ther. Med. 2019, 17, 1085–1090. [Google Scholar] [CrossRef]
- Kumar, A.; Dogra, S.; Prakash, A. Protective effect of curcumin (Curcuma longa), against aluminium toxicity: Possible behavioral and biochemical alterations in rats. Behav. Brain Res. 2009, 205, 384–390. [Google Scholar] [CrossRef]
- Rezaee, R.; Momtazi, A.A.; Monemi, A.; Sahebkar, A. Curcumin: A potentially powerful tool to reverse cisplatin-induced toxicity. Pharmacol. Res. 2017, 117, 218–227. [Google Scholar] [CrossRef]
- Soetikno, V.; Sari, F.R.; Veeraveedu, P.T.; Thandavarayan, R.A.; Harima, M.; Sukumaran, V.; Lakshmanan, A.P.; Suzuki, K.; Kawachi, H.; Watanabe, K. Curcumin ameliorates macrophage infiltration by inhibiting NF-κB activation and proinflammatory cytokines in streptozotocin induced-diabetic nephropathy. Nutr. Metab. 2011, 8, 1–11. [Google Scholar] [CrossRef]
- Shishodia, S.; Sethi, G.; Aggarwal, B.B. Curcumin: Getting back to the roots. Ann. N. Y. Acad. Sci. 2005, 1056, 206–217. [Google Scholar] [CrossRef]
- Shishodia, S.; Singh, T.; Chaturvedi, M.M. Modulation of transcription factors by curcumin. Mol. Targets Ther. Uses Curcumin Health Dis. 2007, 595, 127–148. [Google Scholar]
- Gonzales, A.M.; Orlando, R.A. Curcumin and resveratrol inhibit nuclear factor-kappaB-mediated cytokine expression in adipocytes. Nutr. Metab. 2008, 5, 17. [Google Scholar] [CrossRef] [PubMed]
- JIANG, C.-X. Curcumin analog exhibited anti-inflammatory activity through inhibiting ERK/JNK and NF-κB signaling pathway. Chin. Tradit. Herb. Drugs 2016, 24, 2871–2876. [Google Scholar]
- Han, S.-S.; Keum, Y.-S.; Seo, H.-J.; Surh, Y.-J. Curcumin suppresses activation of NF-κB and AP-1 induced by phorbol ester in cultured human promyelocytic leukemia cells. BMB Rep. 2002, 35, 337–342. [Google Scholar] [CrossRef]
- Kocaadam, B.; Şanlier, N. Curcumin, an active component of turmeric (Curcuma longa), and its effects on health. Crit. Rev. Food Sci. Nutr. 2017, 57, 2889–2895. [Google Scholar] [CrossRef] [PubMed]
- Lao, C.D.; Ruffin, M.T.; Normolle, D.; Heath, D.D.; Murray, S.I.; Bailey, J.M.; Boggs, M.E.; Crowell, J.; Rock, C.L.; Brenner, D.E. Dose escalation of a curcuminoid formulation. BMC Complement. Altern. Med. 2006, 6, 1–4. [Google Scholar] [CrossRef]
- Sharma, R.A.; Euden, S.A.; Platton, S.L.; Cooke, D.N.; Shafayat, A.; Hewitt, H.R.; Marczylo, T.H.; Morgan, B.; Hemingway, D.; Plummer, S.M. Phase I clinical trial of oral curcumin: Biomarkers of systemic activity and compliance. Clin. Cancer Res. 2004, 10, 6847–6854. [Google Scholar] [CrossRef]
- Srinivas, G.; Babykutty, S.; Sathiadevan, P.P.; Srinivas, P. Molecular mechanism of emodin action: Transition from laxative ingredient to an antitumor agent. Med. Res. Rev. 2007, 27, 591–608. [Google Scholar] [CrossRef]
- Zhang, B.; Shi, Y.; Lei, T.C. Detection of active P-glycoprotein in systemic lupus erythematosus patients with poor disease control. Exp. Ther. Med. 2012, 4, 705–710. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Herrera-Bravo, J.; Kamiloglu, S.; Petroni, K.; Mishra, A.P.; Monserrat-Mesquida, M.; Sureda, A.; Martorell, M.; Aidarbekovna, D.S.; Yessimsiitova, Z. Recent advances in the therapeutic potential of emodin for human health. Biomed. Pharmacother. 2022, 154, 113555. [Google Scholar] [CrossRef]
- Akkol, E.K.; Tatlı, I.I.; Karatoprak, G.Ş.; Ağar, O.T.; Yücel, Ç.; Sobarzo-Sánchez, E.; Capasso, R. Is emodin with anticancer effects completely innocent? Two sides of the coin. Cancers 2021, 13, 2733. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Sheng, L.; Chen, Y.; Li, Z.; Wu, H.; Ma, J.; Zhang, D.; Chen, X.; Zhang, S. Total coumarin derivates from Hydrangea paniculata attenuate renal injuries in cationized-BSA induced membranous nephropathy by inhibiting complement activation and interleukin 10-mediated interstitial fibrosis. Phytomedicine 2022, 96, 153886. [Google Scholar] [CrossRef]
- Tubaro, A.; Del Negro, P.; Ragazzi, E.; Zampiron, S.; Della Loggia, R. Anti-inflammatory and peripheral analgesic activity of esculetin in vivo. Pharmacol. Res. Commun. 1988, 20, 83–85. [Google Scholar] [CrossRef] [PubMed]
- Lum, P.T.; Sekar, M.; Gan, S.H.; Jeyabalan, S.; Bonam, S.R.; Rani, N.N.I.M.; Ku-Mahdzir, K.-M.; Seow, L.J.; Wu, Y.S.; Subramaniyan, V. Therapeutic potential of mangiferin against kidney disorders and its mechanism of action: A review. Saudi J. Biol. Sci. 2022, 29, 1530–1542. [Google Scholar] [CrossRef] [PubMed]
- Jangra, A.; Arora, M.K.; Kisku, A.; Sharma, S. The multifaceted role of mangiferin in health and diseases: A review. Adv. Tradit. Med. 2021, 21, 619–643. [Google Scholar] [CrossRef]
- Morozkina, S.N.; Nhung Vu, T.H.; Generalova, Y.E.; Snetkov, P.P.; Uspenskaya, M.V. Mangiferin as new potential anti-cancer agent and mangiferin-integrated polymer systems—A novel research direction. Biomolecules 2021, 11, 79. [Google Scholar] [CrossRef]
- Mei, S.; Ma, H.; Chen, X. Anticancer and anti-inflammatory properties of mangiferin: A review of its molecular mechanisms. Food Chem. Toxicol. 2021, 149, 111997. [Google Scholar] [CrossRef]
- Reddeman, R.A.; Glávits, R.; Endres, J.R.; Clewell, A.E.; Hirka, G.; Vértesi, A.; Béres, E.; Szakonyiné, I.P. A toxicological evaluation of mango leaf extract (Mangifera indica) containing 60% mangiferin. J. Toxicol. 2019, 2019, 4763015. [Google Scholar] [CrossRef] [PubMed]
- Hamidpour, M.; Hamidpour, R.; Hamidpour, S.; Shahlari, M. Chemistry, pharmacology, and medicinal property of sage (Salvia) to prevent and cure illnesses such as obesity, diabetes, depression, dementia, lupus, autism, heart disease, and cancer. J. Tradit. Complement. Med. 2014, 4, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.-Y.; Song, Z.-Y.; Gan, H.-L.; Zheng, M.-H.; Liu, Q.; Meng, X.-T.; Pan, T.; Li, Z.-Y.; Peng, R.-X.; Liu, K. Non-clinical safety evaluation of salvianolic acid A: Acute, 4-week intravenous toxicities and genotoxicity evaluations. BMC Pharmacol. Toxicol. 2022, 23, 83. [Google Scholar] [CrossRef]
- Du, G.; Song, J.; Du, L.; Zhang, L.; Qiang, G.; Wang, S.; Yang, X.; Fang, L. Chemical and pharmacological research on the polyphenol acids isolated from Danshen: A review of salvianolic acids. Adv. Pharmacol. 2020, 87, 1–41. [Google Scholar] [PubMed]
- Wang, Q.; Meng, J.; Dong, A.; Yu, J.-z.; Zhang, G.-X.; Ma, C.-G. The pharmacological effects and mechanism of Tripterygium wilfordii Hook F in central nervous system autoimmunity. J. Altern. Complement. Med. 2016, 22, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.-L.; Yang, Y.-X.; Ding, J.; Li, Y.-C.; Miao, Z.-H. Triptolide: Structural modifications, structure–activity relationships, bioactivities, clinical development and mechanisms. Nat. Prod. Rep. 2012, 29, 457–475. [Google Scholar] [CrossRef] [PubMed]
- Crews, G.; Erickson, L.; Pan, F.; Fisniku, O.; Jang, M.-S.; Wynn, C.; Benediktsson, H.; Kobayashi, M.; Jiang, H. Down-regulation of TGF-β and VCAM-1 is associated with successful treatment of chronic rejection in rats. Transplant. Proc. 2005, 1926–1928. [Google Scholar] [CrossRef]
- Hong, Y.; Zhou, W.; Li, K.; Sacks, S.H. Triptolide is a potent suppressant of C3, CD40 and B7h expression in activated human proximal tubular epithelial cells. Kidney Int. 2002, 62, 1291–1300. [Google Scholar] [CrossRef]
- Zhou, Y.; Hong, Y.; Huang, H. Triptolide attenuates inflammatory response in membranous glomerulo-nephritis rat via downregulation of NF-κB signaling pathway. Kidney Blood Press. Res. 2016, 41, 901–910. [Google Scholar] [CrossRef]
- Li, Y.; Yu, C.; Zhu, W.-M.; Xie, Y.; Qi, X.; Li, N.; Li, J.-S. Triptolide ameliorates IL-10-deficient mice colitis by mechanisms involving suppression of IL-6/STAT3 signaling pathway and down-regulation of IL-17. Mol. Immunol. 2010, 47, 2467–2474. [Google Scholar] [CrossRef]
- Qi, Q.; Li, H.; Lin, Z.-M.; Yang, X.-Q.; Zhu, F.-H.; Liu, Y.-T.; Shao, M.-J.; Zhang, L.-Y.; Xu, Y.-S.; Yan, Y.-X. (5 R)-5-hydroxytriptolide ameliorates anti-glomerular basement membrane glomerulonephritis in NZW mice by regulating Fcγ receptor signaling. Acta Pharmacol. Sin. 2018, 39, 107–116. [Google Scholar] [CrossRef]
- Fan, D.; Guo, Q.; Shen, J.; Zheng, K.; Lu, C.; Zhang, G.; Lu, A.; He, X. The effect of triptolide in rheumatoid arthritis: From basic research towards clinical translation. Int. J. Mol. Sci. 2018, 19, 376. [Google Scholar] [CrossRef]
- Xi, C.; Peng, S.; Wu, Z.; Zhou, Q.; Zhou, J. Toxicity of triptolide and the molecular mechanisms involved. Biomed. Pharmacother. 2017, 90, 531–541. [Google Scholar] [CrossRef]
- Zhao, M.; Liang, G.-P.; Tang, M.-N.; Luo, S.-Y.; Zhang, J.; Cheng, W.-J.; Chan, T.-M.; Lu, Q.-J. Total glucosides of paeony induces regulatory CD4+ CD25+ T cells by increasing Foxp3 demethylation in lupus CD4+ T cells. Clin. Immunol. 2012, 143, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wei, W. Anti-inflammatory and immunoregulatory effects of paeoniflorin and total glucosides of paeony. Pharmacol. Ther. 2020, 207, 107452. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, L.; Cao, Y.; Li, N. Total glucosides of Paeonia lactiflora for safely reducing disease activity in systemic lupus erythematosus: A systematic review and meta-analysis. Front. Pharmacol. 2022, 13, 834947. [Google Scholar] [CrossRef] [PubMed]
- Jun, L.; Junshan, L.; Aiwu, Z.; Minzhu, C.; Shuyun, X. Modulatory effects of total glucosides of paeony on B lymphocyte proliferation and interleukin 1 production. Chin. J. Pharmacol. Toxicol. 1994, 8, 53–55. [Google Scholar]
- Wang, X.W.; Chen, M.Z.; Xu, S.Y. The effects of total glucosides of paeony (TGP) on T lymphocyte subsets. Chin. Pharmacol. Bull. 1992, 8, 340–343. [Google Scholar]
- Wang, X.; Cheng, M.; Xu, S. Effects of total glucosides of paeony on immune system. Zhongguo Bing Li Sheng Li Za Zhi 1991, 7, 609–611. [Google Scholar]
- Wang, X.; Chen, M.; Xu, S. The effects of total glucosides’ of paeony (TGP) on T lymphocyte subsets. Zhongguo Yao Li Xue Tong Bao 1992, 8, 340–343. [Google Scholar]
- Li, M.; Li, Y.; Xiang, L.; Li, L. Efficacy and safety of total glucosides of paeony as an add-on treatment in adolescents and adults with chronic urticaria: A systematic review and meta-analysis. Front. Pharmacol. 2022, 13, 961371. [Google Scholar] [CrossRef]
- Goh, E.; Tan, L.; Chow, S.; Teh, L.; Yeap, S. Use of complementary medicine in systemic lupus erythematosus patients in Malaysia. APLAR J. Rheumatol. 2003, 6, 21–25. [Google Scholar] [CrossRef]
- Hamedi, A.; Sohrabpour, M.; Zarshenas, M.M.; Pasdaran, A. Phytochemical investigation and quantitative analysis of the fatty acids and sterol compounds of seven pharmaceutical valuable seeds. Curr. Pharm. Anal. 2018, 14, 475–482. [Google Scholar] [CrossRef]
- Leiba, A.; Amital, H.; Gershwin, M.E.; Shoenfeld, Y. Diet and lupus. Lupus 2001, 10, 246–248. [Google Scholar] [CrossRef] [PubMed]
- Maki, P.A.; Newberne, P.M. Dietary lipids and immune function. J. Nutr. 1992, 122, 610–614. [Google Scholar] [CrossRef] [PubMed]
- Harbige, L.S. Fatty acids, the immune response, and autoimmunity: A question of n − 6 essentiality and the balance between n − 6 and n − 3. Lipids 2003, 38, 323–341. [Google Scholar] [CrossRef] [PubMed]
- Ramessar, N.; Borad, A.; Schlesinger, N. The effect of Omega-3 fatty acid supplementation in systemic lupus erythematosus patients: A systematic review. Lupus 2022, 31, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Hejr, H.; Ghareghani, M.; Zibara, K.; Ghafari, M.; Sadri, F.; Salehpour, Z.; Hamedi, A.; Negintaji, K.; Azari, H.; Ghanbari, A. The ratio of 1/3 linoleic acid to alpha linolenic acid is optimal for oligodendrogenesis of embryonic neural stem cells. Neurosci. Lett. 2017, 651, 216–225. [Google Scholar] [CrossRef]
- Wei, Y.; Meng, Y.; Li, N.; Wang, Q.; Chen, L. The effects of low-ratio n-6/n-3 PUFA on biomarkers of inflammation: A systematic review and meta-analysis. Food Funct. 2021, 12, 30–40. [Google Scholar] [CrossRef]
- Borges, M.C.; Santos, F.d.M.M.; Telles, R.W.; Correia, M.I.T.D.; Lanna, C.C.D. Polyunsaturated omega-3 fatty acids and systemic lupus erythematosus: What do we know? Rev. Bras. Reumatol. 2014, 54, 459–466. [Google Scholar] [CrossRef]
- MacLean, C.H.; Mojica, W.A.; Morton, S.C.; Pencharz, J.; Garland, R.H.; Tu, W.; Newberry, S.J.; Jungvig, L.K.; Grossman, J.; Khanna, P. Effects of omega-3 fatty acids on lipids and glycemic control in type II diabetes and the metabolic syndrome and on inflammatory bowel disease, rheumatoid arthritis, renal disease, systemic lupus erythematosus, and osteoporosis: Summary. In AHRQ Evidence Report Summaries; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2004. [Google Scholar]
- Pestka, J.J. n-3 polyunsaturated fatty acids and autoimmune-mediated glomerulonephritis. Prostaglandins Leukot. Essent. Fat. Acids (PLEFA) 2010, 82, 251–258. [Google Scholar] [CrossRef]
- Fassett, R.G.; Gobe, G.C.; Peake, J.M.; Coombes, J.S. Omega-3 polyunsaturated fatty acids in the treatment of kidney disease. Am. J. Kidney Dis. 2010, 56, 728–742. [Google Scholar] [CrossRef]
- Tam, L.S.; Li, E.K.; Leung, V.Y.; Griffith, J.F.; Benzie, I.F.; Lim, P.L.; Whitney, B.; Lee, V.W.; Lee, K.K.; Thomas, G.N. Effects of vitamins C and E on oxidative stress markers and endothelial function in patients with systemic lupus erythematosus: A double blind, placebo controlled pilot study. J. Rheumatol. 2005, 32, 275–282. [Google Scholar]
- Kamen, D.L.; Cooper, G.S.; Bouali, H.; Shaftman, S.R.; Hollis, B.W.; Gilkeson, G.S. Vitamin D deficiency in systemic lupus erythematosus. Autoimmun. Rev. 2006, 5, 114–117. [Google Scholar] [CrossRef] [PubMed]
- Solovastru, L.G.; Vâta, D.; Statescu, L.; Constantin, M.M.; Andrese, E. Skin cancer between myth and reality, yet ethically constrained. Rev. Romana Bioet. 2014, 12, 47–52. [Google Scholar]
- Klack, K.; Bonfa, E.; Borba Neto, E.F. Diet and nutritional aspects in systemic lupus erythematosus. Rev. Bras. Reumatol. 2012, 52, 395–408. [Google Scholar] [CrossRef]
- Falcão, S.; Barros, R.; Mateus, M.; Nero, P.; De Matos, A.A.; Pimentão, J.B.; Ribeiro, I.; Weigert, A.; Branco, J. Lúpus eritematoso sistiémico e anemia. Acta Reumatol. Port. 2007, 32, 73–79. [Google Scholar]
- Sahebari, M.; Rezaieyazdi, Z.; Khodashahi, M. Selenium and autoimmune diseases: A review article. Curr. Rheumatol. Rev. 2019, 15, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.C. Lupus erythematosus and nutrition: A review of the literature. J. Ren. Nutr. 2000, 10, 170–183. [Google Scholar] [CrossRef] [PubMed]
- Selmi, C.; Tsuneyama, K. Nutrition, geoepidemiology, and autoimmunity. Autoimmun. Rev. 2010, 9, A267–A270. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-X.; Sun, H.-X. Immunosuppressive effect of ethanol extract of Artemisia annua on specific antibody and cellular responses of mice against ovalbumin. Immunopharmacol. Immunotoxicol. 2009, 31, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X. Effects of Astragalus membranaceus and Tripterygium hypoglancum on natural killer cell activity of peripheral blood mononuclear in systemic lupus erythematosus. Zhongguo Zhong Xi Yi Jie He Za Zhi Zhongguo Zhongxiyi Jiehe Zazhi = Chin. J. Integr. Tradit. West. Med. 1992, 12, 669–671, 645. [Google Scholar]
- Nurdiana; Dantara, T.W.I.; Syaban, M.F.R.; Mustafa, S.A.; Ikhsani, H.; Syafitri, F.E.; Hapsari, N.K.; Khoirunnisa, A. Efficacy and side effects studies of Bryophyllum pinnatum leaves ethanol extract in pristane-induced SLE BALB/c mice model. AIP Conf. Proc. 2019, 2108, 020016. [Google Scholar] [CrossRef]
- Dantara, T.W.I.; Syaban, M.F.R.; Mustafa, S.A.; Ikhsani, H.; Syafitri, F.E.; Hapsari, N.K.; Khoirunnisa, A. Effect of Bryophyllum pinnatum leaves Ethanol extract in TNF-α and TGF-β as candidate therapy of SLE in pristane-induced SLE BALB/c mice model. Res. J. Pharm. Technol. 2021, 14, 1069–1072. [Google Scholar]
- Handono, K.; Dantara, T.W.; Dewi, E.S.; Pratama, M.Z.; Nurdiana, N. Bryophyllum pinnatum leaves ethanol extract inhibit maturation and promote apoptosis of systemic lupus erythematosus BALB/c mice B cells. Med. J. Indones. 2017, 26, 253–260. [Google Scholar] [CrossRef]
- Indriyanti, N.; Garmana, A.N.; Setiawan, F. Repairing effects of aqueous extract of Kalanchoe pinnata (Lmk) Pers. on lupus nephritis mice. Pharmacogn. J. 2018, 10, 548–552. [Google Scholar] [CrossRef]
- Shamekhi, Z.; Amani, R.; Habibagahi, Z.; Namjoyan, F.; Ghadiri, A.; Saki Malehi, A. A Randomized, Double-blind, Placebo-controlled Clinical Trial Examining the Effects of Green Tea Extract on Systemic Lupus Erythematosus Disease Activity and Quality of Life. Phytother. Res. 2017, 31, 1063–1071. [Google Scholar] [CrossRef] [PubMed]
- Khajehdehi, P.; Zanjaninejad, B.; Aflaki, E.; Nazarinia, M.; Azad, F.; Malekmakan, L.; Dehghanzadeh, G.-R. Oral supplementation of turmeric decreases proteinuria, hematuria, and systolic blood pressure in patients suffering from relapsing or refractory lupus nephritis: A randomized and placebo-controlled study. J. Ren. Nutr. 2012, 22, 50–57. [Google Scholar] [CrossRef]
- Cai, Z.; Wong, C.K.; Dong, J.; Jiao, D.; Chu, M.; Leung, P.C.; San Lau, C.B.; Lau, C.P.; Tam, L.S.; Lam, C.W.K. Anti-inflammatory activities of Ganoderma lucidum (Lingzhi) and San-Miao-San supplements in MRL/lpr mice for the treatment of systemic lupus erythematosus. Chin. Med. 2016, 11, 23. [Google Scholar] [CrossRef] [PubMed]
- Lai, N.; Lin, R.; Lai, R.; Kun, U.; Leu, S. Prevention of autoantibody formation and prolonged survival in New Zealand Black/New Zealand White F1 mice with an ancient Chinese herb, Ganoderma tsugae. Lupus 2001, 10, 461–465. [Google Scholar] [CrossRef]
- Yuan, Z.; Feng, J. Observation on the treatment of systemic lupus erythematous with a Gentiana macrophylla complex tablet and a minimal dose of prednisone. Zhong Xi Yi Jie He Za Zhi = Chin. J. Mod. Dev. Tradit. Med. 1989, 9, 156–157, 133. [Google Scholar]
- Sheu, M.-J.; Chiu, C.-C.; Yang, D.-J.; Hsu, T.-C.; Tzang, B.-S. The root extract of Gentiana macrophylla Pall. alleviates B19-NS1-exacerbated liver injuries in NZB/W F1 mice. J. Med. Food 2017, 20, 56–64. [Google Scholar] [CrossRef]
- Clark, W.F.; Kortas, C.; Heidenheim, A.P.; Garland, J.; Spanner, E.; Parbtani, A. Flaxseed in lupus nephritis: A two-year nonplacebo-controlled crossover study. J. Am. Coll. Nutr. 2001, 20, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Clark, W.F.; Parbtani, A.; Huff, M.W.; Spanner, E.; de Salis, H.; Chin-Yee, I.; Philbrick, D.J.; Holub, B.J. Flaxseed: A potential treatment for lupus nephritis. Kidney Int. 1995, 48, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-L.; Chen, Y.-C.; Yang, S.-H.; Ko, Y.-F.; Chen, S.-Y. Immunological alterations in lupus-prone autoimmune (NZB/NZW) F1 mice by mycelia Chinese medicinal fungus Cordyceps sinensis-induced redistributions of peripheral mononuclear T lymphocytes. Clin. Exp. Med. 2009, 9, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-R.; Yen, J.-H.; Lin, C.-C.; Tsai, W.-J.; Liu, W.-J.; Tsai, J.-J.; Lin, S.-F.; Liu, H.-W. The effects of Chinese herbs on improving survival and inhibiting anti-ds DNA antibody production in lupus mice. Am. J. Chin. Med. 1993, 21, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Cao, L.; Wang, X.; Fan, Y. Radix Paeoniae Rubra Ameliorates Lupus Nephritis in Lupus-Like Symptoms of Mrl Mice by Reducing Intercellular Cell Adhesion Molecule-1, Vascular Cell Adhesion Molecule-1, and Platelet Endothelial Cell Adhesion Molecule-1 Expression. Comb. Chem. High Throughput Screen. 2020, 23, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Yine, H.; Dai, S.; Wang, B.; Wei, Q. Effect of moutan cortex extract on Th17 cells of patients with systemic lupus erythematosus. Chin. J. Biochem. Pharm. 2015, 6, 119–121. [Google Scholar]
- Ming, L.; Jingjing, M.; Zhao, X.; Zhu, Y. Clinical study of Radix Astragali, Radix Rehmanniae combined with glucocorticoid in treating systemic lupus erythematosus. Int. J. Tradit. Chin. Med. 2012, 34, 203–206. [Google Scholar]
- Chae, B.-S.; Yang, J.-H. Regulatory effect of fresh rehmanniae radix extract on the in vitro production of proinflammatory cytokines in pristane-induced lupus mice. Nat. Prod. Sci. 2007, 13, 322–327. [Google Scholar]
- Shin, T.-Y.; Oh, C.-H.; Kim, D.-K.; Eun, J.-S.; Jeon, H.; Park, J.-S.; Kim, M.-S.; Yang, J.-H.; Chae, B.-S. Regulatory effect of Scutellariae radix on the proinflammatory cytokine production and abnormal T-cell activation in vitro in pristane-induced lupus mice. Nat. Prod. Sci. 2007, 13, 207–213. [Google Scholar]
- Wang, B.; Yuan, Z. A tablet of Tripterygium wilfordii in treating lupus erythematosus. Zhong Xi Yi Jie He Za Zhi = Chin. J. Mod. Dev. Tradit. Med. 1989, 9, 407–408, 389. [Google Scholar]
- Qin, W.Z.; Zhu, G.D.; Yang, S.M.; Han, K.Y.; Wang, J. Clinical observations on Tripterygium wilfordii in the treatment of 26 cases of discoid lupus erythematosus. J. Trad. Chin. Med. 1983, 3, 131–132. [Google Scholar]
- Treasure, J. Urtica semen reduces serum creatinine levels. J. Am. Herb. Guild 2003, 4, 22–25. [Google Scholar]
- Musette, P.; Galelli, A.; Chabre, H.; Callard, P.; Peumans, W.; Truffa-Bachi, P.; Kourilsky, P.; Gachelin, G. Urtica dioica agglutinin, a Vβ8. 3-specific superantigen, prevents the development of the systemic lupus erythematosus-like pathology of MRL lpr/lpr mice. Eur. J. Immunol. 1996, 26, 1707–1711. [Google Scholar] [CrossRef]
- Tao, X.; Davis, L.S.; Lipsky, P.E. Effect of an extract of the Chinese herbal remedy Tripterygium wilfordii Hook F on human immune responsiveness. Arthritis Rheum. Off. J. Am. Coll. Rheumatol. 1991, 34, 1274–1281. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.; Lipsky, P.E. The Chinese anti-inflammatory and immunosuppressive herbal remedy Tripterygium wilfordii Hook F. Rheum. Dis. Clin. North Am. 2000, 26, 29–50. [Google Scholar] [CrossRef] [PubMed]
- Setty, A.R.; Sigal, L.H. Herbal medications commonly used in the practice of rheumatology: Mechanisms of action, efficacy, and side effects. Semin. Arthritis Rheum. 2005, 773–784. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-Y. Anti-inflammatory and immunosuppressive components of Tripterygium wilfordii Hook F. Int. J. Immunother. 1993, 9, 181–187. [Google Scholar]
- Takaishi, Y.; Shishido, K.; Wariishi, N.; Shibuya, M.; Goto, K.; Kido, M.; Taka, M.; Ono, Y. Triptoquinone A and B novel interleukin-1 inhibitors from Tripterygium wilfordii var Regeli. Tetrahedron Lett. 1992, 33, 7177–7180. [Google Scholar] [CrossRef]
- Juling, G.; Shixiang, Y.; Xichun, W.; Shixi, X.; Deda, L. Tripterygium wilfordii Hook, f. in rheumatoid arthritis and ankylosing spondylitis. Zhonghua Yixue Zazhi 1981, 94, 405–412. [Google Scholar]
- Wong, K.-F.; Yuan, Y.; Luk, J.M. Tripterygium wilfordii bioactive compounds as anticancer and anti-inflammatory agents. Clin. Exp. Pharmacol. Physiol. 2012, 39, 311–320. [Google Scholar] [CrossRef]
- Qin, W.; Wanzhang, Q.; Chenghuang, L.; Shumei, Y.; Guangdou, Z. Tripterygium wilfordii Hook F in systemic lupus erythematosus: Report of 103 cases. Chin. Med. J. 1981, 94, 827–834. [Google Scholar]
- Xu, G.; Tu, W.; Jiang, D.; Xu, C. Tripterygium wilfordii Hook F treatment for idiopathic refractory nephrotic syndrome in adults: A meta-analysis. Nephron Clin. Pract. 2009, 111, c223–c228. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Xie, H.; Li, S.; Jin, B.; Hou, J.; Zhang, H.; Shi, M.; Liu, Z. Treatment of diabetic nephropathy with Tripterygium wilfordii Hook F extract: A prospective, randomized, controlled clinical trial. J. Transl. Med. 2013, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.-M.; Wang, Q.-W.; Chen, J.-S.; Sha, G.-Z.; Liu, Z.-H.; Li, L.-S. Clinical trial of Tripterygium wilfordii Hook F. in human kidney transplantation in China. Transplant. Proc. 2006, 1274–1279. [Google Scholar] [CrossRef] [PubMed]
- Baral, B.; Shrestha, B.; da Silva, J.T. A review of Chinese Cordyceps with special reference to Nepal, focusing on conservation. Environ. Exp. Biol. 2015, 13, 61–73. [Google Scholar]
- Xu, J.; Huang, Y.; Chen, X.X.; Zheng, S.C.; Chen, P.; Mo, M.H. The mechanisms of pharmacological activities of Ophiocordyceps sinensis fungi. Phytother. Res. 2016, 30, 1572–1583. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, Q.-Z.; Li, L.-D.-J.; Zhou, X.-W. Immunostimulatory effects of the intracellular polysaccharides isolated from liquid culture of Ophiocordyceps sinensis (Ascomycetes) on RAW264.7 cells via the MAPK and PI3K/Akt signaling pathways. J. Ethnopharmacol. 2021, 275, 114130. [Google Scholar] [CrossRef] [PubMed]
- Qian, G.-M.; Pan, G.-F.; Guo, J.-Y. Anti-inflammatory and antinociceptive effects of cordymin, a peptide purified from the medicinal mushroom Cordyceps sinensis. Nat. Prod. Res. 2012, 26, 2358–2362. [Google Scholar] [CrossRef] [PubMed]
- Ng, T.B.; Wong, J.H.; Fang, E.F. Recent Research on Pharmacological Activities of the Medicinal Fungus Cordyceps sinensis. In Antitumor Potential and other Emerging Medicinal Properties of Natural Compounds; Fang, E.F., Ng, T.B., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 303–314. [Google Scholar]
- Yang, M.-L.; Kuo, P.-C.; Hwang, T.-L.; Wu, T.-S. Anti-inflammatory principles from Cordyceps sinensis. J. Nat. Prod. 2011, 74, 1996–2000. [Google Scholar] [CrossRef]
- Tong, X.; Guo, J. High Throughput Identification of the Potential Antioxidant Peptides in Ophiocordyceps sinensis. Molecules 2022, 27, 438. [Google Scholar] [CrossRef]
- Xiang, F.; Lin, L.; Hu, M.; Qi, X. Therapeutic efficacy of a polysaccharide isolated from Cordyceps sinensis on hypertensive rats. Int. J. Biol. Macromol. 2016, 82, 308–314. [Google Scholar] [CrossRef]
- Ding, C.; Tian, P.; Xue, W.; Ding, X.; Yan, H.; Pan, X.; Feng, X.; Xiang, H.; Hou, J.; Tian, X. Efficacy of Cordyceps sinensis in long term treatment of renal transplant patients. Front. Biosci.-Elite 2011, 3, 301–307. [Google Scholar]
- Chen, B.; Sun, Y.; Luo, F.; Wang, C. Bioactive metabolites and potential mycotoxins produced by Cordyceps fungi: A review of safety. Toxins 2020, 12, 410. [Google Scholar] [CrossRef] [PubMed]
- Maczynska, I.; Millo, B.; Ratajczak-Stefańska, V.; Maleszka, R.; Szych, Z.; Kurpisz, M.; Giedrys-Kalemba, S. Proinflammatory cytokine (IL-1β, IL-6, IL-12, IL-18 and TNF-α) levels in sera of patients with subacute cutaneous lupus erythematosus (SCLE). Immunol. Lett. 2006, 102, 79–82. [Google Scholar] [CrossRef]
- Umare, V.; Pradhan, V.; Nadkar, M.; Rajadhyaksha, A.; Patwardhan, M.; Ghosh, K.K.; Nadkarni, A.H. Effect of proinflammatory cytokines (IL-6, TNF-α, and IL-1β) on clinical manifestations in Indian SLE patients. Mediat. Inflamm. 2014, 2014, 385297. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.X.; Wong, K.C.; Li, E.K.M.; Tam, S.L.; Leung, C.P.; Yin, B.Y.; Lam, C.W.K. Immunomodulatory effects of lingzhi and san-miao-san supplementation on patients with rheumatoid arthritis. Immunopharmacol. Immunotoxicol. 2006, 28, 197–200. [Google Scholar] [CrossRef]
- Pazzi, F.; Fraile-Fabero, R. Effects of Ganoderma Lucidum on Pain in Women with Fibromyalgia. Fibrom Open Access 2017, 2, 2. [Google Scholar]
- Tang, W.; Gao, Y.; Chen, G.; Gao, H.; Dai, X.; Ye, J.; Chan, E.; Huang, M.; Zhou, S. A randomized, double-blind and placebo-controlled study of a Ganoderma lucidum polysaccharide extract in neurasthenia. J. Med. Food 2005, 8, 53–58. [Google Scholar] [CrossRef]
- Zhong, L.; Yan, P.; Lam, W.C.; Yao, L.; Bian, Z. Coriolus versicolor and Ganoderma lucidum related natural products as an adjunct therapy for cancers: A systematic review and meta-analysis of randomized controlled trials. Front. Pharmacol. 2019, 10, 703. [Google Scholar] [CrossRef]
- Klupp, N.L.; Kiat, H.; Bensoussan, A.; Steiner, G.Z.; Chang, D.H. A double-blind, randomised, placebo-controlled trial of Ganoderma lucidum for the treatment of cardiovascular risk factors of metabolic syndrome. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef]
- Noguchi, M.; Kakuma, T.; Tomiyasu, K.; Yamada, A.; Itoh, K.; Konishi, F.; Kumamoto, S.; Shimizu, K.; Kondo, R.; Matsuoka, K. Randomized clinical trial of an ethanol extract of Ganoderma lucidum in men with lower urinary tract symptoms. Asian J. Androl. 2008, 10, 777–785. [Google Scholar] [CrossRef]
- Henao, S.L.D.; Urrego, S.A.; Cano, A.M.; Higuita, E.A. Randomized Clinical Trial for the Evaluation of Immune Modulation by Yogurt Enriched with β-Glucans from Lingzhi or Reishi Medicinal Mushroom, Ganoderma lucidum (Agaricomycetes), in Children from Medellin, Colombia. Int. J. Med. Mushrooms 2018, 20, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Hamedi, A.; Rezaei, H.; Azarpira, N.; Jafarpour, M.; Ahmadi, F. Effects of Malva sylvestris and its isolated polysaccharide on experimental ulcerative colitis in rats. J. Evid.-Based Complement. Altern. Med. 2016, 21, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Shen, M.; Song, Q.; Xie, J. Biological activities and pharmaceutical applications of polysaccharide from natural resources: A review. Carbohydr. Polym. 2018, 183, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; He, R.; Sun, P.; Zhang, F.; Linhardt, R.J.; Zhang, A. Molecular mechanisms of bioactive polysaccharides from Ganoderma lucidum (Lingzhi), a review. Int. J. Biol. Macromol. 2020, 150, 765–774. [Google Scholar] [CrossRef]
- Ren, L.; Zhang, J.; Zhang, T. Immunomodulatory activities of polysaccharides from Ganoderma on immune effector cells. Food Chem. 2021, 340, 127933. [Google Scholar] [CrossRef] [PubMed]
- Koo, M.H.; Chae, H.-J.; Lee, J.H.; Suh, S.-S.; Youn, U.J. Antiinflammatory lanostane triterpenoids from Ganoderma lucidum. Nat. Prod. Res. 2021, 35, 4295–4302. [Google Scholar] [CrossRef]
- Liang, C.; Tian, D.; Liu, Y.; Li, H.; Zhu, J.; Li, M.; Xin, M.; Xia, J. Review of the molecular mechanisms of Ganoderma lucidum triterpenoids: Ganoderic acids A, C2, D, F, DM, X and Y. Eur. J. Med. Chem. 2019, 174, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Sharma, C.; Bhardwaj, N.; Sharma, A.; Tuli, H.S.; Batra, P.; Beniwal, V.; Gupta, G.K.; Sharma, A.K. Bioactive metabolites of Ganoderma lucidum: Factors, mechanism and broad spectrum therapeutic potential. J. Herb. Med. 2019, 17, 100268. [Google Scholar] [CrossRef]
- Ahmad, M.F. Ganoderma lucidum: Persuasive biologically active constituents and their health endorsement. Biomed. Pharmacother. 2018, 107, 507–519. [Google Scholar] [CrossRef]
- Dewi, S.C.; Sargowo, D.; Widodo, M.A.; Wihastuti, T.A.; Heriansyah, T.; Hartanto, M.A.T.; Pambayun, I.D.A.; Bakhri, S.; A’ini, N.Q.; Sahudi, D.P. Ganoderma lucidum subchronic toxicity on the liver as anti-oxidant and anti-inflamatory agent for cardivascular disease. J. Hypertens. 2015, 33, e30. [Google Scholar] [CrossRef]
- Fattahi, S.; Golpour, M.; Akhavan Niaki, H. Urtica Dioica, An emerauld in the medical Kingdom. Int. Biol. Biomed. J. 2016, 2, 1–10. [Google Scholar]
- Yarnell, E.; Abascal, K. Lupus erythematosus and herbal medicine. Altern. Complement. Ther. 2008, 14, 9–12. [Google Scholar] [CrossRef]
- Francišković, M.; Gonzalez-Pérez, R.; Orčić, D.; Sanchez de Medina, F.; Martínez-Augustin, O.; Svirčev, E.; Simin, N.; Mimica-Dukić, N. Chemical Composition and Immuno-Modulatory Effects of Urtica dioica L. (Stinging Nettle) Extracts. Phytother. Res. 2017, 31, 1183–1191. [Google Scholar] [CrossRef] [PubMed]
- Akbay, P.; Basaran, A.A.; Undeger, U.; Basaran, N. In vitro immunomodulatory activity of flavonoid glycosides from Urtica dioica L. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2003, 17, 34–37. [Google Scholar] [CrossRef] [PubMed]
- Semalty, M.; Adhikari, L.; Semwal, D.; Chauhan, A.; Mishra, A.; Kotiyal, R.; Semalty, A. A comprehensive review on phytochemistry and pharmacological effects of stinging nettle (Urtica dioica). Curr. Tradit. Med. 2017, 3, 156–167. [Google Scholar] [CrossRef]
- Grauso, L.; de Falco, B.; Lanzotti, V.; Motti, R. Stinging nettle, Urtica dioica L.: Botanical, phytochemical and pharmacological overview. Phytochem. Rev. 2020, 19, 1341–1377. [Google Scholar] [CrossRef]
- Taheri, Y.; Quispe, C.; Herrera-Bravo, J.; Sharifi-Rad, J.; Ezzat, S.M.; Merghany, R.M.; Shaheen, S.; Azmi, L.; Prakash Mishra, A.; Sener, B. Urtica dioica-derived phytochemicals for pharmacological and therapeutic applications. Evid.-Based Complement. Altern. Med. 2022, 2022, 4024331. [Google Scholar] [CrossRef]
- Showkat, Q.A.; Rather, J.A.; Jabeen, A.; Dar, B.; Makroo, H.A.; Majid, D. Bioactive components, physicochemical and starch characteristics of different parts of lotus (Nelumbo nucifera Gaertn.) plant: A review. Int. J. Food Sci. Technol. 2021, 56, 2205–2214. [Google Scholar] [CrossRef]
- Limwachiranon, J.; Huang, H.; Shi, Z.; Li, L.; Luo, Z. Lotus flavonoids and phenolic acids: Health promotion and safe consumption dosages. Compr. Rev. Food Sci. Food Saf. 2018, 17, 458–471. [Google Scholar] [CrossRef]
- Bishayee, A.; Patel, P.A.; Sharma, P.; Thoutireddy, S.; Das, N. Lotus (Nelumbo nucifera Gaertn.) and its bioactive phytocompounds: A tribute to cancer prevention and intervention. Cancers 2022, 14, 529. [Google Scholar] [CrossRef]
- Ekiert, H.; Świątkowska, J.; Klin, P.; Rzepiela, A.; Szopa, A. Artemisia annua–Importance in Traditional Medicine and Current State of Knowledge on the Chemistry, Biological Activity and Possible Applications. Planta Med. 2021, 87, 584–599. [Google Scholar] [CrossRef] [PubMed]
- Rolta, R.; Salaria, D.; Sharma, P.; Sharma, B.; Kumar, V.; Rathi, B.; Verma, M.; Sourirajan, A.; Baumler, D.J.; Dev, K. Phytocompounds of Rheum emodi, Thymus serpyllum, and Artemisia annua Inhibit Spike Protein of SARS-CoV-2 Binding to ACE2 Receptor: In Silico Approach. Curr. Pharmacol. Rep. 2021, 7, 135–149. [Google Scholar] [CrossRef]
- Law, S.; Leung, A.W.; Xu, C. Is the traditional Chinese herb “Artemisia annua” possible to fight against COVID-19? Integr. Med. Res. 2020, 9, 100474. [Google Scholar] [CrossRef]
- Fuzimoto, A.D. An overview of the anti-SARS-CoV-2 properties of Artemisia annua, its antiviral action, protein-associated mechanisms, and repurposing for COVID-19 treatment. J. Integr. Med. 2021, 19, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Yang, L. 杨岚; Zhang, D. 张东. 双氢青蒿素及其红斑狼疮新适应症研究概述. 科学通报 2017, 62, 2007–2012. [Google Scholar]
- Willcox, M.; Bodeker, G.; Rasoanaivo, P.; Addae-Kyereme, J. Traditional Medicinal Plants and Malaria; CRC Press: Boca Raton, FL, USA, 2004; Volume 4. [Google Scholar]
- Ashok, P.K.; Upadhyaya, K. Preliminary Phytochemical Screening and Physico-Chemical Parameters of Artemisia absinthium and Artemisia annua. J. Pharmacogn. Phytochem. 2013, 1, 229–235. [Google Scholar]
- Zhuang, G. Clinical study on the treatment of lupus erythematosus with Artemisia apiacea Hce. Zhonghua Yi Xue Za Zhi 1982, 62, 365–367. [Google Scholar]
- Guokang, Z. Discoid Lupus Erythematosus Treated by Artemisia Annua. Altern. Complement. Ther. 2008, 14, 9–12. [Google Scholar]
- Jun, D.Y.; Dong, L.W.; You, T.Y.; Na, Z.H.; Zhong, Z.W.; Lan, Y.; Bin, L.Z. The effects of DQHS on the pathologic changes in BXSB mice lupus nephritis and the effect mechanism. Chin. Pharmacol. Bull. 2003, 19, 1125–1128. [Google Scholar]
- Dong, Y.; Li, W.; Tu, Y. Effect of dihydro-qinghaosu on auto-antibody production, TNF alpha secretion and pathologic change of lupus nephritis in BXSB mice. Zhongguo Zhong Xi Yi Jie He Za Zhi Zhongguo Zhongxiyi Jiehe Zazhi = Chin. J. Integr. Tradit. West. Med. 2003, 23, 676–679. [Google Scholar]
- Yang, J.; Wen, C.; Duan, Y.; Deng, Q.; Peng, D.; Zhang, H.; Ma, H. The composition, extraction, analysis, bioactivities, bioavailability and applications in food system of flaxseed (Linum usitatissimum L.) oil: A review. Trends Food Sci. Technol. 2021, 118, 252–260. [Google Scholar] [CrossRef]
- Akter, Y.; Junaid, M.; Afrose, S.S.; Nahrin, A.; Alam, M.S.; Sharmin, T.; Akter, R.; Hosen, S. A Comprehensive Review on Linum usitatissimum Medicinal Plant: Its Phytochemistry, Pharmacology, and Ethnomedicinal Uses. Mini Rev. Med. Chem. 2021, 21, 2801–2834. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Li, X.; Ma, X.; Li, A.; Wang, Y.; Reaney, M.J.; Shim, Y.Y. A flaxseed heteropolysaccharide stimulates immune responses and inhibits hepatitis B virus. Int. J. Biol. Macromol. 2019, 136, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, I.; Shalaby, A.; Abdallah, H.; El-Zohairy, N.; Bahr, H. Immunomodulatory and anti-angiogenic properties of Linum usitatissium (flaxseed) seeds ethanolic extract in atherogenic diet treated rats. Adv. Anim. Vet. Sci 2020, 8, 18–25. [Google Scholar] [CrossRef]
- Touré, A.; Xu, X. Flaxseed lignans: Source, biosynthesis, metabolism, antioxidant activity, bio-active components, and health benefits. Compr. Rev. Food Sci. Food Saf. 2010, 9, 261–269. [Google Scholar] [CrossRef]
- Yuan, B.; Han, J.; Cheng, Y.; Cheng, S.; Huang, D.; Mcclements, D.J.; Cao, C. Identification and characterization of antioxidant and immune-stimulatory polysaccharides in flaxseed hull. Food Chem. 2020, 315, 126266. [Google Scholar]
- Bashir, S.; Ali, S.; Khan, F. Partial reversal of obesity-induced insulin resistance owing to anti-inflammatory immunomodulatory potential of flaxseed oil. Immunol. Investig. 2015, 44, 451–469. [Google Scholar] [CrossRef]
- Chytilová, M.; Mudroňová, D.; Nemcová, R.; Gancarčíková, S.; Buleca, V.; Koščová, J.; Tkáčiková, Ľ. Anti-inflammatory and immunoregulatory effects of flax-seed oil and Lactobacillus plantarum—Biocenol™ LP96 in gnotobiotic pigs challenged with enterotoxigenic Escherichia coli. Res. Vet. Sci. 2013, 95, 103–109. [Google Scholar] [CrossRef]
- Herchi, W.; Harrabi, S.; Sebei, K.; Rochut, S.; Boukhchina, S.; Pepe, C.; Kallel, H. Phytosterols accumulation in the seeds of Linum usitatissimum L. Plant Physiol. Biochem. 2009, 47, 880–885. [Google Scholar] [CrossRef]
- Vilahur, G.; Ben-Aicha, S.; Diaz-Riera, E.; Badimon, L.; Padró, T. Phytosterols and inflammation. Curr. Med. Chem. 2019, 26, 6724–6734. [Google Scholar] [CrossRef]
- Hamedi, A.; Ghanbari, A.; Razavipour, R.; Saeidi, V.; Zarshenas, M.M.; Sohrabpour, M.; Azari, H. Alyssum homolocarpum seeds: Phytochemical analysis and effects of the seed oil on neural stem cell proliferation and differentiation. J. Nat. Med. 2015, 69, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Ogborn, M.R.; Nitschmann, E.; Bankovic-Calic, N.; Weiler, H.A.; Aukema, H. Dietary flax oil reduces renal injury, oxidized LDL content, and tissue n− 6/n− 3 FA ratio in experimental polycystic kidney disease. Lipids 2002, 37, 1059–1065. [Google Scholar] [CrossRef] [PubMed]
- Naqshbandi, A.; Rizwan, S.; Khan, F. Dietary supplementation of flaxseed oil ameliorates the effect of cisplatin on rat kidney. J. Funct. Foods 2013, 5, 316–326. [Google Scholar] [CrossRef]
- Al Za’abi, M.; Ali, H.; Ali, B.H. Effect of flaxseed on systemic inflammation and oxidative stress in diabetic rats with or without chronic kidney disease. PLoS ONE 2021, 16, e0258800. [Google Scholar] [CrossRef]
- Ogborn, M.R. Flaxseed and flaxseed products in kidney disease. In Flaxseed in Human Nutrition; AOCS Press: Champaign, IL, USA, 2003; pp. 301–318. [Google Scholar]
- Parikh, M.; Maddaford, T.G.; Austria, J.A.; Aliani, M.; Netticadan, T.; Pierce, G.N. Dietary flaxseed as a strategy for improving human health. Nutrients 2019, 11, 1171. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Yook, T.-H.; Kim, J.-U. Rehmanniae radix, an effective treatment for patients with various inflammatory and metabolic diseases: Results from a review of Korean publications. J. Pharmacopunct. 2017, 20, 81. [Google Scholar]
- Li, M.; Ma, J.-J.; Zhao, X.-L.; Zhu, Y. Treating lupus nephritis by a drug pair of radix astragali and rehmanniae radix combined with glucocorticoid: A preliminary clinical study. Zhongguo Zhong Xi Yi Jie He Za Zhi Zhongguo Zhongxiyi Jiehe Zazhi = Chin. J. Integr. Tradit. West. Med. 2014, 34, 956–959. [Google Scholar]
- Li, M.; Jiang, H.; Hao, Y.; Du, K.; Du, H.; Ma, C.; Tu, H.; He, Y. A systematic review on botany, processing, application, phytochemistry and pharmacological action of Radix Rehmnniae. J. Ethnopharmacol. 2022, 285, 114820. [Google Scholar] [CrossRef]
- Lu, M.-K.; Chang, C.-C.; Chao, C.-H.; Hsu, Y.-C. Structural changes, and anti-inflammatory, anti-cancer potential of polysaccharides from multiple processing of Rehmannia glutinosa. Int. J. Biol. Macromol. 2022, 206, 621–632. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, S.; Feng, W.; Zhang, Z.; Li, H. Structural characterization and immunomodulatory activities of two polysaccharides from Rehmanniae Radix Praeparata. Int. J. Biol. Macromol. 2021, 186, 385–395. [Google Scholar] [CrossRef]
- Choi, Y.H. Catalpol attenuates lipopolysaccharide-induced inflammatory responses in BV2 microglia through inhibiting the TLR4-mediated NF-κB pathway. Gen. Physiol. Biophys. 2019, 38, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Chi, X.; Wang, S.; Baloch, Z.; Zhang, H.; Li, X.; Zhang, Z.; Zhang, H.; Dong, Z.; Lu, Y.; Yu, H. Research progress on classical traditional Chinese medicine formula Lily Bulb and Rehmannia Decoction in the treatment of depression. Biomed. Pharmacother. 2019, 112, 108616. [Google Scholar] [CrossRef] [PubMed]
- Song, W.-H.; Cheng, Z.-H.; Chen, D.-F. Anticomplement monoterpenoid glucosides from the root bark of Paeonia suffruticosa. J. Nat. Prod. 2014, 77, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-J.; Li, Y.-X.; Li, S.; He, W.; Wang, Z.-R.; Zhan, T.-P.; Lv, C.-Y.; Liu, Y.-P.; Yang, Y.; Zeng, X.-X. Progress in traditional Chinese medicine and natural extracts for the treatment of lupus nephritis. Biomed. Pharmacother. 2022, 149, 112799. [Google Scholar] [CrossRef] [PubMed]
- Ekiert, H.; Klimek-Szczykutowicz, M.; Szopa, A. Paeonia × suffruticosa (Moutan Peony)—A review of the chemical composition, traditional and professional use in medicine, position in cosmetics industries, and biotechnological studies. Plants 2022, 11, 3379. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.-Q.; Chen, H.-W.; Li, J.; Wu, Q.-J. Efficacy, chemical constituents, and pharmacological actions of Radix Paeoniae rubra and Radix Paeoniae alba. Front. Pharmacol. 2020, 11, 1054. [Google Scholar] [CrossRef]
- Parker, S.; May, B.; Zhang, C.; Zhang, A.L.; Lu, C.; Xue, C.C. A pharmacological review of bioactive constituents of Paeonia lactiflora Pallas and Paeonia veitchii Lynch. Phytother. Res. 2016, 30, 1445–1473. [Google Scholar] [CrossRef]
- Pasdaran, A.; Butovska, D.; Kerr, P.; Naychov, Z.; Aneva, I.; Kozuharova, E. Gentians, natural remedies for future of visceral pain control; an ethnopharmacological review with an in silico approach. Biol. Futur. 2022, 73, 219–227. [Google Scholar] [CrossRef]
- Cui, Y.; Jiang, L.; Shao, Y.; Mei, L.; Tao, Y. Anti-alcohol liver disease effect of Gentianae macrophyllae extract through MAPK/JNK/p38 pathway. J. Pharm. Pharmacol. 2019, 71, 240–250. [Google Scholar] [CrossRef]
- Lohar, A.V.; Wankhade, A.M.; Faisal, M.; Jagtap, A. A review on Glycyrrhiza glabra linn (liquorice)—An excellent medicinal plant. Eur. J. Biomed. 2020, 7, 330–334. [Google Scholar]
- Pandey, S.; Verma, B.; Arya, P. A review on constituents, pharmacological activities and medicinal uses of Glycyrrhiza glabra. Pharm. Res. 2017, 2, 26–31. [Google Scholar] [CrossRef]
- Damle, M. Glycyrrhiza glabra (Liquorice)—A potent medicinal herb. Int. J. Herb. Med. 2014, 2, 132–136. [Google Scholar]
- Kwon, Y.-J.; Son, D.-H.; Chung, T.-H.; Lee, Y.-J. A review of the pharmacological efficacy and safety of licorice root from corroborative clinical trial findings. J. Med. Food 2020, 23, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Yuan, B.-C.; Ma, Y.-S.; Zhou, S.; Liu, Y. The anti-inflammatory activity of licorice, a widely used Chinese herb. Pharm. Biol. 2017, 55, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Mollica, L.; De Marchis, F.; Spitaleri, A.; Dallacosta, C.; Pennacchini, D.; Zamai, M.; Agresti, A.; Trisciuoglio, L.; Musco, G.; Bianchi, M.E. Glycyrrhizin binds to high-mobility group box 1 protein and inhibits its cytokine activities. Chem. Biol. 2007, 14, 431–441. [Google Scholar] [CrossRef]
- Li, X.; Yue, Y.; Zhu, Y.; Xiong, S. Extracellular, but not intracellular HMGB1, facilitates self-DNA induced macrophage activation via promoting DNA accumulation in endosomes and contributes to the pathogenesis of lupus nephritis. Mol. Immunol. 2015, 65, 177–188. [Google Scholar] [CrossRef]
- Nazari, S.; Rameshrad, M.; Hosseinzadeh, H. Toxicological effects of Glycyrrhiza glabra (licorice): A review. Phytother. Res. 2017, 31, 1635–1650. [Google Scholar] [CrossRef] [PubMed]
- Wahab, S.; Ahmad, I.; Irfan, S.; Siddiqua, A.; Usmani, S.; Ahmad, M.P. Pharmacological Efficacy and Safety of Glycyrrhiza glabra in the treatment of respiratory tract infections. Mini Rev. Med. Chem. 2022, 22, 1476–1494. [Google Scholar] [CrossRef]
- Liu, T.; Qiao, X.; Kong, K.; Wang, X.; Li, R.; Zhang, X. Bioinformatics-based Identification of the Mechanism Whereby Astragalus Membranaceus Inhibits Inflammation and Autophagy in Lupus Nephritis. preprint 2021. [Google Scholar] [CrossRef]
- Sheik, A.; Kim, K.; Varaprasad, G.L.; Lee, H.; Kim, S.; Kim, E.; Shin, J.-Y.; Oh, S.Y.; Huh, Y.S. The anti-cancerous activity of adaptogenic herb Astragalus membranaceus. Phytomedicine 2021, 91, 153698. [Google Scholar] [CrossRef]
- Kamboj, A.; Saluja, A. Bryophyllum pinnatum (Lam.) Kurz.: Phytochemical and pharmacological profile: A review. Pharmacogn. Rev. 2009, 3, 364. [Google Scholar]
- Handono, K.; Dantara, T.; Dewi, E.; Pratama, M.; Nurdiana, N. 94 Development of therapy using bryophyllum pinnatum to decrease maturation and increase apoptotic b cells from balb/c lupus mice: In silico and in vitro study approach. Lupus Sci. Med. 2017, 4. [Google Scholar] [CrossRef]
- Kalsum, U.; Nurdiana, N.; Pratama, M.; Kalim, H.; Handono, K. 91 Potential novel natural b cell depleting and immunosuppression agent in lupus treapment using bryophyllum pinnatum. in silico and in pristane induced lupus mice. Lupus Sci. Med. 2017, 4. [Google Scholar] [CrossRef]
- Indriyanti, N.; Soeroso, J.; Khotib, J. T-cell activation controlling effects of ethyl acetate fraction of Kalanchoe pinnata (lmk) pers on tmpd-treated lupus mice. Int. J. Pharm. Sci. Res. 2018, 9, 475–482. [Google Scholar]
- Indriyanti, N.; Soeroso, J.; Khotib, J. The benefits of active compounds in Kalanchoe pinnata (LMK) pers ethyl acetate fraction on lupus arthritis mice. Asian J. Pharm. Clin. Res. 2017, 10, 1–5. [Google Scholar] [CrossRef]
- Indriyanti, N.; Soeroso, J.; Khotib, J. Positive Impact of Ethyl Acetate Fraction of Kalanchoe pinnata on Anti-Smith Antibody and T Reg in Lupus Mice. J. Ilmu Kefarmasian Indones. 2017, 15, 57–62. [Google Scholar]
- Indriyanti, N.; Garmana, A.N. Ekstrak Daun Cocor Bebek (Kalanchoe pinnata) Untuk Terapi Preventif Lupus pada Mencit yang Diinduksi dengan 2, 6, 10, 14 Tetramethylpentadecane. J. Trop. Pharm. Chem. 2011, 1, 221–226. [Google Scholar] [CrossRef]
- Fernandes, J.M.; Cunha, L.M.; Azevedo, E.P.; Lourenço, E.M.; Fernandes-Pedrosa, M.F.; Zucolotto, S.M. Kalanchoe laciniata and Bryophyllum pinnatum: An updated review about ethnopharmacology, phytochemistry, pharmacology and toxicology. Rev. Bras. Farmacogn. 2019, 29, 529–558. [Google Scholar] [CrossRef]
- Liu, T.; An, X.-N.; Liu, D.-L.; Wei, Y.-J. A comparison of several second-order algorithms for simultaneous determination of neomangiferin and mangiferin with severe spectral overlapping in Anemarrhenae Rhizoma. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 208, 172–178. [Google Scholar] [CrossRef]
- Singh, K.G.; Sonia, S.; Konsoor, N. In-vitro and ex-vivo studies on the antioxidant, anti-inflammatory and antiarthritic properties of Camellia sinensis, Hibiscus rosa sinensis, Matricaria chamomilla, Rosa SP., Zingiber officinale tea extracts. Inflammation 2018, 49, 50. [Google Scholar]
- Mawarti, H.; Nugraha, J.; Purwanto, D.A.; Soeroso, J. Identifying and Revealing Active Compound from Green Tea (Camellia sinensis) for Curing Systemic Lupus Erythematosus by Acting as CASPASE 1 Inhibitor. Med. -Leg. Update 2020, 20, 323–329. [Google Scholar]
- Bedrood, Z.; Rameshrad, M.; Hosseinzadeh, H. Toxicological effects of Camellia sinensis (green tea): A review. Phytother. Res. 2018, 32, 1163–1180. [Google Scholar] [CrossRef] [PubMed]
- Labban, L. Medicinal and pharmacological properties of Turmeric (Curcuma longa): A review. Int. J. Pharm. Biomed. Sci. 2014, 5, 17–23. [Google Scholar]
- Gurung, P.; De, P. Spectrum of biological properties of Cinchona alkaloids: A brief review. J. Pharmacogn. Phytochem. 2017, 6, 162–166. [Google Scholar]
- Kinsley-Scott, T.R.; Norton, S.A. Useful plants of dermatology. VII: Cinchona and antimalarials. J. Am. Acad. Dermatol. 2003, 49, 499–502. [Google Scholar] [CrossRef]
- Weng, J.-K. Plant solutions for the COVID-19 pandemic and beyond: Historical reflections and future perspectives. Mol. Plant 2020, 13, 803–807. [Google Scholar] [CrossRef]
- Shah, R.R. Chloroquine and hydroxychloroquine for COVID-19: Perspectives on their failure in repurposing. J. Clin. Pharm. Ther. 2021, 46, 17–27. [Google Scholar] [CrossRef]
- Wu, G.-C.; Wu, H.; Fan, L.-Y.; Pan, H.-F. Saikosaponins: A potential treatment option for systemic lupus erythematosus. Ir. J. Med. Sci. 2011, 180, 259–261. [Google Scholar] [CrossRef]
- Brinkhaus, B.; Lindner, M.; Schuppan, D.; Hahn, E. Chemical, pharmacological and clinical profile of the East Asian medical plant Centella aslatica. Phytomedicine 2000, 7, 427–448. [Google Scholar] [CrossRef]
- Kundu, P.; Sharma, P.; Mahato, R.; Saha, M.; Das, S.; Ghosh, P. A brief review for the development of bio-nanoparticles using some important Indian ethnomedicinal plants. J. Med. Plants 2020, 8, 26–33. [Google Scholar] [CrossRef]
- Islam, M. A Review on Tinospora cordifolia and its Immunomodulatory Activities. Ph.D. Thesis, Brac University, Dhaka, Bangladesh, 2020. [Google Scholar]
- Abrantes, V.E.F.; Matias da Rocha, B.A.; Batista da Nóbrega, R.; Silva-Filho, J.C.; Teixeira, C.S.; Cavada, B.S.; de Almeida Gadelha, C.A.; Ferreira, S.H.; Figueiredo, J.G.; Santi-Gadelha, T. Molecular modeling of Lectin-like protein from Acacia farnesiana reveals a possible anti-inflammatory mechanism in carrageenan-induced inflammation. BioMed Res. Int. 2013, 2013, 253483. [Google Scholar] [CrossRef]
- Delgadillo Puga, C.; Cuchillo Hilario, M.; Navarro Ocaña, A.; Medina Campos, O.N.; Nieto Camacho, A.; Ramírez Apan, T.; López Tecpoyotl, Z.G.; Díaz Martínez, M.; Álvarez Izazaga, M.A.; Cruz Martínez, Y.R. Phenolic Compounds in Organic and Aqueous Extracts from Acacia farnesiana Pods Analyzed by ULPS-ESI-Q-oa/TOF-MS. In Vitro Antioxidant Activity and Anti-Inflammatory Response in CD-1 Mice. Molecules 2018, 23, 2386. [Google Scholar]
- Leal, L.S.S.; Silva, R.O.; Araújo, T.d.S.L.; Silva, V.G.d.; Barbosa, A.L.d.R.; Medeiros, J.V.R.; Oliveira, J.S.; Ventura, C.A. The anti-inflammatory and antinociceptive effects of proteins extracted from Acacia farnesiana seeds. Rev. Bras. Plantas Med. 2016, 18, 38–47. [Google Scholar] [CrossRef]
- Almeida, É.S.; de Oliveira, D.; Hotza, D. Properties and applications of Morinda citrifolia (noni): A review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 883–909. [Google Scholar] [CrossRef] [PubMed]
- Dixon, A.R.; McMillen, H.; Etkin, N.L. Ferment this: The transformation of Noni, a traditional Polynesian medicine (Morinda citrifolia, Rubiaceae). Econ. Bot. 1999, 53, 51–68. [Google Scholar] [CrossRef]
- McClatchey, W. From Polynesian healers to health food stores: Changing perspectives of Morinda citrifolia (Rubiaceae). Integr. Cancer Ther. 2002, 1, 110–120. [Google Scholar] [CrossRef]
- Dong, Y.; Feng, Z.-L.; Chen, H.-B.; Wang, F.-S.; Lu, J.-H. Corni Fructus: A review of chemical constituents and pharmacological activities. Chin. Med. 2018, 13, 1–20. [Google Scholar] [CrossRef]
- Sung, Y.-H.; Chang, H.-K.; Kim, S.-E.; Kim, Y.-M.; Seo, J.-H.; Shin, M.-C.; Shin, M.-S.; Yi, J.-W.; Shin, D.-H.; Kim, H. Anti-inflammatory and analgesic effects of the aqueous extract of corni fructus in murine RAW 264.7 macrophage cells. J. Med. Food 2009, 12, 788–795. [Google Scholar] [CrossRef]
- Park, C.H.; Tanaka, T.; Yokozawa, T. Anti-diabetic action of 7-O-galloyl-D-sedoheptulose, a polyphenol from Corni Fructus, through ameliorating inflammation and inflammation-related oxidative stress in the pancreas of type 2 diabetics. Biol. Pharm. Bull. 2013, 36, 723–732. [Google Scholar] [CrossRef]
- Tao, J.-H.; Zhao, M.; Jiang, S.; Pu, X.-L.; Wei, X.-Y. Comparative metabolism of two major compounds in Fructus Corni extracts by gut microflora from normal and chronic nephropathy rats in vitro by UPLC-Q-TOF/MS. J. Chromatogr. B 2018, 1073, 170–176. [Google Scholar] [CrossRef]
- Londhe, V.; Gavasane, A.; Nipate, S.; Bandawane, D.; Chaudhari, P. Role of garlic (Allium sativum) in various diseases: An overview. Angiogenesis 2011, 12, 13. [Google Scholar]
- Abdou, I.; Abou-Zeid, A.; El-Sherbeeny, M.; Abou-El-Gheat, Z. Antimicrobial activities of Allium sativum, Allium cepa, Raphanus sativus, Capsicum frutescens, Eruca sativa, Allium kurrat on bacteria. Qual. Plant. Mater. Veg. 1972, 22, 29–35. [Google Scholar] [CrossRef]
- Zou, Y.-T.; Zhou, J.; Wu, C.-Y.; Zhang, W.; Shen, H.; Xu, J.-D.; Zhang, Y.-Q.; Long, F.; Li, S.-L. Protective effects of Poria cocos and its components against cisplatin-induced intestinal injury. J. Ethnopharmacol. 2021, 269, 113722. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; He, Y.; Zeng, P.; Liu, Y.; Zhang, M.; Hao, C.; Wang, H.; Lv, Z.; Zhang, L. Molecular basis for Poria cocos mushroom polysaccharide used as an antitumour drug in China. J. Cell. Mol. Med. 2019, 23, 4–20. [Google Scholar] [CrossRef]
- Bae, M.-J.; See, H.-J.; Choi, G.; Kang, C.-Y.; Shon, D.-H.; Shin, H.S. Regulatory T cell induced by Poria cocos bark exert therapeutic effects in murine models of atopic dermatitis and food allergy. Mediat. Inflamm. 2016, 2016, 3472608. [Google Scholar] [CrossRef]
- Knorr, R.; Hamburger, M. Quantitative analysis of anti-inflammatory and radical scavenging triterpenoid esters in evening primrose oil. J. Agric. Food Chem. 2004, 52, 3319–3324. [Google Scholar] [CrossRef]
- Ismail, M.F.; El-Maraghy, S.A.; Sadik, N.A. Study of the immunomodulatory and anti-inflammatory effects of evening primrose oil in adjuvant arthritis. Afr. J. Biochem. Res. 2008, 2, 074–080. [Google Scholar]
- Belch, J.J.; Hill, A. Evening primrose oil and borage oil in rheumatologic conditions. Am. J. Clin. Nutr. 2000, 71, 352s–356s. [Google Scholar] [CrossRef]
- Chao, W.-W.; Lin, B.-F. Isolation and identification of bioactive compounds in Andrographis paniculata (Chuanxinlian). Chin. Med. 2010, 5, 17. [Google Scholar] [CrossRef]
- Bukoye, O.; Musbau, A. Immune modulation potentials of aqueous extract of Andrographis paniculata leaves in male rat. Researcher 2011, 3, 48–57. [Google Scholar]
- Jantan, I.; Haque, M.; Ilangkovan, M.; Arshad, L. An insight into the modulatory effects and mechanisms of action of phyllanthus species and their bioactive metabolites on the immune system. Front. Pharmacol. 2019, 10, 878. [Google Scholar] [CrossRef] [PubMed]
- Ishida, M.; Nishi, K.; Kunihiro, N.; Onda, H.; Nishimoto, S.; Sugahara, T. Immunostimulatory effect of aqueous extract of Coriandrum sativum L. seed on macrophages. J. Sci. Food Agric. 2017, 97, 4727–4736. [Google Scholar] [CrossRef]
- Efferth, T.; Oesch, F. Anti-inflammatory and anti-cancer activities of frankincense: Targets, treatments and toxicities. Semin. Cancer Biol. 2022, 80, 39–57. [Google Scholar] [CrossRef] [PubMed]
- Maksimović, Z. On Frankincense. Arch. Pharm. 2021, 71, 1–21. [Google Scholar]
- WERZ, O. Gender Medicine and Frankincense: Novel Findings in Inflammation Research. Sci. Pharm. 2009, 77, 163. [Google Scholar] [CrossRef]
- Soni, A.; Bohra, N. Boswellia serrata-Propogation and uses—A Review. Int. J. Adv. Res. Biol. Sci. 2021, 8, 35–46. [Google Scholar]
- Stürner, K.H.; Stellmann, J.-P.; Dörr, J.; Paul, F.; Friede, T.; Schammler, S.; Reinhardt, S.; Gellissen, S.; Weissflog, G.; Faizy, T.D. A standardised frankincense extract reduces disease activity in relapsing-remitting multiple sclerosis (the SABA phase IIa trial). J. Neurol. Neurosurg. Psychiatry 2018, 89, 330–338. [Google Scholar] [CrossRef]
- Lim, J.S.; Hahn, D.; Gu, M.J.; Oh, J.; Lee, J.S.; Kim, J.-S. Anti-inflammatory and antioxidant effects of 2, 7-dihydroxy-4, 6-dimethoxy phenanthrene isolated from Dioscorea batatas Decne. Appl. Biol. Chem. 2019, 62, 1–9. [Google Scholar]
- Chen, Y.-F.; Zhu, Q.; Wu, S. Preparation of oligosaccharides from Chinese yam and their antioxidant activity. Food Chem. 2015, 173, 1107–1110. [Google Scholar] [CrossRef]
- Jiao, B.; Gao, J. Intensive research on the prospective use of complementary and alternative medicine to treat systemic lupus erythematosus. Drug Discov. Ther. 2013, 7, 167–171. [Google Scholar] [CrossRef]
- Alabi, Q.K.; Akomolafe, R.O.; Akomolafe, J.G.; Aturamu, A.; Ige, M.S.; Kayode, O.O.; Kajewole, D.I. Polyphenol-rich extract of Ocimum gratissimum leaves Prevented Toxic Effects of Cyclophosphamide on the kidney Function of Wistar rats. BMC Complement. Med. Ther. 2021, 21, 274. [Google Scholar] [CrossRef] [PubMed]
- Mahapatra, S.K.; Chakraborty, S.P.; Roy, S. Immunomodulatory role of Ocimum gratissimum and ascorbic acid against nicotine-induced murine peritoneal macrophages in vitro. Oxidative Med. Cell. Longev. 2011, 2011, 734319. [Google Scholar] [CrossRef] [PubMed]
- Batiha, G.E.-S.; Magdy Beshbishy, A.; Wasef, L.; Elewa, Y.H.; El-Hack, A.; Mohamed, E.; Taha, A.E.; Al-Sagheer, A.A.; Devkota, H.P.; Tufarelli, V. Uncaria tomentosa (Willd. ex Schult.) DC.: A review on chemical constituents and biological activities. Appl. Sci. 2020, 10, 2668. [Google Scholar] [CrossRef]
- Kemper, K.J. Cat’s Claw (Uncaria tomentosa); The Center for Holistic Pediatric Education and Research: Boston, MA, USA, 1999. [Google Scholar]
- Hilepo, J.N.; Bellucci, A.G.; Mossey, R.T. Acute renal failure caused by’cat’s claw’herbal remedy in a patient with systemic lupus erythematosus. Nephron 1997, 77, 361. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Kim, S.; Jung, M.Y.; Ham, I.H.; Whang, W.K. Anti-inflammatory phenylpropanoid glycosides from Clerodendron trichotomum leaves. Arch. Pharmacal Res. 2009, 32, 7–13. [Google Scholar] [CrossRef]
- Chae, S.; Kim, J.S.; Kang, K.A.; Bu, H.D.; Lee, Y.; Hyun, J.W.; Kang, S.S. Antioxidant activity of jionoside D from Clerodendron trichotomum. Biol. Pharm. Bull. 2004, 27, 1504–1508. [Google Scholar] [CrossRef]
- Ren, D.; Shen, Z.-Y.; Qin, L.-P.; Zhu, B. Pharmacology, phytochemistry, and traditional uses of Scrophularia ningpoensis Hemsl. J. Ethnopharmacol. 2020, 269, 113688. [Google Scholar] [CrossRef]
- Chang, C.-M.; Chu, H.-T.; Wei, Y.-H.; Chen, F.-P.; Wang, S.; Wu, P.-C.; Yen, H.-R.; Chen, T.-J.; Chang, H.-H. The core pattern analysis on chinese herbal medicine for Sjögren’s syndrome: A nationwide population-based study. Sci. Rep. 2015, 5, 1–11. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).