Dementia Is Induced via the AGEs/Iba1/iNOS Pathway in Aged KK-Ay/Tajcl Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Experiments
2.2. Open Field Test
2.3. Step-Through Passive Avoidance Test
2.4. Measurement of AGEs, TNF-α, RAGE, Iba1, CCR7, iNOS, NO, CD163, and Arginase-1 in the Hippocampus
2.5. Statistical Analysis
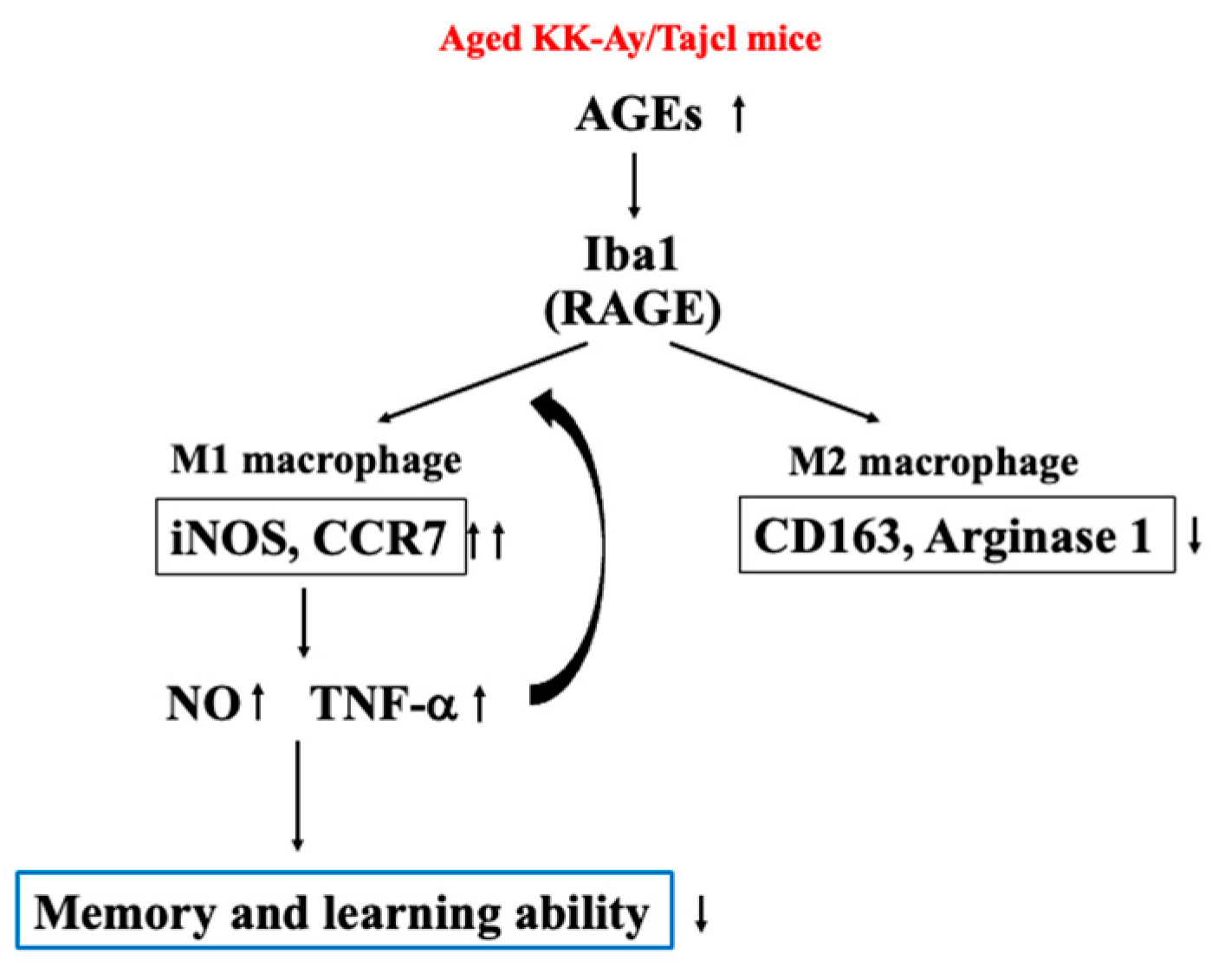
3. Results
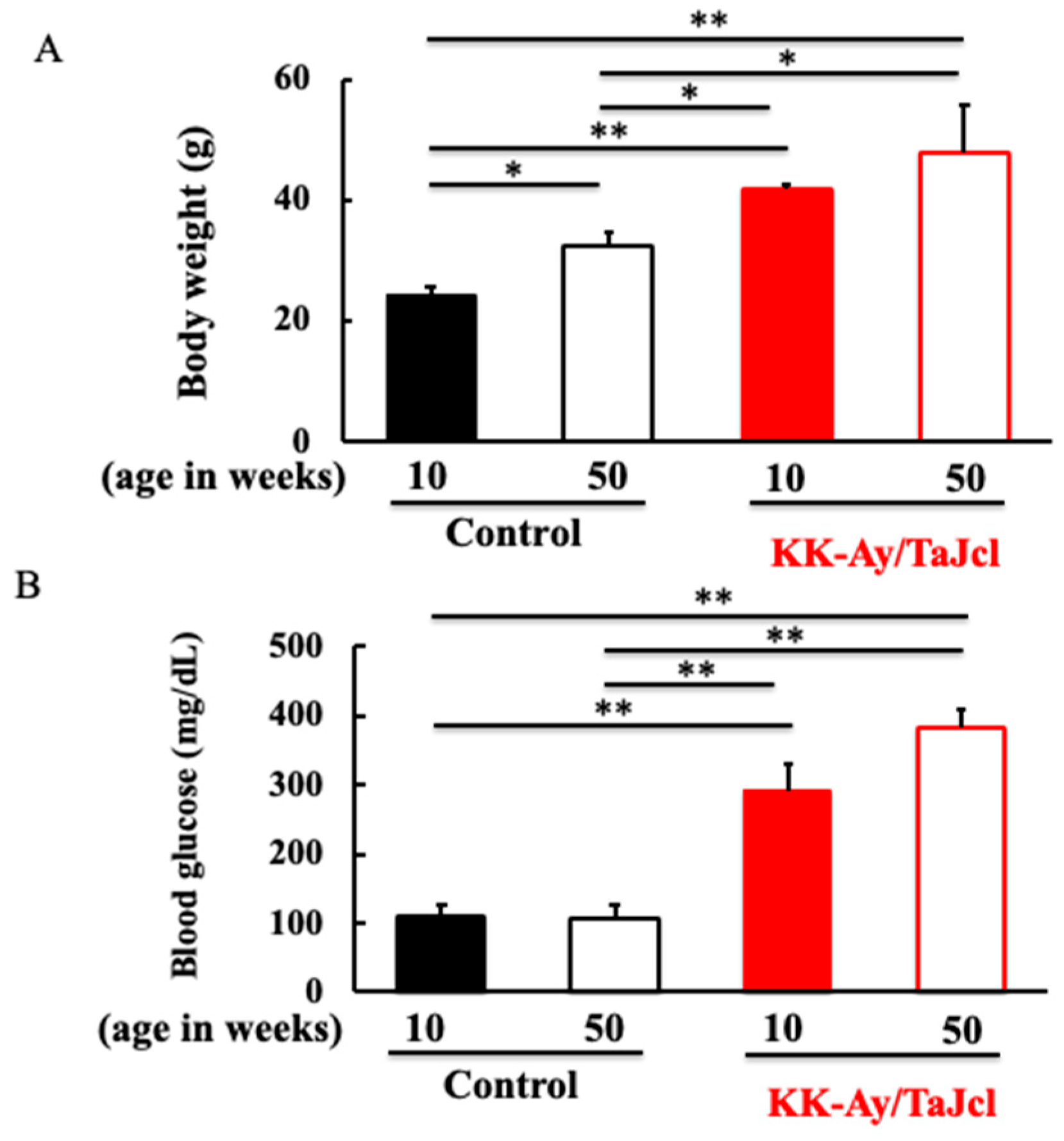
3.1. Effect of Aging on Body Weight and Blood Glucose Level in KK-Ay/Tajcl Mice
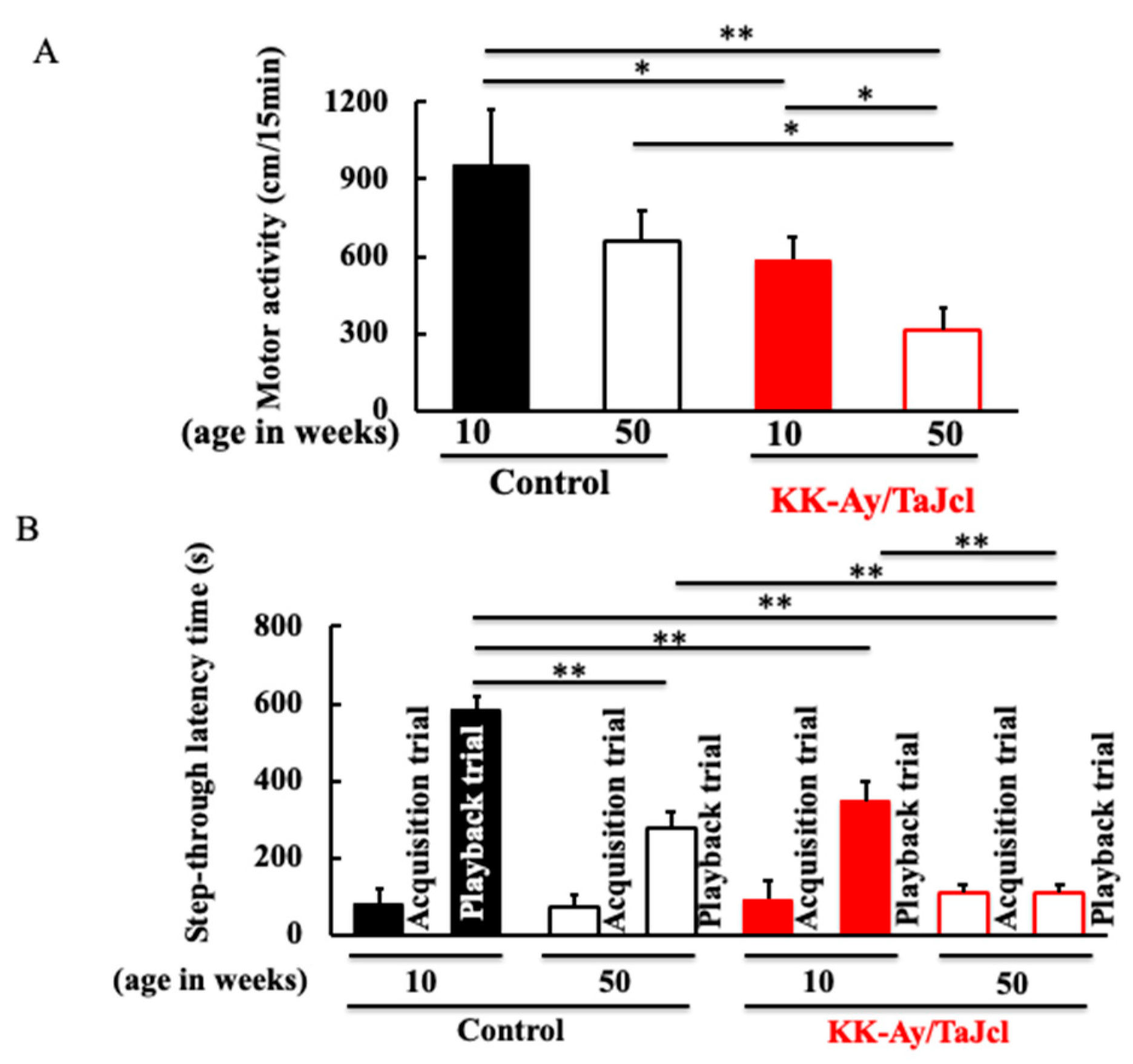
3.2. Behavioral Effects on Aging in KK-Ay/TaJcl Mice
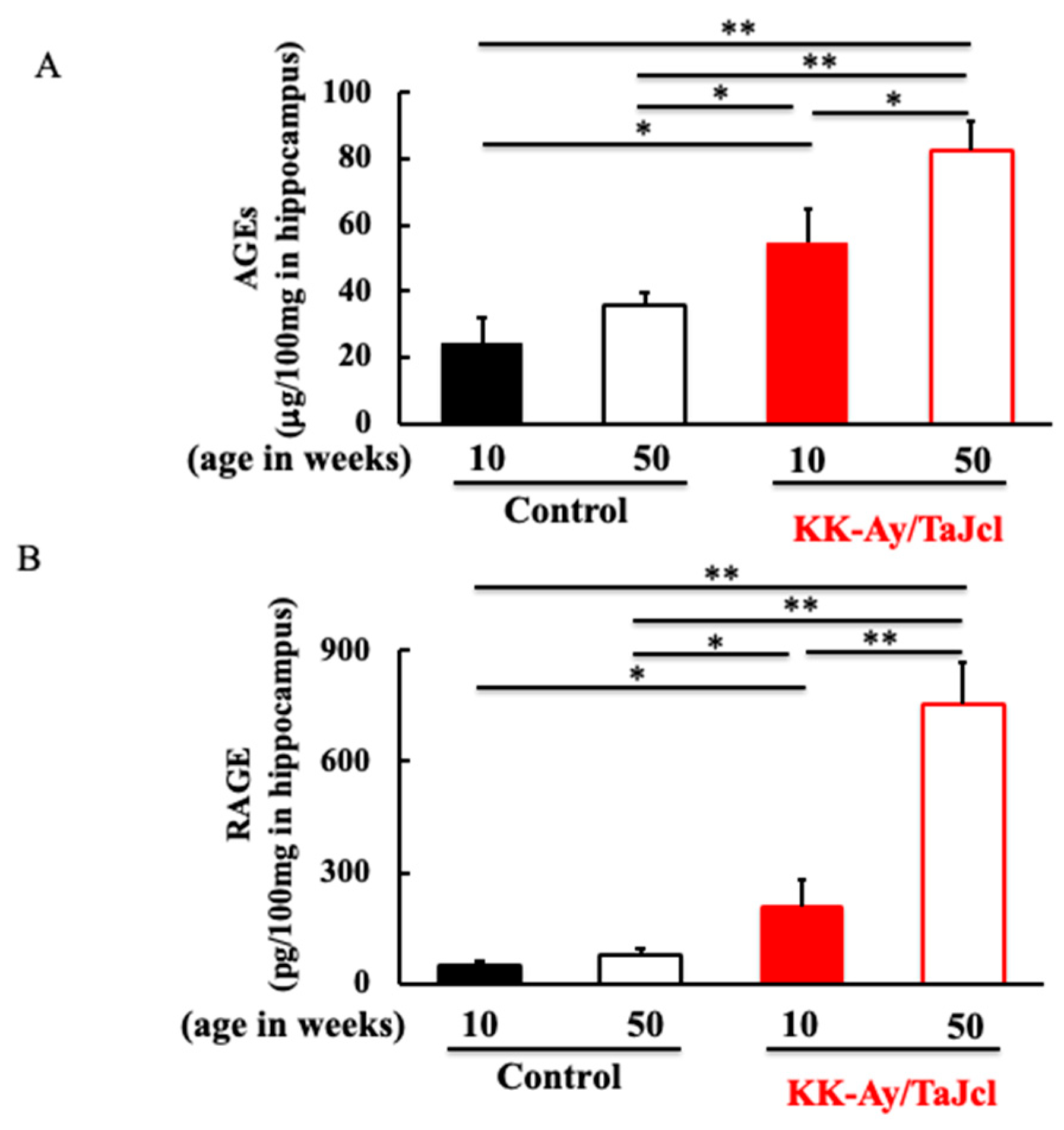
3.3. Effect of Aging on AGEs and RAGE in KK-Ay/TaJcl Mice
3.4. Effect of Aging on Iba1, CCR7, iNOS, TNF-α, and NO in KK-Ay/TaJcl Mice
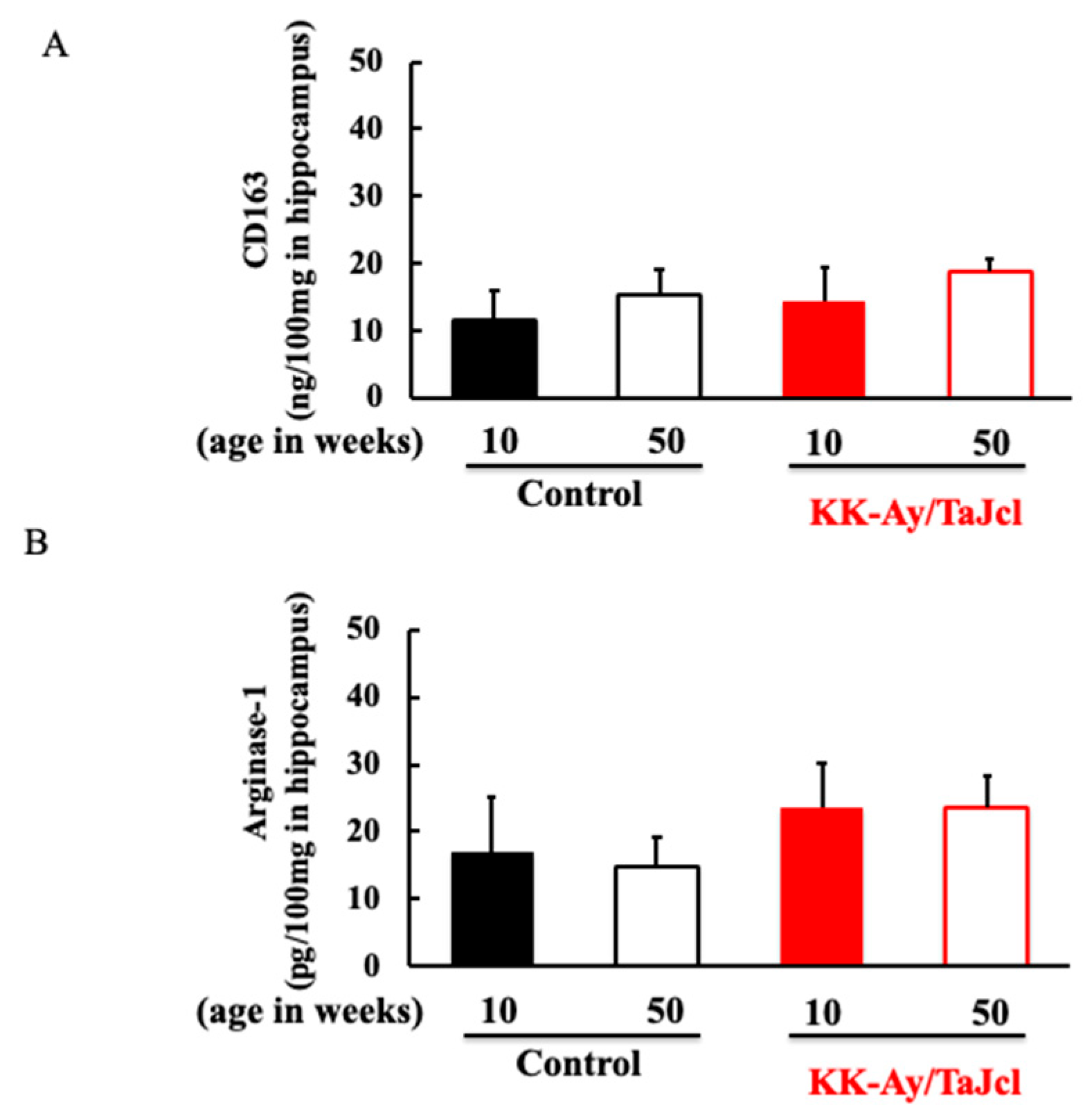
3.5. Effect of Aging on CD163 and Arginase-1 in KK-Ay/Tajcl Mice
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fratiglioni, L.; Paillard-Borg, S.; Winblad, B. An active and socially integrated lifestyle in late life might protect against dementia. Lancet Neurol. 2004, 3, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Kimura, R.; Tomiyasu, H.; Takeuchi, T.; Shimizu, M.; Hayashi, Y.; Okayama, N.; Kamiya, Y.; Joh, T. Prevalence of Alzheimer’s disease with diabetes in the Japanese population. Psychogeriatrics 2008, 8, 73–78. [Google Scholar] [CrossRef]
- Ott, A.; Stolk, R.P.; van Harskamp, F.; Pols, H.A.P.; Hofman, A.; Breteler, M.M.B. Diabetes mellitus and the risk of dementia: The Rotterdam Study. Neurology 1999, 53, 1937–1942. [Google Scholar] [CrossRef] [PubMed]
- Cukierman, T.; Gerstein, H.C.; Williamson, J.D. Cognitive decline and dementia in diabetes—Systematic overview of prospective observational studies. Diabetologia 2005, 48, 2460–2469. [Google Scholar] [CrossRef]
- Cheng, G.; Huang, C.; Deng, H.; Wang, H. Diabetes as a risk factor for dementia and mild cognitive impairment: A meta-analysis of longitudinal studies. Intern. Med. J. 2012, 42, 484–491. [Google Scholar] [CrossRef]
- Sato, N.; Morishita, R. Roles of vascular and metabolic components in cognitive dysfunction of Alzheimer disease: Short- and long-term modification by non-genetic risk factors. Front. Aging Neurosci. 2013, 5, 64. [Google Scholar] [CrossRef]
- Takeda, S.; Sato, N.; Uchio-Yamada, K.; Sawada, K.; Kunieda, T.; Takeuchi, D.; Kurinami, H.; Shinohara, M.; Rakugi, H.; Morishita, R. Diabetes-accelerated memory dysfunction via cerebrovascular inflammation and Aβ deposition in an Alzheimer mouse model with diabetes. Proc. Natl. Acad. Sci. USA 2010, 107, 7036–7041. [Google Scholar] [CrossRef]
- Francis, P.T.; Palmer, A.M.; Snape, M.; Wilcock, G.K. The cholinergic hypothesis of Alzheimer’s disease: A review of progress. J. Neurol. Neurosurg. Psychiatry 1999, 66, 137–147. [Google Scholar] [CrossRef]
- Srikanth, V.; Maczurek, A.; Phan, T.; Steele, M.; Westcott, B.; Juskiw, D.; Münch, G. Advanced glycation endproducts and their receptor RAGE in Alzheimer’s disease. Neurobiol. Aging 2011, 32, 763–777. [Google Scholar] [CrossRef]
- Dukic-Stefanovic, S.; Gasic-Milenkovic, J.; Deuther-Conrad, W.; Münch, G. Signal transduction pathways in mouse microglia N-11 cells activated by advanced glycation endproducts (AGEs). J. Neurochem. 2003, 87, 44–55. [Google Scholar] [CrossRef]
- Takeuchi, M.; Bucala, R.; Suzuki, T.; Ohkubo, T.; Yamazaki, M.; Koike, T.; Kameda, Y.; Makita, Z. Neurotoxicity of Advanced Glycation End-Products for Cultured Cortical Neurons. J. Neuropathol. Exp. Neurol. 2000, 59, 1094–1105. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-S.; Kim, C.-J.; Shin, M.-S.; Lim, B.-V. Treadmill exercise ameliorates memory impairment through ERK-Akt-CREB-BDNF signaling pathway in cerebral ischemia gerbils. J. Exerc. Rehabil. 2020, 16, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Leak, R.K.; Shi, Y.; Suenaga, J.; Gao, Y.; Zheng, P.; Chen, J. Microglial and macrophage polarization—New prospects for brain repair. Nat. Rev. Neurol. 2015, 11, 56–64. [Google Scholar] [CrossRef]
- Chen, G.Y.; Nuñez, G. Sterile inflammation: Sensing and reacting to damage. Nat. Rev. Immunol. 2010, 10, 826–837. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Sawada, M.; Haneda, M.; Fujii, S.; Oh-Hashi, K.; Kiuchi, K.; Takahashi, M.; Isobe, K.-I. Amyloid-β peptides induce cell proliferation and macrophage colony-stimulating factor expression via the PI3-kinase/Akt pathway in cultured Ra2 microglial cells. FEBS Lett. 2005, 579, 1995–2000. [Google Scholar] [CrossRef] [PubMed]
- Yagi, M.; Yonei, Y. Glycative stress and antiaging: 12. Glycative stress and dementia. Glycative Stress Res. 2019, 6, 87–91. [Google Scholar]
- Takeda, A.; Yasuda, T.; Miyata, T.; Goto, Y.; Wakai, M.; Watanabe, M.; Yasuda, Y.; Horie, K.; Inagaki, T.; Doyu, M.; et al. Advanced glycation end products co-localized with astrocytes and microglial cells in Alzheimer’s disease brain. Acta Neuropathol. 1998, 95, 555–558. [Google Scholar] [CrossRef]
- Block, M.L.; Hong, J.-S. Microglia and inflammation-mediated neurodegeneration: Multiple triggers with a common mechanism. Prog. Neurobiol. 2005, 76, 77–98. [Google Scholar] [CrossRef]
- Lenz, K.M.; Nugent, B.M.; Haliyur, R.; McCarthy, M.M. Microglia Are Essential to Masculinization of Brain and Behavior. J. Neurosci. 2013, 33, 2761–2772. [Google Scholar] [CrossRef]
- Gordon, S. Alternative activation of macrophages. Nat. Rev. Immunol. 2003, 3, 23–35. [Google Scholar] [CrossRef]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef] [PubMed]
- Okuno, A.; Tamemoto, H.; Tobe, K.; Ueki, K.; Mori, Y.; Iwamoto, K.; Umesono, K.; Akanuma, Y.; Fujiwara, T.; Horikoshi, H.; et al. Troglitazone increases the number of small adipocytes without the change of white adipose tissue mass in obese Zucker rats. J. Clin. Investig. 1998, 101, 1354–1361. [Google Scholar] [CrossRef]
- Hiramoto, K.; Goto, K.; Tanaka, S.; Horikawa, T.; Ooi, K. Skin, Liver, and Kidney Interactions Contribute to Skin Dryness in Aging KK-Ay/Tajcl Mice. Biomedicines 2022, 10, 2648. [Google Scholar] [CrossRef]
- Jahan, H.; Choudhary, M.I. Gliclazide alters macrophages polarization state in diabetic atherosclerosis in vitro via blocking AGE-RAGE/TLR4-reactive oxygen species-activated NF-kβ nexus. Eur. J. Pharmacol. 2021, 894, 173874. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Wu, Z.; Peterts, C.; Yamamoto, K.; Qing, H.; Nakanishi, H. The Critical Role of Proteolytic Relay through Cathepsins B and E in the Phenotypic Change of Microglia/Macrophage. J. Neurosci. 2015, 35, 12488–12501. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.K.; Mantovani, A. Macrophage plasticity and interaction with lymphocyte subsets: Cancer as a paradigm. Nat. Immunol. 2010, 11, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Toda, N.; Ayajiki, K.; Okamura, T. Cerebral blood flow regulation by nitric oxide in neurological disorders. Can. J. Physiol. Pharmacol. 2009, 87, 581–594. [Google Scholar] [CrossRef] [PubMed]
- Cho, D.-H.; Nakamura, T.; Fang, J.; Cieplak, P.; Godzik, A.; Gu, Z.; Lipton, S.A. S-Nitrosylation of Drp1 Mediates β-Amyloid-Related Mitochondrial Fission and Neuronal Injury. Science 2009, 324, 102–105. [Google Scholar] [CrossRef]
- Taddei, S.; Virdis, A.; Mattei, P.; Natali, A.; Ferrannini, E.; Salvetti, A. Effect of Insulin on Acetylcholine-Induced Vasodilation in Normotensive Subjects and Patients With Essential Hypertension. Circulation 1995, 92, 2911–2918. [Google Scholar] [CrossRef]
- Higashi, Y.; Sasaki, S.; Nakagawa, K.; Kimura, M.; Noma, K.; Hara, K.; Jitsuiki, D.; Goto, C.; Oshima, T.; Chayama, K.; et al. Tetrahydrobiopterin improves aging-related impairment of endothelium-dependent vasodilation through increase in nitric oxide production. Atherosclerosis 2006, 186, 390–395. [Google Scholar] [CrossRef]
- Fukazawa, R.; Hanyu, H.; Sato, T.; Shimizu, S.; Koyama, S.; Kanetaka, H.; Sakurai, H.; Iwamoto, T. Subgroups of Alzheimer’s Disease Associated with Diabetes Mellitus Based on Brain Imaging. Dement. Geriatr. Cogn. Disord. 2013, 35, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.J.; Kim, K.E.; Jeong, E.A.; An, H.S.; Lee, S.J.; Lee, J.; Roh, G.S. Amyloid b oligomer promotes microglial galection-3 and astrocytic lipocalin-2 levels in the hippocampus of mice fed a high-fat diet. Biochem. Biophy. Res. Commun. 2023, 667, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Natunen, T.; Martiskainen, H.; Marttinen, M.; Gabbouj, A.; Koivisto, H.; Kemppainen, S.; Kaipainen, S.; Takalo, M.; Svo-bodova, H.; Leppanen, L.; et al. Diabetic phenotype in mouse and humans reduces the number of microglia atound b-amyloid plaques. Mol. Neurodegener. 2020, 15, 66. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hiramoto, K.; Imai, M.; Tanaka, S.; Ooi, K. Dementia Is Induced via the AGEs/Iba1/iNOS Pathway in Aged KK-Ay/Tajcl Mice. Life 2023, 13, 1540. https://doi.org/10.3390/life13071540
Hiramoto K, Imai M, Tanaka S, Ooi K. Dementia Is Induced via the AGEs/Iba1/iNOS Pathway in Aged KK-Ay/Tajcl Mice. Life. 2023; 13(7):1540. https://doi.org/10.3390/life13071540
Chicago/Turabian StyleHiramoto, Keiichi, Masashi Imai, Shota Tanaka, and Kazuya Ooi. 2023. "Dementia Is Induced via the AGEs/Iba1/iNOS Pathway in Aged KK-Ay/Tajcl Mice" Life 13, no. 7: 1540. https://doi.org/10.3390/life13071540
APA StyleHiramoto, K., Imai, M., Tanaka, S., & Ooi, K. (2023). Dementia Is Induced via the AGEs/Iba1/iNOS Pathway in Aged KK-Ay/Tajcl Mice. Life, 13(7), 1540. https://doi.org/10.3390/life13071540





