Abstract
The Sardinian grass snake, Natrix helvetica cetti, is an endangered endemic snake subspecies with a restricted and highly fragmented geographic distribution. Information on its ecology and detailed geographic distribution are scarce and may negatively impact on its conservation status. Therefore, a literature review on its taxonomy, morphology, ecology, and conservation is presented here. Moreover, field records from the authors, citizen science and the existing literature provide an updated geographic distribution highlighting its presence within 13 new and 7 historic 10 × 10 km cells. Bioclimatic niche modelling was then applied to explore patterns of habitat suitability and phenotypic variation within N. h. cetti. The geographic distribution of the species was found to be positively correlated with altitude and precipitation values, whereas temperature showed a negative correlation. Taken together, these outcomes may explain the snake’s presence, particularly in eastern Sardinia. In addition, analysis of distribution overlap with the competing viperine snake (N. maura) and the urodeles as possible overlooked trophic resources (Speleomantes spp. and Euproctus platycephalus) showed overlaps of 66% and 79%, respectively. Finally, geographical or bioclimatic correlations did not explain phenotypic variation patterns observed in this highly polymorphic taxon. Perspectives on future research to investigate N. h. cetti’s decline and support effective conservation measures are discussed.
Keywords:
Colubridae; habitat suitability; island endemic; Natricidae; Natrix; helvetica; cetti; potential distribution; Reptilia; Sardinia 1. Introduction
Endemism, together with species rarity and diversity, are among the most used criteria to establish conservation priorities [1,2]. Habitat alteration, pollution, climate change, and introduction of invasive species are the main threats to endemics, which are highly adapted to their specific habitats [2,3,4,5].
Among the factors affecting the probability of extinction of a species, rarity is the predominant one, and species become rare before going extinct. Hence, endemic species with a limited distribution range and low dispersal rate are most susceptible to extinction [2]. It is, therefore, important to focus efforts on the conservation of endemic taxa, and one of the preliminary steps is to assess their known and potential distribution in order to be able to detect populations and consequently study them appropriately. Indeed, understanding species distribution is fundamental to tracking and predicting changes in range dynamics and thus setting conservation priorities [6]. Nevertheless, this process is complex and in order to provide meaningful and practical conservation insights, it requires taking into consideration multiple layers of information in addition to pure presence/absence data [7,8,9]. This allows us to better understand the real extent of the geographic range of cryptic and rare species, overcoming the constraints correlated with field surveys, and in some cases, leading to the discovery of new and valuable populations (e.g., [10]).
The island of Sardinia (in red in Figure 1A) is situated in the central-western part of the Mediterranean Sea, and it is the second largest island of the Mediterranean Basin (extension: 23,812.6 km2; max altitude: 1834 m a.s.l. [11]) where, together with Corsica, it is one of the major biodiversity hotspots [12,13]. As for the herpetofauna, Sardinia has 19 species of reptiles, including the loggerhead sea turtle Caretta caretta since it is nesting on the island (see Table 1; [12,14,15,16]). The Sardinian snake fauna consists of four species, half of which share a very recent origin from North Africa, determined by probable introductions in historical times. These are the horseshoe whip snake Hemorrhois hippocrepis [17] and the viperine snake Natrix maura (for the latter, even a transmarine dispersal cannot be excluded; see [18] and Section 3.1.4). The other two snake species have colonised the Sardinian-Corsican block from the Italian peninsula in relatively recent times. These are the western whip snake Hierophis viridiflavus (see [19]) and the barred grass snake N. helvetica. The Sardinian-Corsican populations of N. helvetica then differentiated from the continental ones, resulting in a separate subspecific taxon [20,21].

Table 1.
List of reptiles of Sardinia. The subspecific framework is in agreement with [16].
The Sardinian grass snakes and the Corsican grass snakes could be considered synonyms since they have been demonstrated to be genetically undifferentiated [22]. However, pending studies include a larger sample from both islands, and considering the high threat status of the Sardinian populations, it has been suggested to tentatively keep two separate subspecies -N. h. cetti and N. h. corsa-([22,23]; see Section 3.1.1). For this reason, in the present work, the taxon name N. h. cetti is applied in reference to the Sardinian populations only.
Natrix h. cetti (Figure 1B) is irregularly distributed only on Sardinia’s main island and is considered to be in decline, with a smaller distribution than in the past [22,23,24,25], as well as being categorised as “Endangered” in the last IUCN Red List of Italian vertebrates [26].
Low detectability of individuals (possibly due to their elusiveness, limited distribution, and rarefaction) and the lack of proper field surveys are factors that could have negatively influenced the knowledge of the distribution of N. h. cetti [24,25]. The major aim of this work is to provide updated distribution data as well as an estimate of the potential distribution for the Sardinian grass snake. These aspects are fundamental to assess the current status of the populations and to potentially better understand the ecology of the taxon in order to refine conservation efforts.
Firstly, the available information on the various zoological aspects of the taxon is collected from a range of literature sources and presented here. This work may thus be used as a starting point for conservation planning. Furthermore, updated distribution data derived from personal field surveys and citizen science as well as potential distribution data obtained using bioclimatic niche modelling are presented. In addition, the range overlap of N. h. cetti and that of N. maura, a potential ecological competitor that colonized the island relatively recently (see [18,27] and Section 3.1.4), is also considered as a starting point to evaluate how much, from a spatial point of view, the presence of the non-native taxon may pose a threat to the insular endemic grass snake. Similarly, the range overlap of N. h. cetti with that of the six Sardinian endemic urodelan species (Speleomantes spp. and Euproctus platycephalus) is assessed, as the latter could represent an overlooked trophic resource for the Sardinian grass snake (see Section 3.1.3). Finally, an attempt is made to characterise the variability in the dorsal pattern (i.e., the dark marks on the ground colour), given that the only work concerning chromatic variability of the Sardinian grass snake essentially referred to the dorsal ground colouration (see [28]). The influence of distributional and environmental variables on dorsal pattern variation is also investigated.
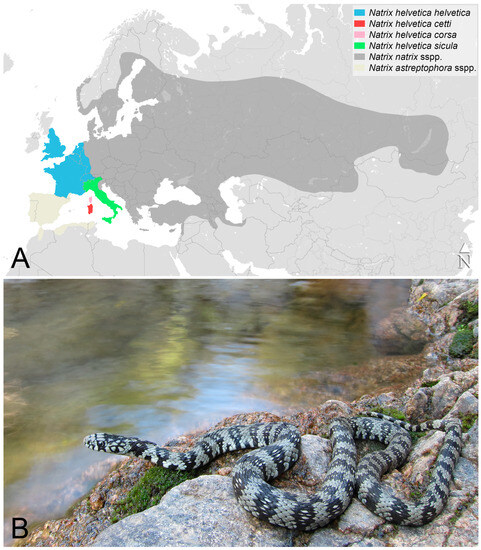

Figure 1.
Western and Central Eurasia, with the approximate* distribution ranges of the Natrix natrix complex species and N. helvetica subspecies according to [21,22,23,29]. *Admixture areas between different taxa are not shown (A); Adult individual of Natrix helvetica cetti in its natural habitat in the “Sette Fratelli” area, South Sardinia (B). Photo credit: Matteo R. Di Nicola.
2. Materials and Methods
2.1. Natural History Review
All available literature on the Sardinian grass snake (i.e., scientific articles, thematic books, and guides) was gathered by consulting the PubMed and Scopus databases, the social network ResearchGate, and by using the Google Scholar web search engine.
To perform the search, the following query was used, applying every possible combination of the following keywords: [<Sardinia> OR <Sardinian> AND <snakes> OR <reptiles> OR <herpetofauna> OR (<grass> AND <snake>)] OR [<Natrix> OR <Tropidonotus> AND <cetti> OR <cettii> OR (<natrix> AND <cetti>) OR (<natrix> AND <cettii>) OR (<natrix> AND <corsus>) OR (<natrix> AND <corsa>) OR (<helvetica> AND <cetti>) OR (<helvetica> AND <corsa>)].
The most relevant information found in the literature was summarised to compose a brief review divided into taxonomy and hypothesis on the origins, morphology, ecology, and conservation.
2.2. Distribution Update
The distribution update of Natrix helvetica cetti in Sardinia was produced on the basis of field observations carried out by two of the authors (MRDN and SM) during opportunistic surveys conducted in the period July 2016–May 2022 (33.3%), and combined with data obtained from citizen science, updated until May 2023 (66.7%). For each snake found by the authors, the date, time, coordinates, and altitude were recorded (via Garmin Etrex 32X GPS device), and a photo of the dorsal pattern was taken. As for the records obtained through citizen science, the data were requested from herpetologist/herpetophile collaborators and observations were also sought on the following Social Networks: Inaturalist.it, Flickr.com, and the Facebook group “Identificazione Anfibi e Rettili”, administered by MRDN. The authors of each observation were then contacted, and only the records with coordinates recorded live (or for which they remembered the place of discovery with an accuracy of at least 300 m) and accompanied by a photo with sufficient resolution were considered. The altitudes of the citizen science records were obtained using the Google Earth Software (ver. 9.194.0.0).
The obtained records were then compared and added to those present in the scientific literature (i.e., [12,14,30,31,32,33,34,35]).
The new data were used to compile both a point map and an updated distribution map-UTM (Universal Transverse Mercator Projection, Coordinate Reference System WGS84/UTM Zone 32N) 10 × 10 km square grid system, which divided the island into 312 squares, similar to the one used by Corti et al. [12].
The N. h. cetti updated UTM distribution map was then overlaid with the map for N. maura. The latter was obtained by merging the data published by Corti et al. [12] with the records collected by two of the authors (MRDN and SM) and citizen science.
Furthermore, the N. h. cetti updated UTM distribution map was also overlaid on those from the six Sardinian species of urodeles (Speleomantes spp. and Euproctus platycephalus), based on data from the publication by Corti et al. [12].
2.3. Habitat Suitability Estimation
The software MaxEnt 3.4.1 [36] was used to model the bioclimatic niche of Natrix helvetica cetti in Sardinia. Only occurrence records with precise coordinates were used for this purpose. In order to avoid pseudo-replication, these were spatially filtered to retain one record per grid cell (30 arcsec spatial resolution). Predictor layers were composed of 19 bioclimatic variables plus altitude downloaded from the WorldClim 2.1 database [37], and a land cover raster obtained from the Global Land Cover National Mapping Organizations (GLCNMO version 3 available at https://globalmaps.github.io/glcnmo.html, accessed on 5 June 2023). The bioclimatic variables were downloaded at a 30 arc-sec spatial resolution and rescaled to match the resolution of the land cover layer (15 arc-sec). Values for all 19 bioclimatic variables were extracted at each presence location and pairwise Pearson’s correlation coefficients were calculated in order to assess multicollinearity between predictors. Among highly correlated variables (|r| > 0.75), those deemed as most ecologically meaningful for the distribution of the taxon were retained, while the others were dropped. The selected variables were: mean annual temperature (bio1), temperature seasonality (bio4), annual precipitation (bio12), precipitation seasonality (bio15), and precipitation of the warmest quarter (bio18). Altitude and land cover were added in a step-wise procedure to observe their effect on model output. In order to assess the influence of variable selection on the models, we compared the performances of our dataset before and after variable selection. A total of six model combinations were tested: (1) all bioclimatic variables (n = 19); (2) bioclimatic variables plus altitude (n = 20); (3) bioclimatic variables plus altitude and land cover (n = 21); (4) only selected bioclimatic variables (n = 5); (5) selected bioclimatic variables plus altitude (n = 6); (6) selected bioclimatic variables plus altitude and land cover (n = 7). A mask layer of Sardinia was used to draw background points (n = 10,000) for modelling in MaxEnt (default settings; logistic outputs). Ten bootstrap replicates were computed for every model combination, each replicate randomly selecting 70% of the data points for model training and the remaining 30% for validation. Jackknife analysis was used to estimate the relative contribution of each predictor variable to the models. A model’s performance was evaluated based on the area under the receiver operating curve (AUC) and omission rate (OR). The former is a measure of the model’s ability to discriminate between presence and background points and can range from 0.5 (no better than chance) to 1 (perfect discrimination; [38]). The latter expresses the proportion of records predicted to fall outside the area predicted as suitable by the model, based on various theoretical thresholds. For the purpose of this study, the 10th percentile training presence omission rate (OR10) was adopted. This sets the minimum suitability threshold at a value allowing only 10% of possible predicted occurrences to be rejected; models that are significantly higher that OR10 are, therefore, considered overfit [39]. The OR10 logistic threshold was also used to set the minimum suitability threshold to MaxEnt continuous suitability outputs. Finally, an average of the six model combinations tested was computed and used to represent the bioclimatic suitability of N. h. cetti in Sardinia. All analyses were performed in R 3.6.1 [40] and QGIS 3.14 [41].
2.4. Dorsal Pattern Characterisation
The Sardinian grass snake is characterised by a high level of colour polymorphism (e.g., [15,25,28]). In reptiles, this interspecific colour variation may be correlated with geographic, bioclimatic, and environmental factors (e.g., [42,43,44]). Therefore, we carried out a tentative characterisation of the dorsal patterns, based on both personally acquired and citizen science photographs (the unavailability of photos taken in a standardized way for all snakes, has not allowed the use of computer-based colour assessment methods). In this endeavour, only observations with photographs deemed suitable for the purpose were considered (i.e., with sufficient resolution, correctly exposed, and showing most of the dorsal pattern of the snake). Since the colouration in Natrix natrix sensu lato is possibly subject to ontogenetic variations (see [45]; e.g., melanism in N. helvetica becomes apparent in adulthood-Faraone, unpublished data), we excluded individuals identified as clearly young (i.e., newborn or no more than yearling, based on body proportions, such as the size of the eyes in relation to the head and the size of the latter in relation to the body) from the evaluation. In some cases, the dorsal pattern does not unequivocally fall into a given category. Hence, we considered the prevailing trend of each dorsal motif and the evaluation was made independently by three of the authors (MRDN, AP, and SM), and this was followed by a collective assessment to discuss any discordant data. Overall, the dorsal patterns of snakes that had coordinates were geographically mapped and the distribution of the different dorsal patterns was visualised using the 10 × 10 km UTM map described in Section 2.2.
Dorsal pattern characterisation was used to explore patterns of geographic segregation and to assess the influence of bioclimatic variables via principal component analysis (see the next section).
2.5. Principal Component Analysis
A principal component analysis (PCA) was computed in order to explore the bioclimatic niche occupied by individuals of Natrix helvetica cetti in Sardinia, differentiating them across various dorsal patterns. To do so, both presence and background points used to compute the above-mentioned MaxEnt models (see Section 2.3) were plotted across the first two principal component dimensions, representing their variation along the reduced set of continuous variables used for modelling (bio1, bio4, bio12, bio15, and bio18). The statistical analyses and visual representation were performed in R 3.6.1 using the packages FactoMineR [46], factoextra [47] and ggplot2 [48].
3. Results
3.1. Natural History Review
3.1.1. Taxonomy and Hypothesis on the Origins
Grass snakes (Natrix natrix complex) are medium-sized to large semi-aquatic Natricidae ophidians distributed from the Maghreb region and the Iberian Peninsula throughout most of Europe to Lake Baikal in central Asia ([21,23,49,50]; Figure 1A). Three different parapatric species are currently recognised [the red-eyed or Iberian grass snake Natrix astreptophora (Seoane, 1884); the barred grass snake Natrix helvetica (Lacépède, 1789); and the common or Eastern grass snake Natrix natrix (Linnaeus, 1758)], and between their distribution ranges, there are narrow hybridization areas in which both hybrids and parental species are present [22,51,52,53,54,55,56,57]. Different subspecies have been described within the N. natrix complex, and Fritz and Schmidtler [23] and Fritz and Ihlow [57] have provided a list of those currently considered valid (Table S1).
The grass snakes from Sardinia and Corsica have been described as a new species Natrix cetti by Gené [58] on the basis of an adult male from Monte di San Giovanni d’Iglesias (southern Sardinia), a pregnant female from Fonni (central Sardinia), and a juvenile from southern Sardinia Corsica (see [23] for more information on syntypes). Mertens and Muller [59] restricted the type locality of the species only to the Monte di San Giovanni d’Iglesias. Hence, N. cetti has generally been considered “the grass snake from Sardinia”. Anyway, the authors did not designate a lectotype and their restriction of the type locality is not valid: the lectotype will then be designated only by Fritz and Schmidtler [23], starting from the female syntype from Fonni. Leunis [60] ascribed the species to the genus Tropidonotus Boie, 1826, while Jan [61], Camerano [62], and Mertens and Müller [59] considered it a subspecies, while reusing the genus Natrix. Hecht [63], in a review of the genus Tropidonotus, considered the Corsican populations as a separate subspecies Tropidonotus natrix subsp. Corsus. Mertens [64] puts the Corsican taxon back into synonymy with the Sardinian one but later [65], investigating further samples from Corsica, reconsidered the separation of the subspecies (using Natrix natrix corsa). Roger S. Thorpe, starting from a PhD project on the intraspecific variation in N. natrix [66], carried out a review of the species based on biometrics. The author recognised a total of three subspecies for N. natrix (i.e., N. n. natrix, N. n. helvetica and N. n. cetti), considering the Corsican populations “phenetically intermediate” between the Sardinian and the continental ones. Hence, in this analysis, the two Sardinian-Corsican taxa were kept partially separate, indicating N. n. cetti for Sardinia and “N. n. helvetica-N. n. cetti” for Corsica ([67]; see also [68]). Later, the same author produced a more comprehensive work in which he recognised four N. natrix subspecies (i.e., N. n. natrix, N. n. helvetica, N. n. cetti and N. n. corsa) and reconfirmed the separation between the Sardinian and Corsican populations [45]. Still Thorpe [69], stated the following: “The four main forms, eastern, western Sardinian and Corsican, although treated as conspecific are obviously not far below the level of species”. In Lanza [70], a work by Vanni and Lanza indicated as “in preparation” (but no longer published in any other form) proposed the taxon N. n. cetti for both Sardinia and Corsica, following the examination of more than 800 Italian specimens as well as material from other regions. Based on the existing morphological and biogeographic differences from the other N. natrix and following the karyological data presented by Aprea et al. [71], Vanni and Cimmaruta [24] proposed the specific status for Sardinian-Corsican populations. Furthermore, given the morphological and chromatic differences between the Sardinian and Corsican populations, the authors indicated a possible subspecific distinction (with the names N. cetti cetti and N. c. corsa); although, this conclusion would still require confirmation at the molecular level. However, studies on a molecular basis have not confirmed the hypothesis of specific status: Fritz et al. [20] analysed Sardinian and Corsican mitochondrial DNA sequences, comparing them with sequences from the rest of the range, to reach the conclusion that the subspecific level has to be reconsidered for Sardinian-Corsican populations. Moreover, phylogenetic analyses by Kindler et al. [72] show that the two island populations share the same mtDNA lineage (named “B”), and they both fall into the third of the three major clades identified [i.e., (i) Iberian Peninsula, adjacent France and North Africa; (ii) East Europe and Asia; (iii) West Europe including Corso-Sardinia, the Apennine Peninsula and Sicily]. A study by Kindler et al. [52] raised N. natrix helvetica to the species level: since the recognition of N. helvetica as a full species implies that all nominal subspecies assigned to the same major clade (the third major clade of [72]) have to be transferred to N. helvetica, the Sardinian and Corsican grass snakes have been involved in the consequent taxonomic update, passing respectively from N. natrix cetti and N. n. corsa to N. helvetica cetti and N. h. corsa.
Further molecular analyses show that N. h. cetti and N. h. corsa constitute together only one microsatellite cluster [22]. Hence, the two taxa are considered genetically undifferentiated with respect to both nuclear and mitochondrial markers and could be synonymous (and they should, therefore, be considered a single Sardinian-Corsican endemic taxon). Overall, from the studies presented above, few samples from Sardinian and Corsican snakes were analysed and since the Sardinian grass snake is the most seriously threatened and apparently less abundant than its Corsican counterpart, a synonymisation of the two taxa could have a negative impact on the conservation of the Sardinian populations. For these reasons, the authors tentatively recognised both subspecies, referring to the need for further research [22,23].
Natricids likely originated ~35–47.1 million years ago in Asia [50,73,74], where most of the extant Natricidae species live, and dispersed to Australo-Melanesia, sub-Saharan Africa, Europe/North Africa, and North/Central America [74]. The Natrix lineage dispersed to the western Palearctic, where the genus Natrix diversified [21]. According to Deepak et al. [74], extant western Palearctic natricids started to diverge ~ 26 Mya, and extant Natrix spp. ~16 Mya with N. maura. Schöneberg et al. [21], who first used a genomics approach for the genus Natrix, indicate for the extant Natrix spp. the following divergence ages: N. maura ~21.5 Mya; N. tessellata ~18.7 Mya; N. natrix ~13.7 Mya; N. astreptophora split from N. helvetica ~7.3 Mya.
With regards to the origins of the Sardinian-Corsican taxon, Lanza [75,76,77] hypothesised a Messinian provenance (~5,2 Mya), or even a pre-Miocene one, whether the taxon is considered as full species; instead, arrival in the Quaternary is hypothesised during the Cassia regression (~1 Mya) if the taxon is considered at a subspecific level [24]. Fritz et al. [20], based on phylogenetic analysis, indicate a divergence from the continental forms between 4.4 and 4.3 Mya, during the lower Pliocene, probably derived from the isolation due to the post-Messinian floods of the Mediterranean basin, which occurred 5.33 Mya (see [78]). The most recent genomic-based phylogenetic analysis confirmed that the divergence would have occurred in the Pliocene, ~4.5 Mya [21]. On the other hand, the Sardinian and Corsican populations appear to be weakly differentiated from each other [20,22]. Fritz et al. [20] indicated an estimated mean time of divergence between 0.41 and 0.35 mya, finding a possible explanation in the intermittent land connections between the two islands, caused by sea level fluctuations in the Pleistocene (according to [79]). Similarly, Schultze et al. [22] argued that Sardinia and Corsica were repeatedly connected during the Pliocene and during the Pleistocene low sea-level stands (see [80]) and that the last connection between the two islands was interrupted by the rising sea in the early Holocene. Overall, from the body of evidence presented above, Corsican and Sardinian grass snakes have most likely repeatedly formed a contiguous population system and their morphological differences may derive from a recent divergence.
3.1.2. Morphology
As highlighted by various authors (e.g., [24,28,45,69]), Natrix helvetica cetti includes grass snake populations with highly distinctive phenotypes. The chromatic pattern presents a peculiar expansion and fusion of its dark elements. Unlike other populations of N. helvetica, the dorsal and lateral series of transverse blotches often tend to merge and form large rings that split on the sides [28,45]. The neck is usually adorned with a large dark ring and the typical yellow or whitish nuchal collar, often present in mainland populations, is completely missing, even in the youngest individuals [24,45]. A large and irregular dark spot is generally present in the area of the parietal scales; furthermore, the remaining cephalic scales have dark edges of variable thickness. Cases of melanism are known for the Sardinian grass snake [15,25,28,81,82].
The Sardinian grass snake also possesses some highly distinctive pholidotic characters [45,69]. Ventral scales have a range of 160–178 in males and 158–173 in females [24] and are on average fewer than those from mainland populations [45]. Subcaudal scales are 56–65 in males and 47–53 in females ([24]; Di Nicola, unpublished records). This is much fewer than those found in mainland populations, which on average have about ten more units in both sexes [24,45]. On average, in Sardinian grass snakes, the sublabials and temporal scales are also about one unit less than those from mainland grass snakes [24,45].
Body proportions in N. h. cetti are different compared to those from other adjacent populations of barred grass snakes, which are characterised by proportionally smaller head characters, a thinner body, and a much shorter tail [45]. With regards to body size, Sardinian grass snakes usually do not reach 100 cm in total length [24,62,83], with a maximum of 109.5 cm for a female, as reported by Di Nicola and Mezzadri [15]. The total length of this subspecies is, therefore, smaller than that of mainland populations, reaching about 100 cm in males and 180 cm in females [25,84].
Natrix helvetica cetti also possesses some unique features of the internal organs, which are generally placed in a more caudal position compared to that of Italian mainland samples [45]. Amongst other differences, the right lung is considerably longer in the Sardinian population and the hemipenial retractor muscle much shorter compared to that reported for the other samples. Finally, the dentition of N. h. cetti have generally lower values than Italian mainland population [45].
3.1.3. Ecology
The vast majority of the ecological aspects of the Sardinian grass snake are extremely understudied. The reasons could be the low density at which this snake seems to occur in the wild, its secretive nature, or most likely, a combination of both factors [25,83,85].
According to some authors, the ecology of Natrix helvetica cetti differs substantially from its mainland counterparts. Sardinian endemism seems to be less associated with water bodies, while it seems to inhabit rocky and dry habitats characterised by scarce vegetation and relevant sun exposure [86,87]. In agreement with the saxicolous nature of N. h. cetti, Lunghi and colleagues [28] observed 15 specimens (out of a total sample size of 18 individuals) within rocky habitats at a distance greater than 1 km from any water sources. On the other hand, Capula et al. [83], in a 38-day field study with a total sample size of 18 specimens, didn’t find any snakes further than 10 m away from water bodies such as mountain streams and lakes. It is then plausible that N. h. cetti is able to exploit a wider range of habitats compared to its mainland relatives [25,28]. The Sardinian grass snake is also considered to be primarily restricted to mid-elevation montane areas [34,86]. Despite this fact, individuals have been found from the sea level up to 1407 m a.s.l. ([15,28]; this study).
Another interesting aspect of the ecology of N. h. cetti, which might contribute to the knowledge gap that characterises this snake, regards its activity pattern. While grass snakes (N. natrix s. l.) tend to be predominantly diurnal, shifting to a nocturnal activity pattern during the hottest part of the year, N. h. cetti seems to be characterised by a nocturnal lifestyle [83]. In fact, despite searching for N. h. cetti in early spring and late summer (April and September), Capula and colleagues [83] only observed four individuals during the daytime, each of which was found hiding under natural refugia. The authors highlighted how active individuals were found at night-time while looking for potential prey during early spring at air temperatures lower than 15 °C. Despite that, De Pous et al. [34], during a series of short field trips to Sardinia that took place between 1999 and 2012, recorded just two specimens of N. h. cetti, both found basking during the daytime. Similarly, Lunghi and colleagues [28] observed 17 individuals active during the day between 10:08 and 16:42. Finally, the observations of the present study made by the authors, excluding individuals under shelters or found road-killed, all occurred in a daytime context. Since these surveys were taking place only in the daytime, the authors can’t rule out or quantify the extent of the nocturnal nature of N. h. cetti. It is plausible that, like the vast majority of the other members of the genus, the Sardinian grass snake alternates between a diurnal and nocturnal activity pattern according to the variation in biotic and abiotic factors. Adopting a nocturnal lifestyle, especially during summer, would allow N. h. cetti to decrease the risk of predation, loss of body fluids, and overheating, while increasing its foraging success (see [88]).
Various species of amphibians, which tend to be predominantly nocturnal, made up the vast proportion of the Sardinian grass snake’s diet [15,25,89]. Based on the limited data available, the dietary habits of N. h. cetti seem to differ from the habits of mainland grass snakes [89], where the diet of the latter includes a broader variety of prey items, such as fish and other terrestrial vertebrates (e.g., [89,90,91,92,93,94,95,96,97]). In fact, among 12 food items collected from specimens of N. h. cetti, Capula and colleagues [83] retrieved a single non-amphibian prey (Tyrrhenian wall lizard -Podarcis tiliguerta-). The rest of the prey items consisted of six adults and five tadpoles of the Sardinian tree frog (Hyla sarda). Interestingly, tree frogs (Hyla spp.) seem to be rarely preyed on by mainland adults N. natrix s.l., while they appear as part of the diet of juveniles (40–50 cm in length) [89,98]. Tyrrhenian painted frogs (Discoglossus sardus) do not appear in the few food items analysed, but it is probable to expect them in the diet of the subspecies, as also generically indicated in thematic books (e.g., [15,24,25,99]). Caudata amphibians can also be part of the diet of the Sardinian grass snake. Natrix h. cetti has been found within the same cave systems colonised by cave salamanders (Speleomantes spp.) [100]. Gené [58] reported a case of predation involving a brown cave salamander (S. genei) and a juvenile specimen of Sardinian grass snake. Furthermore, Lunghi and colleagues [85] recorded four specimens of N. h. cetti at various distances (15 m, 5.4 m, 75 m, 12 m) from the entrance of sub-horizontal caves, potentially foraging for Speleomantes spp. Natrix h. cetti has also been recorded within rivers inhabited by the Sardinian brook newt (Euproctus platycephalus) [101]. It is then likely that the Sardinian grass snake might represent a potential predator of this endangered amphibian [25]. Data regarding the diet of N. h. cetti are still quite scarce, so it’s possible that this endemic snake might feed up on a wider range of native and introduced species.
There is a tremendous knowledge gap regarding the reproductive biology of N. h. cetti. The taxon is oviparous, and its reproduction cycle likely resembles the one of the mainland N. natrix s.l. [25]. Natricidae snakes are known to form mating balls during early spring, where both male and female reproductive success seems to be positively correlated with body size [102]. At birth, hatchlings of N. h. cetti measure around 15 cm in length [27].
Data regarding predation upon Sardinian grass snakes are lacking. Despite that, generalist predators, such as the wild boar (Sus scrofa) and the hedgehog (Erinaceus europaeus), are known to feed on snakes as do snake-specialists such as the snake eagle (Circaetus gallicus) (e.g., [103,104,105,106]). These may represent potential predators of N. h. cetti.
Natrix natrix s.l. are aglyphous snakes [107] and the presence of oral glands secreting toxins is still under debate (See [108]). In this regard, studies addressing the Sardinian grass snake are totally lacking. With regards to the defence mechanisms towards humans, if disturbed, N. h. cetti may primarily try to flee and, in some cases (e.g., when manipulated and particularly stressed), it adopts behaviours such as hissing, death feigning, and emission of foul-smelling cloacal secretions (MRDN pers. obs.). Although very rare, cases of N. helvetica biting humans exist [108], but to date, they have never been reported for N. h. cetti.
3.1.4. Conservation
Data providing the accurate population and conservation status of the Sardinian grass snake are still scarce, and this is further complicated by the intricate systematic history of this taxon.
This endemic subspecies was classified by the IUCN as “Critically Endangered” due to its restricted distribution and small population size [109]. Ozinga et al. [110] also listed the Sardinian grass snake as “Critically Endangered”. In the same way, Vanni and Cimmaruta [24] reported Natrix helvetica cetti to be “Critically Endangered” based on a population reduction of over 80% (A1ac), a fragmented distribution and occupancy decline (B1 + B2b (i, ii, iv, v)), and a declining effective population size of fewer than 250 individuals (C2a). Within a subsequent national assessment, Andreone et al. [111] listed N. n. cetti as “Vulnerable” to extinction due to its low area of occupancy (less than 2000 km2), its fragmented distribution, and its declining population (B2ab (ii, iv)). In fact, between 1985 and 2006, the occurrence of N. h. cetti declined by half, while later surveys that took place between 2008 and 2013 highlighted an occupancy decline of almost 90% [111]. Andreone and colleagues [111], based on previous taxonomic frameworks, considered the N. h. cetti populations of grass snakes from both Sardinia and Corsica, the latter now classified as N. h. corsa (see taxonomy insights in Section 3.1.1). As the Corsican population appears to harbour a large number of specimens, this could have affected the conservation status of N. n. cetti proposed by Andreone et al. [111]. In the last IUCN national assessment [26], N. h cetti is listed as “Endangered” due to its low and declining area of occupancy (<500 km2) (B2ab (ii)). The Sardinian grass snake is also currently listed under the EU Habitat Directive within Annex IV (92/43/CE) [24].
The exact threats responsible for the decline of N. h. cetti are yet to be defined. One of them seems to be represented by the ecological competition with the possibly introduced viperine snake (N. maura) [24,27]. Indeed, the causes behind the presence of N. maura in Sardinia have been debated for decades. Lanza [75] suggested either a potential anthropogenic introduction onto the island or a natural colonisation during the Messinian event when, due to the desiccation of the Mediterranean Sea, Sardinia and Corsica were connected to mainland Italy and Tunisia [112]. Based on morphological similarities between the Sardinian populations and the African one, Schätti [113] proposed a human-mediated introduction of the former population. In the same way, an African origin and an anthropogenic introduction were suggested by Poggesi and colleagues [114]. More recent genetic analyses highlighted the presence of shared mitochondrial haplotypes between the Sardinian populations and the Tunisian ones [115]. Further sampling confirmed the recent colonisation of Sardinia by N. maura, but this did not produce enough evidence to differentiate between a natural colonisation and a human-mediated one [18]. Regardless, the ecological impact of N. maura on N. h. cetti could be relevant. Although there is no evidence of correlation, in Corsica, where N. maura is not present, N. n. corsa is abundant and widely distributed [15,27], while in Sardinia, N. h. cetti is scarce and generally restricted to areas lacking N. maura [27]. On the island, the two species are characterised by overlapping diets [89], with Hyla sarda representing the main prey item for both N. maura and N. h. cetti [83,116]. Where the two snakes are sympatric, competition for resources seems to favour N. maura, affecting N. h. cetti in terms of abundance, body mass, and growth [27].
Habitat alteration represents another potential threat to the survival of this rare reptile. Within a Habitat Report [117], agricultural intensification has been highlighted as a “highly important pressure” for N. h. cetti. Similarly, Falcucci et al. [118] recorded an increase in heterogeneous agriculture, coupled with the abandonment of pastoral lands and an increase in montane forest cover. Furthermore, Puddu and colleagues [119] showed a significant decrease in agropastoral practices during the last decades, which has led to an exponential increase in the forest cover with detrimental consequences on a large portion of Sardinian biodiversity. Moreover, due to its touristic power, Sardinia has undergone a radical urbanisation process, especially in the proximity of coastal areas [120]. These factors are likely to reduce even more the already fragmented distributional range of N. h. cetti.
Another potential threat considered critical for the survival of the Sardinian grass snake is represented by its potentially reduced genetic diversity [117]. This could be associated with an increase in inbreeding, which could lead to a decline in fitness, reproductive potential, and even local extirpation [121,122]. The effects caused by a loss in genetic diversity are stronger in taxa characterised by small and isolated populations, where the levels of gene flow among populations are scarce [123,124]. Based on current distributional data, N. h. cetti persist in small and isolated populations, thus making this taxon particularly susceptible to the detrimental effects of genetic loss [24,25]. Despite that, no studies have so far investigated the genetic status of the Sardinian grass snakes. The authors believe that the use of cutting-edge genomic techniques should be implemented in order to assess the population structure and the genetic health of this cryptic and endemic reptile. This should be coupled with extensive field investigations aimed at better understanding the ecological habits and exact distribution of N. h. cetti. The implementation of such actions would allow to promptly individuate potential threats to the survival of the Sardinian grass snake and to establish effective conservation measures.
3.2. Distribution Update
We collected a total of 66 verified observations of Natrix helvetica cetti (22 through personal surveys, 44 through collaborators and citizen science), carried out between 2004 and 2023, of which 92% (n = 61) were from 2010 onwards (Table S2). Among these, 97% (n = 64) were eligible for geographic mapping (Figure 2A) and were carried out in an altitude range between 20 e 1407 m a.s.l. (mean value = 586.8 m a.s.l.; see Figure S1). The collected data were merged with those presented by Corti et al. [12] in a 10 × 10 km UTM map: the 10 × 10 km cells affected by the presence of the taxon are 47, of which 13 are from pre-2010 and 34 are from 2010-onwards (Figure 2B).
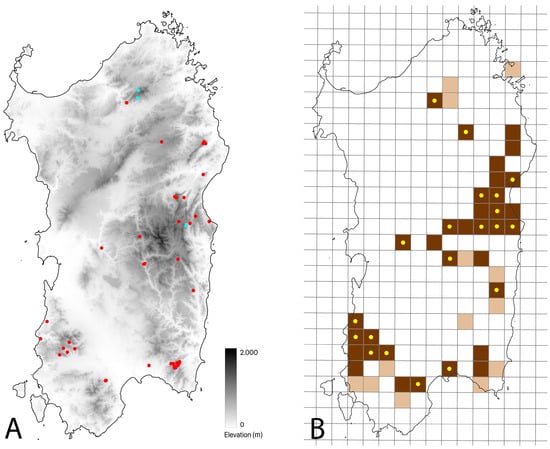
Figure 2.
Map of Sardinia with the 64 observations of Natrix helvetica cetti provided with geographical indications: the red dots represent the post-2010 records, and the blue dots represent the pre-2010 ones (A). A 10 × 10 km UTM map of Sardinia with the updated distribution of N. h. cetti: the brown squares represent records from 2010 onwards, the beige squares represent pre-2010 records; brown squares with yellow dots represent new squares or reconfirmations of pre-2010 data with respect to what is indicated in [12] (B).
Natrix maura occupies 131 UTM cells (of which 98 are known from Corti et al. [12] and 33 from personal observations and citizen science). From the overlay of the UTM distribution maps of N. h. cetti and N. maura, a co-presence emerges in 31 cells, equal to 66% of the total number of cells occupied by N. h. cetti and to 24% of the total number of cells occupied by N. maura (Figure S2).
According to Corti et al. [12], cave salamanders occupy a total of 61 UTM cells (Speleomantes flavus = 4, S. supramontis = 12; S. imperialis = 23; S. sarrabusensis = 5; S. genei = 17). From the overlay of the UTM distribution maps of N. h. cetti and Speleomantes spp., a co-presence emerges in 33 cells, equal to 70% of the total number of cells occupied by N. h. cetti and to 54% of the total number of cells occupied by Speleomantes spp. Specifically, N. h. cetti occurs in 3 out of 4 cells (75%) where S. flavus is present; in 10 out of 12 cells (83%) where S. supramontis is present; in 4 out of 23 cells (17%) where S. imperialis is present; in 3 out of 5 cells (60%) where S. sarrabusensis is present; and in 11 out of 17 cells (65%) where S. genei is present (Figure S3). Still in agreement with Corti et al. [12], the Sardinian brook newt occupies a total of 37 UTM cells. From the overlay of the UTM distribution maps of N. h. cetti and Euproctus platycephalus, a co-presence emerges in 18 cells, equal to 38% of the total number of cells occupied by N. h. cetti and to 48% of the total number of cells occupied by E. platycephalus (Figure S3). Ultimately, the co-presence between the Sardinian grass snake and at least one urodelan species occurs in 37 cells, equal to 79% of the total number of cells occupied by N. h. cetti.
3.3. Habitat Suitability Estimation
The models computed showed high discriminatory ability and low overfitting, with AUC values between 0.839 and 0.912 and OR10 values consistently below the 10% threshold (Table 2). Although all models led to robust statistical results, those including altitude as a predictor variable showed slightly higher AUC and lower OR. When included, altitude was, in fact, the most influential predictor for the distribution of Natrix helvetica cetti, followed by precipitation of the warmest quarter (bio18) and, in models using a reduced set of climatic variables, temperature seasonality (bio4). Based on the occurrences used for training the models, the distribution of the taxon showed a negative response to temperature variables (bio1–bio11, bio4 excl.) and a positive response to precipitation variables (bio12–bio19; bio15 excl.), whereas the most suitable land classes where the ones related to forested and herbaceous habitats (see Figure S4 for details).

Table 2.
Statistical results of the six bioclimatic suitability models computed in MaxEnt. Mean (±SD) area under curve (AUC) values higher than 0.75 indicate good model quality, with values over 0.9 indicating excellent predictive performance (Fielding and Bell, 1997 [38]). A mean (±SD) 10th percentile training omission rate (OR10) lower than the predicted 0.1 denotes no overfitting (Boria et al., 2014 [39]). The OR10 threshold was used to set the minimum suitability values for the relative model (Figure S5).
There was high consistency across the six models in the areas predicted as highly suitable for N. h. cetti in Sardinia (Figure S5). Models built upon the reduced set of bioclimatic variables showed a lower OR10 threshold for minimum suitability compared with models built with all bioclimatic variables (Table 2). Therefore, these models predicted a more widespread suitability for the taxon, although with arguably low suitability values (<0.15). These differences were minimised when adding altitude and then land cover as predictor variables to the models. The average model shows areas predicted as suitable for the taxon by all six models, using the mean OR10 (0.138) as the threshold for minimum suitability (Figure 3). This model predicted high suitability in the southeast of the island, specifically in the eastern part of the “Campidano di Cagliari” sub-region bordering the southern sector of the “Sarrabus-Gerrei”, and in the central-east of the island, especially in the “ Ogliastra” and secondly in the “Barbagia di Seulo”, in the southern portion of the “Barbagia di Nuoro” and in the eastern sectors of the “Barbagia di Belvì” and “Mandorlisai”. Further areas of high suitability are found in the northeast of the island, in the centre of the “Baronie” sub-region, and in the north of the island between the “Gallura” and “Monte Acuto” sub-regions. Finally, in the south-western sector of the island, two areas of medium-low suitability were highlighted in the south-east and in the north-west of the “Sulcis-Iglesiente”, with the latter case also encroaching on the southern sector of the “Monreale” (Figure S6). Figure S7 shows the predicted bioclimatic suitability areas compared to the updated distribution of N. h. cetti.
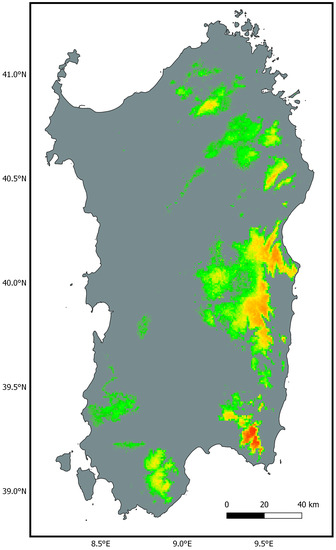
Figure 3.
Predicted bioclimatic suitability for Natrix helvetica cetti in Sardinia. Warmer colours indicate higher suitability and grey areas fall below the minimum suitability threshold.
3.4. Dorsal Pattern Characterisation
On the basis of the prevalent trend of the dorsal motif observed in each considered snake, four different patterns have been identified for the Sardinian grass snake (Figure 4):

Figure 4.
Examples of the four categories into which the dorsal patterns of Natrix helvetica cetti have been grouped: category I (A), category II (B), category III (C) and category IV (D).
(I) Prevalence of thin lateral spots, sometimes light mottled, which do not reach the vertebral line; “third” row of spots along the vertebral line, tendentially offset from the lateral ones.
(II) Prevalence of lateral spots with an evident light central area, which reach the vertebral line, staggered or aligned (predominantly ocelli appearance).
(III) Tending to abundism: prevalence of thick lateral spots with reduced or absent light central area, which reach the vertebral line, staggered or aligned (predominantly bar-like appearance, or ocelli with thick border and narrow centre).
(IV) Melanic: uniformly dark dorsal colouration, with more or less visible dorsal pattern.
A total of 56 snakes were eligible for the pattern characterisation. Of these, 25% (n = 14) fell into cat. I, 46.43% (n = 26) in cat. II, 12.5% (n = 7) in cat. III, and 16.07% (n = 9) in cat. IV.
Out of 56 individuals with the dorsal pattern characterised, 54 had coordinates and have been computed in the 10 × 10 km UTM maps (Figure S8).
3.5. Principal Component Analysis
The result of the principal component analysis (PCA) of Natrix helvetica cetti shows an apparent differentiation of the bioclimatic niche of the taxon compared to the background environment (Figure 5). The first two PCA dimensions explain 52.7% (Dim1) and 28.2% (Dim2) of the variation observed in the data. Mean annual temperature (bio1) and precipitation of the warmest quarter (bio18) contribute mostly to the first dimension, whereas precipitation seasonality (bio15) and, to a lesser extent, temperature seasonality (bio4) explain most of the variation along the second dimension (Figure 5A). The PCA shows a certain degree of bioclimatic variation for the taxon, especially with regard to the first dimension. Individuals of N. h. cetti were found in areas characterised by lower temperatures, higher summer precipitations, lower precipitation seasonality, and higher temperature seasonality compared to the background environment. However, there is no apparent separation between the bioclimatic niche occupied by individuals with different dorsal patterns (Figure 5B).
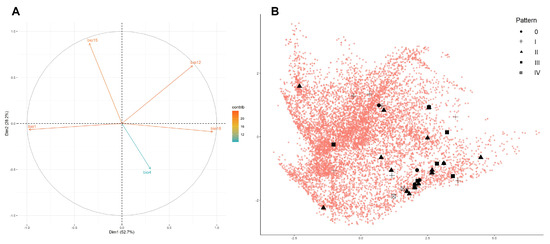
Figure 5.
Principal component analysis of Natrix helvetica cetti in Sardinia. The first and second dimensions explain 52.7% and 28.2% of the variation observed in the data, respectively. (A) Bioclimatic variables used for defining the environmental space (warmer colours indicate higher variable contribution). (B) Occurrence records divided by dorsal pattern (black shapes) and represented in bidimensional environmental space against randomly sampled background points (red dots).
4. Discussion
4.1. Natural History Review
Natrix natrix s.l. is a taxon with a complex taxonomic history, but various molecular studies carried out in recent years, including a recent genomic work, have helped to shed light on the phylogenetic relationships (e.g., [20,21,22,51,52,53,54,55]). This also applies to N. helvetica cetti, a taxon currently considered Sardinian endemic, but which could undergo updates regarding the inclusion of Corsican populations, currently kept separate due to the greater rarefaction of the Sardinian ones, which would need greater conservation attention [22,23].
From a morphological point of view, there are data for Sardinian populations mainly concerning morphometry, pholidosis, and even internal anatomy, while information on chromatic variability is still not very thorough (see [15,24,25,28,45,69,70,83]).
The ecological aspects of the Sardinian grass snake are still largely unknown and the little information in our possession comes from a limited number of studies, also concerning a small number of snakes. For example, more information on phenology, diet, and reproduction would be needed.
From a conservation point of view, N. h. cetti is a taxon adequately protected both by laws and categorisations in the global and national IUCN red lists. However, a greater effort in monitoring the populations would be useful in order to gain more precise information on their status. To date, there is no health and epidemiological information for Sardinian grass snakes. Recently, the European Food Safety Authority’s funder project “AMPHIDEB” aims to develop biologically-based models for environmental risk assessment and to assess the impact of chemicals and pathogenic fungi on amphibian and reptile populations (see [125]). In this context, individuals of N. h. cetti are being monitored for ophidiomycosis, an emerging infectious disease caused by an ascomycete fungus (see [126]) whose presence has recently been ascertained in Italy, involving the genus Natrix [127].
4.2. Distribution Update
Species geographic distribution is largely used during conservation assessments [128,129] and understanding the real extent of species’ geographic ranges is thus fundamental in order to reliably determine their conservation status and, if necessary, designate appropriate protected areas [7,130,131]. In the case of elusive and rare taxa occurring at low densities, the extent of their geographic range may be underestimated due to their low detectability and the occurrence of observer biases [132,133,134,135]. Here, combining personal survey effort, citizen science records and literature searches, we provided the most up-to-date geographic distribution of the Sardinian grass snake.
The Sardinian grass snake is characterised by a highly restricted and fragmented geographic range that mainly encompasses the mountainous areas of eastern and southern Sardinia [15,24,25]. Recent work by Corti and colleagues [12] aimed to update the distribution of N. h. cetti, as well as other Sardinian reptile and amphibian species, using distributional records extrapolated from the literature and surveys conducted between 2005 and July 2022. The authors reported the presence of Natrix helvetica cetti within 34 UTM cells, 14 (41.18%) of which were characterised by recent (from 2010 onwards) observational records, while the remaining 20 UTM cells (58.82%) included older (before 2010) observational records.
Our results highlighted the presence of the taxon within a total of 47 UTM cells (+13 UTM cells, an increase of +38.2%) (Figure 2B). The number of cells containing old unconfirmed observational records (before 2010) slightly differed from what was recorded by Corti et al. [12] (13 vs. 20, respectively). In addition, our dataset was characterised by more than a twofold increase in the number of cells harbouring recent (from 2010 onwards) observational data in comparison with what was highlighted by Corti and colleagues [12] (34 vs. 14, respectively). Notably, we were able to re-confirm the presence of N. h. cetti within 7 cells containing old observational data [12] and record the presence of the taxon in 13 previously overlooked cells, expanding in this way its known geographic occurrence quite significantly.
Natrix h. cetti is known in an altitudinal range between sea level and about 1000 m a.s.l., with a prevalent distribution in hilly and medium-mountainous areas [15,24,25], and the altitude record was 1029 m a.s.l. [28]. From the data available in the present study, the altitude limit rises to 1407 m a.s.l., while most of the records are within an altitude range between 440 and 730 m a.s.l. (Figure S1), confirming a mainly hilly and medium-mountainous distribution.
The discrepancy found between our results and those from previous studies may be due to a series of interacting factors. In the first place, there is no doubt that N. h. cetti occurs at low densities within its geographic range, making it extremely difficult to detect specimens even during tailored surveys ([31,85,134,136]; Di Nicola pers. obs.). Furthermore, the low detectability of the taxon may be also correlated with its still poorly understood activity pattern (see Section 3.1.3). In particular, the potential nocturnal nature of N. h. cetti may negatively affect the detectability and the encounter rate of the taxon [83]. The implementation of nocturnal surveys could provide conservation managers with new critical geographic data and contribute to exploring variations in the activity pattern of the Sardinian grass snakes throughout the active season, as has been conducted with other snake taxa (e.g., [137]). Finally, we believe that, aside from the authors’ personal survey effort, the richness of our dataset was partly associated with the exploitation of observational data obtained from citizen science (constituting 66.7% of the records). The exponential growth of these platforms during the last few years has in some cases allowed them to fill critical distributional knowledge gaps, even in the case of rare and rarely recorded species [138,139,140,141]. Accordingly, the authors acknowledge that citizen science platforms have an enormous potential for better understanding the real extent of the geographic distribution of N. h. cetti, especially taking into consideration the fact that Sardinia is becoming a popular destination for nature-oriented tourism [142,143].
4.3. Habitat Suitability Estimation
Understanding the role of bioclimatic variables in shaping rare and elusive species’ geographic range via the implementation of niche modelling methods is fundamental to better direct survey efforts towards unoccupied areas of potentially suitable habitat [144,145,146].
Our bioclimatic niche modelling analysis recovered the variable “altitude” (bio20) as the strongest predictor for the geographic distribution of Natrix helvetica cetti. This seems to agree with what is currently known about the ecology of the taxon. The Sardinian grass snakes seem in fact to mainly inhabit mid-elevation montane areas [25,28,34,86], as further highlighted by our altitudinal records (see Section 4.2). Nevertheless, specimens of Sardinian grass snakes have been found from the sea level up to over 1000 m a.s.l (see novel altitudinal record) [15,28].
When the variable “altitude” was excluded from the analysis, the “precipitation of the warmest quarter” (bio18) variable appeared as the most influential predictor for the distribution of the taxon (Figure S4). Taking into consideration that a vast proportion of Sardinia is characterised by dry conditions during the warmest quarter of the year, with levels of evaporations that surpass the precipitations [147], the tendency of N. h. cetti to inhabit areas with high rates of precipitations during the warmer period of the year may be correlated with the presence of water-dependent trophic resources (amphibians) [27].
More generally, the positive correlation with precipitation variables highlighted by our model may explain the predominantly eastern-restricted geographic distribution of N. h. cetti [15,24]. The eastern side of Sardinia is, in fact, characterised by higher rates of precipitation (mean annual precipitation and mean annual number of rainy days) compared to the western side of the island [148].
On the other hand, our model recovered a negative correlation with temperature bioclimatic variables. In particular, the distribution of N. h. cetti was negatively correlated with the mean annual temperature (bio1) and the temperature seasonality (bio4) variables. The latter negative correlation is likely linked to the high evaporation rate discussed above and consequently to the availability of trophic resources.
Finally, forested and herbaceous land cover categories were highlighted by our model as the most suitable for N. h. cetti (Figure S4). Individuals of Sardinian grass snakes are commonly found within forested areas [15,24,25] where high levels of humidity and soil moisture correlated with evapotranspiration patterns may influence amphibian abundance [149,150]. In contrast, herbaceous habitats seem to be poorly exploited by N. h. cetti. In fact, these habitats are highly subject to humidity loss and aridification [150,151]. Thus, we believe that the high response towards herbaceous land cover recovered by our model may be due to the resolution of the available land cover raster, which does not allow the distinction of microhabitats within larger herbaceous openings [152].
Indeed, the present results constitute a conservative, coarse-scale representation of the climatic suitability of the taxon on the island. Future studies should focus on gathering additional information regarding the local environment in which individuals are found. This would allow us to further refine the current models by implementing data on microhabitats (i.e., distance from small water courses, canopy cover, soil type, etc.), ecology (i.e., presence/absence of local prey/predators), and human impacts (i.e., land cover change, habitat disturbance, etc.) at a finer scale.
During the past decades, Sardinia has undergone a drastic decline in the rate of precipitation and a critical increase in temperature [153,154,155]. This has potentially reduced the suitable habitat for N. h. cetti, with consequent drastic population decline (red list). A further decrease in precipitation and a further increase in temperature are expected for Sardinia under future climatic scenarios [156]. Furthermore, future climatic variations are expected to negatively impact the distribution of the forested areas in Sardinia, thus further reducing the available habitat for N. h. cetti [156].
Climate change represents a serious threat to the persistence of N. h. cetti, and we argue that intensive monitoring is necessary to assess future distributional shifts and population contractions.
4.4. Geographic Distribution of Potential Competitors and Trophic Resources
In addition to climatic factors, species’ geographic distribution is limited by competition with other taxa and influenced by the presence of trophic resources.
Here we compared distributional data regarding Natrix helvetica cetti with those from a potential competitor, the viperine snake (N. maura), and a potentially overlooked trophic resource, urodeles (Speleomantes spp. and Euproctus platycephalus).
Regarding the potential ecological competitor, our analyses, based on distributional data from Corti et al. [12], highlighted a striking overlap between the geographic range of N. h. cetti and the graphic range of N. maura. In fact, 31 UTM cells appeared to be shared between the two taxa (Figure S2), corresponding to 66% of the geographic range of the endangered Sardinian grass snake. It’s expected that niche partition and differences in habitat choice should reduce the competition by limiting sympatric occurrence between the endemic N. h. cetti and the possibly introduced N. maura [27]. Among these factors, altitude seems to play a crucial role, with the former taxon mainly inhabiting hilly and mid-mountainous areas and the latter species preferring humid areas from the sea level up to 660 m a.s.l. [15,24,25,157]. When the two taxa are found sympatrically, N. maura seems to outcompete N. h. cetti, with negative consequences on the fitness of the latter (see Section 3.1.4; [27]). Furthermore, changes in climatic conditions are likely to produce an altitudinal shift in the geographic range of N. maura, enhancing the sympatry between the two reptiles [158,159]. Under this scenario, the interspecific competition between the two taxa is likely to increase with potentially detrimental consequences on the conservation status of the already declining N. h. cetti [27]. While the use of UTM cells to investigate the overlapping range of the two snake species is limited by the spatial resolution of the data, our results still advocate for the need for surveys aimed at assessing shifts in the geographic range of N. maura and the consequent ecological and geographical response of the endemic N. h. cetti.
Additionally, we assessed the geographic overlap between the Sardinian grass snake and the six species of urodelan amphibians (five species of cave salamanders, Speleomantes spp., and the Sardinian brook newt, Euproctus platycephalus) endemic to the island (Figure S3).
The results highlighted how a vast proportion of the geographic range of N. h. cetti, 33 out of the 47 UTM cells (70%), was interested in the presence of Speleomantes spp.
Unsurprisingly, a third of the communal UTM cells shared between the Sardinian grass snake and cave salamanders were interested in the presence of the brown cave salamander (S. genei) (11/33 UTM cells). The latter species is in fact characterised by one of the largest distributional ranges among all the Sardinian cave salamanders, in an altitude range between 8 and 600 m [12,15]. On the other hand, an unexpectedly high level of geographic overlap was found between N. h. cetti and the Supramonte cave salamander (S. supramontis) (10/33 UTM cells). This latter species is characterised by a highly restricted geographic range where it inhabits a wide variety of habitats from 106 to 1360 m a.s.l. [15], potentially suitable for the sympatric presence of N. h. cetti. The high level of geographic overlap between N. h. cetti and the five species of Sardinian cave salamanders may highlight the presence of an overlooked and crucial trophic resource for the former taxon. Individuals of Sardinian grass snake have been found within cave areas inhabited by Speleomantes spp. [85,100] and predatory episodes have been recorded [58]. Despite the limitation of UTM cells, we believe that further work aimed to investigate the tropic relationship between N. h. cetti and Speleomantes spp. may have drastic implications on the conservation of all the involved taxa.
The Sardinian brook newt occupies 38% of the cells where N. h. cetti is reported. This amphibian prefers aquatic environments with fresh and well oxygenated water, in an altitude range from 50 to 1,800 m a.s.l. (especially between 400 and 800 m a.s.l.) [15], contexts that are also suitable for N. h. cetti, for which the newt probably represent a prey, although not yet ascertained.
Anyway, the overlapping distribution between the Sardinian grass snake and the six species of urodelan ampbibians may be correlated with the biogeographic history of the island and the formation of significant biogeographic barriers. This peculiar distributional pattern of endemic taxa has been further highlighted by the recently published work of Corti and colleagues [12].
The highlighted overlap geographical pattern may represent a unique conservation opportunity. In fact, the institution of protected areas for the Sardinia grass snake is likely to have beneficial impacts on the endemic and threatened six urodelan species.
4.5. Geographic and Environmental Influence on Dorsal Pattern Variation
Snakes’ dorsal patterns have been shown to provide crucial antipredatory functions, such as crypsis, aposematism, and mimicry [160,161,162,163,164,165]. Furthermore, the amount of dark pigments within dorsal patterns has been demonstrated to influence an individual’s thermoregulatory abilities [166].
Consequently, an interplay of various selective forces is expected to shape intraspecific pattern variability, acting on a trade-off between predator avoidance and thermal benefits [158,167,168].
Here, we tentatively investigated the influence of distributional and environmental variables on the high dorsal pattern variation observed in the Sardinian grass snake. Nevertheless, taking into consideration the low encounter rate that characterises Natrix helvetica cetti, we potentially produced and analysed the largest dataset regarding the phenotypic variation in the Sardinian grass snake to date.
Firstly, no geographic correlation was highlighted by our analyses as the assessed dorsal pattern categories seem to be randomly distributed across the geographic range of the taxon (Figure S8). Furthermore, geographical overlapping, in terms of shared UTM cells, between individuals characterised by different dorsal patterns was frequently observed. The “ocellated” dorsal pattern (cat. II) appeared as the most common dorsal pattern category, characterising 46% of the assessed individuals across 13 UTM cells. This was followed by the “thinly banded” dorsal pattern (cat. I) (11 UTM cells). The historical presence of Vipera aspis complex members on the island until the late Pliocene [169,170] is highly unlikely to explain a potential mimicry function of these widely diffused dorsal patterns (e.g., [164]). On the other hand, it is possible that these dorsal pattern categories may be beneficial in terms of crypsis, promoting predator avoidance [162,171]. In this regard, the “banded” dorsal pattern (cat. III) may balance the trade-off between thermal advantages and predator detection [166]. Nevertheless, the lack of a defined bioclimatic niche (Figure 5) and the low geographic distribution of the latter dorsal pattern category (7 UTM cells), seem to not support this hypothesis.
Furthermore, our PCA analysis failed to find any evident distinction between the assessed dorsal pattern categories based on the evaluated bioclimatic variables (Figure 5B). It is possible that this phenomenon may be due to the active maintenance of polymorphism within populations [172,173,174]. Moreover, predation avoidance rather than climatic niche segregation may represent another potential explanation for our results [175].
Interestingly, despite the close geographic clustering observed between individuals characterised by the melanistic dorsal pattern (cat. IV) (Figure S8), our PCA analysis did not highlight any significant distinction between this and the other dorsal patterns based on the bioclimatic variables considered. Melanism in snakes has been largely associated with thermoregulatory benefits [176,177,178]. For example, Bury et al. [168] found a negative correlation between melanistic individuals of N. natrix and bioclimatic variables such as spring and winter temperatures. Nevertheless, harder-to-detect selective forces have been shown to influence the intrapopulation abundance of melanistic snakes [179]. Data density represents a crucial factor for the reliability of PCA analysis [180]. The scarce number of melanistic individuals within our dataset may explain the inconclusive results of our PCA analysis. Despite this, taking into consideration the restricted geographic area where the vast majority of the melanistic N. h. cetti occur, it is possible that other mechanisms rather than the influence of bioclimatic factors may be responsible for the abundance of melanistic individuals (e.g., [176]).
5. Conclusions
Natrix helvetica cetti is an insular endemic grass snake subspecies for which many ecological aspects, as well as the actual distribution, are still poorly understood. The low detectability of the taxon and the scarcity of targeted field surveys probably led to this lack of information.
Exploiting some herpetological surveys by two of the authors and citizen science records, the present work had the main purpose of providing updated distribution data for the taxon, as well as giving information on habitat suitability and on potential distribution through bioclimatic niche modelling. Second, a preliminary characterization of the dorsal pattern was made with an attempt to explore the pattern of geographic segregation and to evaluate the influence of bioclimatic variables.
The fact that much of the data comes from citizen science has limited its potential for use. For example, it was not possible to take advantage of photos taken in standardized conditions or to know the morphometries, sex, microhabitat, and precise context of discovery for all the animals. Therefore, it will be important to carry out further field surveys aimed at acquiring this data and at implementing the ecological knowledge of the species, also carrying out investigations on the diet, of which little is known, and on the reproductive biology, for which information is almost totally lacking. The non-invasive acquisition of a set of environmental parameters will also be important (see [181]), such as body temperature, environmental temperature, illumination, and ultraviolet flux at the place of detection of an individual, as well as carrying out parasitological investigations (in addition to those underway for ophidiomycosis; see Section 4.1). Other ecological aspects, the knowledge of which will be fundamental for planning conservation strategies for the Sardinian grass snake, are the sheltering behaviour, the seasonal activity, the habitat trophism, the predation pressure, and the possible human mortality rate. By obtaining all the above information, it would be possible to provide targeted suggestions on the maintenance of populations, e.g., supporting the food supply and competition, controlling the competition with allochthonous and synanthropic animals, reducing the influence of nuisance factors, creating shelters and, although it is probably a limited phenomenon given the elusiveness and distribution of the species, preventing death on the roads.
As highlighted by our mapping results, the Sardinian grass snake seems to be characterised by a highly fragmented and limited geographic distribution. Unfortunately, the lack of historical data on the past distribution and abundance of this snake may have led to underestimating its current decline, and therefore, its current conservation status. Furthermore, this phenomenon may be even worsened by the almost complete lack of quantitative data regarding patterns of intraspecific competition with the possibly introduced viperine snake.
Therefore, we believe that an integrative approach is needed in order to assess the real extent of the decline of Natrix helvetica cetti and, if necessary, to put in place effective conservation measures.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/life13091867/s1, Table S1: Valid Natrix natrix complex subspecies, and their approximate distribution ranges, according to Fritz and Schmidtler (2020) and Fritz and Ihlow (2022); Table S2: List of verified observations of Natrix helvetica cetti considered in the study; Figure S1: Altitudinal plot of the 64 Natrix helvetica cetti observations provided with geographic information; Figure S2: 10 × 10 km UTM map of Sardinia with the distribution of Natrix helvetica cetti (brown squares: post-2010 records; beige squares: pre-2010 records) and N. maura (yellow circles); Figure S3: 10 × 10 km UTM map of Sardinia with the distribution of Natrix helvetica cetti (brown squares: post-2010 records; beige squares: pre-2010 records) and urodeles (blue dots: Speleomantes flavus; red dots: S. supramontis; green dots: S. imperialis; yellow dots: S. sarrabusensis; violet dots: S. genei; yellow circles: Euproctus platycephalus).; Figure S4: Response curves showing the change in bioclimatic suitability for Natrix helvetica cetti (y axes) in response to variation in the predictor variables (x axes) used in the models; Figure S5: Predicted bioclimatic suitability for Natrix helvetica cetti in Sardinia based on six model combinations; Figure S6: Predicted bioclimatic suitability for Natrix helvetica cetti in Sardinia (see Figure 3 in the main document) superimposed on the map of Sardinian sub-region; Figure S7: Predicted bioclimatic suitability for Natrix helvetica cetti in Sardinia (see Figure 3 in the main document) superimposed on the 10 × 10 km UTM map with the updated distribution of the taxon (see Figure 2A in the main document); Figure S8: Distribution of the four different Natrix helvetica cetti dorsal patterns in the 10 × 10 km UTM map of Sardinia.
Author Contributions
Conceptualization, M.R.D.N.; methodology, M.R.D.N., A.V.P., G.R. and G.M.; data collection, M.R.D.N. and S.M.; formal analysis, M.R.D.N. and G.M.; writing—original draft, M.R.D.N., A.V.P., S.M., F.P.F. and G.M.; writing—review and editing, M.R.D.N., A.V.P., F.P.F., J.L.M.C.D. and G.M.; visualization, M.R.D.N., A.V.P., J.L.M.C.D. and G.M.; supervision, M.R.D.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Records by the authors were carried out in field surveys authorized by the following permits: MATTM reg. 0012442, 13.06.2017; Prot. ISPRA 25913, 26.5.2017. Regione Autonoma della Sardegna Prot. N. 14071 Rep. N. 390 del 04.07.2017; MITE reg. 0024526, 28.2.2022; Prot ISPRA 0009384/2022 del 23.02.2022; Regione Autonoma della Sardegna Prot. N. 6747 del 14-03-2022.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data (i.e., exact find coordinates) are not publicly available due to conservation reasons for the taxon involved.
Acknowledgments
We would like to thank all the people who have kindly provided us with their finding data of the Sardinian grass snakes as well as the photos of the individuals, which were used to confirm the identification and for the categorization of patterns: Ciro Amata, Bobby Bok, Henrik Bringsøe, Giacomo Calvia, Mara Casti, Ettore Cavalli, Davide Cocco, Corrado Conca, Marco Colombo, Gottardo Crameri, Dino Deiana, Luca Deiana, Chiara Delugas, Francesca Fiori, Giovanni Giuseppe Floris, Umberto Fusini, Lorenzo Laddaga, Manuela Lai, Manuela Mulargia, Manu Nijenhuis, Gianluca Onnis, Denis Orrù, Silvia Pelti, Cinzia Petrangeli, Marta Pinna, Silvia Piroddi, Pier Ivano Podda, Fabiano Rota, Ivana Scalas, Andre Schmid, Fabio Secci, Sabrina Sois, Jeroen Speybroeck, Matteo Tauriello, Giulia Tessa, Davide Tolu and Laurent Vallotton.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Schmeller, D.S.; Gruber, B.; Budrys, E.; Framsted, E.; Lengyel, S.; Henle, K. National Responsibilities in European Species Conservation: A Methodological Review. Conserv. Biol. 2008, 22, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Burlakova, L.E.; Karatayev, A.Y.; Karatayev, V.A.; May, M.E.; Bennett, D.L.; Cook, M.J. Endemic Species: Contribution to Community Uniqueness, Effect of Habitat Alteration, and Conservation Priorities. Biol. Conserv. 2011, 144, 155–165. [Google Scholar] [CrossRef]
- Le Galliard, J.F.; Massot, M.; Baron, J.-P.; Clobert, J. Ecological Effects of Climate Change on European Reptiles. Wildl. Conserv. Chang. Clim. 2012, 179, 203. [Google Scholar]
- Maiorano, L.; Amori, G.; Capula, M.; Falcucci, A.; Masi, M.; Montemaggiori, A.; Pottier, J.; Psomas, A.; Rondinini, C.; Russo, D.; et al. Threats from Climate Change to Terrestrial Vertebrate Hotspots in Europe. PLoS ONE 2013, 8, e74989. [Google Scholar] [CrossRef]
- Manes, S.; Costello, M.J.; Beckett, H.; Debnath, A.; Devenish-Nelson, E.; Grey, K.-A.; Jenkins, R.; Khan, T.M.; Kiessling, W.; Krause, C.; et al. Endemism Increases Species’ Climate Change Risk in Areas of Global Biodiversity Importance. Biol. Conserv. 2021, 257, 109070. [Google Scholar] [CrossRef]
- Syfert, M.M.; Joppa, L.; Smith, M.J.; Coomes, D.A.; Bachman, S.P.; Brummitt, N.A. Using Species Distribution Models to Inform IUCN Red List Assessments. Biol. Conserv. 2014, 177, 174–184. [Google Scholar] [CrossRef]
- Mota-Vargas, C.; Rojas-Soto, O.R. The Importance of Defining the Geographic Distribution of Species for Conservation: The Case of the Bearded Wood-Partridge. J. Nat. Conserv. 2012, 20, 10–17. [Google Scholar] [CrossRef]
- Marcer, A.; Sáez, L.; Molowny-Horas, R.; Pons, X.; Pino, J. Using Species Distribution Modelling to Disentangle Realised versus Potential Distributions for Rare Species Conservation. Biol. Conserv. 2013, 166, 221–230. [Google Scholar] [CrossRef]
- Guisan, A.; Tingley, R.; Baumgartner, J.B.; Naujokaitis-Lewis, I.; Sutcliffe, P.R.; Tulloch, A.I.T.; Regan, T.J.; Brotons, L.; McDonald-Madden, E.; Mantyka-Pringle, C.; et al. Predicting Species Distributions for Conservation Decisions. Ecol. Lett. 2013, 16, 1424–1435. [Google Scholar] [CrossRef]
- Mizsei, E.; Üveges, B.; Vági, B.; Szabolcs, M.; Lengyel, S.; Pfliegler, W.P.; Nagy, Z.T.; Tóth, J.P. Species Distribution Modelling Leads to the Discovery of New Populations of One of the Least Known European Snakes, Vipera ursinii graeca, in Albania. Amphibia-Reptilia 2016, 37, 55–68. [Google Scholar] [CrossRef]
- Camarda, I.; Falchi, S.; Nudda, G. Aspetti Geografici Della Sardegna Nell’ambito Del Mediterraneo. In L’ambiente Naturale in Sardegna; Libreria Della Natura: Milano, Italy, 1988; pp. 35–36. ISBN 978-88-7138-131-2. [Google Scholar]
- Corti, C.; Biaggini, M.; Nulchis, V.; Cogoni, R.; Cossu, I.M.; Frau, S.; Mulargia, M.; Lunghi, E.; Bassu, L. Species Diversity and Distribution of Amphibians and Reptiles in Sardinia, Italy. Acta Herpetol. 2022, 17, 125–133. [Google Scholar] [CrossRef]
- Blondel, J.; Aronson, J.; Bodiou, J.-Y.; Boeuf, G. (Eds.) The Mediterranean Region: Biological Diversity in Space and Time, 2nd ed.; Oxford Biology; Oxford University Press: Oxford, UK; New York, NY, USA, 2010; ISBN 978-0-19-955799-8. [Google Scholar]
- Salvi, D.; Bombi, P. Reptiles of Sardinia: Updating the Knowledge on Their Distribution. Acta Herpetol. 2010, 5, 161–177. [Google Scholar] [CrossRef]
- Di Nicola, M.R.; Mezzadri, S. Anfibi e Rettili Di Sardegna: Guida Fotografica; Libreria Della Natura: Milano, Italy, 2018; ISBN 88-909788-7-2. [Google Scholar]
- Sindaco, R.; Razzetti, E. An Updated Check-List of Italian Amphibians and Reptiles. Nat. Hist. Sci. 2021, 8, 35–46. [Google Scholar] [CrossRef]
- Faraone, F.P.; Melfi, R.; Di Nicola, M.R.; Giacalone, G.; Lo Valvo, M. Phylogenetic Relationships of the Italian Populations of Horseshoe Whip Snake Hemorrhois hippocrepis (Serpentes, Colubridae). Acta Herpetol. 2020, 15, 129–135. [Google Scholar] [CrossRef]
- Guicking, D.; Joger, U.; Wink, M. Molecular Phylogeography of the Viperine Snake Natrix maura (Serpentes: Colubridae): Evidence for Strong Intraspecific Differentiation. Org. Divers. Evol. 2008, 8, 130–145. [Google Scholar] [CrossRef][Green Version]
- Mezzasalma, M.; Dall’Asta, A.; Loy, A.; Cheylan, M.; Lymberakis, P.; Zuffi, M.A.L.; Tomović, L.; Odierna, G.; Guarino, F.M. A Sisters’ Story: Comparative Phylogeography and Taxonomy of Hierophis viridiflavus and H. gemonensis (Serpentes, Colubridae). Zool. Scr. 2015, 44, 495–508. [Google Scholar] [CrossRef]
- Fritz, U.; Corti, C.; Päckert, M. Mitochondrial DNA Sequences Suggest Unexpected Phylogenetic Position of Corso-Sardinian Grass Snakes (Natrix cetti) and Do Not Support Their Species Status, with Notes on Phylogeography and Subspecies Delineation of Grass Snakes. Org. Divers. Evol. 2012, 12, 71–80. [Google Scholar] [CrossRef]
- Schöneberg, Y.; Winter, S.; Arribas, O.; Di Nicola, M.R.; Master, M.; Owens, J.B.; Rovastos, M.; Wuster, W.; Janke, A.; Fritz, U. Genomics Reveals Broad Hybridization in Deeply Divergent Palearctic Grass and Water Snakes (Natrix spp.). Mol. Phylogenet. Evol. 2023, 184, 107787. [Google Scholar] [CrossRef]
- Schultze, N.; Spitzweg, C.; Corti, C.; Delaugerre, M.; Di Nicola, M.R.; Geniez, P.; Lapini, L.; Liuzzi, C.; Lunghi, E.; Novarini, N.; et al. Mitochondrial Ghost Lineages Blur Phylogeography and Taxonomy of Natrix helvetica and N. natrix in Italy and Corsica. Zool. Scr. 2020, 49, 395–411. [Google Scholar] [CrossRef]
- Fritz, U.; Schmidtler, J.F. The Fifth Labour of Heracles: Cleaning the Linnean Stable of Names for Grass Snakes (Natrix astreptophora, N helvetica, N. natrix Sensu Stricto). Vertebr. Zool. 2020, 70, 621–665. [Google Scholar] [CrossRef]
- Vanni, S.; Cimmaruta, R. Natrix cetti. In Fauna d’Italia Reptilia; Corti, C., Capula, M., Luiselli, L., Sindaco, R., Razzetti, E., Eds.; Calderini: Bologna, Italy, 2010; Volume XLV, pp. 538–545. [Google Scholar]
- Di Nicola, M.R.; Cavigioli, L.; Luiselli, L.; Andreone, F. Anfibi & Rettili d’Italia. Edizione Aggiornata; Edizioni Belvedere: Latina, Italy, 2021. [Google Scholar]
- Rondinini, C.; Battistoni, A.; Teofili, C. Lista Rossa IUCN Dei Vertebrati Italiani 2022; Comitato Italiano IUCN e Ministero dell’Ambiente e della Sicurezza Energetica: Roma, Italy, 2022. [Google Scholar]
- Luiselli, L. Resource Partitioning and Interspecific Competition in Snakes: The Search for General Geographical and Guild Patterns. Oikos 2006, 114, 193–211. [Google Scholar] [CrossRef]
- Lunghi, E.; Giachello, S.; Mulargia, M.; Dore, P.P.; Cogoni, R.; Corti, C. Variability in the Dorsal Pattern of the Sardinian Grass Snake (Natrix natrix Cetti) with Notes on Its Ecology. Acta Herpetol. 2019, 14, 141–145. [Google Scholar] [CrossRef]
- Kindler, C.; Graciá, E.; Fritz, U. Extra-Mediterranean Glacial Refuges in Barred and Common Grass Snakes (Natrix helvetica, N. natrix). Sci. Rep. 2018, 8, 1821. [Google Scholar] [CrossRef] [PubMed]
- Gentilli, A.; Scali, S. Natrix natrix. In Atlante Degli Anfibi e Dei Rettili d’Italia/Atlas of Italian Amphibians and Reptiles. Societas Herpetologica Italica; Sindaco, R., Doria, G., Razzetti, E., Bernini, F., Eds.; Edizioni Polistampa: Firenze, Italy, 2006; pp. 560–565. [Google Scholar]
- Bassu, L.; Nulchis, V.; Satta, M.G.; Fresi, C.; Corti, C. Atlas of Amphibians and Reptiles of Sardinia—State of the Art and General Considerations. In Herpetologia Sardiniae; Corti, C., Ed.; Edizioni Belvedere: Latina, Italy, 2008; pp. 52–58. [Google Scholar]
- Corti, C.; Nistri, A.; Vanni, S.; Lanza, B. Atlante Erpetologico Della Sardegna, Risultati Preliminari. In Proceedings of the Atti I Congresso Nazionale Societas Herpetologica Italica, Turin, Italy, 2–6 October 1996; Bollettino Museo Regionale di Scienze Naturali: Turin, Italy, 2000; pp. 573–576. [Google Scholar]
- Bassu, L.; Nulchis, V.; Satta, M.G.; Fresi, C.; Corti, C. Atlas of Amphibians and Reptiles of Sardinia Part III, Reptilia. Anfibi e Rettili di Sardegna III, Reptilia. In Proceedings of the Atti IX Congresso Nazionale Della Societas Herpetologica Italica, Rende-Cosenza, Italy, 1–5 October 2018; Tipolitografia Pineta: Bari, Italy, 2013; pp. 108–113. [Google Scholar]
- de Pous, P.; Speybroeck, J.; Bogaerts, S.; Pasmans, F.; Beukema, W. A Contribution to the Atlas of the Terrestrial Herpetofauna of Sardinia. Herpetol. Notes 2012, 5, 391–405. [Google Scholar]
- Mulargia, M.; Corti, C.; Lunghi, E. The Herpetofauna of the Monte Albo, Sardinia (Italy). Russ. J. Herpetol. 2018, 25, 172. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Dudík, M.; Schapire, R.E.; Blair, M.E. Opening the Black Box: An Open-Source Release of Maxent. Ecography 2017, 40, 887–893. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km Spatial Resolution Climate Surfaces for Global Land Areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Fielding, A.H.; Bell, J.F. A Review of Methods for the Assessment of Prediction Errors in Conservation Presence/Absence Models. Environ. Conserv. 1997, 24, 38–49. [Google Scholar] [CrossRef]
- Boria, R.A.; Olson, L.E.; Goodman, S.M.; Anderson, R.P. Spatial Filtering to Reduce Sampling Bias Can Improve the Performance of Ecological Niche Models. Ecol. Model. 2014, 275, 73–77. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- QGIS Development Team. QGIS Geographic Information System. Open Source Geospatial Foundation Project. 2020. Available online: http://qgis.osgeo.org (accessed on 5 June 2023).
- Sau, S.; Smolinský, R.; Martínková, N. Environment Drives Color Pattern Polymorphism in Sand Lizards beyond the Gloger’s Rule. J. Zool. 2023. [Google Scholar] [CrossRef]
- Storniolo, F.; Mangiacotti, M.; Zuffi, M.A.L.; Scali, S.; Sacchi, R. Large Scale Phenotypic Characterisation of Hierophis viridiflavus (Squamata: Serpentes): Climatic and Environmental Drivers Suggest the Role of Evolutionary Processes in a Polymorphic Species. Evol. Ecol. 2023, 37, 419–434. [Google Scholar] [CrossRef]
- Broennimann, O.; Ursenbacher, S.; Meyer, A.; Golay, P.; Monney, J.-C.; Schmocker, H.; Guisan, A.; Dubey, S. Influence of Climate on the Presence of Colour Polymorphism in Two Montane Reptile Species. Biol. Lett. 2014, 10, 20140638. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, R.S. Multivariate Analysis of the Population Systematics of the Ringed Snake, Natrix natrix (L). Proc. R. Soc. Edinb. Sect. B Biol. Sci. 1979, 78, 1–62. [Google Scholar] [CrossRef]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R Package for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Kassambara, A.; Mundt, F. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses. R Package Version 1.0.7. 2020. Available online: https://cran.r-project.org/package=factoextra (accessed on 5 June 2023).
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis, 2nd ed.; Use R! Springer International Publishing: Cham, Switzerland, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
- Geniez, P. Snakes of Europe, North Africa and the Middle East. A Photographic Guide; Princeton University Press: Princeton, NJ, USA, 2018. [Google Scholar]
- Zaher, H.; Murphy, R.W.; Arredondo, J.C.; Graboski, R.; Machado-Filho, P.R.; Mahlow, K.; Montingelli, G.G.; Quadros, A.B.; Orlov, N.L.; Wilkinson, M.; et al. Large-Scale Molecular Phylogeny, Morphology, Divergence-Time Estimation, and the Fossil Record of Advanced Caenophidian Snakes (Squamata: Serpentes). PLoS ONE 2019, 14, e0216148. [Google Scholar] [CrossRef]
- Pokrant, F.; Kindler, C.; Ivanov, M.; Cheylan, M.; Geniez, P.; Böhme, W.; Fritz, U. Integrative Taxonomy Provides Evidence for the Species Status of the Ibero-Maghrebian Grass Snake Natrix astreptophora. Biol. J. Linn. Soc. 2016, 118, 873–888. [Google Scholar] [CrossRef]
- Kindler, C.; Chèvre, M.; Ursenbacher, S.; Böhme, W.; Hille, A.; Jablonski, D.; Vamberger, M.; Fritz, U. Hybridization Patterns in Two Contact Zones of Grass Snakes Reveal a New Central European Snake Species. Sci. Rep. 2017, 7, 7378. [Google Scholar] [CrossRef]
- Schultze, N.; Laufer, H.; Kindler, C.; Fritz, U. Distribution and Hybridisation of Barred and Common Grass Snakes (Natrix helvetica, N. natrix) in Baden-Württemberg, South-Western Germany. Herpetozoa 2019, 32, 229–236. [Google Scholar] [CrossRef]
- Asztalos, M.; Schultze, N.; Ihlow, F.; Geniez, P.; Berroneau, M.; Delmas, C.; Guiller, G.; Legentilhomme, J.; Kindler, C.; Fritz, U. How Often Do They Do It? An in-Depth Analysis of the Hybrid Zone of Two Grass Snake Species (Natrix astreptophora and Natrix helvetica). Biol. J. Linn. Soc. 2020, 131, 756–773. [Google Scholar] [CrossRef]
- Asztalos, M.; Glaw, F.; Franzen, M.; Kindler, C.; Fritz, U. Transalpine Dispersal: Italian Barred Grass Snakes in Southernmost Bavaria—This Far but No Further! J. Zool. Syst. Evol. Res. 2021, 59, 1136–1148. [Google Scholar] [CrossRef]
- Speybroeck, J.; Beukema, W.; Dufresnes, C.; Fritz, U.; Jablonski, D.; Lymberakis, P.; Martínez-Solano, I.; Razzetti, E.; Vamberger, M.; Vences, M.; et al. Species List of the European Herpetofauna—2020 Update by the Taxonomic Committee of the Societas Europaea Herpetologica. Amphib. Reptil. 2020, 41, 139–189. [Google Scholar] [CrossRef]
- Fritz, U.; Ihlow, F. Citizen Science, Taxonomy and Grass Snakes: INaturalist Helps to Clarify Variation of Coloration and Pattern in Natrix natrix Subspecies. Vertebr. Zool. 2022, 72, 533–549. [Google Scholar] [CrossRef]
- Gené, C.G. Synopsis Reptilium: Sardiniae Indigenorum; Reale Academia Delle Scienze: Torino, Italy, 1839. [Google Scholar]
- Mertens, R.; Müller, L. Liste der Amphibien und Reptilien Europas. Senckenberg. Naturforschenden Ges. 1928, 41, 1–62. [Google Scholar]
- Leunis, J. Synopsis der Drei Naturreiche. I. Zoologie. Zweite Auflage; Hahn’sche Hofbuchhandlung: Hannover, Italy, 1860. [Google Scholar]
- Jan, G. Elenco Sistematico Degli Ofidi Descritti e Disegnati per L’iconografia Generale Edita Dal Prof. G. Jan Direttore Del Museo Civico Di Milano; Tip. di A. Lombardi: Milano, Italy, 1863. [Google Scholar]
- Camerano, L. Monografia Degli Ofidi Italiani. Parte Seconda. Colubridi e Monografia Dei Cheloni Italiani. Mem. R. Accad. Sci. Fis. Mat. Torino Ser. 1891, 2, 403–481. [Google Scholar]
- Hecht, G. Systematik, Ausbreitungsgeschichte und Ökologie der Europäischen Arten der Gattung Tropidonotus (Kuhl) H. Boie. Mitt. Zool. Mus. Berl. 1930, 16, 244–393. [Google Scholar]
- Mertens, R. Studien zur Eidonomie und Taxonomie der Ringelnatter-(Natrix natrix). Senckenberg. Naturforschenden Ges. 1947, 476, 1–38. [Google Scholar]
- Mertens, R. Die Amphibien und Reptilien Korsikas. Senckenberg. Biol. 1957, 38, 175–192. [Google Scholar]
- Thorpe, R.S. Intraspecific Variation of the Ringed Snake, Natrix natrix (L.). Ph. D. Thesis, Council for National Academic Awards, London, UK, 1973. [Google Scholar]
- Thorpe, R.S. Biometric Analysis of Incipient Speciation in the Ringed Snake, Natrix natrix (L.). Experientia 1975, 31, 180–182. [Google Scholar] [CrossRef]
- Thorpe, R.S. Quantitative Handling of Characters Useful in Snake Systematics with Particular Reference to Intraspecific Variation in the Ringed Snake Natrix natrix (L.). Biol. J. Linn. Soc. 1975, 7, 27–43. [Google Scholar] [CrossRef]
- Thorpe, R.S. Microevolution and Taxonomy of European Reptiles with Particular Reference to the Grass Snake Natrix natrix and the Wall Lizards Podarcis sicula and P. melisellensis. Biol. J. Linn. Soc. 1980, 14, 215–233. [Google Scholar] [CrossRef]
- Lanza, B. Guide per il Riconoscimento Delle Specie Animali Nelle Acque Interne Italiane. 27. Anfibi, Rettili (Amphibia, Reptilia); Collana del Progetto Finalizzato “Promozione della Qualità dell’Ambiente” AQ/1/205; Consiglio Nazionale delle Ricerche: Roma, Italy, 1983; pp. 156–185. [Google Scholar]
- Aprea, G.; Odierna, G.; Capriglione, T.; Caputo, V.; Guarino, F.M. Analisi Cromosomica in Tre Specie del Genere Natrix Duméril (Reptilia, Squamata). In Atti del I Congresso Nazionale della Societas Herpetologica Italica, Torino, 1996; Museo Regionale di Scienze Nataturali: Turin, Italy, 2000; pp. 423–428. [Google Scholar]
- Kindler, C.; Böhme, W.; Corti, C.; Gvoždík, V.; Jablonski, D.; Jandzik, D.; Metallinou, M.; Široký, P.; Fritz, U. Mitochondrial Phylogeography, Contact Zones and Taxonomy of Grass Snakes (Natrix natrix, N. megalocephala). Zool. Scr. 2013, 42, 458–472. [Google Scholar] [CrossRef]
- Burbrink, F.T.; Grazziotin, F.G.; Pyron, R.A.; Cundall, D.; Donnellan, S.; Irish, F.; Keogh, J.S.; Kraus, F.; Murphy, R.W.; Noonan, B.; et al. Interrogating Genomic-Scale Data for Squamata (Lizards, Snakes, and Amphisbaenians) Shows No Support for Key Traditional Morphological Relationships. Syst. Biol. 2020, 69, 502–520. [Google Scholar] [CrossRef]
- Deepak, V.; Cooper, N.; Poyarkov, N.A.; Kraus, F.; Burin, G.; Das, A.; Narayanan, S.; Streicher, J.W.; Smith, S.-J.; Gower, D.J. Multilocus Phylogeny, Natural History Traits and Classification of Natricine Snakes (Serpentes: Natricinae). Zool. J. Linn. Soc. 2022, 195, 279–298. [Google Scholar] [CrossRef]
- Lanza, B. Ipotesi Sulle Origini Del Popolamento Erpetologico della Sardegna. Lavori della Società Italiana di Biogeografia. Biogeogr. J. Integr. Biogeogr. 1983, 8, 723–744. [Google Scholar] [CrossRef]
- Lanza, B. Hypotheses on the Origins of Corsican Herpetofauna (p. 2). In Proceedings of the Colloque International sur les Vertébrés Terrestres et Dulçaquicoles des Îles Méditerranéennes, Evisa, France, 10–16 October 1983; p. 53. [Google Scholar]
- Lanza, B. Hypothèses Sur Les Origines de la Faune Herpétologique Corse. Actes du Colloque International Sur Les Vertébrés Terrestres et Dulçaquicoles des Îles Méditerranéennes, Evisa (Corse), 10–16 October 1983. Bull. Ecol. 1988, 19, 163–170. [Google Scholar]
- Garcia-Castellanos, D.; Estrada, F.; Jiménez-Munt, I.; Gorini, C.; Fernàndez, M.; Vergés, J.; De Vicente, R. Catastrophic Flood of the Mediterranean after the Messinian Salinity Crisis. Nature 2009, 462, 778–781. [Google Scholar] [CrossRef] [PubMed]
- Lambeck, K.; Antonioli, F.; Purcell, A.; Silenzi, S. Sea-Level Change along the Italian Coast for the Past 10,000 yr. Quat. Sci. Rev. 2004, 23, 1567–1598. [Google Scholar] [CrossRef]
- Marchetti, M.; Soldati, M.; Vandelli, V. The Great Diversity of Italian Landscapes and Landforms: Their Origin and Human Imprint. In Landscapes and Landforms of Italy; Soldati, M., Marchetti, M., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 7–20. ISBN 978-3-319-26192-8. [Google Scholar]
- Lunghi, E.; Deschandol, F.; Cornago, L.; Cogoni, R. Dark Coloration in Sardinian Grass Snakes (Natrix natrix cetti). Herpetol. Bull. 2016, 137, 28–29. [Google Scholar]
- Bruni, G.; Di Nicola, M.R.; Banfi, F.; Faraone, F.P. Distribution and Characterization of Melanism in Grass Snakes from Italy. Nat. Sicil. 2022, 46, 41–48. [Google Scholar] [CrossRef]
- Capula, M.; Rugiero, L.; Luiselli, L. Ecological Observations on the Sardinian Grass Snake, Natrix natrix cetti. Amphib. Reptil. 1994, 15, 221–224. [Google Scholar] [CrossRef]
- Scali, S.; Gentilli, A.; Lanza, B. Natrix natrix. In Fauna d’Italia, Reptilia; Corti, C., Capula, M., Luiselli, L., Sindaco, R., Razzetti, E., Eds.; Calderini: Bologna, Italy, 2010; Volume 45, pp. 552–562. [Google Scholar]
- Lunghi, E.; Mascia, C.; Mulargia, M.; Corti, C. Is the Sardinian Grass Snake (Natrix natrix cetti) an Active Hunter in Underground Environments. Spixiana 2018, 41, 160. [Google Scholar]
- Stefani, R. La Natrice Del Cetti. Biogeogr. J. Integr. Biogeogr. 1983, 8, 745–755. [Google Scholar] [CrossRef]
- Lanza, B.; Camarda, I.; Falchi, S.; Nudda, G. (Eds.) I Rettili e Gli Anfibi. In L’ambiente Naturale in Sardegna (Elementi di Base per la Conoscenza e la Gestione del Territorio); Carlo Delfino Editore: Sassari, Italy, 1986. [Google Scholar]
- Mebert, K.; Trapp, B.; Kreiner, G.; Billing, H.; Speybroeck, J.; Henggeler, M. Nocturnal Activity in Natrix tessellata, a Neglected Aspect of Its Behavioral Repetoire. Mertensiella 2011, 18, 234–236. [Google Scholar]
- Luiselli, L.; Filippi, E.; Capula, M. Geographic Variation in Diet Composition of the Grass Snake (Natrix natrix) along the Mainland and an Island of Italy: The Effects of Habitat Type and Interference with Potential Competitors. Herpetol. J. 2005, 15, 221–230. [Google Scholar]
- Filippi, E.; Capula, M.; Luiselli, L.; Agrimi, U. The Prey Spectrum of Natrix natrix (Linnaeus, 1758) and Natrix tessellata (Laurenti, 1768) in Sympatric Populations. Herpetozoa 1996, 8, 155–164. [Google Scholar]
- Reading, C.J.; Davies, J.L. Predation by Grass Snakes (Natrix natrix) at a Site in Southern England. J. Zool. 1996, 239, 73–82. [Google Scholar] [CrossRef]
- Gregory, P.T.; Isaac, L.A. Food Habits of the Grass Snake in Southeastern England: Is Natrix natrix a Generalist Predator? J. Herpetol. 2004, 38, 88–95. [Google Scholar] [CrossRef]
- Faraone, F.P.; Giacalone, G.; Lo Valvo, M. Dati Preliminari Sulla Biometria, Il Cromatismo e la Dieta di una Popolazione di Natrix natrix della Sicilia Occidentale. In Proceedings of the Atti VIII Congresso Nazionale Societas Herpetologica Italica, Chieti, Italy, 22–26 September 2010; pp. 247–252. [Google Scholar]
- Šukalo, G.; Đorđević, S.; Gvozdenović, S.; Simović, A.; Anđelković, M.; Blagojević, V.; Tomović, L. Intra-and Inter-Population Variability of Food Preferences of Two Natrix Species on the Balkan Peninsula. Herpetol. Conserv. Biol. 2014, 9, 123–136. [Google Scholar]
- Di Nicola, M.R.; Bruni, G. A Case of Active Predation of Natrix helvetica (Serpentes: Colubridae) on Sturnus vulgaris (Passeriformes: Sturnidae). Herpetol. Notes 2020, 13, 461–462. [Google Scholar]
- Di Nicola, M.R.; Zabbia, T. Natrix helvetica (Barred Grass Snake). Diet. Herpetol. Rev. 2021, 52, 877–878. [Google Scholar]
- Luiselli, L.; Rugiero, L. Food Niche Partitioning by Water Snakes (Genus Natrix) at a Freshwater Environment in Central Italy. J. Freshw. Ecol. 1991, 6, 439–444. [Google Scholar] [CrossRef]
- Kaczmarski, M. Arboreal Foraging and Ambush by Grass Snakes Natrix natrix on European Treefrogs Hyla Arborea. Herpetol. Bull. 2020, 154, 39–40. [Google Scholar] [CrossRef]
- Puddu, F.; Viarengo, M.; Erminio, C. Animali di Sardegna. Gli Anfibi e i Rettili; Edizioni Della Torre: Cagliari, Italy, 1988; ISBN 0-01-870169-8. [Google Scholar]
- Lanza, B.; Pastorelli, C.; Laghi, P.; Cimmaruta, R. A Review of Systematics, Taxonomy, Genetics, Biogeography and Natural History of the Genus Speleomantes Dubois, 1984 (Amphibia Caudata Plethodontidae). Atti Mus. Civ. Stor. Nat. Trieste 2006, 52, 5–135. [Google Scholar]
- Van Rooy, P.T.; Stumpel, A.H. Ecological Impact of Economic Development on Sardinian Herpetofauna. Conserv. Biol. 1995, 9, 263–269. [Google Scholar] [CrossRef]
- Luiselli, L. Individual Success in Mating Balls of the Grass Snake, Natrix natrix: Size Is Important. J. Zool. 1996, 239, 731–740. [Google Scholar] [CrossRef]
- Cauli, F.; Di Nicola, M.R.; Audisio, P.; Petretti, F.; Faraone, F.P. Feeding Habits of the Short-Toed Eagle Circaetus gallicus during the Breeding Period in Central Italy. Avocetta 2022, 46, 1. [Google Scholar] [CrossRef]
- Filippi, E.; Luiselli, L. Negative Effect of the Wild Boar (Sus scrofa) on the Populations of Snakes at a Protected Mountainous Forest in Central Italy. Ecol. Mediterr. 2002, 28, 93–98. [Google Scholar] [CrossRef]
- Schoenfeld, M.; Yom-Tov, Y. The Biology of Two Species of Hedgehogs, Erinaceus europaeus Concolor and Hemiechinus auritus aegyptius, in Israel. Mammalia 1985, 49, 339–356. [Google Scholar] [CrossRef]
- Di Nicola, M.R.; Costa, W.; Mori, E. An Evidence of Asp Viper (Vipera aspis) Consumption by a Western European Hedgehog (Erinaceus europaeus) on Elba Island (Italy). Nat. Hist. Sci. 2021, 9, 35–38. [Google Scholar] [CrossRef]
- Petrilla, V.; Flešárová, S.; Šulla, I. Poison Apparatus in Snakes. Folia Vet. 2008, 52, 22–23. [Google Scholar]
- Di Nicola, M.R.; Chiara, R.; Colnaghi, S.; Valvo, M.L.; Faraone, F.P. First Documented Cases of Defensive Biting Behaviour towards Humans in the Italian Barred Grass Snake Natrix helvetica sicula (Cuvier, 1829). Herpetol. Notes 2023, 16, 229–232. [Google Scholar]
- European Reptile & Amphibians Specialist Group. Natrix natrix ssp. Cetti. The IUCN Red List of Threatened Species. 1996, e.T14364A4436077. Available online: https://doi.org/10.2305/IUCN.UK.1996.RLTS.T14364A4436077.en (accessed on 17 August 2023).
- Ozinga, W.A.; de Heer, M.; Hennekens, S.M.; van Opstal, A.; Schaminee, J.H.J.; Sierdsema, H.; Smits, N.A.C.; Stumpel, A.H.P.; van Swaay, C. Target Species-Species of European Concern; a Database Driven Selection of Plant and Animal Species for the Implementation of the Pan European Ecological Network; Alterra: Wageningen, The Netherlands, 2005. [Google Scholar]
- Andreone, F.; Corti, C.; Ficetola, G.F.; Razzetti, E.; Romano, A.; Sindaco, R. Natrix natrix ssp. cetti; IUCN Comitato Italiano: Grand, Switzerland, 2013. [Google Scholar]
- Krijgsman, W.; Hilgen, F.J.; Raffi, I.; Sierro, F.J.; Wilson, D.S. Chronology, Causes and Progression of the Messinian Salinity Crisis. Nature 1999, 400, 652–655. [Google Scholar] [CrossRef]
- Schätti, B. Bemerkungen zur Ökologie, Verbreitung und Intraspezifischen Variation der Vipernatter, Natrix maura (Linné, 1758) (Reptilia, Serpentes). Rev. Suisse Zool. 1982, 89, 521–542. [Google Scholar] [CrossRef]
- Poggesi, M.; Agnelli, P.; Borri, M.; Corti, C.; Finotello, P.; Lanza, B.; Tosini, G. Erpetologia delle Isole Circumsarde. Biogeogr. J. Integr. Biogeogr. 1996, 18, 583–618. [Google Scholar] [CrossRef]
- Guicking, D.; Griffiths, R.A.; Moore, R.D.; Joger, U.; Wink, M. Introduced Alien or Persecuted Native? Resolving the Origin of the Viperine Snake (Natrix maura) on Mallorca. Biodivers. Conserv. 2006, 15, 3045–3054. [Google Scholar] [CrossRef]
- Rugiero, L.; Capula, M.; Persichetti, D.; Luiselli, L.; Angelici, F.M. Life-History and Diet of Two Populations of Natrix maura (Reptilia, Colubridae) from Contrasted Habitats in Sardinia. Miscel Lània Zool. 2000, 23, 41–51. [Google Scholar]
- European Environment Agency. Natrix natrix cetti; European Environment Agency: Copenhagen, Denmark, 2012. [Google Scholar]
- Falcucci, A.; Maiorano, L.; Boitani, L. Changes in Land-Use/Land-Cover Patterns in Italy and Their Implications for Biodiversity Conservation. Landsc. Ecol. 2007, 22, 617–631. [Google Scholar] [CrossRef]
- Puddu, G.; Falcucci, A.; Maiorano, L. Forest Changes over a Century in Sardinia: Implications for Conservation in a Mediterranean Hotspot. Agrofor. Syst. 2012, 85, 319–330. [Google Scholar] [CrossRef]
- Fiorini, L.; Zullo, F.; Romano, B. Urban Development of the Coastal System of the Italian Largest Islands: Sicily and Sardinia. Ocean Coast. Manag. 2017, 143, 184–194. [Google Scholar] [CrossRef]
- Reed, D.H.; Frankham, R. Correlation between Fitness and Genetic Diversity. Conserv. Biol. 2003, 17, 230–237. [Google Scholar] [CrossRef]
- Charlesworth, D.; Willis, J.H. The Genetics of Inbreeding Depression. Nat. Rev. Genet. 2009, 10, 783–796. [Google Scholar] [CrossRef] [PubMed]
- Frankham, R. Genetic Rescue of Small Inbred Populations: Meta-Analysis Reveals Large and Consistent Benefits of Gene Flow. Mol. Ecol. 2015, 24, 2610–2618. [Google Scholar] [CrossRef] [PubMed]
- Phillipsen, I.C.; Kirk, E.H.; Bogan, M.T.; Mims, M.C.; Olden, J.D.; Lytle, D.A. Dispersal Ability and Habitat Requirements Determine Landscape-Level Genetic Patterns in Desert Aquatic Insects. Mol. Ecol. 2015, 24, 54–69. [Google Scholar] [CrossRef] [PubMed]
- Di Nicola, M.R.; Ortiz-Santaliestra, M.E.; Bosch, J.; Pasmans, F.; Martel, A.; Focks, A.; Dorne, J.L.C.M.; Marini, D. Indagine Sull’ofidiomicosi Nei Serpenti Italiani Nell’ambito del Progetto EFSA “AMPHIDEB”. In Proceedings of the XIV Congresso Nazionale Societas Herpetologica Italica, Turin, Italy, 13–17 September 2022. [Google Scholar]
- Di Nicola, M.R.; Coppari, L.; Notomista, T.; Marini, D. Ophidiomyces ophidiicola Detection and Infection: A Global Review on a Potential Threat to the World’s Snake Populations. Eur. J. Wildl. Res. 2022, 68, 64. [Google Scholar] [CrossRef]
- Marini, D.; Di Nicola, M.R.; Crocchianti, V.; Notomista, T.; Iversen, D.; Coppari, L.; Di Criscio, M.; Brouard, V.; Dorne, J.-L.C.M.; Rüegg, J.; et al. Pilot Survey Reveals Ophidiomycosis in Dice Snakes Natrix tessellata from Lake Garda, Italy. Vet. Res. Commun. 2023. Online ahead of print. [Google Scholar] [CrossRef]
- Mace, G.M.; Collar, N.J.; Gaston, K.J.; Hilton-Taylor, C.; Akçakaya, H.R.; Leader-Williams, N.; Milner-Gulland, E.J.; Stuart, S.N. Quantification of Extinction Risk: IUCN’s System for Classifying Threatened Species. Conserv. Biol. 2008, 22, 1424–1442. [Google Scholar] [CrossRef]
- Gaston, K.J.; Fuller, R.A. The Sizes of Species’ Geographic Ranges. J. Appl. Ecol. 2009, 46, 1–9. [Google Scholar] [CrossRef]
- Margules, C.R.; Pressey, R.L. Systematic Conservation Planning. Nature 2000, 405, 243–253. [Google Scholar] [CrossRef]
- Trindade-Filho, J.; de Carvalho, R.A.; Brito, D.; Loyola, R.D. How Does the Inclusion of Data Deficient Species Change Conservation Priorities for Amphibians in the Atlantic Forest? Biodivers. Conserv. 2012, 21, 2709–2718. [Google Scholar] [CrossRef]
- Royle, J.A.; Link, W.A. Generalized Site Occupancy Models Allowing for False Positive and False Negative Errors. Ecology 2006, 87, 835–841. [Google Scholar] [CrossRef]
- Durso, A.M.; Willson, J.D.; Winne, C.T. Needles in Haystacks: Estimating Detection Probability and Occupancy of Rare and Cryptic Snakes. Biol. Conserv. 2011, 144, 1508–1515. [Google Scholar] [CrossRef]
- McCarthy, M.A.; Moore, J.L.; Morris, W.K.; Parris, K.M.; Garrard, G.E.; Vesk, P.A.; Rumpff, L.; Giljohann, K.M.; Camac, J.S.; Bau, S.S. The Influence of Abundance on Detectability. Oikos 2013, 122, 717–726. [Google Scholar] [CrossRef]
- Pacifici, K.; Reich, B.J.; Dorazio, R.M.; Conroy, M.J. Occupancy Estimation for Rare Species Using a Spatially-adaptive Sampling Design. Methods Ecol. Evol. 2016, 7, 285–293. [Google Scholar] [CrossRef]
- Gu, W.; Swihart, R.K. Absent or Undetected? Effects of Non-Detection of Species Occurrence on Wildlife–Habitat Models. Biol. Conserv. 2004, 116, 195–203. [Google Scholar] [CrossRef]
- Dyugmedzhiev, A.; Naumov, B.; Tzankov, N. Sex-and Age-Related Variations in Seasonal and Circadian Activity of the Nose-Horned Viper Vipera ammodytes (Linnaeus, 1758). Belg. J. Zool. 2022, 152, 139–156. [Google Scholar] [CrossRef]
- Wilson, J.S.; Pan, A.D.; General, D.E.M.; Koch, J.B. More Eyes on the Prize: An Observation of a Very Rare, Threatened Species of Philippine Bumble Bee, Bombus irisanensis, on INaturalist and the Importance of Citizen Science in Conservation Biology. J. Insect Conserv. 2020, 24, 727–729. [Google Scholar] [CrossRef]
- Mesaglio, T.; Soh, A.; Kurniawidjaja, S.; Sexton, C. ‘First Known Photographs of Living Specimens’: The Power of INaturalist for Recording Rare Tropical Butterflies. J. Insect Conserv. 2021, 25, 905–911. [Google Scholar] [CrossRef]
- Roberts, C.J.; Vergés, A.; Callaghan, C.T.; Poore, A.G.B. Many Cameras Make Light Work: Opportunistic Photographs of Rare Species in INaturalist Complement Structured Surveys of Reef Fish to Better Understand Species Richness. Biodivers. Conserv. 2022, 31, 1407–1425. [Google Scholar] [CrossRef]
- Gaier, A.G.; Resasco, J. Does Adding Community Science Observations to Museum Records Improve Distribution Modeling of a Rare Endemic Plant? Ecosphere 2023, 14, e4419. [Google Scholar] [CrossRef]
- Balletto, G.; Borruso, G.; Ladu, M.; Milesi, A. Smart and Slow Tourism. Evaluation and Challenges in Sardinia (Italy). In Innovation in Urban and Regional Planning; La Rosa, D., Privitera, R., Eds.; Springer International Publishing: Cham, Switzerland, 2022; Volume 242, pp. 175–182. ISBN 978-3-030-96984-4. [Google Scholar]
- Bussu, E. Sardegna, Un Turismo Con Un Futuro Diverso. AMMENTU-Boll. Stor. Arch. Mediterr. Americhe 2022, 1, 283–296. [Google Scholar]
- Guisan, A.; Broennimann, O.; Engler, R.; Vust, M.; Yoccoz, N.G.; Lehmann, A.; Zimmermann, N.E. Using Niche-based Models to Improve the Sampling of Rare Species. Conserv. Biol. 2006, 20, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Marino, J.; Bennett, M.; Cossios, D.; Iriarte, A.; Lucherini, M.; Pliscoff, P.; Sillero-Zubiri, C.; Villalba, L.; Walker, S. Bioclimatic Constraints to Andean Cat Distribution: A Modelling Application for Rare Species. Divers. Distrib. 2011, 17, 311–322. [Google Scholar] [CrossRef]
- Ferrer-Sánchez, Y.; Rodríguez-Estrella, R. How Rare Species Conservation Management Can Be Strengthened with the Use of Ecological Niche Modelling: The Case for Endangered Endemic Gundlach’s Hawk and Cuban Black-Hawk. Glob. Ecol. Conserv. 2016, 5, 88–99. [Google Scholar] [CrossRef]
- Pedrotta, T.; Gobet, E.; Schwörer, C.; Beffa, G.; Butz, C.; Henne, P.D.; Morales-Molino, C.; Pasta, S.; van Leeuwen, J.F.; Vogel, H. 8000 Years of Climate, Vegetation, Fire and Land-Use Dynamics in the Thermo-Mediterranean Vegetation Belt of Northern Sardinia (Italy). Veg. Hist. Archaeobot. 2021, 30, 789–813. [Google Scholar] [CrossRef]
- Caloiero, T.; Coscarelli, R.; Gaudio, R. Spatial and Temporal Variability of Daily Precipitation Concentration in the Sardinia Region (Italy). Int. J. Climatol. 2019, 39, 5006–5021. [Google Scholar] [CrossRef]
- Rodríguez, M.Á.; Belmontes, J.A.; Hawkins, B.A. Energy, Water and Large-Scale Patterns of Reptile and Amphibian Species Richness in Europe. Acta Oecologica 2005, 28, 65–70. [Google Scholar] [CrossRef]
- Detto, M.; Montaldo, N.; Albertson, J.D.; Mancini, M.; Katul, G. Soil Moisture and Vegetation Controls on Evapotranspiration in a Heterogeneous Mediterranean Ecosystem on Sardinia, Italy. Water Resour. Res. 2006, 42, W08419. [Google Scholar] [CrossRef]
- Montaldo, N.; Corona, R.; Albertson, J.D. On the Separate Effects of Soil and Land Cover on Mediterranean Ecohydrology: Two Contrasting Case Studies in Sardinia, Italy. Water Resour. Res. 2013, 49, 1123–1136. [Google Scholar] [CrossRef]
- Kobayashi, T.; Tateishi, R.; Alsaaideh, B.; Sharma, R.C.; Wakaizumi, T.; Miyamoto, D.; Bai, X.; Long, B.D.; Gegentana, G.; Maitiniyazi, A.; et al. Production of Global Land Cover Data—GLCNMO2013. J. Geogr. Geol. 2017, 9, 1. [Google Scholar] [CrossRef]
- Bird, D.N.; Benabdallah, S.; Gouda, N.; Hummel, F.; Koeberl, J.; La Jeunesse, I.; Meyer, S.; Prettenthaler, F.; Soddu, A.; Woess-Gallasch, S. Modelling Climate Change Impacts on and Adaptation Strategies for Agriculture in Sardinia and Tunisia Using AquaCrop and Value-at-Risk. Sci. Total Environ. 2016, 543, 1019–1027. [Google Scholar] [CrossRef]
- Lai, S.; Leone, F.; Zoppi, C. Spatial Distribution of Surface Temperature and Land Cover: A Study Concerning Sardinia, Italy. Sustainability 2020, 12, 3186. [Google Scholar] [CrossRef]
- Caloiero, T.; Guagliardi, I. Climate Change Assessment: Seasonal and Annual Temperature Analysis Trends in the Sardinia Region (Italy). Arab. J. Geosci. 2021, 14, 2149. [Google Scholar] [CrossRef]
- Cipolla, S.S.; Montaldo, N. On the Impacts of Historical and Future Climate Changes to the Sustainability of the Main Sardinian Forests. Remote Sens. 2022, 14, 4893. [Google Scholar] [CrossRef]
- Gentilli, A.; Scali, S. Natrix maura. In Atlante Degli Anfibi e dei Rettili D’italia/Atlas of Italian Amphibians and Reptiles. Societas Herpetologica Italica; Sindaco, R., Doria, G., Razzetti, E., Bernini, F., Eds.; Edizioni Polistampa: Firenze, Italy, 2006; pp. 556–559. [Google Scholar]
- Martínez-Freiría, F.; i de Lanuza, G.P.; Pimenta, A.A.; Pinto, T.; Santos, X. Aposematism and Crypsis Are Not Enough to Explain Dorsal Polymorphism in the Iberian Adder. Acta Oecologica 2017, 85, 165–173. [Google Scholar] [CrossRef]
- Souchet, J.; Bossu, C.; Darnet, E.; Le Chevalier, H.; Poignet, M.; Trochet, A.; Bertrand, R.; Calvez, O.; Martinez-Silvestre, A.; Mossoll-Torres, M.; et al. High Temperatures Limit Developmental Resilience to High-Elevation Hypoxia in the Snake Natrix maura (Squamata: Colubridae). Biol. J. Linn. Soc. 2021, 132, 116–133. [Google Scholar] [CrossRef]
- Wüster, W.; Allum, C.S.; Bjargardottir, I.B.; Bailey, K.L.; Dawson, K.J.; Guenioui, J.; Lewis, J.; McGurk, J.; Moore, A.G.; Niskanen, M. Do Aposematism and Batesian Mimicry Require Bright Colours? A Test, Using European Viper Markings. Proc. R. Soc. Lond. B Biol. Sci. 2004, 271, 2495–2499. [Google Scholar] [CrossRef] [PubMed]
- Valkonen, J.K.; Nokelainen, O.; Mappes, J. Antipredatory Function of Head Shape for Vipers and Their Mimics. PLoS ONE 2011, 6, e22272. [Google Scholar] [CrossRef]
- Allen, W.L.; Baddeley, R.; Scott-Samuel, N.E.; Cuthill, I.C. The Evolution and Function of Pattern Diversity in Snakes. Behav. Ecol. 2013, 24, 1237–1250. [Google Scholar] [CrossRef]
- Rajabizadeh, M.; Adriaens, D.; Kaboli, M.; Sarafraz, J.; Ahmadi, M. Dorsal Colour Pattern Variation in Eurasian Mountain Vipers (Genus Montivipera): A Trade-off between Thermoregulation and Crypsis. Zool. Anz. J. Comp. Zool. 2015, 257, 1–9. [Google Scholar] [CrossRef]
- Santos, X.; Azor, J.S.; Cortés, S.; Rodríguez, E.; Larios, J.; Pleguezuelos, J.M. Ecological Significance of Dorsal Polymorphism in a Batesian Mimic Snake. Curr. Zool. 2018, 64, 745–753. [Google Scholar] [CrossRef]
- Pizzigalli, C.; Banfi, F.; Ficetola, G.F.; Falaschi, M.; Mangiacotti, M.; Sacchi, R.; Zuffi, M.A.; Scali, S. Eco-Geographical Determinants of the Evolution of Ornamentation in Vipers. Biol. J. Linn. Soc. 2020, 130, 345–358. [Google Scholar] [CrossRef]
- Martínez-Freiría, F.; Toyama, K.S.; Freitas, I.; Kaliontzopoulou, A. Thermal Melanism Explains Macroevolutionary Variation of Dorsal Pigmentation in Eurasian Vipers. Sci. Rep. 2020, 10, 16122. [Google Scholar] [CrossRef] [PubMed]
- Andrén, C.; Nilson, G. Reproductive Success and Risk of Predation in Normal and Melanistic Colour Morphs of the Adder, Vipera berus. Biol. J. Linn. Soc. 1981, 15, 235–246. [Google Scholar] [CrossRef]
- Bury, S.; Mazgajski, T.D.; Najbar, B.; Zając, B.; Kurek, K. Melanism, Body Size, and Sex Ratio in Snakes—New Data on the Grass Snake (Natrix natrix) and Synthesis. Sci. Nat. 2020, 107, 22. [Google Scholar] [CrossRef] [PubMed]
- Venczel, M.; Sanchiz, B. Lower Miocene Amphibians and Reptiles from Oschiri (Sardinia, Italy). Hantkeniana 2006, 5, 72–75. [Google Scholar]
- Delfino, M.; Bailon, S.; Pitruzzella, G. The Late Pliocene Amphibians and Reptiles from “Capo Mannu D1 Local Fauna”(Mandriola, Sardinia, Italy). Geodiversitas 2011, 33, 357–382. [Google Scholar] [CrossRef]
- Jackson, J.F.; Ingram, W., III; Campbell, H.W. The Dorsal Pigmentation Pattern of Snakes as an Antipredator Strategy: A Multivariate Approach. Am. Nat. 1976, 110, 1029–1053. [Google Scholar] [CrossRef]
- Ford, E.B. Genetic Polymorphism. Proc. R. Soc. Lond. B Biol. Sci. 1966, 164, 350–361. [Google Scholar]
- Cox, C.L.; Davis Rabosky, A.R. Spatial and Temporal Drivers of Phenotypic Diversity in Polymorphic Snakes. Am. Nat. 2013, 182, E40–E57. [Google Scholar] [CrossRef]
- Schielzeth, H. Phylogenetic, Geographic and Habitat Distribution of the Green-Brown Polymorphism in European Orthopterans. Evol. Biol. 2020. [Google Scholar] [CrossRef]
- Matthews, G.; Goulet, C.T.; Delhey, K.; Atkins, Z.S.; While, G.M.; Gardner, M.G.; Chapple, D.G. Avian Predation Intensity as a Driver of Clinal Variation in Colour Morph Frequency. J. Anim. Ecol. 2018, 87, 1667–1684. [Google Scholar] [CrossRef] [PubMed]
- Bittner, T.D.; King, R.B.; Kerfin, J.M. Effects of Body Size and Melanism on the Thermal Biology of Garter Snakes (Thamnophis sirtalis). Copeia 2002, 2002, 477–482. [Google Scholar] [CrossRef]
- Clusella Trullas, S.; van Wyk, J.H.; Spotila, J.R. Thermal Melanism in Ectotherms. J. Therm. Biol. 2007, 32, 235–245. [Google Scholar] [CrossRef]
- Hantak, M.M.; Guralnick, R.P.; Cameron, A.C.; Griffing, A.H.; Harrington, S.M.; Weinell, J.L.; Paluh, D.J. Colour Scales with Climate in North American Ratsnakes: A Test of the Thermal Melanism Hypothesis Using Community Science Images. Biol. Lett. 2022, 18, 20220403. [Google Scholar] [CrossRef] [PubMed]
- Madsen, T.; Stille, B.; Ujvari, B.; Bauwens, D.; Endler, J.A. Negative Frequency-Dependent Selection on Polymorphic Color Morphs in Adders. Curr. Biol. 2022, 32, 3385–3388.e3. [Google Scholar] [CrossRef]
- Osborne, J.W.; Costello, A.B. Sample Size and Subject to Item Ratio in Principal Components Analysis. Pract. Assess. Res. Eval. 2004, 9, 11. [Google Scholar]
- Huey, R.B. Temperature, Physiology, and the Ecology of Reptiles. Physiol. Ecol. 1982, 25–95. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).