Abstract
Obstructive sleep apnea is considered a risk factor for erectile dysfunction. The aim of this study was to determine sleep architecture and assess daytime sleepiness in patients with erectile dysfunction. The study group included 280 patients. The 107 enrolled patients had reported erectile dysfunction. The control group consisted of 173 patients who had no history of erectile dysfunction. The Epworth sleepiness scale (ESS) was used to measure the subjects’ level of daytime sleepiness. All patients underwent a standardized overnight, single-night polysomnography in sleep laboratory. In the erectile dysfunction group, we observed increased ESS total score and N1 sleep phase duration. Mean and minimal oxygen saturation and mean oxygen desaturation were decreased in comparison to the control group. In summary, subjects with erectile dysfunction have altered sleep architecture, oxygen saturation parameters and increased daytime sleepiness.
1. Introduction
Erectile dysfunction (ED) is defined as the consistent inability to obtain and/or maintain a penile erection during sexual activity [1]. ED affects millions of middle-aged to elderly men worldwide [2]. There are many causes of ED including diabetes, ischemic heart disease, medications (e.g., thiazides, b-blockers, spironolactone, and antidepressants), neurogenic disorders, atherosclerosis, tobacco use, hyperlipidemia, hypogonadism, lower urinary tract symptoms, metabolic syndrome, and depression [3,4]. The prevalence of ED increases with age, particularly after the age of 60 years [4,5,6]. There also data suggesting that obstructive sleep apnea (OSA) may have an independent association with sexual dysfunction and impotence [7,8]. OSA is a common sleep disorder characterized by the collapse of the upper airway leading to the cessation of airflow, intermittent arterial oxygen desaturation and arousals during sleep. Recent evidence showed that one in five adults suffer from at least a mild degree of OSA, 936 million adults aged 30–69 years have mild-to-severe OSA and 425 million (399–450) adults aged 30–69 years have moderate-to-severe obstructive sleep apnea globally [9,10]. Thus, this is one of the most common sleep disorders. Male sex and obesity are known risk factors for sleep apnea [11]. Several studies confirmed the increased prevalence of ED in patients with OSA [12,13].
Overnight polysomnography (PSG) is a gold standard in diagnosis of OSA [14]. Due to limited accessibility of PSG and its high expenditure, alternative tools for screening purposes have been developed including the Epworth sleepiness scale, Berlin Questionnaire or STOB-BANG Questionnaire. One of the most widely used is the Epworth sleepiness scale (ESS), which measures general level of sleepiness. The scale is self-administered: patients estimate their probability of falling asleep during different situations. The tool has been used in normal subjects [15], as well as in those with OSA [16], narcolepsy [17], stroke [18], coronary artery disease [19], heart failure [20], epilepsy [21], Parkinson’s disease [22], hemodialysis [23], diabetes [24], rheumatoid arthritis [25] and obesity [24]. However, the data concerning daytime sleepiness in erectile dysfunctions are limited.
The aims of this study were as follows: 1. to determine sleep architecture in ED patients; and to 2. assess sleepiness scores using the Epworth sleepiness scale (ESS) in ED patients.
2. Materials and Methods
A summary of the study protocol is shown in Figure 1.
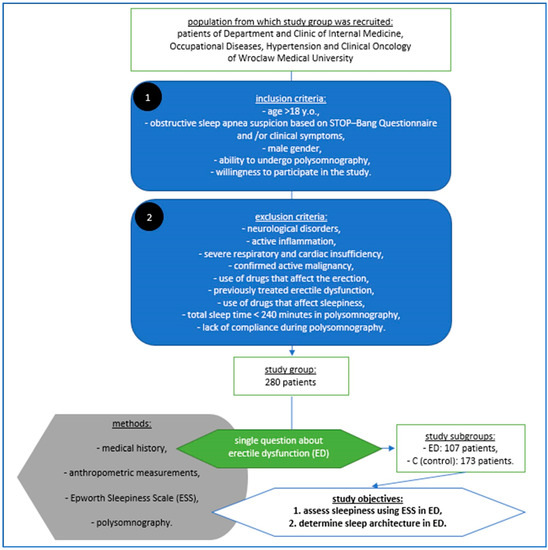
Figure 1.
Material, methods and objectives of the study according to its protocol.
The study group included 280 male patients of Department and Clinic of Internal Medicine, Occupational Diseases, Hypertension and Clinical Oncology hospitalized for the assessment of possible obstructive sleep apnea. The inclusion criteria obtain age > 18 years old, obstructive sleep apnea suspicion based on STOP–Bang Questionnaire and/or clinical symptoms, male gender, ability to undergo polysomnography and willingness to participate in the study, while the exclusion criteria included the presence of neurological disorders, active inflammation, severe respiratory and cardiac insufficiency, confirmed active malignancy, the use of drugs that affect the erection, previously treated erectile dysfunction, the use of drugs that affect sleepiness, in polysomnography total sleep time < 240 min and a lack of compliance during the study. The 107 enrolled patients had reported erectile dysfunction (ED). The control group (C) consisted of 173 patients who had no history of erectile dysfunction. ED was assessed via a single question during a clinical interview [26,27]. The Epworth sleepiness scale (ESS) was used to measure the subjects’ level of daytime sleepiness. The ESS was developed by Murray Johns at Epworth Hospital in Australia and was first reported in 1991. In the Epworth scale, the patients rate dozing in eight different situations. The minimum score of 0 indicates “would never doze”, while a maximum score of 3 indicates “high chance of dozing”. The total score can range from a minimum of 0 to a maximum of 24. Scores ≥ 10 on the ESS were indicative of excessive daytime sleepiness [15,16].
Height and weight were recorded using a nursing calibrated scale. The body mass index (BMI, calculated as weight in kilogram divided by square of height in meter) was calculated. Besides ESS, a questionnaire on OSA symptoms, OSA comorbidities, smoking status was performed. In the study group, 37.50% were smokers (n = 105), 78.21% hypertensives (n = 219), 12.85% patients had coronary heart disease (n = 36), 6.7% patients had a history of myocardial infarction (n = 19), and 8.2% were assessed after a stroke (n = 23). The mean score of the Epworth scale was 9.33 ± 5.40. The mean PSG parameters of the entire study group are presented in Table 1.

Table 1.
The PSG parameters of the entire study group.
All patients underwent a standardized overnight, single-night polysomnography in a sleep laboratory. We used the NOXA1 (NOX Medical) PSG system. Polysomnograms were assessed in 30 s epochs according to the AASM (American Academy of Sleep Medicine) standard criteria for sleep scoring. PSG outcome variables included sleep latency, total sleep time (TST) and sleep efficiency (%), the ratio of N1, N2, N3 and the stage of REM. Abnormal respiratory events were scored from the pressure airflow signal evaluated according to the standard criteria of the American Academy of Sleep Medicine Task Force [28]. Apneas were defined as the absence of airflow for ≥10 s. Hypopnea was defined as a reduction in the amplitude of breathing by ≥30% for ≥10 s with a decline ≥3% in blood oxygen saturation or an arousal. A NONIN WristOx2 3150 pulse oximeter (Nonin Medical Inc., Plymouth, MN, USA), coupled with the PSG system, was used to record the oxygen saturation level. To analyze the full polysomnography recording, the Noxturnal software (Nox Medical, Reykjavík, Iceland) was used. A certified, qualified physician (H.M.) from the sleep laboratory scored and manually analyzed the data in accordance with the AASM guidelines.
Statistical analysis was conducted using the statistical software Statistica 12 PL, Statistica, Tulsa, US. For quantitative variables, arithmetic means and standard deviations were calculated for the estimated parameters in the studied groups. The distribution of the variables was tested using the Shapiro–Wilk test. In cases of quantitative variables manifesting the normal distribution in further statistical analysis, the t test for unlinked variables was used. In cases of variables manifesting distribution distinct from the normal one, the nonparametric equivalent of the t test, i.e., the Mann–Whitney U test was used. In order to detect relationships between the studied variables, univariate regression analysis was performed. The results at the level of p < 0.05 were accepted as statistically significant.
This study was approved by the Ethical Committee of Wroclaw Medical University (ID KB-227/2015) and was conducted in accordance with the Declaration of Helsinki. All patients signed an informed consent form for this study.
3. Results
The mean BMI was higher in the ED group compared to the that of the control. The mean age, BMI, height, body mass and smoking status are shown in Table 2.

Table 2.
The height, body mass, BMI (body mass index), and smoking status of patients with erectile dysfunction (ED) and control (C) subjects.
In the ED group, statistically significantly AHI (apnea/hypopnea index) < 5 (AHI excluding OSA) was observed less frequently than in the control group. The number of subjects according to AHI in patients with erectile dysfunction (ED) and control (C) subjects is shown in Table 3.

Table 3.
The number of subjects according to AHI in patients with erectile dysfunction (ED) and control (C) subjects.
In the erectile dysfunction group (ED), we observed a higher ESS total score and lower mean and minimal oxygen saturation levels compared to those of the control group. The mean oxygen desaturation levels were lower in the ED group in comparison with the control group. The N1 sleep phase was increased in comparison with the control group. The polysomnographic parameters and ESS (Epworth sleepiness scale) score in patients with erectile dysfunction (ED) and control (C) subjects are presented in Table 4.

Table 4.
The polysomnographic parameters and ESS (Epworth sleepiness scale) score in patients with erectile dysfunction (ED) and control (C) subjects.
Then, we performed an additional analysis considering the occurrence of hypertension. Hypertension is an important risk factor for erectile dysfunction and may also affect the sleep architecture and the level of sleepiness. Therefore, it might affect the results of the study. We divided all participants into two subgroups (normotensive and hypertensives). In univariate regression analysis, in the hypertensive group, we observed a relationship between the presence of erectile dysfunction and mean saturation (r = −0.20; p < 0.05), minimal saturation (r = −0.14; p < 0.05), and N1 (%TST) (r = 0.31, p < 0.05). In the normotensive group, we observed a relationship between ED and AHI (apnea/hypopnea index) (r = 0.43, p < 0.05), ED and ODI (r = 0.45, p < 0.05) and ED and TST (r = −0.82, p < 0.05). In this group, we also observed a relationship between ED and mean saturation (r = −0.44, p < 0.05) and minimal saturation (r = −0.45, p < 0.05). Interestingly, in the normotensive group, we observed a relationship between ED and N2 (%TST) (r = −0.57, p < 0.05), N3 (%TST) (r = 0.62, p < 0.05) and REM (rapid eye movement) sleep (%TST) (r = −0.61, p < 0.05). The result of the univariate regression analyses in the hypertensive and normotensive groups are summarized in Table 5.

Table 5.
The results of univariate regression analyzes between erectile dysfunction (ED) and polysomnographic parameters in hypertensive and normotensive groups.
4. Discussion
Obstructive sleep apnea affects both the patient and the sexual partner [29,30,31]. In a cross-sectional study of 401 men referred to a sleep lab for suspected OSA, 92% were diagnosed with OSA, and ED was present in 69% of those with OSA and 34% without OSA [8]. The evidence for the association between OSA and ED remains inconclusive, with some studies suggesting no association, or an association being shown for severe OSA only [32,33]. The pathogenesis of ED in OSA is complex. The factors implicated include increased sympathetic activity, oxidative stress, endothelial dysfunction a reduction in nitric oxide formation [34,35]. Sleep fragmentation and a lack of rapid eye movement (REM) periods related to OSA may be involved in ED pathogenesis because physiological erections appear to help maintain erectile function through cavernous tissue oxygenation during the REM sleep period [36,37]. ED is also related to changes in the hormonal axis caused by sleep pattern changes associated with OSA [38].
The main result of this study is an increased ESS total score in patients with erectile dysfunction compared to those of the control group, although the AHI and ODI (oxygen desaturation index) were similar in both groups. It is worth noting that this is a novel observation. In the ED group, the mean saturation, minimal saturation and mean desaturation levels were decreased compared to those of control group. Interestingly, we also observed increased N1 sleep duration (%TST) in the ED group. N1 is the lighter stage of NREM sleep, which usually occurs at the beginning of sleep and often alternates with brief arousal episodes. N1 is the period of transition from unsynchronized beta and gamma brain waves to more synchronized but slower alpha waves, and then to theta waves with slow rolling eye movement. This stage comprises only about 5% of the total sleep time. In our study, N1 comprises as much as 17.3% of TST in patients with erectile dysfunction. This phenomenon may be a consequence of increased activity of the sympathetic nervous system, which is common in OSA due to repeated hypoxemia. Additionally, fatigue and a decrease in rapid eye movement (REM) sleep period may provoke the deterioration in the quality of erections [39,40]. These results agree with those of our study. We observed a negative relationship between ED and the REM (rapid eye movement) period (%TST); however, we did not observe statistical differences between the REM period in ED and controls.
In our study, the patients with ED had lower mean oxygen saturation, minimal saturation and mean desaturation levels compared to the control group. These results are in agreement with those of previous studies, which showed that recurrent apnea attacks in patients with OSA cause hypoxia reperfusion injury and oxidative stress, endothelial dysfunction and, consequently, ED [41]. Severe and moderate OSA was more prevalent in the ED group than in the control group, which may be a possible explanation for these observations.
OSA is a known risk factor for erectile dysfunction; however, the mechanism underlying ED in patients with OSA is complex and remains unclear. The data on EDS (excessive daytime sleepiness) and ED are limited and often contradictory. Surprisingly, it was demonstrated that ED subjects had significantly lower ESS and SaO2 [42]. Recently. Jeon also showed that ESS is inversely correlated with the International Index of Erectile Function (KIIEF-5) [43]. We have shown that patients with ED have increased total ESS scores compared to those of the control group, which is a novel observation. This is most likely the result of increased frequency of severe and moderate OSA in the ED group. However, we have not observed a statistically significant difference in AHI between the ED and control groups; thus, other mechanisms may be involved.
The most unexpected result of our study is the presence of a relationship between ED and N3 sleep (%TST) in normotensive patients; however, the duration of N3 was similar between the ED group and the control group. We did not observe this relationship in hypertensive patients, which might suggest different relationships between the structure of sleep and ED depending on the blood pressure levels. We have also observed a positive relationship between AHI, ODI (oxygen desaturation index), and erectile dysfunction, and a negative relationship between mean saturation, minimal saturation, TST and erectile dysfunction in the normotensive group. The relationship between AHI, ODI, TST and ED was not observed in hypertensive patients.
The present study confirms the strong role of hypoxia in erectile dysfunction. Firstly, we observed decreased mean oxygen saturation as well as minimal oxygen saturation levels in the erectile dysfunction group compared to those of the control group. Secondly, both in hypertensive and normotensive patients, mean and minimal oxygen saturation were related to erectile dysfunction. Chronic hypoxia may be observed in physiological conditions such as aging, as well as in many pathological conditions such as smoking and chronic obstructive pulmonary disease (COPD), heart and respiratory failure, obstructive sleep apnea, atherosclerosis, diabetes, and hypertension. It is worth noting that CPAP (continuous positive airway pressure), when used with testosterone replacement therapy, can increase total testosterone and cause an improvement in the indicators of altered nocturnal penile erection episodes [44]. In the present study’s regression analysis, the oxygen desaturation index was associated with ED only in the normotensive group. These results may indicate the role of oxygen level drops in normotensive but not in hypertensive patients. Recently, Feng demonstrated that minimum oxygen saturation and average oxygen saturation may predict the occurrence of ED in obstructive sleep apnea patients [45]. The possible mechanisms involved in hypoxia on erectile dysfunction mainly include excessive reactive-oxygen-species-mediated oxidative stress, hypoxia-inducible factors-1α mediated endothelial cell apoptosis and proliferation inhibition, endothelial dysfunction, reduced nitric oxide production and systematic inflammation [46].
Short sleep duration is considered a risk factor for erectile dysfunction. The sleep duration recommended by the AASM is between 7 and 9 h of sleep. A short sleep duration is defined as habitual sleep time of less than 6 h [47]. It was previously shown that short sleep is associated with a large group of disorders, such as hypertension, diabetes, major depressive disorder, and other morbidities [48,49]. Sleep duration is decreasing in modern societies, as large cohorts studies have shown [50,51,52]. As much as 29.1% of adults suffered from short sleep duration is United States [53]. The pathomechanisms of erectile dysfunction in short sleepers are complex and lead to hypothalamic–pituitary–adrenal axis overactivity, resulting in cortisol release as well as an autonomic nervous system imbalance, consequently resulting in catecholamines release and vasoconstriction. Moreover, short sleep duration may reduce the testosterone level and the frequency of REM sleep and sleep-related erections (SREs) [54]. SREs or nocturnal penile tumescence (NPT) can be measured using PSG. The majority of NPT occurrences, which are a physiological and spontaneous phenomenon, are related to REM sleep [55]. The erection initiates during the shift from NREM to REM sleep with full tumescence throughout REM; however, in the present study, we did not measure NPT. It is worth noting that short sleep duration is related to many negative health outcomes such as cardiovascular diseases, increased morbidity and mortality [56,57]. In the present study, we did not observe statistically significant differences in total sleep time between the patients with erectile dysfunction and the control group. Although sleep time was comparable, we noticed an increased stage N1 sleep duration. A high percentage of the stage N1 sleep is usually a result of frequent arousals caused by sleep disorders or environmental disturbances. Thus, in the present study, altered sleep structure, but not sleep duration, was related to erectile dysfunction.
Summarizing, we studied the sleep structure and level of sleepiness of a relatively large ED cohort using polysomnography, which is the gold standard for sleep assessment. We have confirmed many observations that have been described before, especially the relationship between OSA and ED; however, this study has some novel conclusions. We have also observed an increased sleepiness level in ED subjects in comparison to that of the control group. We have shown the importance of oxygen saturation parameters and altered sleep architecture in erectile dysfunction. We have also confirmed the relationship between ED and sleep parameters in normotensive but not in hypertensive patients. These results indicate a different mechanism of ED in hypertension; thus, further studies are needed to explain these observations.
The strengths of this study are its large population and use of the gold standard in sleep disorders diagnosis (avPSG). However, several limitations of the present study should be highlighted. Firstly, we did not use any scales for measuring erectile dysfunction, including the International Index of Erectile Dysfunction (IIED), which may be a major confounder. The cause, type and degree of severity of ED was not studied. We did not collect data on hypotensive therapy, which could affect erectile dysfunction. In addition, there was a small number of patients in the group without OSA compared to the OSA group.
5. Conclusions
Patients with erectile dysfunction are more likely to be affected by obstructive sleep apnea, altered sleep architecture and oxygen saturation parameters compared to the control group. The sleepiness measured using ESS in patients with erectile dysfunction is increased compared to that of the control group.
Author Contributions
Conceptualization, H.M.; Investigation, H.M., T.W., Z.D., A.W., S.W. and P.M.; Methodology, H.M.; Project administration, H.M.; Software, R.P. and P.G.; Supervision, H.M., R.P., R.S., G.M. and P.G.; Writing—original draft, H.M.; Writing—review and editing, P.G. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Wroclaw Medical University (project number as recorded in the Simple system SUBZ.E264.23.039).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of Wroclaw Medical University.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author. The data are not publicly available.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rosen, R.C.; Riley, A.; Wagner, G.; Osterloh, I.H.; Kirkpatrick, J.; Mishra, A. The International Index of Erectile Function (IIEF): A multidimensional scale for assessment of erectile dysfunction. Urology 1997, 49, 822–830. [Google Scholar] [CrossRef] [PubMed]
- Grant, P.; Jackson, G.; Baig, I.; Quin, J. Erectile dysfunction in general medicine. Clin. Med. 2013, 13, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Kałka, D.; Gebala, J.; Borecki, M.; Pilecki, W.; Rusiecki, L. Return to sexual activity after myocardial infarction—An analysis of the level of knowledge in men undergoing cardiac rehabilitation. Eur. J. Intern. Med. 2017, 37, e31–e33. [Google Scholar] [CrossRef]
- Shamloul, R.; Ghanem, H. Erectile dysfunction. Lancet 2013, 381, 153–165. [Google Scholar] [CrossRef]
- McVary, K.T. Clinical practice. Erectile dysfunction. N. Engl. J. Med. 2007, 357, 2472–2481. [Google Scholar] [CrossRef] [PubMed]
- Johannes, C.B.; Araujo, A.B.; Feldman, H.A.; Derby, C.A.; Kleinman, K.P.; McKinlay, J.B. Incidence of erectile dysfunction in men 40 to 69 years old: Longitudinal results from the Massachusetts male aging study. J. Urol. 2000, 163, 460–463. [Google Scholar] [CrossRef] [PubMed]
- Stannek, T.; Hürny, C.; Schoch, O.D.; Bucher, T.; Münzer, T. Factors affecting self-reported sexuality in men with obstructive sleep apnea syndrome. J. Sex Med. 2009, 6, 3415–3424. [Google Scholar] [CrossRef]
- Budweiser, S.; Enderlein, S.; Jörres, R.A.; Hitzl, A.P.; Wieland, W.F.; Pfeifer, M.; Arzt, M. Sleep apnea is an independent correlate of erectile and sexual dysfunction. J. Sex Med. 2009, 6, 3147–3157. [Google Scholar] [CrossRef]
- Young, T.; Peppard, P.E.; Gottlieb, D.J. Epidemiology of obstructive sleep apnea: A population health perspective. Am. J. Respir. Crit Care Med. 2002, 165, 1217–1239. [Google Scholar] [CrossRef]
- Benjafield, A.V.; Ayas, N.T.; Eastwood, P.R.; Heinzer, R.; Ip, M.S.M.; Morrell, M.J.; Nunez, C.M.; Patel, S.R.; Penzel, T.; Pépin, J.L.; et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: A literature-based analysis. Lancet Respir. Med. 2019, 7, 687–698. [Google Scholar] [CrossRef]
- Young, T.; Palta, M.; Dempsey, J.; Skatrud, J.; Weber, S.; Badr, S. The occurrence of sleep-disordered breathing among middle-aged adults. N. Engl. J. Med. 1993, 328, 1230–1235. [Google Scholar] [CrossRef] [PubMed]
- Margel, D.; Cohen, M.; Livne, P.M.; Pillar, G. Severe, but not mild, obstructive sleep apnea syndrome is associated with erectile dysfunction. Urology 2004, 63, 545–549. [Google Scholar] [CrossRef] [PubMed]
- Perimenis, P.; Konstantinopoulos, A.; Karkoulias, K.; Markou, S.; Perimeni, P.; Spyropoulos, K. Sildenafil combined with continuous positive airway pressure for treatment of erectile dysfunction in men with obstructive sleep apnea. Int. Urol. Nephrol. 2007, 39, 547–552. [Google Scholar] [CrossRef]
- Malhotra, A.; White, D.P. Obstructive sleep apnoea. Lancet 2002, 360, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Johns, M.W. A new method for measuring daytime sleepiness—The epworth sleepiness scale. Sleep 1991, 14, 540–545. [Google Scholar] [CrossRef]
- Fong, S.Y.; Ho, C.K.; Wing, Y.K. Comparing MSLT and ESS in the measurement of excessive daytime sleepiness in obstructive sleep apnoea syndrome. J. Psychosom. Res. 2005, 58, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Van der Heide, A.; van Schie, M.K.; Lammers, G.J.; Dauvilliers, Y.; Arnulf, I.; Mayer, G.; Bassetti, C.; Ding, C.-L.; Lehert, P.; Van Dijk, J.G. Comparing Treatment Effect Measurements in Narcolepsy: The Sustained Attention to Response Task, Epworth Sleepiness Scale and Maintenance of Wakefulness Test. Sleep 2015, 38, 1051–1058. [Google Scholar] [CrossRef]
- Mills, R.J.; Koufali, M.; Sharma, A.; Tennant, A.; Young, C.A. Is the Epworth sleepiness scale suitable for use in stroke? Top. Stroke Rehabil. 2013, 20, 493–499. [Google Scholar] [CrossRef]
- Lee, C.H.; Ng, W.Y.; Hau, W.; Ho, H.H.; Tai, B.C.; Chan, M.Y.; Richards, A.M.; Tan, H.-C. Excessive daytime sleepiness is associated with longer culprit lesion and adverse outcomes in patients with coronary artery disease. Sleep Med. 2013, 15, 1267–1272. [Google Scholar] [CrossRef]
- Wu, X.; Fu, C.; Zhang, S.; Liu, Z.; Li, S.; Jiang, L. Adaptive servoventilation improves cardiac dysfunction and prognosis in heart failure patients with sleep-disordered breathing: A meta-analysis. Clin. Respir. J. 2015, 25, 547–557. [Google Scholar] [CrossRef]
- Quigg, M.; Gharai, S.; Ruland, J.; Schroeder, C.; Hodges, M.; Ingersoll, K.S.; Thorndike, F.P.; Yan, G.; Ritterband, L.M. Insomnia in epilepsy is associated with continuing seizures and worse quality of life. Epilepsy Res. 2016, 122, 91–96. [Google Scholar] [CrossRef]
- Cochen De Cock, V.; Bayard, S.; Jaussent, I.; Charif, M.; Grini, M.; Langenier, M.C.; Yu, H.; Lopez, R.; Geny, C.; Carlander, B.; et al. Daytime sleepiness in Parkinson’s disease: A reappraisal. PLoS ONE 2014, 9, e107278. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Huang, X.; Luo, Z.; Xu, X.; Zhao, X.; He, Q. Sleep quality, daytime sleepiness and health-related quality-of-life in maintenance haemodialysis patients. J. Int. Med. Res. 2016, 44, 698–709. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Peralta, F.; Abreu, C.; Castro, J.C.; Alcarria, E.; Cruz-Bravo, M.; Garcia-Llorente, M.J.; Albornos, C.; Moreno, C.; Cepeda, M.; Almódovar, F. An association between liraglutide treatment and reduction in excessive daytime sleepiness in obese subjects with type 2 diabetes. BMC Endocr. Disord. 2015, 4, 78. [Google Scholar] [CrossRef] [PubMed]
- Purabdollah, M.; Lakdizaji, S.; Rahmani, A.; Hajalilu, M.; Ansarin, K. Relationship between Sleep Disorders, Pain and Quality of Life in Patients with Rheumatoid Arthritis. J. Caring Sci. 2015, 4, 233–241. [Google Scholar] [CrossRef]
- Lopez, D.S.; Wang, R.; Tsilidis, K.K.; Zhu, H.; Daniel, C.R.; Sinha, A.; Canfield, S. Role of Caffeine Intake on Erectile Dysfunction in US Men: Results from NHANES 2001–2004. PLoS ONE 2015, 28, e0123547. [Google Scholar] [CrossRef]
- Chew, S.K.; Taouk, Y.; Xie, J.; Nicolaou, T.E.; Wang, J.J.; Wong, T.Y.; Lamoureux, E.L. Relationship between diabetic retinopathy, diabetic macular oedema and erectile dysfunction in type 2 diabetics. Clin. Exp. Ophthalmol. 2013, 41, 683–689. [Google Scholar] [CrossRef]
- Berry, R.B.; Budhiraja, R.; Gottlieb, D.J.; Gozal, D.; Iber, C.; Kapur, V.K.; Marcus, C.L.; Mehra, R.; Parthasarathy, S.; Quan, S.F.; et al. American Academy of Sleep Medicine. Rules for scoring respiratory events in sleep: Update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J. Clin. Sleep Med. 2012, 8, 597–619. [Google Scholar]
- Broström, A.; Johansson, P.; Albers, J.; Wiberg, J.; Svanborg, E.; Fridlund, B. 6-month CPAP-treatment in a young male patient with severe obstructive sleep apnoea syndrome—A case study from the couple’s perspective. Eur. J. Cardiovasc. Nurs. 2008, 7, 103–112. [Google Scholar] [CrossRef]
- Broström, A.; Johansson, P.; Strömberg, A.; Albers, J.; Mårtensson, J.; Svanborg, E. Obstructive sleep apnoea syndrome-patients’ perceptions of their sleep and its effects on their life situation. J. Adv. Nurs. 2007, 57, 318–327. [Google Scholar] [CrossRef]
- Stålkrantz, A.; Broström, A.; Wiberg, J.; Svanborg, E.; Malm, D. Everyday life for the spouses of patients with untreated OSA syndrome. Scand. J. Caring Sci. 2012, 26, 324–332. [Google Scholar] [CrossRef]
- Hanak, V.; Jacobson, D.; McGree, M.; Sauver, J.S.; Lieber, M.M.; Olson, E.J.; Somers, V.K.; Gades, N.M.; Jacobsen, S.J. Snoring as a risk factor for sexual dysfunction in community men. J. Sex Med. 2008, 5, 898–908. [Google Scholar] [CrossRef]
- Seftel, A.D.; Strohl, K.P.; Loye, T.L.; Bayard, D.; Kress, J.; Netzer, N.C. Erectile dysfunction and symptoms of sleep disorders. Sleep 2002, 25, 643–647. [Google Scholar] [CrossRef] [PubMed]
- Husnu, T.; Ersoz, A.; Bulent, E.; Tacettin, O.; Remzi, A.; Bulent, A.; Aydin, M. Obstructive sleep apnea syndrome and erectile dysfunction: Does long term continuous positive airway pressure therapy improve erections? Afr. Health Sci. 2015, 15, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Szymański, F.; Puchalski, B.; Filipiak, K. Obstructive sleep apnea, atrial fibrillation and erectile dysfunction: Are they only coexisting conditions or a new clinical syndrome? The concept of the OSAFED syndrome. Pol. Arch. Med. Wewn. 2013, 123, 701–707. [Google Scholar] [CrossRef] [PubMed]
- Andersen, M.L.; Santos-Silva, R.; Bittencourt, L.R.; Tufik, S. Prevalence of erectile dysfunction complaints associated with sleep disturbances in Sao Paulo, Brazil: A population-based survey. Sleep Med. 2010, 11, 1019–1024. [Google Scholar] [CrossRef]
- Teloken, P.E.; Smith, E.B.; Lodowsky, C.; Freedom, T.; Mulhall, J.P. Defining association between sleep apnea syndrome and erectile dysfunction. Urology 2006, 67, 1033–1037. [Google Scholar] [CrossRef]
- Luboshitzky, R.; Aviv, A.; Hefetz, A.; Herer, P.; Shen-Orr, Z.; Lavie, L.; Lavie, P. Decreased pituitary-gonadal secretion in men with obstructive sleep apnea. J. Clin. Endocrinol. Metab. 2002, 87, 3394–3398. [Google Scholar] [CrossRef]
- Somers, V.K.; Dyken, M.E.; Clary, M.P.; Abboud, F.M. Sympathetic neural mechanisms in obstructive sleep apnea. J. Clin. Investig. 1995, 96, 1897–1904. [Google Scholar] [CrossRef]
- Wolf, J.; Lewicka, J.; Narkiewicz, K. Obstructive sleep apnea: An update on mechanisms and cardiovascular consequences. Nutr. Metab. Cardiovasc. Dis. 2007, 17, 233–240. [Google Scholar] [CrossRef]
- Christou, K.; Markoulis, N.; Moulas, A.N.; Pastaka, C.; Gourgoulianis, K.I. Reactive oxygen metabolites (ROMs) as an index of oxidative stress in obstructive sleep apnea patients. Sleep Breath 2003, 7, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, M.A.; Guilleminault, C.; Ramos, E.; Palha, A.; Paiva, T. Erectile dysfunction, obstructive sleep apnea syndrome and nasal CPAP treatment. Sleep Med. 2005, 6, 333–339. [Google Scholar] [CrossRef]
- Jeon, Y.J.; Yoon, D.W.; Han, D.H.; Won, T.B.; Kim, D.Y.; Shin, H.W. Low Quality of Life and Depressive Symptoms as an Independent Risk Factor for Erectile Dysfunction in Patients with Obstructive Sleep Apnea. J. Sex Med. 2015, 12, 2168–2177. [Google Scholar] [CrossRef] [PubMed]
- Madaeva, I.M.; Berdina, O.N.; Semenova, N.V.; Madaev, V.V.; Rychkova, L.V.; Kolesnikova, L.I. Sindrom obstruktivnogo apnoé sna i vozrastnoĭ gipogonadizm [Obstructive sleep apnea syndrome and age-related hypohonadism]. Zhurnal Nevrol. Psikhiatrii Im. SS Korsakova. 2017, 117, 79–83. (In Russian) [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Yang, Y.; Chen, L.; Guo, R.; Liu, H.; Li, C.; Wang, Y.; Dong, P.; Li, Y. Prevalence and Characteristics of Erectile Dysfunction in Obstructive Sleep Apnea Patients. Front. Endocrinol. 2022, 13, 812974. [Google Scholar] [CrossRef]
- Konstantinovsky, A.; Tamir, S.; Katz, G.; Tzischinsky, O.; Kuchersky, N.; Blum, N.; Blum, A. Erectile Dysfunction, Sleep Disorders, and Endothelial Function. Isr. Med. Assoc. J. 2019, 21, 408–411. [Google Scholar]
- Hirshkowitz, M.; Whiton, K.; Albert, S.M.; Alessi, C.; Bruni, O.; DonCarlos, L.; Hazen, N.; Herman, J.; Adams Hillard, P.J.; Katz, E.S.; et al. National Sleep Foundation’s updated sleep duration recommendations: Final report. Sleep Health 2015, 1, 233–243. [Google Scholar] [CrossRef]
- Itani, O.; Jike, M.; Watanabe, N.; Kaneita, Y. Short sleep duration and health outcomes: A systematic review, meta-analysis, and meta-regression. Sleep Med. 2017, 32, 246–256. [Google Scholar] [CrossRef]
- Dashti, H.S.; Scheer, F.A.; Jacques, P.F.; Lamon-Fava, S.; Ordovás, J.M. Short sleep duration and dietary intake: Epidemiologic evidence, mechanisms, and health implications. Adv. Nutr. 2015, 6, 648–659. [Google Scholar] [CrossRef] [PubMed]
- National Center for Health Statistics. Quick-Stats: Percentage of adults who reported an average of ≤6 h of sleep per 24-h period, by sex and age group—United States, 1985 and 2004. MMWR Morb. Mortal. Wkly. Rep. 2005, 54, 933. [Google Scholar]
- Kronholm, E.; Partonen, T.; Laatikainen, T.; Peltonen, M.; Härmä, M.; Hublin, C.; Kaprio, J.; Aro, A.R.; Partinen, M.; Fogelholm, M.; et al. Trends in self-reported sleep duration and insomnia-related symptoms in Finland from 1972 to 2005, a comparative review and re-analysis of Finnish population samples. J. Sleep Res. 2008, 17, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Jean-Louis, G.; Williams, N.J.; Sarpong, D.; Pandey, A.; Youngstedt, S.; Zizi, F.; Ogedegbe, G. Associations between inadequate sleep and obesity in the US population: Analysis of national health interview survey (1977–2009). BMC Public Health 2014, 14, 290. [Google Scholar] [CrossRef] [PubMed]
- Vgontzas, A.N.; Fernandez-Mendoza, J.; Liao, D.; Bixler, E.O. Insomnia with objective short sleep duration: The most biologically severe phenotype of the disorder. Sleep Med. Rev. 2013, 17, 241–254. [Google Scholar] [CrossRef]
- Zhang, F.; Xiong, Y.; Qin, F.; Yuan, J. Short Sleep Duration and Erectile Dysfunction: A Review of the Literature. Nat. Sci. Sleep 2022, 27, 1945–1961. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Lin, H.; Zhang, Y.; Wang, R. The Role of Nocturnal Penile Tumescence and Rigidity (NPTR) Monitoring in the Diagnosis of Psychogenic Erectile Dysfunction: A Review. Sex Med. Rev. 2019, 7, 442–454. [Google Scholar] [CrossRef] [PubMed]
- Ai, S.; Zhang, J.; Zhao, G.; Wang, N.; Li, G.; So, H.-C.; Liu, Y.; Chau, S.W.-H.; Chen, J.; Tan, X.; et al. Causal associations of short and long sleep durations with 12 cardiovascular diseases: Linear and nonlinear Mendelian randomization analyses in UK Biobank. Eur. Heart J. 2021, 42, 3349–3357. [Google Scholar] [CrossRef]
- Grandner, M.A.; Patel, N.P.; Gehrman, P.R.; Perlis, M.L.; Pack, A.I. Problems associated with short sleep: Bridging the gap between laboratory and epidemiological studies. Sleep Med. Rev. 2010, 14, 239–247. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).