The Impact of Systemic Oncological Treatments on the Fertility of Adolescents and Young Adults—A Systematic Review
Abstract
1. Introduction
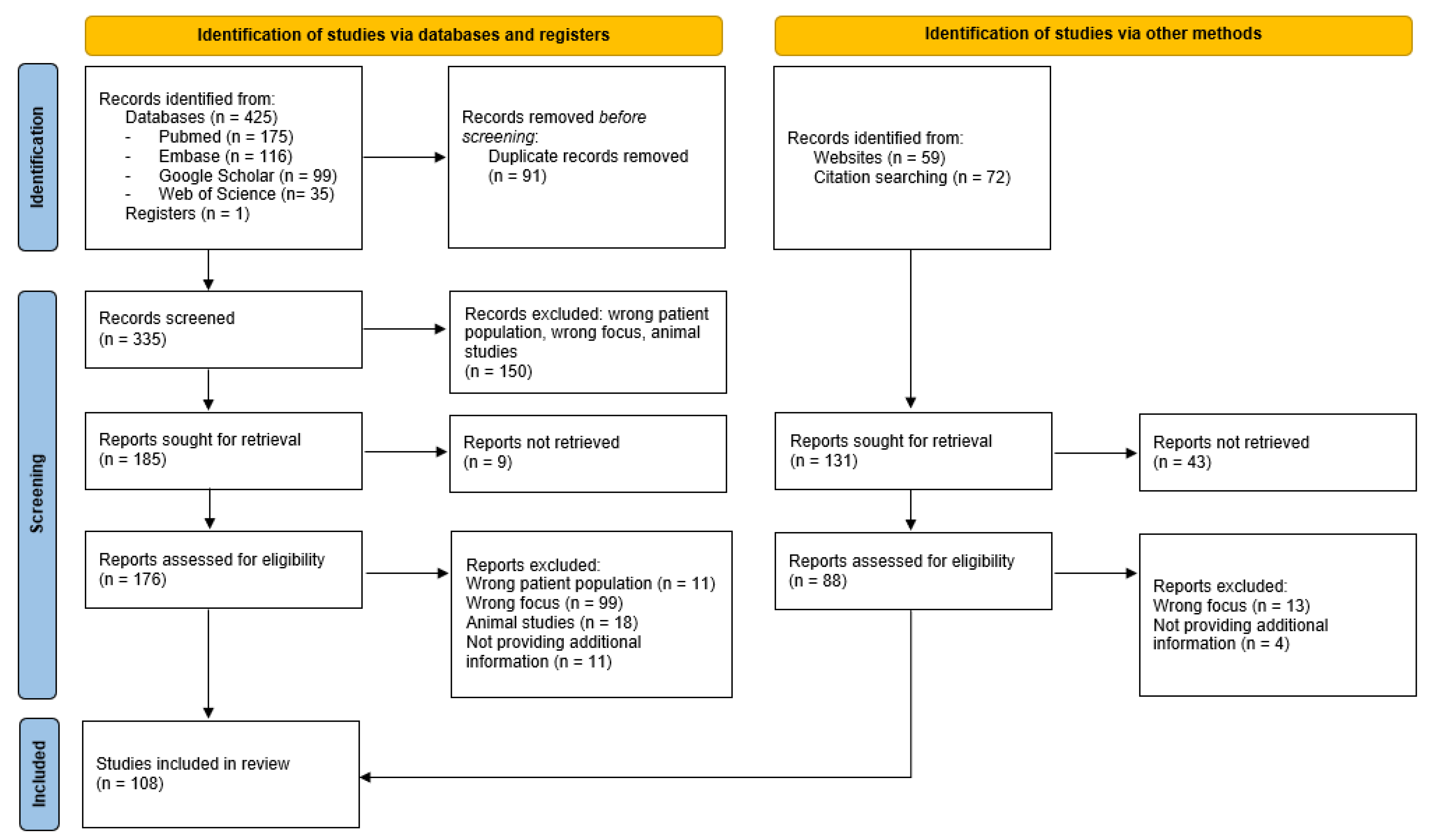
2. Materials and Methods
3. Results
3.1. Women
3.1.1. Folliculogenesis and Measuring Gonadal Function
3.1.2. Mechanisms of Gonadotoxicity
Chemotherapy
Targeted Therapy
- Monoclonal antibodies
- Tyrosine kinase inhibitors
- Other
Immunotherapy
3.1.3. Gonadotoxic Effect of Systemic Oncological Treatments
Chemotherapy
- Alkylating agents
- Anthracyclines
- Taxanes
- Platinum agents
- Antimetabolites
Targeted Therapy
- Monoclonal antibodies
- Tyrosine kinase inhibitors
- Others
Immunotherapy
3.2. Men
3.2.1. Spermatogenesis and Measuring Gonadal Function
3.2.2. Mechanisms of Gonadotoxicity
Chemotherapy
Targeted Therapy
Immunotherapy
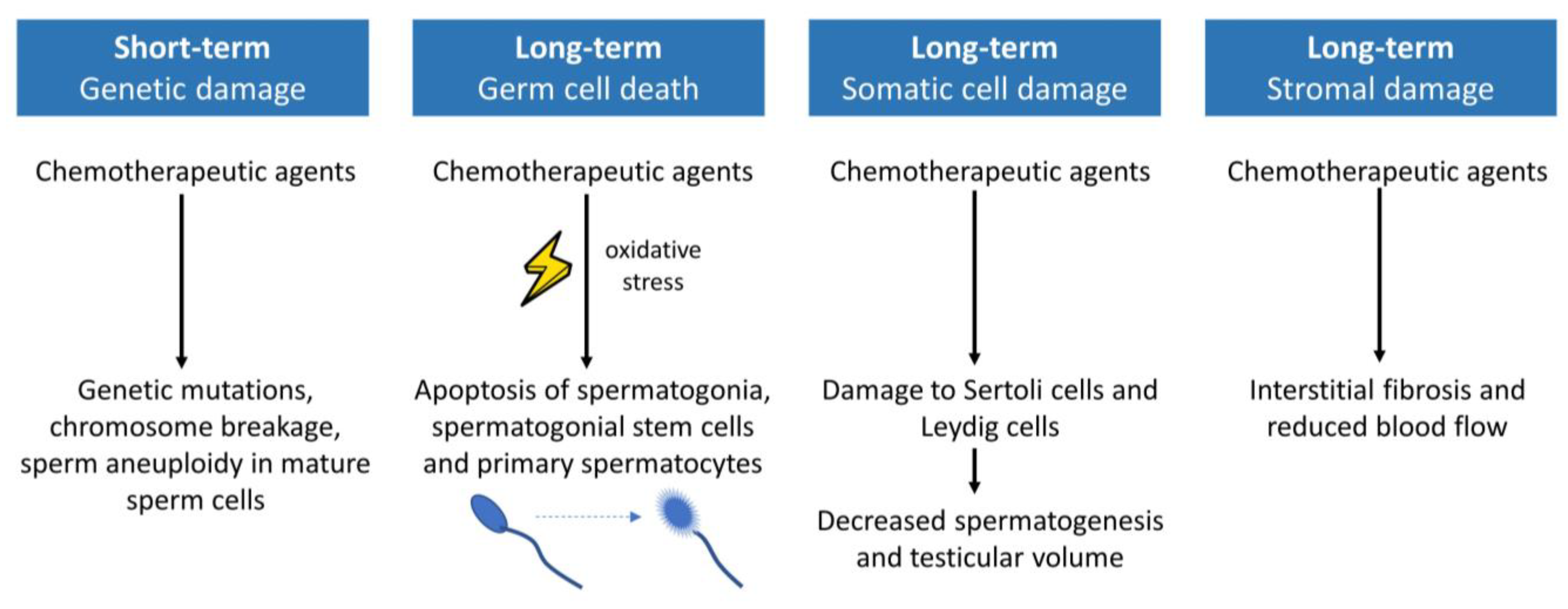
3.2.3. Gonadotoxic Effect of Systemic Oncological Treatments
Chemotherapy
- Alkylating agents
- Platinum agents
- Anthracyclines
- Taxanes
Targeted Therapy
Immunotherapy
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ferrari, A.; Stark, D.; Peccatori, F.A.; Fern, L.; Laurence, V.; Gaspar, N.; Bozovic-Spasojevic, I.; Smith, O.; de Munter, J.; Derwich, K.; et al. Adolescents and Young Adults (AYA) with Cancer: A Position Paper from the AYA Working Group of the European Society for Medical Oncology (ESMO) and the European Society for Paediatric Oncology (SIOPE). ESMO Open 2021, 6, 100096. [Google Scholar] [CrossRef]
- Anderson, B.; Albritton, K. Closing the Gap: Research and Care Imperatives for Adolescents and Young Adults with Cancer; 2 0608020F; Overview of the Adolescent and Young Adult Oncology Progress Review Group (AYAO PRG); Priority Recommendations from the AYAO PRG Roundtable; National Cancer Institute: Bethesda, MD, USA, 2006. [Google Scholar]
- Miller, K.D.; Fidler-Benaoudia, M.; Keegan, T.H.; Hipp, H.S.; Jemal, A.; Siegel, R.L. Cancer Statistics for Adolescents and Young Adults, 2020. CA Cancer J. Clin. 2020, 70, 443–459. [Google Scholar] [CrossRef] [PubMed]
- You, L.; Lv, Z.; Li, C.; Ye, W.; Zhou, Y.; Jin, J.; Han, Q. Worldwide Cancer Statistics of Adolescents and Young Adults in 2019: A Systematic Analysis of the Global Burden of Disease Study 2019. ESMO Open 2021, 6, 100255. [Google Scholar] [CrossRef] [PubMed]
- Sodergren, S.C.; Husson, O.; Robinson, J.; Rohde, G.E.; Tomaszewska, I.M.; Vivat, B.; Dyar, R.; Darlington, A.S. Systematic Review of the Health-Related Quality of Life Issues Facing Adolescents and Young Adults with Cancer. Qual. Life Res. 2017, 26, 1659. [Google Scholar] [CrossRef] [PubMed]
- Husson, O.; Huijgens, P.C.; van der Graaf, W.T.A. Psychosocial Challenges and Health-Related Quality of Life of Adolescents and Young Adults with Hematologic Malignancies. Blood 2018, 132, 385–392. [Google Scholar] [CrossRef]
- Anderson, R.A.; Amant, F.; Braat, D.; D’Angelo, A.; Chuva de Sousa Lopes, S.M.; Demeestere, I.; Dwek, S.; Frith, L.; Lambertini, M.; Maslin, C.; et al. ESHRE Guideline: Female Fertility Preservation†. Hum. Reprod. Open 2020, 2020, hoaa052. [Google Scholar] [CrossRef]
- Oktay, K.; Harvey, B.E.; Partridge, A.H.; Quinn, G.P.; Reinecke, J.; Taylor, H.S.; Hamish Wallace, W.; Wang, E.T.; Loren, A.W. Fertility Preservation in Patients with Cancer: ASCO Clinical Practice Guideline Update. J. Clin. Oncol. 2018, 36, 1994–2001. [Google Scholar] [CrossRef]
- Lambertini, M.; Peccatori, F.A.; Demeestere, I.; Amant, F.; Wyns, C.; Stukenborg, J.B.; Paluch-Shimon, S.; Halaska, M.J.; Uzan, C.; Meissner, J.; et al. Fertility Preservation and Post-Treatment Pregnancies in Post-Pubertal Cancer Patients: ESMO Clinical Practice Guidelines†. Ann. Oncol. 2020, 31, 1664–1678. [Google Scholar] [CrossRef] [PubMed]
- Guida, M.; Antonietta Castaldi, M.; Rosamilio, R.; Giudice, V.; Orio, F.; Selleri, C. Reproductive Issues in Patients Undergoing Hematopoietic Stem Cell Transplantation: An Update. J. Ovarian Res. 2016, 9, 72. [Google Scholar] [CrossRef]
- Janssen, S.H.M.; van der Graaf, W.T.A.; van der Meer, D.J.; Manten-Horst, E.; Husson, O. Adolescent and Young Adult (Aya) Cancer Survivorship Practices: An Overview. Cancers 2021, 13, 4847. [Google Scholar] [CrossRef]
- Coccia, P.F.; Pappo, A.S.; Beaupin, L.; Borges, V.F.; Borinstein, S.C.; Chugh, R.; Dinner, S.; Folbrecht, J.; Frazier, A.L.; Goldsby, R.; et al. Adolescent and Young Adult Oncology, Version 2.2018, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc Netw. 2018, 16, 66–97. [Google Scholar] [CrossRef] [PubMed]
- Santaballa, A.; Márquez-Vega, C.; Rodríguez-Lescure, Á.; Rovirosa, Á.; Vázquez, L.; Zeberio-Etxetxipia, I.; Andrés, M.; Bassas, L.; Ceballos-Garcia, E.; Domingo, J.; et al. Multidisciplinary Consensus on the Criteria for Fertility Preservation in Cancer Patients. Clin. Transl. Oncol. 2022, 24, 227–243. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, C.M.; Salani, R. Ovarian Effects of Radiation and Cytotoxic Chemotherapy Damage. Best Pract. Res. Clin. Obs. Gynaecol. 2019, 55, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Sonigo, C.; Beau, I.; Binart, N.; Grynberg, M. The Impact of Chemotherapy on the Ovaries: Molecular Aspects and the Prevention of Ovarian Damage. Int. J. Mol. Sci. 2019, 20, 5342. [Google Scholar] [CrossRef]
- Anderson, R.A.; Cameron, D.; Clatot, F.; Demeestere, I.; Lambertini, M.; Nelson, S.M.; Peccatori, F. Anti-Müllerian Hormone as a Marker of Ovarian Reserve and Premature Ovarian Insufficiency in Children and Women with Cancer: A Systematic Review. Hum. Reprod. Update 2022, 28, 417–434. [Google Scholar] [CrossRef]
- Bedoschi, G.; Navarro, P.A.; Oktay, K. Chemotherapy-Induced Damage to Ovary: Mechanisms and Clinical Impact. Future Oncol. 2016, 12, 2333. [Google Scholar] [CrossRef]
- Christian, N.; Gemignani, M.L. Issues with Fertility in Young Women with Breast Cancer. Curr. Oncol. Rep. 2019, 21, 58. [Google Scholar] [CrossRef]
- Miller, J.J.; Williams, G.F.; Leissring, J.C. Multiple Late Complications of Therapy with Cyclophosphamide, Including Ovarian Destruction. Am. J. Med. 1971, 50, 530–535. [Google Scholar] [CrossRef]
- Chow, E.J.; Stratton, K.L.; Leisenring, W.M.; Oeffinger, K.C.; Sklar, C.A.; Donaldson, S.S.; Ginsberg, J.P.; Kenney, L.B.; Levine, J.M.; Robison, L.L.; et al. Pregnancy after Chemotherapy in Male and Female Survivors of Childhood Cancer Treated between 1970 and 1999: A Report from the Childhood Cancer Survivor Study Cohort. Lancet Oncol. 2016, 17, 567–576. [Google Scholar] [CrossRef]
- Anderson, R.A.; Brewster, D.H.; Wood, R.; Nowell, S.; Fischbacher, C.; Kelsey, T.W.; Wallace, W.H.B. The Impact of Cancer on Subsequent Chance of Pregnancy: A Population-Based Analysis. Hum. Reprod. 2018, 33, 1281–1290. [Google Scholar] [CrossRef]
- Di Meglio, A.; Vaz-Luis, I.; Pistilli, B.; Di Meglio, A.; Vaz-Luis, I.; Pistilli, B. Impact of Systemic Anticancer Therapy on Fertility. In Fertility Challenges and Solutions in Women with Cancer; Springer: Berlin/Heidelberg, Germany, 2020; pp. 67–80. [Google Scholar] [CrossRef]
- Dinas, K.D. Impact of Breast Cancer Treatment on Fertility. In Diseases of the Breast during Pregnancy and Lactation; Springer: Berlin/Heidelberg, Germany, 2020; p. 1252. [Google Scholar]
- Vo, K.C.T.; Kawamura, K. Female Oncofertility: Current Understandings, Therapeutic Approaches, Controversies, and Future Perspectives. J. Clin. Med. 2021, 10, 5690. [Google Scholar] [CrossRef] [PubMed]
- Martelli, V.; Latocca, M.M.; Ruelle, T.; Perachino, M.; Arecco, L.; Beshiri, K.; Razeti, M.G.; Tagliamento, M.; Cosso, M.; Fregatti, P.; et al. Comparing the Gonadotoxicity of Multiple Breast Cancer Regimens: Important Understanding for Managing Breast Cancer in Pre-Menopausal Women. Breast Cancer Targets Ther. 2021, 13, 341. [Google Scholar] [CrossRef] [PubMed]
- Reddy, N.; Furness, C.L.; Davies, M.C. Fertility in the Adolescent and Young Adult Patient with Cancer. In A Practical Approach to the Care of Adolescents and Young Adults with Cancer; Springer: Berlin/Heidelberg, Germany, 2018; pp. 153–178. [Google Scholar] [CrossRef]
- Chan, J.L.; Wang, E.T. Oncofertility for Women with Gynecologic Malignancies. Gynecol. Oncol. 2017, 144, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Alesi, L.R.; Winship, A.L.; Hutt, K.J. Evaluating the Impacts of Emerging Cancer Therapies on Ovarian Function. Curr. Opin. Endocr. Metab. Res. 2021, 18, 15–28. [Google Scholar] [CrossRef]
- Szymanska, K.J.; Tan, X.; Oktay, K. Unraveling the Mechanisms of Chemotherapy-Induced Damage to Human Primordial Follicle Reserve: Road to Developing Therapeutics for Fertility Preservation and Reversing Ovarian Aging. Mol. Hum. Reprod. 2020, 26, 553–566. [Google Scholar] [CrossRef]
- Silva, C.; Cristina, A.; Rama, R.; Soares, S.R.; Moura-Ramos, M.; Almeida-Santos, T. Adverse Reproductive Health Outcomes in a Cohort of Young Women with Breast Cancer Exposed to Systemic Treatments. J. Ovarian Res. 2019, 12, 102. [Google Scholar] [CrossRef]
- Moftakhar, B.; Vitek, W.; Huston, A. Impact of Breast Cancer Systemic Therapies on Fertility. Curr. Breast Cancer Rep. 2020, 12, 367–374. [Google Scholar] [CrossRef]
- Lasica, M.; Taylor, E.; Bhattacharyya, P.; Bennett, A.; Cooke, R.E.; Stern, C.; Agresta, F.; Ayton, R.; Grigg, A. Fertility in Premenopausal Women Post Autologous Stem Cell Transplant with BEAM Conditioning. Eur. J. Haematol. 2016, 97, 348–352. [Google Scholar] [CrossRef]
- Overbeek, A.; van den Berg, M.H.; van Leeuwen, F.E.; Kaspers, G.J.L.; Lambalk, C.B.; van Dulmen-den Broeder, E. Chemotherapy-Related Late Adverse Effects on Ovarian Function in Female Survivors of Childhood and Young Adult Cancer: A Systematic Review. Cancer Treat. Rev. 2017, 53, 10–24. [Google Scholar] [CrossRef]
- Goodwin, P.J.; Ennis, M.; Pritchard, K.I.; Trudeau, M.; Hood, N. Risk of Menopause during the First Year after Breast Cancer Diagnosis. J. Clin. Oncol. 1999, 17, 2365–2370. [Google Scholar] [CrossRef]
- Loren, A.W.; Senapati, S. Fertility Preservation in Patients with Hematologic Malignancies and Recipients of Hematopoietic Cell Transplants. Blood 2019, 134, 746–760. [Google Scholar] [CrossRef] [PubMed]
- Hoelder, S.; Clarke, P.A.; Workman, P. Discovery of Small Molecule Cancer Drugs: Successes, Challenges and Opportunities. Mol. Oncol. 2012, 6, 155. [Google Scholar] [CrossRef]
- Bussies, P.L.; Richards, E.G.; Rotz, S.J.; Falcone, T. Targeted Cancer Treatment and Fertility: Effect of Immunotherapy and Small Molecule Inhibitors on Female Reproduction. Reprod. Biomed. Online 2022, 44, 81–92. [Google Scholar] [CrossRef]
- Bang, Y.J.; Van Cutsem, E.; Feyereislova, A.; Chung, H.C.; Shen, L.; Sawaki, A.; Lordick, F.; Ohtsu, A.; Omuro, Y.; Satoh, T.; et al. Trastuzumab in Combination with Chemotherapy versus Chemotherapy Alone for Treatment of HER2-Positive Advanced Gastric or Gastro-Oesophageal Junction Cancer (ToGA): A Phase 3, Open-Label, Randomised Controlled Trial. Lancet 2010, 376, 687–697. [Google Scholar] [CrossRef]
- von Minckwitz, G.; Procter, M.; de Azambuja, E.; Zardavas, D.; Benyunes, M.; Viale, G.; Suter, T.; Arahmani, A.; Rouchet, N.; Clark, E.; et al. Adjuvant Pertuzumab and Trastuzumab in Early HER2-Positive Breast Cancer. N. Engl. J. Med. 2017, 377, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Cameron, D.; Piccart-Gebhart, M.J.; Gelber, R.D.; Procter, M.; Goldhirsch, A.; de Azambuja, E.; Castro, G.; Untch, M.; Smith, I.; Gianni, L.; et al. 11 Years’ Follow-up of Trastuzumab after Adjuvant Chemotherapy in HER2-Positive Early Breast Cancer: Final Analysis of the HERceptin Adjuvant (HERA) Trial. Lancet 2017, 389, 1195–1205. [Google Scholar] [CrossRef]
- Richani, D.; Gilchrist, R.B. The Epidermal Growth Factor Network: Role in Oocyte Growth, Maturation and Developmental Competence. Hum. Reprod. Update 2018, 24, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Rambhatla, A.; Strug, M.R.; De Paredes, J.G.; Cordoba Munoz, M.I.; Thakur, M. Fertility Considerations in Targeted Biologic Therapy with Tyrosine Kinase Inhibitors: A Review. J. Assist. Reprod. Genet. 2021, 38, 1897–1908. [Google Scholar] [CrossRef]
- Dosiou, C. Thyroid and Fertility: Recent Advances. Thyroid 2020, 30, 479–486. [Google Scholar] [CrossRef]
- Robert, C. A Decade of Immune-Checkpoint Inhibitors in Cancer Therapy. Nat. Commun. 2020, 11, 3801. [Google Scholar] [CrossRef]
- Schmid, P.; Cortes, J.; Dent, R.; Pusztai, L.; McArthur, H.; Kümmel, S.; Bergh, J.; Denkert, C.; Park, Y.H.; Hui, R.; et al. Event-Free Survival with Pembrolizumab in Early Triple-Negative Breast Cancer. N. Engl. J. Med. 2022, 386, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Eggermont, A.M.M.; Blank, C.U.; Mandalà, M.; Long, G.V.; Atkinson, V.G.; Dalle, S.; Haydon, A.M.; Meshcheryakov, A.; Khattak, A.; Carlino, M.S.; et al. Adjuvant Pembrolizumab versus Placebo in Resected Stage III Melanoma (EORTC 1325-MG/KEYNOTE-054): Distant Metastasis-Free Survival Results from a Double-Blind, Randomised, Controlled, Phase 3 Trial. Lancet Oncol. 2021, 22, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Ribas, A.; Wolchok, J.D. Cancer Immunotherapy Using Checkpoint Blockade. Science 2018, 359, 1350–1355. [Google Scholar] [CrossRef]
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Sosman, J.A.; Atkins, M.B.; Leming, P.D.; et al. Five-Year Survival and Correlates Among Patients with Advanced Melanoma, Renal Cell Carcinoma, or Non-Small Cell Lung Cancer Treated with Nivolumab. JAMA Oncol. 2019, 5, 1411–1420. [Google Scholar] [CrossRef]
- Robert, C.; Marabelle, A.; Herrscher, H.; Caramella, C.; Rouby, P.; Fizazi, K.; Besse, B. Immunotherapy Discontinuation—How, and When? Data from Melanoma as a Paradigm. Nat. Rev. Clin. Oncol. 2020, 17, 707–715. [Google Scholar] [CrossRef] [PubMed]
- Garutti, M.; Lambertini, M.; Puglisi, F. Checkpoint Inhibitors, Fertility, Pregnancy, and Sexual Life: A Systematic Review. ESMO Open 2021, 6, 100276. [Google Scholar] [CrossRef]
- Duma, N.; Lambertini, M. It Is Time to Talk About Fertility and Immunotherapy. Oncologist 2020, 25, 277–278. [Google Scholar] [CrossRef]
- Tanda, E.T.; Croce, E.; Spagnolo, F.; Zullo, L.; Spinaci, S.; Genova, C.; Rossi, G. Immunotherapy in Adolescents and Young Adults: What Remains in Cancer Survivors? Front. Oncol. 2021, 11, 3709. [Google Scholar] [CrossRef]
- Postow, M.A.; Sidlow, R.; Hellmann, M.D. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N. Engl. J. Med. 2018, 378, 158–168. [Google Scholar] [CrossRef]
- Traila, A.; Dima, D.; Achimas-Cadariu, P.; Micu, R. Fertility Preservation in Hodgkin’s Lymphoma Patients That Undergo Targeted Molecular Therapies: An Important Step Forward from the Chemotherapy Era. Cancer Manag. Res. 2018, 10, 1517–1526. [Google Scholar] [CrossRef]
- Berjeb, K.K.; Debbabi, L.; Braham, M.; Zemni, Z.; Chtourou, S.; Hannachi, H.; Hamdoun, M.; Ayadi, M.; Kacem, K.; Zhioua, F.; et al. Evaluation of Ovarian Reserve before and after Chemotherapy. J. Gynecol. Obs. Hum. Reprod. 2021, 50, 102035. [Google Scholar] [CrossRef] [PubMed]
- Salama, M.; Woodruff, T.K. Anticancer Treatments and Female Fertility: Clinical Concerns and Role of Oncologists in Oncofertility Practice. Expert Rev. Anticancer. Ther. 2017, 17, 687–692. [Google Scholar] [CrossRef] [PubMed]
- Algarroba, G.N.; Sanfilippo, J.S.; Valli-Pulaski, H. Female Fertility Preservation in the Pediatric and Adolescent Cancer Patient Population. Best. Pract. Res. Clin. Obs. Gynaecol. 2018, 48, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Balachandren, N.; Davies, M. Fertility, Ovarian Reserve and Cancer. Maturitas 2017, 105, 64–68. [Google Scholar] [CrossRef]
- Oncofertility Consensus Document Compiled by Members of the CCLG Late Effects Group. Subfertility Risk Consensus Document: Update 2008. 2019. Available online: cclg.org.uk (accessed on 30 December 2022).
- Lambertini, M.; Del Mastro, L.; Pescio, M.C.; Andersen, C.Y.; Azim, H.A.; Peccatori, F.A.; Costa, M.; Revelli, A.; Salvagno, F.; Gennari, A.; et al. Cancer and Fertility Preservation: International Recommendations from an Expert Meeting. BMC Med. 2016, 14, 1. [Google Scholar] [CrossRef] [PubMed]
- van Dorp, W.; Haupt, R.; Anderson, R.A.; Mulder, R.L.; van den Heuvel-Eibrink, M.M.; van Dulmen-den Broeder, E.; Su, H.I.; Winther, J.F.; Hudson, M.M.; Levine, J.M.; et al. Reproductive Function and Outcomes in Female Survivors of Childhood, Adolescent, and Young Adult Cancer: A Review. J. Clin. Oncol. 2018, 36, 2169–2180. [Google Scholar] [CrossRef]
- van den Berg, M.H.; van Dijk, M.; Byrne, J.; Berger, C.; Dirksen, U.; Winther, J.F.; Fossa, S.D.; Grabow, D.; Grandage, V.L.; Haupt, R.; et al. Treatment-Related Fertility Impairment in Long-Term Female Childhood, Adolescent and Young Adult Cancer Survivors: Investigating Dose-Effect Relationships in a European Case-Control Study (PanCareLIFE). Hum. Reprod. 2021, 36, 1561–1573. [Google Scholar] [CrossRef]
- Cardoso, F.; Kyriakides, S.; Ohno, S.; Penault-Llorca, F.; Poortmans, P.; Rubio, I.T.; Zackrisson, S.; Senkus, E. Early Breast Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up†. Ann. Oncol. 2019, 30, 1194–1220. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, J.; Chen, K.; Li, S.; Wang, Y.; Yang, Y.; Deng, H.; Jia, W.; Rao, N.; Liu, Q.; et al. What Lies behind Chemotherapy-Induced Amenorrhea for Breast Cancer Patients: A Meta-Analysis. Breast Cancer Res. Treat. 2014, 145, 113–128. [Google Scholar] [CrossRef]
- Bines, J.; Oleske, D.M.; Cobleigh, M.A. Ovarian Function in Premenopausal Women Treated with Adjuvant Chemotherapy for Breast Cancer. J. Clin. Oncol. 1996, 14, 1718–1729. [Google Scholar] [CrossRef]
- Goldhirsch, A.; Gelber, R.D.; Castiglione, M. The Magnitude of Endocrine Effects of Adjuvant Chemotherapy for Premenopausal Breast Cancer Patients. The International Breast Cancer Study Group. Ann. Oncol. 1990, 1, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Parulekar, W.R.; Day, A.G.; Ottaway, J.A.; Shepherd, L.E.; Trudeau, M.E.; Bramwell, V.; Levine, M.; Pritchard, K.I. Incidence and Prognostic Impact of Amenorrhea during Adjuvant Therapy in High-Risk Premenopausal Breast Cancer: Analysis of a National Cancer Institute of Canada Clinical Trials Group Study—NCIC CTG MA.5. J. Clin. Oncol. 2005, 23, 6002–6008. [Google Scholar] [CrossRef] [PubMed]
- Ganz, P.A.; Land, S.R.; Geyer, C.E.; Cecchini, R.S.; Costantino, J.P.; Pajon, E.R.; Fehrenbacher, L.; Atkins, J.N.; Polikoff, J.A.; Vogel, V.G.; et al. Menstrual History and Quality-of-Life Outcomes in Women with Node-Positive Breast Cancer Treated with Adjuvant Therapy on the NSABP B-30 Trial. J. Clin. Oncol. 2011, 29, 1110–1116. [Google Scholar] [CrossRef] [PubMed]
- Elis, A.; Tevet, A.; Yerushalmi, R.; Blickstein, D.; Bairy, O.; Dann, E.; Blumenfeld, Z.; Abraham, A.; Manor, Y.; Shpilberg, O.; et al. Fertility Status among Women Treated for Aggressive Non-Hodgkin’s Lymphoma. Leuk. Lymphoma 2006, 47, 623–627. [Google Scholar] [CrossRef]
- Behringer, K.; Mueller, H.; Goergen, H.; Thielen, I.; Eibl, A.D.; Stumpf, V.; Wessels, C.; Wiehlpütz, M.; Rosenbrock, J.; Halbsguth, T.; et al. Gonadal Function and Fertility in Survivors after Hodgkin Lymphoma Treatment within the German Hodgkin Study Group HD13 to HD15 Trials. J. Clin. Oncol. 2013, 31, 231–239. [Google Scholar] [CrossRef]
- Eichenauer, D.A.; Aleman, B.M.P.; André, M.; Federico, M.; Hutchings, M.; Illidge, T.; Engert, A.; Ladetto, M. Hodgkin Lymphoma: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2018, 29, iv19–iv29. [Google Scholar] [CrossRef]
- Budman, D.R.; Berry, D.A.; Cirrincione, C.T.; Henderson, I.C.; Wood, W.C.; Weiss, R.B.; Ferree, C.R.; Muss, H.B.; Green, M.R.; Norton, L.; et al. Dose and Dose Intensity as Determinants of Outcome in the Adjuvant Treatment of Breast Cancer. J. Natl. Cancer Inst. 1998, 90, 1205–1211. [Google Scholar] [CrossRef]
- Venturini, M.; Del Mastro, L.; Aitini, E.; Baldini, E.; Caroti, C.; Contu, A.; Testore, F.; Brema, F.; Pronzato, P.; Cavazzini, G.; et al. Dose-Dense Adjuvant Chemotherapy in Early Breast Cancer Patients: Results from a Randomized Trial. J. Natl. Cancer Inst. 2005, 97, 1724–1733. [Google Scholar] [CrossRef]
- Lambertini, M.; Ceppi, M.; Cognetti, F.; Cavazzini, G.; De Laurentiis, M.; De Placido, S.; Michelotti, A.; Bisagni, G.; Durando, A.; Valle, E.; et al. Dose-Dense Adjuvant Chemotherapy in Premenopausal Breast Cancer Patients: A Pooled Analysis of the MIG1 and GIM2 Phase III Studies. Eur. J. Cancer 2017, 71, 34. [Google Scholar] [CrossRef]
- Engert, A.; Plütschow, A.; Eich, H.T.; Lohri, A.; Dörken, B.; Borchmann, P.; Berger, B.; Greil, R.; Willborn, K.C.; Wilhelm, M.; et al. Reduced Treatment Intensity in Patients with Early-Stage Hodgkin’s Lymphoma. N. Engl. J. Med. 2010, 363, 640–652. [Google Scholar] [CrossRef]
- Decanter, C.; Morschhauser, F.; Pigny, P.; Lefebvre, C.; Gallo, C.; Dewailly, D. Anti-Müllerian Hormone Follow-up in Young Women Treated by Chemotherapy for Lymphoma: Preliminary Results. Reprod. Biomed. Online 2010, 20, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.; Pienkowski, T.; Mackey, J.; Pawlicki, M.; Guastalla, J.-P.; Weaver, C.; Tomiak, E.; Al-Tweigeri, T.; Chap, L.; Juhos, E.; et al. Adjuvant Docetaxel for Node-Positive Breast Cancer. N. Engl. J. Med. 2005, 352, 2302–2313. [Google Scholar] [CrossRef]
- Silva, C.; Caramelo, O.; Almeida-Santos, T.; Rama, A.C.R. Factors Associated with Ovarian Function Recovery after Chemotherapy for Breast Cancer: A Systematic Review and Meta-Analysis. Hum. Reprod. 2016, 31, 2737–2749. [Google Scholar] [CrossRef] [PubMed]
- Lambertini, M.; Campbell, C.; Bines, J.; Korde, L.A.; Izquierdo, M.; Fumagalli, D.; Del Mastro, L.; Ignatiadis, M.; Pritchard, K.; Wolff, A.C.; et al. Adjuvant Anti-HER2 Therapy, Treatment-Related Amenorrhea, and Survival in Premenopausal HER2-Positive Early Breast Cancer Patients. JNCI J. Natl. Cancer Inst. 2019, 111, 86. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.L.; Klitus, M.; Mintzer, D.M. Chemotherapy-Induced Amenorrhea from Adjuvant Breast Cancer Treatment: The Effect of the Addition of Taxanes. Clin. Breast Cancer 2005, 6, 421–424. [Google Scholar] [CrossRef]
- Tham, Y.L.; Sexton, K.; Weiss, H.; Elledge, R.; Friedman, L.C.; Kramer, R. The Rates of Chemotherapy-Induced Amenorrhea in Patients Treated with Adjuvant Doxorubicin and Cyclophosphamide Followed by a Taxane. Am. J. Clin. Oncol. 2007, 30, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Gast, K.C.; Cathcart-Rake, E.J.; Norman, A.D.; Eshraghi, L.; Obidegwu, N.; Nichols, H.B.; Rosenberg, S.; Su, H.I.; Stewart, E.A.; Couch, F.J.; et al. Regimen-Specific Rates of Chemotherapy-Related Amenorrhea in Breast Cancer Survivors. JNCI Cancer Spectr. 2019, 3, pkz081. [Google Scholar] [CrossRef]
- Morarji, K.; McArdle, O.; Hui, K.; Gingras-Hill, G.; Ahmed, S.; Greenblatt, E.M.; Warner, E.; Sridhar, S.; Ali, A.M.F.; Azad, A.; et al. Ovarian Function after Chemotherapy in Young Breast Cancer Survivors. Curr. Oncol. 2017, 24, e494–e502. [Google Scholar] [CrossRef] [PubMed]
- Ruddy, K.J.; Guo, H.; Barry, W.; Dang, C.T.; Yardley, D.A.; Moy, B.; Marcom, P.K.; Albain, K.S.; Rugo, H.S.; Ellis, M.J.; et al. Chemotherapy-Related Amenorrhea after Adjuvant Paclitaxel-Trastuzumab (APT Trial). Breast Cancer Res. Treat. 2015, 151, 589. [Google Scholar] [CrossRef]
- Lambertini, M.; Ceppi, M.; Anderson, R.A.; Cameron, D.A.; Bruzzone, M.; Franzoi, M.A.; Massarotti, C.; El-Abed, S.; Wang, Y.; Lecocq, C.; et al. Impact of Anti-HER2 Therapy Alone and With Weekly Paclitaxel on the Ovarian Reserve of Young Women With HER2-Positive Breast Cancer. J. Natl. Compr. Cancer Netw. 2023, 21, 33–41. [Google Scholar] [CrossRef]
- Ruddy, K.J.; Zheng, Y.; Tayob, N.; Hu, J.; Dang, C.T.; Yardley, D.A.; Isakoff, S.J.; Valero, V.V.; Faggen, M.G.; Mulvey, T.M.; et al. Chemotherapy-Related Amenorrhea (CRA) after Adjuvant Ado-Trastuzumab Emtansine (T-DM1) Compared to Paclitaxel in Combination with Trastuzumab (TH) (TBCRC033: ATEMPT Trial). Breast Cancer Res. Treat. 2021, 189, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Lorenzi, E.; Simonelli, M.; Persico, P.; Dipasquale, A.; Santoro, A. Risks of Molecular Targeted Therapies to Fertility and Safety during Pregnancy: A Review of Current Knowledge and Future Needs. Expert Opin. Drug Saf. 2021, 20, 503–521. [Google Scholar] [CrossRef] [PubMed]
- Allegra, C.J.; Yothers, G.; O’Connell, M.J.; Sharif, S.; Petrelli, N.J.; Colangelo, L.H.; Atkins, J.N.; Seay, T.E.; Fehrenbacher, L.; Goldberg, R.M.; et al. Phase III Trial Assessing Bevacizumab in Stages II and III Carcinoma of the Colon: Results of NSABP Protocol C-08. J. Clin. Oncol. 2011, 29, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Gharwan, H.; Lai, C.; Grant, C.; Dunleavy, K.; Steinberg, S.M.; Shovlin, M.; Fojo, T.; Wilson, W.H. Female Fertility Following Dose-Adjusted EPOCH-R Chemotherapy in Primary Mediastinal B-Cell Lymphomas. Leuk. Lymphoma 2016, 57, 1616–1624. [Google Scholar] [CrossRef]
- Christopoulos, C.; Dimakopoulou, V.; Rotas, E. Primary Ovarian Insufficiency Associated with Imatinib Therapy. N. Engl. J. Med. 2008, 358, 1079–1080. [Google Scholar] [CrossRef]
- de Sanctis, R.; Lorenzi, E.; Agostinetto, E.; D’Amico, T.; Simonelli, M.; Santoro, A. Primary Ovarian Insufficiency Associated with Pazopanib Therapy in a Breast Angiosarcoma Patient: A CARE-Compliant Case Report. Medicine 2019, 98, e18089. [Google Scholar] [CrossRef]
- De Filette, J.; Andreescu, C.E.; Cools, F.; Bravenboer, B.; Velkeniers, B. A Systematic Review and Meta-Analysis of Endocrine-Related Adverse Events Associated with Immune Checkpoint Inhibitors. Horm. Metab. Res. 2019, 51, 145–156. [Google Scholar] [CrossRef]
- Duma, N.; Abdel-Ghani, A.; Yadav, S.; Hoversten, K.P.; Reed, C.T.; Sitek, A.N.; Enninga, E.A.L.; Paludo, J.; Aguilera, J.V.; Leventakos, K.; et al. Sex Differences in Tolerability to Anti-Programmed Cell Death Protein 1 Therapy in Patients with Metastatic Melanoma and Non-Small Cell Lung Cancer: Are We All Equal? Oncologist 2019, 24, e1148–e1155. [Google Scholar] [CrossRef]
- Chatzidarellis, E.; Makrilia, N.; Giza, L.; Georgiadis, E.; Alamara, C.; Syrigos, K.N. Effects of Taxane-Based Chemotherapy on Inhibin B and Gonadotropins as Biomarkers of Spermatogenesis. Fertil. Steril. 2010, 94, 558–563. [Google Scholar] [CrossRef]
- Pallotti, F.; Pelloni, M.; Faja, F.; Di Chiano, S.; Di Rocco, A.; Lenzi, A.; Lombardo, F.; Paoli, D. Semen Quality in Non-Hodgkin Lymphoma Survivors: A Monocentric Retrospective Study. Hum. Reprod. 2021, 36, 16–25. [Google Scholar] [CrossRef]
- Wasilewski-Masker, K.; Seidel, K.D.; Leisenring, W.; Mertens, A.C.; Shnorhavorian, M.; Ritenour, C.W.; Stovall, M.; Green, D.M.; Sklar, C.A.; Armstrong, G.T.; et al. Male Infertility in Long-Term Survivors of Pediatric Cancer: A Report from the Childhood Cancer Survivor Study. J. Cancer Surviv. 2014, 8, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Vakalopoulos, I.; Dimou, P.; Anagnostou, I.; Zeginiadou, T. Impact of Cancer and Cancer Treatment on Male Fertility. Hormones 2015, 14, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Delessard, M.; Saulnier, J.; Rives, A.; Dumont, L.; Rondanino, C.; Rives, N. Exposure to Chemotherapy During Childhood or Adulthood and Consequences on Spermatogenesis and Male Fertility. Int. J. Mol. Sci. 2020, 21, 1454. [Google Scholar] [CrossRef] [PubMed]
- Ghafouri-Fard, S.; Shoorei, H.; Abak, A.; Seify, M.; Mohaqiq, M.; Keshmir, F.; Taheri, M.; Ayatollahi, S.A. Effects of Chemotherapeutic Agents on Male Germ Cells and Possible Ameliorating Impact of Antioxidants. Biomed. Pharmacother. 2021, 142, 112040. [Google Scholar] [CrossRef]
- Bahadur, G.; Ozturk, O.; Muneer, A.; Wafa, R.; Ashraf, A.; Jaman, N.; Patel, S.; Oyede, A.W.; Ralph, D.J. Semen Quality before and after Gonadotoxic Treatment. Hum. Reprod. 2005, 20, 774–781. [Google Scholar] [CrossRef]
- Meistrich, M.L.; Wilson, G.; Brown, B.W.; Da Cunha, M.F.; Lipshultz, L.I. Impact of Cyclophosphamide on Long-Term Reduction in Sperm Count in Men Treated with Combination Chemotherapy for Ewing and Soft Tissue Sarcomas. Cancer 1992, 70, 2703–2712. [Google Scholar] [CrossRef]
- Pryzant, R.M.; Meistrich, M.L.; Wilson, G.; Brown, B.; McLaughlin, P. Long-Term Reduction in Sperm Count after Chemotherapy with and without Radiation Therapy for Non-Hodgkin’s Lymphomas. J. Clin. Oncol. 1993, 11, 239–247. [Google Scholar] [CrossRef]
- Amin, A.; Brunckhorst, O.; Scott, C.; Wrench, D.; Gleeson, M.; Kazmi, M.; Ahmed, K. ABVD and BEACOPP Regimens’ Effects on Fertility in Young Males with Hodgkin Lymphoma. Clin. Transl. Oncol. 2021, 23, 1067–1077. [Google Scholar] [CrossRef]
- Van Der Kaaij, M.A.E.; Heutte, N.; Van Echten-Arends, J.; Raemaekers, J.M.M.; Carde, P.; Noordijk, E.M.; Fermé, C.; Thomas, J.; Eghbali, H.; Brice, P.; et al. Sperm Quality before Treatment in Patients with Early Stage Hodgkin’s Lymphoma Enrolled in EORTC-GELA Lymphoma Group Trials. Haematologica 2009, 94, 1691–1697. [Google Scholar] [CrossRef]
- Bujan, L.; Walschaerts, M.; Moinard, N.; Hennebicq, S.; Saias, J.; Brugnon, F.; Auger, J.; Berthaut, I.; Szerman, E.; Daudin, M.; et al. Impact of Chemotherapy and Radiotherapy for Testicular Germ Cell Tumors on Spermatogenesis and Sperm DNA: A Multicenter Prospective Study from the CECOS Network. Fertil. Steril. 2013, 100, 673–680.e2. [Google Scholar] [CrossRef]
- Lampe, H.; Horwich, A.; Norman, A.; Nicholls, J.; Dearnaley, D.P. Fertility after Chemotherapy for Testicular Germ Cell Cancers. J. Clin. Oncol. 1997, 15, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Viviani, S.; Santoro, A.; Ragni, G.; Bonfante, V.; Bestetti, O.; Bonadonna, G. Gonadal Toxicity after Combination Chemotherapy for Hodgkin’s Disease. Comparative Results of MOPP vs ABVD. Eur. J. Cancer Clin. Oncol. 1985, 21, 601–605. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Zhou, L.; Chen, X.; Xu, B.; Cheng, Y.; Sun, S.; Fang, M.; Xiang, Y. Impact of Imatinib on the Fertility of Male Patients with Chronic Myelogenous Leukaemia in the Chronic Phase. Target. Oncol. 2017, 12, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Gambacorti-Passerini, C.; Tornaghi, L.; Cavagnini, F.; Rossi, P.; Pecori-Giraldi, F.; Mariani, L.; Cambiaghi, N.; Pogliani, E.; Corneo, G.; Gnessi, L. Gynaecomastia in Men with Chronic Myeloid Leukaemia after Imatinib. Lancet 2003, 361, 1954–1956. [Google Scholar] [CrossRef]
- Brunet-Possenti, F.; Opsomer, M.A.; Gomez, L.; Ouzaid, I.; Descamps, V. Immune Checkpoint Inhibitors-Related Orchitis. Ann. Oncol. 2017, 28, 906–907. [Google Scholar] [CrossRef]
- Quach, H.T.; Robbins, C.J.; Balko, J.M.; Chiu, C.Y.; Miller, S.; Wilson, M.R.; Nelson, G.E.; Johnson, D.B. Severe Epididymo-Orchitis and Encephalitis Complicating Anti-PD-1 Therapy. Oncologist 2019, 24, 872–876. [Google Scholar] [CrossRef]
- Scovell, J.M.; Benz, K.; Samarska, I.; Kohn, T.P.; Hooper, J.E.; Matoso, A.; Herati, A.S. Association of Impaired Spermatogenesis with the Use of Immune Checkpoint Inhibitors in Patients with Metastatic Melanoma. JAMA Oncol. 2020, 6, 1297–1299. [Google Scholar] [CrossRef]
- Salzmann, M.; Tosev, G.; Heck, M.; Schadendorf, D.; Maatouk, I.; Enk, A.H.; Hartmann, M.; Hassel, J.C. Male Fertility during and after Immune Checkpoint Inhibitor Therapy: A Cross-Sectional Pilot Study. Eur. J. Cancer 2021, 152, 41–48. [Google Scholar] [CrossRef]
- Peters, M.; Pearlman, A.; Terry, W.; Mott, S.L.; Monga, V. Testosterone Deficiency in Men Receiving Immunotherapy for Malignant Melanoma. Oncotarget 2021, 12, 199–208. [Google Scholar] [CrossRef]
- Rabinowitz, M.J.; Kohn, T.P.; Peña, V.N.; Samarska, I.v.; Matoso, A.; Herati, A.S. Onset of Azoospermia in Man Treated with Ipilimumab/Nivolumab for BRAF Negative Metastatic Melanoma. Urol. Case Rep. 2020, 34, 101488. [Google Scholar] [CrossRef]
- Davies, A.; Naderpoor, N.; Parakh, S. Isolated Hypogonadotropic Hypogonadism Secondary to Anti-Programmed Death Ligand 1 Inhibitor. J. Thorac. Oncol. 2019, 14, e147–e148. [Google Scholar] [CrossRef] [PubMed]
- Tulchiner, G.; Pichler, R.; Ulmer, H.; Staudacher, N.; Lindner, A.K.; Brunner, A.; Zelger, B.; Steinkohl, F.; Aigner, F.; Horninger, W.; et al. Sex-Specific Hormone Changes during Immunotherapy and Its Influence on Survival in Metastatic Renal Cell Carcinoma. Cancer Immunol. Immunother. 2021, 70, 2805–2817. [Google Scholar] [CrossRef] [PubMed]
- Practice Committee of the American Society for Reproductive Medicine. Fertility Preservation in Patients Undergoing Gonadotoxic Therapy or Gonadectomy: A Committee Opinion. Fertil. Steril. 2019, 112, 1022–1033. [Google Scholar] [CrossRef] [PubMed]
- Lambertini, M.; Horicks, F.; del Mastro, L.; Partridge, A.H.; Demeestere, I. Ovarian Protection with Gonadotropin-Releasing Hormone Agonists during Chemotherapy in Cancer Patients: From Biological Evidence to Clinical Application. Cancer Treat. Rev. 2019, 72, 65–77. [Google Scholar] [CrossRef]
- Spears, N.; Lopes, F.; Stefansdottir, A.; Rossi, V.; De Felici, M.; Anderson, R.A.; Klinger, F.G. Ovarian Damage from Chemotherapy and Current Approaches to Its Protection. Hum. Reprod. Update 2019, 25, 673. [Google Scholar] [CrossRef] [PubMed]
- Morgan, S.; Lopes, F.; Gourley, C.; Anderson, R.A.; Spears, N. Cisplatin and Doxorubicin Induce Distinct Mechanisms of Ovarian Follicle Loss; Imatinib Provides Selective Protection Only against Cisplatin. PLoS ONE 2013, 8, e70117. [Google Scholar] [CrossRef]
- Oktay, K.; Turan, V.; Titus, S.; Stobezki, R.; Liu, L. BRCA Mutations, DNA Repair Deficiency, and Ovarian Aging. Biol. Reprod. 2015, 93, 67. [Google Scholar] [CrossRef]

| High Risk (>80%) | Intermediate Risk (20–80%) | Low/Very Low Risk (<20%) | Unknown Risk | |
|---|---|---|---|---|
| Alkylating Agents | ||||
| Cyclophosphamide | ✓ | |||
| Carmustine | ✓ | |||
| Ifosfamide | ✓ | |||
| Busulfan | ✓ | |||
| Chlorambucil | ✓ | |||
| Melphalan | ✓ | |||
| Procarbazine | ✓ | |||
| Nitrogen mustard | ✓ | |||
| Antimetabolites | ||||
| Cytarabine | ✓ | ✓ | ||
| Methotrexate | ✓ | |||
| Mercaptopurine | ✓ | |||
| Fluorouracil | ✓ | |||
| Gemcitabine | ✓ | |||
| Antimitotic Cytostatics | ||||
| Vinblastine | ✓ | ✓ | ||
| Vincristine | ✓ | |||
| Taxanes | ✓ | ✓ | ||
| Anti-tumor Antibiotics | ||||
| Bleomycin | ✓ | ✓ | ||
| Dactinomycin | ✓ | |||
| Daunorubicin | ✓ | |||
| Doxorubicin | ✓ | ✓ | ||
| Epirubicin | ✓ | |||
| Mitomycin | ✓ | |||
| Topo-isomerase Inhibitors | ||||
| Etoposide | ✓ | |||
| Irinotecan | ✓ | |||
| Platinum-based Drugs | ||||
| Cisplatin | ✓ >600 mg/m2 | ✓ <600 mg/m2 | ||
| Carboplatin | ✓ | ✓ | ||
| Oxaliplatin | ✓ | ✓ | ||
| Combinations | ||||
| CMF (6 cycles) * | ✓ >40 years old | ✓ 30–39 years old | ✓ <30 years old | |
| CEF (6 cycles) * | ✓ >40 years old | ✓ 30–39 years old | ✓ <30 years old | |
| CAF (6 cycles) * | ✓ >40 years old | ✓ 30–39 years old | ✓ <30 years old | |
| AC (4 cycles) * | ✓ >40 years old | ✓ <40 years old | ||
| EC (4 cycles) * | ✓ >40 years old | ✓ <40 years old | ||
| ABVD * | ✓ | |||
| CHOP (4–6 cycles) * | ✓ | |||
| TAC * | ✓ | |||
| BEACOPP * | ✓ | |||
| FOLFOX * | ✓ | |||
| Anthracycline/ cytarabine | ✓ | |||
| EURAMOS * | ✓ | ✓ | ||
| EuroEWING 12 * | ✓ |
| High Risk (>75%) | Intermediate Risk (25–75%) | Low/Very Low Risk (<25%) | Unknown Risk | |
|---|---|---|---|---|
| Alkylating Agents | ||||
| Cyclophosphamide (19 g/m2) | ✓ | |||
| Carmustin (300 mg/m2) | ✓ | |||
| Ifosfamide (42 g/m2) | ✓ | |||
| Busulfan (600 mg/kg) | ✓ | |||
| Chlorambucil (1.4 g/m2) | ✓ | |||
| Melphalan (140 mg/m2) | ✓ | |||
| Procarbazine (4 g/m2) | ✓ | |||
| Antimetabolites | ||||
| Cytarabine (1 g/m2) | ✓ | |||
| Methotrexate | ✓ | |||
| Fluorouracil | ✓ | |||
| Antimitotic Cytostatics | ||||
| Vinblastine (50 g/m2) | ✓ | |||
| Vincristine (8 g/m2) | ✓ | |||
| Anti-tumor Antibiotics | ||||
| Bleomycin | ✓ | |||
| Dactinomycin | ✓ | |||
| Daunorubicin | ✓ | |||
| Doxorubicin (770 mg/m2) | ✓ | |||
| Topo-isomerase Inhibitors | ||||
| Etoposide | ✓ | |||
| Platinum-based Drugs | ||||
| Cisplatin | ✓ >600 mg/m2 | ✓ <600 mg/m2 | ||
| Carboplatin (2 g/m2) | ✓ | |||
| Combinations | ||||
| ABVD * | ✓ | |||
| CHOP * | ✓ | |||
| FOLFOX * | ✓ | |||
| BEACOPP * | ✓ | |||
| EURAMOS * | ✓ | |||
| EuroEWING 12 * | ✓ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Himpe, J.; Lammerant, S.; Van den Bergh, L.; Lapeire, L.; De Roo, C. The Impact of Systemic Oncological Treatments on the Fertility of Adolescents and Young Adults—A Systematic Review. Life 2023, 13, 1209. https://doi.org/10.3390/life13051209
Himpe J, Lammerant S, Van den Bergh L, Lapeire L, De Roo C. The Impact of Systemic Oncological Treatments on the Fertility of Adolescents and Young Adults—A Systematic Review. Life. 2023; 13(5):1209. https://doi.org/10.3390/life13051209
Chicago/Turabian StyleHimpe, Justine, Sander Lammerant, Lore Van den Bergh, Lore Lapeire, and Chloë De Roo. 2023. "The Impact of Systemic Oncological Treatments on the Fertility of Adolescents and Young Adults—A Systematic Review" Life 13, no. 5: 1209. https://doi.org/10.3390/life13051209
APA StyleHimpe, J., Lammerant, S., Van den Bergh, L., Lapeire, L., & De Roo, C. (2023). The Impact of Systemic Oncological Treatments on the Fertility of Adolescents and Young Adults—A Systematic Review. Life, 13(5), 1209. https://doi.org/10.3390/life13051209






