Outcomes of Different In Vitro Maturation Procedures for Oocyte Cryopreservation for Fertility Preservation and yet Another Live Birth in a Cancer Patient
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. Ovarian Stimulation (OS)-FP Treatment
3. IVM-FP Treatment
3.1. OPU-IVM
3.2. OTO-IVM
3.3. Oocyte Warming and Embryo Transfer Procedure
4. Statistical Analysis
5. Results
5.1. Patients’ Characteristics
5.2. Comparison of Collection Parameters among OTO-IVM, OPU-IVM and OS Procedures
5.3. Oocytes Collection and Maturation
5.4. Outcomes of Cryopreserved Oocytes
5.5. Pregnancy Case from OPU-IVM
6. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anderson, R.A.; Brewster, D.H.; Wood, R.; Nowell, S.; Fischbacher, C.; Kelsey, T.W.; Wallace, W.H.B. The Impact of Cancer on Subsequent Chance of Pregnancy: A Population-Based Analysis. Hum. Reprod. 2018, 33, 1281–1290. [Google Scholar] [CrossRef]
- ESHRE Guideline Group on Female Fertility Preservation; Anderson, R.A.; Amant, F.; Braat, D.; D’Angelo, A.; Chuva de Sousa Lopes, S.M.; Demeestere, I.; Dwek, S.; Frith, L.; Lambertini, M.; et al. ESHRE Guideline: Female Fertility Preservation. Hum. Reprod. Open 2020, 2020, hoaa052. [Google Scholar] [CrossRef]
- Cakmak, H.; Katz, A.; Cedars, M.I.; Rosen, M.P. Effective Method for Emergency Fertility Preservation: Random-Start Controlled Ovarian Stimulation. Fertil. Steril. 2013, 100, 1673–1680. [Google Scholar] [CrossRef] [PubMed]
- De Vos, M.; Smitz, J.; Woodruff, T.K. Fertility Preservation in Women with Cancer. Lancet 2014, 384, 1302–1310. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F. International Society for Fertility Preservation–ESHRE–ASRM Expert Working Group Update on Fertility Preservation from the Barcelona International Society for Fertility Preservation-ESHRE-ASRM 2015 Expert Meeting: Indications, Results and Future Perspectives. Fertil. Steril. 2017, 108, 407–415.e11. [Google Scholar] [CrossRef]
- Silber, S.J.; DeRosa, M.; Goldsmith, S.; Fan, Y.; Castleman, L.; Melnick, J. Cryopreservation and Transplantation of Ovarian Tissue: Results from One Center in the USA. J. Assist. Reprod. Genet. 2018, 35, 2205–2213. [Google Scholar] [CrossRef]
- Hourvitz, A.; Yerushalmi, G.M.; Maman, E.; Raanani, H.; Elizur, S.; Brengauz, M.; Orvieto, R.; Dor, J.; Meirow, D. Combination of ovarian tissue harvesting and immature oocyte collection for fertility preservation increases preservation yield. Reprod. Biomed. Online 2015, 31, 497–505. [Google Scholar] [CrossRef]
- Grynberg, M.; Poulain, M.; le Parco, S.; Sifer, C.; Fanchin, R.; Frydman, N. Similar in Vitro Maturation Rates of Oocytes Retrieved during the Follicular or Luteal Phase Offer Flexible Options for Urgent Fertility Preservation in Breast Cancer Patients. Hum. Reprod. 2016, 31, 623–629. [Google Scholar] [CrossRef]
- Creux, H.; Monnier, P.; Son, W.-Y.; Tulandi, T.; Buckett, W. Immature Oocyte Retrieval and in Vitro Oocyte Maturation at Different Phases of the Menstrual Cycle in Women with Cancer Who Require Urgent Gonadotoxic Treatment. Fertil. Steril. 2017, 107, 198–204. [Google Scholar] [CrossRef]
- Creux, H.; Monnier, P.; Son, W.-Y.; Buckett, W. Thirteen Years’ Experience in Fertility Preservation for Cancer Patients after in Vitro Fertilization and in Vitro Maturation Treatments. J. Assist. Reprod. Genet. 2018, 35, 583–592. [Google Scholar] [CrossRef]
- Delattre, S.; Segers, I.; Van Moer, E.; Drakopoulos, P.; Mateizel, I.; Enghels, L.; Tournaye, H.; De Vos, M. Combining Fertility Preservation Procedures to Spread the Eggs across Different Baskets: A Feasibility Study. Hum. Reprod. 2020, 35, 2524–2536. [Google Scholar] [CrossRef] [PubMed]
- Fasano, G.; Dechène, J.; Antonacci, R.; Biramane, J.; Vannin, A.S.; Van Langendonckt, A.; Devreker, F.; Demeestere, I. Outcomes of immature oocytes collected from ovarian tissue for cryopreservation in adult and prepubertal patients. Reprod. Biomed. Online 2017, 34, 575–582. [Google Scholar] [CrossRef]
- Segers, I.; Bardhi, E.; Mateizel, I.; Van Moer, E.; Schots, R.; Verheyen, G.; Tournaye, H.; De Vos, M. Live Births Following Fertility Preservation Using In-Vitro Maturation of Ovarian Tissue Oocytes. Hum. Reprod. 2020, 35, 2026–2036. [Google Scholar] [CrossRef]
- Prasath, E.B.; Chan, M.L.H.; Wong, W.H.W.; Lim, C.J.W.; Tharmalingam, M.D.; Hendricks, M.; Loh, S.F.; Chia, Y.N. First Pregnancy and Live Birth Resulting from Cryopreserved Embryos Obtained from in Vitro Matured Oocytes after Oophorectomy in an Ovarian Cancer Patient. Hum. Reprod. 2014, 29, 276–278. [Google Scholar] [CrossRef] [PubMed]
- Uzelac, P.S.; Nakajima, S.T. Live Birth Following in Vitro Maturation of Oocytes Retrieved from Extracorporeal Ovarian Tissue Aspiration and Embryo Cryopreservation for 5 Years. Fertil. Steril. 2015, 104, 3. [Google Scholar] [CrossRef] [PubMed]
- Segers, I.; Mateizel, I.; Van Moer, E.; Smitz, J.; Tournaye, H.; Verheyen, G.; De Vos, M. In Vitro Maturation (IVM) of Oocytes Recovered from Ovariectomy Specimens in the Laboratory: A Promising “Ex Vivo” Method of Oocyte Cryopreservation Resulting in the First Report of an Ongoing Pregnancy in Europe. J. Assist. Reprod. Genet. 2015, 32, 1221–1231. [Google Scholar] [CrossRef]
- Kedem, A. Outcome of Immature Oocytes Collection of 119 Cancer Patients during Ovarian Tissue Harvesting for Fertility Preservation. J. Assist. Reprod. Genet. 2018, 6, 851–856. [Google Scholar] [CrossRef]
- Mayeur, A.; Puy, V.; Windal, V.; Hesters, L.; Gallot, V.; Benoit, A.; Grynberg, M.; Sonigo, C.; Frydman, N. Live Birth Rate after Use of Cryopreserved Oocytes or Embryos at the Time of Cancer Diagnosis in Female Survivors: A Retrospective Study of Ten Years of Experience. J. Assist. Reprod. Genet 2021, 38, 1767–1775. [Google Scholar] [CrossRef]
- Rodrigues, P. Oncofertility Case Report: Live Birth 10 Years after Oocyte in Vitro Maturation and Zygote Cryopreservation. J. Assist. Reprod. Genet. 2020, 6, 1767–1775. [Google Scholar] [CrossRef]
- Takae, S.; Sugishita, Y.; Yoshioka, N.; Hoshina, M.; Horage, Y.; Sato, Y.; Nishijima, C.; Kawamura, K.; Suzuki, N. The Role of Menstrual Cycle Phase and AMH Levels in Breast Cancer Patients Whose Ovarian Tissue Was Cryopreserved for Oncofertility Treatment. J. Assist. Reprod. Genet. 2015, 32, 305–312. [Google Scholar] [CrossRef]
- Yin, H.; Jiang, H.; Kristensen, S.G.; Andersen, C.Y. Vitrification of in Vitro Matured Oocytes Collected from Surplus Ovarian Medulla Tissue Resulting from Fertility Preservation of Ovarian Cortex Tissue. J. Assist. Reprod. Genet. 2016, 33, 741–746. [Google Scholar] [CrossRef] [PubMed]
- Silber, S.J.; Goldsmith, S.; Castleman, L.; Hurlbut, K.; Fan, Y.; Melnick, J.; Hayashi, K. In-Vitro Maturation and Transplantation of Cryopreserved Ovary Tissue: Understanding Ovarian Longevity. Reprod. BioMed. Online 2022, 44, 504–514. [Google Scholar] [CrossRef] [PubMed]
- El Hachem, H.; Sonigo, C.; Benard, J.; Presse, M.; Sifer, C.; Sermondade, N.; Grynberg, M. Comparison of GnRH Agonist and HCG for Priming in Vitro Maturation Cycles in Cancer Patients Undergoing Urgent Fertility Preservation. PLoS ONE 2018, 13, e0208576. [Google Scholar] [CrossRef] [PubMed]
- Sermondade, N.; Sonigo, C.; Sifer, C.; Valtat, S.; Ziol, M.; Eustache, F.; Grynberg, M. Serum Antimüllerian Hormone Is Associated with the Number of Oocytes Matured in Vitro and with Primordial Follicle Density in Candidates for Fertility Preservation. Fertil. Steril. 2019, 111, 357–362. [Google Scholar] [CrossRef]
- Sonigo, C.; Simon, C.; Boubaya, M.; Benoit, A.; Sifer, C.; Sermondade, N.; Grynberg, M. What Threshold Values of Antral Follicle Count and Serum AMH Levels Should Be Considered for Oocyte Cryopreservation after in Vitro Maturation? Hum. Reprod. 2016, 31, 1493–1500. [Google Scholar] [CrossRef]
- Rienzi, L.; Cobo, A.; Paffoni, A.; Scarduelli, C.; Capalbo, A.; Vajta, G.; Remohí, J.; Ragni, G.; Ubaldi, F.M. Consistent and Predictable Delivery Rates after Oocyte Vitrification: An Observational Longitudinal Cohort Multicentric Study. Hum. Reprod. 2012, 27, 1606–1612. [Google Scholar] [CrossRef]
- Grynberg, M.; Mayeur Le Bras, A.; Hesters, L.; Gallot, V.; Frydman, N. First Birth Achieved after Fertility Preservation Using Vitrification of in Vitro Matured Oocytes in a Woman with Breast Cancer. Ann. Oncol. 2020, 31, 541–542. [Google Scholar] [CrossRef]
- Gellert, S.E.; Pors, S.E.; Kristensen, S.G.; Bay-Bjørn, A.M.; Ernst, E.; Yding Andersen, C. Transplantation of frozen-thawed ovarian tissue: An update on worldwide activity published in peer-reviewed papers and on the Danish cohort. J. Assist. Reprod. Genet. 2018, 35, 561–570. [Google Scholar] [CrossRef]
- Donnez, J.; Dolmans, M.M.; Demylle, D.; Jadoul, P.; Pirard, C.; Squifflet, J.; Martinez-Madrid, B.; Langendonckt, A.V. Livebirth after Orthotopic Transplantation of Cryopreserved Ovarian Tissue. Lancet 2004, 364, 1405–1410. [Google Scholar] [CrossRef]
- Donnez, J.; Dolmans, M.-M. Fertility Preservation in Women. Available online: https://www.nejm.org/doi/10.1056/NEJMra1614676 (accessed on 28 May 2021).
- Diaz-Garcia, C.; Domingo, J.; Garcia-Velasco, J.A.; Herraiz, S.; Mirabet, V.; Iniesta, I.; Cobo, A.; Remohí, J.; Pellicer, A. Oocyte Vitrification versus Ovarian Cortex Transplantation in Fertility Preservation for Adult Women Undergoing Gonadotoxic Treatments: A Prospective Cohort Study. Fertil. Steril. 2018, 109, 478–485.e2. [Google Scholar] [CrossRef]
- Silber, S.; Kagawa, N.; Kuwayama, M.; Gosden, R. Duration of Fertility after Fresh and Frozen Ovary Transplantation. Fertil. Steril. 2010, 94, 2191–2196. [Google Scholar] [CrossRef] [PubMed]
- Son, W.-Y.; Henderson, S.; Cohen, Y.; Dahan, M.; Buckett, W. Immature Oocyte for Fertility Preservation. Front. Endocrinol. 2019, 10, 464. [Google Scholar] [CrossRef] [PubMed]
- Mohsenzadeh, M.; Tabibnejad, N.; Vatanparast, M.; Anbari, F.; Ali Khalili, M.; Karimi-Zarchi, M. Vitrification Has Detrimental Effects on Maturation, Viability, and Subcellular Quality of Oocytes Post IVM in Cancerous Women: An Experimental Study. Int. J. Reprod. Biomed. 2019, 17, 175–184. [Google Scholar] [CrossRef] [PubMed]
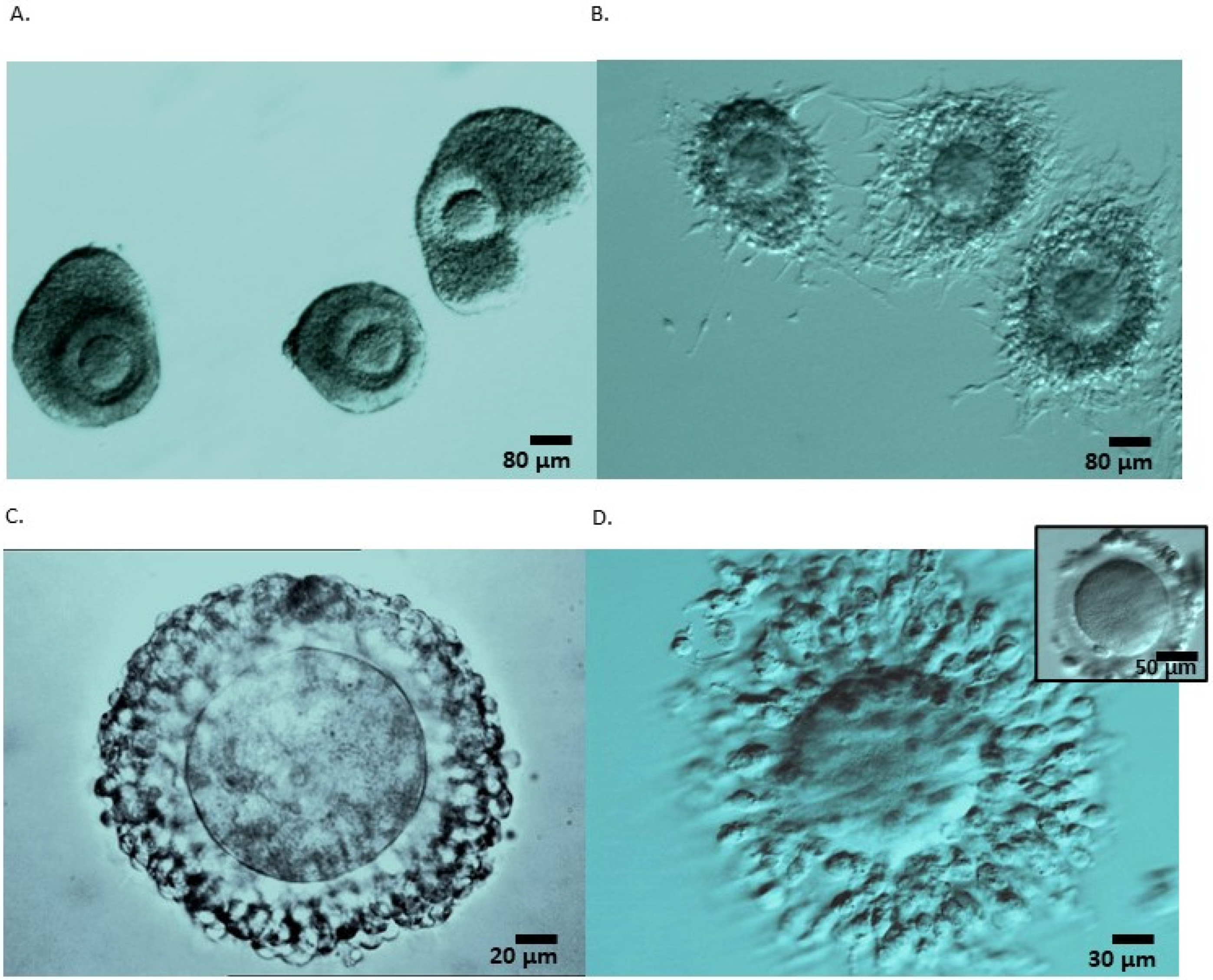
| IVM-FP | |||
|---|---|---|---|
| OTO-IVM | OPU-IVM | OS-FP | |
| Total patients (%) | 22 (100%) | 73 (100%) | 27 (100%) |
| Breast cancer | 1 (4%) | 71 (97%) | 19 (70%) |
| Borderline ovarian tumors | 6 (27%) | - | 5 (19%) |
| Haematologic malignancy or disorder | 4 (18%) | - | 2 (7%) |
| Osteosarcoma | 2 (9%) | 1 (1.5%) | - |
| Gastro-intestinal cancer | 3 (14%) | - | 1 (4%) |
| Ewing sarcoma | 3 (14%) | - | - |
| Sarcoma other | 3 (14%) | 1 (1.5%) | - |
| IVM-FP | |||
|---|---|---|---|
| OTO-IVM | OPU–IVM | OS-FP | |
| Total no. of patients | 22 | 73 | 27 |
| Total no. of patients with vitrified oocytes | 20 | 69 | 26 |
| Age at OTC, years ± SD (range) | 23.6 ± 6.4 a (16–32) | 30.9 ± 4.2 b (20–39) | 31.8 ± 4.9 b (19–41) |
| FSH IU/L, mean ± SD (range) | 5.9 ± 2.4 a (2.1–9.3) | 6.4 ± 3.5 a (2.3–12.0) | 7.9 ± 2.3 a (3.7–12.3) |
| AMH ng/mL, mean ± SD (range) | 5.1 ± 3.7 a (1.2–15.0) | 3.5 ± 3.0 ab (0.5–13.3) | 2.5 ± 1.6 b (0.4–5.8) |
| AFC, mean ± SD (range) | 17.0 ± 10.5 a (6–40) | 15.6 ± 8.4 a (7–41) | 13.0 ± 5.1 a (6–23) |
| BMI (kg/m2), mean ± SD (range) | 24.1 ± 2.9 a (21–28) | 22.7 ± 5.8 a (17–38) | 23.4 ± 3.7 a (20–34) |
| Total no. of oocytes collected (mean ± SD) | 223 (11.1 ± 8.1) a | 392 (5.4 ± 5.0) b | 246 (7.7 ± 5.2) a |
| Total no. of MI/MII oocytes collected (mean ± SD) | 0 (0.0) a | 82 (1.2 ± 2.8) b | 217 (6.9 ± 4.4) c |
| Total no. of GV oocytes collected (mean ± SD) | 223 (11.1 ± 8.1) a | 310 (4.4 ± 3.7) b | 29 (1.1 ± 1.8) c |
| Total no. of MII vitrified oocytes/GV after IVM (mean ± SD) | 152 (7.6 ± 5.7) a | 254 (3.8 ± 2.8) b | - |
| % maturation at 24–28 h | 57% a | 73% b | - |
| % maturation at 44–48 h | 70% a | 82% b | - |
| Total no. of MII vitrified oocytes (mean ± SD) | 152 (7.6 ± 5.7) ac | 324 (4.6 ± 4.9) b | 212 (6.8 ± 4.6) c |
| IVM–FP | ||||
|---|---|---|---|---|
| Patients Return | OTO–IVM | OPU–IVM | Total IVM-FP | OS-FP |
| Total no. of patients returned (%) | 2 (10%) | 4 (6%) | 6 | 3 (12%) |
| Total no. of oocytes warmed | 11 | 32 | 43 | 20 |
| % of oocyte survival | 45% | 57% | 54% | 56% |
| % of fertilization after ICSI | 80% | 61% | 65% | 77% |
| No. of embryos obtained | 4 | 11 | 15 | 10 |
| No. of patients with embryo transfer | 1 | 3 | 4 | 2 |
| No of embryos transferred | 1 | 6 | 7 | 2 |
| Total no. of deliveries | 0 | 1 | 1 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nogueira, D.; Fajau-Prevot, C.; Clouet, M.; Assouline, P.; Deslandres, M.; Montagut, M. Outcomes of Different In Vitro Maturation Procedures for Oocyte Cryopreservation for Fertility Preservation and yet Another Live Birth in a Cancer Patient. Life 2023, 13, 1355. https://doi.org/10.3390/life13061355
Nogueira D, Fajau-Prevot C, Clouet M, Assouline P, Deslandres M, Montagut M. Outcomes of Different In Vitro Maturation Procedures for Oocyte Cryopreservation for Fertility Preservation and yet Another Live Birth in a Cancer Patient. Life. 2023; 13(6):1355. https://doi.org/10.3390/life13061355
Chicago/Turabian StyleNogueira, Daniela, Carole Fajau-Prevot, Muriel Clouet, Patrick Assouline, Marion Deslandres, and Marie Montagut. 2023. "Outcomes of Different In Vitro Maturation Procedures for Oocyte Cryopreservation for Fertility Preservation and yet Another Live Birth in a Cancer Patient" Life 13, no. 6: 1355. https://doi.org/10.3390/life13061355
APA StyleNogueira, D., Fajau-Prevot, C., Clouet, M., Assouline, P., Deslandres, M., & Montagut, M. (2023). Outcomes of Different In Vitro Maturation Procedures for Oocyte Cryopreservation for Fertility Preservation and yet Another Live Birth in a Cancer Patient. Life, 13(6), 1355. https://doi.org/10.3390/life13061355






