Association of Piriformis Thickness, Hip Muscle Strength, and Low Back Pain Patients with and without Piriformis Syndrome in Malaysia
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Population, and Location
2.2. Sampling Method
2.3. Inclusion and Exclusion Criteria
2.4. Sample Size Calculation
2.5. Ethics Approval
2.6. Instrument and Outcome Measure
2.6.1. Anthropometry
2.6.2. Identification of Piriformis
2.6.3. Screening for Piriformis Syndrome
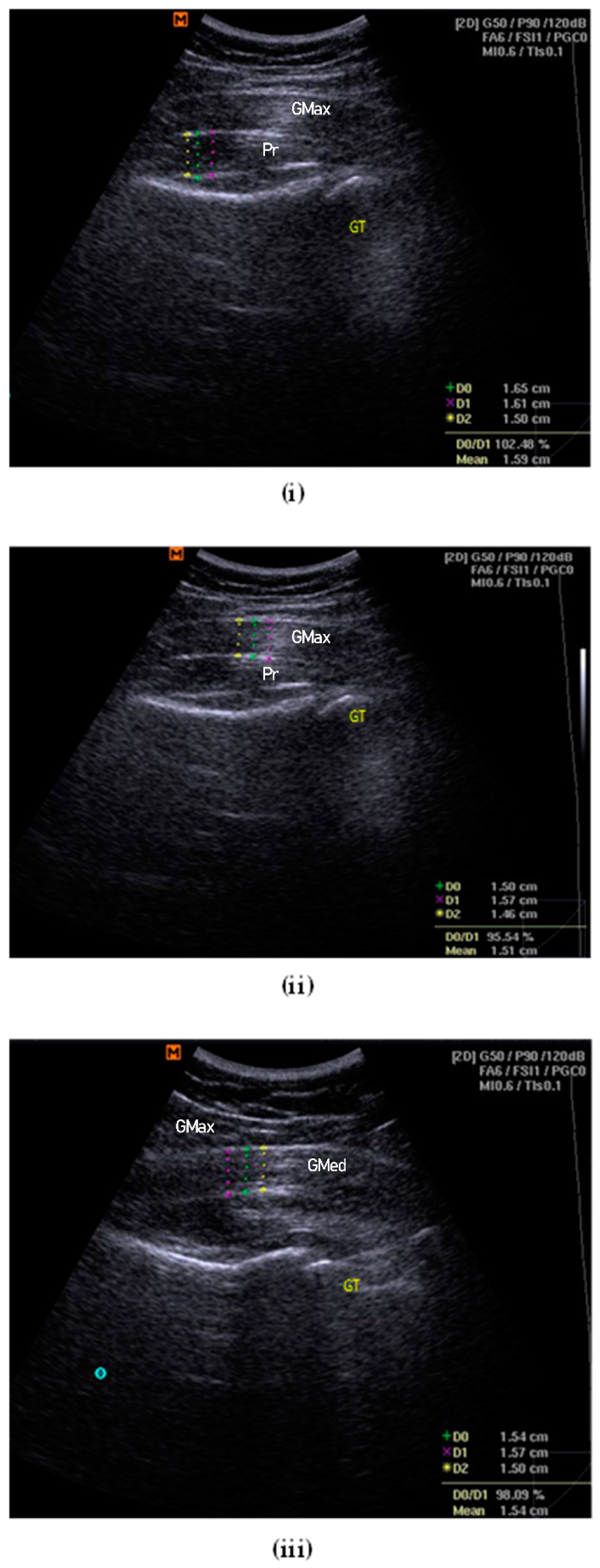
2.7. Ultrasonography Examination
2.7.1. Intraclass Correlation Coefficient (ICC)
2.7.2. Intraclass Correlation Coefficient of USG
2.7.3. Ultrasonography Measurement of Muscle Thickness
2.7.4. Piriformis Thickness
2.7.5. Gluteus Maximus Muscle Thickness
2.7.6. Gluteus Medius Muscle Thickness
2.7.7. Measurement of Muscle Activation Using an Electromyogram (EMG)
2.7.8. Intraclass Correlation Coefficient (ICC) of the sEMG and Manual Resistance for Maximum Voluntary Contraction
2.7.9. Muscle Strength
2.7.10. Muscle Activation
2.8. Data Analysis
3. Results
3.1. Participants’ Anthropometric and Sociodemographic Characteristics
3.2. Participants’ Physical Examinations and Exposure to Physical Activities
3.3. Between-Group Comparisons of Piriformis and Gluteus Muscles Thickness
3.4. Comparisons of Gluteus Muscles Strength and Activation
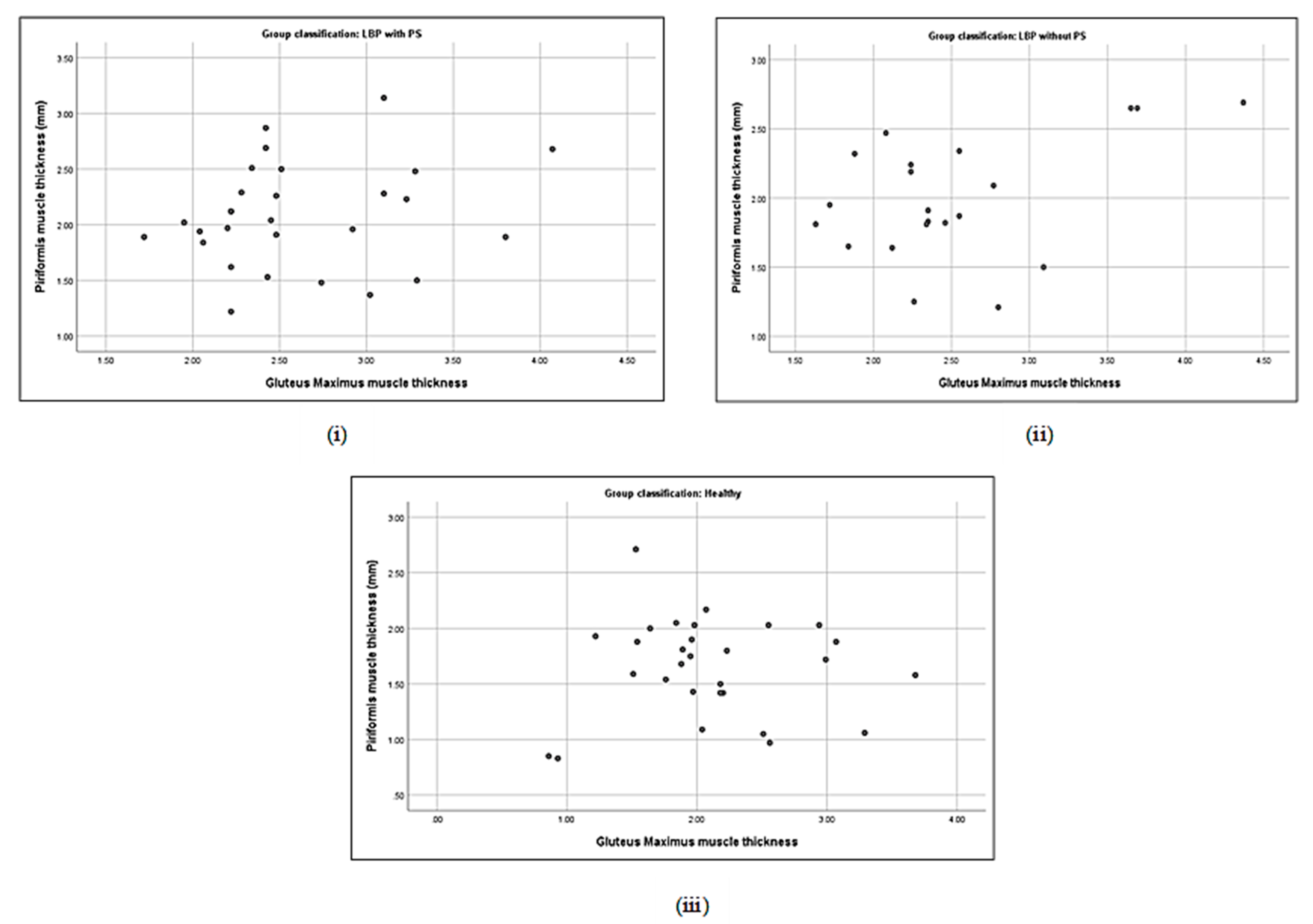
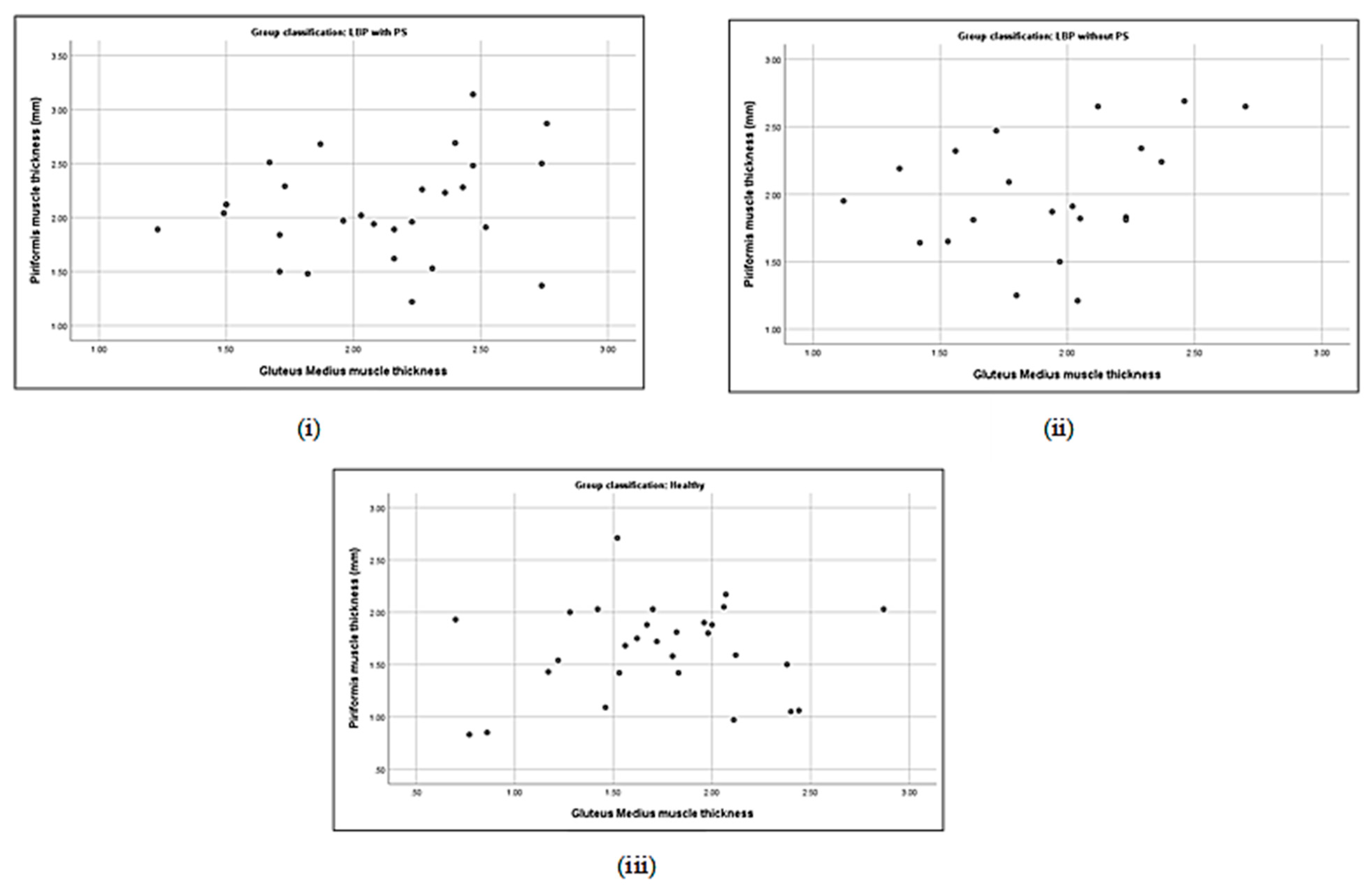
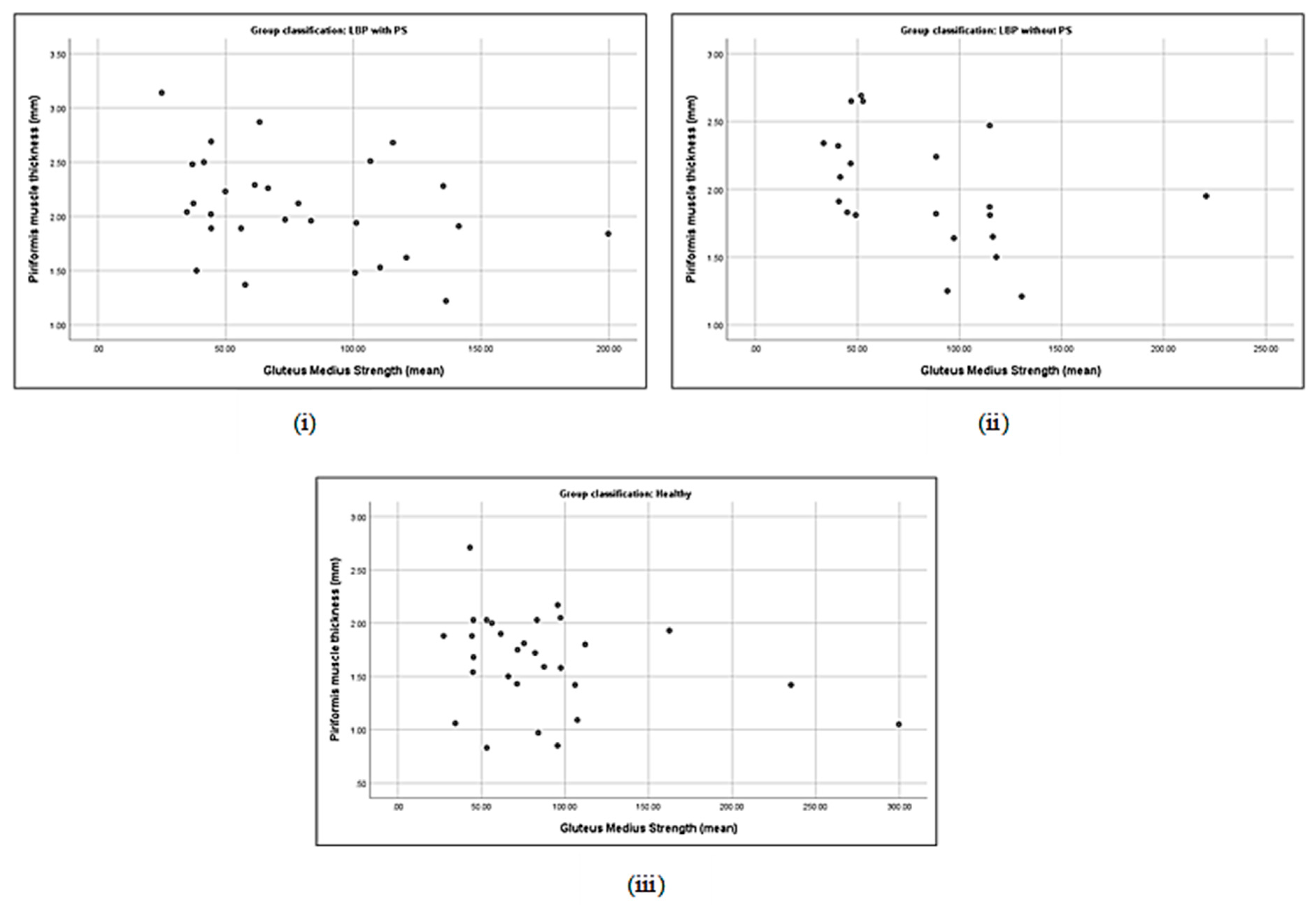
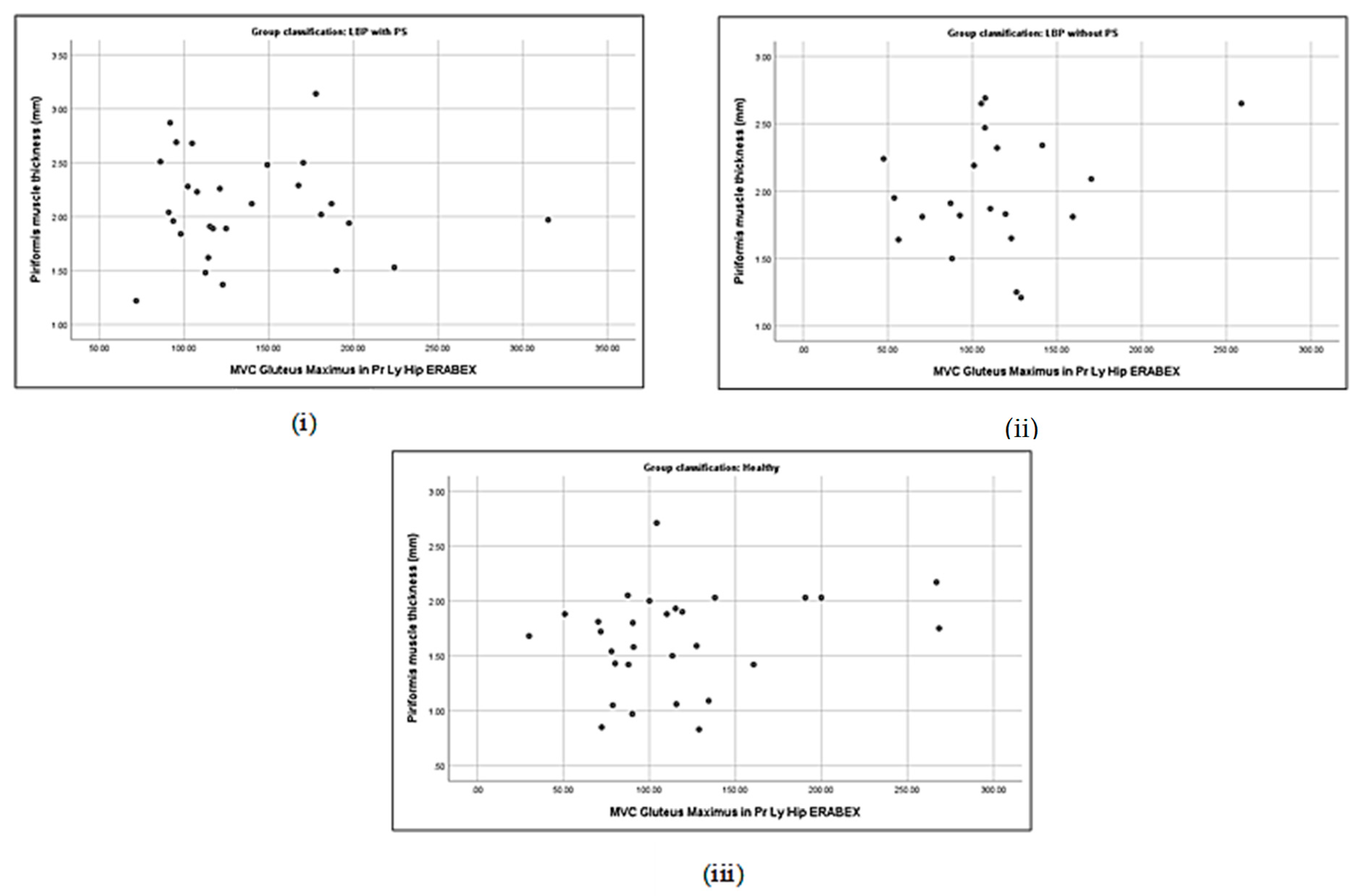
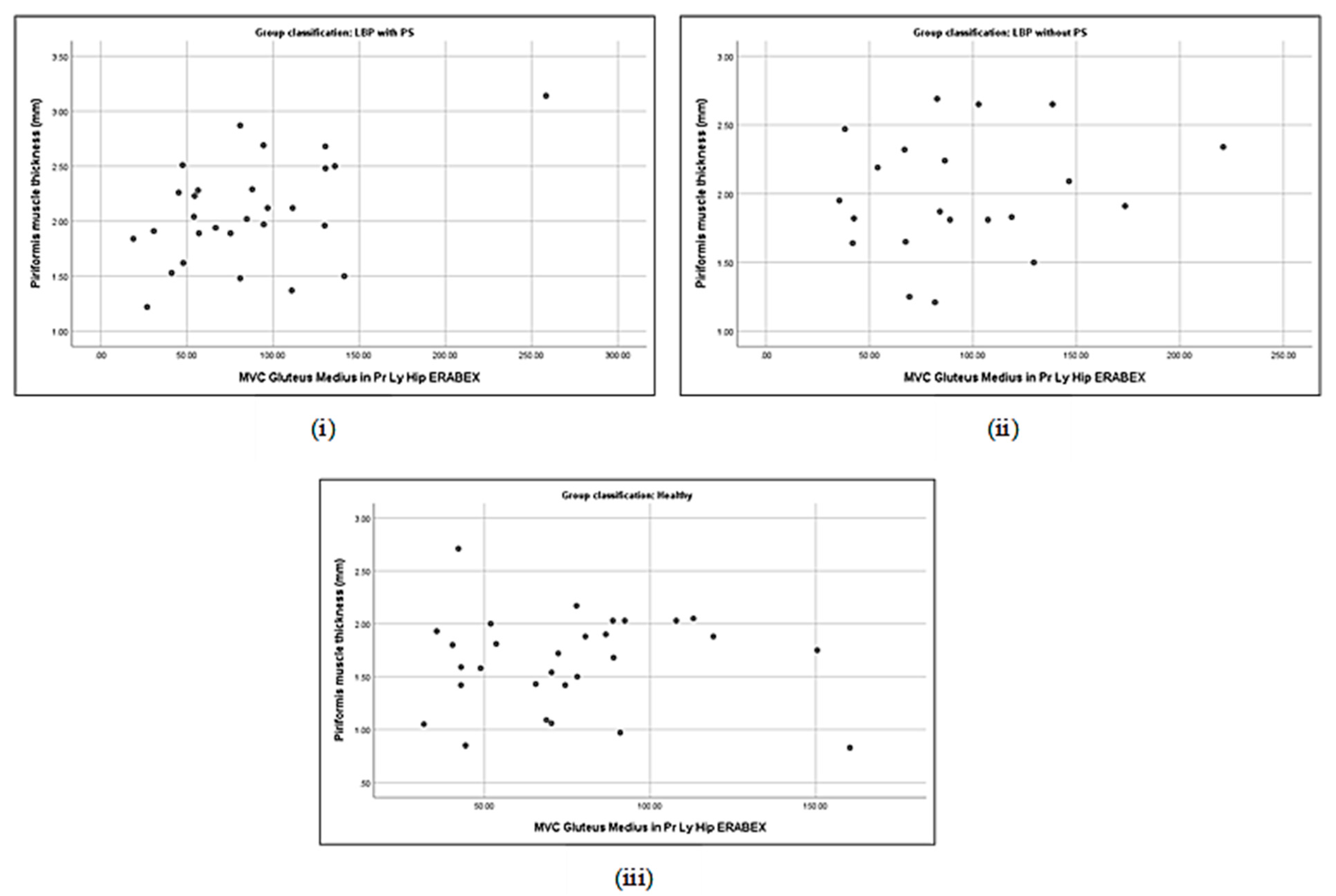
3.5. Correlation between Piriformis Thickness and Gluteus Muscle Strength
3.6. Association between Piriformis Thickness and Gluteus Thickness, Strength, and Activation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hartvigsen, J.; Hancock, M.J.; Kongsted, A.; Louw, Q.; Ferreira, M.L.; Genevay, S.; Hoy, D.; Karppinen, J.; Pransky, G.; Sieper, J.; et al. What Low Back Pain is and why We need to Pay Attention. Lancet 2018, 391, 2356–2367. [Google Scholar] [CrossRef] [PubMed]
- Vos, T.; Allen, C.; Arora, M.; Barber, R.M.; Bhutta, Z.A.; Brown, A.; Carter, A.; Casey, D.C.; Charlson, F.J.; Chen, A.Z.; et al. Global, Regional, and National Incidence, Prevalence, and Years Lived with Disability for 310 Diseases and Injuries, 1990–2015: A Systematic Analysis For The Global Burden of Disease Study 2015. Lancet 2016, 388, 1545–1602. [Google Scholar] [CrossRef] [PubMed]
- Maher, C.; Underwood, M.; Buchbinder, R. Non-Specific Low Back Pain. Lancet 2016, 389, 736–747. [Google Scholar] [CrossRef]
- Malik, M.H.A.; Singh, D.; Mansor, M.; Mohamed Kamil, O.I.; Yin Choy, C.; Cardosa, M.S.; Hasnan, N.; Vijayan, R. The Malaysian Low Back Pain Guideline; Malaysian Association for the Study of Pain and Spine Society: Kuala Lumpur, Malaysia, 2014. [Google Scholar]
- Ikram, M.A.; Burud, I.; Gobu, S.G.; Shantiyaa, K.; Lin, P.J.; Aisyah, S.; Adibi, M. Prevalence and Risk Factors Associated with Low Back Pain: A Cross-Sectional Study. Med. Sci. 2020, 24, 1677–1683. [Google Scholar]
- Cass, S.P. Piriformis syndrome: A Cause of Nondiscogenic Sciatica. Curr. Sport. Med. Rep. 2015, 14, 41–44. [Google Scholar] [CrossRef]
- Chen, C.K.; Nizar, A.J. Prevalence of Piriformis Syndrome in Chronic Low Back Pain Patients. A Clinical Diagnosis with Modified FAIR Test. Pain Pract. 2013, 13, 276–281. [Google Scholar] [CrossRef]
- Windisch, G.; Braun, E.M.; Anderhuber, F. Piriformis muscle: Clinical anatomy and consideration of the piriformis syndrome. Surg. Radiol. Anat. 2007, 29, 37–45. [Google Scholar] [CrossRef]
- Knudsen, J.S.; Mei-Dan, O.; Brick, M.J. Piriformis Syndrome and Endoscopic Sciatic Neurolysis. Sport. Med. Arthrosc. Rev. 2016, 24, e1–e7. [Google Scholar] [CrossRef]
- Singh, U.S.; Meena, R.K.; Singh, C.A.K.; Singh, A.K.J.; Singh, A.M.; Langshong, R. Prevalence of Piriformis Syndrome among the Cases of Low Back/Buttock Pain with Sciatica: A prospective study. J. Med. Soc. 2013, 27, 2–7. [Google Scholar] [CrossRef]
- Kumar, A.P.; Nidhi, A. Piriformis Syndrome: A Case Report. Int. J. Physiother. Res. 2017, 5, 1926–1929. [Google Scholar] [CrossRef]
- Neumann, D.A. Kinesiology of the Hip: A Focus on Muscular Actions. J. Orthop. Sport. Phys. Ther. 2010, 40, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Kibler, W.B.; Press, J.; Sciascia, A. The Role of Core Stability in Athletic Function. Sport. Med. 2006, 36, 189–198. [Google Scholar] [CrossRef]
- Nelson-Wong, E.; Gallant, P.; Alexander, S.; Dehmer, K.; Ingvalson, S.; McClenahan, B.; Piatte, A.; Poupore, K.; Davis, A.M. Multiplanar Lumbopelvic Control In Patients With Low Back Pain: Is Multiplanar Assessment Better Than Single Plane Assessment In Discriminating Between Patients And Healthy Controls? J. Man. Manip. Ther. 2016, 24, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Giphart, J.E.; Stull, J.D.; Laprade, R.F.; Wahoff, M.S.; Philippon, M.J. Recruitment and Activity of the Pectineus and Piriformis Muscles during Hip Rehabilitation Exercises: An electromyography study. Am. J. Sport. Med. 2012, 40, 1654–1663. [Google Scholar] [CrossRef] [PubMed]
- Michel, F.; Decavel, P.; Toussirot, E.; Tatu, L.; Aleton, E.; Monnier, G.; Garbuio, P.; Parratte, B. The Piriformis Muscle Syndrome: An Exploration of Anatomical Context, Pathophysiological Hypotheses and Diagnostic Criteria. Ann. Phys. Rehabil. Med. 2013, 56, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Siddiq, A.B.; Hossain, S.M.; Uddin, M.M.; Jahan, I.; Khasru, M.R.; Haider, N.M.; Rasker, J.J. Piriformis Syndrome: A Case Series of 31 Bangladeshi People with Literature Review. Eur. J. Orthop. Surg. Traumatol. 2017, 27, 193–203. [Google Scholar] [CrossRef]
- Kankaanpää, M.; Taimela, S.; Laaksonen, D.; Hänninen, O.; Airaksinen, O. Back and hip extensor fatigability in chronic low back pain patients and controls. Arch. Phys. Med. Rehabil. 1998, 79, 412–417. [Google Scholar] [CrossRef]
- Park, J.C.; Shim, J.H.; Chung, S.H. The Effects of Three Types of Piriform Muscle Stretching on Muscle Thickness and the Medial Rotation Angle Of The Coxal Articulation. J. Phys. Ther. Sci. 2017, 29, 1811–1814. [Google Scholar] [CrossRef]
- Hopayian, K.; Danielyan, A. Four Symptoms Define the Piriformis Syndrome: An Updated Systematic Review of its Clinical Features. Eur. J. Orthop. Surg. Traumatol. 2018, 28, 155–164. [Google Scholar] [CrossRef]
- Boyajian-O’Neill, L.A.; Mcclain, R.L.; Coleman, M.K.; Thomas, P.P. Diagnosis and Management of Piriformis Syndrome: An Osteopathic Approach. J. Am. Osteopath. Assoc. 2008, 108, 657–664. [Google Scholar] [CrossRef]
- Miller, T.A.; White, K.P.; Ross, D.C. The Diagnosis and Management of Piriformis Syndrome: Myths and Facts. Can. J. Neurol. Sci. 2012, 39, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Jankovic, D.; Peng, P.; van Zundert, A. Brief Review: Piriformis Syndrome: Aetiology, Diagnosis, and Management. Can. J. Anesth. 2013, 60, 1003–1012. [Google Scholar] [CrossRef]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Waldner, A.; Monsrud, J.; Franklin, M.; Bernath, D.; Clemente, F.R.; Fabrizio, P.A. Effect of Hip Rotation Stretch on the Piriformis Muscle: A Pilot Study. J. Stud. Phys. Ther. Res. 2015, 8, 111–122. [Google Scholar]
- Reichert, B.; Stelzenmüller, W.; Matthijs, O. Palpation Techniques: Surface Anatomy for Physical Therapists; Thieme: Stuttgart, Germany, 2011; p. 432. [Google Scholar]
- Zhang, W.; Luo, F.; Sun, H.; Ding, H. Ultrasound appears to be a reliable technique for the diagnosis of piriformis syndrome. Muscle Nerve 2019, 16766, 395–396. [Google Scholar] [CrossRef] [PubMed]
- Nunes, G.S.; Barton, C.J.; Serrão, F.V. Hip Rate of Force Development and Strength are Impaired in Females with Patellofemoral Pain without Signs of Altered Gluteus Medius and Maximus Morphology. J. Sci. Med. Sport 2018, 21, 123–128. [Google Scholar] [CrossRef]
- Fukumoto, Y.; Ikezoe, T.; Tateuchi, H.; Tsukagoshi, R.; Akiyama, H.; So, K.; Kuroda, Y.; Yoneyama, T.; Ichihashi, N. Muscle Mass and Composition of the Hip, Thigh and Abdominal Muscles in Women With and Without Hip Osteoarthritis. Ultrasound Med. Biol. 2012, 38, 1540–1545. [Google Scholar] [CrossRef]
- Whittaker, J.L.; Emery, C.A. Sonographic Measures of the Gluteus Medius, Gluteus Minimus, and Vastus Medialis Muscles. J. Orthop. Sport. Phys. Ther. 2014, 44, 627–632. [Google Scholar] [CrossRef]
- Ikezoe, T.; Mori, N.; Nakamura, M.; Ichihashi, N. Age-Related Muscle Atrophy in the Lower Extremities and Daily Physical Activity in Elderly Women. Arch. Gerontol. Geriatr. 2011, 53, e153–e157. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Gong, Y.; Cheng, Y.; Gao, X.; Chen, X. Motor Function Evaluation of Hemiplegic Upper-Extremities Using Data Fusion from Wearable Inertial and Surface EMG Sensors. Sensors 2017, 17, 582. [Google Scholar] [CrossRef]
- Hermens, H.J.; Freriks, B.; Merletti, R.; Stegeman, D.; Blok, J.; Rau, G.; Disselhorst-Klug, C.; Hägg, G. European Recommendations for Surface Electromyography. Roessingh Res. Dev. 1999, 8, 13–54. [Google Scholar]
- Penney, T.; Ploughman, M.; Austin, M.W.; Behm, D.G.; Byrne, J.M. Determining the Activation of Gluteus Medius and the Validity of the Single-Leg Stance Test in Chronic, Nonspecific Low Back Pain. Arch. Phys. Med. Rehabil. 2014, 95, 1969–1976. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.Y.; Guo, X.Y.; Chen, K.; He, F.D.; Quan, J.R. Feasibility and Reliability of an Ultrasound Examination to Diagnose Piriformis Syndrome. World Neurosurg. 2020, 134, e1085–e1092. [Google Scholar] [CrossRef]
- Kanakis, D.N.; Lazaris, A.C.; Papadopoulos, E.C.; Kallitsis, E.A.; Patsouris, E.S.; Paraskevakou, H.A. Piriformis syndrome--an attempt to understand its pathology. Clin. Neuropathol. 2010, 29, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Leung, F.T.; Mendis, M.D.; Stanton, W.R.; Hides, J.A. The Relationship between the Piriformis Muscle, Low Back Pain, Lower Limb Injuries and Motor Control Training Among Elite Football Players. J. Sci. Sport 2015, 18, 407–411. [Google Scholar] [CrossRef]
- Aboufazeli, M.; Akbari, M.; Jamshidi, A.A.; Jafarpisheh, M.S. Comparison of Selective Local and Global Muscle Thicknesses in Females with and Without Chronic Low Back Pain. Ortop. Traumatol. Rehabil. 2018, 20, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Kamaz, M.; Kiresi, D.; Oguz, H.; Emlik, D.; Levendoglu, F. CT Measurement of Trunk Muscle Areas in Patients With Chronic Low Back Pain. Diagn. Interv. Radiol. 2007, 13, 144. [Google Scholar]
- Amabile, A.H.; Bolte, J.H.; Richter, S.D. Atrophy of Gluteus Maximus among Women with A History Of Chronic Low Back Pain. PLoS ONE 2017, 12, e0177008. [Google Scholar] [CrossRef]
- Nourbakhsh, M.R.; Arab, A.M. Relationship between Mechanical Factors and Incidence Of Low Back Pain. J. Orthop. Sport. Phys. Ther. 2002, 32, 447–460. [Google Scholar] [CrossRef]
- Narouei, S.; Barati, A.; Akuzawa, H.; Talebian, S.; Ghiasi, F.; Akbari, A.; Alizadeh, M. Effects of core stabilization exercises on thickness and activity of trunk and hip muscles in subjects with nonspecific chronic low back pain. J. Bodyw. Mov. Ther. 2020, 24, 138–146. [Google Scholar] [CrossRef]
- Lee, S.-W.; Kim, S.-Y.; Yang, J.-M.; Park, S.-D. Comparison of Difference of the Gluteus Medius Muscle Fibre Thickness during maximum muscle contraction between Chronic Low Back Pain with Gluteus Medius Weakness and Healthy Subject. J. Korean Soc. Phys. Med. 2015, 10, 71–82. [Google Scholar] [CrossRef]
- Cooper, N.A.; Scavo, K.M.; Strickland, K.J.; Tipayamongkol, N.; Nicholson, J.D.; Bewyer, D.C.; Sluka, K.A. Prevalence of gluteus medius weakness in people with chronic low back pain compared to healthy controls. Eur. Spine J. 2016, 25, 1258–1265. [Google Scholar] [CrossRef] [PubMed]
- Pourahmadi, M.; Asadi, M.; Dommerholt, J.; Yeganeh, A. Changes in the Macroscopic Morphology of Hip Muscles In Low Back Pain. J. Anat. 2020, 236, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Al-Dirini, R.M.; Reed, M.P.; Thewlis, D. Deformation of the gluteal soft tissues during sitting. Clin. Biomech. 2015, 30, 662–668. [Google Scholar] [CrossRef]
- Sadler, S.; Cassidy, S.; Peterson, B.; Spink, M.; Chuter, V. Gluteus medius muscle function in people with and without low back pain: A systematic review. BMC Musculoskelet. Disord. 2019, 20, 463. [Google Scholar] [CrossRef]
- Wattananon, P.; Sinsurin, K.; Somprasong, S. Association between lumbopelvic motion and muscle activation in patients with non-specific low back pain during forward bending task: A cross-sectional study. Hong Kong Physiother. J. 2020, 40, 29–37. [Google Scholar] [CrossRef]
- Santos, F.G.; Carmo, C.M.; Fracini, A.C.; Pereira, R.R.; Takara, K.S.; Tanaka, C. Chronic low back pain in women: Muscle activation during task performance. J. Phys. Ther. Sci. 2013, 25, 1569–1573. [Google Scholar] [CrossRef]
- Strasser, E.M.; Draskovits, T.; Praschak, M.; Quittan, M.; Graf, A. Association between ultrasound measurements of muscle thickness, pennation angle, echogenicity and skeletal muscle strength in the elderly. Age 2013, 35, 2377–2388. [Google Scholar] [CrossRef]
- Franchi, M.V.; Longo, S.; Mallinson, J.; Quinlan, J.I.; Taylor, T.; Greenhaff, P.L.; Narici, M.V. Muscle Thickness Correlates to Muscle Cross-Sectional Area in the Assessment of Strength Training-Induced Hypertrophy. Scand. J. Med. Sci. Sport. 2018, 28, 846–853. [Google Scholar] [CrossRef]
- Kim, C.Y.; Choi, J.D.; Kim, S.Y.; Oh, D.W.; Kim, J.K.; Park, J.W. Comparison between Muscle Activation Measured by Electromyography and Muscle Thickness Measured using Ultrasonography for Effective Muscle Assessment. J. Electromyogr. Kinesiol. 2014, 24, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Warnica, M.J.; Weaver, T.B.; Prentice, S.D.; Laing, A.C. The Influence of Ankle Muscle Activation on Postural Sway during Quiet Stance. Gait Posture 2014, 39, 1115–1121. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.; Karppinen, J.; Samartzis, D. Low back pain in older adults: Risk factors, management options and future directions. Scoliosis Spinal Disord. 2017, 12, 14. [Google Scholar] [CrossRef] [PubMed]
- Bento, T.P.F.; Genebra, C.V.D.S.; Maciel, N.M.; Cornelio, G.P.; Simeão, S.F.A.P.; Vitta, A.D. Low back pain and some associated factors: Is there any difference between genders? Braz. J. Phys. Ther. 2020, 24, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Imtiaz, S.; Shah, H.; Muneer, S.; Hussain Shah, S.I.; Zahra, G.; Muneer, S. Prevalence of Piriformis Syndrome in Working and Non Working Women with Low Back Pain. J. Riphah Coll. Rehabil. Sci. 2014, 2, 6–11. [Google Scholar]
- Miller, R.M.; Freitas, E.D.S.; Heishman, A.D.; Peak, K.M.; Buchanan, S.R.; Kellawan, J.M.; Pereira, H.M.; Bemben, D.A.; Bemben, M.G. Muscle performance changes with age in active women. Int. J. Environ. Res. Public Health 2021, 18, 4477. [Google Scholar] [CrossRef]
- Mondal, M.; Sarkar, B.; Alam, S.; Das, S.; Malik, K.; Kumar, P.; Sahay, P. Prevalence of Piriformis Tightness in Healthy Sedentary Individuals: A Cross-Sectional Study. Int. J. Health Sci. Res. 2017, 7, 134. [Google Scholar]



| Variables | LBP + PS (n = 36; 40%) | LBP − PS (n = 25; 27%) | Healthy (n = 30; 33%) | p-Value |
|---|---|---|---|---|
| Gender | 0.79 | |||
| Male | 12 (41.38) | 8 (27.59) | 9 (31.03) | |
| Female | 24 (38.72) | 17 (27.42) | 21 (33.87) | |
| Age, years | 36.72 ± 9.26 | 34.44 ± 8.21 | 28.48 ± 9.95 | <0.001 *** |
| Height, m | 1.61 ± 0.09 | 1.59 ± 0.08 | 1.58 ± 0.07 | 0.59 |
| Weight, kg | 74.35 ± 17.38 | 72.34 ± 19.44 | 63.95 ± 20.00 | 0.02 * |
| BMI, kg/m2 | 28.83 ± 6.76 | 28.27 ± 6.23 | 25.09 ± 7.06 | 0.01 ** |
| Body fat, % | 37.65 ± 11.72 | 37.20 ± 9.80 | 32.45 ± 8.29 | 0.02 * |
| Muscle mass, % | 29.06 ± 7.37 | 26.92 ± 6.08 | 25.72 ± 5.86 | 0.09 |
| Muscle thickness, cm | ||||
| Piriformis | 2.09 ± 0.50 | 1.99 ± 0.43 | 1.64 ± 0.44 | <0.001 *** |
| Gluteus maximus | 2.62 ± 0.54 | 2.52 ± 0.69 | 2.10 ± 0.66 | <0.001 *** |
| Gluteus medius | 2.08 ± 0.43 | 1.92 ± 0.39 | 1.73 ± 0.51 | 0.02 * |
| Muscle strength, μV | ||||
| Gluteus maximus | 50.69 ± 29.89 | 72.59 ± 27.12 | 59.27 ± 18.52 | 0.04 * |
| Gluteus medius | 73.44 ± 32.93 | 82.64 ± 32.98 | 73.27 ± 34.84 | 0.53 |
| Smoking | 6 (85.71) | 1 (14.29) | 0.19 | |
| Yes | 30 (54.56) | 24 (43.67) | 1 (1.82) | |
| No | 2 (5.71) | 5 (14.29) | 28 (80.00) | |
| Physical level | 0.27 | |||
| Active (>2 strenuous/week) | 34 (57.63) | 20 (33.90) | 5 (8.47) | |
| Inactive (<2 strenuous/week) | 2 (6.45) | 5 (16.13) | 24 (77.41) | |
| Marital status | 24 (58.54) | 17 (41.46) | <0.001 *** | |
| Married | 12 (41.38) | 8 (27.59) | 9 (31.03) | |
| Unmarried | 20 (100.00) | |||
| Employment status | 27 (60.00) | 18 (40.00) | <0.001 *** | |
| Employed | 4 (28.57) | 3 (21.43) | 7 (50.00) | |
| Unemployed | 4 (14.81) | 1 (3.70) | 22 (81.48) |
| Variables | LBP + PS (n = 36; 40%) | LBP − PS (n = 25; 27%) | Healthy (n = 30; 33%) | p-Value |
|---|---|---|---|---|
| Radiculopathy | ||||
| Yes | 25 (69.40) | 7 (28.00) | 0 | <0.001 *** |
| No | 11 (30.60) | 18 (72.00) | 30 (97.00) | |
| Current history: Trauma/Fall/Heavy lifting | 28 (77.80) | 13 (52.00) | 2 (6.70) | <0.001 *** |
| Prolonged driving/sitting | 2 (5.60) | 1 (4.00) | 0 | <0.001 *** |
| Presence of gluteal tenderness | 36 (100.00) | 15 (60.00) | 6 (20.00) | <0.001 *** |
| Presence of a sausage-shaped mass | 15 (41.70) | 1 (4.00) | 0 | <0.001 *** |
| Positive special test | <0.001 *** | |||
| SLR | 21 (58.30) | 3 (12.00) | 0 | |
| HCLK | 24 (66.70) | 1 (4.00) | 0 | |
| Active Piriformis test | 34 (94.40) | 12 (48.00) | 2 (6.70) | |
| Laseuge sign | 28 (77.80) | 3 (12.00) | 2 (6.70) | |
| FAIR | 36 (100.00) | 5 (20.00) | 2 (6.70) | |
| Job field | <0.01 ** | |||
| White collar | 11 (45.80) | 9 (37.50) | 4 (16.70) | |
| Agriculture/Forestry | 1 (2.78) | 0 | 0 | |
| Transportation | 0 | 1 (100.00) | 0 | |
| Metal/Machine/Construction | 3 (60.00) | 2 (40.00) | 0 | |
| Clinician/Nurse/Healthcare | 4 (44.40) | 4 (44.40) | 1 (11.10) | |
| Sales work/Entrepreneurship | 3 (60.00) | 1 (20.00) | 1 (20.00) | |
| Services or other occupations | 5 (50.00) | 4 (40.00) | 1 (10.00) | |
| Sitting duration in a day | 0.66 | |||
| <4 h | 8 (32.00) | 9 (36.00) | 8 (32.00) | |
| 4–6 h | 23 (46.00) | 11 (22.00) | 16 (32.00) | |
| >6 h | 5 (13.89) | 5 (20.00) | 5 (17.24) | |
| Standing duration in a day | 0.41 | |||
| <4 h | 10 (27.78) | 12 (36.40) | 11 (33.30) | |
| 4–6 h | 13 (36.11) | 7 (21.90) | 12 (37.50) | |
| >6 h | 13 (36.11) | 6 (24.00) | 6 (24.00) | |
| Tasks required prolonged sitting | <0.01 ** | |||
| Occupational sitting | 18 (60.00) | 8 (27.70) | 4 (13.30) | |
| Religious activity | 1 (50.00) | 0 | 1 (50.00) | |
| Social media (i.e.,: games, Facebook, Whatsapp) | 4 (28.6) | 3 (21.40) | 7 (50.00) | |
| Leisure (i.e.,: reading/watching television) | 4 (14.80) | 7 (25.90) | 16 (59.30) | |
| House chores | 9 (56.30) | 6 (37.50) | 1 (6.30) | |
| Task required prolonged standing | 0.29 | |||
| Occupational standing | 21 (43.80) | 14 (29.20) | 13 (27.10) | |
| Religious activity | 1 (50.00) | 1 (50.00) | 0 | |
| Sport activities | 0 | 3 (42.90) | 4 (57.10) | |
| Leisure (farming/gardening/sports) | 1 (25.00) | 0 | 3 (75.00) | |
| House chores | 12 (42.90) | 7 (25.00) | 9 (32.10) |
| (i) | |||||||||
| Thickness (cm) | LBP + PS Mean ± SD | LBP-PS Mean ± SD | Healthy Mean ± SD | p-Value | Sum of Squares | df | Mean Square | F | Partial ETA Squared |
| Piriformis | 2.09 ± 0.50 | 1.99 ± 0.43 | 1.64 ± 0.44 | p < 0.01 | 2.65 | 2 | 1.33 | 5.03 | 0.11 |
| Gluteus maximus | 2.62 ± 0.54 | 2.52 ± 0.69 | 2.10 ± 0.66 | 0.02 * | 3.64 | 2 | 1.82 | 3.96 | 0.09 |
| Gluteus medius | 2.08 ± 0.43 | 1.92 ± 0.39 | 1.73 ± 0.51 | 0.06 | 1.40 | 2 | 0.70 | 2.79 | 0.09 |
| (ii) | |||||||||
| Thickness (cm) | Group Classification | Std. Error | p-Value | 95% CI for Difference | |||||
| Lower Bound | Upper Bound | ||||||||
| Piriformis | LBP − PS | Healthy | 0.12 | 1.00 | −0.18 | 0.38 | |||
| LBP + PS | Healthy | 0.11 | 0.00 *** | 0.08 | 0.61 | ||||
| LBP − PS | LBP + PS | 0.12 | 1.00 | −0.39 | 0.19 | ||||
| Gluteus maximus | LBP − PS | Healthy | 0.18 | 0.15 | −0.08 | 0.79 | |||
| LBP + PS | Healthy | 0.16 | 0.01 ** | 0.07 | 0.84 | ||||
| LBP − PS | LBP + PS | 0.17 | 1.00 | −0.31 | 0.51 | ||||
| Gluteus medius | LBP − PS | Healthy | 0.13 | 0.54 | −0.15 | 0.50 | |||
| LBP + PS | Healthy | 0.12 | 0.10 | −0.05 | 0.62 | ||||
| LBP − PS | LBP + PS | 0.13 | 0.62 | −0.15 | 0.46 | ||||
| (i) | ||||||||
| Variables | PS Mean ± SD | Non-PS Mean ± SD | Healthy Mean ± SD | p-Value | Sum of Squares | df | Mean Square | F |
| Muscle strength (μV) | ||||||||
| Gluteus maximus | 47.43 ± 26.54 | 72.59 ± 27.12 | 59.27 ± 18.52 | 0.01 * | 5441.96 | 2 | 2720.98 | 4.53 |
| Gluteus medius | 70.29 ± 30.39 | 82.64 ± 32.98 | 73.27 ± 34.84 | 0.53 | 1364.52 | 2 | 682.26 | 0.64 |
| Muscle activation prone lying with hip ERABEX (μV) | ||||||||
| Gluteus maximus | 105.70 ± 48.64 | 83.35 ± 22.98 | 103.79 ± 51.20 | 0.28 | 4927.54 | 2 | 2463.77 | 1.29 |
| Gluteus medius | 65.81 ± 6.17 | 68.53 ± 7.30 | 61.17 ± 7.11 | 0.52 | 1103.12 | 2 | 551.56 | 0.64 |
| Muscle activation in SLS (μV) | ||||||||
| Gluteus maximus | 73.48 ± 64.48 | 63.12 ± 39.56 | 58.85 ± 36.21 | 0.66 | 2061.09 | 2 | 1030.54 | 0.40 |
| Gluteus medius | 45.30 ± 28.24 | 58.75 ± 34.97 | 56.17 ± 30.71 | 0.39 | 1837.67 | 2 | 918.83 | 0.95 |
| (ii) | ||||||||
| Variables | Group Classification | Std. Error | p-Value | 95% CI for Difference | ||||
| Lower Bound | Upper Bound | |||||||
| Gluteus maximus strength | LBP − PS | Healthy | 8.80 | 0.41 | −8.53 | 35.16 | ||
| LBP + PS | Healthy | 8.21 | 0.46 | −32.24 | 8.54 | |||
| LBP − PS | LBP + PS | 8.37 | 0.01 ** | −45.93 | −4.40 | |||
| Gluteus medius strength | LBP − PS | Healthy | 11.71 | 1.00 | −19.69 | 38.43 | ||
| LBP + PS | Healthy | 10.93 | 1.00 | −30.09 | 24.14 | |||
| LBP − PS | LBP + PS | 11.13 | 0.81 | −39.96 | 15.27 | |||
| Gluteus maximus activation in STS | LBP − PS | Healthy | 18.03 | 1.00 | −40.47 | 49.02 | ||
| LBP + PS | Healthy | 16.83 | 1.00 | −27.12 | 56.39 | |||
| LBP − PS | LBP + PS | 17.14 | 1.00 | −32.17 | 52.88 | |||
| Gluteus maximus activation in STS | LBP − PS | Healthy | 10.62 | 0.63 | −12.90 | 39.80 | ||
| LBP + PS | Healthy | 10.43 | 0.90 | −36.75 | 15.00 | |||
| LBP − PS | LBP + PS | 10.62 | 0.63 | −39.80 | 12.90 | |||
| Gluteus maximus activation in hip ERABEX | LBP − PS | Healthy | 15.95 | 0.57 | −60.69 | 18.53 | ||
| LBP + PS | Healthy | 15.07 | 0.44 | −59.64 | 15.18 | |||
| LBP − PS | LBP + PS | 15.07 | 0.44 | −15.18 | 59.64 | |||
| Gluteus medius activation in hip ERABEX | LBP − PS | Healthy | 10.11 | 1.00 | −17.59 | 32.64 | ||
| LBP + PS | Healthy | 9.48 | 1.00 | −16.80 | 30.26 | |||
| LBP − PS | LBP + PS | 9.55 | 1.00 | −24.52 | 22.93 | |||
| Gluteus Maximus | Gluteus Maximus Activation | Gluteus Medius | Gluteus Medius Activation | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | LBP | Thickness (cm) | Strength (μV) | Prone Lying with Hip ERABEX (μV) | SLS (μV) | Thickness (cm) | Strength (μV) | Prone Lying with Hip ERABEX (μV) | SLS (μV) |
| Piriformis thickness (cm) | with PS | 0.19 | −0.40 * | −0.06 | 0.16 | 0.21 | −0.33 | 0.48 ** | 0.18 |
| without PS | 0.44 * | −0.35 | 0.24 | 0.19 | 0.33 | −0.43 * | 0.17 | 0.18 | |
| Gluteus maximus thickness (cm) | with PS | 1.00 | 0.25 | −0.17 | −0.15 | .31 | −0.18 | 0.37 * | 0.26 |
| without PS | −0.35 | 0.33 | 0.02 | 0.69 ** | −0.38 | 0.29 | 0.29 | ||
| Gluteus maximus activation prone lying with hip ERABE (μV) | with PS | 1.00 | −0.45 * | −0.55 ** | 0.13 | 0.44 * | −0.14 | 0.11 | |
| without PS | 1.00 | 0.35 | −0.42 | −0.34 | 0.64 ** | −0.33 | −0.32 | ||
| Gluteus maximus activation in SLS (μV) | with PS | 1.00 | 0.08 | −0.07 | −0.45 | 0.29 | −0.03 | ||
| without PS | 1.00 | 0.12 | 0.35 | −0.30 | 0.31 | −0.05 | |||
| Gluteus maximus activation in SLS (μV) | with PS | 1.00 | −0.04 | 0.15 | −0.24 | ||||
| without PS | 1.00 | −0.51 * | 0.47 * | 0.32 | |||||
| Gluteus medius thickness (μV) | with PS | 1.00 | −0.58 ** | −0.47 * | |||||
| without PS | 1.00 | −0.52 * | −0.37 | ||||||
| Gluteus medius activation prone lying with hip ERABEX (μV) | with PS | 1.00 | 0.31 | ||||||
| without PS | 1.00 | 0.59 ** | |||||||
| B | Β | Adjusted R2 | p-Value | Tolerance | VIF | |
|---|---|---|---|---|---|---|
| Model 1 | ||||||
| Constant | 1.69 | |||||
| MVC gluteus medius in prone lying with hip ERABEX (μV) | 0.00 | 0.48 ** | 0.20 | 0.01 | 1.00 | 1.00 |
| Model 2 | ||||||
| Constant | 2.04 | |||||
| MVC gluteus medius in prone lying with hip ERABEX (μV) | 0.01 | 0.43 * | 0.29 | 0.01 | 0.98 | 1.02 |
| Gluteus maximus strength (μV) | −0.01 | −0.34 * | 0.04 | 0.98 | 1.02 |
| Covariates | Coefficient | p Value | T | VIF |
|---|---|---|---|---|
| a Gender | 0.06 | 0.75 | 0.91 | 1.10 |
| Age (year) | 0.07 | 0.71 | 0.90 | 1.11 |
| Gluteus maximus strength (mean) (μV) | −0.31 | 0.09 | 0.88 | 1.13 |
| MVC gluteus medius in a prone lying position with hip ERABEX | 0.43 | 0.02 * | 0.94 | 1.06 |
| b Age (year) | 0.08 | 0.66 | 0.92 | 1.09 |
| Gluteus maximus strength (mean) (μV) | −0.33 | 0.07 | 0.93 | 1.07 |
| MVC gluteus medius in a prone lying position with hip ERABEX (μV) | 0.42 | 0.02 * | 0.96 | 1.05 |
| c Gluteus Maximus Strength (mean) (μV) | −0.34 | 0.04 * | 0.98 | 1.02 |
| MVC gluteus medius in prone lying hip ERABEX (μV) | 0.43 | 0.01 ** | 0.98 | 1.02 |
| Covariates | Coefficient | p-Value | T | VIF |
|---|---|---|---|---|
| a Gender | −0.19 | 0.40 | 0.90 | 1.11 |
| Age | 0.20 | 0.39 | 0.82 | 1.22 |
| Gluteus maximus muscle thickness | 0.31 | 0.21 | 0.78 | 1.28 |
| b Age | 0.22 | 0.35 | 0.83 | 1.21 |
| Gluteus maximus muscle thickness | 0.35 | 0.14 | 0.83 | 1.21 |
| c Gluteus maximus muscle thickness | 0.44 | 0.04 * | 1.00 | 1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Othman, I.K.; Raj, N.B.; Siew Kuan, C.; Sidek, S.; Wong, L.S.; Djearamane, S.; Loganathan, A.; Selvaraj, S. Association of Piriformis Thickness, Hip Muscle Strength, and Low Back Pain Patients with and without Piriformis Syndrome in Malaysia. Life 2023, 13, 1208. https://doi.org/10.3390/life13051208
Othman IK, Raj NB, Siew Kuan C, Sidek S, Wong LS, Djearamane S, Loganathan A, Selvaraj S. Association of Piriformis Thickness, Hip Muscle Strength, and Low Back Pain Patients with and without Piriformis Syndrome in Malaysia. Life. 2023; 13(5):1208. https://doi.org/10.3390/life13051208
Chicago/Turabian StyleOthman, Ida Kartini, Naresh Bhaskar Raj, Chua Siew Kuan, Sabrilhakim Sidek, Ling Shing Wong, Sinouvassane Djearamane, Annaletchumy Loganathan, and Siddharthan Selvaraj. 2023. "Association of Piriformis Thickness, Hip Muscle Strength, and Low Back Pain Patients with and without Piriformis Syndrome in Malaysia" Life 13, no. 5: 1208. https://doi.org/10.3390/life13051208
APA StyleOthman, I. K., Raj, N. B., Siew Kuan, C., Sidek, S., Wong, L. S., Djearamane, S., Loganathan, A., & Selvaraj, S. (2023). Association of Piriformis Thickness, Hip Muscle Strength, and Low Back Pain Patients with and without Piriformis Syndrome in Malaysia. Life, 13(5), 1208. https://doi.org/10.3390/life13051208










