Abstract
There is little evidence that the already described and accepted taxa of ascarids (Ascaris lumbricoides, A. suum, and A. ovis) infecting individuals of taxonomically distant groups (hominids, pigs, sheep, goats, and dogs) can be genetically or morphologically distinguished. However, despite described morphological differences, e.g., due to intraspecific variation, these are insufficient for species determination and may indicate differences amongst ascarids because of cross infections, hybrid production, and specific adaptations to hosts. Herein, the results of a molecular and morphological analysis of ascarids parasitising Sumatran orangutans (Pongo abelii Lesson, 1827) in native populations are presented. The research took place in the Bukit Lawang area, Indonesia, in 2009. Throughout the year, fresh faecal samples were collected regularly from 24 orangutans, and all were examined for the presence of nematode adults. Only five adult worms from two orangutan females were found during regular collection. Using the integrative taxonomic approach, the nematodes found were identified as A. lumbricoides. The significance of the find and its rarity is documented by the fact that this is the first confirmed finding of adult ascarids from an original orangutan site (not from a zoo) in more than 130 years (including the long-term study spanning the last 20 years focusing on orangutan parasites and natural antiparasitic drugs). More accurate morphometric parameters and genetic differences for the identification of ascarids were established. These parameters will be helpful for other findings in great apes and will also be suitable for further and precise determination of this parasite. The details distinguishing between male and female specimens are also stated and well defined. A comprehensive evaluation of the situation of Ascaris species parasitising orangutans, including a comparison with previously described orangutan parasite (i.e., A. satyri—species inquirenda), is discussed.
1. Introduction
Orangutans (genus Pongo Lacépède, 1799, Hominidae) are the only great ape species found exclusively in Asia [1] and are recognised as Critically Endangered by the IUCN [2]. The health of orangutans can be affected by a wide variety of intestinal parasites, among which nematodes play a significant role, as they have the potential to cause numerous serious health problems [3,4]. Because of this, the accurate identification of nematode species parasitising orangutans—including the genus Ascaris Linnaeus, 1758—has become increasingly important in recent decades [5,6,7,8,9].
According to the literature on ascarid findings in orangutans (Table 1), not considering Ascaris sp. and Ascaris spp., only two species, namely A. lumbricoides (Linnaeus, 1758) and A. satyri Chatin, 1877, have been consistently found. Regarded as a typically cosmopolitan parasite of humans, A. lumbricoides findings from primates, especially from great apes, have been very rare. However, records of this species have increased in the last two decades [7,9,10,11,12,13,14]. The species A. satyri is regarded by the author of its original description as a specialised parasite of Bornean orangutans. The name of this taxon is mentioned in a checklist by Linstow [15], but it is missing in modern monographs and is not mentioned even as a synonymic name of A. lumbricoides in some older literature [16,17,18]. Only Sprent in 1968 states the name of A. satyri with the remark that the inadequacy of the description prevented its inclusion in the genus Ascaris [19]. Later, Kirby with colleagues in 1975 mentioned this species as excluded from the genus Ascaris [20].
Ascarids parasitising orangutans are known mainly from their descriptions in captive hosts (Table 1). All reported ascarids from hosts living in native zoogeographical areas in Kalimantan (Borneo) or Sumatra have been based strictly on coprological analyses and on measurements of egg size; no molecular analyses have been used. The ascarids identified using these methods were all either A. lumbricoides [21,22,23] or Ascaris sp. [7,8,24,25,26]. Ascarids found in orangutans have not yet been morphologically studied, documented, or determined in detail.
This is the first study to present the results of morphological and molecular-genetic research on ascarids found in Sumatran orangutans from native populations. It comprehensively proves the ability of nematode Ascaris spp. to parasitise orangutans as well as different unrelated vertebrates.

Table 1.
List of Ascaris species reported from host of the genus Pongo Lacépède, 1799.
Table 1.
List of Ascaris species reported from host of the genus Pongo Lacépède, 1799.
| Species | Host | Material | Locality | References |
|---|---|---|---|---|
| A.lumbricoides | P. pygmaeus, captive | specimens | France Zoo | [27] |
| A.satyri | P. pygmaeus, captive | specimens | Museum Paris (imported from Borneo) | [28] |
| A.lumbricoides | P. pygmaeus, captive | specimens | Zoo Calcutta (India) | [29] |
| A.lumbricoides | P. pygmaeus, captive | specimens | Zoo Philadelphia (imported from Borneo) | [30] |
| A.lumbricoides | P. pygmaeus, captive | specimens | Zoo (Japan) | [31] |
| A.lumbricoides | P. pygmaeus, captive | specimens | Zoo London (UK) | [32,33] |
| A.lumbricoides | P. pygmaeus, captive | specimens | Zoo Amsterdam Rotterdam | [34] |
| Ascaris spp. | P. pygmaeus, captive | eggs | Primate Center Atlanta (USA) | [35] |
| Ascaris sp. | Pongo spp. captive, semi-wild | eggs | Zoo Jakarta, Sumatra-Bohorok, Kalimantan-Tanjung Puting (Indonesia) | [26] |
| A.lumbricoides | P. abelii, wild | Just simple list | Sumatra-Ketambe (Indonesia) | [36] |
| Ascaris sp. | P. pygmaeus, rehabilitant | eggs | Kalimantan-Tanjung Puting (Indonesia) | [24] |
| Ascaris sp. | P. pygmaeus, captive | eggs | Zoo Jakarta (Indonesia) | [24] |
| Ascaris sp. | P.abelii, rehabilitant | eggs | Sumatra-Bohorok (Indonesia) | [24] |
| “Ascarids” | Pongo spp. captive | eggs and specimens | from 50 international Zoo institutions | [37] |
| Ascaris sp. | P. abelii, wild | eggs | Sumatra-Ketambe (Indonesia) | [25] |
| A.lumbricoides | P. pygmaeus, during rehabilitation | eggs | Kalimantan-Tanjung Puting (Indonesia) | [21] |
| Ascaris sp. | P. pygmaeus, captive, semi-wild | eggs | Kalimantan-Wanariset (Indonesia) | [38] |
| A. lumbricoides | P. abelii, semi-wild | eggs | Sumatra-Bohorok (Indonesia) | [22] |
| A. lumbricoides | P. pygmaeus, captive | eggs | Zoo Wroclaw (Poland) | [39] |
| Ascaris sp. | P. abelii, captive | eggs | Sumatra (Indonesia) | [8] |
| Ascaris sp. | P. abelii, captive | eggs | Sumatra-quarantine Batu Mbelin (Indonesia) | [40] |
| Ascaris sp. | Pongo spp., captive | eggs | Zoo Rizal (Philippines) | [41] |
| Ascaris sp. | P. pygmaeus, captive | eggs | Kalimantan-Nyaru Menteng, Wanariset (Indonesia) | [7] |
| A. lumbricoides | P. pygmaeus, wild | eggs | Kalimantan-Sebangau (Indonesia) | [23] |
| Ascaris spp. | P. pygmaeus, wild, urban, captive | eggs | Peninsular Malaysia and Malaysian Borneo (Sabah and Sarawak) | [10] |
| Ascaris sp. | P. pygmaeus, captive | eggs | Matang Wildlife Centre, Kuching Division, Sarawak (Malaysia) | [9] |
2. Methods
2.1. Sample Collection
The location of this study, the village of Bukit Lawang (the former site of a rehabilitation centre for Sumatran orangutans), is situated on the southwest border of the Gunung Leuser National Park, North Sumatra, Indonesia (N 03°32.983′ E 098°06.908′), at an altitude of 323 m. Temperatures in this region range between 21 °C and 28 °C, with humidity levels ranging between 80% and 100%. Annual rainfall ranges from 2000 mm to 3200 mm.
The majority of Sumatran orangutans (Pongo abelii Lesson, 1827) in this area belong to a semi-wild population. At the time of this study (2009), nine orangutans were in cages in the quarantine area and fifteen orangutans were roaming freely within the vicinity of the quarantine area, so contact between these individuals was possible. Continuously throughout the whole year, the faecal samples were collected regularly and examined for the presence of nematode adults. In total, five (n = 5) adult specimens were found from the fresh faeces of two semi-wild adult females and immediately fixed in 70% ethanol.
2.2. Morphological Analyses
For microscopic examination, sections of the nematode body were cleared gradually in glycerine and examined for morphometric analysis under a light microscope (Olympus BX51) equipped with differential interference contrast (DIC), a digital image analysis system (Micro Image 4.0 for Windows), and a drawing attachment. All provided measurements are given in micrometers (µm) unless stated otherwise, and are given as the range followed by the mean in parentheses. The parasite specimen (one female, anterior part of the body) selected for scanning electron microscopy (SEM) was further dehydrated in ethanol, dried in a CPD 030 critical point drying apparatus (Bal-tec) using liquid CO2, mounted on aluminium stubs with a double-sided adhesive disc, coated with gold in a SCD 040 sputter coating unit (Balzers), and examined in a MIRA (Tescan) scanning electron microscope operating at 15 kV.
2.3. DNA Extraction, PCR Amplification, and Gene Characterisation
Five adult worms collected from two orangutans in Sumatra were processed for molecular analyses. A middle part of the body of approximately 10–12 mm in length was cut off and fixed in 96% ethanol. In addition, one ascarid specimen collected from domestic pigs (Sus scrofa domesticus Erxleben, 1777) in the Czech Republic and one specimen of Baylisascaris columnaris (Leidy, 1856) obtained from the captive striped skunk (Mephitis mephitis (Schreber, 1776)) in the Czech Republic were also analysed, and obtained sequences were included into phylogenetic analyses.
Prior to DNA extraction, parasites were dried, and genomic DNA was isolated from each specimen using a DNeasy Blood and Tissue Kit (Qiagen, Hilden, Germany) following the recommended protocol with slight modification (overnight incubation at room temperature during lysis with proteinase K until tissues were fully digested). For molecular characterisation, partial sequences of one mitochondrial gene (CO1) and four nuclear regions for rRNA genes (18S, 5.8S, 28S and both internal transcribe spacers (ITS1, ITS2)) were amplified by using the primer sets listed in Table 2. Each polymerase chain reaction (PCR) was performed in 30 μL of reaction volume using 20–50 ng of genomic DNA as a template. For amplification of the CO1 fragment, a PCR reaction mixture consisted of 1× PPP Master Mix (Top-Bio, Vestec, Czech Republic), 0.3 μM of each primer, template DNA in a stated concentration, and nuclease-free water. Amplification ran under the following conditions: initial denaturation at 95 °C for 5 min, then 35 cycles of 95 °C for 30 s, 55 °C for 30 s, and 72 °C for 40 s; a final extension ran at 72 °C for 10 min. The reaction mixture for 18S rDNA amplification was comprised of DNA template, nuclease-free water, 1× Taq buffer with KCl, 1.5 mM MgCl2, 0.2 mM of each dNTP, 0.5 μM of each primer, and 1 U of Taq DNA polymerase (Fermentas by Thermo Fisher Scientific, Waltham, MA, USA, hereinafter Fermentas). Amplification ran in 39 cycles under the following conditions: initial denaturation at 94 °C for 5 min; cycling at 94 °C for 30 s, 54 °C for 30 s, and 72 °C for 1 min; a final extension ran at 72 °C for 10 min. Amplification of the 18S-ITS1-5.8S-ITS2-28S region (referred to below as ITS only) was performed using four primer combinations: (1) TW81–AB28 [42]; (2) 18SNemF–28SNemR [43]; (3) ITS2-F–ITS2-R [44]; and (4) Ascaris ITS F1–Ascaris ITSR1 [45] (for details, see Table 2). Each reaction mixture was comprised of DNA template, nuclease-free water, 1× Taq buffer with KCl, 2 mM MgCl2, 0.2 mM of each dNTP, 0.5 μM of each primer, and 2 U of Taq DNA polymerase (Fermentas). Cycling parameters started with 5 min denaturation at 94 °C, then 39 cycles of 94 °C for 40 s, AT °C (Table 2) for 40 s, 72 °C for 1 min, and a final extension of 10 min at 72 °C. For TW81–AB28 primer combination, the elongation at 72 °C in cycling was prolonged up to 2 min. All PCR reactions were performed in a Mastercycler EP gradient S thermocycler (Eppendorf, Hamburg, Germany). The quality and yield of isolates, as well as PCR products, were verified via agarose gel electrophoresis on 1.5% gel stained with GoodView dye (SBS Genetech, Beijing, China).

Table 2.
List of primers used for PCR amplification of mitochondrial and nuclear markers in the present study.
For automatic sequencing, the obtained PCR products were purified according to manufacturers’ recommendations using the High Pure PCR Product Purification Kit (Roche, Basel, Switzerland) or enzymatic approach with ExoSAP-IT PCR cleanup reagent (Thermo Fisher Scientific, Waltham, MA, USA). Amplification was performed using the fluorescent chemistry of BigDye Terminator v3.1 Ready Reaction Cycle Sequencing Kit (Applied Biosystems by Life Technologies, Carlsbad, CA, USA; hereinafter Applied Biosystems). Subsequently, the products of the sequencing reactions were purified using BigDye X-Terminator Purification Kit (Applied Biosystems) according to the manufacturer’s protocol and sequenced in both directions using PCR primers on an ABI 3130 Genetic Analyzer (Applied Biosystems) under the appropriate run module. Raw sequencing data were analysed with Sequencing Analysis software v.5.2 (Applied Biosystems) and subjected to processing via Sequencer v.5.4.6 software (Gene Codes Corporation, MI, USA) to obtain contigs. The obtained sequences were deposited in GenBank database (IDs: LN600399–LN600409, and OQ539679–OQ539680–OQ539681). The Basic Local Alignment Search Tool (BLAST; https://blast.ncbi.nlm.nih.gov/Blast.cgi; accessed on 16 February 2023) searches were performed to verify the similarity of the sequences obtained in this study with other sequences of ascarid nematodes.
2.4. Sequence Alignment and Phylogenetic Analyses
Two sequence alignments were built to assess the phylogenetic placement of newly obtained sequences within Ascarididae. The first CO1 alignment included one sequence of Ascaris lumbricoides isolated from P. abelii in Sumatra, 23 sequences representing the most common haplotypes of Ascaris species, and 13 sequences of other representatives of the family Ascarididae (details and accession numbers are stated in Table 3). According to previous published studies [48,49,50,51] and the GenBank/BLAST data search, the consensus haplotype sequences (i.e., HapA1-7, HapB1-4, and HapC1-4) were generated for analysis purposes for this study. The CO1 sequences from these study (LN600399–LN600401) were trimmed from primer sequences before alignment. As outgroup, the sequences of four Toxocara species (AJ9220055; AJ920057; AJ920062; AM412316) were used. The second ITS alignment comprised three sequences generated in the present study (access. no. OQ539679–A. lumbricoides from P. abelli; OQ539680–A. lumbricoides from S. scrofa domesticus and OQ539681–B. columnaris from striped skunk) and 24 sequences downloaded from Genbank representing Ascaris species isolated from different host and selected representatives of Ascarididae (for details, sequence origin, and accession numbers see Figure 5). The sequences containing partial regions of 18S and 28S were trimmed before alignment to the ITS region only. Two sequences of Toxocara species (JF837169 and JF837171) were used as outgroup.

Table 3.
List of CO1 sequences of selected representatives of Ascarididae used in the phylogenetic analyses with host species, countries of collection, and GenBank accession numbers.
Sequences in both datasets were aligned in MAFFT v.7 [54,55] with default parameters and manually edited in BioEdit [56]. For the CO1 sequences, the presence of stop codons and indels was verified with MEGA 11 [57], using the invertebrate mitochondrial code. Highly variable regions, extensive gaps, and poorly aligned positions present in the ITS alignment were eliminated using Gblocks 0.91b [58], allowing all options for a less stringent selection. Phylogenetic relationships were analysed using Bayesian inference (BI) and Maximum likelihood (ML) approaches. The best fitting models of nucleotide substitution were determined via ModelFinder [59] for each gene dataset. According to the Bayesian information criterion (BIC), the TIM3 + F + G model was selected as the most appropriate evolutionary model for CO1 dataset and K2P + G4 for ITS1, K2P + I for 5.8S, and HKY + F + G4 for ITS2 dataset. BI analysis was conducted using MrBayes 3.2.6 [60]. Two simultaneous runs with four independent Markov chains were performed for 5 million generations, sampling every 100 generations. After the average standard deviation of split frequencies fell below 0.01, the first 25% of samples were discarded as burn-in, and the remaining trees were used to generate a consensus tree and calculate the posterior probabilities. The ML analysis was performed in IQ-TREE v.2 [61]. To obtain node support statistics, 1000 bootstrap replicates using ultrafast bootstrap approximation (UFBoot) [62] and the Shimodaira–Hasegawa approximate likelihood ratio test (1000 replicates) were chosen. The obtained trees for BL and ML were visualised in FigTree v.1.4.3 [63].
2.5. Ethical Note
All the research reported in this manuscript adhered to the legal requirements of the country in which the work took place (SIP No 0056/FRP/SM/III/2009, 0008/EXT/FRP/SM/II/2010) CITES permit 08411/IV/SATS-LN2011). Since the collection of faecal samples from orangutans was non-invasive and did not involve interaction with or distress to the animals, the study was not reviewed by an animal ethics committee. No interaction with animals was conducted for this study.
3. Results
3.1. Morphological Analysis
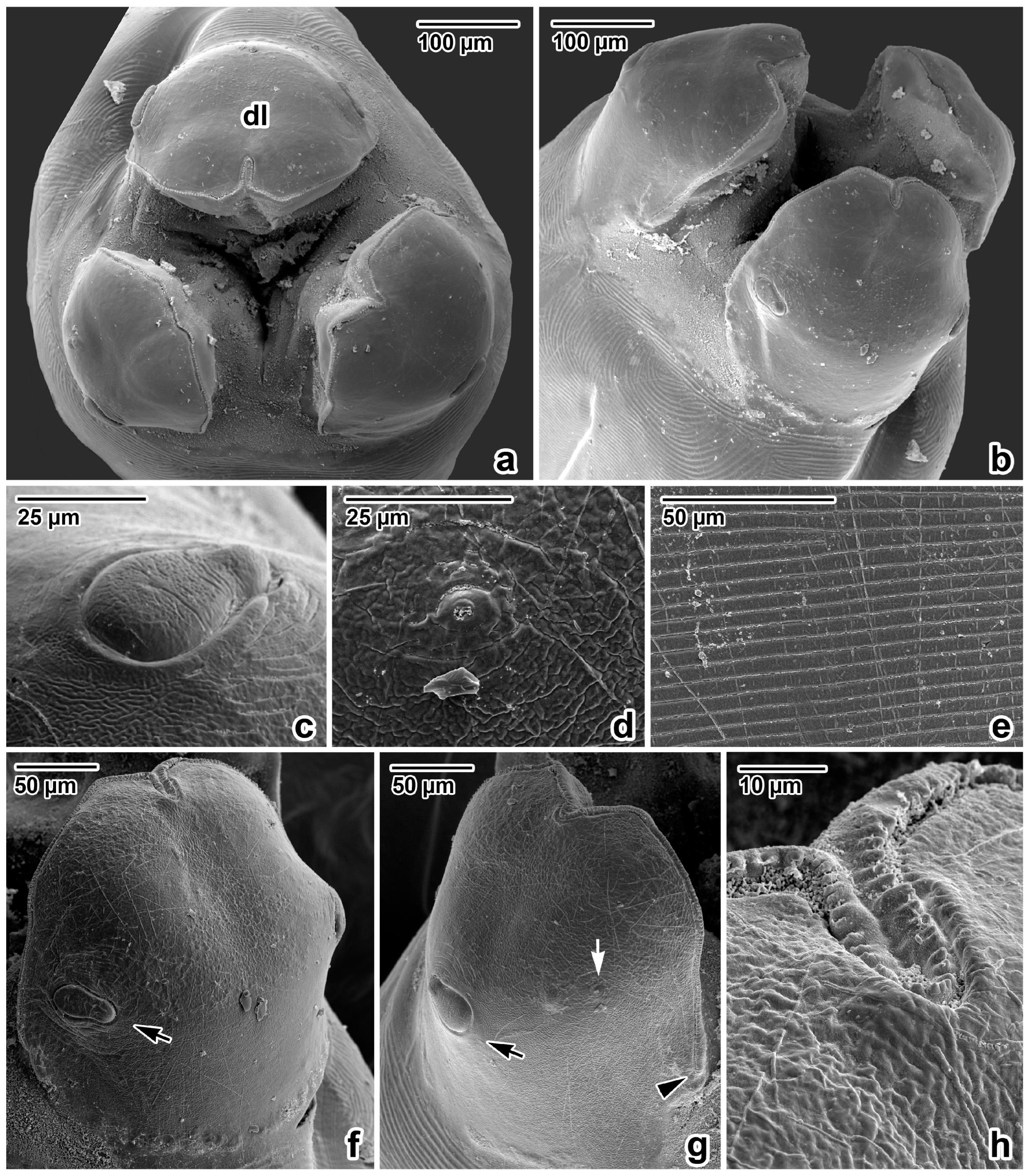
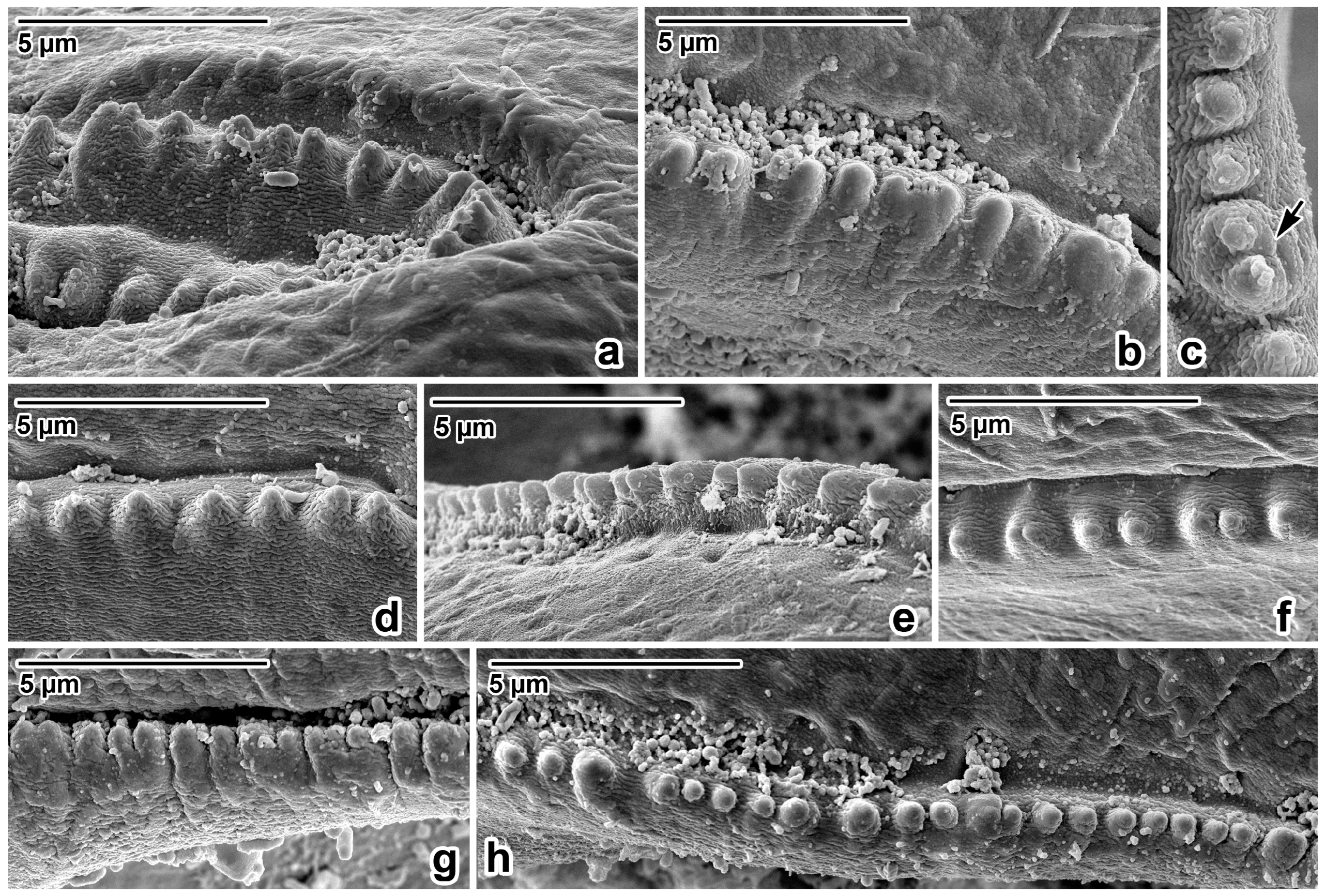
Nematodes were robust, tapering at both ends and white to yellowish in colour. Anterior end (Figure 1a and Figure 2a,b) exhibited a terminal triangular mouth surrounded by three prominent lips: dorsal lip with two double elliptical papillae (Figure 1b and Figure 2c,f) and two ventrolateral lips each with one double elliptical papilla and amphid adjacent to externolateral papilla (Figure 2d,g). Lips were slightly hexagonal with rounded anglěěes. Central upper rims of lips more or less inflexed (Figure 2h). The height of the lips was 200–240 (227) and the width was 220–280 (249). One ring of denticles (Figure 3) was situated on the inside of each lip edge, terminating 20–38 from the base of lip (Figure 2g arrowhead). Each lip was reinforced by labial pulp with two main branches ending and dividing anteriorly into two parts. Bifurcation was very slight. Interlabia and cuticular lateral alae were absent. Denticles were located close to each other and varied in shape and size. Denticles were mainly pyramidal at anterior and lateral edges of lips; rarely, some peaks were broadly rounded or bicuspid (Figure 3c,f,h). The height and width varied in the ranges 2.0–5.0 (3.41) and 1.5–2.8 (2.38), respectively. Denticles had double peak maximum width 4.0. Denticles on lateral sides were smaller with height 2.0–3.7 (2.73) and width 1.2–2.9 (2.37). Denticles on the distal line of the lip base were spaced closer together and had mostly rounded tops with height 1.2–2.2 (1.68) and width 1.2–1.9 (1.54). On a section of anterior margin of lip 60 wide, the number of denticles varied between 17 and 31; the number of denticles at the base of the lips (the same width) varied between 27 and 39. Cuticle was clearly transversely grooved in intervals of 5–8 (Figure 2e). Oesophagus was muscular, cylindrical, and slightly broader posteriorly than anteriorly. Excretory pore was slightly posterior to the nerve ring and stood on a slightly elevated cuticle, with an opening surrounded by circular muscle.
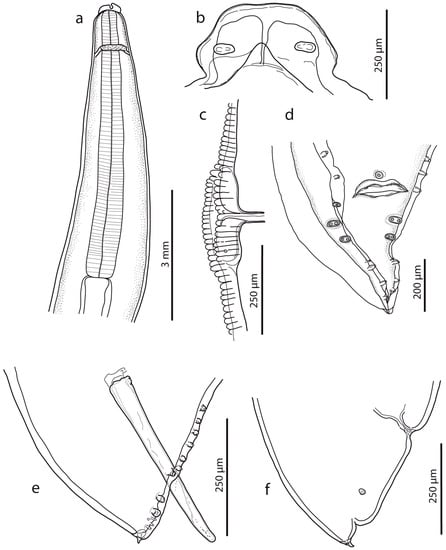
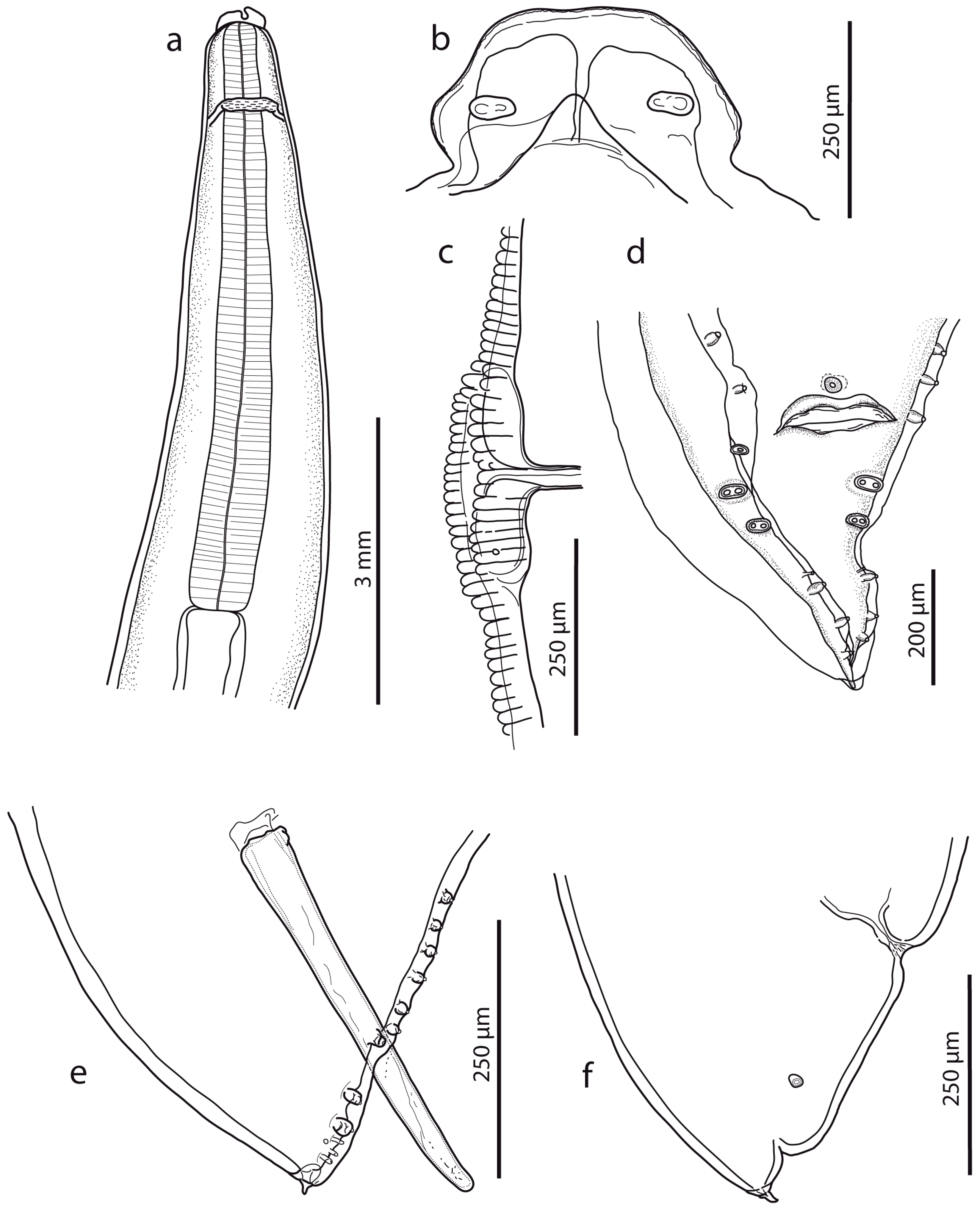
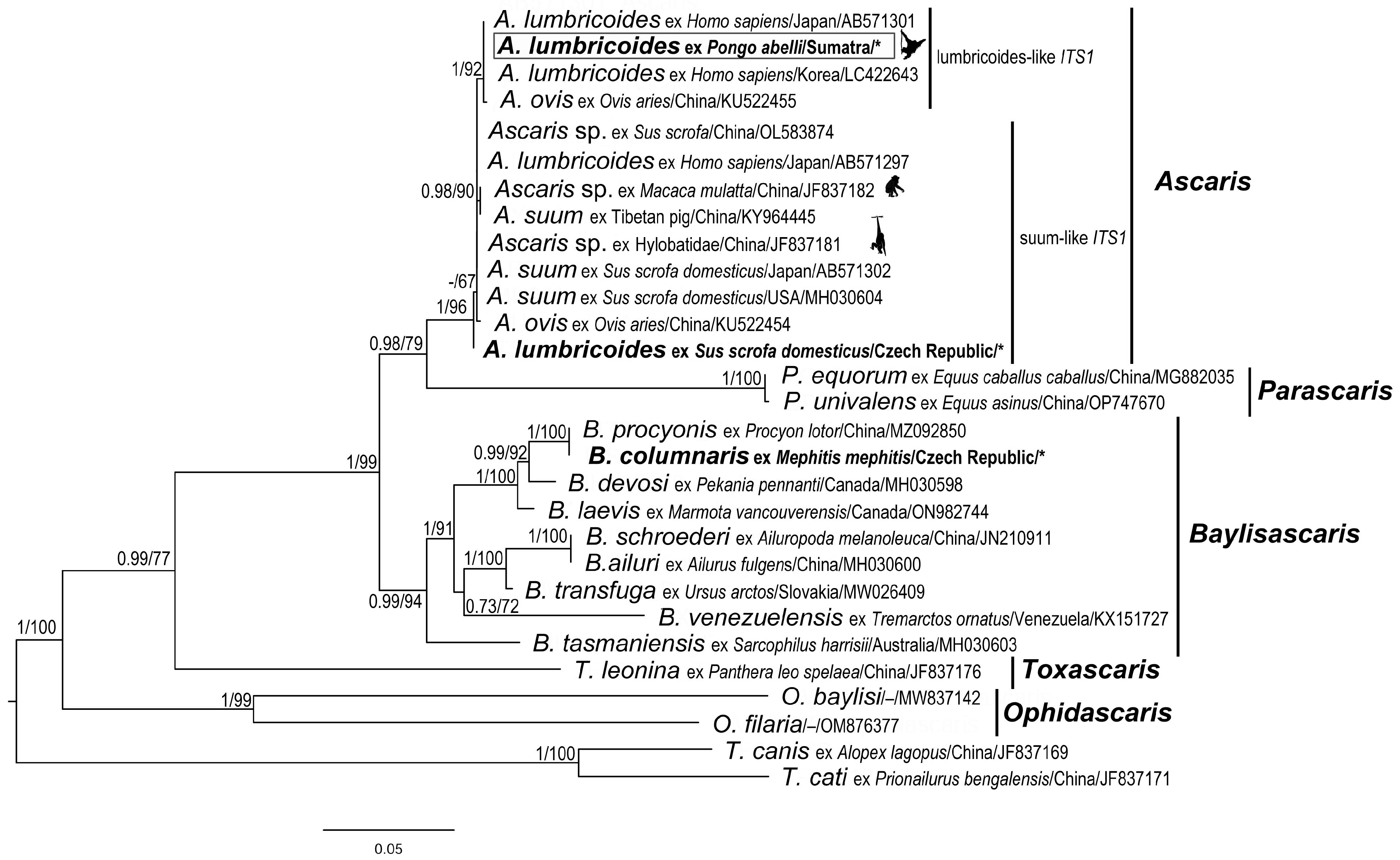
Figure 1.
Ascaris lumbricoides, line drawings. (a) Anterior end with oesophageal region (lateral view). (b) Dorsal lip (detail). (c) Vulval region (lateral view). (d) Female caudal end (lateral view). (e) Male caudal end with papillae (ventral view) (for comparison, see [16]). (f) Male caudal end with papillae and spicule (lateral view).
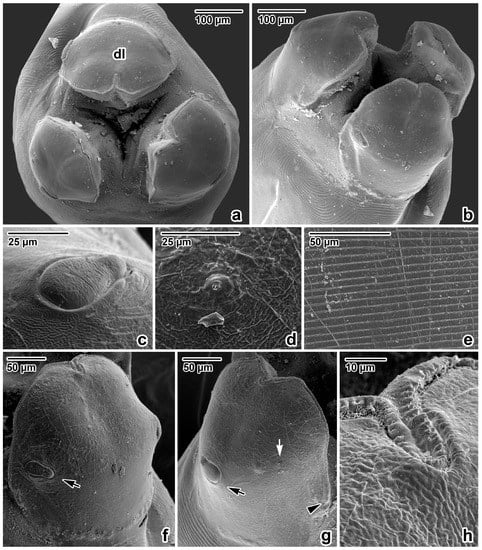
Figure 2.
Anterior extremity of Ascaris lumbricoides, female, SEM micrographs. (a) Anterior extremity, apical view. (b) Anterior extremity, dorsolateral view. (c) Detail of the dorsal labial papilla. (d) Detail of the amphidial pore in lateroventral lip. (e) Detail of the cuticle, transverse striation in oesophageal part of body. (f) Dorsal lip, total view. (g) Lateroventral lip, total view. (h) Inflexed line of denticles in anterior extremity of dorsal lip (detail); dl—dorsal lip; arrow—papilla; white arrow—amphidal pore; arrowhead—end of denticle row.
Figure 3.
Lip rim details of Ascaris lumbricoides, female, SEM micrographs. (a,b) Anterior part of lip rim, lateral view. (c) Denticle with double peak, apical view. (d,e) Lateral part of lip rim, lateral view. (f) Lateral part of lip rim, apical view. (g) Posterior part of lip rim, lateral view. (h) Posterior part of lip rim, apical view; arrow—double peak denticle.
Male (based on two specimens): Body length 9.3–10.1 cm, maximum body width 2.40–2.50 mm. The width of the anterior extremity at the base of the lips was 458–479; the width of the posterior extremity at the level of the cloaca was 318–549. The distances of the nerve ring and the excretory pore from the anterior extremity were 1.05–1.20 mm and 2.67–3.08 mm, respectively. The tail length was 236–390, tapered conically, bearing terminal mucron 19–28 long (Figure 1d,e). There were five pairs of post-cloacal papillae, the first two pairs doubled; there was one single papilla on the upper cloacal lip (Figure 1d,e). The total number of pre-cloacal simple papillae was 64–75 pairs, formed into two longitudinal lateral rows on the sides of the body towards the cloacal opening. In the bottom part of the rows, the span between the papillae was narrow and in the anterior part of the rows, the distances between the papillae were greater. The pre-cloacal papillae end extends anteriorly 3.92–4.67 mm to the tail tip. There were two slightly curved and relatively massive spicules, equal in form and length (1.065–1.223); there were no spicular alae. Proximal ends of spicules had flat endings of width 105–169; there was a distal part with an obtuse tip of width 47–49. There was no gubernaculum.
Female (based on three juvenile specimens): Body length 11.9–19.2 cm, maximum body width 3.1–4.6 mm. The width of the anterior extremity at the base of the lips was 620–753; the width of the posterior extremity at the level of the anus was 1.07–1.19 mm. The length of the oesophagus was 6.23–7.76 mm; the maximum width was 0.98–1.03 mm. The distances of the nerve ring and the excretory pore from the anterior extremity were 1.14–2.33 mm and 1.46–2.15 mm, respectively. The vulva (Figure 1c) was in the form of a transverse slit with rounded non-prominent lips, situated at about the first third of the body. The vagina separates into two posteriorly directed uteri just posterior to the vulva. The tail (700–978 long) tapered conically (Figure 1f), with mucron 52–69 long. The distance of the phasmids from the posterior extremity was 294–339. Eggs were not developed.
3.2. Molecular-Genetic Analysis
Characterisation of 18S rDNA Region
Two partial 18S rDNA sequences (LN600406 (n = 2; 843 bp) and LN600407 (n = 3; 839 bp)) were obtained for A. lumbricoides isolated from P. abelii in Sumatra. Both sequences are almost identical, but LN600407 has a heterozygote site (S, G/C) in the position of 457. No interspecies genetic variation among 18S rDNA sequences generated in the present study was found (the identical 18S rDNA sequences were also obtained from pigs’ ascarids (A. lumbricoides–access. no. LN600408) and from ascarid parasitising the striped skunk in the Czech Republic (B. columnaris–access. no. LN600409)). The sequence comparison through NCBI BLAST (February 2023) showed 100% identity with three sequences of A. suum (MN558962 (dog; China), AF036587 (host not stated) and U94367 (pig; USA)), one sequence of Ascaris sp. (JN256985 (hoolock gibbon; China), two sequences of B. procyonis (U94368 (raccoon; USA), and KC172105 (raccoon dog; Norway)).
3.3. Characterisation of ITS Region
No intra-individual variation for the ITS region was detected in all five Ascaris specimens isolated from P. abelii in Sumatra. The resulting ITS sequence (ID OQ539679) had a total length of 1080 bp (the lengths of the partial 18S, complete ITS1–5.8S–ITS2 and partial 28S sequences were detected in positions 1–145 bp, 146–593 bp, 594–750 bp, 751–1024 bp, and 1024–1080, respectively). BLASTn search (February 2023) revealed 100% identity with two sequences of the whole ITS region (LC422642, LC422643) obtained from A. lumbricoides parasitising humans in Japan, whereas the ITS1 sequence (diagnostic region) corresponded to the previously characterised genotype G1 (AJ554036 [64]), which is the most prevalent Ascaris ITS1 genotype in humans [64,65]. For the 5.8S and ITS2 region, no informative nucleotide differences were detected.
3.4. Characterisation of CO1 mtDNA Region
One CO1 sequence variant (LN600399; 441 bp) was obtained from five specimens of A. lumbricoides isolated from P. abelii in Sumatra. BLASTn search (February 2023) revealed 100% identity with the sequences representing the most frequent CO1 haplotype of A. lumbricoides/suum parasitising humans and pigs (also indicated in dog; Mohd-Shaharuddin unpublished) with worldwide distribution (named HapA1 in the present study; H12 in [49]; H1 in [48]; H29 in [51]; and US4 in [50]; for detailed information see Table 3).
3.5. Phylogenetic Analyses
The tree topologies obtained through BI and ML phylogenetic analyses of both datasets (CO1 and ITS) were almost identical, and the phylogenetic reconstructions inferred from ML analyses are shown in Figure 4 and Figure 5.
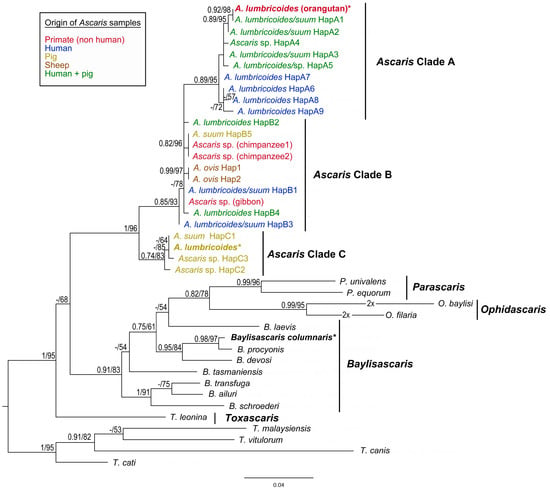
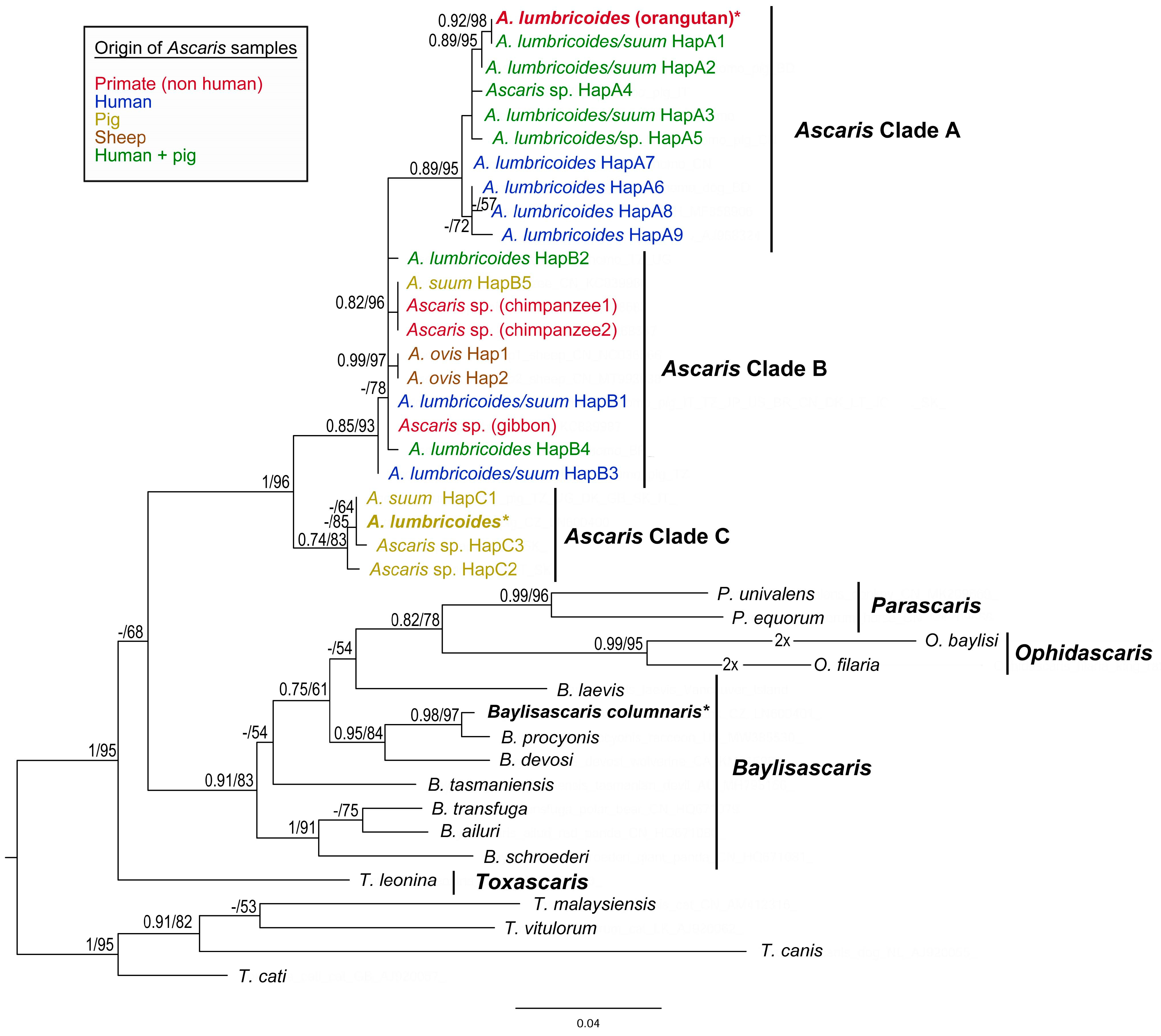
Figure 4.
Maximum likelihood (ML) tree of Ascarididae species generated from partial CO1 mtDNA sequence alignment. Values along the nodes indicate posterior probabilities from BI and bootstrap values from ML analyses. Dashes indicate values below 0.70 and 50, respectively. Sequences obtained in the present study are shown in bold and highlighted with asterisks. Host origin of the CO1 haplotypes of Ascaris species is marked by different colours.
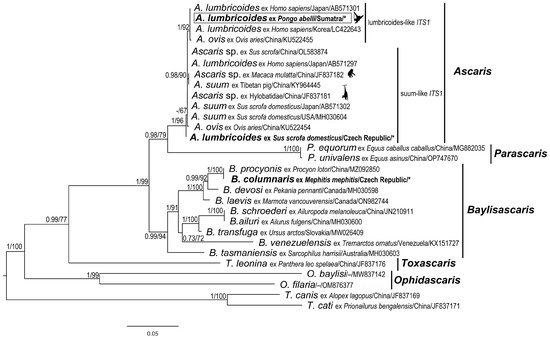
Figure 5.
Maximum likelihood (ML) tree of Ascarididae species generated from complete ITS (ITS1 –5.8S–ITS2) sequence alignment. Values along the nodes indicate posterior probabilities from BI and bootstrap values from ML analyses. Dashes indicate values below 0.70 and 50, respectively. Sequences obtained in the present study are shown in bold and marked with an asterisk. The sequence from orangutan host in this study is in the rectangle.
The final alignment of CO1 sequences yields 384 characters (117 variable, 88 parsimony-informative). Results of the present phylogenetic analyses based on CO1 sequences are congruent with the previous phylogenetic studies of Ascaris species [47,48,66,67]. The CO1 haplotypes of A. lumbricoides/suum form three main clades (A, B and C), with clade C being basal to the two other clusters (Figure 4). The sequence of A. lumbricoides from P. abelii obtained in the present study clusters together with CO1 haplotypes of clade A. The CO1 sequence of A. lumbricoides isolated from domestic pig in Czech Republic belonged to clade C and it is identical as the most frequent “pig” haplotype (named HapC1 in the present study, H64 in [48], H1 in [49], and H3 in [51]) with distribution in Europe and Africa.
The final alignment of ITS sequences yielded 857 characters (453 variable, 309 parsimony-informative). The resulting phylogenetic tree based on the ITS sequences is shown in Figure 5. The ITS sequence (orangutan) obtained in the present study from orangutans was nested within the lumbricoides-like ITS1 clade, which was highly supported by both analyses (PP = 1; BS = 92%).
4. Discussion
It was difficult to identify the agents of Ascaris infections among primates with the species level before [19,68]. In this research, it was possible to establish the identity of ascarids from Sumatran orangutans by means of a morphological and metrical study. Moreover, some details (anterior extremity) using SEM were also determined and compared with descriptions of A. lumbricoides from man [68,69,70,71].
Although the number of samples studied might seem very low for description, in practice, even when hosts are massively parasitised, they excrete huge numbers of parasite eggs, but the detected presence of adult worms is very sporadic in faecal samples. Therefore, the adult ascarids found in the present study represent a significant and rare finding. Up to date, the last documented record of nematode adults shed by orangutans living in the native locality date was in 1877 [28]. It is also obvious that the infection was really massive because five worms from two hosts were obtained in a very short period (one year of a 20-year long-term study focusing on orangutan parasites and natural antiparasitic drugs).
Former findings and the determinations of ascarids in orangutans are not accompanied by information on their morphometry except length of bodies at A. satyri by Chatin [28] and A. lumbricoides by Canavan [30]. Canavan reported A. lumbricoides from the Bornean orangutan, describing one male with a body length of 144 mm and two females with body lengths of 148 mm and 165 mm [30]. Many authorities consider A. lumbricoides and A. suum Goeze, 1782, to be morphometrically indistinguishable [72,73,74]. On the other hand, several authors found a specific morphological point of difference when studying the rows of denticles in these species’ mouths ending at the lips [75,76,77,78]. Others documented the great variability in the shape and form of the denticles [79,80,81,82]. Based on the morphology of the denticles documented via SEM, present results were compared with data on measurements of previously mentioned authors. So far, only Hartwich reported that the denticles of A. lumbricoides in the upper-centre of the lips reach a height of 2–7 µm and a width of 2–6 µm [69]. Borkovcová found the heights and widths of all forms of denticles in the lips of A. lumbricoides to be in the range of 0.7–4.2 µm and 1.3–2.7 µm, respectively [80]. The dimensions of the denticles of ascarids in this study material from Sumatra (height range: 1.2–5.0; width range: 1.2–2.9) correspond with data in Borkovcová [80].
The main features for differentiating and determining morphospecies of A. lumbricoides established by previously mentioned authors are as follows: (1) the shape (morphology) of pulps of lips, the anterior lobes of which are entirely divided; (2) males possessing five pairs of post-cloacal papillae, the first two pairs (located closest to the cloaca) being doubles. This feature was already mentioned by Schneider [83] and confirmed by Baylis and Daubney [29]. Using an SEM study, Uni and Takada also documented the number and distribution of papillae, including phasmidial openings situated between the second (double) and third (simple) post-cloacal papillae [84]; (3) the proximal ends of spicules are consistently straight; (4) in females, the vulva is located at the beginning of the second third of the body length (the vulva divides the body in the ratio range 1:2.0–1:2.1). A comparison of the main values characterising morphospecies of A. lumbricoides can be seen in Table 4.

Table 4.
Selected measurement of the A. lumbricoides (males and females) parasitising man (Homo sapiens) and Sumatran orangutan (P. abelii).
In the opinion of Sprent, the inadequacy of the description of the species A. satyri prevents its inclusion in the genus Ascaris [19,77]. After studying the original description, authors of the presented work do not agree with this opinion and suggest considering it as species inquirenda. According to Chatin’s description without species illustrations [28], males do not exceed 90–100 mm in length, while females have an average body length of 120 mm; the colour is milk white, or slightly yellowish; the transverse cuticle striation and layer of subcutaneous musculature are thick; the description of the digestive system refers to the shape of the oral opening with three lips (the presence of denticles on the edge of the lips) and the shape of the oesophagus. Chatin also stresses that the eggs (elliptical in shape, 0.069 mm) are smaller than those of A. lumbricoides [28]. Even if these features do not differentiate it from A. lumbricoides this taxon should clearly belong to the genus Ascaris.
For the differentiation of A. satyri females in connection with the opinion of Blanchard [27], Chatin states the morphology of the reproductive organs to be the determining feature [28]. According to Baylis and Daubney, the taxonomic importance of this feature is small [29]. This opinion is generally accepted and thus this feature cannot be used to differentiate these species. It needs to be also noted that the taxon A. satyri is not morphologically or molecularly distinguishable from A. lumbricoides and it A. satyri is classified as a doubtful species with inadequate description.
Several approaches in molecular biology have been used before now with the aim of distinguishing ascarids to the species level (e.g., PCR-RFLP, sequencing of PCR products of selected DNA markers, microsatellites polymorphism, completion of the mitochondrial genome, NGS) [14,65,66,85,86,87]. In this work, ascarids from Sumatran orangutans were studied at the routine molecular level for the first time.
Even though the 18S is very often considered to be good and provable barcoding marker for species identification, it could not be used for comparison or phylogenetic analyses due to the very low variability inside the genus Ascaris. It is proved to be not suitable for identification of ascaridoid nematodes and for the phylogeny of low level taxa. Null or very low variability in the 18S region confirms the claims of Li, Blaxter and their colleagues [88,89] that rDNA cannot provide entirely satisfactory solutions for species and genus classification (talking about the genera Toxocara and Baylisascaris), mainly because rDNA contains fewer informative sites and is less useful at a lower level. Presented findings in Ascaris confirmed this stage. Two obtained 18S ascarid variants from orangutan hosts differed only by one SNP (mixed base S in position 547 in LN600407) and no other variability was observed even between ascarids from different hosts (domestic pig and stripped skunk). The variant containing pure cytosine in this position was not detected.
Concerning the ITS region, ITS2 and 5.8S are highly conserved inside the Ascaris spp. and therefore are considered to be unsuitable markers for distinguishing Ascaris species. The ITS2 was regarded as not informative for differentiating between two species in the past [51]. However, inside the ITS1 region, considered to be the suitable diagnostic region for distinguishing A. suum and A. lumbricoides, two from six diagnostic sites (indicated in the past [64,90]) were demonstrated in our study. Differences at positions 274 (G/C) and 389 (T/A) (ID OQ539679) seem to be the most usable to distinguish between the species (lumbricoides/suum) or better lumbricoides-like and suum-like variants. The observed variability in the number of T nucleotide repeats at position 262 (ID OQ539679)—8–14 bases in Ascaris spp., 4 bases in Baylisascaris—was not considered to be an infallible diagnostic variability. This variability appears to be strongly affected by sequencing discrepancies; in this study, it was even necessary to use internal primers to obtain reliable sequences of this region of ITS1. According to the present findings in ITS1 variability, all samples from Sumatran orangutans were identified as A. lumbricoides representing the G1 genotype of lumbricoides-like ITS1 clade characterised by Peng and colleagues [64]. To date, it has been identified mainly from humans in Asia (Iraq, Korea, China, India, Bangladesh, Laos, Myanmar, South Korea, Thailand, Japan) but also as a dominant haplotype from humans in Brazil [65]. The second clade, suum-like ITS1, is characterised well by the C and A nucleotides in the aforementioned positions, respectively. Moreover, the obtained ITS variant from pigs in this study (ID OQ539680) is unique, confirmed in two specimens, and not described before. It is well characterised and distinguishable from other pig variants by CG deletion in the ITS2 region (observable in position 909–910 bp in ID OQ539679 and in other isolates from pigs), by noticeably longer T-nucleotide repetition (14 bases at position 102, ID OQ539680), and more unique SNPs compared to the other sequences from suum-like Ascaris sequences (position in ID OQ539680-nucleotide: 51-T, 101-C, 647-C).
Concerning the variability of CO1 sequences in the database, a huge range of those defined as A. suum or A. lumbricoides are 100% identical and no interspecific differences are evident even in validated reliable genetic markers. After the sequencing of the complete mitochondrial genomes of A. lumbricoides and A. suum from China, very little divergence was found comparing them: 1.9% in mitochondrial genomes of Chinese isolates of these two species and 1.5% comparing isolates of A. suum from China and the USA [88,91]. CO1 marker is used frequently, and it appears to be a good candidate for the identification of genera, and with a reasonable degree of stringency, even for the distinguishing of species.
The present phylogenetic findings highly correspond with the previous reports [47,48,66,92,93,94]. Ascaris lumbricoides isolated from Sumatran orangutans showed a very close relationship to the human genotypes representing the most frequent lumbricoides-like genotypes worldwide (HapA1 for CO1, G1 for ITS1). For a comparison of ape host effects (Pan troglodytes Blumenbach, 1775, Hylobates hoolock Harlan, 1834, Macaca mulatta Zimmermann, 1780, Pongo abelii), several different sequences were applied for phylogenetic analysis. Relationships with other isolates from non-human primates (chimpanzees, gibbons) were not as close as one could expect. The CO1 sequences from chimpanzees’ ascarids were identical to sequences obtained from Ascaris parasitising pigs in China (marked as HapB5). In gibbons, the most frequent haplotype HapB1 was identified. It occurs very often in humans and pigs, and its distribution is also very wide (Table 3). Two non-human primates’ Ascaris sequences were included also in the ITS region analysis. Meanwhile, concerning Sumatran orangutans linked to lumbricoides-like genotypes, the sequence from gibbon parasites was identical with G3 ITS1 genotype (JF837181) according to Peng and colleagues [64]. The one from macaque (JF837182) was also in a suum-like ITS1 clade with little sequence differences (indels) compared to the G3 genotype.
The present study on Sumatran ascarids brings a hint of evidence that A. lumbricoides and A. suum can be (partially) genetically distinguished. According to present results on ITS and CO1 variabilities and presented molecular phylogenetic findings, the Ascaris species should be, for now, primarily divided into two main genotypes: lumbricoides-like genotype and suum-like genotype, or possibly human-like and pig-like types suggested by Zhou and colleagues [87]. However, the trend of host-based naming (lumbricoides/suum/ovis) should finally be abandoned as it is misleading. The present results support the conclusions of Leles and others [95], who formally synonymised A. suum as a junior name of A. lumbricoides. Simply Ascaris lumbricoides should be considered the valid name. All possible discrepancies observable in the found ascarid genotypes can result from host switching of parasite and hybridisation, leading to forming hybrid genotypes [72,87,96].
Future perspectives should lead to combined analyses of concatenated datasets of especially both of the provable genetic markers for Ascaris species—ITS1, CO1—and possibly others [93]. Only thus can it be confirmed that lumbricoides-like genotypes may be represented by CO1 clades A and B, and suum-like genotype may be represented by the CO1 Ascaris clade C and by corresponding ITS genotypes, respectively. Moreover, the detailed morphological descriptions of these species should be performed firstly, and the diagnostic features should be defined well. Morphological and molecular diagnostics would be performed in combined matrix analyses. Only after this will it be possible to give inevitable proof of distinguishing A. lumbricoides and A. suum at a species level.
Author Contributions
I.F. coordinated field work, sample and data collection and supervised the project. W.N. contributed to field study design and field coordination. K.C.K. and M.S. designed and performed the complete molecular-genetic study, analysed all molecular data, and performed and interpreted the phylogenetic analyses. I.F., V.B. and Š.M. performed the morphological analyses. I.H. performed SEM analyses. Š.M. and I.H. prepared microscopic slides, figures and plates. K.C.K. and V.B. drafted the first version of the manuscript. V.B., Š.M. and I.F. drafted the whole discussion of morphological findings. M.S. and I.F. contributed to drafting the manuscript and revising it. Authors K.C.K., M.S., V.B., I.H., Š.M. and I.F. have been involved in revising the manuscript critically for important intellectual content. K.C.K. formatted references and revised final reference list. All authors have read and agreed to the published version of the manuscript.
Funding
The study was financially supported by the “UMI–Saving of Pongidae” Foundation project “Parasites and Natural Antiparasitics in the Orang-utan”, by the Czech Science Foundation, Grant No. GA23-06571S and by Masaryk University in Brno, Faculty of Science, Department of Botany and Zoology.
Institutional Review Board Statement
All the research reported in this manuscript adhered to the legal requirements of the country in which the work took place (SIP No 0056/FRP/SM/III/2009, 0008/EXT/FRP/SM/II/2010) CITES permit 08411/IV/SATS-LN2011). Since the collection of faecal samples from orangutans was non-invasive and did not involve interaction with or distress to the animals, the study was not reviewed by an animal ethics committee. No interaction with animals was conducted for this study.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets supporting the conclusions of this article are included within the article (and its additional files). DNA sequences generated and analysed during the current study are available in GenBank repository under accession numbers.
Acknowledgments
The authors would like to thank the State Ministry of Research and Technology (RISTEK) and the Directorate General of Forest Protection and Nature Conservation (PHKA) for their cooperation and permission to conduct research in the Gunung Leuser National Park. The authors are also grateful to Martha Betson, Peter Nejsum, and Darwin Murrell for the providing of CO1 haplotype sequences of their samples, and to Matthew Nicholls for English correction of the draft. Finally, the authors would like to thank Mallory Abel and David Bartholomew for editing and formatting the final draft.
Conflicts of Interest
V.B. passed away before editing the final draft took place and was unable to read or approve it. He was a larger-than-life personality and will stay in our minds and hearts forever. The life and work of Professor Vlastimil Baruš will continue to guide us after his death.
References
- Brandon-Jones, D.; Eudey, A.A.; Geissmann, T.; Groves, C.P.; Melnick, D.J.; Morales, J.C.; Shekelle, M.; Stewart, C.B. Asian primate classification. Int. J. Primatol. 2004, 25, 97–164. [Google Scholar] [CrossRef]
- IUCN. IUCN Red List of Threatened Species. Version 2022.2. 2022. Available online: www.iucnredlist.org (accessed on 16 February 2023).
- Foitová, I.; Huffman, M.A.; Nurcahyo, W.; Olšanský, M. Parasites and their impacts on orangutan health. In Orangutans–Geographic Variation in Behavioral Ecology and Conservation, 1st ed.; Wish, S.A., Suci, S.U., Setia, T.M., van Schaik, C.P., Eds.; Oxford University Press: New York, NY, USA, 2009; pp. 157–169. [Google Scholar]
- Nurcahyo, W.; Konstanzová, V.; Foitová, I. Parasites of orangutans (primates: Ponginae): An overview. Am. J. Primatol. 2017, 79, e22650. [Google Scholar] [CrossRef] [PubMed]
- Foitová, I.; Baruš, V.; Hodová, I.; Koubková, B.; Nurcahyo, W. Two remarkable pinworms (Nematoda: Enterobiinae) parasitizing orangutan (Pongo abelii) in the Sumatra (Indonesia) including Lemuricola pongoi n. sp. Helminthologia 2008, 45, 162–168. [Google Scholar] [CrossRef]
- Foitová, I.; Baruš, V.; Koubková, B.; Mašová, Š.; Nurcahyo, W. Description of Lemuricola (Lemuricola) pongoi—Male (Nematoda: Enterobiinae) parasitising orangutan Pongo abelii. Parasitol. Res. 2010, 106, 817–820. [Google Scholar] [CrossRef] [PubMed]
- Labes, E.M.; Hegglin, D.; Grimm, F.; Nurcahyo, W.; Harrison, M.E.; Bastian, M.L.; Deplazes, P. Intestinal parasites of endangered orangutans (Pongo pygmaeus) in East Kalimantan, Borneo, Indonesia. Parasitology 2010, 137, 123–135. [Google Scholar] [CrossRef]
- Mul, I.F.; Paembonan, W.; Singleton, I.; Wich, S.A.; van Bolhuis, H.G. Intestinal parasites of free-ranging, semicaptive and captive Pongo abelii in Sumatra, Indonesia. Int. J. Primatol. 2007, 28, 407–420. [Google Scholar] [CrossRef][Green Version]
- Teo, S.Z.; Tuen, A.A.; Madinah, A.; Aban, S.; Chong, Y.L. Occurrence of gastrointestinal nematodes in captive non-human primates at Matang Wildlife Centre, Sarawak. Trop. Biomed. 2019, 36, 594–603. [Google Scholar]
- Adrus, M.; Zainudin, R.; Ahamad, M.; Jayasilan, M.A.; Abdullah, M.T. Gastrointestinal parasites of zoonotic importance observed in the wild, urban, and captive populations of non-human primates in Malaysia. J. Med. Primatol. 2019, 48, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Drakulovski, P.; Bertout, S.; Locatelli, S.; Butel, C.; Pion, S.; Mpoudi-Ngole, E.; Delaporte, E.; Peeters, M.; Mallié, M. Assessment of gastrointestinal parasites in wild chimpanzees (Pan troglodytes troglodytes) in southeast Cameroon. Parasitol. Res. 2014, 113, 2541–2550. [Google Scholar] [CrossRef]
- Kalema-Zilusoka, G.; Rothman, J.M.; Fox, M.T. Intestinal parasites and bacteria of mountain gorillas (Gorilla beringei beringei) in Bwindi Impenetrable National Park, Uganda. Primates 2005, 46, 59–63. [Google Scholar] [CrossRef]
- Mafuyai, H.B.; Barshep, Y.; Audu, B.S.; Kumbak, D.; Ojobe, T.O. Baboons as potential reservoirs of zoonotic gastrointestinal parasite infections at Yankari National Park, Nigeria. Afr. Health Sci. 2013, 13, 252–254. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xie, Y.; Niu, L.; Zhao, B.; Wang, Q.; Nong, X.; Chen, L.; Zhou, X.; Gu, X.; Wang, S.; Peng, X.; et al. Complete mitochondrial genomes of chimpanzee- and gibbon-derived Ascaris isolated from a zoological garden in Southwest China. PLoS ONE 2013, 8, e82795. [Google Scholar] [CrossRef] [PubMed]
- von Linstow, O. Compendium der Helminthologie; Nachtrag: Die Litteratur der Jahre 1878–1889; Hahnsche Buchhandlung: Hannover, Germany, 1889; p. 151. [Google Scholar]
- Mozgovoy, A.A. Fundamentals of Nematodology. In Volume 1, Ascaridata of Animals and Man and the Diseases They Caused by Them, 1st ed.; Publication House Akademii Nauk SSSR: Moscow, Russia, 1953; p. 616. [Google Scholar]
- Skryabin, K.I.; Shikhobalova, N.P.; Mozgovoy, A.A. Key to Parasitic Nematodes Volume II: Oxyurata and Ascaridata, 1st ed.; Akademiya Nauk SSSR Publishers: Moscow, Russia, 1951; p. 631. [Google Scholar]
- Yamaguti, S. Systema Helminthum III: The Nematodes of Vertebrates (Part 1 and 2), 1st ed.; Interscience Publishers: New York, NY, USA, 1961; p. 1261. [Google Scholar]
- Sprent, J.F.A. Notes on Ascaris and Toxascaris with a definition of Baylisascaris gen. nov. Parasitology 1968, 58, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Kirby, M.D.; Podani, J.M.; Shaw, J.H.; Edwards, S.J.; Rayburn, J.D.; Hood, M.W. Supplement 19, Part 4, Parasite-Subject Catalogue: Parasites: Nematoda and Acanthocephala; United States Government Printing Office: Washington, DC, USA, 1975. [Google Scholar]
- Djojoasmoro, R.; Purnomo, A. Prevalensi Nematoda Usus pada Orangutan di Taman Nasional Tanjung Puting Kalimantan Tengah. J. Primatol. Indones. 1998, 2, 9–12. [Google Scholar]
- Foitová, I. Parasites in Orangutans (Pongo pygmaeus Linnaeus, 1760) with Connection to the Ecology and Ethology Factors. Ph.D. Thesis, Veterinary and Pharmaceutical University Brno, Brno, Czech Republic, 2002. [Google Scholar]
- Hilser, H.B.; Cheyne, S.M.; Ehlers-Smith, D.A. Socioecology and gastro-intestinal parasites of sympatric primate species inhabiting the Sabangau peat-swamp forest, Central Kalimantan. Am. J. Primatol. 2011, 74, 31–49. [Google Scholar]
- Collet, J.; Galdikas, B.M.F.; Sugarjito, J.; Jojosudharmo, S. A coprological study of parasitism in orangutans (Pongo pygmaeus) in Indonesia. J. Med. Primatol. 1986, 15, 121–129. [Google Scholar] [CrossRef]
- Djojosudharmo, S.; Gibson, A.T. Parasit intestinalis pada primata liar di Taman Nasional Gunung Leuser, Sumatra. In Proceedings of the Simposium and Seminar Nasional Primata, Bandung, Indonesia, 13–14 October 1993; pp. 13–14. [Google Scholar]
- Stafford, E.E.; Moede, A.L.; Brown, R.J.; Galdikas-Brindamour, B. Enteric parasites of orangutans (Pongo pygmaeus) in Indonesia; Special Report; Naval Aerospace Medical Research Laboratory: Pensacola, FL, USA, 1978. [Google Scholar]
- Blanchard, E. Recherches sur l’Organisation des Vers. In Annales des Sciences Naturelles, 3rd ed.; Place de l’Ecole de Médecine: Paris, France, 1848; pp. 106–202. [Google Scholar]
- Chatin, J.M. Étude anatomique et zoologique de l’ascaride de l’Orang-outang. Comptes Rendus Soc. Biol. 1877, 26, 384–387. [Google Scholar]
- Baylis, H.A.; Daubney, M. Report of the parasitic nematodes in the collection of the Zoological Survey of India. Mem. Indian Mus. Calcutta 1922, 7, 263–347. [Google Scholar] [CrossRef]
- Canavan, W.P.N. Nematode parasites of vertebrates in the Philadelphia Zoological Garden and vicinity. Parasitology 1931, 23, 196–229. [Google Scholar] [CrossRef]
- Matsubayashi, H. On Ascaris and Trichuris found in orangutans with some remarks on specific characters on Trichuris. Keio Igaku 1934, 14, 965–976. [Google Scholar]
- Sandosham, A.A. On Enterobius vermicularis (Linnaeus, 1758) and some related species from primates and rodents. J. Helminthol. 1950, 24, 171–204. [Google Scholar] [CrossRef]
- Sandosham, A.A. On two helminthes from the orang utan, Leipertrema rewelli n.g., n.sp. and Dirofilaria immitis (Leidy, 1856). J. Helminthol. 1951, 25, 19–26. [Google Scholar] [CrossRef]
- Swierstra, D.; Jansen, J.; Van Den Broek, E. Parasites of zoo–animals in the Netherlands. Tijdschr. Diergeneesk 1959, 84, 1301–1305. [Google Scholar]
- Cummins, L.B.; Keeling, M.E.; McClure, H.M. Preventive medicine in anthropoids: Parasite control. Lab. Anim. Sci. 1973, 23, 819–821. [Google Scholar]
- Rijksen, H.D. A Field Study in Sumatran Orangutans (Pongo pygmaeus abelii Lesson 1827): Ecology, Behaviour and Conservation, 1st ed.; Veenman & B.V. Zonen: Wageningen, The Netherlands, 1978; pp. 1–416. [Google Scholar]
- Frazier-Taylor, H.; Galdikas, B.; Karesh, W.B. A Survey of Intestinal Parasites in Wild, Ex-Captive and Captive Orangutans (Pongo pygmaeus): An American Association of ZOO Keepers Research Project–Part One; Animal Keepers Forum 8: Seattle, WA, USA, 1987; pp. 245–254. [Google Scholar]
- Warren, K.S. Orangutan Conservation-Epidemiological Aspects of Health Management and Population Genetics. Ph.D. Thesis, Murdoch University, Murdoch, Australia, 2001. [Google Scholar]
- Okulewicz, A.; Lonc, E.; Borgesteede, F.H.M. Ascarid nematodes in domestic and wild terrestrial mammals. Pol. J. Vet. Sci. 2002, 5, 277–281. [Google Scholar]
- Wahyudi, R.; Singleton, I.; Saraswati, Y. Medical record in Sumatran orangutan quarantine Batu Mbelin–Sibolangit Medan North Sumatra. In Proceeding of the 3rd Meeting of the Asian Zoo Wildlife Medicine and Conservation, Bogor, Indonesia, 19–22 August 2008. [Google Scholar]
- Velasco, N.J.; Claveria, F.G. Intestinal parasites identified in caged orangutans (Pongo spp.) at the Avilon Zoo, Montalban Zoological Park, Rizal, Philippines. J. Protozool. Res. 2009, 19, 12–15. [Google Scholar]
- Joyce, S.A.; Reid, A.; Driver, F.; Curran, J. Application of polymerase chain reaction (PCR) methods to identification of entomo-pathogenic nematodes. In COST 812 Biotechnology: Genetics of Entomopathogenic Nematode-Bacterium Complexes; Burnell, A.M., Ehlers, R.U., Masson, J.P., Eds.; Proceedings Symposium Workshop, St. Patrick’s College, Maynooth, Ireland, DG XII; European Commission: Luxembourg, 1994; pp. 178–187. [Google Scholar]
- Vrain, T.C.; Wakarchuk, D.A.; Levesque, A.C.; Hamilton, R.I. Intraspecific rDNA restriction fragment length polymorphism in the Xiphinema americanum group. Fundam. Appl. Nematol. 1992, 15, 563–573. [Google Scholar]
- Floyd, R.M.A.; Rogers, D.; Lambshead, J.D.; Smith, C.R. Nematodes specific PCR primers for the 18S small subunit rRNA gene. Mol. Ecol. Notes 2005, 5, 611–612. [Google Scholar] [CrossRef]
- Das, K.; Chowdhury, P.; Ganguly, S. Internal Transcribed Spacer 1 (ITS1) based sequence typing reveals phylogenetically distinct Ascaris population. Comp. Struct. Biotechnol. J. 2015, 13, 478–483. [Google Scholar] [CrossRef]
- Nakano, T.; Okamoto, M.; Ikeda, Y.; Hasegawa, H. Mitochondrial cytochrome c oxidase subunit 1 gene and nuclear rDNA regions of Enterobius vermicularis parasitic in captive chimpanzees with special reference to its relationship with pinworms in humans. Parasitol. Res. 2006, 100, 51–57. [Google Scholar] [CrossRef]
- Grehan, J.R.; Schwartz, J.H. Evolution of the second orangutan: Phylogeny and biogeography of hominid origins. J. Biogeogr. 2009, 36, 1823–1844. [Google Scholar] [CrossRef]
- Betson, M.; Nejsum, P.; Bendall, R.P.; Deb, R.M.; Stothard, J.R. Molecular epidemiology of ascariasis: A global perspective on the transmission dynamics of Ascaris in people and pigs. J. Infect. Dis. 2014, 210, 932–941. [Google Scholar] [CrossRef] [PubMed]
- Cavallero, S.; Snabel, V.; Pacella, F.; Perrone, V.; D’Amelio, S. Phylogeographical studies of Ascaris spp. based on ribosomal and mitochondrial DNA sequences. PLoS Neglect. Trop. Dis. 2013, 7, e2170. [Google Scholar] [CrossRef] [PubMed]
- Jesudoss Chelladurai, J.; Murphy, K.; Snobl, T.; Bader, C.; West, C.; Thompson, K.; Brewer, M.T. Molecular epidemiology of Ascaris infecting pigs in Iowa. J. Infect. Dis. 2016, 215, 131–138. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sadaow, L.; Sanpool, O.; Phosuk, I.; Rodpai, R.; Thanchomnang, T.; Wijit, A.; Anamnart, W.; Laymanivong, S.; Aung, W.P.P.; Janwan, P.; et al. Molecular identification of Ascaris lumbricoides and Ascaris suum recovered from humans and pigs in Thailand, Lao PDR, and Myanmar. Parasitol. Res. 2018, 117, 2427–2436. [Google Scholar] [CrossRef]
- Betson, M.; Halstead, F.D.; Nejsum, P.; Imison, E.; Khamis, I.S.; Sousa–Figueiredo, J.C.; Rollinson, D.; Stothard, J.R. A molecular epidemiological investigation of Ascaris on Unguja, Zanzibar using isoenzyme analysis, DNA barcoding and microsatellite DNA profiling. Trans. R. Soc. Trop. Med. Hyg. 2011, 105, 370–379. [Google Scholar] [CrossRef]
- Cavallero, S.; Rondón, S.; Monterrosa, I.A.; Šnábel, V.; Papajová, I.; Goldová, M.; Štrkolcová, D.; Caraballo, L.; Acevedo, N.; D’Amelio, S. Genotyping of Ascaris spp. infecting humans and pigs in Italy, Slovakia and Colombia. Infect. Genet. Evol. 2021, 94, 104997. [Google Scholar] [CrossRef]
- Katoh, K.; Misawa, K.; Kuma, K.I.; Miyata, T. MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform. 2019, 20, 1160–1166. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Castresana, J. Selection of Conserved Blocks from Multiple Alignments for Their Use in Phylogenetic Analysis. Mol. Biol. Evol. 2000, 17, 540–552. [Google Scholar] [CrossRef] [PubMed]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.; Von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Teslenko, M.; Van Der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchrd, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.; Schmidt, H.A.; Von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Hoang, D.T.; Chernomor, O.; Von Haeseler, A.; Minh, B.Q.; Vinh, L.S. UFBoot2: Improving the ultrafast bootstrap approximation. Mol. Biol. Evol. 2018, 35, 518–522. [Google Scholar] [CrossRef]
- Rambaut, A. FigTree-Version 1.4. 3, A Graphical Viewer of Phylogenetic Trees. Computer Program Distributed by the Author. Available online: http://tree.bio.ed.ac.uk/software/figtree (accessed on 25 October 2017).
- Peng, W.; Yuan, K.; Zhou, X.; Hu, M.; Abs EL-Osta, Y.G.; Gasser, R.B. Molecular epidemiological investigation of Ascaris genotypes in China based on single-strand conformation polymorphism analysis of ribosomal DNA. Electrophoresis 2003, 24, 2308–2315. [Google Scholar] [CrossRef]
- Leles, D.; Araújo, A.; Vicente, A.C.P.; Iñiguez, A.M. ITS1 intra-individual variability of Ascaris isolates from Brazil. Parasitol. Int. 2010, 59, 93–96. [Google Scholar] [CrossRef]
- Nejsum, P.; Hawash, M.B.F.; Betson, M.; Stothard, J.R.; Gasser, R.B.; Andersen, L.O. Ascaris phylogeny based on multiple whole mtDNA genomes. Infect. Genet. Evol. 2017, 48, 4–9. [Google Scholar] [CrossRef]
- Šnábel, V.; Taira, K.; Cavallero, S.; D’Amelio, S.; Rudohradská, P.; Saitoh, Y. Genetic structure of Ascaris roundworm in Japan and patterns of its geographical variation. JPN J. Infect. Dis. 2012, 65, 179–183. [Google Scholar] [CrossRef]
- Cromton, D.W.T. Ascaris and ascariasis. Adv. Parasitol. 2001, 48, 285–375. [Google Scholar]
- Hartwich, G. Die Tierwelt Deutschlands. 62. In Teil, I. Rhabditida und Ascaridida; VEB Gustav Fischer Verlag: Jena, Germany, 1975; p. 256. [Google Scholar]
- Mozgovoy, A.A.; Popova, T.I.; Shalaeva, N.M.; Smitova, G.Y. The validity of some species of Ascaridata of man and animals. Trudy GELAN 1960, 10, 153–165. [Google Scholar]
- Mozgovoy, A.A. Helminths Domestic and Wild Suidae and Diseases Caused by Them, 1st ed.; Publication House Nauka: Moscow, Russia, 1967; p. 540. [Google Scholar]
- Arizono, N.; Yoshimura, Y.; Tohzaka, N.; Yamaga, M.; Tegoshi, T.; Onishi, K.; Uchikawa, R. Ascariasis in Japan: Is pig–derived ascaris infecting humans? JPN J. Infect. Dis. 2010, 63, 447–448. [Google Scholar] [CrossRef] [PubMed]
- Dold, C.; Holland, C.V. Ascaris and ascariasis. Microbes Infect. 2011, 13, 632–637. [Google Scholar] [CrossRef]
- Lamson, P.D.; Ward, C.B. Earth Worms as Test Objects for Determining the Value of Drugs to Be Used in Human Intestinal Helminth Infestations. Science 1936, 84, 293–294. [Google Scholar] [CrossRef]
- Abdulrachman, S.; Lie, K.J. Morphometrical differences between Ascaris from man and pigs. Doc. Med. Geogr. Trop. 1954, 6, 342–344. [Google Scholar]
- Madden, P.A.; Tromba, F.G.; Vetterling, J.M. En face views of Ascaris suum with the scanning electron microscope. J. Parasitol. 1970, 56, 202–203. [Google Scholar] [CrossRef]
- Sprent, J.F.A. Anatomical distinction between human and pig strains of Ascaris. Nature 1952, 170, 627–628. [Google Scholar] [CrossRef]
- Ubelaker, J.E.; Allison, V.F. Scanning electron microscopy of denticles and eggs of Ascaris lumbricoides and Ascaris suum. In Proceedings of the 30th Annual Proceeding Electron Microscopy Society of America, Los Angeles, CA, USA, 14 August 1972; pp. 1–2. [Google Scholar]
- Barry, J.M.; O’Rourke, F.J. Ascariasis in pig and man. Sci. Proc. R. Dublin Soc. (Ser. A) 1967, 3, 39–55. [Google Scholar]
- Borkovcová, M. Scanning electron microscopy of denticles of Ascaris lumbricoides Linné, 1758. Acta Univ. Agric. Silvic. Mendel. Brun. 1992, 3, 245–261. [Google Scholar]
- Lýsek, H. Contribution to the morphological problem of differences between Ascaris lumbricoides Linné 1758 and Ascaris suum Goeze 1782. Acta Soc. Zool. Bohemoslov. 1963, 27, 97–101. [Google Scholar]
- Prokopič, J. Scanning electron microscopic study of the morphology of Ascaris suum Goeze, 1782. Folia Parasitol. 1979, 2, 377–380. [Google Scholar]
- Schneider, A. Monographie der Nematoden, 1st ed.; Reimer: Berlin, Germany, 1866; p. 357. [Google Scholar]
- Uni, S.; Takada, S. Comparison of Scanning Electron Microscopy on Baylisascaris transfuga, Toxascaris leonina and Ascaris lumbricoides (Nematoda: Ascarididae). JPN J. Parasitol. 1981, 30, 187–195. [Google Scholar]
- Nadler, S.A. Phylogeny of some ascaridoid nematodes, inferred from comparison of 18S and 28S rRNA sequences. Mol. Biol. Evol. 1992, 9, 932–944. [Google Scholar]
- Nejsum, P.; Bertelsen, M.F.; Betson, M.; Stothard, J.R.; Murrell, K.D. Molecular evidence for sustained transmission of zoonotic Ascaris suum among zoo chimpanzees (Pan troglodytes). Vet. Parasitol. 2010, 17, 273–276. [Google Scholar] [CrossRef]
- Zhou, C.; Guo, T.; Deng, Y.; He, J.; Ouyang, S.; Wu, X. Mitochondrial phylogenomics of human-type Ascaris, pig-type Ascaris, and hybrid Ascaris populations. Vet. Parasitol. 2020, 287, 109256. [Google Scholar] [CrossRef]
- Li, Y.; Niu, L.; Wang, Q.; Zhang, Z.; Chen, Z.; Gu, X.; Xie, Y.; Yan, N.; Wang, S.; Peng, X.; et al. Molecular characterization and phylogenetic analysis of ascarid nematodes from twenty-one species of captive wild mammals based on mitochondrial and nuclear sequences. Parasitology 2012, 139, 1329–1338. [Google Scholar] [CrossRef]
- Blaxter, M.L.; Ley, P.D.; Garey, J.R.; Liu, L.Y.; Scheldeman, P.; Vierstraete, A.; Vanfleteren, J.R.; Mackey, L.Y.; Dorris, M.; Frisse, L.M.; et al. A molecular evolutionary framework for the phylum Nematoda. Nature 1998, 392, 71–75. [Google Scholar] [CrossRef]
- Zhu, X.; Chilton, N.B.; Jacobs, D.E.; Boes, J.; Gasser, R.B. Characterisation of Ascaris from human and pighosts by nuclear ribosomal DNA sequences. Int. J. Parasit. 1999, 29, 469–478. [Google Scholar] [CrossRef]
- Liu, G.H.; Wu, C.H.Y.; Song, H.Q.; Wei, S.J.; Xu, M.J.; Lin, R.Q.; Zhao, G.H.; Huang, S.Y.; Zhu, Y.Q. Comparative analyses of the complete mitochondrial genomes of Ascaris lumbicoides and Ascaris suum from humans and pigs. Gene 2012, 492, 110–116. [Google Scholar] [CrossRef]
- Eamsobhana, P.; Yong, H.S.; Boonyong, S.; Wanachiwanawin, D.; Tungtrongchitr, A. Genetic diversity and identity of Ascaris worms from human and pig hosts in Thailand. Vet. Parasitol. Reg. Stud. Rep. 2022, 33, 100752. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.H.; Oh, C.S.; Seo, M.; Chai, J.Y.; Shin, D.H. Ancient Ascaris DNA sequences of cytochrome B, cytochrome C oxidase subunit 1, NADH dehydrogenase subunit 1, and internal transcribed spacer 1 genes from Korean Joseon mummy feces. J. Parasitol. 2017, 103, 795–800. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Funayama, K.; Hoshi, R.; Takatsuka, H.; Sato, M.O. Ascaris lumbricoides found in ashore corpses from Korean peninsula to Japan. Parasitol. Int. 2019, 70, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Leles, D.; Gardner, S.L.; Reinhard, K.; Iñigues, A.; Araujo, A. Are Ascaris lumbricoides and Ascaris suum a single species? Parasites Vectors 2012, 5, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Criscione, C.D.; Anderson, J.D.; Sudimack, D.; Peng, W.; Jha, B.; Williams-Blangero, S.; Anderson, T.J. Disentangling hybridization and host colonization in parasitic roundworms of humans and pigs. Proc. R. Soc. B Biol. Sci. 2007, 274, 2669–2677. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).