Complete Mitochondrial Genome Assembly of an Upland Wild Rice Species, Oryza granulata and Comparative Mitochondrial Genomic Analyses of the Genus Oryza
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material Collection and Genome Sequencing
2.2. Mitochondrial Genome Assembly and Annotation
2.3. SSRs and Repeat Sequences
2.4. Prediction of RNA Editing Sites
2.5. Mitochondrial Plastid Sequences and Collinearity Analysis
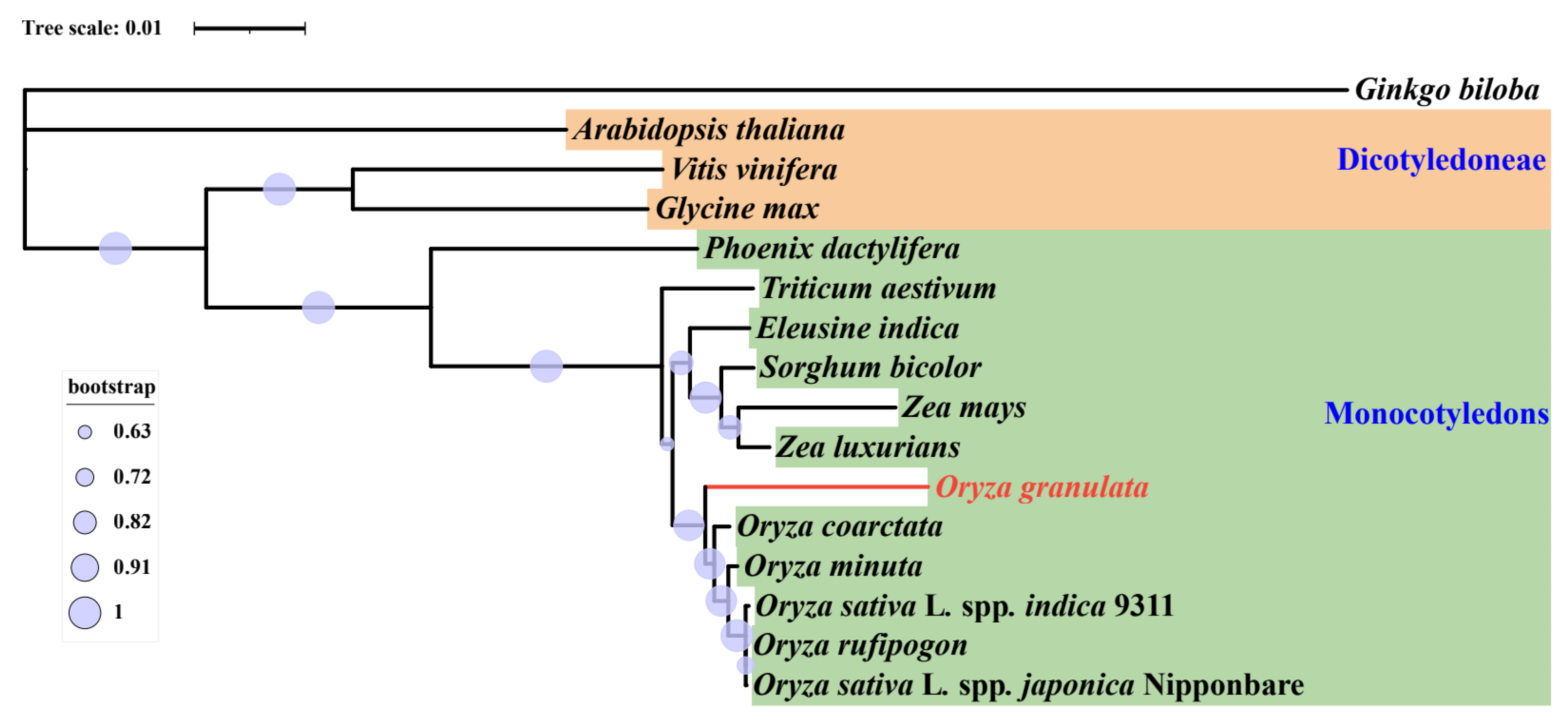
2.6. Phylogenetic Analysis
3. Results
3.1. Structural Characteristics of the O. Granulata Mitogenome
3.2. Repeat Sequences
3.3. Prediction of RNA Editing Sites
3.4. Homologous Sequence Analysis of Mitochondrial and Plastid Genomes
3.5. Comparative Mitochondrial Genomic Analyses of the Oryza Species
3.6. Phylogenetic Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stein, J.C.; Yu, Y.; Copetti, D.; Zwickl, D.J.; Zhang, L.; Zhang, C.; Chougule, K.; Gao, D.; Iwata, A.; Goicoechea, J.L.; et al. Genomes of 13 domesticated and wild rice relatives highlight genetic conservation, turnover and innovation across the genus Oryza. Nat. Genet. 2018, 50, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Wing, R.A.; Purugganan, M.D.; Zhang, Q. The rice genome revolution: From an ancient grain to Green Super Rice. Nat. Rev. Genet. 2018, 19, 505–517. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhu, T.; Xia, E.; Shi, C.; Liu, Y.; Zhang, Y.; Liu, Y.; Jiang, W.; Zhao, Y.; Mao, S.; et al. Rapid diversification of five Oryza AA genomes associated with rice adaptation. Proc. Natl. Acad. Sci. USA 2014, 111, E4954–E4962. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Sang, T.; Lu, B.R.; Hong, D.Y. Phylogeny of rice genomes with emphasis on origins of allotetraploid species. Proc. Natl. Acad. Sci. USA 1999, 96, 14400–14405. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.H.; Zhang, F.M.; Zhang, J.G.; Zang, L.L.; Tang, L.; Wang, J.; Sang, T.; Ge, S. Analysis of 142 genes resolves the rapid diversification of the rice genus. Genome Biol. 2008, 9, R49. [Google Scholar] [CrossRef]
- Aggarwal, R.K.; Brar, D.S.; Khush, G.S. Two new genomes in the Oryza complex identified on the basis of molecular divergence analysis using total genomic DNA hybridization. Mol. Gen. Genet. 1997, 254, 1–12. [Google Scholar] [CrossRef]
- Vaughan, D.A.; Morishima, H.; Kadowaki, K. Diversity in the Oryza genus. Curr. Opin. Plant Biol. 2003, 6, 139–146. [Google Scholar] [CrossRef]
- Qian, W.; Ge, S.; Hong, D. Genetic Diversity in Accessions of Wild Rice Oryza granulata from South and Southeast Asia. Genet. Resour. Crop Evol. 2006, 53, 197–204. [Google Scholar] [CrossRef]
- Yu, H.; Lin, T.; Meng, X.; Du, H.; Zhang, J.; Liu, G.; Chen, M.; Jing, Y.; Kou, L.; Li, X.; et al. A route to de novo domestication of wild allotetraploid rice. Cell 2021, 184, 1156–1170. [Google Scholar] [CrossRef]
- Yang, C.; Shen, S.; Zhou, S.; Li, Y.; Mao, Y.; Zhou, J.; Shi, Y.; An, L.; Zhou, Q.; Peng, W.; et al. Rice metabolic regulatory network spanning the entire life cycle. Mol. Plant. 2022, 15, 258–275. [Google Scholar] [CrossRef]
- Jing, C.; Zhang, F.; Wang, X.; Wang, M.; Zhou, L.; Cai, Z.; Han, J.; Geng, M.; Yu, W.; Jiao, Z.; et al. Multiple domestications of Asian rice. Nat. Plants 2023, 9, 1221–1235. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, K.; Zhang, Q.J.; Zhu, T.; Zhang, Y.; Shi, C.; Liu, Y.L.; Xia, E.H.; Jiang, J.J.; Shi, C.; et al. Improved hybrid de novo genome assembly and annotation of African wild rice, Oryza longistaminata, from Illumina and PacBio sequencing reads. Plant Genome 2020, 13, e20001. [Google Scholar] [CrossRef]
- Li, W.; Zhang, Q.J.; Zhu, T.; Tong, Y.; Li, K.; Shi, C.; Zhang, Y.; Liu, Y.L.; Jiang, J.J.; Liu, Y.; et al. Draft genomes of two outcrossing wild rice, Oryza rufipogon and O. longistaminata, reveal genomic features associated with mating-system evolution. Plant Direct 2020, 4, e00232. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, K.; Huang, Y.; Shi, C.; Hu, W.; Zhang, Y.; Zhang, Q.; Xia, E.; Hutang, G.; Zhu, X.; et al. SMRT sequencing of the Oryza rufipogon genome reveals the genomic basis of rice adaptation. Commun. Biol. 2020, 3, 167. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Zhang, S.; Zhou, Y.; Ge, S.; Hong, D. A survey of the current status of wild rice in China. Chin. Biodivesity 1996, 4, 160–166. [Google Scholar]
- Gao, L.; Ge, S.; Hong, D. Low levels of genetic diversity within populations and high differentiation among populations of a wild rice, Oryza granulata nees et arn. ex watt., from china. Int. J. Plant Sci. 2000, 161, 691. [Google Scholar] [CrossRef][Green Version]
- Gao, L.; Ge, S.; Hong, D.; Zhang, J.; Luo, Q.; Tao, G.; Xu, Z. A study on population genetic structure of Oryza meyeriana (Zoll. et Mor. ex Steud.) Baill. from Yunnan and its in situ conservation significance. Sci. China Ser. C Life Sci. 1999, 42, 102–108. [Google Scholar] [CrossRef]
- Klingenberg, M. The ADP and ATP transport in mitochondria and its carrier. Biochim. Et Biophys. Acta (BBA)—Biomembr. 2008, 1778, 1978–2021. [Google Scholar] [CrossRef]
- Waltz, F.; Giegé, P. Striking Diversity of Mitochondria-Specific Translation Processes across Eukaryotes. Trends Biochem. Sci. 2020, 45, 149–162. [Google Scholar] [CrossRef]
- Chen, Z.; Nie, H.; Grover, C.E.; Wang, Y.; Li, P.; Wang, M.; Pei, H.; Zhao, Y.; Li, S.; Wendel, J.F.; et al. Entire nucleotide sequences of Gossypium raimondii and G. arboreum mitochondrial genomes revealed A-genome species as cytoplasmic donor of the allotetraploid species. Plant Biol. 2017, 19, 484–493. [Google Scholar] [CrossRef]
- Sibbald, S.J.; Lawton, M.; Archibald, J.M. Mitochondrial Genome Evolution in Pelagophyte Algae. Genome Biol. Evol. 2021, 13, evab018. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Liu, F.; Jia, Q.; Du, H.; Chen, W.; Ruan, J.; Lei, J.; Li, D.Z.; Mower, J.P.; Zhu, A. Fragaria mitogenomes evolve rapidly in structure but slowly in sequence and incur frequent multinucleotide mutations mediated by micro-inversions. New Phytol. 2022, 236, 745–759. [Google Scholar] [CrossRef] [PubMed]
- Petersen, G.; Anderson, B.; Braun, H.; Meyer, E.H.; Møller, I.M. Mitochondria in parasitic plants. Mitochondrion 2020, 52, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Li, L.; Feng, H.; Tu, W.; Bao, Z.; Xiong, C.; Wang, X.; Qing, Y.; Huang, W. Characterization of the Complete Mitochondrial Genome of Basidiomycete Yeast Hannaella oryzae: Intron Evolution, Gene Rearrangement, and Its Phylogeny. Front. Microbiol. 2021, 12, 646567. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Ni, Y.; Lin, Z.; Yang, L.; Chen, G.; Nijiati, N.; Hu, Y.; Chen, X. De novo assembly of the complete mitochondrial genome of sweet potato (Ipomoea batatas [L.] Lam) revealed the existence of homologous conformations generated by the repeat-mediated recombination. BMC Plant Biol. 2022, 22, 285. [Google Scholar] [CrossRef]
- Yu, R.; Sun, C.; Zhong, Y.; Liu, Y.; Sanchez-Puerta, M.V.; Mower, J.P.; Zhou, R. The minicircular and extremely heteroplasmic mitogenome of the holoparasitic plant Rhopalocnemis phalloides. Curr. Biol. 2021, 32, 470–479. [Google Scholar] [CrossRef]
- Kubo, T.; Kitazaki, K.; Matsunaga, M.; Kagami, H.; Mikami, T. Male Sterility-Inducing Mitochondrial Genomes: How Do They Differ? Crit. Rev. Plant Sci. 2011, 30, 378–400. [Google Scholar] [CrossRef]
- Liao, X.; Wei, M.; Khan, A.; Zhao, Y.; Kong, X.; Zhou, B.; Li, M.; Peng, S.; Munsif, F.; Ullah, A.; et al. Comparative analysis of mitochondrial genome and expression variation between UG93A and UG93B reveals a candidate gene related to cytoplasmic male sterility in kenaf. Ind. Crop. Prod. 2020, 152, 112502. [Google Scholar] [CrossRef]
- Liberatore, K.L.; Dukowic-Schulze, S.; Miller, M.E.; Chen, C.; Kianian, S.F. The role of mitochondria in plant development and stress tolerance. Free. Radic. Biol. Med. 2016, 100, 238–256. [Google Scholar] [CrossRef]
- Chevigny, N.; Schatz-Daas, D.; Lotfi, F.; Gualberto, J.M. DNA Repair and the Stability of the Plant Mitochondrial Genome. Int. J. Mol. Sci. 2020, 21, 328. [Google Scholar] [CrossRef]
- Wu, Z.; Fang, D.; Yang, R.; Gao, F.; An, X.; Zhuo, X.; Li, Y.; Yi, C.; Zhang, T.; Liang, C.; et al. De novo genome assembly of Oryza granulata reveals rapid genome expansion and adaptive evolution. Commun. Biol. 2018, 1, 84. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Li, W.; Zhang, Q.; Zhang, Y.; Tong, Y.; Li, K.; Liu, Y.; Gao, L. The draft genome sequence of an upland wild rice species, Oryza granulata. Sci. Data 2020, 7, 131. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Liu, Y.; Zhang, D.; Li, W.; Gao, J.; Liu, Y.; Li, K.; Shi, C.; Zhao, Y.; Zhao, Y.; et al. Evolution of Oryza chloroplast genomes promoted adaptation to diverse ecological habitats. Commun. Biol. 2019, 2, 278. [Google Scholar] [CrossRef]
- Porebski, S.; Bailey, L.G.; Baum, B.R. Modification of a CTAB DNA extraction protocol for plants containing high polysaccharide and polyphenol components. Plant Mol. Biol. Rep. 1997, 15, 8–15. [Google Scholar] [CrossRef]
- Notsu, Y.; Masood, S.; Nishikawa, T.; Kubo, N.; Akiduki, G.; Nakazono, M.; Hirai, A.; Kadowaki, K. The complete sequence of the rice (Oryza sativa L.) mitochondrial genome: Frequent DNA sequence acquisition and loss during the evolution of flowering plants. Mol. Genet. Genom. 2002, 268, 434–445. [Google Scholar] [CrossRef]
- Li, H.; Durbin, R. Fast and accurate long-read alignment with Burrows–Wheeler transform. Bioinformatics 2010, 26, 589–595. [Google Scholar] [CrossRef]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R. The Sequence Alignment/Map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef]
- Koren, S.; Walenz, B.P.; Berlin, K.; Miller, J.R.; Bergman, N.H.; Phillippy, A.M. Canu: Scalable and accurate long-read assembly via adaptive k-mer weighting and repeat separation. Genome Res. 2017, 27, 722–736. [Google Scholar] [CrossRef]
- Walker, B.J.; Abeel, T.; Shea, T.; Priest, M.; Abouelliel, A.; Sakthikumar, S.; Cuomo, C.A.; Zeng, Q.; Wortman, J.; Young, S.K.; et al. Pilon: An integrated tool for comprehensive microbial variant detection and genome assembly improvement. PLoS ONE 2014, 9, e112963. [Google Scholar] [CrossRef]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef]
- Alverson, A.J.; Wei, X.; Rice, D.W.; Stern, D.B.; Barry, K.; Palmer, J.D. Insights into the evolution of mitochondrial genome size from complete sequences of Citrullus lanatus and Cucurbita pepo (Cucurbitaceae). Mol. Biol. Evol. 2010, 27, 1436–1448. [Google Scholar] [CrossRef] [PubMed]
- Lowe, T.M.; Eddy, S.R. tRNAscan-SE: A program for improved detection of transfer RNA genes in genomic sequence. Nucleic. Acids. Res. 1997, 25, 955–964. [Google Scholar] [CrossRef]
- Lagesen, K.; Hallin, P.; Rødland, E.A.; Stærfeldt, H.; Rognes, T.; Ussery, D.W. RNAmmer: Consistent and rapid annotation of ribosomal RNA genes. Nucleic Acids Res. 2007, 35, 3100–3108. [Google Scholar] [CrossRef] [PubMed]
- Greiner, S.; Lehwark, P.; Bock, R. OrganellarGenomeDRAW (OGDRAW) version 1.3.1: Expanded toolkit for the graphical visualization of organellar genomes. Nucleic Acids Res. 2019, 47, W59–W64. [Google Scholar] [CrossRef] [PubMed]
- Beier, S.; Thiel, T.; Münch, T.; Scholz, U.; Mascher, M. MISA-web: A web server for microsatellite prediction. Bioinformatics 2017, 33, 2583–2585. [Google Scholar] [CrossRef]
- Zhang, Z.; Schwartz, S.; Wagner, L.; Miller, W. A greedy algorithm for aligning DNA sequences. J. Comput. Biol. 2000, 7, 203. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, S.; Choudhuri, J.V.; Ohlebusch, E.; Schleiermacher, C.; Stoye, J.; Giegerich, R. REPuter: The manifold applications of repeat analysis on a genomic scale. Nucleic. Acids. Res. 2001, 29, 4633–4642. [Google Scholar] [CrossRef]
- Edera, A.A.; Small, I.; Milone, D.H.; Sanchez-Puerta, M.V. Deepred-Mt: Deep representation learning for predicting C-to-U RNA editing in plant mitochondria. Comput. Biol. Med. 2021, 136, 104682. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant. 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Krzywinski, M.; Schein, J.; Birol, I.; Connors, J.; Gascoyne, R.; Horsman, D.; Jones, S.J.; Marra, M.A. Circos: An information aesthetic for comparative genomics. Genome Res. 2009, 19, 1639–1645. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Castresana, J. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol. Biol. Evol. 2000, 17, 540–552. [Google Scholar] [CrossRef] [PubMed]
- Capella-Gutiérrez, S.; Silla-Martínez, J.M.; Gabaldón, T. trimAl: A tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 2009, 25, 1972–1973. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian Phylogenetic Inference and Model Choice Across a Large Model Space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic. Acids. Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef]
- Fujii, S.; Kazama, T.; Yamada, M.; Toriyama, K. Discovery of global genomic re-organization based on comparison of two newly sequenced rice mitochondrial genomes with cytoplasmic male sterility-related genes. BMC Genom. 2010, 11, 209. [Google Scholar] [CrossRef]
- Tian, X.; Zheng, J.; Hu, S.; Yu, J. The Rice Mitochondrial Genomes and Their Variations. Plant Physiol. 2006, 140, 401–410. [Google Scholar] [CrossRef]
- Asaf, S.; Khan, A.L.; Khan, A.R.; Waqas, M.; Kang, S.; Khan, M.A.; Shahzad, R.; Seo, C.; Shin, J.; Lee, I. Mitochondrial Genome Analysis of Wild Rice (Oryza minuta) and Its Comparison with Other Related Species. PLoS ONE 2016, 11, e152937. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Xu, P.; Liu, J.; Peng, S.; Mo, X.; Gao, L. Phylogenetic relationships and genome divergence among the AA- genome species of the genus Oryza as revealed by 53 nuclear genes and 16 intergenic regions. Mol. Phylogenet. Evol. 2014, 70, 348–361. [Google Scholar] [CrossRef]
- Qiu, L.; Yang, C.; Tian, B.; Yang, J.; Liu, A. Exploiting EST databases for the development and characterization of EST-SSR markers in castor bean (Ricinus communis L.). BMC Plant Biol. 2010, 10, 278. [Google Scholar] [CrossRef]
- Powell, W.; Machray, G.C.; Provan, J. Polymorphism revealed by simple sequence repeats. Trends Plant Sci. 1996, 1, 215–222. [Google Scholar] [CrossRef]
- Malek, O.; Lattig, K.; Hiesel, R.; Brennicke, A.; Knoop, V. RNA editing in bryophytes and a molecular phylogeny of land plants. EMBO J. 1996, 15, 1403–1411. [Google Scholar] [CrossRef]
- Brennicke, A.; Marchfelder, A.; Binder, S. RNA editing. FEMS Microbiol. Rev. 1999, 23, 297–316. [Google Scholar] [CrossRef] [PubMed]
- Kozik, A.; Rowan, B.A.; Lavelle, D.; Berke, L.; Schranz, M.E.; Michelmore, R.W.; Christensen, A.C. The alternative reality of plant mitochondrial DNA: One ring does not rule them all. PLoS Genet. 2019, 15, e1008373. [Google Scholar] [CrossRef] [PubMed]
- Alverson, A.J.; Rice, D.W.; Dickinson, S.; Barry, K.; Palmer, J.D. Origins and Recombination of the Bacterial-Sized Multichromosomal Mitochondrial Genome of Cucumber. Plant Cell 2011, 23, 2499–2513. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, K.; Cottrell, J.; Ennos, R.A.; Vendramin, G.G.; A’Hara, S.; King, S.; Perry, A.; Wachowiak, W.; Cavers, S. Reconstructing the plant mitochondrial genome for marker discovery: A case study using Pinus. Mol. Ecol. Resour. 2017, 17, 943–954. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Xiang, K.; Chen, C.; Wang, J.; Wu, Z. Master graph: An essential integrated assembly model for the plant mitogenome based on a graph-based framework. Brief. Bioinform. 2023, 24, bbac522. [Google Scholar] [CrossRef]
- Du, H.; Yu, Y.; Ma, Y.; Gao, Q.; Cao, Y.; Chen, Z.; Ma, B.; Qi, M.; Li, Y.; Zhao, X.; et al. Sequencing and de novo assembly of a near complete indica rice genome. Nat. Commun. 2017, 8, 15324. [Google Scholar] [CrossRef]
- Mondal, T.K.; Rawal, H.C.; Gaikwad, K.; Sharma, T.R.; Singh, N.K. First de novo draft genome sequence of Oryza coarctata, the only halophytic species in the genus Oryza. F1000Res 2017, 6, 1750. [Google Scholar] [CrossRef]
- Mondal, T.K.; Rawal, H.C.; Chowrasia, S.; Varshney, D.; Panda, A.K.; Mazumdar, A.; Kaur, H.; Gaikwad, K.; Sharma, T.R.; Singh, N.K. Draft genome sequence of first monocot-halophytic species Oryza coarctata reveals stress-specific genes. Sci. Rep. 2018, 8, 13698. [Google Scholar] [CrossRef]
- Zhang, F.; Li, W.; Gao, C.; Zhang, D.; Gao, L. Deciphering tea tree chloroplast and mitochondrial genomes of Camellia sinensis var. assamica. Sci. Data 2019, 6, 209. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Tang, H.; Luo, H.; Tang, J.; Zhong, N.; Xiao, L. Complete mitochondrial genome assembly and comparison of Camellia sinensis var. Assamica cv. Duntsa. Front. Plant Sci. 2023, 14, 1117002. [Google Scholar] [CrossRef] [PubMed]
- Sloan, D.B.; Wu, Z.; Sharbrough, J. Correction of Persistent Errors in Arabidopsis Reference Mitochondrial Genomes. Plant Cell 2018, 30, 525–527. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Zhu, W.; Sloan, D.B.; Wu, Z. Long-read sequencing characterizes mitochondrial and plastid genome variants in Arabidopsis msh1 mutants. Plant. J. 2022, 112, 738–755. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Grewe, F.; Fan, W.; Young, G.J.; Knoop, V.; Palmer, J.D.; Mower, J.P. Ginkgo and Welwitschia Mitogenomes Reveal Extreme Contrasts in Gymnosperm Mitochondrial Evolution. Mol. Biol. Evol. 2016, 33, 1448. [Google Scholar] [CrossRef]
- Bi, C.; Paterson, A.H.; Wang, X.; Xu, Y.; Wu, D.; Qu, Y.; Jiang, A.; Ye, Q.; Ye, N. Analysis of the Complete Mitochondrial Genome Sequence of the Diploid Cotton Gossypium raimondii by Comparative Genomics Approaches. Biomed Res. Int. 2016, 2016, 5040598. [Google Scholar] [CrossRef]
- Liao, X.; Zhao, Y.; Kong, X.; Khan, A.; Zhou, B.; Liu, D.; Kashif, M.H.; Chen, P.; Wang, H.; Zhou, R. Complete sequence of kenaf (Hibiscus cannabinus) mitochondrial genome and comparative analysis with the mitochondrial genomes of other plants. Sci. Rep. 2018, 8, 12714. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; He, X.; Priyadarshani, S.V.G.N.; Wang, Y.; Ye, L.; Shi, C.; Ye, K.; Zhou, Q.; Luo, Z.; Deng, F.; et al. Assembly and comparative analysis of the complete mitochondrial genome of Suaeda glauca. BMC Genom. 2021, 22, 167. [Google Scholar] [CrossRef]
- Khera, P.; Saxena, R.; Sameerkumar, C.V.; Saxena, K.; Varshney, R.K. Mitochondrial SSRs and their utility in distinguishing wild species, CMS lines and maintainer lines in pigeonpea (Cajanus cajan L.). Euphytica 2015, 206, 737–746. [Google Scholar] [CrossRef]
- Bi, C.; Lu, N.; Xu, Y.; He, C.; Lu, Z. Characterization and Analysis of the Mitochondrial Genome of Common Bean (Phaseolus vulgaris) by Comparative Genomic Approaches. Int. J. Mol. Sci. 2020, 21, 3778. [Google Scholar] [CrossRef]
- Yang, H.; Li, W.; Yu, X.; Zhang, X.; Zhang, Z.; Liu, Y.; Wang, W.; Tian, X. Insights into molecular structure, genome evolution and phylogenetic implication through mitochondrial genome sequence of Gleditsia sinensis. Sci. Rep. 2021, 11, 14850. [Google Scholar] [CrossRef] [PubMed]
- Palmer, J.D.; Adams, K.L.; Cho, Y.; Parkinson, C.L.; Qiu, Y.L.; Song, K. Dynamic evolution of plant mitochondrial genomes: Mobile genes and introns and highly variable mutation rates. Proc. Natl. Acad. Sci. USA 2000, 97, 6960–6966. [Google Scholar] [CrossRef] [PubMed]
- Sloan, D.B. One ring to rule them all? Genome sequencing provides new insights into the ‘master circle’ model of plant mitochondrial DNA structure. New Phytol. 2013, 200, 978–985. [Google Scholar] [CrossRef] [PubMed]
- Gualberto, J.M.; Mileshina, D.; Wallet, C.; Niazi, A.K.; Weber-Lotfi, F.; Dietrich, A. The plant mitochondrial genome: Dynamics and maintenance. Biochimie 2014, 100, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Small, I.D.; Schallenberg Rüdinger, M.; Takenaka, M.; Mireau, H.; Ostersetzer Biran, O. Plant organellar RNA editing: What 30 years of research has revealed. Plant J. 2020, 101, 1040–1056. [Google Scholar] [CrossRef]
- Picardi, E.; Horner, D.S.; Chiara, M.; Schiavon, R.; Valle, G.; Pesole, G. Large-scale detection and analysis of RNA editing in grape mtDNA by RNA deep-sequencing. Nucleic. Acids. Res. 2010, 38, 4755–4767. [Google Scholar] [CrossRef]
- Edera, A.A.; Gandini, C.L.; Sanchez-Puerta, M.V. Towards a comprehensive picture of C-to-U RNA editing sites in angiosperm mitochondria. Plant Mol. Biol. 2018, 97, 215–231. [Google Scholar] [CrossRef]
- Bock, R. Taming plastids for a green future. Trends. Biotechnol. 2004, 22, 311–318. [Google Scholar] [CrossRef]
- Chen, H.; Deng, L.; Jiang, Y.; Lu, P.; Yu, J. RNA Editing Sites Exist in Protein-coding Genes in the Chloroplast Genome of Cycas taitungensis. J. Integr. Plant Biol. 2011, 53, 961–970. [Google Scholar] [CrossRef]
- Raman, G.; Park, S. Analysis of the Complete Chloroplast Genome of a Medicinal Plant, Dianthus superbus var. longicalyncinus, from a Comparative Genomics Perspective. PLoS ONE 2015, 10, e141329. [Google Scholar] [CrossRef]
- Li, J.; Li, J.; Ma, Y.; Kou, L.; Wei, J.; Wang, W. The complete mitochondrial genome of okra (Abelmoschus esculentus): Using nanopore long reads to investigate gene transfer from chloroplast genomes and rearrangements of mitochondrial DNA molecules. BMC Genom. 2022, 23, 481. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.R. Mitochondrion-to-plastid DNA transfer: It happens. New Phytol. 2014, 202, 736–738. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Rousseau-Gueutin, M.; Timmis, J.N. Plastid Sequences Contribute to Some Plant Mitochondrial Genes. Mol. Biol. Evol. 2012, 29, 1707–1711. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Grover, C.E.; Chen, Z.; Wendel, J.F.; Hua, J. Intergenomic gene transfer in diploid and allopolyploid Gossypium. BMC Plant Biol. 2019, 19, 492. [Google Scholar] [CrossRef]
- Wallis, G.P. Do animal mitochondrial genomes recombine? Trends Ecol. Evol. 1999, 14, 209–210. [Google Scholar] [CrossRef]
- Du, Y.; Zhang, C.; Dietrich, C.H.; Zhang, Y.; Dai, W. Characterization of the complete mitochondrial genomes of Maiestas dorsalis and Japananus hyalinus (Hemiptera: Cicadellidae) and comparison with other Membracoidea. Sci. Rep. 2017, 7, 14197. [Google Scholar] [CrossRef]
- Wu, Z.; Ge, S. The phylogeny of the BEP clade in grasses revisited: Evidence from the whole-genome sequences of chloroplasts. Mol. Phylogenet. Evol. 2012, 62, 573–578. [Google Scholar] [CrossRef]
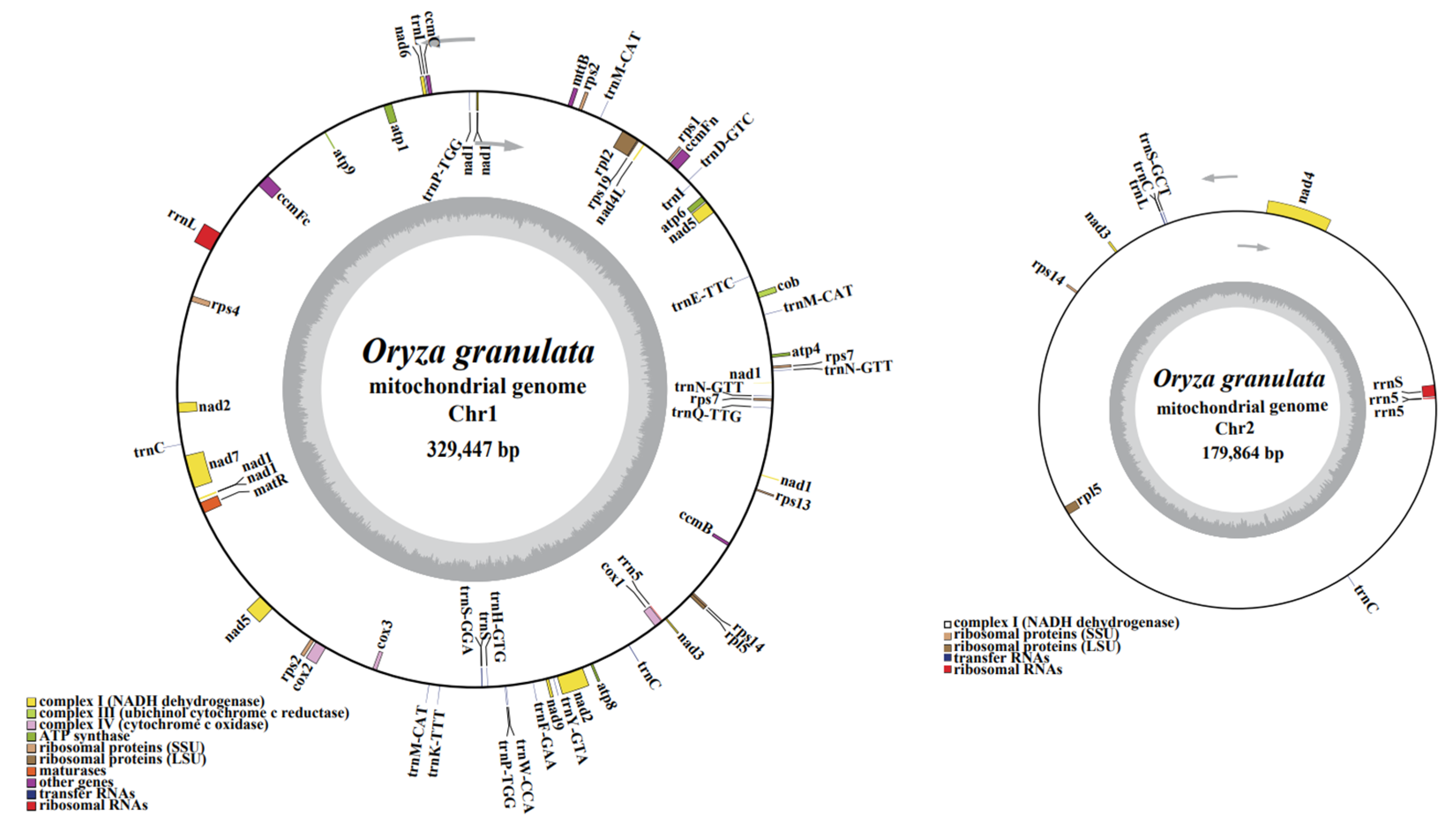
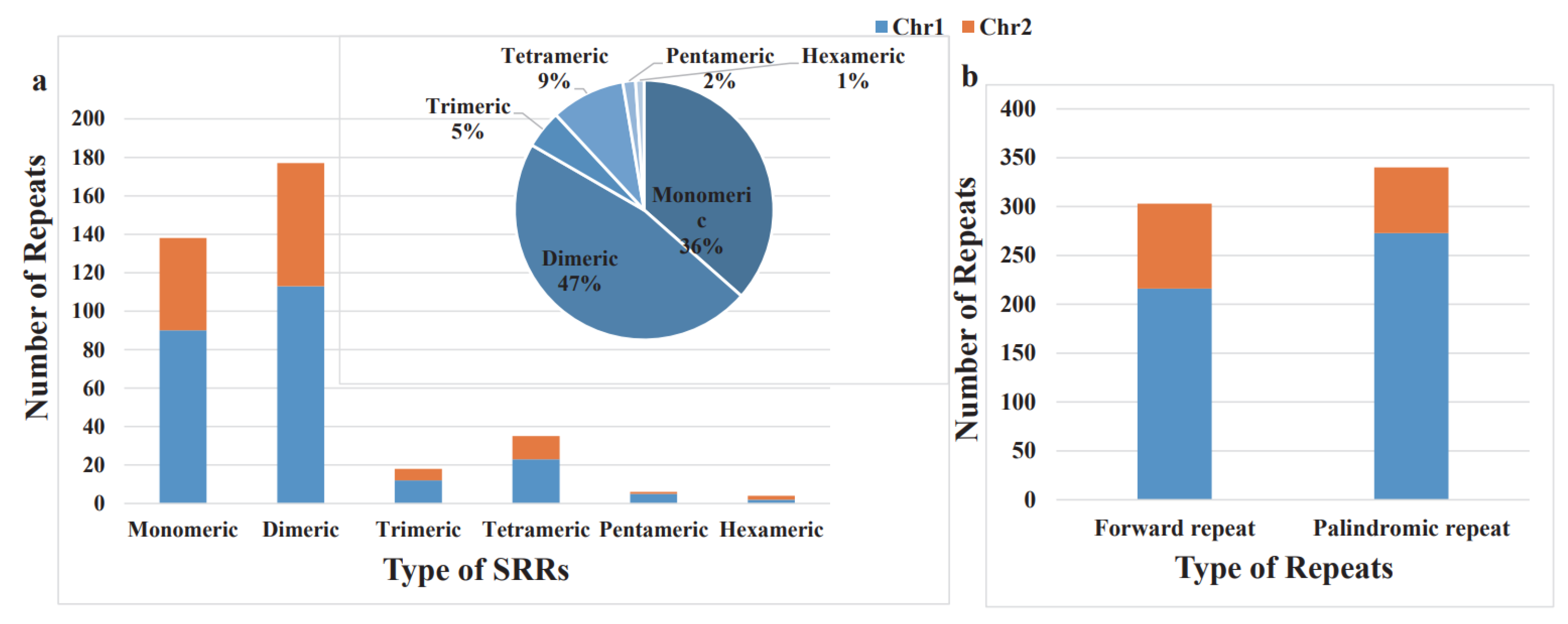

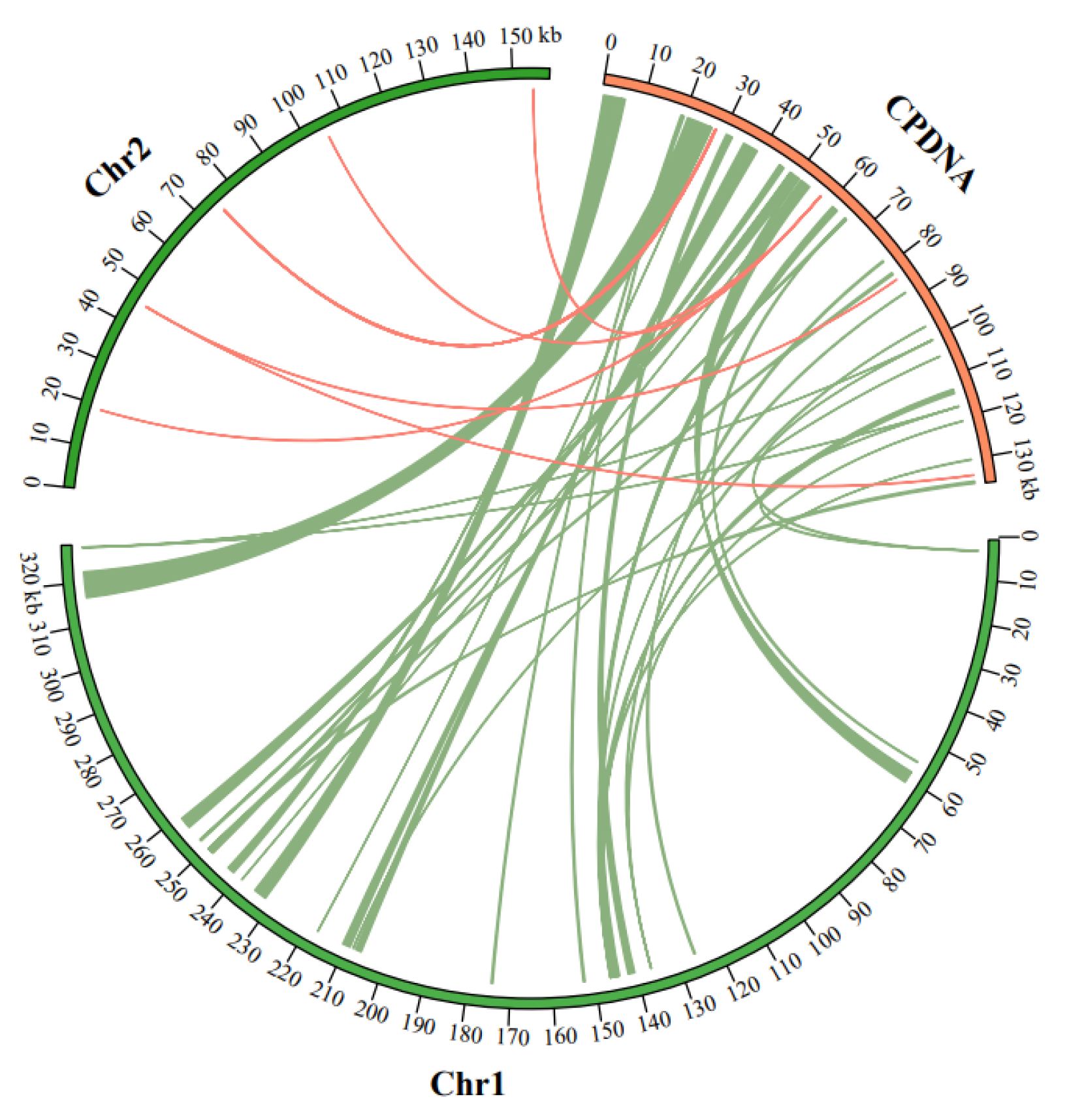
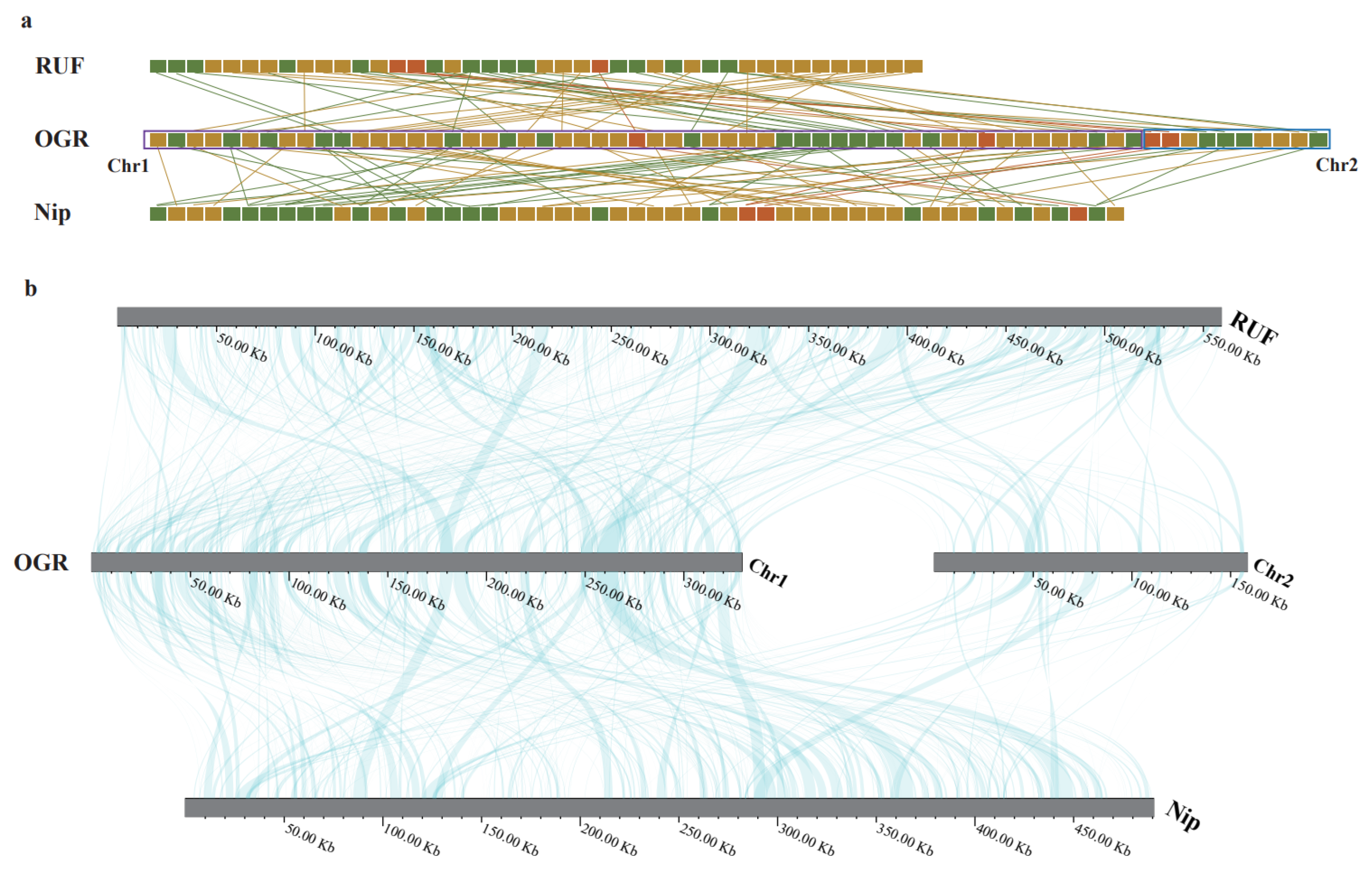
| Group of Genes | Genes | |
|---|---|---|
| Chr1 | Chr2 | |
| Complex Ⅰ NADH dehydrogenase | nad1, nad5, nad4L, Nad6, nad2, nad7, nad9, nad3 | nad4, nad3 |
| Complex Ⅲ Cytochrome c biogenesis | cob | |
| Complex Ⅳ Cytochrome c oxidase | cox2, cox3, cox1 | |
| Complex Ⅴ ATP synthase | atp4, atp6, atp1, atp9, atp8 | |
| Ubiquinol cytochrome c biogenesis | ccmFn, ccmC, ccmFc, ccmB | |
| Ribosome large subunit | rpl2, rpl5 | rpl5 |
| Ribosome small subunit | rps 7*2, rps1, rps19, rps2, rps4, rps14, rps13 | rps14 |
| Ribosome RNA | rrnL, rrn5 | rrnS, rrn5*2 |
| Transfer RNA | trnN-GTT*2, trnM-CAT*2, trnE-TTC, trnI, trnD-GTC, trnP-TGG*2, trnL, trnC*2, trnM-CAT, trnK-TTT, trnS, trnS-GGA, trnH-GTG, trnW-CCA, trnF-GAA, trnY-GTA, trnQ-TTG | trnC*2, trnL, trnS-GCT |
| Maturases | matR | |
| Transport membrane protein | mttB | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, F.; Kang, H.; Gao, L. Complete Mitochondrial Genome Assembly of an Upland Wild Rice Species, Oryza granulata and Comparative Mitochondrial Genomic Analyses of the Genus Oryza. Life 2023, 13, 2114. https://doi.org/10.3390/life13112114
Zhang F, Kang H, Gao L. Complete Mitochondrial Genome Assembly of an Upland Wild Rice Species, Oryza granulata and Comparative Mitochondrial Genomic Analyses of the Genus Oryza. Life. 2023; 13(11):2114. https://doi.org/10.3390/life13112114
Chicago/Turabian StyleZhang, Fen, Haiqi Kang, and Lizhi Gao. 2023. "Complete Mitochondrial Genome Assembly of an Upland Wild Rice Species, Oryza granulata and Comparative Mitochondrial Genomic Analyses of the Genus Oryza" Life 13, no. 11: 2114. https://doi.org/10.3390/life13112114
APA StyleZhang, F., Kang, H., & Gao, L. (2023). Complete Mitochondrial Genome Assembly of an Upland Wild Rice Species, Oryza granulata and Comparative Mitochondrial Genomic Analyses of the Genus Oryza. Life, 13(11), 2114. https://doi.org/10.3390/life13112114







