Additive Effect of the Composition of Endophytic Bacteria Bacillus subtilis on Systemic Resistance of Wheat against Greenbug Aphid Schizaphis graminum Due to Lipopeptides
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Objects
2.2. Bioassay of the Endophytic Properties of the Bacillus Strains
2.3. Bioassay of the Antagonistic Activity of the Bacillus Strains
2.4. Bioassay of the Phytohormone Content in the Liquid Culture Medium of B. subtilis
2.5. Isolation of DNA and Identification of Lipopeptide Synthetase Genes in the B. subtilis Strains by PCR
2.6. Isolation and Purification of the Lipopeptide-Rich Fraction (LRF) from the Liquid Culture Medium of B. subtilis Strains
2.7. High-Performance Liquid Chromatography (HPLC) Analysis of the Bacillus Strains LRF
2.8. Screening of Growth-Promoting Concentrations for the Bacillus Strains and Their LRFs
2.9. Experimental Design of Tripartite Bacteria-Aphid-Plant Interaction
2.10. Bioassay of Aphicidal Activity of the Bacillus Strains and Their LRF
2.11. Bioanalysis of the Different Types of Resistance to Aphids—Antibiosis and Endurance
2.12. Biochemical Parameters
2.13. Isolation of RNA and Performing the Quantitative Real-Time Polymerase Chain Reaction (qPCR)
2.14. Statistical Analysis
3. Results
3.1. Characterization of B. subtilis 26D and B. subtilis 11VM Strains
3.1.1. Production of Phytohormones by the Strain B. subtilis 11VM
3.1.2. Endophytic Rate and Antagonism of B. subtilis Strains to Each Other
3.1.3. Identification of Cyclic Lipopeptide Synthetases Genes of Endophytic Strains of B. subtilis
3.1.4. Identification of the Cyclic Lipopeptides Produced by Endophytic Strains of B. subtilis
3.2. Screening of Wheat Growth-Promoting Concentrations of Suspensions of B. subtilis and Their LRFs
3.3. Aphicidal Activity of Endophytic Strains of B. subtilis and Their LRF
3.4. Different Types of Defense against Aphids—Antibiosis and Endurance
3.5. The Effect of B. subtilis Strains, LRFs and Their Compositions on the Induction of Systemic Resistance in Wheat Plants Populated by Greenbug Aphid
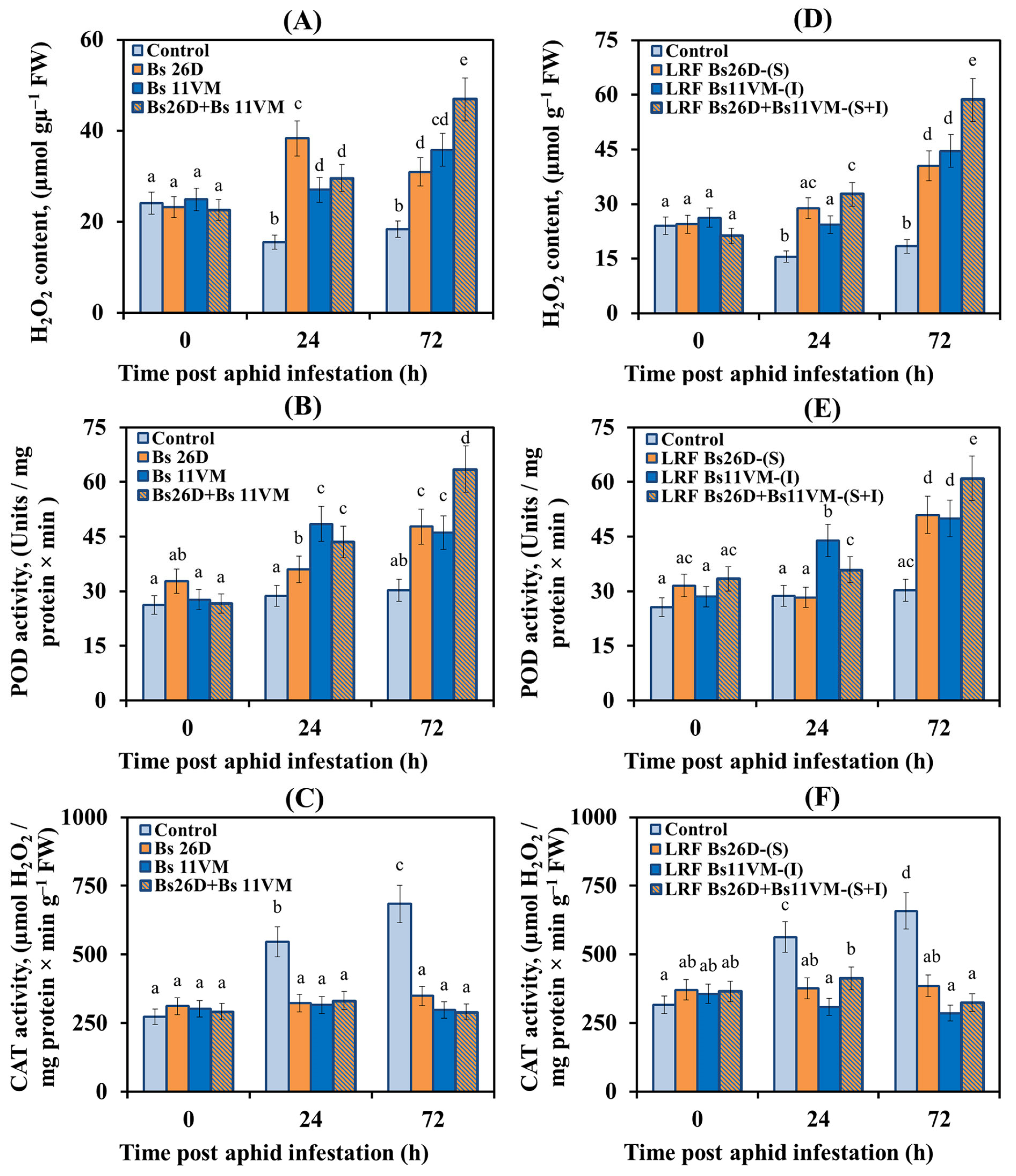
3.5.1. The Content of Hydrogen Peroxide and Activity of Redox Enzymes in Wheat Plants
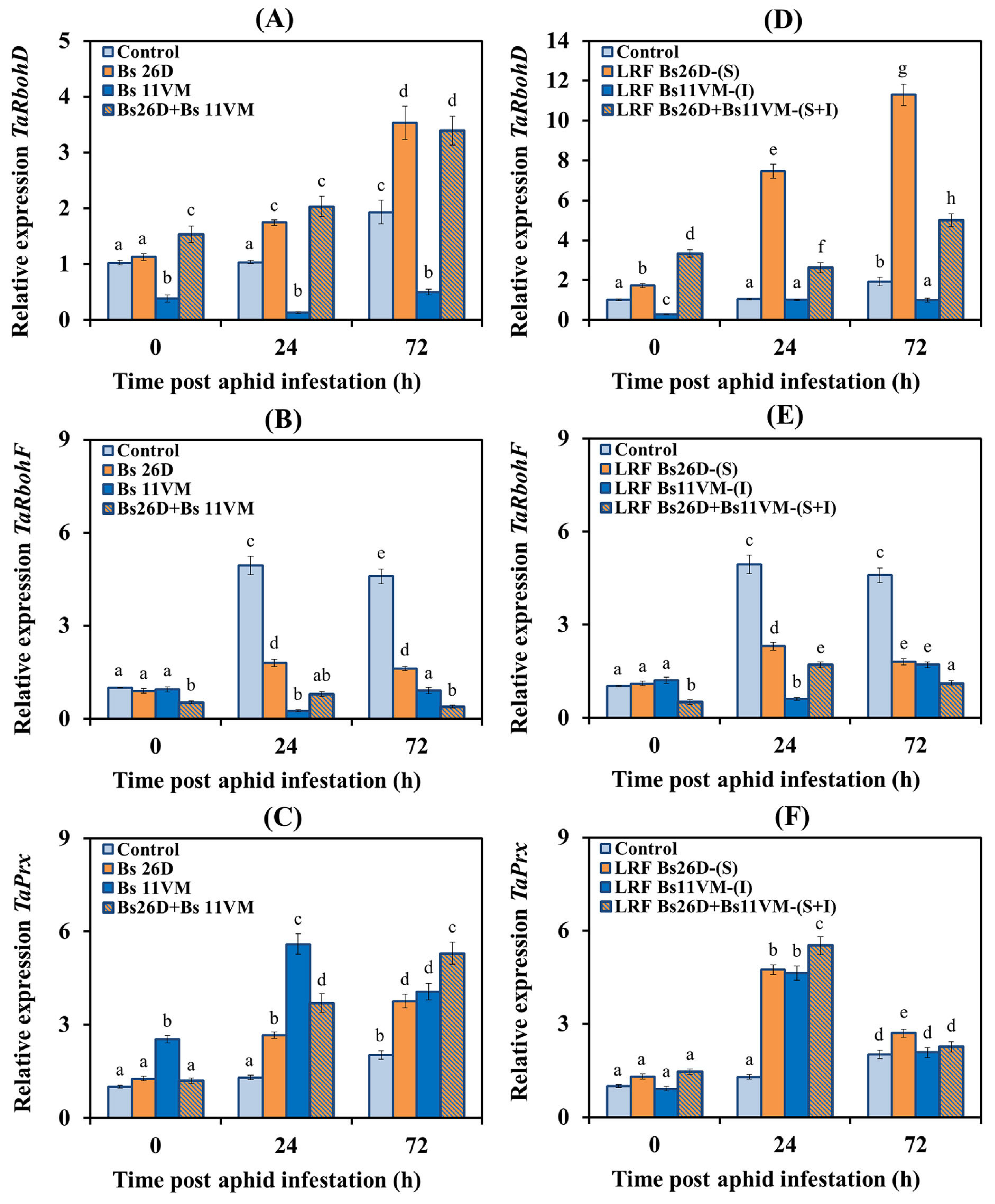
3.5.2. Expression of Redox Enzyme Genes
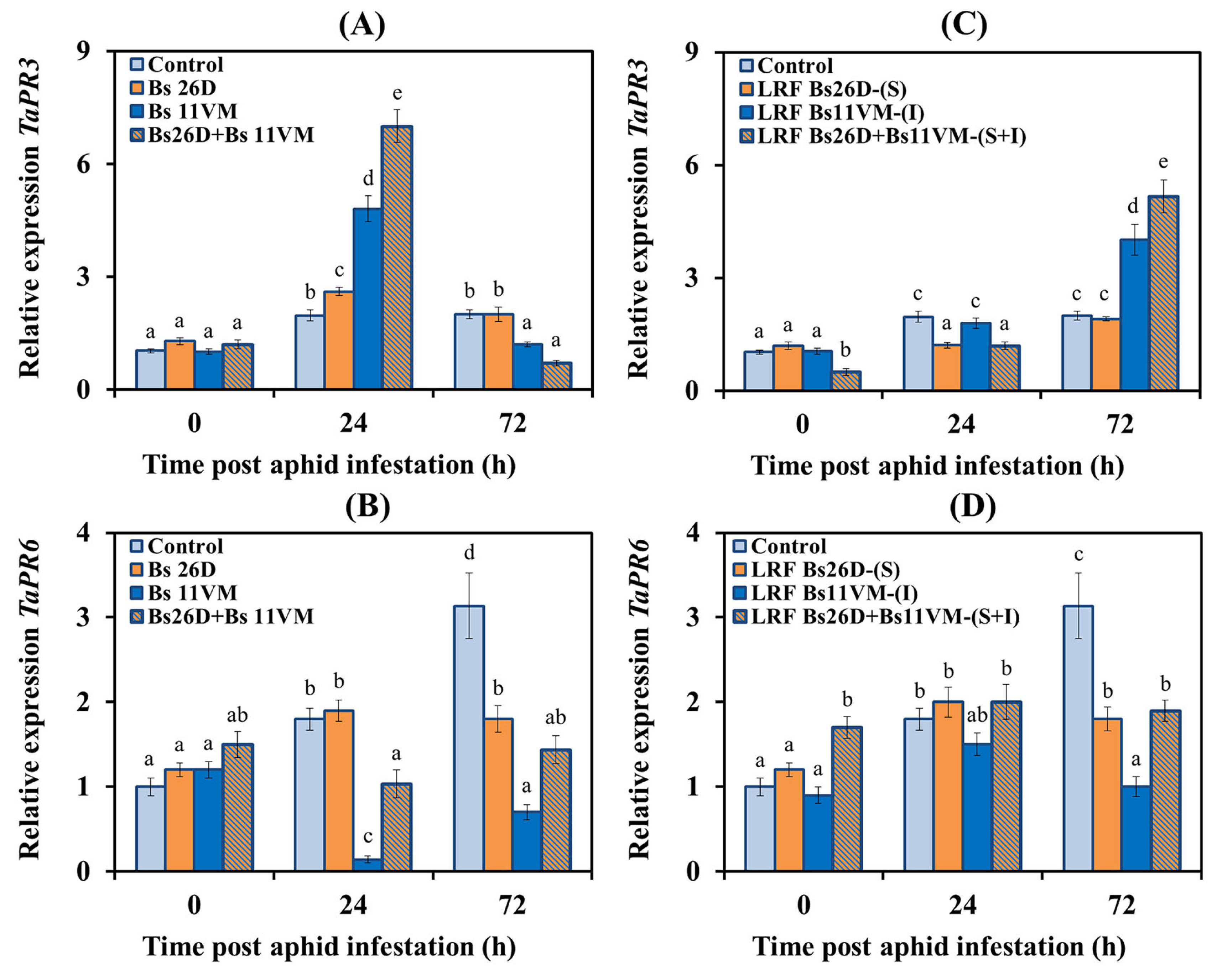
3.5.3. Expression of PR Proteins Genes Relating to Plant Hormone Signaling Pathways
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Crespo-Herrera, L.; Singh, R.P.; Reynolds, M.; Huerta-Espino, J. Genetics of greenbug resistance in synthetic hexaploid wheat derived germplasm. Front. Plant Sci. 2019, 10, 782. [Google Scholar] [CrossRef] [PubMed]
- Radchenko, E.E.; Abdullaev, R.A.; Anisimova, I.N. Genetic resources of cereal crops for aphid resistance. Plants 2022, 11, 1490. [Google Scholar] [CrossRef] [PubMed]
- Conrath, U.; Beckers, G.J.; Flors, V.; García-Agustín, P.; Jakab, G.; Mauch, F.; Newman, M.A.; Pieterse, C.M.; Poinssot, B.; Pozo, M.J.; et al. Priming: Getting ready for battle. Mol. Plant Microbe Interact. 2006, 19, 1062–1071. [Google Scholar] [CrossRef] [PubMed]
- Maksimov, I.V.; Veselova, S.V.; Nuzhnaya, T.V.; Sarvarova, E.R.; Khairullin, R.M. Plant growth-promoting bacteria in regulation of plant resistance to stress factors. Russ. J. Plant Physiol. 2015, 62, 715–726. [Google Scholar] [CrossRef]
- Rashid, M.H.; Chung, Y.R. Induction of systemic resistance against insect herbivores in plants by beneficial soil microbes. Front. Plant Sci. 2017, 8, 1816. [Google Scholar] [CrossRef]
- Xia, Y.; Liu, J.; Chen, C.; Mo, X.; Tan, Q.; He, Y.; Wang, Z.; Yin, J.; Zhou, G. The multifunctions and future prospects of endophytes and their metabolites in plant disease management. Microorganisms 2022, 23, 1072. [Google Scholar] [CrossRef]
- Rani, S.; Kumar, P.; Dahiya, P.; Maheshwari, R.; Dang, A.S.; Suneja, P. Endophytism: A multidimensional approach to plant–prokaryotic microbe interaction. Front. Microbiol. 2022, 13, 861235. [Google Scholar] [CrossRef]
- Disi, J.; Simmons, J.; Zebelo, S. Plant growth-promoting rhizobacteria-induced defense against insect herbivores. In Field Crops: Sustainable Management by PGPR. Sustainable Development and Biodiversity; Maheshwari, D., Dheeman, S., Eds.; Springer Nature: Cham, Switzerland, 2019; Volume 23, pp. 154–287. [Google Scholar] [CrossRef]
- Oukala, N.; Aissat, K.; Pastor, V. Bacterial endophytes: The hidden actor in plant immune responses against biotic stress. Plants 2021, 19, 1012. [Google Scholar] [CrossRef]
- Miljaković, D.; Marinković, J.; Balešević-Tubić, S. The significance of Bacillus spp. in disease suppression and growth promotion of field and vegetable crops. Microorganisms 2020, 13, 1037. [Google Scholar] [CrossRef]
- Maksimov, I.V.; Singh, B.P.; Cherepanova, E.A.; Burkhanova, G.F.; Khairullin, R.M. Prospects and applications of lipopeptide-producing bacteria for plant protection (Review). Appl. Biochem. Microbiol. 2020, 14, 15–28. [Google Scholar] [CrossRef]
- De Vleesschauwer, D.; Höfte, M. Rhizobacteria-induced systemic resistance. Adv. Bot. Res. 2009, 51, 223–281. [Google Scholar] [CrossRef]
- Pieterse, C.M.; Zamioudis, C.; Berendsen, R.L.; Weller, D.M.; Van Wees, S.C.; Bakker, P.A. Induced systemic resistance by beneficial microbes. Annu. Rev. Phytopathol. 2014, 52, 347–375. [Google Scholar] [CrossRef]
- Ongena, M.; Jacques, P. Bacillus lipopeptides: Versatile weapons for plant disease biocontrol. Trends Microbiol. 2008, 16, 115–125. [Google Scholar] [CrossRef]
- Andrić, S.; Meyer, T.; Rigolet, A.; Prigent-Combaret, C.; Höfte, M.; Balleux, G.; Steels, S.; Hoff, G.; De Mot, R.; McCann, A.; et al. Lipopeptide interplay mediates molecular interactions between soil Bacilli and Pseudomonads. Microbiol. Spectr. 2021, 9, e0203821. [Google Scholar] [CrossRef]
- Lee, J.H.; Anderson, A.J.; Kim, Y.C. Root-associated bacteria are biocontrol agents for multiple plant pests. Microorganisms 2022, 10, 1053. [Google Scholar] [CrossRef]
- Cawoy, H.; Debois, D.; Franzil, L.; De Pauw, E.; Thonart, P.; Ongena, M. Lipopeptides as main ingredients for inhibition of fungal phytopathogens by Bacillus subtilis/amyloliquefaciens. Microb. Biotechnol. 2015, 2, 281–295. [Google Scholar] [CrossRef]
- Yang, S.Y.; Lim, D.J.; Noh, M.Y.; Kim, J.C.; Kim, Y.C.; Kim, I.S. Characterization of biosurfactants as insecticidal metabolites produced by Bacillus subtilis Y9. Entomol. Res. 2017, 47, 55–59. [Google Scholar] [CrossRef]
- Rodríguez, M.; Marín, A.; Torres, M.; Béjar, V.; Campos, M.; Sampedro, I. Aphicidal activity of surfactants produced by Bacillus atrophaeus L193. Front. Microbiol. 2018, 18, 3114–3123. [Google Scholar] [CrossRef]
- Maksimov, I.V.; Blagova, D.K.; Veselova, S.V.; Sorokan, A.V.; Burkhanova, G.F.; Cherepanova, E.A.; Sarvarova, E.R.; Rumyantsev, S.D.; Alekseev, V.Yu.; Khayrullin, R.M. Recombinant Bacillus subtilis 26DCryChS line with gene Btcry1Ia encoding Cry1Ia toxin from Bacillus thuringiensis promotes integrated wheat defense against pathogen Stagonospora nodorum Berk. and greenbug Schizaphis graminum Rond. Biol. Control. 2020, 144, 104242. [Google Scholar] [CrossRef]
- Denoirjean, T.; Ameline, A.; Couty, A.; Dubois, F.; Coutte, F.; Doury, G. Effects of surfactins, Bacillus lipopeptides, on the behavior of an aphid and host selection by its parasitoid. Pest Manag. Sci. 2022, 78, 929–937. [Google Scholar] [CrossRef]
- Rashid, M.H.; Kim, H.-J.; Yeom, S.-I.; Yu, H.-A.; Manir, M.M.; Moon, S.-S.; Kang, Y.J.; Chung, Y.R. Bacillus velezensis YC7010 enhances plant defenses against brown planthopper through transcriptomic and metabolic changes in rice. Front. Plant Sci. 2018, 9, 1904. [Google Scholar] [CrossRef] [PubMed]
- Tunsagool, P.; Leelasuphakul, W.; Jaresitthikunchai, J.; Phaonakrop, N.; Roytrakul, S.; Jutidamrongphan, W. Targeted transcriptional and proteomic studies explicate specific roles of Bacillus subtilis iturin A, fengycin, and surfactin on elicitation of defensive systems in mandarin fruit during stress. PLoS ONE 2019, 14, e0217202. [Google Scholar] [CrossRef] [PubMed]
- Chandler, S.; Van Hese, N.; Coutte, F.; Jacques, P.; Höfte, M.; De Vleesschauwer, D. Role of cyclic lipopeptides produced by Bacillus subtilis in mounting induced immunity in rice (Oryza sativa L.). Physiol. Mol. Plant Pathol. 2015, 91, 20–30. [Google Scholar] [CrossRef]
- Waewthongrak, W.; Pisuchpen, S.; Leelasuphakul, W. Effect of Bacillus subtilis and chitosan applications on green mold (Penicilium digitatum Sacc.) decay in citrus fruit. Postharvest Biol. Technol. 2015, 99, 44–49. [Google Scholar] [CrossRef]
- Jiang, M.; Pang, X.; Liu, H.; Lin, F.; Lu, F.; Bie, X.; Lu, Z.; Lu, Y. Iturin A induces resistance and improves the quality and safety of harvested cherry tomato. Molecules 2021, 26, 6905. [Google Scholar] [CrossRef]
- Farace, G.; Fernandez, O.; Jacquens, L.; Coutte, F.; Krier, F.; Jacques, P.; Clement, C.; Barka, E.A.; Jacquard, C.; Dorey, S. Cyclic lipopeptides from Bacillus subtilis activate distinct patterns of defence responses in grapevine. Mol. Plant Pathol. 2015, 16, 177–187. [Google Scholar] [CrossRef]
- Rumyantsev, S.D.; Veselova, S.V.; Burkhanova, G.F.; Maksimov, I.V. Induced resistance to the greenbug aphid Schizaphis graminum Rond. in species of the genus Triticum. Vavilov J. Genet. Breed. 2019, 23, 865–872. [Google Scholar] [CrossRef]
- Sorokan, A.; Cherepanova, E.; Burkhanova, G.; Veselova, S.; Rumyantsev, S.; Alekseev, V.; Mardanshin, I.; Sarvarova, E.; Khairullin, R.; Benkovskaya, G.; et al. Endophytic Bacillus spp. as a prospective biological tool for control of viral diseases and non-vector Leptinotarsa decemlineata Say. In Solanum tuberosum L. Front. Microbiol. 2020, 11, 569457. [Google Scholar] [CrossRef]
- Burkhanova, G.F.; Veselova, S.V.; Sorokan’, A.V.; Blagova, D.K.; Nuzhnaya, T.V.; Maksimov, I.V. Strains of Bacillus ssp. regulate wheat resistance to Septoria nodorum Berk. Appl. Biochem. Microbiol. 2017, 53, 346–352. [Google Scholar] [CrossRef]
- Sorokan, A.V.; Benkovskaya, G.V.; Blagova, D.K.; Maksimova, T.I.; Maksimov, I.V. Defense responses and changes in symbiotic gut microflora in the Colorado Potato Beetle Leptinotarsa decemlineata under the effect of endophytic bacteria from the genus Bacillus. J. Evol. Biochem. Physiol. 2018, 54, 300–307. [Google Scholar] [CrossRef]
- Veselov, S.Y.; Timergalina, L.N.; Akhiyarova, G.R.; Kudoyarova, G.R.; Korobova, A.V.; Ivanov, I.; Arkhipova, T.N.; Prinsen, E. Study of cytokinin transport from shoots to roots of wheat plants is informed by a novel method of differential localization of freecytokinin bases or their ribosylated forms by means of their specific fixation. Protoplasma 2018, 255, 1581–1594. [Google Scholar] [CrossRef]
- Veselova, S.V.; Sorokan, A.V.; Burkhanova, G.F.; Rumyantsev, S.D.; Cherepanova, E.A.; Alekseev, V.Y.; Sarvarova, E.R.; Kasimova, A.R.; Maksimov, I.V. By modulating the hormonal balance and ribonuclease activity of tomato plants Bacillus subtilis induces defense response against Potato Virus X and Potato Virus Y. Biomolecules 2022, 12, 288. [Google Scholar] [CrossRef]
- Kudoyarova, G.R.; Veselov, D.S.; Sharipova, G.V.; Akhiyarova, R.G.; Dodd, I.C.; Veselov, S.Y. Water relations and growth oforiginal barley plants and its ABA-deficient mutants at increased air temperature. Russ. J. Plant Physiol. 2014, 61, 188–193. [Google Scholar] [CrossRef]
- Kudoyarova, G.R.; Korobova, A.V.; Akhiyarova, G.R.; Arkhipova, T.N.; Zaytsev, D.Y.; Prinsen, E.; Egutkin, N.L.; Medvedev, S.S.; Veselov, S.Y. Accumulation of cytokinins in roots and their export to the shoots of durum wheat plants treated with theprotonophore carbonyl cyanide m-chlorophenylhydrazone (CCCP). J. Exp. Bot. 2014, 65, 2287–2294. [Google Scholar] [CrossRef]
- Cao, Y.; Xu, Z.H.; Ling, N.; Yuan, Y.J.; Yang, X.M.; Chen, L.H.; Shen, B.; Shen, Q.R. Isolation and identification of lipopeptides produced by B. subtilis SQR 9 for suppressing Fusarium wilt of cucumber. Sci. Hortic. 2013, 135, 32–39. [Google Scholar] [CrossRef]
- Veselova, S.V.; Burkhanova, G.F.; Rumyantsev, S.D.; Blagova, D.K.; Maksimov, I.V. Strains of Bacillus spp. regulate wheat resistance to greenbug aphid Schizaphis graminum Rond. Appl. Biochem. Microbiol. 2019, 55, 41–47. [Google Scholar] [CrossRef]
- Bindschedler, L.V.; Dewdney, J.; Blee, K.A. Peroxidase-dependent apoplastic oxidative burst in Arabidopsis required for pathogen resistance. Plant J. 2006, 47, 851–863. [Google Scholar] [CrossRef]
- Egorshina, A.A.; Khairullin, R.M.; Lukyantsev, M.A.; Kuramshina, Z.M.; Smirnova, Y.V. Phosphate-mobilizing activity of the endophytic Bacillus subtilis strains and their effect on wheat roots micorrhization ratio. J. Sib. Fed. Univ. Biol. 2011, 4, 172–182. [Google Scholar] [CrossRef]
- Kudoyarova, G.; Arkhipova, T.; Korshunova, T.; Bakaeva, M.; Loginov, O.; Dodd, I.C. Phytohormone mediation of interactions between plants and non-symbiotic growth promoting bacteria under edaphic stresses. Front. Plant Sci. 2019, 10, 1368. [Google Scholar] [CrossRef]
- Koch, K.G.; Palmer, N.A.; Donze-Reiner, T.; Scully, E.D.; Seravalli, J.; Amundsen, K.; Twigg, P.; Louis, J.; Bradshaw, J.D.; Heng-Moss, T.M.; et al. Aphid-responsive defense networks in hybrid switchgrass. Front. Plant Sci. 2020, 11, 1145. [Google Scholar] [CrossRef]
- Sorokan, A.V.; Iskandarova, Z.F.; Blagova, D.K.; Sarvarova, E.V.; Maximov, I.V. The role of surfactine produced by endophitic bacteria Bacillus subtilis 26D in the development of symbiotic relations with potato plant. Ecobiotech 2019, 2, 257–261. (In Russian) [Google Scholar] [CrossRef]
- Zhaogao, L.; Weie, W.; Ming, Q.; Yuqi, H.; Delin, X.; Lin, L. Biosynthetic mechanisms of secondary metabolites promoted by the interaction between endophytes and plant hosts. Front. Microbiol. 2022, 13, 928967. [Google Scholar] [CrossRef]
- Fitzpatrick, C.R.; Salas-González, I.; Conway, J.M.; Finkel, O.M.; Gilbert, S.; Russ, D.; Teixeira, O.M.P.L.; Dangl, J.L. The plant microbiome: From ecology to reductionism and beyond. Annu. Rev. Microbiol. 2020, 74, 81–100. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.-L.; Wan, B.; Xiao, S.-J.; Allen, S.; Gu, Y.-C.; Ding, L.-S.; Zhou, Y. Cyclic lipopeptides with herbicidal and insecticidal activities produced by Bacillus clausii DTM1. Nat. Prod. Commun. 2015, 10, 2151–2153. [Google Scholar] [CrossRef] [PubMed]
- Leconte, A.; Tournant, L.; Muchembled, J.; Paucellier, J.; Héquet, A.; Deracinois, B.; Deweer, C.; Krier, F.; Deleu, M.; Oste, S.; et al. Assessment of lipopeptide mixtures produced by Bacillus subtilis as biocontrol products against apple scab (Venturia inaequalis). Microorganisms 2022, 10, 1810. [Google Scholar] [CrossRef]
- Eid, A.M.; Fouda, A.; Abdel-Rahman, M.A.; Salem, S.S.; Elsaied, A.; Oelmüller, R.; Hijri, M.; Bhowmik, A.; Elkelish, A.; Hassan, S.E.-D. Harnessing bacterial endophytes for promotion of plant growth and biotechnological applications: An overview. Plants 2021, 10, 935. [Google Scholar] [CrossRef]
- Chaudhary, P.; Singh, S.; Chaudhary, A.; Sharma, A.; Kumar, G. Overview of biofertilizers in crop production and stress management for sustainable agriculture. Front. Plant Sci. 2022, 13, 930340. [Google Scholar] [CrossRef]
- Serteyn, L.; Quaghebeur, C.; Ongena, M.; Cabrera, N.; Barrera, A.; Molina-Montenegro, M.A.; Francis, F.; Ramírez, C.C. Induced systemic resistance by a plant growth-promoting rhizobacterium impacts development and feeding behavior of aphids. Insects 2020, 11, 234. [Google Scholar] [CrossRef]
- Zhu-Salzman, K.; Salzman, R.A.; Ahn, J.-E.; Koiwa, H. Transcriptional regulation of sorghum defense determinants against a phloem-feeding aphid. Plant Physiol. 2004, 134, 420–431. [Google Scholar] [CrossRef]
- Lei, J.; Zhu-Salzman, K. Enhanced aphid detoxification when confronted by a host with elevated ROS production. Plant Signal. Behav. 2015, 10, 4. [Google Scholar] [CrossRef]
- Bano, A.; Muqarab, R. Plant defence induced by PGPR against Spodoptera litura in tomato (Solanum lycopersicum L.). Plant Biol. 2016, 19, 3. [Google Scholar] [CrossRef]
- Ling, S.; Zhao, Y.; Sun, S.; Zheng, D.; Sun, X.; Zeng, R.; Chen, D.; Song, Y. Enhanced anti-herbivore defense of tomato plants against Spodoptera litura by their rhizosphere bacteria. BMC Plant Biol. 2022, 22, 254. [Google Scholar] [CrossRef]
- Farzand, A.; Moosa, A.; Zubair, M.; Khan, A.R.; Massawe, V.C.; Tahir, H.A.S.; Sheikh, S.M.M.; Ayaz, M.; Gao, X. Suppression of Sclerotinia sclerotiorum by the induction of systemic resistance and regulation of antioxidant pathways in tomato using fengycin produced by Bacillus amyloliquefaciens FZB42. Biomolecules 2019, 9, 613. [Google Scholar] [CrossRef]
- Zhang, Y.; Fu, Y.; Wang, Q.; Liu, X.; Li, Q.; Chen, J. Transcriptome analysis reveals rapid defence responses in wheat induced by phytotoxic aphid Schizaphis graminum feeding. BMC Genom. 2020, 21, 339. [Google Scholar] [CrossRef]
- Podgórska, A.; Burian, M.; Szal, B. Extra cellular but extra-ordinarily important for cells: Apoplastic reactive oxygen species metabolism. Front. Plant Sci. 2017, 8, 1353. [Google Scholar] [CrossRef]
- Mersmann, S.; Bourdais, G.; Rietz, S.; Robatzek, S. Ethylene signaling regulates accumulation of the FLS2 receptor and is required for the oxidative burst contributing to plant immunity. Plant Physiol. 2010, 154, 391–400. [Google Scholar] [CrossRef]
- Pangesti, N.; Reichelt, M.; van de Mortel, J.E.; Kapsomenou, E.; Gershenzon, J.; van Loon, J.J.; Dicke, M.; Pineda, A. Jasmonic acid and ethylene signaling pathways regulate glucosinolate levels in plants during rhizobacteria-induced systemic resistance against a leaf-chewing herbivore. J. Chem. Ecol. 2016, 42, 1212–1225. [Google Scholar] [CrossRef]
- Morkunas, I.; Narozna, D.; Nowak, W.; Samardakiewicz, S.; Remlein-Starosta, D. Cross-talk interactions of sucrose and Fusarium oxysporum on the phenylpropanoid pathway and the accumulation and localization of flavonoids in embryo axes of yellow lupine. J. Plant Physiol. 2011, 168, 424–433. [Google Scholar] [CrossRef]
- Argandona, V.H.; Chaman, M.; Cardemil, L.; Munoz, O.; Zuniga, G.E.; Corcuera, L.J. Ethylene production and peroxidase activity in aphid-infested barley. J. Chem. Ecol. 2001, 27, 53–68. [Google Scholar] [CrossRef]
- Wu, C.; Avila, C.A.; Goggin, F.L. The ethylene response factor Pti5 contributes to potato aphid resistance in tomato independent of ethylene signaling. J. Exp. Bot. 2015, 66, 559–570. [Google Scholar] [CrossRef]
- Valenzuela-Soto, J.H.; Estrada-Hernández. M., G.; Ibarra-Laclette., E.; Délano-Frier, J.P. Inoculation of tomato plants (Solanum lycopersicum) with growth-promoting Bacillus subtilis retards whitefly Bemisia tabaci development. Planta 2010, 231, 397–410. [Google Scholar] [CrossRef] [PubMed]
- Pangesti, N.; Pineda, A.; Pieterse, C.M.J.; Dicke, M.; van Loon, J.J.A. Two-way plant-mediated interactions between root associated microbes and insects: From ecology to mechanisms. Front. Plant Sci. 2013, 4, 414. [Google Scholar] [CrossRef] [PubMed]



| Strain | Phytohormone Level, µg/mL of Culture Medium | ||
|---|---|---|---|
| IAA | ABA | Cytokinins * | |
| B. subtilis 11VM | 0.31 ± 0.04 | 0.0 | 0.07 ± 0.006 |
| Parameter | Part of Plant | Strain | ||
|---|---|---|---|---|
| B. subtilis 26D | B. subtilis 11VM | B. subtilis 26D + B. subtilis 11VM | ||
| Endophytic Rate, CFU × 103/g of fresh weight | shoot | 1728.8 ± 231.74 a | 60.3 ± 5.9 b | 3267.8 ± 316.09 c |
| root | 297.7 ± 80.26 a | 424.7 ± 78.54 b | 240.1 ± 56.29 a | |
| Strain | Distance from the Antagonist Colony, mm | |
|---|---|---|
| Antagonist | ||
| B. subtilis 26D | B. subtilis 11VM | |
| B. subtilis 26D | 0 | 3.5 ± 3.6 a |
| B. subtilis 11VM | 3.5 ± 0.12 a | 0 |
| Strain or Strains Composition | Growth-Promoting Concentrations | |
|---|---|---|
| Strain Concentration, µL/g Seeds | LRF Concentration, µg/mL | |
| B. subtilis 26D | 2.0 | 2.5 |
| B. subtilis 11VM | 1.0 | 1.5 |
| B. subtilis 26D + B. subtilis 11VM | 1.5 + 0.5 | 2.0 + 1.5 |
| Variant of Treatment | Concentration of Bacterial Suspension, µl/g Seeds | Aphid Viability Indicators (Antibiosis) | Plants Endurance | |||
|---|---|---|---|---|---|---|
| Aphid Amount, (Nymphs/Seedling) | Mortality, % | Propagation Coefficient | Growth Rate of the 1st Leaf, % of Control * | Growth Rate of the 2nd Leaf, % of Control * | ||
| Water | - | 36.8 ± 3.9 a | 6.9 ± 1.7 a | 2.45 a | 81.8 ± 6.2 a | 70.2 ± 5.1 a |
| B. subtilis 26D | 2.0 | 19.8 ± 2.2 b | 31.5 ± 2.2 b | 1.32 b | 114.7 ± 7.3 b | 142.0 ± 12.9 b |
| B. subtilis 11VM | 1.0 | 22.7 ± 2.8 b | 24.3 ± 3.4 c | 2.1 c | 103.2 ± 5.6 c | 115.0 ± 9.2 c |
| B. subtilis 26D + B. subtilis 11VM | 1.5 + 0.5 | 14.7 ± 1.9 c | 28.2 ± 2.6 b | 1.18 d | 120.5 ±6.8 d | 116 ± 5.3 c |
| LRF from Strain and Their Mixture | Concentration of LRF, µg/mL | Aphid Viability Indicators (Antibiosis) | Plants Endurance | |||
|---|---|---|---|---|---|---|
| Aphid amount, (nymphs/Seedling) | Mortality, % | Propagation Coefficient | Growth Rate of the 1st Leaf, % of Control | Growth Rate of the 2nd Leaf, % of Control | ||
| Water | - | 36.8 ± 3.9 a | 6.9 ± 1.7 a | 2.45 a | 81.8 ± 6.2 a | 70.2 ± 5.1 a |
| LRF of B. subtilis 26D | 2.5 * | 20.0 ± 3.1 b | 24.9 ± 2.3 b | 1.3 b | 106.7 ± 5.7 b | 102.1 ± 4.8 b |
| 5 | 19.4 ± 2.9 b | 33.7 ± 4.3 c | 1.26 b | 87.6 ± 5.8 c | 100.0 ± 4.1 b | |
| 10 | 15.6 ± 2.7 c | 37.3 ± 4.8 d | 1.26 b | 86.5 ± 6.1 c | 94.7 ± 3.2 c | |
| LRF of B. subtilis 11VM | 1.5 * | 17.3 ± 3.3 bc | 20.9 ± 2.6 b | 1.2 b | 98.1 ± 6.2 d | 102.3 ± 7.6 b |
| 2.5 | 16.3 ± 4.1 c | 29.3 ± 2.7 c | 1.3 b | 96.9 ± 6.3 d | 101.8 ± 4.5 b | |
| 3.5 | 16.8 ± 1.8 c | 30.5 ± 4.1 c | 1.06 c | 91.2 ± 6.0 c | 90.8 ± 4.6 c | |
| 5 | 14.1 ± 3.3 c | 34.9 ± 3.9 c | 1.09 c | 75.0 ± 4.4 e | 72.3 ± 3.3 d | |
| 10 | 10.6 ± 3.2 d | 39.7 ± 5.1 d | 1.07 c | 73.4 ± 4.2 e | 71.5 ± 4.1 d | |
| LRF of B. subtilis 26D + B. subtilis 11VM | 2.0 + 1.5 * | 16.3 ± 1.2 c | 26.3 ± 3.1 b | 1.06 c | 110.2 ± 5.1 b | 100.3 ± 3.8 b |
| 2.5 + 1.5 | 16.5 ± 2.8 c | 34.6 ± 3.5 c | 1.07 c | 95.0 ± 5.8 d | 103.9 ± 4.8 b | |
| 2.5 + 2.5 | 20.6 ± 3.5 b | 34.2 ± 3.7 c | 1.6 d | 90.8 ± 5.6 c | 93.8 ± 4.2 c | |
| 2.5 + 3.5 | 16.4 ± 1.9 c | 31.5 ± 4.1 c | 1.06 c | 94.1 ± 6.1 cd | 94.8 ± 4.5 c | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rumyantsev, S.D.; Alekseev, V.Y.; Sorokan, A.V.; Burkhanova, G.F.; Cherepanova, E.A.; Garafutdinov, R.R.; Maksimov, I.V.; Veselova, S.V. Additive Effect of the Composition of Endophytic Bacteria Bacillus subtilis on Systemic Resistance of Wheat against Greenbug Aphid Schizaphis graminum Due to Lipopeptides. Life 2023, 13, 214. https://doi.org/10.3390/life13010214
Rumyantsev SD, Alekseev VY, Sorokan AV, Burkhanova GF, Cherepanova EA, Garafutdinov RR, Maksimov IV, Veselova SV. Additive Effect of the Composition of Endophytic Bacteria Bacillus subtilis on Systemic Resistance of Wheat against Greenbug Aphid Schizaphis graminum Due to Lipopeptides. Life. 2023; 13(1):214. https://doi.org/10.3390/life13010214
Chicago/Turabian StyleRumyantsev, Sergey D., Valentin Y. Alekseev, Antonina V. Sorokan, Guzel F. Burkhanova, Ekaterina A. Cherepanova, Ravil R. Garafutdinov, Igor V. Maksimov, and Svetlana V. Veselova. 2023. "Additive Effect of the Composition of Endophytic Bacteria Bacillus subtilis on Systemic Resistance of Wheat against Greenbug Aphid Schizaphis graminum Due to Lipopeptides" Life 13, no. 1: 214. https://doi.org/10.3390/life13010214
APA StyleRumyantsev, S. D., Alekseev, V. Y., Sorokan, A. V., Burkhanova, G. F., Cherepanova, E. A., Garafutdinov, R. R., Maksimov, I. V., & Veselova, S. V. (2023). Additive Effect of the Composition of Endophytic Bacteria Bacillus subtilis on Systemic Resistance of Wheat against Greenbug Aphid Schizaphis graminum Due to Lipopeptides. Life, 13(1), 214. https://doi.org/10.3390/life13010214








