1. Introduction
The coconut leaf beetle,
Brontispa longissima Gestro (Coleoptera: Chrysomelidae), is thought to be native to Indonesia and Papua New Guinea. The nipa palm hispid beetle,
Octodonta nipae Maulik (Coleoptera: Chrysomelidae), is native to Malaysia. These species have invaded China with the international trade in seedlings and are currently wreaking havoc in southern China [
1,
2,
3,
4]. The two beetles attack young leaf fronds of different palm plants, leaving behind small brown spots that run parallel to veins and can even kill the entire plant [
3,
5]. As a result, the decorative palm sector in China suffers large palm losses every year [
3,
6].
For these two beetles, chemical and biological control are currently significant control methods. The high stems of palm plants combined with the characteristics of these two beetles, such as feeding and lodging in the tightly furled fronds and trunk fibers, render typical chemical control ineffectual [
7]. There have been numerous successful reports of using local natural enemies or introducing natural enemies to manage these two invasive beetles, which is why many nations and areas focus on this approach [
8].
Different insects can be parasitized by parasitic wasps; however, not all parasitized insects can produce parasitoid offspring. Adaptive hosts are insects that parasitic wasps may effectively parasitize and breed in, whereas non-adaptive hosts are insects that are incapable of producing progeny [
9,
10,
11,
12]. After being parasitized, insect immune systems react right away [
13,
14]. There are two types of immunological responses in insects: cellular immunity and humoral immunity. Cellular immunity uses hemocytes to enclose, phagocytose, and nodulate foreign compounds [
15,
16]. The encapsulation in host cellular immunity is the initial immunological response that the parasitic wasp encounters [
12,
17]. The encapsulation response requires a sufficient number of immunological hemocytes [
18,
19]. The type, quantity, and composition of hemocytes are significant indicators in depicting the strength of host cell immunity [
20,
21,
22,
23].
A gregarious and koinobiont endoparasitoid native to Western Samoa and Papua New Guinea [
24],
Asecodes hispinarum Bouček (Hymenoptera: Eulophidae) demonstrates an enhanced potential in the biocontrol of
B. longissima larvae [
8,
25,
26,
27]. Understanding how
A. hispinarum manipulates the physiology and biochemistry of
B. longissima larvae to generate an environment favorable for the development of its progeny is important for developing an efficient pest-management approach [
23,
28,
29,
30,
31].
We observed that
O. nipae larvae were also parasitized, but the eggs of
A. hispinarum that were encapsulated cannot develop normally. Invasive pests can be managed with the help of parasitic wasps in a long-lasting and environmentally responsible manner. The parasitic wasp and the host engage in interaction, and the parasitic aspect of the parasitoid engages the immune system of the host. The focus of most research has always been on the aforementioned biological phenomena and mechanisms [
32,
33,
34,
35].
This study compared the interactions between parasitoid-adaptive host and parasitoid-non-adaptive host from the perspectives of biological phenomenon and the function of immune hemocytes in order to better understand the developmental and immune interactions between parasitoids and their hosts. A. hispinarum, its adaptive host B. longissima, and its non-adaptive host O. nipae were used as research objects.
2. Materials and Methods
2.1. Experimental Insects
B. longissima and O. nipae samples were obtained in July 2017 from the diseased Phoenix canariensis Hort.ex Chabaud host tree in Xiamen City (Fujian Province) (24.52° N, 118.18° E) and were introduced alive with a natural food source into the laboratory (fresh leaves of P. canariensis). The F4 laboratory generations were used for the experiment. The Chinese Academy of Tropical Agricultural Sciences provided the A. hispinarum. All insects were kept at (25 ± 1) °C, (70 ± 5)% RH, and 12 h:12 h photoperiod (light:dark).
2.2. A. hispinarum Parasite Selection on B. longissima and O. nipae Larvae
2.2.1. Non-Selective Parasitism
In a plastic box measuring 15 cm long, 6 cm wide, and 4.5 cm high, 30 B. longissima larvae in their fourth instar were chosen and fed on fresh P. canariensis leaves. After 0.5 days of rearing, the larvae were parasitized with 30 mated one-day-old females of A. hispinarum, and the parasitic wasps were fed cotton dipped in 10% sucrose in plastic boxes. After 24 h, the parasitized larvae were dissected. The average parasitism rate and fecundity per female were obtained by counting the number of parasitized larvae and eggs per larva. The experiment was repeated in 40 groups, but only 10 groups had the A. hispinarum eggs counted.
O. nipae used the same experimental procedure as B. longissima.
2.2.2. Selective Parasitism
In the same plastic box, thirty 4th instar larvae of B. longissima and thirty 4th instar larvae of O. nipae were chosen and fed on fresh P. canariensis leaves, respectively. Sixty mated one-day-old female A. hispinarum were introduced to the parasitoid wasps after 0.5 days of upbringing, and the parasitic wasps were fed cotton dipped in 10% sucrose in plastic containers. After 24 h, the parasitized larvae were dissected. The average parasitism rate and fecundity per female were obtained by counting the number of parasitized larvae and eggs per larva. The experiment was repeated in 40 groups, but only 10 groups had the A. hispinarum eggs counted.
2.3. Developmental Interaction of A. hispinarum with B. longissima and O. nipae Larvae
2.3.1. The Development of A. hispinarum Eggs in the Body of B. longissima and O. nipae Larvae
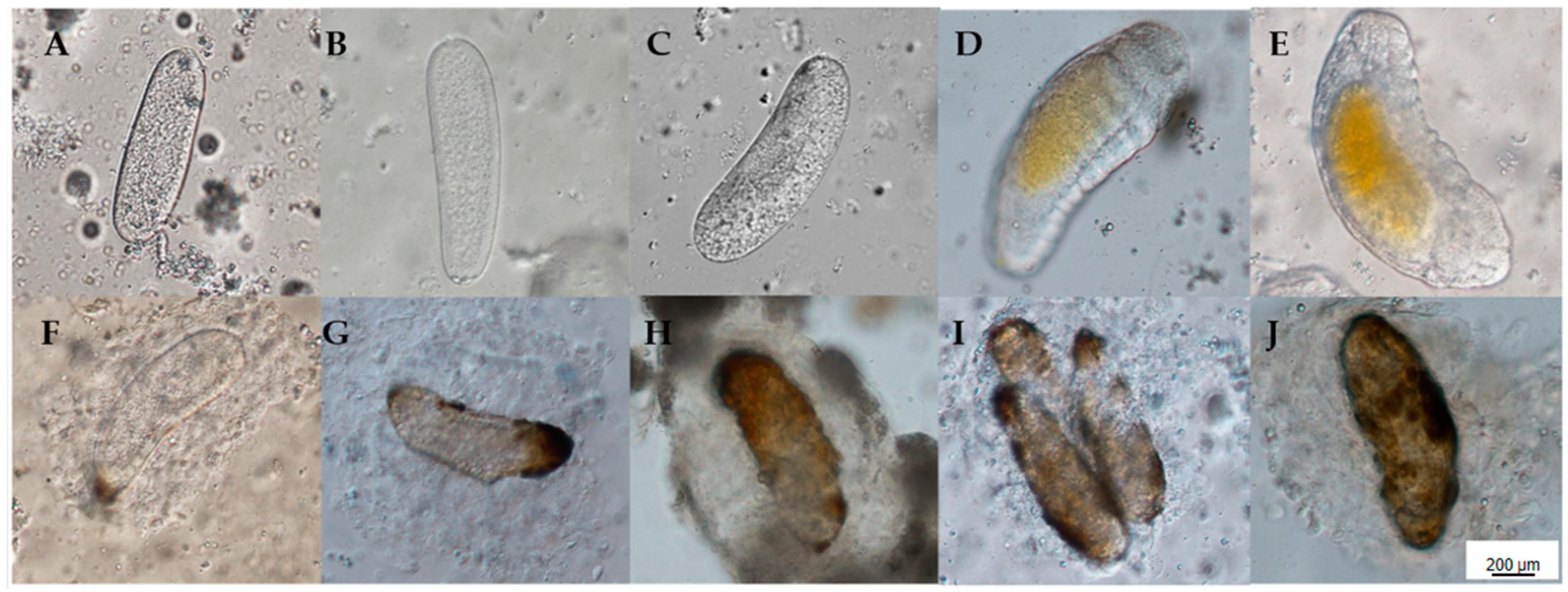
B. longissima larvae in their fourth instar were selected, placed in separate 9 cm petri dishes, and parasitized by fifteen A. hispinarum mating females in each dish. We dissected B. longissima larvae that were infected by A. hispinarum, using a differential interference microscope. We also observed their progress and captured images at 12, 24, 48, 72, and 96 h after the two insects lay their eggs. Each time point involved the repetition of three groups. The same experimental protocol as B. longissima was followed by O. nipae.
The eggs were stained with rhodamine phalloidin and DAPI and photographed using a differential interference microscope within 24 h. These are the precise steps: The eggs were transferred into a slide coated with poly-L-lysine after 10 μL of PBS was poured upon it. Remove the liquid, absorb 10 μL of PBS rinse, repeat three times, add 10 μL of 4% paraformaldehyde, and then place the wet box in a fixed-temperature environment for 15 min. Rinse three times with 10 μL of PBS, then add 10 μL of 0.1% Triton X-100 and incubate for five minutes in a moist box. Following three rinses with 10 μL of PBS, 10 μL of 1% BSA was added to the wet box for one hour. After three rinses with 10 μL PBS, 10 μL Rhodamine phalloidin and 5 μL (1 μg/μL) DAPI were added and stained in a wet box for 45 min. Rinse three times with 10 μL PBS, then add another 10 μL PBS, cover with a coverslip, and photograph with a differential interference microscope.
2.3.2. Effects of A. hispinarum Parasitism on B. longissima and O. nipae Larvae Development
B. longissima and O. nipae larvae that were parasitized by A. hispinarum were observed and captured under a stereo microscope; 30 B. longissima 4th instar larvae and 30 O. nipae 4th instar larvae were chosen and placed in separate 9 cm petri dishes. One-day-old, mated female A. hispinarum parasitized each larva one at a time. A. hispinarum was removed 24 h later. The parasitized larvae were constantly fed until they either pupated or became stiff. To count the number of larval deaths, B. longissima and O. nipae fourth instar larvae that were not parasitized throughout the same period were utilized as controls. The experiment was carried out in 30 groups. Ten fourth-instar O. nipae larvae were chosen, and 30 mated female A. hispinarum were inserted to perform one-for-one parasitism. The larvae were used as a control, and the pupal and fourth instar larvae of the palmar anise were counted. The experiment was carried out in thirty groups.
2.4. Comparison of the Encapsulation Rates of A. hispinarum Eggs with B. longissima and O. nipae Larvae
Thirty B. longissima 4th instar larvae and thirty O. nipae 4th instar larvae were dissected, and the total number of eggs in each larva and the number of eggs encapsulated were counted.
2.5. The Effect of A. hispinarum Parasitism on the Number and Proportion of B. longissima and O. nipae Larvae Hemocytes
The hemolymph of B. longissima fourth instar larvae was collected at 12, 24, 48, 72, and 96 h after parasitization, and the hemolymph of non-parasitic B. longissima larvae was used as a control at each time. The hemocytes were counted with a blood cell counter (25 × 16) under an optical microscope, including the number of cells and blood cell types; this was repeated five times. At each time point, 30 larvae were taken as replicates, and the experimental method was the same for O. nipae larvae.
2.6. Satistical Analysis
The mortality of of B. longissima and O. nipae larvae under non-parasitization and parasitization, the selective parasitism rate of A. hispinarum to B. longissima and O. nipae larvae, and the egg encapsulation rate of these two hosts after parasitization were analyzed using the Chi-square test. The fecundity of B. longissima and O. nipae larvae after parasitization, the duration of the larvae’s fourth instar and pupal stage, the hemocyte count, and the proportion of different hemocyte types under parasitization and non-parasitization were compared using an independent sample t-test. The changes in the proportion of hemocyte types at different time points post-parasitization and non-parasitization were analyzed using ANOVA. All statistical analysis mentioned above were performed on SPSS 21, and the graphs were drawn using GraphPad Prism 7.
4. Discussion
In nature, parasitoid wasps and hosts have a balanced struggle and restraint interaction [
35,
36]. Researchers from all around the world have been investigating the immunological and developmental interactions between parasitoids and hosts in recent decades [
37,
38,
39,
40,
41]. Depending on whether the parasitoid can develop and enclose successfully in the host, the parasitoid’s host can be classified as adaptive or non-adaptive [
42]. In contrast to non-adaptive hosts, in which the host is unable to overcome the host’s immune system, adaptable hosts allow the host to suppress or avoid the immune system. During the parasitic process, the host must first contend with cellular immunity, with the host hemocytes’ envelope reaction serving as the initial hurdle [
16,
18].
Studies have been conducted on the selection of
A. hispinarum as a parasite, the development interaction between
A. hispinarum and the hosts
B. longissima and
O. nipae, and the hemocyte response of these hosts. The findings demonstrated that
A. hispinarum exhibited no preference for
B. longissima or
O. nipae parasitic selection, but that it preferred to deposit more eggs in
B. longissima larvae. This might be connected to how big
B. longissima and
O. nipae are physically. The body size of the
B. longissima is larger than that of the
O. nipae in all life stages, including larvae, pupa, and adults. During the parasitism process, parasitic wasps typically choose the most suitable host in which to lay eggs and tend to lay more eggs into the most suitable hosts [
43]. Host density, body size, nutritional status, and developmental stage are the main influencing factors [
44,
45,
46,
47].
However, the eggs laid by
A. hispinarum into its adaptive host
B. longissima larvae could develop normally, whereas the eggs laid into its non-adaptive host
O. nipae larvae were encapsulated to death and could not develop normally. In fact, in the
O. nipae larvae, there are some
A. hispinarum eggs that can develop into
A. hispinarum, but this phenomenon is extremely rare; the current experiment found only one case. Non-adaptive hosts will still be affected by this process, even though parasitic wasps cannot successfully parasitize them. The parasitism of
A. hispinarum in this experiment can cause a death rate of 15.31% of the
O. nipae larvae and extend the larval stage by 5 days and the pupal stage by 1 day.
O. nipae damages the young leaves of palm trees by feeding adults and larvae [
48]. Field chemical control often uses beta-cypermethrin, imidacloprid, and other drugs to spray leaves [
49,
50]. Thus, the extension of the larval stage may allow the insect to absorb the drug more fully, thereby achieving a good control effect. Additionally, we discovered through ongoing field research that though
B. longissima and
O. nipae can coexist in one area, they rarely feed on the same plants. As a result, utilizing
A. hispinarum to regulate
B. longissima can have some negative consequences on
O. nipae.
The encapsulation response of the host is the first thing the
A. hispinarum eggs encounter after being deposited into the host. In this experiment, hemolymph from
B. longissima larvae could only encapsulate 1.14% of
A. hispinarum eggs, but hemolymph from
O. nipae larvae could contain 99.05% of
A. hispinarum eggs. Studies have shown a strong correlation between the overall number of host hemocytes and the number of differential hemocytes and the success of parasitic wasps [
51]. Statistics show that
O. nipae larvae had 6.08 times as many total hemocytes than
B. longissima larvae. The amount of circulating hemocytes in the host hemolymph may have a significant role in controlling the capacity to trigger cyst response [
52].
The immune system of the host is also suppressed or avoided by parasitic wasps, ensuring the normal development of the offspring [
22,
29,
30]. The hemocytes in the
B. longissima larvae did not react significantly to the attack by
A. hispinarum, and only a small increase in the total number of hemocytes and an increase in the proportion of granulocytes and oenocytoids were visible in the early stages of the parasitization. This demonstrates that the cell’s immune system can act quickly, but it may be slowed down by
A. hispinarum’s parasitic elements or rendered ineffective by the parasite’s eggs, which prevent the hemocytes from encasing the foreign substance. However, after
O. nipae larvae were attacked by
A. hispinarum, the total number of hemocytes grew, and the proportion of plasma and granular hemocytes increased, which formed the basis for the success of
O. nipae larvae’s cellular immunity. According to research, the capacity of hemocytes is positively correlated with the number of circulating hemocytes in
Drosophila melanogaster [
20]. Plasmatocytes and granulocytes are key players in the encapsulation reaction, and oenocytoid cells can produce phenoloxidase to help blacken and kill bee eggs. Additionally, the original hemocytes can change into different types of hemocytes. Since the likelihood of
A. hispinarum eggs being encapsulated by the two hosts differ significantly, it may be concluded that
A. hispinarum eggs are capable of passive escape, which can only happen in
B. longissima larvae.