Intraoperative Catastrophes during Robotic Lung Resection: A Single-Center Experience and Review of the Literature
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection, Criteria, and Data Collection
2.2. Surgical Technique
2.3. Statistics
3. Results
3.1. Patient Characteristics and Outcomes
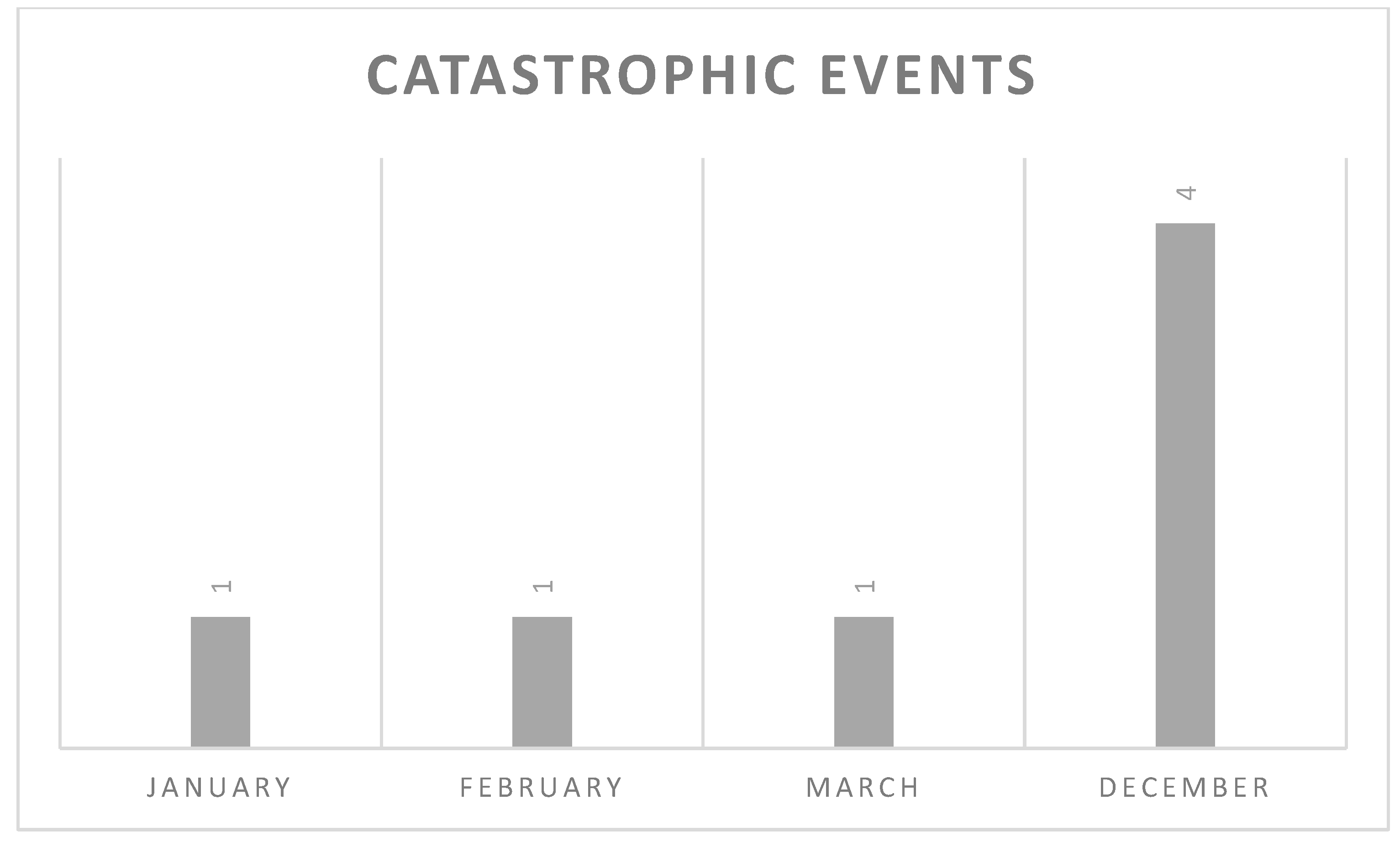
3.2. Catastrophic Events and Their Management
- Planned and executed procedure: left upper lobectomy. An accidental rupture of an anterior branch of the left upper lobe artery during its dissection with fenestrated bipolar forceps was dealt with endoscopic stapler positioning;
- Planned and executed procedure: left upper lobectomy. Principal pulmonary artery bleeding during lymph nodal dissection required undocking and posterolateral thoracotomy in attempt to control the bleeding with hemostatic, followed by tangential resection and suture of the artery;
- Planned and executed procedure: right upper lobectomy. An accidental rupture of the small branch of the right upper lobe artery at its origin during its section with the robotic stapler took place with consequent conversion to open surgery and control of the bleeding with hemostatic;
- Planned and executed procedure: left upper lobectomy. The tumor, in this case, was in hilar position, strongly adherent to the origin of the superior lobar bronchus. Left lower lobe artery bleeding during bronchotomy required undocking, posterolateral thoracotomy, and suture of the artery;
- Planned and executed procedure: right lower lobectomy. Bleeding due to the accidental rupture of the right lower lobe artery during its dissection was solved by endoscopic stapler positioning;
- Planned and executed procedure: right lower lobectomy. An accidental lesion of the thoracic aorta with fenestrated bipolar forceps during mobilization of the lung required undocking, posterolateral thoracotomy, suture, and reinforcement with a pledget;
- Planned and executed procedure: left upper lobectomy. Accidental rupture of the dorsal branch of the left upper lobe artery during its dissection required undocking, posterolateral thoracotomy, suture, and section of the artery.
4. Discussion
- The presence of a rolled gauze nearby during the hilar dissection available to perform immediate compression;
- The application of compression on the injury site for a few minutes, the subsequent verification that hemostasis had been obtained, and the use of the waiting time to plan the strategy to solve the disaster;
- The leadership of the surgeon at the console and clear communication with the equipe at the table was paramount to optimize the management of critical situations;
- The close collaboration between the surgical and the anesthesia team, which was fundamental in immediately managing the catastrophe from the hemodynamic point of view;
- We found that in case of conversion to open surgery, it is advisable to keep a robotic grasper to compress for hemostasis control while the team at the table performs the undocking and prepares the instruments for the thoracotomy. It is also useful to practice undocking to reduce the time needed;
- In case of conversion to thoracotomy, it is important to be able to alert an experienced surgeon, if not already present in the operating room; an expert colleague can certainly help to act rationally and without panic;
- In conclusion, it is important to perform a debriefing with the surgical team after each procedure marked by a catastrophic event to identify critical issues and encourage ideas to improve the management of these events thus reducing their incidence.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Melfi, F.M.; Menconi, G.F.; Mariani, A.M.; Angeletti, C.A. Early experience with robotic technology for thoracoscopic surgery. Eur. J. Cardio-Thorac. Surg. 2002, 21, 864–868. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Manganas, C.; Ang, S.C.; Yan, T.D. A systematic review and meta-analysis on pulmonary resections by robotic video-assisted thoracic surgery. Ann. Cardiothorac. Surg. 2012, 1, 3–10. [Google Scholar] [CrossRef]
- Park, B.J.; Melfi, F.; Mussi, A.; Maisonneuve, P.; Spaggiari, L.; Da Silva, R.K.C.; Veronesi, G. Robotic lobectomy for non–small cell lung cancer (NSCLC): Long-term oncologic results. J. Thorac. Cardiovasc. Surg. 2012, 143, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Zirafa, C.C.; Romano, G.; Key, T.H.; Davini, F.; Melfi, F. The evolution of robotic thoracic surgery. Ann. Cardiothorac. Surg. 2019, 8, 210–217. [Google Scholar] [CrossRef]
- Cerfolio, R.; Ghanim, A.F.; Dylewski, M.; Veronesi, G.; Spaggiari, L.; Park, B.J. The long-term survival of robotic lobectomy for non–small cell lung cancer: A multi-institutional study. J. Thorac. Cardiovasc. Surg. 2018, 155, 778–786. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Liang, W.; Zhao, L.; Chen, D.; Zhang, J.; Zhang, Y.; Tang, S.; He, J. Robotic Versus Video-assisted Lobectomy/Segmentectomy for Lung Cancer: A Meta-analysis. Ann Surg. 2018, 268, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Chen, Y.; Dai, J.; Zhu, X.; Gonzalez-Rivas, D.; Jiang, G.; Li, H.; Zhang, P. Perioperative outcomes of robot-assisted vs. video-assisted and traditional open thoracic surgery for lung cancer: A systematic review and network meta-analysis. Int. J. Med. Robot. Comput. Assist. Surg. 2020, 16, 1–14. [Google Scholar] [CrossRef]
- Zheng, L.; Song, P.; Jiang, Y.; Fan, X.; Yang, C.; Zhang, L.; Wang, Q. Outcomes and quality of life after Robot-assisted lobectomy/segmentectomy for lung cancer compared to video-assisted thoracoscopic surgery: Both three-port procedures performed by a single surgeon. J. Thorac. Dis. 2022, 14, 689–698. [Google Scholar] [CrossRef]
- Flores, R.M.; Ihekweazu, U.; Dycoco, J.; Rizk, N.P.; Rusch, V.W.; Bains, M.S.; Downey, R.J.; Finley, D.; Adusumilli, P.; Sarkaria, I.; et al. Video-assisted thoracoscopic surgery (VATS) lobectomy: Catastrophic intraoperative complications. J. Thorac. Cardiovasc. Surg. 2011, 142, 1412–1417. [Google Scholar] [CrossRef]
- Decaluwe, H.; Petersen, R.H.; Hansen, H.; Piwkowski, C.; Augustin, F.; Brunelli, A.; Schmid, T.; Papagiannopoulos, K.; Moons, J.; Gossot, D. Major intraoperative complications during video-assisted thoracoscopic anatomical lung resections: An intention-to-treat analysis. Eur. J. Cardio-Thorac. Surg. 2015, 48, 588–598. [Google Scholar] [CrossRef]
- Dunning, J.; Walker, W.S. Pulmonary artery bleeding caused during VATS lobectomy. Ann. Cardiothorac. Surg. 2012, 1, 109–110. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Rivas, D.; Stupnik, T.; Fernandez, R.; de la Torre, M.; Velasco, C.; Yang, Y.; Lee, W.; Jiang, G. Intraoperative bleeding control by uniportal video-assisted thoracoscopic surgery. Eur. J. Cardio-Thorac. Surg. 2016, 49, i17–i24. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Cerfolio, R.J.; Louie, B.E.; Melfi, F.; Veronesi, G.; Razzak, R.; Romano, G.; Novellis, P.; Shah, S.; Ranganath, N.; et al. Incidence, Management, and Outcomes of Intraoperative Catastrophes During Robotic Pulmonary Resection. Ann. Thorac. Surg. 2019, 108, 1498–1504. [Google Scholar] [CrossRef]
- Cerfolio, R.J.; Bess, K.M.; Wei, B.; Minnich, D.J. Incidence, Results, and Our Current Intraoperative Technique to Control Major Vascular Injuries During Minimally Invasive Robotic Thoracic Surgery. Ann. Thorac. Surg. 2016, 102, 394–399. [Google Scholar] [CrossRef]
- Louie, B.E. Catastrophes and complicated intraoperative events during robotic lung resection. J. Vis. Surg. 2017, 3, 52. [Google Scholar] [CrossRef]
- Cao, C.; Manganas, C.; Ang, S.C.; Peeceeyen, S.; Yan, T.D. Video-assisted thoracic surgery versus open thoracotomy for non-small cell lung cancer: A meta-analysis of propensity score-matched patients. Interact. Cardiovasc. Thorac. Surg. 2013, 16, 244–249. [Google Scholar] [CrossRef]
- Toker, A.; Özyurtkan, M.O.; Kaba, E.; Ayalp, K.; Demirhan, Ö.; Uyumaz, E. Robotic anatomic lung resections: The initial experience and description of learning in 102 cases. Surg. Endosc. 2016, 30, 676–683. [Google Scholar] [CrossRef]
- Adams, R.D.; Bolton, W.D.; Stephenson, J.E.; Henry, G.; Robbins, E.T.; Sommers, E. Initial Multicenter Community Robotic Lobectomy Experience: Comparisons to a National Database. Ann. Thorac. Surg. 2014, 97, 1893–1898. [Google Scholar] [CrossRef]
- Dylewski, M.R.; Ohaeto, A.C.; Pereira, J.F. Pulmonary Resection Using a Total Endoscopic Robotic Video-Assisted Approach. Semin. Thorac. Cardiovasc. Surg. 2011, 23, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.-X.; Woo, K.M.; Sima, C.S.; Bains, M.S.; Adusumilli, P.S.; Huang, J.; Finley, D.J.; Rizk, N.P.; Rusch, V.; Jones, D.R.; et al. Long-term Survival Based on the Surgical Approach to Lobectomy for Clinical Stage I Nonsmall Cell Lung Cancer: Comparison of Robotic, Video-assisted Thoracic Surgery, and Thoracotomy Lobectomy. Ann. Surg. 2017, 265, 431–437. [Google Scholar] [CrossRef]
- Mei, J.; Pu, Q.; Liao, H.; Ma, L.; Zhu, Y.; Liu, L. A novel method for troubleshooting vascular injury during anatomic thoracoscopic pulmonary resection without conversion to thoracotomy. Surg. Endosc. 2013, 27, 530–537. [Google Scholar] [CrossRef]
- Xiao, Z.-L.; Mei, J.-D.; Pu, Q.; Liu, L.-X. Technical strategy for dealing with bleeding during thoracoscopic lung surgery. Ann. Cardiothorac. Surg. 2014, 3, 213–215. [Google Scholar] [CrossRef] [PubMed]
- Sakakura, N.; Nakada, T.; Shirai, S.; Takahara, H.; Suzuki, A.; Takahashi, Y.; Kuroda, H. Emergency rollout and conversion procedures during the three-arm robotic open-thoracotomy-view approach. Interact. Cardiovasc. Thorac. Surg. 2022, 34, 1045–1051. [Google Scholar] [CrossRef] [PubMed]
- Sarsam, M.; Mordojovich, G.; Bottet, B.; Baste, J.M. Robotic development: ‘Patients’ safety always comes first’. Interact. Cardiovasc. Thorac. Surg. 2022, 34, 1052–1053. [Google Scholar] [CrossRef] [PubMed]
| All Patients (n = 486) | Catastrophic Event (n = 7) | No Catastrophic Event (N = 479) | |
|---|---|---|---|
| Age (years) | 68 (32–86) | 72 (65–82) | 68 (32–86) |
| Male | 279 (57%) | 4 (57%) | 275 (57%) |
| History of smoking | 393 (81%) | 5 (71%) | 388 (81%) |
| ASA class ≥ 2 | 462 (95%) | 7 (100%) | 455 (95%) |
| FEV1 predicted | 96 (42–188) | 88 (65–107) | 96 (42–188) |
| FEV1/FVC predicted | 93 (45–140) | 91 (76–109) | 93 (45–140) |
| BMI | 26 (16–44) | 25 (17–34) | 26 (16–44) |
| Pathological stage | |||
| I | 278 (57%) | 3 (43%) | 277 (58%) |
| II | 109 (22%) | 1 (14%) | 108 (23%) |
| III | 59 (12%) | 2 (29%) | 57 (12%) |
| IV | 2 (0.4%) | 2 (0.4%) | |
| Histopathology | |||
| Adenocarcinoma | 314 (65%) | 3 (43%) | 311 (65%) |
| Squamous carcinoma | 77 (16%) | 2 (29%) | 75 (16%) |
| Adenosquamous | 4 (1%) | 1 (14%) | 3 (1%) |
| Neuroendocrine tumor | 53 (11%) | 53 (11%) | |
| Others | 38 (8%) | 1 (14%) | 37 (7%) |
| All Patients (n = 486) | Catastrophic Event (n = 7) | No Catastrophic Event (N = 479) | |
|---|---|---|---|
| Procedure | |||
| RUL lobectomy | 154 (32%) | 1 (14%) | 153 (32%) |
| RML lobectomy | 36 (7%) | 36 (7%) | |
| RLL lobectomy | 81 (16%) | 2 (29%) | 79 (16%) |
| LUL lobectomy | 79 (16%) | 4 (57%) | 75 (16%) |
| LLL lobectomy | 70 (15%) | 70 (15%) | |
| Segmentectomy | 59 (12%) | 59 (12%) | |
| Bilobectomy | 4 (1%) | 4 (1%) | |
| Pneumonectomy | 3 (1%) | 3 (1%) | |
| Surgical time (min) | 232 (75–615) | 322 (175–530) | 230 (75–615) |
| Hospital stay mean (days) | 6 (2–28) | 7 (4–19) | 6 (2–28) |
| Postoperative complications | 144 (30%) | 4 (57%) | 140 (29%) |
| Prolonged air leak | 84 (17%) | 2 (29%) | 82 (17%) |
| Respiratory failure | 8 (2%) | 1 (14%) | 7 (1%) |
| Atrial fibrillation | 19 (4%) | 1 (14%) | 18 (4%) |
| Anemization with blood transfusion | 26 (5%) | 26 (5%) | |
| Pneumothorax | 21 (4%) | 21 (4%) | |
| Others | 8 (2%) | 1 (14%) | 7 (1%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manfredini, B.; Zirafa, C.C.; Romano, G.; Bagalà, E.; Cariello, C.; Davini, F.; Melfi, F. Intraoperative Catastrophes during Robotic Lung Resection: A Single-Center Experience and Review of the Literature. Life 2023, 13, 215. https://doi.org/10.3390/life13010215
Manfredini B, Zirafa CC, Romano G, Bagalà E, Cariello C, Davini F, Melfi F. Intraoperative Catastrophes during Robotic Lung Resection: A Single-Center Experience and Review of the Literature. Life. 2023; 13(1):215. https://doi.org/10.3390/life13010215
Chicago/Turabian StyleManfredini, Beatrice, Carmelina Cristina Zirafa, Gaetano Romano, Elena Bagalà, Claudia Cariello, Federico Davini, and Franca Melfi. 2023. "Intraoperative Catastrophes during Robotic Lung Resection: A Single-Center Experience and Review of the Literature" Life 13, no. 1: 215. https://doi.org/10.3390/life13010215
APA StyleManfredini, B., Zirafa, C. C., Romano, G., Bagalà, E., Cariello, C., Davini, F., & Melfi, F. (2023). Intraoperative Catastrophes during Robotic Lung Resection: A Single-Center Experience and Review of the Literature. Life, 13(1), 215. https://doi.org/10.3390/life13010215





