Associations of Peak-Width Skeletonized Mean Diffusivity and Post-Stroke Cognition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Clinical and Cognitive Assessments
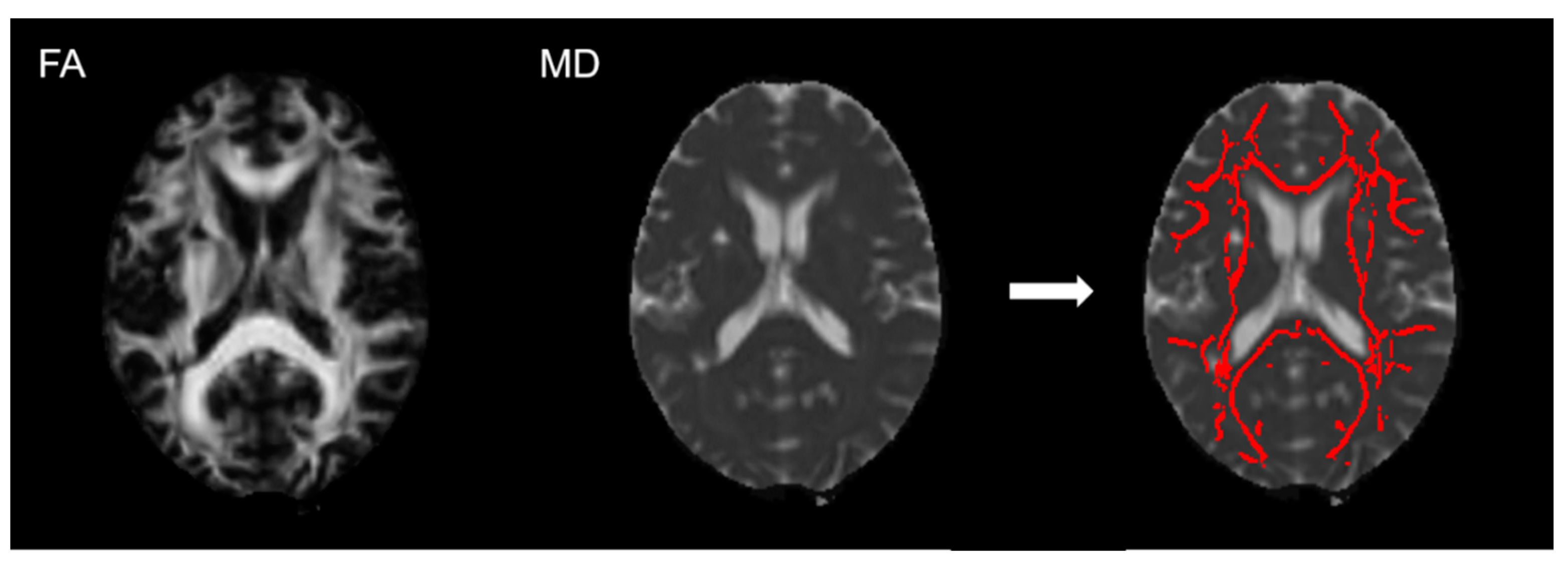
2.3. Image Acquisition
2.4. Imaging Analysis
2.5. Statistical Analysis
3. Results
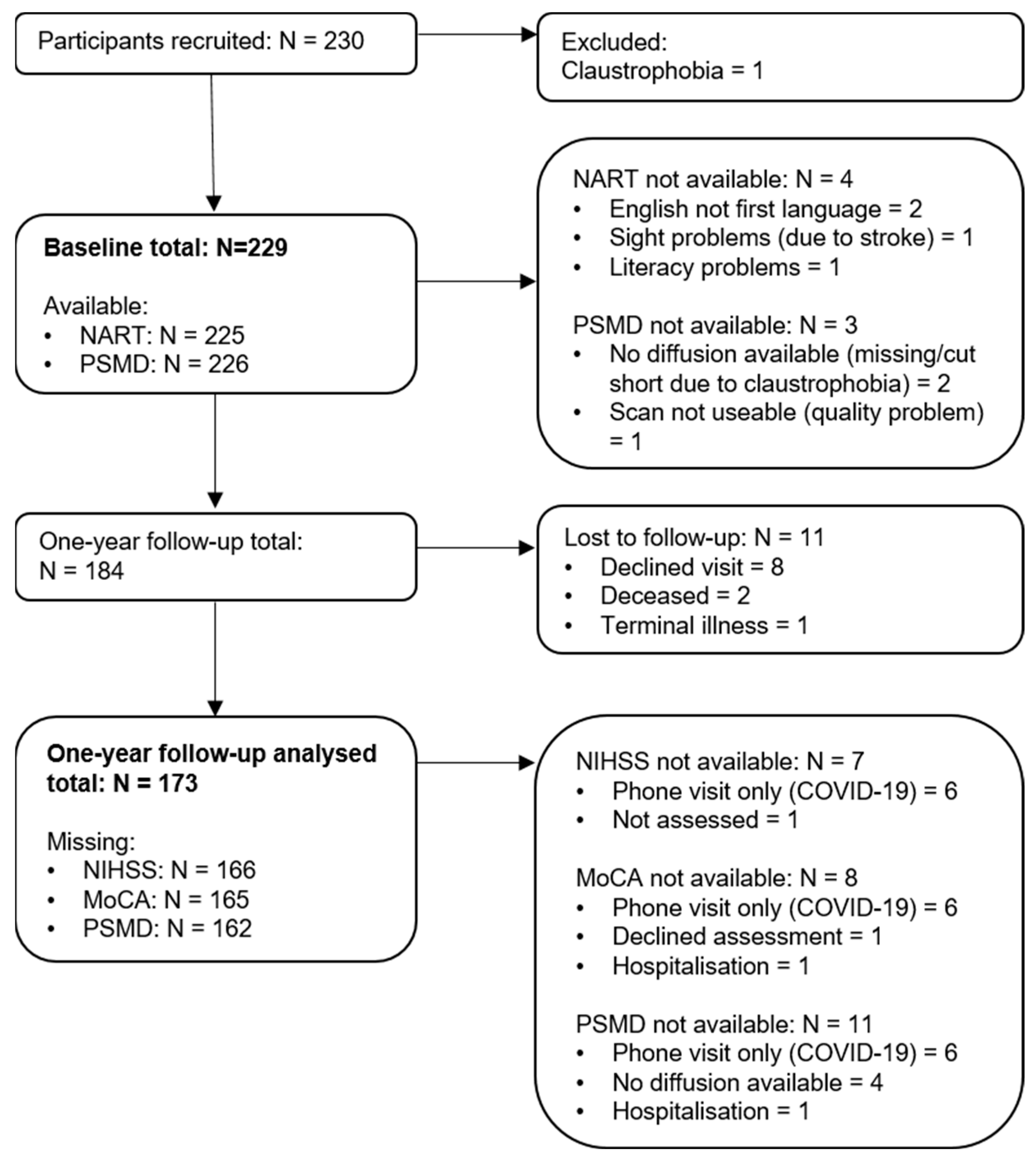
3.1. Participants
3.2. Cross-Sectional Associations between PSMD and Global Cognition at Baseline and 1-Year Visit
3.3. Longitudinal Analysis of Baseline PSMD and 1-Year Global Cognition
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jokinen, H.; Melkas, S.; Ylikoski, R.; Pohjasvaara, T.; Kaste, M.; Erkinjuntti, T.; Hietanen, M. Post-stroke cognitive impairment is common even after successful clinical recovery. Eur. J. Neurol. 2015, 22, 1288–1294. [Google Scholar] [CrossRef] [PubMed]
- Arba, F.; Quinn, T.; Hankey, G.J.; Inzitari, D.; Ali, M.; Lees, K.R.; Collaboration, T.V. Determinants of post-stroke cognitive impairment: Analysis from VISTA. Acta Neurol. Scand. 2017, 135, 603–607. [Google Scholar] [CrossRef]
- Crichton, S.L.; Bray, B.D.; McKevitt, C.; Rudd, A.G.; Wolfe, C.D.A. Patient outcomes up to 15 years after stroke: Survival, disability, quality of life, cognition and mental health. J. Neurol. Neurosurg. Psychiatry 2016, 87, 1091–1098. [Google Scholar] [CrossRef]
- McHutchison, C.A.; Cvoro, V.; Makin, S.; Chappell, F.M.; Shuler, K.; Wardlaw, J.M. Functional, cognitive and physical outcomes 3 years after minor lacunar or cortical ischaemic stroke. J. Neurol. Neurosurg. Psychiatry 2019, 90, 436–443. [Google Scholar] [CrossRef]
- Ezekiel, L.; Collett, J.; Mayo, N.E.; Pang, L.; Field, L.; Dawes, H. Factors Associated With Participation in Life Situations for Adults With Stroke: A Systematic Review. Arch. Phys. Med. Rehabil. 2019, 100, 945–955. [Google Scholar] [CrossRef] [PubMed]
- Della Vecchia, C.; Viprey, M.; Haesebaert, J.; Termoz, A.; Giroudon, C.; Dima, A.; Rode, G.; Préau, M.; Schott, A.-M. Contextual determinants of participation after stroke: A systematic review of quantitative and qualitative studies. Disabil. Rehabil. 2021, 43, 1786–1798. [Google Scholar] [CrossRef] [PubMed]
- Adamson, J.; Beswick, A.; Ebrahim, S. Is stroke the most common cause of disability? J. Stroke Cerebrovasc. Dis. 2004, 13, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Selves, C.; Stoquart, G.; Lejeune, T. Gait rehabilitation after stroke: Review of the evidence of predictors, clinical outcomes and timing for interventions. Acta Neurol. Belg. 2020, 120, 783–790. [Google Scholar] [CrossRef]
- Stinear, C.M. Prediction of motor recovery after stroke: Advances in biomarkers. Lancet Neurol. 2017, 16, 826–836. [Google Scholar] [CrossRef]
- Brainin, M.; Tuomilehto, J.; Heiss, W.-D.; Bornstein, N.M.; Bath, P.M.W.; Teuschl, Y.; Richard, E.; Guekht, A.; Quinn, T.; the Post Stroke Cognition Study Group. Post-stroke cognitive decline: An update and perspectives for clinical research. Eur. J. Neurol. 2015, 22, e216–e229. [Google Scholar] [CrossRef]
- Kalaria, R.N.; Akinyemi, R.; Ihara, M. Stroke injury, cognitive impairment and vascular dementia. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2016, 1862, 915–925. [Google Scholar] [CrossRef] [PubMed]
- Baykara, E.; Gesierich, B.; Adam, R.; Tuladhar, A.M.; Biesbroek, J.M.; Koek, H.L.; Ropele, S.; Jouvent, E.; Initiative, A.s.D.N.; Chabriat, H.; et al. A Novel Imaging Marker for Small Vessel Disease Based on Skeletonization of White Matter Tracts and Diffusion Histograms. Ann. Neurol. 2016, 80, 581–592. [Google Scholar] [CrossRef]
- Tofts, P.S.; Davies, G.R.; Dehmeshki, J. Histograms: Measuring Subtle Diffuse Disease. In Quantitative MRI of the Brain; Wiley: Chichester, UK, 2003; pp. 581–610. [Google Scholar] [CrossRef]
- Debette, S.; Markus, H. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: Systematic review and meta-analysis. BMJ 2010, 341, c3666. [Google Scholar] [CrossRef] [PubMed]
- Debette, S.; Schilling, S.; Duperron, M.-G.; Larsson, S.C.; Markus, H.S. Clinical Significance of Magnetic Resonance Imaging Markers of Vascular Brain Injury: A Systematic Review and Meta-analysis. JAMA Neurol. 2019, 76, 81–94. [Google Scholar] [CrossRef]
- Hamilton, O.K.; Backhouse, E.V.; Janssen, E.; Jochems, A.C.; Maher, C.; Ritakari, T.E.; Stevenson, A.J.; Xia, L.; Deary, I.J.; Wardlaw, J.M. Cognitive impairment in sporadic cerebral small vessel disease: A systematic review and meta-analysis. Alzheimer’s Dement. 2021, 17, 665–685. [Google Scholar] [CrossRef] [PubMed]
- Beaudet, G.; Tsuchida, A.; Petit, L.; Tzourio, C.; Caspers, S.; Schreiber, J.; Pausova, Z.; Patel, Y.; Paus, T.; Schmidt, R.; et al. Age-Related Changes of Peak Width Skeletonized Mean Diffusivity (PSMD) Across the Adult Lifespan: A Multi-Cohort Study. Front. Psychiatry 2020, 11, 342. [Google Scholar] [CrossRef]
- Etherton, M.R.; Schirmer, M.D.; Zotin, M.C.Z.; Rist, P.M.; Boulouis, G.; Lauer, A.; Wu, O.; Rost, N.S. Global white matter structural integrity mediates the effect of age on ischemic stroke outcomes. Int. J. Stroke 2021, 17474930211055906. [Google Scholar] [CrossRef]
- Deary, I.J.; Ritchie, S.J.; Muñoz Maniega, S.; Cox, S.R.; Valdés Hernández, M.C.; Luciano, M.; Starr, J.M.; Wardlaw, J.M.; Bastin, M.E. Brain Peak Width of Skeletonized Mean Diffusivity (PSMD) and Cognitive Function in Later Life. Front. Psychiatry 2019, 10, 524. [Google Scholar] [CrossRef]
- Lam, B.Y.K.; Leung, K.T.; Yiu, B.; Zhao, L.; Biesbroek, J.M.; Au, L.; Tang, Y.; Wang, K.; Fan, Y.; Fu, J.-H.; et al. Peak width of skeletonized mean diffusivity and its association with age-related cognitive alterations and vascular risk factors. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2019, 11, 721–729. [Google Scholar] [CrossRef]
- Wei, N.; Deng, Y.; Yao, L.; Jia, W.; Wang, J.; Shi, Q.; Chen, H.; Pan, Y.; Yan, H.; Zhang, Y.; et al. A Neuroimaging Marker Based on Diffusion Tensor Imaging and Cognitive Impairment Due to Cerebral White Matter Lesions. Front. Neurol. 2019, 10, 81. [Google Scholar] [CrossRef]
- Oberlin, L.E.; Respino, M.; Victoria, L.; Abreu, L.; Hoptman, M.J.; Alexopoulos, G.S.; Gunning, F.M. Late-life depression accentuates cognitive weaknesses in older adults with small vessel disease. Neuropsychopharmacology 2021, 47, 580–587. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Zhu, H.; Yu, L.; Lu, P.; Qiu, Y.; Zhou, Y.; Cao, W.; Lu, D.; Zhao, W.; Yang, J. Multi-Dimensional Diffusion Tensor Imaging Biomarkers for Cognitive Decline From the Preclinical Stage: A Study of Post-stroke Small Vessel Disease. Front. Neurol. 2021, 12, 1151. [Google Scholar] [CrossRef] [PubMed]
- Low, A.; Mak, E.; Stefaniak, J.D.; Malpetti, M.; Nicastro, N.; Savulich, G.; Chouliaras, L.; Markus, H.S.; Rowe, J.B.; O’Brien, J.T. Peak Width of Skeletonized Mean Diffusivity as a Marker of Diffuse Cerebrovascular Damage. Front. Neurosci. 2020, 14, 238. [Google Scholar] [CrossRef]
- Clancy, U.; Makin, S.D.J.; McHutchison, C.A.; Cvoro, V.; Chappell, F.M.; Hernandez, M.; Sakka, E.; Doubal, F.; Wardlaw, J.M. Impact of Small Vessel Disease Progression on Long-term Cognitive and Functional Changes After Stroke. Neurology 2022, 98, e1459–e1469. [Google Scholar] [CrossRef] [PubMed]
- Clancy, U.; Jaime Garcia, D.; Stringer, M.S.; Thrippleton, M.J.; Valdés-Hernández, M.C.; Wiseman, S.; Hamilton, O.K.; Chappell, F.M.; Brown, R.; Blair, G.W. Rationale and design of a longitudinal study of cerebral small vessel diseases, clinical and imaging outcomes in patients presenting with mild ischaemic stroke: Mild Stroke Study 3. Eur. Stroke J. 2021, 6, 81–88. [Google Scholar] [CrossRef]
- Swieten, J.C.v.; Koudstaal, P.J.; Visser, M.C.; Schouten, H.J.; Gijn, J.v. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988, 19, 604–607. [Google Scholar] [CrossRef]
- Brott, T.; Adams, H.P.; Olinger, C.P.; Marle, J.R.; Barsan, W.G.; Biller, J.; Spilker, J.; Holleran, R.; Eberle, R.; Hertzberg, V.; et al. Measurements of acute cerebral infarction: A clinical examination scale. Stroke 1989, 20, 864–870. [Google Scholar] [CrossRef]
- Makin, S.D.; Doubal, F.N.; Shuler, K.; Chappell, F.M.; Staals, J.; Dennis, M.S.; Wardlaw, J.M. The impact of early-life intelligence quotient on post stroke cognitive impairment. Eur. Stroke J. 2018, 3, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Nelson, H.E.; Willison, J. National Adult Reading Test (NART); Nfer-Nelson Windsor: Windsor, UK, 1991. [Google Scholar]
- McHutchison, C.A.; Chappell, F.M.; Makin, S.; Shuler, K.; Wardlaw, J.M.; Cvoro, V. Stability of estimated premorbid cognitive ability over time after minor stroke and its relationship with post-stroke cognitive ability. Brain Sci. 2019, 9, 117. [Google Scholar] [CrossRef] [Green Version]
- McGurn, B.; Starr, J.M.; Topfer, J.A.; Pattie, A.; Whiteman, M.C.; Lemmon, H.A.; Whalley, L.J.; Deary, I.J. Pronunciation of irregular words is preserved in dementia, validating premorbid IQ estimation. Neurology 2004, 62, 1184–1186. [Google Scholar] [CrossRef]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A Brief Screening Tool For Mild Cognitive Impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Chertkow, H.; Nasreddine, Z.; Johns, E.; Phillips, N.; McHenry, C. The Montreal Cognitive Assessment (MoCA): Validation of alternate forms and new recommendations for education corrections. Alzheimer’s Dement. 2011, 7, S157. [Google Scholar] [CrossRef]
- Costa, A.S.; Fimm, B.; Friesen, P.; Soundjock, H.; Rottschy, C.; Gross, T.; Eitner, F.; Reich, A.; Schulz, J.B.; Nasreddine, Z.S.; et al. Alternate-Form Reliability of the Montreal Cognitive Assessment Screening Test in a Clinical Setting. Dement. Geriatr. Cogn. Disord. 2012, 33, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Clayden, J.D.; Maniega, S.M.; Storkey, A.J.; King, M.D.; Bastin, M.E.; Clark, C.A. TractoR: Magnetic resonance imaging and tractography with R. J. Stat. Softw. 2011, 44, 1–18. [Google Scholar] [CrossRef]
- Clayden, J.D. divest: Get Images Out of DICOM Format Quickly. 2017. Available online: https://CRAN.R-project.org/package=divest (accessed on 11 January 2022).
- Andersson, J.L.; Skare, S.; Ashburner, J. How to correct susceptibility distortions in spin-echo echo-planar images: Application to diffusion tensor imaging. NeuroImage 2003, 20, 870–888. [Google Scholar] [CrossRef]
- Andersson, J.L.; Sotiropoulos, S.N. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. NeuroImage 2016, 125, 1063–1078. [Google Scholar] [CrossRef]
- Smith, S.M.; Jenkinson, M.; Woolrich, M.W.; Beckmann, C.F.; Behrens, T.E.; Johansen-Berg, H.; Bannister, P.R.; De Luca, M.; Drobnjak, I.; Flitney, D.E. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage 2004, 23, S208–S219. [Google Scholar] [CrossRef]
- Smith, S.M. Fast robust automated brain extraction. Hum. Brain Mapp. 2002, 17, 143–155. [Google Scholar] [CrossRef]
- Salvador, R.; Peña, A.; Menon, D.K.; Carpenter, T.A.; Pickard, J.D.; Bullmore, E.T. Formal characterization and extension of the linearized diffusion tensor model. Hum. Brain Mapp. 2005, 24, 144–155. [Google Scholar] [CrossRef]
- R Core Team R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Viena, Austria, 2020.
- Jacquin, A.; Binquet, C.; Rouaud, O.; Graule-Petot, A.; Daubail, B.; Osseby, G.-V.; Bonithon-Kopp, C.; Giroud, M.; Béjot, Y. Post-Stroke Cognitive Impairment: High Prevalence and Determining Factors in a Cohort of Mild Stroke. J. Alzheimer’s Dis. 2014, 40, 1029–1038. [Google Scholar] [CrossRef]
- Patel, M.D.; Coshall, C.; Rudd, A.G.; Wolfe, C.D.A. Cognitive Impairment after Stroke: Clinical Determinants and Its Associations with Long-Term Stroke Outcomes. J. Am. Geriatr. Soc. 2002, 50, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Nys, G.M.S.; Van Zandvoort, M.J.E.; De Kort, P.L.M.; Jansen, B.P.W.; Van Der Worp, H.B.; Kappelle, L.J.; De Haan, E.H.F. Domain-specific cognitive recovery after first-ever stroke: A follow-up study of 111 cases. J. Int. Neuropsychol. Soc. 2005, 11, 795–806. [Google Scholar] [CrossRef] [PubMed]
- Sachdev, P.S.; Brodaty, H.; Valenzuela, M.J.; Lorentz, L.; Looi, J.C.L.; Berman, K.; Ross, A.; Wen, W.; Zagami, A.S. Clinical Determinants of Dementia and Mild Cognitive Impairment following Ischaemic Stroke: The Sydney Stroke Study. Dement. Geriatr. Cogn. Disord. 2006, 21, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Backhouse, E.V.; Shenkin, S.D.; McIntosh, A.M.; Bastin, M.E.; Whalley, H.C.; Valdez Hernandez, M.; Muñoz Maniega, S.; Harris, M.A.; Stolicyn, A.; Campbell, A.; et al. Early life predictors of late life cerebral small vessel disease in four prospective cohort studies. Brain 2021, 144, 3769–3778. [Google Scholar] [CrossRef]
- McHutchison, C.A.; Backhouse, E.V.; Cvoro, V.; Shenkin, S.D.; Wardlaw, J.M. Education, socioeconomic status, and intelligence in childhood and stroke risk in later life. Epidemiology 2017, 28, 608–618. [Google Scholar] [CrossRef] [PubMed]
- Penke, L.; Maniega, S.M.; Bastin, M.E.; Valdés Hernández, M.C.; Murray, C.; Royle, N.A.; Starr, J.M.; Wardlaw, J.M.; Deary, I.J. Brain white matter tract integrity as a neural foundation for general intelligence. Mol. Psychiatry 2012, 17, 1026–1030. [Google Scholar] [CrossRef]
- Sharma, R.; Mallick, D.; Llinas, R.H.; Marsh, E.B. Early Post-stroke Cognition: In-hospital Predictors and the Association With Functional Outcome. Front. Neurol. 2020, 11, 613607. [Google Scholar] [CrossRef]
- Taylor-Rowan, M.; Keir, R.; Cuthbertson, G.; Shaw, R.; Drozdowska, B.; Elliott, E.; Evans, J.; Stott, D.; Quinn, T.J. Pre-Stroke Frailty Is Independently Associated With Post-Stroke Cognition: A Cross-Sectional Study. J. Int. Neuropsychol. Soc. 2019, 25, 501–506. [Google Scholar] [CrossRef]
- Pendlebury, S.T.; Rothwell, P.M. Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: A systematic review and meta-analysis. Lancet Neurol. 2009, 8, 1006–1018. [Google Scholar] [CrossRef]
- Nys, G.M.S.; van Zandvoort, M.J.E.; de Kort, P.L.M.; Jansen, B.P.W.; de Haan, E.H.F.; Kappelle, L.J. Cognitive Disorders in Acute Stroke: Prevalence and Clinical Determinants. Cerebrovasc. Dis. 2007, 23, 408–416. [Google Scholar] [CrossRef]
- Phan, H.T.; Reeves, M.J.; Blizzard, C.L.; Thrift, A.G.; Cadilhac, D.A.; Sturm, J.; Otahal, P.; Rothwell, P.; Bejot, Y.; Cabral, N.L.; et al. Sex Differences in Severity of Stroke in the INSTRUCT Study: A Meta-Analysis of Individual Participant Data. J. Am. Heart Assoc. 2019, 8, e010235. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Sánchez, L.; Hamilton, O.K.L.; Clancy, U.; Backhouse, E.V.; Stewart, C.R.; Stringer, M.S.; Doubal, F.N.; Wardlaw, J.M. Sex Differences in Cerebral Small Vessel Disease: A Systematic Review and Meta-Analysis. Front. Neurol. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
| N | Baseline | N | 1 Year | ||
|---|---|---|---|---|---|
| Age, mean ± SD (range) | 229 | 65.9 ± 11.1 (32.7–86.3) | 173 | 67.5 ± 11.0 (33.7–87.4) | |
| Sex, female (%) | 229 | 77 (33.6) | 173 | 55 (31.8) | |
| Final diagnosis, lacunar stroke (%) * | 229 | 130 (56.8) | 173 | 94 (54.3) | |
| Days between stroke and baseline visit, median IQR (range) | 229 | 61, 43–76 (11–105) | |||
| Hypertension, yes (%) * | 229 | 157 (68.6) | 173 | 116 (67.1) | |
| Smoking, yes (%) * | 229 | 173 | |||
| Never | 108 (47.2) | 84 (48.6) | |||
| Ex, >1 year | 82 (35.8) | 62 (35.8) | |||
| Ex, <1 year | 12 (5.2) | 9 (5.2) | |||
| Current | 27 (11.8) | 18 (10.4) | |||
| Diabetes, yes (%) * | 229 | 50 (21.8) | 173 | 38 (22.0) | |
| Hypercholesterolaemia, yes (%) * | 229 | 171 (74.7) | 173 | 124 (71.7) | |
| Atrial fibrillation, yes (%) * | 229 | 21 (9.2) | 173 | 15 (8.7) | |
| NIHSS, median, IQR (range) | 229 | 1, 0–2 (0–7) | 166 | 1, 0–2 (0–14) | |
| mRS, median, IQR (range) | 229 | 1, 0–1 (0–2) | 173 | 1, 0–1 (0–5) | |
| MoCA, mean ± SD (range) | 229 | 24.3 ± 3.6 (11–30) | 165 | 25.0 ± 3.8 (10–30) | |
| NART correct, mean ± SD (range) * | 225 | 32.7 ± 9.6 (6–50) | 170 | 32.2 ± 9.7 (7–48) | |
| PSMD, mean ± SD (range), mm2/s × 10−4 | 226 | 0.238 ± 0.066 (0.141–0.595) | 162 | 0.254 ± 0.086 (0.137–0.766) | |
| Standardized β | Standardized 95% CI | p Value | |
|---|---|---|---|
| Age | −0.309 | −0.433, −0.185 | <0.001 |
| Sex, male | 0.031 | −0.197, 0.259 | 0.790 |
| NIHSS | −0.201 | −0.321, −0.082 | 0.001 |
| NART | 0.417 | 0.310, 0.525 | <0.001 |
| mRS | −0.037 | −0.152, 0.078 | 0.526 |
| PSMD | −0.116 | −0.241, 0.009 | 0.069 |
| Standardized β | Standardized 95% CI | p Value | |
|---|---|---|---|
| Age | −0.218 | −0.350, −0.085 | 0.001 |
| Sex, male * | 0.211 | −0.054, 0.477 | 0.117 |
| NIHSS | −0.220 | −0.357, −0.084 | 0.002 |
| NART * | 0.406 | 0.286, 0.526 | <0.001 |
| mRS | −0.004 | −0.142, 0.135 | 0.958 |
| PSMD | −0.301 | −0.434, −0.168 | <0.001 |
| Standardized β | Standardized 95% CI | p Value | |
|---|---|---|---|
| Age | −0.111 | −0.242, 0.020 | 0.095 |
| Sex, male | 0.008 | −0.232, 0.248 | 0.945 |
| NIHSS | −0.058 | −0.189, 0.072 | 0.379 |
| Baseline MoCA | 0.502 | 0.360, 0.644 | <0.001 |
| NART | 0.144 | 0.017, 0.271 | 0.026 |
| mRS | −0.064 | −0.187, 0.059 | 0.304 |
| PSMD | −0.182 | −0.308, −0.056 | 0.005 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jochems, A.C.C.; Muñoz Maniega, S.; Clancy, U.; Jaime Garcia, D.; Arteaga, C.; Hewins, W.; Penman, R.; Hamilton, O.K.L.; Czechoń, A.; Backhouse, E.V.; et al. Associations of Peak-Width Skeletonized Mean Diffusivity and Post-Stroke Cognition. Life 2022, 12, 1362. https://doi.org/10.3390/life12091362
Jochems ACC, Muñoz Maniega S, Clancy U, Jaime Garcia D, Arteaga C, Hewins W, Penman R, Hamilton OKL, Czechoń A, Backhouse EV, et al. Associations of Peak-Width Skeletonized Mean Diffusivity and Post-Stroke Cognition. Life. 2022; 12(9):1362. https://doi.org/10.3390/life12091362
Chicago/Turabian StyleJochems, Angela C. C., Susana Muñoz Maniega, Una Clancy, Daniela Jaime Garcia, Carmen Arteaga, Will Hewins, Rachel Penman, Olivia K. L. Hamilton, Agnieszka Czechoń, Ellen V. Backhouse, and et al. 2022. "Associations of Peak-Width Skeletonized Mean Diffusivity and Post-Stroke Cognition" Life 12, no. 9: 1362. https://doi.org/10.3390/life12091362
APA StyleJochems, A. C. C., Muñoz Maniega, S., Clancy, U., Jaime Garcia, D., Arteaga, C., Hewins, W., Penman, R., Hamilton, O. K. L., Czechoń, A., Backhouse, E. V., Thrippleton, M. J., Stringer, M. S., Bastin, M. E., Valdés Hernández, M. d. C., Wiseman, S., Chappell, F. M., Doubal, F. N., & Wardlaw, J. M. (2022). Associations of Peak-Width Skeletonized Mean Diffusivity and Post-Stroke Cognition. Life, 12(9), 1362. https://doi.org/10.3390/life12091362






