Abstract
Mounting concern over the misuse of chemical pesticides has sparked broad interest for safe and effective alternatives to control plant pests and pathogens. Xenorhabdus bacteria, as pesticidal symbionts of the entomopathogenic nematodes Steinernema species, can contribute to this solution with a treasure trove of insecticidal compounds and an ability to suppress a variety of plant pathogens. As many challenges face sound exploitation of plant–phytonematode interactions, a full useful spectrum of such interactions should address nematicidal activity of Xenorhabdus. Steinernema–Xenorhabdus complex or Xenorhabdus individually should be involved in mechanisms underlying the favorable side of plant–nematode interactions in emerging cropping systems. Using Xenorhabdus bacteria should earnestly be harnessed to control not only phytonematodes, but also other plant pests and pathogens within integrated pest management plans. This review highlights the significance of fitting Xenorhabdus-obtained insecticidal, nematicidal, fungicidal, acaricidal, pharmaceutical, antimicrobial, and toxic compounds into existing, or arising, holistic strategies, for controlling many pests/pathogens. The widespread utilization of Xenorhabdus bacteria, however, has been slow-going, due to costs and some issues with their commercial processing. Yet, advances have been ongoing via further mastering of genome sequencing, discovering more of the beneficial Xenorhabdus species/strains, and their successful experimentations for pest control. Their documented pathogenicity to a broad range of arthropods and pathogens and versatility bode well for useful industrial products. The numerous beneficial traits of Xenorhabdus bacteria can facilitate their integration with other tactics for better pest/disease management programs.
1. Introduction
Upgrading the methods and materials used to control plant pests and pathogens via more effective and safer approaches is one of the current challenges in the field of agriculture. Growing discontent with many chemical pesticides has been elevating, due to their unacceptable impacts on human health and environmental contamination, non-target organisms, and plant-resistance development [1,2,3]. Therefore, successes of these approaches are deeply entrenched in the available opportunities to phase out such unhealthy chemicals and to displace them by friendly ecologically safe products. In this respect, challenges to traditional plant-parasitic nematode (PPN) control measures have become evident due to accumulated knowledge on plant–PPN interactions. This situation has clearly exposed the limitations of classical studies on plant–nematode interactions, and surfaced the necessity to adopt novel interdisciplinary specialty views for effective integrated pest management. Interestingly, while bacterial use has promising effects on suppressing PPNs, it enhances growth parameters and yields of the PPN-infected plants [4]. This is not to say that such traditional studies of plant–nematode interaction cannot help scientists meet challenges imposed by PPNs. I am simply arguing that these studies alone will not suffice as many interdisciplinary challenges face sound exploitation of indirect plant–nematode interactions. A full useful spectrum of such interactions should address nematicidal activity of Xenorhabdus (Enterobacteriales: Morganellaceae) individually or Steinernema (Steinernematidae: Rhabditida)–Xenorhabdus complex in emerging cropping systems for integrated pest management. These latter should be exploited not only for crop pest/pathogen management but also for sustainable agriculture.
Admittedly, the significance of utilizing beneficial bacteria in various programs for pest/pathogen control has been increasing. While these bacteria can substitute the toxic and frequently undesirable chemicals employed for plant protection, they can offer benign ecological technologies [4]. Among them, the genus Xenorhabdus comprises entomopathogenic bacterial species that can be promising alternatives for boosting the biocontrol of numerous plant pests/pathogens. These bacteria can secrete diverse arrays of operative bioactive metabolites against these pests [2,5,6,7,8,9]. Their efficacy as biopesticides is based on a massive variety of Xenorhabdus genes that encode for generating related compounds such as antibiotics, enzymes, bacteriocins, and toxins. They are established in the shape of pathogenicity islands on the chromosomes of different Xenorhabdus species.
Therefore, the current review addresses the diversity of endosymbiotic bacterial species from EPNs and their function in biological control against many plant pests and pathogens for the agro-environmental sustainability. It throws light on the recent discoveries of new species of Xenorhabdus, with presupposed further genes encoding for beneficial attributes that are being detected in addition to presenting genes in the Xenorhabdus species that are already described so that it should be further exploited or their expressed produced compounds be improved [1,8,9,10,11]. While this article offers the relevant framework of the state-of-the-art knowledge regarding Xenorhabdus spp., it focuses on their up-to-date biotechnology to unlock more approaches of discovery that function for boosting durable agricultural systems. This review deals with the latest discoveries on their identification, taxonomy, diversity, and utilization in the present or future management plans, especially against plant pests/pathogens.
2. Identification, Taxonomy, Lifestyle, and Diversity of Xenorhabdus spp.
2.1. Their Identification/Taxonomy
As Xenorhabdus and Photorhabdus bacteria (Enterobacterales: Morganellaceae) are phylogenetically close, it is not surprising that at first they were of the same genus [12]. Thus, two bacterial species in this genus (that is, Xenorhabdus nematophila (type species) and X. luminesces) including symbionts of the nematode genera Steinernema and Heterorhabditis, respectively, were exclusively present until 1993 [12]. Yet, the important variations in the phenotypic and molecular traits could distinguish X. nematophila from X. luminesces, leading to the transfer of all symbionts related to Heterorhabditis into Photorhabdus as a new genus with the type species Photorhabdus luminescens [13]. Before splitting into the two genera, some phenotypic features and symbiotic properties were utilized to characterize Xenorhabdus and Photorhabdus bacteria as two recognized groups; P. luminescens distinctly had a DNA relatedness group unlike all Xenorhabdus strains with significant variations in phenotypic traits [14]. Xenorhabdus bacteria are obviously separated from Photorhabdus species/strains by the 16S rDNA signature sequences [15]. Yet, both Photorhabdus luminescens and X. nematophila received much research work, due to being type species of their two genera, with high insecticidal activities and a global distribution.
Interestingly, the genus Xenorhabdus still has more homogenous species than Photorhabdus, but the former genus possesses a higher number of species than the latter. For example, Sajnaga and Kazimierczak [16] reported 26 Xenorhabdus spp. versus 19 Photorhabdus spp. This is possibly due to higher number of the current Steinernema species (the mutualistic partner of Xenorhabdus spp.) than Heterorhabditis spp. (the mutualistic partner of Photorhabdus spp.), i.e., >100 Steinernema spp. but >20 Heterorhabditis spp. [17]. Admittedly, there are other undescribed species related to both Xenorhabdus and their Steinernema partner which are recognized or are likely to become recognized soon. In this respect, the number of their close relatives, Photorhabdus species, has recently doubled, from four to twenty in the last few years [18].
Initially, three X. nematophila subspecies were raised to the species level via further description using a polyphasic technique, i.e., X. poinarii, X. beddingi, and X. bovienii [19,20]. Identification of novel Xenorhabdus species as EPN symbionts continued relying on sequence data of single gene sequences until the year 2007, when a compilation of 20 Xenorhabdus species was published [21]. Thereafter, new Xenorhabdus species were identified via multi-locus sequence analysis and whole genome sequence data. Some recent references, e.g., [16,22], stated that the genus Xenorhabdus includes the following 26 species: X. beddingii, X. budapestensis, X. ehlersii, X. cabanillasii, X. doucetiae, X. eapokensis, X. griffiniae, X. bovienii, X. hominickii, X. innexi, X. indica, X. japonica, X. ishibashii, X. khoisanae, X. koppenhoeferi, X. magdalenensis, X. kozodoii, X. mauleonii, X. nematophila, X. miraniensis, X. poinarii, X. szentirmaii, X. romanii, X. stockiae, X. vietnamensis, and X. thuongxuanensis. Nonetheless, other novel species are still in the pipeline. For example, X. lircayensis linked to its partner S. unicornum was recently described [23] to elevate the number to 27 species. Comprehensive biochemical, physiological, and chemotaxonomic bioassays of the bacterial strain VLST demonstrated consistent differences from the other described Xenorhabdus species. In addition, the authors presented phylogenetic approaches to prove that VLST is a novel strain of X. lircayensis. While they presented phylogenetic relationship placing X. lircayensis within the genus Xenorhabdus based on the 16S rRNA gene analysis, a phylogenetic reconstruction was also established to include X. lircayensis based on core genome sequences [23]. Ultimately, species of Xenorhabdus are anticipated to be raised. This expectation is based on both their importance, especially against many pests/pathogens, and the recent physiological, biochemical, and molecular techniques to identify them as mutualistic or symbiotic bacteria of Steinernema spp. Therefore, it could be concluded that the progressive increment in the number of novel Xenorhabdus species and strains and new technological methods to characterize them have led to a well-established Xenorhabdus taxa with finer and easier profile to study than before. Consequently, sound genome-constructed phylogenetic trees, enriched with precise sequence comparative studies, could perfectly define their diversity and relationship. These efforts for their characterization and identification should also take part in further research of the related Xenorhabdus spp.-bioactive complexes that can be utilized in agricultural and industrial products [2,17,24].
2.2. The Lifestyle and Diversity of Xenorhabdus spp.
Basically, the bacterial species in the genus Xenorhabdus have a mutualistic relationship with the entomopathogenic nematodes (EPNs) of the genus Steinernema. The bacteria live symbiotically in the specialized intestinal vesicles of Steinernema. The two partners naturally form an antagonist sharing mainly against their insect hosts. The EPN-third-stage infective juveniles (IJs) conserve the bacteria in their body from the outer environmental stresses until these IJs release them within the insect body. In addition, after EPN infection and depleting the host resources, the IJs vector the bacteria from one susceptible host to another. In turn, Xenorhabdus spp. generate antimicrobial compounds and secondary metabolites into the insect. These materials can not only kill the insect host and prepare the contents of its body to feed the nematodes for their development and reproduction, but also protect the insect cadaver from soil scavengers and saprobes [5]. During their feeding, the nematodes also swallow the bacteria in order to grow and reproduce.
Mentioning Xenorhabdus is sometimes linked to its counterpart Photorhabdus bacteria, since much similarity is generally found between them in the frame of living. Xenorhabdus and Photorhabdus comprise symbiotic bacteria of EPN-IJs, of the genera Steinernema and Heterorhabditis, respectively. Nonetheless, each of them possesses particular bacterial traits that differentiate its identity. Xenorhabdus spp. survive within a receptacle exhibiting biological specialization at the anterior part of the intestine. Photorhabdus spp. are found at the mucosa of their nematode guts [25,26]. Steinernema spp.-infective juveniles mature to amphimictic males and females. Therefore, the dyad (males/females) must infect their susceptible host to mate then reproduce within the insect hemocele. Heterorhabditis–IJs develop into hermaphrodites (females) and then, in subsequent generations, to males/females. As a result, a single hermaphroditic–IJ has the merit to infect and reproduce in the host insect. Moreover, the demeanor of bacterial colonization of Xenorhabdus and Photorhabdus differs within their nematode partners [26]. An important attribute utilized to discern the two EPN genera, after infecting their host insect, is the pigment of Photorhabdus (bacterium) to tinge the insect host’s body in a reddish tint. Photorhabdus can glow so frequently that its entire infected host usually glows in unlighted sites. To counteract the insect host-immune system, Photorhabdus can modify the lipopolysaccharide to tolerate the influence of the host-derived antimicrobial generation of peptides, but Xenorhabdus disorganizes the creation of these peptides [25]. Both genera have more than 94% identical 16S rRNA genes, but the genome may be disrupted by many inversions, translocations, deletions, and insertions [27]. Photorhabdus species can go through major transcriptional reformation in their host, EPN, intestine. They can stimulate general starvation mechanisms, switch to the pathway of pentose phosphate to comply with nutrition shortage and oxidative stress, cellular acidification to slacken growth, and shape biofilms to firmly last in the nematode intestine for subsequent transmission to the hemolymph of the insect [28]. These bacterial behavior can back them while lasting in the EPN gut and secure fitting transmission of the nematode–bacterium complex from one susceptible host to another. Strikingly, species of both genera are capable of growing in vitro as free-living bacteria, without their EPN partners, on artificial media with specific terms, i.e., no competition in adequate nutrient media [5,18].
It is well established that Xenorhabdus bacteria have essential services in the biocontrol processes against pests [5]. They, via their metabolites and exoenzymes, are in charge of the bioconversion of the infected host into nutrient soups as ideal for the development and multiplication of their nematode partner. On the other hand, recent transcriptomic analysis of both S. carpocapsae–X. nematophila and S. puntauvense–X. bovienii indicated important metabolic shifts related to the rearing conditions as well as bacterial (presence versus absence). Nematode genes involved in the production of amino acid, carbohydrate, and lipid were downregulated as IJs were cultured in vitro, in the presence or absence of the symbiotic bacteria [29]. The downregulation seems to influence the longevity of the relevant metabolism pathways. Furthermore, IJs of both S. carpocapsae and S. puntauvense displayed a unique mechanism to adjust their virulence when their symbionts were absent. Both axenic nematode species showed a differential manifestation of the toxic protein that they secrete, i.e., absence of their symbiotic bacteria raises the expression of their secreted venom protein. The study suggested that IJs of these nematode species may have a unique mechanism to adjust infecting their hosts in the absence of their Xenorhabdus partners [29]. This does not mean, in any way, that the fundamental role played by these mutualistic bacteria in the reproduction of nematodes and even their production on a commercial scale can be neglected or may be abandoned.
Steinernema–bacterial symbiont specificity and their coevolution have been thoroughly studied for many involved axenic (free of bacteria) and monoxenic (having a Xenorhabdus species) Steinernema species [16]. While a Steinernema species can presumably set up symbiosis with only one species of Xenorhabdus, any of numerous Xenorhabdus species are able to associate with several Steinernema. On the contrary, the symbiotic Heterorhabditis–Photorhabdus associations are more adaptable as many species of each partner can engage in symbiotic relationships with multiple species of the other partner [30]. These facts have recently been reviewed and backed by plenty of data [31]. However, the mechanisms underlying these relationships remain to be clarified [16]. These associations do not negate the fact that the robust specificity that favors symbionts with the most useful attributes facilitates effective transfer of such a nematode–bacterium pair from a susceptible insect pest to another. Generally, Sajnaga and Kazimierczak [16] concluded that there is a possibility of horizontal transfer of Xenorhabdus bacteria between different Steinernema species, relying on the species of Xenorhabdus–Steinernema pair used. However, such switching in the bacterium–nematode pair may have its pros and cons. On the positive side, associations of Steinernema species with new Xenorhabdus partner may validate colonization of novel niches or expand one by offering considerable fitness benefits [32,33]. These favorable results may occur when the introduced bacteria/symbiont is closely related to its native Steinernema species. On the negative side, the Xenorhabdus partner switching frequently has a harmful effect on the Steinernema host in terms of a reduction in their fitness, reproduction, and symbiont carriage as well as virulence. For example, Xenorhabdus bovienii is the native symbiotic bacteria of S. feltiae. However, using X. nematophila strain HGB315, not the native symbiont of S. feltiae, this nematode species developed and turned into gravid much faster at approximately 4 days on X. bovienii (versus 5–6 days on X. nematophila HGB315) post-seeding [34]. These detrimental outputs are usually associated with non-cognate and phylogenetically distant symbionts [16]. Eventually, researchers and stakeholders should be aware of the fact that the Steinernema host diversity substantially impacts coadaptation between various Xenorhabdus–Steinernema partners [35,36] for their further wise application. In this respect, Tailliez et al. [37] could identify two main groups of Xenorhabdus strains based on phenotypic analysis. A group included bacterial strains that can commonly grow at 35–42 °C, while the other group included Xenorhabdus strains that grow below 35 °C. Hence, Xenorhabdus bacteria may be adapted to temperate, subtropical, or tropical regions. They are also differently impacted by the growth temperature of their Steinernema host [37]. Moreover, the wide host range of their nematode host along with their major attributes can prove their diversity and global distribution [38] as well as give opportunities to familiarize stakeholders with the potential usage of these symbiotic bacteria [5,17]. With the global spread of the Steinernema–Xenorhabdus complex, we must mention that recent references, e.g., [18], still indicate that EPNs are not discovered in Antarctica. The long-established realization regarding the species-specific characterization for the dyad Steinernema–Xenorhabdus complex as partners for the mutualism is still effective. Thus, it can bode well for more investigations concerning their distribution in diversity and space [39]. Moreover, current research efforts have been focusing on optimizing methods and techniques for EPN surveys and extraction [15,16] to detect novel species/strains that bode well for effective and safe biocontrol of pests and pathogens and adaptation to local conditions. Therefore, it can be mostly presumed that the distribution and diversity of the EPN species is only an artefact of the linked sampling efforts [18]. However, growing interest is mainly dedicated to these bacteria when applied to suppress pests and pathogens independently, i.e., without the EPN partners [5,40,41,42,43,44].
Similar to their near relatives, Photorhabdus species, all the species of Xenorhabdus are exclusively linked symbionts to the Steinernema spp.–IJ stage [13]. The exception of Photorhabdus, materialized in P. asymbiotica as a human pathogen in addition to infecting insects [45], is not found in Xenorhabdus. None of the Xenorhabdus bacteria were found in free-living order in nature; therefore, they had formerly boosted doubts concerning their ability to survive and infect pathogens/pests without the EPN partner.
3. Pathogenicity of Xenorhabdus spp.
3.1. Magnitude and Profile of Pathogenicity
Traditionally, various Steinernema–Xenorhabdus partnerships, to attack and kill numerous arthropod pests, have been marketed and utilized as biocontrol agents [17], with increasing ambition for their expansion to prepare them for reliable alternatives in pest management and plant protection [45,46,47,48,49]. In the original status of the natural Steinernema–Xenorhabdus complex, Xenorhabdus host range is surely limited to the ability of the IJs to locate and infect the host. This is a prerequisite for the development and multiplication of Xenorhabdus spp. to achieve high levels of cells within the host. Factually, both mutualists, Steinernema and Xenorhabdus, can generate bioactive compounds to kill the invaded host [34,41]. Thereafter, the bacterial cells can modify the insect host tissues to become a nutrient diet needed for the IJ development and multiplication. Hence, the pathogenicity depends on the bacterial activity and growth. Thus, the Xenorhabdus rate of growth is tightly related to the time needed to kill the insect host. Clearly, Xenorhabdus spp. are quite virulent pathogens of a broad range of pests/pathogens including insects, fungi, bacteria, protozoa, and nematodes [41,42].
Discovering the competency of Xenorhabdus bacteria to live in fresh water and in soil for 6 days has surely opened a new avenue with fixed timeframe for their further biocontrol usages, apart from their mutualistic Steinernema [48]. Consequently, various formulations and techniques (Figure 1), fundamentally comprising just the bacteria or/and bacterial metabolites, have been used [2,4,5,11,17,41,42,49,50,51,52]. In this regard, boosted pathogenicity islands of the Xenorhabdus chromosome, having numerous genes that encode various antibiotics, insecticidal protein toxins, enzymes, and bacteriocins, were investigated [5,41,53], and more are still to be further characterized, e.g., [22,42,49,54]. For instance, only one Xenorhabdus strain may generate a variety of antifungal and antibacterial compounds. Some of its compounds are active against insects, protozoa, nematodes, and cancer cells, too [41]. All tested X. nematophila strains showed insecticidal activity against representative pests of three insect orders; the cabbage white caterpillar Pieris brassicae (Lepidoptera: Pieridae), the mosquito larva Aedes aegypti (Diptera: Culicidae), and the mustard beetle Phaedon cochleariae (Coleoptera: Chrysomelidae) [52]. In this study and others [40,44,55,56], an important note is the variation in the abilities of different Xenorhabdus species/strains to kill/inhibit the growth of the intended pest or pathogen. These variations are based either on the ability of each Xenorhabdus species/strain to generate effective metabolites or the relative susceptibility/tolerance of the targeted pest/pathogen. These differences are found not only between Xenorhabdus species/strains, but surely exist to varying degrees between bacterial species/strains belonging to different genera. Photorhabdus luminescens showed more suppressing effect against Plutella xylostella (the diamondback moth) than that shown by [56]. Xenorhabdus nematophila and P. luminescens induced 40% and 60% mortality of P. xylostella pupae, with LC50 values of 5.5 × 105 and 5 × 104 cells/mL, respectively. This difference does not mean the constant superiority of Photorhabdus over Xenorhabdus bacteria. On the contrary, the cells of X. budapestensis strain DSM 16342, X. szentirmaii strain DSM 16338, and P. luminescens ssp. laumondi strain TT01 could induce 100%, 88%, and 79.3% mortality rates of the locust bean moth Ectomyelois ceratoniae (Lepidoptera: Pyralidae) larvae, respectively, at three days’ post-exposure [57]. In addition, the cell-free culture media of the strain DSM 16342 induced 53.7% mortality, demonstrating the existence of a robust insecticidal component generated by the strain. They recommended this strain as a promising alternative biocontrol agent against E. ceratoniae to protect pomegranate (Punica granatum) fruit cultivars [57] were more suppressive against second-stage juveniles of Meloidogyne javanica than X. bovienii where the nematode-induced mortalities were 88, 94.7, and 67.7%, respectively, at 24 h post-application. To explain the difference in nematode-mortality percentages, the authors [58] reported that metabolites with nematicidal activity in X. bovienii are different from those of X. nematophila and P. luminescens. Furthermore, among nine bacterial symbionts of EPNs, X. nematophila HB310 showed the best insecticidal activities against Locusta migratoria manilensis (Orthoptera: Acrididae)-fourth instar nymph [50]. The toxicities of its culture broth against different locust instars were examined via the leaves-tip bioassays. The LC50 of the strain HB310 culture broth against adult, fourth instar nymph, and second instar nymph were 6.24 × 105, 4.90 × 105, and 2.97 × 105 CFU/mL at 96 h, respectively. In addition, the LT50 of its culture broth were 64.34, 52.59, and 51.48 h, respectively. Moreover, this strain exerted antifeeding activity to locust where the median antifeedant concentrations (AFC50) of its culture broth were 3.16 × 105, 2.36 × 105, and 1.15 × 105 CFU/mL at 48 h, respectively. The authors [50] concluded that strain HB310 culture broth has high and fast insecticidal activities; it could be used as insecticidal agent against the locust. Apparently, some Xenorhabdus species could be more effective against pests/pathogens than others related to the same genus. The adults of Drosophila melanogaster showed resistance to X. innexi [59] which was also ineffective in the death of Manduca sexta larvae, while X. nematophila proved effective against the two insect species [60].
An obvious technique to circumvent the lack of appropriate efficacy of Xenorhabdus species/strain and/or to increase its potency is to introduce other antagonists in combination with Xenorhabdus bacteria and/or their bioactive compounds. This approach can establish and boost the efficacy of the introduced organism, too. Clearly, synergistic activity to kill the beet army worm Spodoptera exigua could be obtained by mixing growth media supernatants of Xenorhabdus bacteria with B. cereus or B. thuringiensis spores. In this case, while the supernatant of Xenorhabdus bacteria could exert its impact on the insect hemocoel, the Bacillus cells were able to perforate the insect midgut epithelium [61,62]. Later, Eom et al. [62] could develop a “dual Bt-plus” product by mixing B. thuringiensis (Bt) spores and culture broth of X. nematophila (Xn). Although this product demonstrated high toxicity, it has also some modification to widen its efficacy against a diverse insect pest spectrum. Their tests centered on increasing “Bt-Plus” toxicity against a semi-susceptible insect, S. exigua, via adding Xn metabolites. Given the fact that Xn metabolites, benzylideneacetone (BZA) and oxindole (OI), can boost the Bt insecticidal activities, adding each of them (OI or BZA) could significantly enhance Bt-Plus pathogenicity. Moreover, when the freeze-dried Xn culture broth was included into Bt-Plus, a much smaller amount could suffice to raise the toxicity relative to the amount of BZA or OI. High-performance liquid chromatography analysis revealed that there were more than 12 unidentified X. nematophila metabolites in Xn culture broth. Therefore, they [62] proposed that there are other potent biological response modifiers in X. nematophila metabolites, not solely OI and BZA. Likewise, a Xenorhabdus species could induce high mortality of S. exigua third-instar larvae but its pathogenicity was much less for the fifth-instar larvae. Seongchae and Yonggyun [63] speculated that the high mortalities in the third-instar larvae were due to antibiotic activity against B. cereus, a gut symbiont needed to optimize S. exigua development. To enhance the Xenorhabdus species pathogenicity in the fifth instar, the bacteria should be delivered into the hemocoel. Thus, the authors utilized B. thuringiensis aizawai (Bt) as a synergist to back entry of the bacteria from the insect gut lumen into its hemocoel by disrupting the S. exigua gut epithelium. As a result, the applied bacterial mixture was highly synergistic against the S. exigua fifth-instar larvae. This synergism was proved via the successful infection of X. sp. or Bt in the insect hemocoel. Therefore, Xenorhabdus bacteria can be used to kill S. exigua by oral treatment in a mixture with Bt [63].
In other investigations, other insecticidal toxic proteins were purified from Xenorhabdus bacteria and/or heterologously expressed in other bacterial systems [11,64,65]. In this respect, to conquer some of their limitations, Xenorhabdus can be genetically engineered to enhance the potential virulence of its nematode host against more resistant herbivore pests [40]. Differently, expression of a X. bovienii-derived protease inhibitor protein in tobacco (Nicotiana tobacum cv. Samsun NN) plants could increase tolerance against green peach aphid Myzus persicae (Hemiptera: Aphididae). Fusion proteins of a protease inhibitor from X. bovienii strain PIN1 and green fluorescent protein (GFP) were expressed in the plant [11]. The effect of genetic transformation on anti-pest activity for M. persicae was examined by feeding neonate aphids on three independent homozygous lines. When nymphs were fed on PIN1-GFP-expressing plants, no impacts on insect survival were noticed, but fecundity and average insect weight and were considerably decreased. The aphid biomass was reduced by 30–35% relative to those reared on control plants. The effects of PIN1 on M. persicae were tightly related to the decrease of total protease activities of whole insect extracts and the leucine aminopeptidase. Additionally, an elevation in polyphenoloxidase activity was apparent in PIN1-GFP-expressing plants. These results demonstrated that the transgenic expression of PIN1 in tobacco increased plant tolerance against aphids. The study disclosed Xenorhabdus bacteria as other valuable resources of protease inhibitors which can be engineered into plants for insect pest management [11].
Obviously, the aforementioned traits of the Xenorhabdus bacteria—such as the ability to disturb the induction of insect-derived antimicrobial peptides, adjusting and fitting to the settings, particularly under stresses, and global spread with a treasure trove of beneficial materials and compounds (e.g., [16,41,42])—are quite attractive for their multiple usage. They may be a sound ground to materialize their capability in the wide and reliable biocontrol of plant pests/pathogens. Their magnificent array of (primary and secondary) metabolites may be so effective that they are, independently, absolutely capable to suppress a wide profile of pests in many groups, particularly in the orders Lepidoptera (caterpillars, moths, and butterflies), Coleoptera (weevils and beetles), and Diptera (flies, including insects that transmit human and plant diseases) [2,7,42,52]. For instance, X. nematophila cells and their metabolic secretions are lethal to the Galleria mellonella larvae. The bacterial toxic secretion in broth induced 95% mortality within 4 days post-application but the X. nematophila cells brought about 93% mortality at 6 days post-application [66]. The insect mortality was better in sand substrate than filter one. Likewise, X. nematophila cells and secretion in broth showed the highest efficacy at 25 °C and 14% moisture treatments. Full insect mortality (100%) was noted at X. nematophila concentration of 4 × 106 cells/mL. Only within 2 h post-application could maximum X. nematophila cells in broth (95%) penetrate into the G. mellonella larval body. Moreover, when the bacterial toxic secretion was dried and then dissolved either in water or broth, it also proved to be effective. On the other hand, the efficacy of bacterial toxic secretion was declined due to storage. Because the bacterial cells can enjoy a free-living lifetime and invade the insect hemocoele without their nematode partner, the authors confirmed that the Xenorhabdus cells, or their impactful secretions, can be utilized for pest control. Therefore, they [66] suggested that X. nematophila or its toxic secretion can be utilized as a significant component of integrated pest management (IPM) programs against Galleria.
Usually, re-extraction of the Xenorhabdus bacteria from the insect cadavers and comparison with the standard (original) culture can assure Koch’s postulates [41,66]. Although the obtained data confirmed the direct toxicity of the bacteria to definite insect species in nature, e.g., [5,52,66], particular Xenorhabdus bacteria may have wide host range of insect pests. For example, 122 strains of symbiotic bacteria associated with 23 EPNs were gathered from various Chinese localities [67]. These extracted strains displayed oral growth inhibition and/or insecticidal activity against the Ostrinia furnacalis larvae. One of the strains, however, Xenorhabdus sp. SY5, with determined partial toxin gene sequence, exhibited strong insecticidal activity to a variety of economically significant agricultural pests. Their species comprised Plutella xylostella, Ostrinia furnacalis, Tenebrio molitor, S. exigua, and Mythimna separata. The strain isolated from Steinernema sp. SY5 appeared to have seven purified toxins based on DEAE-52 column chromatography. These toxins exhibited, to a certain extent, growth inhibition and/or insecticidal activity to these insect species. The authors [67] stressed the high virulence of this strain as a potential asset for biological pest control.
It is likely that the arsenal of Xenorhabdus spp. still possesses much that has not been discovered yet, for controlling wide categories of many pathogens. In this respect, Hajihassani et al. [68] assessed the efficacy of application timing, that is, 5 days before planting (DBP) and at planting (AP) of X. bovienii and X. szentirmaii metabolites for the root-knot nematode (RKN) Meloidogyne incognita control on cabbage roots in two environmental conditions. At-plant applications of Paecilomyces lilacinus strain 251 (MeloCon WG) and secondary metabolites of Burkholderia rinojensis strain A396 (Majestene) and oxamyl (Vydate) were used for comparison. In the greenhouse, X. szentirmaii and Vydate at 5 DBP had a lower (p < 0.05) root gall rating than the untreated control. Vydate and all metabolite treatments showed significantly lower root galling relative to Majestene, MeloCon, and the control. In addition, the metabolites and Vydate decreased (p < 0.05) RKN egg counts per gram of root compared to the other treatments in the greenhouse. No differences were observed in the egg count between Vydate and the metabolites. At-plant and 5 DBP applications of X. bovienii and X. szentirmaii at decreased the total egg count relative to Majestene and the control in the greenhouse. Thus, the natural metabolites generated by the two Xenorhabdus species can control M. incognita regardless of application timing and are suggested as a potential alternative to nematicides in organic production systems [68]. In addition, direct effect of X. lircayensis, identified using the whole genome, was evaluated on a population of the plant-parasitic nematode Xiphinema index [69]. Supernatants of bacteria were discarded via centrifugation, then X. lircayensis were resuspended in phosphate-buffered saline (PBS) and set to 1 × 106 and 1 × 107 CFU mL−1 for laboratory and semi-field assays, respectively. Cell bacteria (1 × 107 CFU mL−1) were applied in the semi-field assay by 30 min dipping grapevine roots in the bacterial suspension. Afterward, these plants were established in 5 L pots filled with naturally X. index-infested soil and immediately inoculated with 350 mL of the same X. lircayensis suspension. The nematicidal effects of X. lircayensis suspension appeared at 24 h post-inoculation but attained full (100%) X. index mortality after 72 h exposition (p < 0.001) in laboratory assays. In addition, under semi-field conditions, X. lircayensis suspension significantly (p ≤ 0.05) reduced X. index populations. While the study recommended X. lircayensis as a good candidate for further assesses in field conditions, additional analyses must be performed to set the metabolites, enzymes, and mode of action for its nematicidal aptitude [69]. Vicente-Díez et al. [70] tested the antibiotic impact of cell-free supernatants (CFSs) and unfiltered ferments (UFs) of X. nematophila and P. laumondii on another plant pathogenic category represented by the fungus Botrytis cinerea growth and compared the activity of bacteria isolated from a bio-fermenter with the commercial B. amyloliquefaciens (Serenade®ASO, Bayern CropScience). The UF and CFS of X. nematophila suppressed about 95% and 80% of B. cinerea growth, respectively, while both UF and CFS of P. laumondii inhibited only about 40%. These data showed the potential of CFS and UF of X. nematophila for B. cinerea control.
In another study [71], X. bovienii metabolite treatment was comparable to fenbuconazole (a commercial fungicide) in decreasing Fusicladium effusum sporulation on pecan (Carya illinoinensis) terminals. X. bovienii metabolite and broth treatments suppressed development of lesions brought about by Phytophthora cactorum (using pecan tree leaves maintained on agar). The bacterial metabolite treatment was also toxic to Armillaria tabescens, another important pathogen but of peach (Prunus persica) trees, especially in the southeastern United States [71]. These results offer a basis for further investigations on utilizing the bacterial metabolites or broth for suppression of economically significant diseases of pecan and peach. Likewise, X. nematophila generates many metabolites during growth and multiplication. Only one of these secondary metabolites (xenocoumacin 1) proved to have a robust antifungal activity for controlling Rhizoctonia solani [72], Botrytis cinerea [73], Alternaria alternate [74], several Phytophthora species, etc. [73,74,75,76]. These effects suggest, a priori, that other Xenorhabdus species, which are available or are likely to befit broadly soon, are able to control other pests and diseases. Recently, Xenorhabdus budapestensis strain C72 showed remarkable suppressing effect on spore germination and mycelial growth of the fungus Bipolaris maydis which causes the Southern corn leaf blight [77]. The relative control effect of the bacterial cell-free culture media reached 59.15% and 77.96% in greenhouse and field experiments, respectively, which was as efficacious as a commercial fungicide. The in vitro tests also indicated that C72 cell-free culture media with thermostability proved wide-spectrum antifungal efficacy towards other economically significant fungi and pathogens of plants [77]. Chacón-Orozco et al. [78] reported that among 16 strains of EPN-symbiotic bacteria, cell-free supernatants of X. szentrimaii had the highest fungicidal effect on mycelium growth of Sclerotinia sclerotiorum. They reported that X. szentrimaii produces volatile organic compounds that inhibit S. sclerotiorum growth and/or its consequent generation of sclerotia.
The discovery and cloning of additional useful compounds from Xenorhabdus are still in progress [1,22,43,54,57]. Factually, these bacteria can demonstrate metabolites with the major characteristics of safe pesticides. In other words, their effect is boosted with an enhanced dose, but a negative correlation is found between the number or density of pathogen/pest eggs, adult survival of the pest, percentage of hatching, and the Xenorhabdus bacterial dosage [5,41,42].
3.2. Xenorhabdus Bacterial Mechanism via Their Secreted Materials
The Xenorhabdus bacteria are typically famous for killing their hosts via toxemia/septicemia, within the form of the normal Xenorhabdus–Steinernema complex [5]. However, as different Steinernema species carrying specific Xenorhabdus strains can invade a single insect, Xenorhabdus spp. are also engaged in competition with both related strains and nonrelated gut microbes of the insect host [79]. This competition, in addition to Xenorhabdus having the capability to kill the insect host, can explain why Xenorhabdus spp. produce a treasure trove of diverse insecticidal and antimicrobial compounds. Moreover, Ciezki [79] found that X. bovienii and X. nematophila can generate R-type bacteriocins (xenorhabdicins) that are specifically active towards different Xenorhabdus and Photorhabdus species. The latter author stressed that xenorhabdicin activity could be predictive of competitive results between two Xenorhabdus strains, while other determinants, besides xenorhabdicins, were mainly included in the competitive success between the other Xenorhabdus strains. Thus, Ciezki [79] demonstrated that various Xenorhabdus antibiotics could define the output of interspecies competition in a natural host environment.
The mounting ambition to harness Xenorhabdus-derived compounds in industrial products stems from not only their abundance, but also their qualities that enhance their functions. Initially, standalone pathogenicity trials of Xenorhabdus bacteria and/or their released materials usually start with their direct injection into the haemocoel of insects via artificial means [5,66]. Xenorhabdus protein toxins ordinarily have oral or/and injectable toxicity to insects. Xenorhabdus-derived compounds have a variety of modes of action that have been reviewed [41,69,79]. The suggested mode of action of Xenorhabdus–dithiopyrrolone derivatives (comprising the two metabolites xenorhabdins and xenorxides) is inhibition of RNA synthesis [80]. However, Xenorhabdus–indole-containing compounds could show additional mechanism via weak phospholipase A2 inhibitory effects. This latter, phospholipase A2, is necessary for producing eicosanoids. Eicosanoids have substantial role for activating the insect-immune response via mediating and modulating hemocyte behavior [81]. Thus, Dreyer et al. [41] assumed that indole-containing materials produced by X. nematophila can suppress the immune response of the insect host to be more vulnerable to microbial infection. Xenorhabdus budapestensis has two antimicrobial peptides, GP-19 and EP-20, with wide-spectrum antimicrobial activity against bacteria and fungi [82]. The first peptide, with a neutral charge, is suggested to cause a disruptive impact to the host membrane by moving to the cell surface and penetrating the membrane. The second peptide likely has a different mode of action. It is suggested to have an intracellular influence, by inhibiting protein synthesis, cell wall, and nucleic acid [82].
Complete genome sequencing of various Xenorhabdus species/strains has been uncovering the ability of these bacteria to produce numerous secondary metabolites. Thus, it can contribute to comprehensive examination of the molecular basis underlying the biological control activity of this Xenorhabdus strain [83]. Various types of biological molecules have been detected and characterized for Xenorhabdus bacteria. The main antimicrobial materials comprise ribosomal-encoded benzylideneace-tone [84] xenocin and bicornutin [85,86], and non-ribosomally generated xenematides [87], fabclavines [88], xenocoumacin [89], nematophin [90], rhabdopeptides [91], and peptide–antimicrobial–Xenorhabdus lipopeptides [92]. Knowing the attributes of these compounds, e.g., the range of pH and heating needed for their stability, should enable their successful use as alternatives to chemical pesticides in agriculture [40,41]. For example, depsipeptides are peptides that generally have alternating peptide and ester bonds, and five classes of depsipeptides have been characterized. The first class, produced by Xenorhabdus doucetiae and X. mauleonii and known as xenoamicin, are tridecadepsipeptides with hydrophobic amino acids [93]. The genome sequence of X. doucetiae DSM 17909 revealed that xenoamicins are encoded by five non-ribosomal peptide synthetases (NRPSs), XabABCD, and an aspartic acid decarboxylase (XabE). Due to its hydrophobic characteristics, xenoamicin can interact with the host–cytoplasmic membrane. Nevertheless, no antifungal or antibacterial activity has been listed for xenoamicin A, which displays a different mechanism. Xenoamicin A has weak cytotoxic and anti-protozoal activities [93]. The second class of depsipeptides, the lipodepsipeptides produced by X. indica, has supplemental fatty acid chain linked to one of the amino acids [94]. The peptides are named after their amino acid sequence and are known as taxlllaids (A–G). Natural taxlllaid A and synthetic taxlllaids B–G can manifest antiprotozoal activity. Taxlllaid A is optimistically cytotoxic to human carcinoma cells [94]. The third depsipeptides class are grouped as indole-containing xenematides. Xenematide A, secreted by X. nematophila [95], is antibacterial and insecticidal. The other two depsipeptide classes contain szentiamide and xenobactin isolated from X. szentirmaii and Xenorhabdus sp., strain PB30.3 [96,97]. Both compounds are active against Plasmodium falciparum (protozoan parasite of humans) and have some activity against Trypanosoma brucei rhodesiense and Trypanosoma cruzi (parasites of many vertebrates). Szentiamide possesses a weak cytotoxic activity against Galleria mellonella hemocytes. Xenobactin has no cytotoxic activity; yet, it is active against Micrococcus luteus. This antibacterial activity is mostly due to its hydrophobic status where it probably targets the bacterial cell membrane [40]. Eventually, each of the aforementioned groups of toxins has a conceivable role as a biocontrol material, via a particular mode of action against pathogens and arthropod pests such as vector insects. The differential virulence of the candidate toxins can be correlated not only with their interspecies/strain gene sequence diversity of the same EPN-symbiotic bacterial genus but also between the two EPN-symbiotic bacterial genera, Xenorhabdus and Photorhabdus [51,78]. Fabclavine is broadly generated in Xenorhabdus species but Photorhabdus species do not produce fabclavines, except for P. asymbiotica [98]. This can elucidate partially why the tested Photorhabdus species (P. kayaii, P. namnaoensis, P. laumondii, P. akhurstii, P. thracensis) did not show antiprotozoal activity [51]. On the contrary, fabclavines 1a and 1b demonstrate diverse bioactivities against various bacterial, fungal, and protozoal organisms [89]. Other antiprotozoal bioactive materials produced by 22 Xenorhabdus species are xenorhabdins, xenocoumacins, and PAX peptides. Thus, the tested Xenorhabdus species were more effective against the serious human protozoal parasites Entamoeba histolytica, Acanthamoeba castellanii, Trichomonas vaginalis, Trypanosoma cruzi, and Leishmania tropica [51]. Furthermore, it is quite possible that more Xenorhabdus-derived toxins will uncover certain variations among bacterial strains regarding toxicity to these pests. Various features and details concerning the mode of action, structure, and putative function of the Xenorhabdus-bioactive compounds in the process of infection have been clarified [41,42,79].
While many detected metabolic compounds of Xenorhabdus bacteria with their beneficial functions are known (Table 1), the modes of action of their other compounds are still required to be understood, to facilitate their sound utilization in the management of agricultural pests/pathogens [8,51]. Xenorhabdus bacteria can control economically significant endoparasitic species of nematodes inside plant roots via their antibiotic compounds and toxins [99]. Moreover, the bacterium-derived protease inhibitor protein could be genetically transformed into tobacco plants in order to offer protection from the aphids Myzus persicae [11]. Therefore, such genetically engineered techniques are suggested as a promising replacement to the Bt toxin [18], to preclude development of insect resistance [1]. The numerous instances of pathogen and arthropod pest killing induced by Xenorhabdus spp. [5,22,42,46,54,82,99] do not deny the variations in the immune response among their pathogen/pest hosts [6,22,51,52]. In addition, the difference in immune reaction among host species/strains may be based on biologic/genetic and evolutionary/ecological factors set for each pathogen–host system. The various system constituents, including specificity, induction, and memory of the immunity, can determine the cognate resistance mechanism of the intended insect population/species [100]. Generally, physical parameters, especially pH, temperature, and sodium chloride, could variably affect the mortality percentage induced by these metabolites to the G. mellonella larvae [101].

Table 1.
Bioactive compounds generated by Xenorhabdus bacteria *.
4. The Positive and Negative Aspects of Xenorhabdus spp.
4.1. Major and Direct Favorable Aspects
The growing interest in investigating Xenorhabdus bacteria is justified by major testimonies available in the literature, to name but a few: (i) possessing genes that are responsible for encoding low-molecular-weight secondary metabolites/toxins with various activities against many pests/pathogens, e.g., insects [17,42], fungi [78,79], protozoa [49,51], bacteria [41,80], plant-parasitic nematodes (PPNs) [70,121], and other parasites [22,122]; (ii) many research tests indicate the success of Xenorhabdus bacteria in pest control under laboratory conditions [52,123] with some experimentations showing promise in large crop production areas (e.g., [99]) and under field conditions (e.g., [124]); (iii) Xenorhabdus bacteria releases toxins with substantial activities in the insect intestinal epithelium [5,125]; (iv) Xenorhabdus bacteria can have synergistic activity, when added to other biocontrol agents, against serious and intractable insect pests such as the beet army worm [62,63]; (v) specific bacterial proteins could be effective against certain insect pests, e.g., X. ehlersii protein (XeGroEL) is reliable for G. mellonella control [126,127]; (vi) toxins and metabolites of certain Xenorhabdus bacteria could have acaricidal and antibacterial activities, e.g., X. stockiae PB09 [128,129]; (vii) the aforementioned Xenorhabdus-derived bioactive compounds can offer novel templates for commercial use, obtaining reliable and environmentally safe alternatives to currently unsafe pesticides, e.g., to control phytonematodes [54] and mites [130]; (viii) Xenorhabdus nematophila-derived bioactive compounds blocked the feeding of crickets, ants, and wasps [131,132]; (ix) commercial pesticides could operate more effectively via advantageous inputs of specific Xenorhabdus-derived metabolites such as oxindole and benzylideneacetone [63]. Xenorhabdus-derived bioactive compounds could be used not only in agricultural sustainable systems but also in pharmaceutical and industrial products. A mixture of Bacillus thuringiensis israelensis (Bti) spores with X. nematophila culture broth containing metabolites has superior efficacy in controlling Aedes albopictus mosquitoes and Culex pipiens pallens. Based on enhanced Bti toxicity in the mixture against the two insect species, a commercial product was created, named “Dip-Kill” [133]. The culture broths of P. temperate temperata and X. hominickii could also boost the toxicity of Bti against culicides [2,133].
4.2. Favorable Aspects That Need Further Exploration
Several published books and tomes, e.g., [134,135,136], of EPNs and their symbionts mostly focus on their original use as insecticides. Hence, other avenues for other applications of Xenorhabdus bacteria against additional pests and pathogens should be further explored. For instance, Xenorhabdus bacteria proved useful against serious PPN species [69,70,99,121]. X. bovienii played a key role in decreasing PPN populations in turfgrass [137]. Notably, dipping the tomato roots into X. bovienii supernatant immediately before planting to infested soil was the most effective treatment for both M. arenaria and M. incognita control compared to treatments based on EPN-infected insect cadaver formulations using different EPN species, EPN IJs, and the cell-free supernatants of their mutualistic bacteria grown in liquid culture [121]. The treatment with X. bovienii supernatant considerably decreased numbers of RKN egg masses, but increased plant height and enhanced fresh and dry weights relative to the infested control plants. Later, to further test and improve the materials tested by Kepenekci et al. [121], the seedling roots were dipped into 192-hour-old X. bovienii bacterial supernatant just before tomato transplanting to the soil. Another bacterial treatment included applying 10 mL X. bovienii supernatant to the soil surface [99]. The two bacterial treatments were compared to others using Steinernema feltiae–IJs, their infected insect cadavers, and the nematode-parasitic fungus Purpureocillium lilacinum in addition to the RKN-infested check plants. In their greenhouse-2 test in the Kepez region, Turkey, they found that the highest (2.94 ± 0.10 kg tomato fruit/plant) and lowest (2.11 ± 0.09 kg tomato fruit/plant) were harvested from X. bovienii (dipped + topical application) treatment and control, respectively (p < 0.0001). While such results furnish growers with hands-on knowledge for decision-making to determine the financial feasibility of these different RKN control strategies [99], they provide researchers and stakeholders with data to further optimize relevant biocontrol techniques and avoid their inconsistent effects. The authors [99] speculated that such applications of Xenorhabdus bacteria can suppress infection by plant pathogenic fungi [40], in addition to reducing RKN populations. Thus, they assumed that what was measured [99] could sometimes be an underestimate of the value of applying the symbiotic bacteria. Further exploration of Xenorhabdus bacterial efficacy on various pests and pathogens associated with tomato, or other PPNs-susceptible plant species, in specific agricultural systems should lead to conclusive and practical results.
Tomato plants expressing certain genes of X. nematophila gained not only resistance to the cotton bollworm Helicoverpa armigera (Lepidoptera: Noctuidae) but also tolerance against salt and thermal stresses [138]. With such multiple benefits, reassessing social and economic effects1 of genetically engineered crops is challenging and requires further investigations. Such crops may be introduced to growers in rural communities with varying social communities and heterogeneous agricultural systems. On the other hand, there is a great potential of using Xenorhabdus bacteria in pharmaceutical and industrial products. For example, their products can be used to control vector insects of human diseases such as dengue and malaria. In this respect, occurrence of mosquito’s resistance to Bti was documented [2]. Nonetheless, mixing the bacteria with Xenorhabdus nematophila-cultured broth could deter the resistance and strengthen toxicity against such vector insects as Aedes albopictus and Culex pipiens pallens (Diptera: Culicidae) [2,133]. Further research should be directed for using Xenorhabdus bacteria against mosquito-borne arboviruses and other microorganisms of the Plasmodium group.
4.3. Cost-Effective Xenorhabdus Mass Culture, Formulation, and Application
Contrary to P. luminescens, cell phenotypic variation in X. nematophila strains could be controlled via regular selection of primary variants. Consequently, no trait change was detected in the X. nematophila primary variant after prolonged subculture. The genetic basis for this stability is a merit for the bacterial economical and biocontrol applications of X. nematophila production [139] because the tested Xenorhabdus bacteria have superior production (i.e., reduced culture time and scale-up) against the need to preserve traits significant for biocontrol. Furthermore, the fermentation process was recently optimized for xenocoumacin 1 (Xcn 1) production by X. nematophila [75,140]. As Xcn 1 has excellent activity against bacteria, oomycetes, and fungi, the obtained 243.38% increase in Xcn1 production [140] can lay a foundation for its industrial production. In another study, the X. nematophila fermentation production of Xcn1 was raised to 3.4-fold (234.9 mg/L) and 3.6-fold (249.7 mg/L) at 1.0 and 0.5% L-arabinose concentration, respectively [75]. These improvements should be extended to other Xenorhabdus-bioactive compounds.
Various Xenorhabdus formulations have been implemented (Figure 1) with specific examples representing varying degrees of successful and failed/uneconomical formulation and application. Among successful trials, X. stockiae PB09 formulated in wettable powder or liquid cell pellet could economically induce high miticidal mortalities of 90.25, 86.50, and 92.78% for the mushroom mite (Luciaphorus perniciosus). These formulations have potential to be further developed as commercial products for controlling mushroom mites [130]. An opposite example that still has major obstacles to beat before implementation is the use of X. szentirmaii in a powder form against the fungus Botrytis cinerea. This effect of the antifungal metabolites of X. szentirmaii in a powder form was weaker than its corresponding liquid X. szentirmaii supernatant [141]. Because Xenorhabdus powder has longer shelf-life and can be easily and economically formulated and applied compared to liquid supernatants, the author suggested that further studies should optimize the processes for the powder formulation to enhance its efficacy. In such a case, the system should possess all factors for success including high levels of Xenorhabdus virulence, favorable pulverization process, proper contact with the tested fungal pathogen, and compatibility with current practices, e.g., temperature.
4.4. Other Aspects of Xenorhabdus spp.
Contrary to Photorhabdus bacteria, which has the human-pathogenic P. asymbiotica [5], Xenorhabdus bacteria have never been detected from clinical specimens [5]. However, with the exception of some studies in phylogenetics and molecular identifications, Xenorhabdus bacterial studies on their bioactive compounds have somewhat lagged behind those of Photorhabdus bacteria. For instance, the high-molecular-weight insecticidal “toxin complexes” were first identified in P. luminescens strain W14 and subsequently in X. nematophila [142]. To conclude this review, it suffices that this article referred to the above examples that clearly express their counterparts, leaving more relevant details in their original references. It is needless to remind the reader that using these bacteria singly or with their host nematodes are mostly costly and may comprise sometimes inconsistent results due to many biological and ecological factors that should be specified to suit the used biocontrol agents [143]. Yet, the changing scope of crop protection necessitates facing such challenges for sustainable agriculture [144].
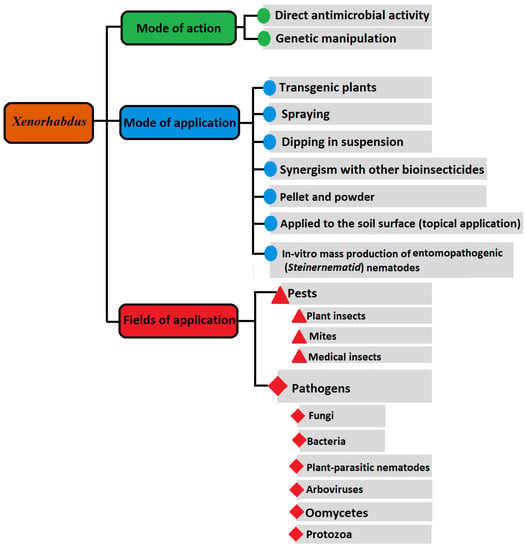
Figure 1.
A sketch of possible framework of Xenorhabdus bacterial application, mode of application, and mechanism of operation to control different groups of pathogens and pests [4,5,8,11,22,24,40,41,42,44,51,52,54,55,57,62,63,67,68,69,71,72,78,79,84,98,99,105,121,125,126,129,130,133,138,141].
5. Conclusions
Accelerated public concerns over the misuse and impact of chemical pesticides on human health and environment create the need to discover alternative methods for management of plant pests and pathogens. Utilizing Xenorhabdus bacteria as biologicals in various sustainable agricultural systems against a wide range of plant pests and pathogens is apparently an attractive and promising approach. This bacterium can be exploited directly (with its nematode partner) or indirectly (without its partner) to neutralize the plant–phytonematode interaction to the advantage of the plant. Such an emerging approach should be tried in earnest in order to control not only PPNs, but also many other plant pests and pathogens within IPM plans. The detection of new Xenorhabdus species and strains globally will occur to widen the pool of these bacterial-related materials that are reliable for managing economically significant pathogens/pests. Developing low-priced techniques for their trade production, formulation, and application may expedite their use in current or future management programs. In such programs, Xenorhabdus bacteria can be used with their Steinernema host or as standalone pesticides for common management strategies. Obviously, these bacteria produce a treasure trove of diverse compounds that can kill many harmful insects and microorganisms, e.g., protozoa, PPNs, fungi, oomycetes, and bacteria. Xenorhabdus bacteria could be applied in various formats for topical application, e.g., pellets, powder, spray, suspension, or supernatant. Furthermore, their toxin genes could be incorporated into transgenic plants to control insect pests. The transgenic plants could also gain tolerance against salt and thermal stress in some cases. Therefore, Xenorhabdus bacteria and their bioactive compounds are highlighted herein; in order to provide a substantial way forward in pest management and crop protection, they should be leveraged to fit into holistic crop management strategies. These may comprise their combination with other additive or synergistic inputs, to enhance their efficacy while blocking, or decreasing, the likelihood of developing pesticide-resistant harmful strains.
Funding
This research was funded by STDF, US–Egyptian project “Preparing and evaluating IPM tactics for increasing strawberry and citrus production” cycle 17 grant number 172 and the NRC help offered via “In-house project headed by Mahfouz Abd-Elgawad in research plan no. 13” is acknowledged.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This article is supported in part by the US-Egypt Project cycle 17 (no. 172).
Conflicts of Interest
The author declares no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Xiao, Y.; Wu, K. Recent progress on the interaction between insects and Bacillus thuringiensis crops. Philos. Trans. R. Soc. 2019, 374, 20180316. [Google Scholar] [CrossRef] [PubMed]
- da Silva, W.J.; Pilz-Júnior, H.L.; Heermann, R.; da Silva, O.S. The great potential of entomopathogenic bacteria Xenorhabdus and Photorhabdus for mosquito control: A review. Parasites Vectors 2020, 13, 376. [Google Scholar] [CrossRef] [PubMed]
- Abd-Elgawad, M.M.M. Status of entomopathogenic nematodes in integrated pest management strategies in Egypt. In Biocontrol Agents: Entomopathogenic and Slug Parasitic Nematodes; Abd-Elgawad, M.M.M., Askary, T.H., Coupland, J., Eds.; CAB International: Wallingford, UK, 2017; pp. 473–501. [Google Scholar]
- Migunova, V.D.; Sasanelli, N. Bacteria as biocontrol tool against phytoparasitic nematodes. Plants 2021, 10, 389. [Google Scholar] [CrossRef]
- Javed, N.; Kamran, M.; Abbas, H. Toxic secretions of Xenorhabdus and their efficacy against crop insect pests. In Biocontrol Agents: Entomopathogenic and Slug Parasitic Nematodes; Abd-Elgawad, M.M.M., Askary, T.H., Coupland, J., Eds.; CAB International: Wallingford, UK, 2017; pp. 223–230. [Google Scholar]
- Eroglu, C.; Cimenb, H.; Ulug, D.; Karagoz, M.; Hazir, S.; Cakmaka, I. Acaricidal effect of cell-free supernatants from Xenorhabdus and Photorhabdus bacteria against Tetranychus urticae (Acari: Tetranychidae). J. Invertebr. Pathol. 2019, 106, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Ffrench-Constant, R.; Waterfield, N.; Daborn, P. Insecticidal toxins from Photorhabdus and Xenorhabdus. In Encyclopedia of Microbiology, 4th ed.; Schmid, T.M., Ed.; Academic Press: New York, NY, USA, 2019; pp. 704–715. [Google Scholar] [CrossRef]
- Muangpat, P.; Suwannaroj, M.; Yimthin, T.; Fukruksa, C.; Sitthisak, S.; Chantratita, N.; Vitta, A.; Thanwisai, A. Antibacterial activity of Xenorhabdus and Photorhabdus isolated from entomopathogenic nematodes against antibiotic-resistant bacteria. PLoS ONE 2020, 15, e0234129. [Google Scholar] [CrossRef]
- Dong, Y.; Li, X.; Duan, J.; Qin, Y.; Yang, X.; Ren, J.; Li, G. Improving the yield of xenocoumacin 1 enabled by in situ product removal. ACS Omega 2020, 5, 20391–20398. [Google Scholar] [CrossRef]
- Hug, J.J.; Krug, D.; Müller, R. Bacteria as genetically programmable producers of bioactive natural products. Nat. Rev. Chem. 2020, 4, 172–193. [Google Scholar] [CrossRef]
- Zhang, H.; Mao, J.; Liu, F.; Zeng, F. Expression of a nematode symbiotic bacterium-derived protease inhibitor protein in tobacco enhanced tolerance against Myzus persicae. Plant Cell Rep. 2012, 31, 1981–1989. [Google Scholar] [CrossRef]
- Thomas, G.M.; Poinar, G.O. Xenorhabdus gen. nov., a genus of entomopathogenic nematophilic bacteria of the family Enterobacteriaceae. Int. J. Syst. Bacteriol. 1979, 29, 352–360. [Google Scholar] [CrossRef] [Green Version]
- Boemare, N.E.; Akhurst, R.J.; Mourant, R.G. DNA relatedness between Xenorhabdus spp. (Enterobacteriaceae), symbiotic bacteria of entomopathogenic nematodes, and a proposal to transfer Xenorhabdus luminescens to a new genus, Photorhabdus gen. nov. Int. J. Syst. Bacteriol. 1993, 43, 249–255. [Google Scholar] [CrossRef]
- Fischer-Le Saux, M.; Viallard, V.; Brunel, B.; Normand, P.; Boemare, N.E. Polyphasic classification of the genus Photorhabdus and proposal of new taxa: P. luminescens subsp. luminescens subsp. nov., P. luminescens subsp. akhurstii subsp. nov., P. luminescens subsp. laumondii subsp. nov., P. temperata sp. nov., P. temperate subsp. temperatas ubsp. nov. and P. asymbiotica sp. nov. Int. J. Syst. Bacteriol. 1999, 49, 1645–1656. [Google Scholar] [PubMed]
- Boemare, N. Biology, taxonomy and systematics of Photorhabdus and Xenorhabdus. In Entomopathogenic Nematology; Gaugler, R., Ed.; CAB International: Wallingford, UK, 2002; pp. 35–56. [Google Scholar]
- Sajnaga, E.; Kazimierczak, W. Evolution and taxonomy of nematode-associated entomopathogenic bacteria of the genera Xenorhabdus and Photorhabdus: An overview. Symbiosis 2020, 80, 1–13. [Google Scholar] [CrossRef]
- Shapiro-Ilan, D.; Hazir, S.; Glazer, I. Advances in use of entomopathogenic nematodes. In Integrated Management of Insect Pests: Current and Future Developments; Kogan, M., Heinrichs, E.A., Eds.; Burleigh Dodds Science Publication: Cambridge, UK, 2020; pp. 1–30. [Google Scholar]
- Abd-Elgawad, M.M.M. Photorhabdus spp.: An overview of the beneficial aspects of mutualistic bacteria of insecticidal nematodes. Plants 2021, 10, 1660. [Google Scholar] [CrossRef] [PubMed]
- Akhurst, R.J. Taxonomic study of Xenorhabdus, a genus of bacteria symbiotically associated with insect pathogenic nematodes. Int. J. Syst. Bacteriol. 1983, 33, 38–45. [Google Scholar] [CrossRef]
- Akhurst, R.J.; Boemare, N.E. A numerical taxonomic study of the genus Xenorhabdus (Enterobacteriaceae) and proposed elevation of the subspecies of X. nematophilus to species. J. Gen. Microbiol. 1988, 134, 1835–1845. [Google Scholar] [CrossRef]
- Koppenhöfer, H.S. Bacterial symbionts of Steinernema and Heterorhabditis. In Entomopathogenic Nematodes: Systematics, Phylogeny and Bacterial Symbionts; Nguyen, K.B., Hunt, D.J., Eds.; Brill Academic Publishers: Leiden, The Netherlands; Boston, MA, USA, 2007; pp. 735–808. [Google Scholar]
- Tomar, P.; Thakur, N.; Yadav, A.N. Endosymbiotic microbes from entomopathogenic nematode (EPNs) and their applications as biocontrol agents for agro-environmental sustainability. Egypt. J. Biol. Pest. Control 2022, 32, 80. [Google Scholar] [CrossRef]
- Castaneda-Alvarez, C.; Prodan, S.; Zamorano, A.; San-Blas, E.; Aballay, E. Xenorhabdus lircayensis sp. nov., the symbiotic bacterium associated with the entomopathogenic nematode Steinernema unicornum. Int. J. Syst. Evol. Microbiol. 2021, 71, 005151. [Google Scholar] [CrossRef]
- Donmez Ozkan, H.; Cimen, H.; Ulug, D.; Wenski, S.; Yigit Ozer, S.; Telli, M.; Aydin, N.; Bode, H.B.; Hazir, S. Nematode-associated bacteria: Production of antimicrobial agent as a presumptive nominee for curing endodontic infections caused by Enterococcus faecalis. Front. Microbiol. 2019, 10, 2672. [Google Scholar] [CrossRef]
- Goodrich-Blair, H.; Clarke, D.J. Mutualism and pathogenesis in Xenorhabdus and Photorhabdus: Two roads to the same destination. Mol. Microbiol. 2007, 64, 260–268. [Google Scholar] [CrossRef]
- Ciche, T.A.; Kim, K.S.; Kaufmann-Daszczuk, B.; Nguyen, K.C.Q.; Hall, D.H. Cell invasion and matricide during Photorhabdus luminescens transmission by Heterorhabditis bacteriophora nematodes. Appl. Environ. Microbiol. 2008, 74, 2275–2287. [Google Scholar] [CrossRef]
- Chaston, J.M.; Suen, G.; Tucker, S.L.; Andersen, A.W.; Bhasin, A.; Bode, E. The entomopathogenic bacterial endosymbionts Xenorhabdus and Photorhabdus: Convergent lifestyles from divergent genomes. PLoS ONE 2011, 6, e27909. [Google Scholar] [CrossRef] [PubMed]
- An, R.; Grewal, P.S. Molecular mechanisms of persistence of mutualistic bacteria Photorhabdus in the entomopathogenic nematode host. PLoS ONE 2010, 5, e13154. [Google Scholar] [CrossRef] [PubMed]
- Lefoulon, E.; McMullen, J.G.; Stock, S.P. Transcriptomic analysis of Steinernema nematodes highlights metabolic costs associated to Xenorhabdus endosymbiont association and rearing conditions. Front. Physiol. 2022, 13, 821845. [Google Scholar] [CrossRef] [PubMed]
- Koppenhöfer, H.S.; Gaugler, R. Entomopathogenic nematode and bacteria mutualism. In Defensive Mutualism in Microbial Symbiosis; White, J., Torres, M., Eds.; CRC Press: Boca Raton, FL, USA, 2009; pp. 99–116. [Google Scholar]
- Hillman, K.; Goodrich-Blair, H. Are you my symbiont? Microbial polymorphic toxins and antimicrobial compounds as honest signals of beneficial symbiotic defensive traits. Curr. Opin. Microbiol. 2016, 31, 184–190. [Google Scholar] [CrossRef]
- Henry, L.M.; Peccoud, J.; Simon, J.C.; Hadfield, J.D.; Maiden, M.J.; Ferrari, J.; Godfray, H.C. Horizontally transmitted symbionts and host colonization of ecological niches. Curr. Biol. 2013, 9, 1713–1717. [Google Scholar] [CrossRef]
- Maher, A.M.D.; Asaiyah, M.A.M.; Brophy, C.; Griffin, C.T. An entomopathogenic nematode extends its niche by associating with different symbionts. Microb. Ecol. 2017, 73, 211–223. [Google Scholar] [CrossRef]
- Chang, D.Z.; Serra, L.; Lu, D.; Mortazavi, A.; Dillman, A.R.A. Core set of venom proteins is released by entomopathogenic nematodes in the genus Steinernema. PLoS Pathog. 2019, 15, e1007626. [Google Scholar] [CrossRef]
- Murfin, K.E.; Whooley, A.C.; Klassen, J.L.; Goodrich-Blair, H. Comparison of Xenorhabdus bovienii bacterial strain genomes reveals diversity in symbiotic functions. BMC Genom. 2015, 16, 889. [Google Scholar] [CrossRef]
- McMullen, J.G.; Peterson, B.F.; Forst, S.; Goodrich-Blair, H.; Stock, S.P. Fitness costs of symbiont switching using entomopathogenic nematodes as a model. BMC Evol. Biol. 2017, 17, 100. [Google Scholar] [CrossRef] [Green Version]
- Tailliez, P.; Laroui, C.; Ginibre, N.; Paule, A.; Pages, S.; Boemare, N. Phylogeny of Photorhabdus and Xenorhabdus based on universally conserved protein-coding sequences and implications for the taxonomy of these two genera. Proposal of new taxa. X. vietnamensis sp. nov., P. luminescens subsp. caribbeanensis subsp. nov., P. luminescens subsp. hainanensis subsp. nov., P. temperata subsp. khanii subsp. nov., P. temperata subsp. tasmaniensis subsp. nov., and the reclassification of P. luminescens subsp. thracensis as P. temperata subsp. thracensis comb. nov. Int. J. Syst. Evol. Microbiol. 2010, 60, 1921–1937. [Google Scholar]
- Askary, T.H.; Abd-Elgawad, M.M.M. Opportunities and challenges of entomopathogenic nematodes as biocontrol agents in their tripartite interactions. Egypt. J. Biol. Pest Cont. 2021, 31, 42. [Google Scholar] [CrossRef]
- Baiocchi, T.; Abd-Elgawad, M.M.M.; Dillman, A.R. Genetic improvement of entomopathogenic nematodes for enhanced biological control. In Biocontrol Agents: Entomopathogenic and Slug Parasitic Nematodes; Abd-Elgawad, M.M.M., Askary, T.H., Coupland, J., Eds.; CAB International: Wallingford, UK, 2017; pp. 505–517. [Google Scholar]
- Hazir, S.; Shapiro-Ilan, D.I.; Bock, C.H.; Hazir, C.; Leite, L.G.; Hotchkiss, M.W. Relative potency of culture supernatants of Xenorhabdus and Photorhabdus spp. on growth of some fungal phytopathogens. Eur. J. Plant Pathol. 2016, 146, 369–381. [Google Scholar] [CrossRef]
- Dreyer, J.; Malan, A.P.; Dicks, L.M.T. Bacteria of the genus Xenorhabdus, a novel source of bioactive compounds. Front. Microbiol. 2018, 9, 3177. [Google Scholar] [CrossRef]
- Cimen, H.; Touray, M.; Gulsen, S.H.; Hazir, S. Natural products from Photorhabdus and Xenorhabdus: Mechanisms and impacts. Appl. Microbiol. Biotechnol. 2022, 106, 4387–4399. [Google Scholar] [CrossRef]
- Booysen, E.; Rautenbach, M.; Stander, M.A.; Dicks, L.M. Profiling the production of antimicrobial secondary metabolites by Xenorhabdus khoisanae J194 under different culturing conditions. Front. Chem. 2021, 9, 626653. [Google Scholar] [CrossRef]
- Nunez-Valdez, M.E.; Lanois, A.; Pages, S.; Duvic, B.; Gaudriault, S. Inhibition of Spodoptera frugiperda phenoloxidase activity by the products of the Xenorhabdus rhabduscin gene cluster. PLoS ONE 2019, 22, e0212809. [Google Scholar]
- Plichta, K.L.; Joyce, S.A.; Clarke, D.; Waterfield, N.; Stock, S.P. Heterorhabditis gerrardi n. sp. (Nematoda: Heterorhabditidae): The hidden host of Photorhabdus asymbiotica (Enterobacteriaceae:g-Proteobacteria). J. Helminthol. 2009, 83, 309–320. [Google Scholar] [CrossRef]
- Abd-Elgawad, M.M.M. Towards optimization of entomopathogenic nematodes for more service in the biological control of insect pests. Egypt. J. Biol. Pest Cont. 2019, 29, 77. [Google Scholar] [CrossRef]
- Koppenhöfer, A.M.; Shapiro-Ilan, D.I.; Hiltpold, I. Entomopathogenic nematodes in sustainable food production. Front. Sustain. Food Syst. 2020, 4, 125. [Google Scholar] [CrossRef]
- Morgan, J.A.W.; Kuntzelmann, V.; Tavernor, S.; Ousley, M.A.; Winstanley, C. Survival of Xenorhabdus nematophilus and Photorhabdus luminescens in water and soil. J. Appl. Microbiol. 1997, 83, 665–670. [Google Scholar] [CrossRef]
- Antonello, A.M.; Sartori, T.; Silva, M.B.; Prophiro, J.S.; Pinge-Filho, P.; Heermann, R. Anti-Trypanosoma activity of bioactive metabolites from Photorhabdus luminescens and Xenorhabdus nematophila. Exp. Parasitol. 2019, 204, 107724. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Nangong, Z.; Kong, F.; Song, P.; Wang, Q. Biological activity of Xenorhabdus nematophila HB310 against Locusta migratoria manilensis. Chin. J. Pest Sci. 2013, 15, 516–522. [Google Scholar]
- Gulsen, S.H.; Tileklioglu, E.; Bode, E.; Cimen, H.; Ertabaklar, H.; Ulug, D.; Ertug, S.; Wenski, S.L.; Touray, M.; Hazir, C.; et al. Antiprotozoal activity of different Xenorhabdus and Photorhabdus bacterial secondary metabolites and identification of bioactive compounds using the easyPACId approach. Sci. Rep. 2022, 12, 10779. [Google Scholar] [CrossRef]
- Sergeant, M.; Baxter, L.; Jarrett, P.; Shaw, E.; Ousley, M.; Winstanley, C.; Alun, J.; Morgan, W. Identification, typing and insecticidal activity of Xenorhabdus isolates from entomopathogenic nematodes in United Kingdom soil and characterization of the xpt toxin loci. Appl. Environ. Microbiol. 2006, 72, 5895–5907. [Google Scholar] [CrossRef]
- Pidot, S.J.; Coyne, S.; Kloss, F.; Hertweck, C. Antibiotics from neglected bacterial sources. Int. J. Med. Microbiol. 2014, 304, 14–22. [Google Scholar] [CrossRef]
- Abebew, D.; Sayedain, F.S.; Bode, E.; Bode, H.B. Uncovering nematicidal natural products from Xenorhabdus bacteria. J. Agric. Food Chem. 2022, 70, 498–506. [Google Scholar] [CrossRef]
- Dreyer, J.; Rautenbach, M.; Booysen, E.; Van Staden, A.; Deane, S.; Dicks, L. Xenorhabdus khoisanae SB10 produces Lys-rich PAX lipopeptides and a Xenocoumacin in its antimicrobial complex. BMC Microbiol. 2019, 19, 1–11. [Google Scholar] [CrossRef]
- Abdel-Razek, A.S. Pathogenic effects of Xenorhabdus nematophilus and Photorhabdus luminescens (Enterobacteriaceae) against pupae of the Diamondback Moth, Plutella xylostella (L.). J. Pest Sci. 2003, 76, 108–111. [Google Scholar] [CrossRef]
- Alotaibi, S.S.; Darwish, H.; Alharthi, S.; Alghamdi, A.; Noureldeen, A.; Fallatah, A.M.; Fodor, A.; Al-Barty, A.; Albogami, B.; Baazeem, A. Control potentials of three entomopathogenic bacterial isolates for the carob moth, Ectomyelois ceratoniae (Lepidoptera: Pyralidae) in pomegranates. Agriculture 2021, 11, 1256. [Google Scholar] [CrossRef]
- Sayedain, F.S.; Ahmadzadeh, M.; Talaei-Hassanloui, R.; Olia, M.; Bode, H.B. Nematicidal effect of cell-free culture filtrates of EPN-symbiotic bacteria on Meloidogyne javanica. Biol. Cont. Pests Pl. Dis. 2019, 8, 17–26. [Google Scholar]
- Sicard, M.; Le Brun, N.; Pages, S.; Godelle, B.; Boemare, N.; Moulia, C. Effect of native Xenorhabdus on the fitness of their Steinernema hosts: Contrasting types of interaction. Parasitol. Res. 2003, 91, 520–524. [Google Scholar] [CrossRef]
- Kim, I.H.; Aryal, S.K.; Aghai, D.T.; Casanova-Torres, Á.M.; Hillman, K.; Kozuch, M.P.; Mans, E.J.; Mauer, T.J.; Ogier, J.C.; Ensign, J.C.; et al. The insect pathogenic bacterium Xenorhabdus innexi has attenuated virulence in multiple insect model hosts yet encodes a potent mosquitocidal toxin. BMC Genom. 2017, 18, 927. [Google Scholar] [CrossRef]
- Askary, T.H.; Abd-Elgawad, M.M.M. Beneficial nematodes in agroecosystems: A global perspective. In Biocontrol Agents: Entomopathogenic and Slug Parasitic Nematodes; Abd-Elgawad, M.M.M., Askary, T.H., Coupland, J., Eds.; CAB International: Wallingford, UK, 2017; pp. 3–25. [Google Scholar]
- Eom, S.; Park, Y.; Kim, H.; Kim, Y. Development of a high efficient“dual Bt-plus” insecticide using a primary form of an entomopathogenic bacterium, Xenorhabdus nematophila. J. Microbiol. Biotechnol. 2014, 24, 507–521. [Google Scholar] [CrossRef]
- Seongchae, J.; Yonggyun, K. Synergistic effect of entomopathogenic bacteria (Xenorhabdus sp. and Photorhabdus temperata ssp. temperata) on the pathogenicity of Bacillus thuringiensis ssp. Aizawai against Spodoptera exigua (Lepidoptera: Noctuidae). Environ. Entomol. 2006, 35, 1584–1589. [Google Scholar]
- Seo, S.; Lee, S.; Hong, Y.; Kim, Y. Phospholipase A2 inhibitors synthesized by two entomopathogenic bacteria, Xenorhabdus nematophila and Photorhabdus temperata subsp. temperata. Appl. Environ. Microbiol. 2012, 78, 3816–3823. [Google Scholar] [CrossRef]
- Mollah, M.I.; Kim, Y. Virulent secondary metabolites of entomopathogenic bacteria genera, Xenorhabdus and Photorhabdus, inhibit phospholipase A2 to suppress host insect immunity. BMC Microbiol. 2020, 20, 359. [Google Scholar] [CrossRef]
- Mahar, A.N.; Munir, M.; Gowen, S.R.; Hague, N.G.M. Role of entomopathogenic bacteria, Photorhabdus luminescens and its toxic secretions against Galleria mellonella larvae. J. Entomol. 2005, 2, 69–76. [Google Scholar] [CrossRef]
- Wang, H.; Dong, H.; Qian, H.; Xia, R.; Cong, B. Isolation, bioassay and characterisation of Xenorhabdus sp. SY5, a highly virulent symbiotic bacterium of an entomopathogenic nematode isolated from China. Nematology 2013, 15, 153–163. [Google Scholar] [CrossRef]
- Hajihassani, A.; Gitonga, D.; Timper, P.; Shapiro-Ilan, D. Effects of application timing on the efficacy of Xenorhabdus and Photorhabdus metabolites for control of Meloidogyne incognita. In Proceedings of the 7th International Congress of Nematology, Antibes Juan-les-Pins, France, 1–5 May 2022; p. 598. [Google Scholar]
- Castaneda-Alvarez, C.; Prodan, S.; San-Blas, E.; Aballay, E. Symbiotic bacteria of entomopathogenic nematodes for the biocontrol of dagger nematode Xiphinema index. In Proceedings of the 7th International Congress of Nematology, Antibes Juan-les-Pins, France, 1–5 May 2022; p. 587. [Google Scholar]
- Vicente-Díez, I.; Blanco-Pérez, R.; Chelkha, M.; Pou1, A.; Campos-Herrera, R. Plasticity in the use of Xenorhabdus nematophila and Photorhabdus laumondii against Botrytis cinerea. In Proceedings of the 7th International Congress of Nematology, Antibes Juan-les-Pins, France, 1–5 May 2022; p. 660. [Google Scholar]
- Shapiro-Ilan, D.I.; Bock, C.H.; Hotchkiss, M.W. Suppression of pecan and peach pathogens on different substrates using Xenorhabdus bovienii and Photorhabdus luminescens. Biol. Cont. 2014, 77, 1–6. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Q.; Han, Y.; Han, J.; Yan, Z.; Wang, Y.; Zhang, X. Nematophin, an antimicrobial dipeptide compound from Xenorhabdus nematophila YL001 as a potent biopesticide for Rhizoctonia solani control. Front. Microbiol. 2019, 10, 1765. [Google Scholar] [CrossRef]
- Fang, X.; Zhang, M.; Tang, Q.; Wang, Y.; Zhang, X. Inhibitory effect of Xenorhabdus nematophila TB on plant pathogens Phytophthora capsici and Botrytis cinerea in vitro and in planta. Sci. Rep. 2014, 4, 4300. [Google Scholar] [CrossRef]
- Qin, Y.; Jia, F.; Li, X.; Li, B.; Ren, J.; Yang, X.; Li, G. Improving the yield of xenocoumacin 1 by PBAD promoter replacement in Xenorhabdus nematophila CB6. Agriculture 2021, 11, 1251. [Google Scholar] [CrossRef]
- Yang, X.; Qiu, D.; Yang, H.; Liu, Z.; Zeng, H.; Yuan, J. Antifungal activity of xenocoumacin 1 from Xenorhabdus nematophilus var. pekingensis against Phytophthora infestans. World J. Microb. Biotechnol. 2011, 27, 523–528. [Google Scholar] [CrossRef]
- Zhou, T.; Yang, X.; Qiu, D.; Zeng, H. Inhibitory effects of xenocoumacin 1 on the different stages of Phytophthora capsici and its control effect on Phytophthora blight of pepper. BioControl 2017, 62, 151–160. [Google Scholar] [CrossRef]
- Li, B.; Kong, L.; Qiu, D.; Francis, F.; Wang, S. Biocontrol potential and mode of action of entomopathogenic bacteria Xenorhabdus budapestensis C72 against Bipolaris maydis. Biol. Cont. 2021, 158, 104605. [Google Scholar] [CrossRef]
- Chacón-Orozco, J.G.; Bueno, C., Jr.; Shapiro Ilan, D.I.; Hazir, S.; Leite, L.G.; Harakava, R. Antifungal activity of Xenorhabdus spp. and Photorhabdus spp. against the soybean pathogenic Sclerotinia sclerotiorum. Sci. Rep. 2020, 10, 20649. [Google Scholar] [CrossRef]
- Ciezki, K.J. New Insights into the Role of Antimicrobials of Xenorhabdus in Interspecies Competition. Ph.D. Dissertation, University of Wisconsin-Milwaukee, Milwaukee, WI, USA, 2017; 103p. [Google Scholar]
- Oliva, B.; O’Neill, A.; Wilson, J.M.; O’Hanlon, P.J.; Chopra, I. Antimicrobial properties and mode of action of the pyrrothine holomycin. Antimicrob. Agents Chemother. 2001, 45, 532–539. [Google Scholar] [CrossRef]
- Shrestha, S.; Kim, Y. An entomopathogenic bacterium, Xenorhabdus nematophila, inhibits hemocyte phagocytosis of Spodoptera exigua by inhibiting phospholipase A2. J. Invertebr. Pathol. 2007, 96, 64–70. [Google Scholar] [CrossRef]
- Xiao, Y.; Meng, F.; Qiu, D.; Yang, X. Two novel antimicrobial peptides purified from the symbiotic bacteria Xenorhabdus budapestensis NMC-10. Peptides 2012, 35, 253–260. [Google Scholar] [CrossRef]
- Li, B.; Qiu, D.; Wang, S. Complete genome sequence data of Xenorhabdus budapestensis strain C72, a candidate biological control agent from China. Plant Dis. 2021, 105, 3276–3278. [Google Scholar] [CrossRef]
- Ji, D.; Yi, Y.; Kang, G.-H.; Choi, Y.-H.; Kim, P.; Baek, N.-I.; Kim, Y. Identification of an antibacterial compound, benzylideneacetone, from Xenorhabdus nematophila against major plant-pathogenic bacteria. FEMS Microbiol. Lett. 2004, 239, 241–248. [Google Scholar] [CrossRef]
- Böszörményi, E.; Ersek, T.; Fodor, A.; Fodor, A.M.; Földes, L.S.; Hevesi, M.; Hogan, J.S.; Katona, Z.; Klein, M.G.; Kormany, A.; et al. Isolation and activity of Xenorhabdus antimicrobial compounds against the plant pathogens Erwinia amylovora and Phytophthora nicotianae. J. Appl. Microbiol. 2009, 107, 746–759. [Google Scholar] [CrossRef]
- Rathore, J.S. Expression, purification, and functional characterization of atypical xenocin, its immunity protein, and their domains from Xenorhabdus nematophila. Int. J. Bacteriol. 2013, 2013, 746862. [Google Scholar] [CrossRef]
- Xi, X.; Lu, X.; Zhang, X.; Bi, Y.; Li, X.; Yu, Z. Two novel cyclic depsipeptides xenematides F and G from the entomopathogenic bacterium Xenorhabdus budapestensis. J. Antibiot. 2019, 72, 736–743. [Google Scholar] [CrossRef]
- Fuchs, S.W.; Grundmann, F.; Kurz, M.; Kaiser, M.; Bode, H.B. Fabclavines: Bioactive peptide-polyketide-polyamino hybrids from Xenorhabdus. ChemBioChem 2014, 15, 512–516. [Google Scholar] [CrossRef]
- Park, D.; Ciezki, K.; van der Hoeven, R.; Singh, S.; Reimer, D.; Bode, H.B.; Forst, S. Genetic analysis of xenocoumacin antibiotic production in the mutualistic bacterium Xenorhabdus nematophila. Mol. Microbiol. 2009, 73, 938–949. [Google Scholar] [CrossRef]
- Li, J.; Chen, G.; Webster, J.M. Nematophin, a novel antimicrobial substance produced by Xenorhabdus nematophilus (Enterobactereaceae). Can. J. Microbiol. 1997, 43, 770–773. [Google Scholar] [CrossRef]
- Zhao, L.; Kaiser, M.; Bode, H.B. Rhabdopeptide/xenortide-like peptides from Xenorhabdus innexi with terminal amines showing potent antiprotozoal activity. Org. Lett. 2018, 20, 5116–5120. [Google Scholar] [CrossRef]
- Fuchs, S.W.; Proschak, A.; Jaskolla, T.W.; Karas, M.; Bode, H.B. Structure elucidation and biosynthesis of lysine-rich cyclic peptides in Xenorhabdus nematophila. Org. Biomol. Chem. 2011, 9, 3130–3132. [Google Scholar] [CrossRef]
- Zhou, Q.; Grundmann, F.; Kaiser, M.; Schiell, M.; Gaudriault, S.; Batzer, A. Structure and biosynthesis of xenoamicins from entomopathogenic Xenorhabdus. Chem. Eur. J. 2013, 19, 16772–16779. [Google Scholar] [CrossRef]
- Kronenwerth, M.; Brachmann, A.O.; Kaiser, M.; Bode, H.B. Bioactive derivatives of isopropylstilbene from mutasynthesis and chemical synthesis. ChemBioChem 2014, 15, 2689–2691. [Google Scholar] [CrossRef]
- Lang, G.; Kalvelage, T.; Peters, A.; Wiese, J.; Imhoff, J.F. Linear and cyclic peptides from the entomopathogenic bacterium Xenorhabdus nematophilus. J. Nat. Prod. 2008, 71, 1074–1077. [Google Scholar] [CrossRef]
- Nollmann, F.I.; Dowling, A.; Kaiser, M.; Deckmann, K.; Grösch, S.; French-Constant, R.; Bode, H.B. Synthesis of szentiamide, a depsipeptide from entomopathogenic Xenorhabdus szentirmaii with activity against Plasmodium falciparum. Beilstein J. Org. Chem. 2012, 8, 528–533. [Google Scholar] [CrossRef]
- Grundmann, F.; Kaiser, M.; Kurz, M.; Schiell, M.; Batzer, A.; Bode, H.B. Structure determination of the bioactive depsipeptide xenobactin from Xenorhabdus sp. PB30. 3. RSC Adv. 2013, 3, 22072–22077. [Google Scholar] [CrossRef]
- Tobias, N.J.; Wolff, H.; Djahanschiri, B.; Grundmann, F.; Kronenwerth, M.; Shi, Y.M. Natural product diversity associated with the nematode symbionts Photorhabdus and Xenorhabdus. Nat. Microbiol. 2017, 2, 1676–1685. [Google Scholar] [CrossRef]
- Kepenekci, I.; Hazir, S.; Oksal, E.; Lewis, E.E. Application methods of Steinernema feltiae, Xenorhabdus bovienii and Purpureocillium lilacinum to control root-knot nematodes in greenhouse tomato systems. Crop Prot. 2018, 108, 31–38. [Google Scholar] [CrossRef]
- Erler, S.; Popp, M.; Lattorff, M. Dynamics of immune system gene expression upon bacterial challenge and wounding in a social insect (Bombus terrestris). PLoS ONE 2011, 6, e18126. [Google Scholar] [CrossRef]
- Abd El-Zaher, F.H.; Abd-Elgawad, M.M.M.; Abd El-Maksoud, H.K. Use of the entomopathogenic nematode symbiont Photorhabdus luminescens as a biocontrol agent. B- Factors affecting the cell-free filtrates from the bacterium. J. Appl. Sci. Res. 2012, 8, 4600–4614. [Google Scholar]
- Boemare, N.; Boyer-Giglio, M.; Thaler, J.; Akhurst, R.; Brehelin, M. Lysogeny and bacteriocinogeny in Xenorhabdus nematophilus and other Xenorhabdus spp. Appl. Environ. Microbiol. 1992, 58, 3032–3037. [Google Scholar] [CrossRef]
- Proschak, A.; Zhou, Q.; Schöner, T.; Thanwisai, A.; Kresovic, D.; Dowling, A.; Ffrench-Constant, R.; Proshack, E.; Bode, H.B. Biosynthesis of the insecticidal xenocyloins in Xenorhabdus bovienii. Chem. Biol. Chem. 2014, 15, 369–372. [Google Scholar] [CrossRef]
- Park, H.B.; Perez, C.E.; Perry, E.K.; Crawford, J.M. Activating and attenuating the amicoumacin antibiotics. Molecules 2016, 21, 824. [Google Scholar] [CrossRef]
- Li, J.; Chen, G.; Webster, J.M.; Czyzewska, E. Antimicrobial metabolites from a bacterial symbiont. J. Nat. Prod. 1995, 58, 1081–1086. [Google Scholar] [CrossRef]
- Wenski, S.L.; Cimen, H.; Berghaus, N.; Fuchs, S.W.; Hazir, S.; Bode, H.B. Fabclavine diversity in Xenorhabdus bacteria. Beilstein J. Org. Chem. 2020, 16, 956–965. [Google Scholar] [CrossRef]
- Gualtieri, M.; Villain-Guillot, P.; Givaudan, A.; Pages, S. Nemaucin, an Antibiotic Produced by Entomopathogenic Xenorhabdus cabanillasii. Google Patents. WO2012085177 A1, 28 June 2012. pp. 1–39. [Google Scholar]
- Reimer, D.; Cowles, K.N.; Proschak, A.; Nollmann, F.I.; Dowling, A.J.; Kaiser, M.; Constant, R.F.; Goodrich-Blair, H.; Bode, H.B. Rhabdopeptides as insect-specific virulence factors from entomopathogenic bacteria. Chem. Biol. Chem. 2013, 14, 1991–1997. [Google Scholar] [CrossRef]
- Houard, J.; Aumelas, A.; Noel, T.; Givaudan, A.; Fitton-Ouhabi, V.; Villain-Guillot, P.; Gualtieri, M. Cabanillasin, a new antifungal metabolite, produced by entomopathogenic Xenorhabdus cabanillasii JM26. J. Antibiot. 2013, 66, 617–620. [Google Scholar] [CrossRef]
- Bode, E.; He, Y.; Vo, T.D.; Schultz, R.; Kaiser, M.; Bode, H.B. Biosynthesis and function of simple amides in Xenorhabdus doucetiae. Environ. Microbiol. 2017, 19, 4564–4575. [Google Scholar] [CrossRef]
- Hacker, C.; Cai, X.; Kegler, C.; Zhao, L.; Weickhmann, A.K.; Wurm, J.P.; Bode, H.B.; Wöhnert, J. Structure-based redesign of docking domain interactions modulates the product spectrum of a rhabdopeptide-synthesizing NRPS. Nat. Commun. 2018, 9, 4366. [Google Scholar] [CrossRef]
- Brachmann, A.O.; Reimer, D.; Lorenzen, W.; Augusto, A.E.; Kopp, Y.; Piel, J.; Bode, H.B. Reciprocal cross talk between fatty acid and antibiotic biosynthesis in a nematode symbiont. Angew. Chem. 2012, 124, 12252–12255. [Google Scholar] [CrossRef]
- Qin, Z.; Huang, S.; Yu, Y.; Deng, H. Dithiolopyrrolone natural products: Isolation, synthesis and biosynthesis. Mar. Drugs 2013, 11, 3970–3997. [Google Scholar] [CrossRef]
- Cai, X.; Nowak, S.; Wesche, F.; Bischoff, I.; Kaiser, M.; Fürst, R.; Bode, H.B. Entomopathogenic bacteria use multiple mechanisms for bioactive peptide library design. Nat. Chem. 2017, 9, 379–386. [Google Scholar] [CrossRef]
- Guo, S.; Zhang, S.; Fang, X.; Liu, Q.; Gao, J.; Bilal, M.; Wang, Y.; Zahng, X. Regulation of antimicrobial activity and xenocoumacins biosynthesis by pH in Xenorhabdus nematophila. Microb. Cell. Fact. 2017, 16, 203. [Google Scholar] [CrossRef]
- Esmati, N.; Maddirala, A.; Hussein, N.; Amawi, H.; Tiwari, A.; Andreana, P. Efficient syntheses and anti-cancer activity of xenortides A-D including ent/epi-stereoisomers. Org. Biomol. Chem. 2018, 16, 5332–5342. [Google Scholar] [CrossRef]
- Crawford, J.M.; Portmann, C.; Zhang, X.; Roeffaers, M.B.; Clardy, J. Small molecule perimeter defense in entomopathogenic bacteria. Proc. Natl. Acad. Sci. USA 2012, 109, 10821–10826. [Google Scholar] [CrossRef]
- Nollmann, F.I.; Dauth, C.; Mulley, G.; Kegler, C.; Kaiser, M.; Waterfield, N.R.; Bode, H.B. Insect-specific production of new GameXPeptides in Photorhabdus luminescens TTO1, widespread natural products in entomopathogenic bacteria. Chem. Biol. Chem. 2015, 16, 205–208. [Google Scholar] [CrossRef]
- Dongare, R.K.; Inamdar, S.N.; Tigote, R.M. Dft based investigations of antibiotic and antifungal activity of allantofuranone and related γ-lactone compounds. J. Adv. Sci. Res. 2021, 12, 336–339. [Google Scholar]
- Fodor, A.; Gualtieri, M.; Zeller, M.; Tarasco, E.; Klein, M.G.; Fodor, A.M.; Haynes, L.; Lengyel, K.; Forst, S.A.; Furgani, G.M.; et al. Type strains of entomopathogenic nematode-symbiotic bacterium species, Xenorhabdus szentirmaii (EMC) and X. budapestensis (EMA), are exceptional sources of non-ribosomal templated, large-target-spectral, thermotolerant-antimicrobial peptides (by both), and iodinin (by EMC). Pathogens 2022, 11, 342. [Google Scholar] [CrossRef]
- Kepenekci, I.; Hazir, S.; Lewis, E.E. Evaluation of entomopathogenic nematodes and the supernatants of the in vitro culture medium of their mutualistic bacteria for the control of the root-knot nematodes Meloidogyne incognita and M. arenaria. Pest Manag. Sci. 2016, 72, 327–334. [Google Scholar] [CrossRef]
- Grundmann, F.; Kaiser, M.; Schiell, M.; Batzer, A.; Kurz, M.; Thanwisai, A.; Chantratita, N.; Bode, H.B. Antiparasitic chaiyaphumines from entomopathogenic Xenorhabdus sp. PB61.4. J. Nat. Prod. 2014, 77, 779–783. [Google Scholar] [CrossRef]
- Ruiu, L.; Satta, A.; Floris, I. Emerging entomopathogenic bacteria for insect pest management. Bull. Insectol. 2013, 66, 181–186. [Google Scholar]
- Hemalatha, D.; Prabhu, S.; Rani, W.B.; Anandham, R. Isolation and characterization of toxins from Xenorhabdus nematophilus against Ferrisia virgata (Ckll.) on tuberose, Polianthes tuberosa. Toxicon 2018, 146, 42–49. [Google Scholar] [CrossRef]
- Vicente-Díez, I.; Blanco-Pérez, R.; Chelkha, M.; Puelles, M.; Pou, A.; Campos-Herrera, R. Exploring the use of entomopathogenic nematodes and the natural products derived from their symbiotic bacteria to control the grapevine moth, Lobesia botrana (Lepidoptera: Tortricidae). Insects 2021, 12, 1033. [Google Scholar] [CrossRef]
- Shi, H.; Zeng, H.; Yang, X.; Zhao, J.; Chen, M.; Qiu, D. An insecticidal protein from Xenorhabdus ehlersii triggers prophenoloxidase activation and hemocyte decrease in Galleria mellonella. Curr. Microbiol. 2012, 64, 604–610. [Google Scholar] [CrossRef]
- Shi, H.X.; Zengm, H.M.; Yang, X.F.; Liu, Z.; Qiu, D. An insecticidal protein from Xenorhabdus ehlersii stimulates the innate immune response in Galleria mellonella. World J. Microbiol. Biotechnol. 2013, 29, 1705–1711. [Google Scholar] [CrossRef]
- Bussaman, P.; Sa-Uth, C.; Rattanasena, P.; Chandrapatya, A. Acaricidal activities of whole cell suspension, cell-free supernatant, and crude cell extract of Xenorhabdus stokiae against mushroom mite (Luciaphorus sp.). J. Zhejiang Univ. Sci. B 2012, 13, 261–266. [Google Scholar] [CrossRef]
- Bussaman, P.; Rattanasena, P. Additional property of Xenorhabdus stockiae for inhibiting cow mastitis-causing bacteria. Biosci. Biotechnol. Res. Asia 2016, 13, 1871–1878. [Google Scholar] [CrossRef]
- Namsena, P.; Bussaman, P.; Rattanasena, P. Bioformulation of Xenorhabdus stockiae PB09 for controlling mushroom mite, Luciaphorus perniciosus Rack. Bioresour. Bioprocess. 2016, 3, 19. [Google Scholar] [CrossRef]
- Zhou, X.; Kaya, H.K.; Heungens, K.; Goodrich-Blair, H. Response of ants to a deterrent factor(s) produced by the symbiotic bacteria of entomopathogenic nematodes. Appl. Environ. Microbiol. 2002, 68, 6202–6209. [Google Scholar] [CrossRef] [Green Version]
- Gulcu, B.; Hazir, S.; Kaya, H.K. Scavenger deterrent factor (SDF) from symbiotic bacteria of entomopathogenic nematodes. J. Invertebr. Pathol. 2012, 110, 326–333. [Google Scholar] [CrossRef]
- Park, Y.; Kyo Jung, J.; Kimm, Y.A. Mixture of Bacillus thuringiensis subsp. israelensis with Xenorhabdus nematophila-cultured broth enhances toxicity against mosquitoes Aedes albopictus and Culex pipiens pallens (Diptera: Culicidae). J. Econ. Entomol. 2016, 109, 1086–1093. [Google Scholar] [CrossRef]
- Gaugler, R. (Ed.) Entomopathogenic Nematology; CAB International: New York, NY, USA, 2002; 365p. [Google Scholar]
- Grewal, P.S.; Ehlers, R.-U.; Shapiro-Ilan, D.I. (Eds.) Nematodes as Biocontrol Agents; CAB International: Wallingford, UK, 2005; 523p. [Google Scholar]
- Abd-Elgawad, M.M.M.; Askary, T.H.; Coupland, J. (Eds.) Biocontrol Agents: Entomopathogenic and Slug Parasitic Nematodes; CAB International: Wallingford, UK, 2005; 662p. [Google Scholar]
- Grewal, P.S.; Martin, W.R.; Miller, R.; Lewis, E.E. Suppression of plant-parasitic nematode populations in turfgrass by application of entomopathogenic nematodes. Biocontrol Sci. Technol. 1997, 7, 393–399. [Google Scholar] [CrossRef]
- Kumari, P.; Mahapatro, G.K.; Banerjee, N.; Sarin, N.B. Ectopic expression of GroEL from Xenorhabdus nematophila in tomato enhances resistance against Helicoverpa armigera and salt and thermal stress. Transgenic Res. 2015, 24, 859–873. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Bilgrami, A.L.; Shapiro-Ilan, D.; Gaugler, R. Stability of entomopathogenic bacteria, Xenorhabdus nematophila and Photorhabdus luminescens, during in vitro culture. J. Int. Microbiol. Biotechnol. 2007, 34, 73–81. [Google Scholar] [CrossRef]
- Han, Y.; Gao, J.; Zhang, S.; Han, J.; Yan, Z.; Ta, Y.; Wang, Y. Increasing the production of xenocoumacin 1 by optimizing the fermentation process of Xenorhabdus nematophila. Res. Square 2021, 1–23. [Google Scholar] [CrossRef]
- Gulcu, B. Comparison of powder and liquid forms of antifungal metabolites produced by Xenorhabdus szentirmaii, the symbionts of entomopathogenic nematodes, against gray mold Botrytis cinerea. J. Agric. Sci. Technol. 2022, 24, 457–464. [Google Scholar]
- Sergeant, M.; Jarrett, P.; Ousely, M.; Morgan, A.W. Interactions of insecticidal toxin gene products from Xenorhabdus nematophila PMFI296. Appl. Environ. Microbiol. 2003, 69, 3344–3349. [Google Scholar] [CrossRef] [PubMed]
- Abd-Elgawad, M.M.M.; Askary, T.H. Factors affecting success of biological agents used in controlling plant-parasitic nematodes. Egypt. J. Biol. Pest Cont. 2020, 30, 17. [Google Scholar] [CrossRef]
- Coupland, J.; Abd-Elgawad, M.M.M.; Askary, T.H. Beneficial nematodes and the changing scope of crop protection. In Biocontrol Agents: Entomopathogenic and Slug Parasitic Nematodes; Abd-Elgawad, M.M.M., Askary, T.H., Coupland, J., Eds.; CAB International: Wallingford, UK, 2017; pp. 26–42. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).