Improving the Tolerance to Salinity Stress in Lettuce Plants (Lactuca sativa L.) Using Exogenous Application of Salicylic Acid, Yeast, and Zeolite
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Experimental Design and Plant Growth
2.3. Treatments Preparation
- Control: Distilled water.
- Salicylic acid: 0.2 g L−1 prepared by dissolving 0.2 grams of salicylic acid, obtained from Sigma-Aldrich Chemical Company in 1 liter of distilled water.
- Yeast: A 3% yeast extract was prepared using a commercial baker’s yeast (Saccharomyces cerevisiae) obtained from Saf-instant® France by dissolving a quantity of dry yeast in distilled water, a 1:1 ratio of sugar was added (as a source of C and N). The culture was grown for 48 h at 28 °C before application to the plants [26].
- Zeolite: To prepare a concentration of 5 g L−1, a quantity of 5 grams of natural zeolite mineral obtained from Rota Mining Corporation was dissolved in 1 liter of distilled water.
2.4. Growth Attributes
2.5. Estimation of Photosynthetic Pigments
2.6. Estimation of Total Leaf Soluble Sugar
2.7. Estimation of Total Leaf Proline
2.8. Statistical Analysis
3. Results
3.1. Growth Attributes
3.1.1. Height of Shoots
3.1.2. Roots
3.1.3. Number of Leaves
3.1.4. Leaf Area
3.1.5. Fresh Weight
3.1.6. Dry Weight
3.2. Biochemical Parameters
3.2.1. Sugar Content
3.2.2. Proline Content
3.2.3. Chlorophyll Content
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Youssef, M.H.M.; Raafat, A.; El-Yazied, A.A.; Selim, S.; Azab, E.; Khojah, E.; El Nahhas, N.; Ibrahim, M.F.M. Exogenous Application of Alpha-Lipoic Acid Mitigates Salt-Induced Oxidative Damage in Sorghum Plants through Regulation Growth, Leaf Pigments, Ionic Homeostasis, Antioxidant Enzymes, and Expression of Salt Stress Responsive Genes. Plants 2021, 10, 2519. [Google Scholar] [CrossRef] [PubMed]
- Dajic, Z. Salt stress. In Physiology and Molecular Biology of Stress Tolerance in Plants; Springer: Berlin/Heidelberg, Germany, 2006; pp. 41–99. [Google Scholar]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Mao, W.; Zhu, Y.; Wu, J.; Ye, M.; Yang, J. Evaluation of effects of limited irrigation on regional-scale water movement and salt accumulation in arid agricultural areas. Agric. Water Manag. 2022, 262, 107398. [Google Scholar] [CrossRef]
- Pitman, M.G.; Läuchli, A. Global impact of salinity and agricultural ecosystems. In Salinity: Environment—Plants—Molecules; Läuchli, A., Lüttge, U., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2004; pp. 3–20. ISBN 978-1-4020-0492-6. [Google Scholar]
- Alsamadany, H.; Mansour, H.; Elkelish, A.; Ibrahim, M.F. Folic Acid Confers Tolerance against Salt Stress-Induced Oxidative Damages in Snap Beans through Regulation Growth, Metabolites, Antioxidant Machinery and Gene Expression. Plants 2022, 11, 1459. [Google Scholar] [CrossRef] [PubMed]
- El Nahhas, N.; AlKahtani, M.D.; Abdelaal, K.A.; Al Husnain, L.; AlGwaiz, H.I.; Hafez, Y.M.; Attia, K.A.; El-Esawi, M.A.; Ibrahim, M.F.; Elkelish, A. Biochar and jasmonic acid application attenuates antioxidative systems and improves growth, physiology, nutrient uptake and productivity of faba bean (Vicia faba L.) irrigated with saline water. Plant Physiol. Biochem. 2021, 166, 807–817. [Google Scholar] [CrossRef]
- Ramadan, K.M.A.; Alharbi, M.M.; Alenzi, A.M.; El-Beltagi, H.S.; Darwish, D.B.; Aldaej, M.I.; Shalaby, T.A.; Mansour, A.T.; El-Gabry, Y.A.; Ibrahim, M.F.M. Alpha Lipoic Acid as a Protective Mediator for Regulating the Defensive Responses of Wheat Plants against Sodic Alkaline Stress: Physiological, Biochemical and Molecular Aspects. Plants 2022, 11, 787. [Google Scholar] [CrossRef] [PubMed]
- Negrão, S.; Schmöckel, S.M.; Tester, M. Evaluating physiological responses of plants to salinity stress. Ann. Bot. 2017, 119, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Costa, S.F.; Martins, D.; Agacka-Mołdoch, M.; Czubacka, A.; de Sousa Araújo, S. Strategies to alleviate salinity stress in plants. In Salinity Responses and Tolerance in Plants, Volume 1; Kumar, V., Wani, S.H., Suprasanna, P., Tran, L.-S.P., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 307–337. ISBN 978-3-319-75670-7. [Google Scholar]
- Zheng, J.; Chen, T.; Wu, Q.; Yu, J.; Chen, W.; Chen, Y.; Siddique, K.H.M.; Meng, W.; Chi, D.; Xia, G. Effect of zeolite application on phenology, grain yield and grain quality in rice under water stress. Agric. Water Manag. 2018, 206, 241–251. [Google Scholar] [CrossRef]
- Yuvaraj, M.; Subramanian, K.S. Zeolites application in agriculture. Adv. Life Sci. 2016, 5, 10899–10904. [Google Scholar]
- Ntanos, E.; Kekelis, P.; Assimakopoulou, A.; Gasparatos, D.; Denaxa, N.-K.; Tsafouros, A.; Roussos, P.A. Amelioration effects against salinity stress in strawberry by bentonite–zeolite mixture, glycine betaine, and Bacillus amyloliquefaciens in terms of plant growth, nutrient content, soil properties, yield, and fruit quality characteristics. Appl. Sci. 2021, 11, 8796. [Google Scholar] [CrossRef]
- Negahban, M.; Saeedfar, S.; Ramezan, D.; Asil, M.H. Effects of natural zeolite to reduce salt stress in kentucky bluegrass (Poa pratensis). Russ. J. Biol. Res. 2014, 1, 38–45. [Google Scholar] [CrossRef]
- Aboul-Magd, M.; Elzopy, K.A.; Zangana, Z.R.M. Effect of zeolite and urea fertilizer on maize grown under saline conditions. Middle East J. Appl. Sci. 2020, 10, 18–25. [Google Scholar] [CrossRef]
- Koo, Y.M.; Heo, A.Y.; Choi, H.W. Salicylic acid as a safe plant protector and growth regulator. Plant Pathol. J. 2020, 36, 1–10. [Google Scholar] [CrossRef] [PubMed]
- El-Taher, A.M.; Abd El-Raouf, H.S.; Osman, N.A.; Azoz, S.N.; Omar, M.A.; Elkelish, A.; Abd El-Hady, M.A.M. Effect of Salt Stress and Foliar Application of Salicylic Acid on Morphological, Biochemical, Anatomical, and Productivity Characteristics of Cowpea (Vigna unguiculata L.) Plants. Plants 2022, 11, 115. [Google Scholar] [CrossRef] [PubMed]
- Ghafoor, M.; Ali, Q.; Malik, A. Effects of salicylic acid priming for salt stress tolerance in wheat. Biol. Clin. Sci. Res. J. 2020, 2020, e024. [Google Scholar] [CrossRef]
- Khodary, S.E.A. Effect of salicylic acid on the growth, photosynthesis and carbohydrate metabolism in salt stressed maize plants. Int. J. Agric. Biol. 2004, 6, 5–8. [Google Scholar]
- Patel, P.K.; Hemantaranjan, A. Salicylic acid induced alteration in dry matter partitioning, antioxidant defence system and yield in chickpea (Cicer arietinum L.) under drought stress. Asian J. Crop Sci. 2012, 4, 86–102. [Google Scholar] [CrossRef]
- Abd-Alrahman, H.A.; Aboud, F.S. Response of sweet pepper plants to foliar application of compost tea and dry yeast under soilless conditions. Bull. Natl. Res. Cent. 2021, 45, 119. [Google Scholar] [CrossRef]
- Dawood, M.G.; Sadak, M.S.; Abdallah, M.M.S.; Bakry, B.A.; Darwish, O.M. Influence of biofertilizers on growth and some biochemical aspects of flax cultivars grown under sandy soil conditions. Bull. Natl. Res. Cent. 2019, 43, 81. [Google Scholar] [CrossRef]
- Lonhienne, T.; Mason, M.G.; Ragan, M.A.; Hugenholtz, P.; Schmidt, S.; Paungfoo-Lonhienne, C. Yeast as a biofertilizer alters plant growth and morphology. Crop Sci. 2014, 54, 785–790. [Google Scholar] [CrossRef]
- Kang, S.-M.; Radhakrishnan, R.; You, Y.-H.; Khan, A.L.; Park, J.-M.; Lee, S.-M.; Lee, I.-J. Cucumber performance is improved by inoculation with plant growth-promoting microorganisms. Acta Agric. Scand. Sect. B—Soil Plant Sci. 2015, 65, 36–44. [Google Scholar] [CrossRef]
- Marzauk, N.M. Effect of vitamin E and Yeast extract foliar application on growth, pod yield and both green pod and seed yield of broad bean. Middle East J. Appl. Sci. 2014, 4, 61–67. [Google Scholar]
- Francesca, S.; Arena, C.; Hay Mele, B.; Schettini, C.; Ambrosino, P.; Barone, A.; Rigano, M.M. The use of a plant-based biostimulant improves plant performances and fruit quality in tomato plants grown at elevated temperatures. Agronomy 2020, 10, 363. [Google Scholar] [CrossRef]
- Darwesh, R.S.S. Improving growth of date palm plantlets grown under salt stress with yeast and amino acids applications. Ann. Agric. Sci. 2013, 58, 247–256. [Google Scholar] [CrossRef]
- El-Yazied, A.A.; Mady, M.A. Effect of boron and yeast extract foliar application on growth, pod setting and both green pod and seed yield of broad bean (Vicia faba L.). J. Am. Sci. 2012, 8, 517–533. [Google Scholar]
- Rehman, A.; Hassan, F.; Qamar, R.; Rehman, A.U. Application of plant growth promoters on sugarcane (Saccharum officinarum L.) budchip under subtropical conditions. Asian J. Agric. Biol. 2021, 2, 1–10. [Google Scholar] [CrossRef]
- Enebe, M.C.; Babalola, O.O. The influence of plant growth-promoting rhizobacteria in plant tolerance to abiotic stress: A survival strategy. Appl. Microbiol. Biotechnol. 2018, 102, 7821–7835. [Google Scholar] [CrossRef]
- Aćamović-DJoković, G.; Pavlović, R.; Mladenović, J.; DJurić, M. Vitamin C content of different types of lettuce varieties. Acta Agric. Serbica 2011, 16, 83–89. [Google Scholar]
- Kim, M.J.; Moon, Y.; Tou, J.C.; Mou, B.; Waterland, N.L. Nutritional value, bioactive compounds and health benefits of lettuce (Lactuca sativa L.). J. Food Compos. Anal. 2016, 49, 19–34. [Google Scholar] [CrossRef]
- Medina-Lozano, I.; Bertolín, J.R.; Díaz, A. Nutritional value of commercial and traditional lettuce (Lactuca sativa L.) and wild relatives: Vitamin C and anthocyanin content. Food Chem. 2021, 359, 129864. [Google Scholar] [CrossRef]
- Mou, B. Nutritional quality of lettuce. Curr. Nutr. Food Sci. 2012, 8, 177–187. [Google Scholar] [CrossRef]
- Noumedem, J.A.K.; Djeussi, D.E.; Hritcu, L. Lactuca sativa. In Medicinal Spices and Vegetables from Africa; Academic Press: Cambridge, MA, USA, 2017; pp. 437–449. [Google Scholar]
- Maroyi, A. Not just minor wild edible forest products: Consumption of pteridophytes in sub-Saharan Africa. J. Ethnobiol. Ethnomed. 2014, 10, 78. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.T.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Troll, W.; Lindsley, J. A photometric method for the determination of proline. J. Biol. Chem. 1955, 215, 655–660. [Google Scholar] [CrossRef]
- Zhu, J. Plant salt stress. In Encyclopedia of Life Sciences (ELS); Wiley Online Library: Chichester, UK, 2007. [Google Scholar] [CrossRef]
- Chinnusamy, V.; Zhu, J.; Zhu, J.-K. Salt stress signaling and mechanisms of plant salt tolerance. In Genetic Engineering; Setlow, J.K., Ed.; Genetic Engineering: Principles and Methods; Kluwer Academic Publishers: Boston, MA, USA, 2006; Volume 27, pp. 141–177. ISBN 978-0-387-25855-3. [Google Scholar]
- Hasamuzzaman, M.; Fujita, M.; Islam, M.N.; Ahamed, K.U.; Nahar, K. Performance of four irrigated rice varieties under different levels of salinity stress. Int. J. Integr. Biol. 2009, 6, 85–90. [Google Scholar]
- Farooq, M.; Hussain, M.; Wakeel, A.; Siddique, K.H.M. Salt Stress in Maize: Effects, resistance mechanisms, and management. A Review. Agron. Sustain. Dev. 2015, 35, 461–481. [Google Scholar] [CrossRef]
- Munns, R.; James, R.A.; Läuchli, A. Approaches to increasing the salt tolerance of wheat and other cereals. J. Exp. Bot. 2006, 57, 1025–1043. [Google Scholar] [CrossRef]
- Oukarroum, A.; Bussotti, F.; Goltsev, V.; Kalaji, H.M. Correlation between reactive oxygen species production and photochemistry of photosystems I and II in Lemna gibba L. Plants under salt stress. Environ. Exp. Bot. 2015, 109, 80–88. [Google Scholar] [CrossRef]
- Schmidt, R.; Mieulet, D.; Hubberten, H.-M.; Obata, T.; Hoefgen, R.; Fernie, A.R.; Fisahn, J.; San Segundo, B.; Guiderdoni, E.; Schippers, J.H. Salt-responsive ERF1 regulates reactive oxygen species–dependent signaling during the initial response to salt stress in rice. Plant Cell 2013, 25, 2115–2131. [Google Scholar] [CrossRef]
- Hnilickova, H.; Kraus, K.; Vachova, P.; Hnilicka, F. Salinity stress affects photosynthesis, malondialdehyde formation, and proline content in Portulaca oleracea L. Plants 2021, 10, 845. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.V. Regulation of chlorophyll biosynthesis and degradation by salt stress in sunflower leaves. Sci. Hortic. 2004, 103, 93–99. [Google Scholar] [CrossRef]
- Netondo, G.W.; Onyango, J.C.; Beck, E. Sorghum and Salinity: II. Gas exchange and chlorophyll fluorescence of sorghum under salt stress. Crop Sci. 2004, 44, 806–811. [Google Scholar] [CrossRef]
- Najar, B.; Pistelli, L.; Marchioni, I.; Pistelli, L.; Muscatello, B.; De Leo, M.; Scartazza, A. Salinity-Induced Changes of Photosynthetic Performance, Lawsone, VOCs, and antioxidant metabolism in Lawsonia Inermis L. Plants 2020, 9, 1797. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Ahmed, S.; Roy, S.K.; Woo, S.H.; Sonawane, K.D.; Shohael, A.M. Effect of salinity on the morphological, physiological and biochemical properties of lettuce (Lactuca sativa L.) in Bangladesh. Open Agric. 2019, 4, 361–373. [Google Scholar] [CrossRef]
- Bohnert, H.J.; Jensen, R.G. Strategies for engineering water-stress tolerance in plants. Trends Biotechnol. 1996, 14, 89–97. [Google Scholar] [CrossRef]
- Munir, N.; Hasnain, M.; Roessner, U.; Abideen, Z. Strategies in improving plant salinity resistance and use of salinity resistant plants for economic sustainability. Crit. Rev. Environ. Sci. Technol. 2021, 10, 2150–2196. [Google Scholar] [CrossRef]
- Yildirim, E.; Turan, M.; Guvenc, I. Effect of foliar salicylic acid applications on growth, chlorophyll, and mineral content of cucumber grown under salt stress. J. Plant Nutr. 2008, 31, 593–612. [Google Scholar] [CrossRef]
- Iqbal, N.; Nazar, R.; Khan, M.I.R.; Masood, A.; Kahan, N.A. Role of gibberellins in regulation of source-sink relations under optimal and limiting environmental conditions. Curr. Sci. 2011, 100, 998–1007. [Google Scholar]
- Appu, M.; Muthukrishnan, S. Foliar application of salicylic acid stimulates flowering and induce defense related proteins in finger millet plants. Univers. J. Plant Sci. 2014, 2, 14–18. [Google Scholar] [CrossRef]
- Erdal, S.; Aydın, M.; Genisel, M.; Taspınar, M.S.; Dumlupinar, R.; Kaya, O.; Gorcek, Z. Effects of salicylic acid on wheat salt sensitivity. Afr. J. Biotechnol. 2011, 10, 5713–5718. [Google Scholar] [CrossRef]
- Jagendorf, A.T.; Takabe, T. Inducers of glycinebetaine synthesis in barley. Plant Physiol. 2001, 127, 1827–1835. [Google Scholar] [CrossRef] [PubMed]
- Bandurska, H.; Stroinski, A. The effect of salicylic acid on barley response to water deficit. Acta Physiol. Plant. 2005, 27, 379–386. [Google Scholar] [CrossRef]
- Jumali, S.S.; Said, I.M.; Ismail, I.; Zainal, Z. Genes induced by high concentration of salicylic acid in ‘Mitragyna speciosa’. Aust. J. Crop Sci. 2011, 5, 296–303. [Google Scholar]
- Khoshbakht, D.; Asgharei, M.R. Influence of foliar-applied salicylic acid on growth, gas-exchange characteristics, and chlorophyll fluorescence in citrus under saline conditions. Photosynthetica 2015, 53, 410–418. [Google Scholar] [CrossRef]
- Horváth, E.; Csiszár, J.; Gallé, Á.; Poór, P.; Szepesi, Á.; Tari, I. Hardening with salicylic acid induces concentration-dependent changes in abscisic acid biosynthesis of tomato under salt stress. J. Plant Physiol. 2015, 183, 54–63. [Google Scholar] [CrossRef]
- Shaki, F.; Maboud, H.E.; Niknam, V. Growth enhancement and salt tolerance of safflower (Carthamus tinctorius L.), by salicylic acid. Curr. Plant Biol. 2018, 13, 16–22. [Google Scholar] [CrossRef]
- Kováčik, J.; Klejdus, B.; Hedbavny, J.; Bačkor, M. Salicylic acid alleviates NaCl-induced changes in the metabolism of Matricaria chamomilla plants. Ecotoxicology 2009, 18, 544–554. [Google Scholar] [CrossRef]
- Palma, F.; Lluch, C.; Iribarne, C.; García-Garrido, J.M.; Tejera García, N.A. Combined effect of salicylic acid and salinity on some antioxidant activities, oxidative stress and metabolite accumulation in Phaseolus vulgaris. Plant Growth Regul. 2009, 58, 307–316. [Google Scholar] [CrossRef]
- Khalifa, G.S.; Abdelrassoul, M.; Hegazi, A.M.; Elsherif, M.H. Attenuation of negative effects of saline stress in two lettuce cultivars by salicylic acid and glycine betaine. Gesunde Pflanz. 2016, 68, 177–189. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Fujita, M. Plant response to salt stress and role of exogenous protectants to mitigate salt-induced damages. In Ecophysiology and Responses of Plants under Salt Stress; Azooz, M.M., Prasad, M.N.V., Eds.; Springer New York: New York, NY, USA, 2013; pp. 25–87. ISBN 978-1-4614-4746-7. [Google Scholar]
- El-Greadly, N. physiological responses, growth, yield and quality of snap beans in response to foliar application of yeast, vitamin E and zinc under sandy soil conditions. Aust. J. Basic Appl. Sci. 2007, 1, 294–299. [Google Scholar]
- Zaghloul, R.A.; Abou-Aly, H.E.; El-Meihy, R.M.; El-Saadony, M.T. Improvement of growth and yield of pea plants using integrated fertilization management. Univers. J. Agric. Res. 2015, 3, 135–143. [Google Scholar] [CrossRef]
- Shalaby, T.A.; El-Ramady, H. Effect of foliar application of bio-stimulants on growth, yield, components, and storability of garlic (Allium sativum L.). Aust. J. Crop Sci. 2014, 8, 271–273. [Google Scholar]
- Fu, S.-F.; Sun, P.-F.; Lu, H.-Y.; Wei, J.-Y.; Xiao, H.-S.; Fang, W.-T.; Cheng, B.-Y.; Chou, J.-Y. Plant Growth-Promoting traits of yeasts isolated from the phyllosphere and rhizosphere of Drosera spatulata Lab. Fungal Biol. 2016, 120, 433–448. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Verma, J.P.; Gaurav, A.K.; Chouhan, G.K.; Patel, J.S.; Hesham, A.E.-L. Yeast a potential bio-agent: Future for plant growth and postharvest disease management for sustainable agriculture. Appl. Microbiol. Biotechnol. 2020, 104, 1497–1510. [Google Scholar] [CrossRef]
- Taha, R.S.; Seleiman, M.F.; Alhammad, B.A.; Alkahtani, J.; Alwahibi, M.S.; Mahdi, A.H.A. Activated yeast extract enhances growth, anatomical structure, and productivity of Lupinus termis L. plants under actual salinity conditions. Agronomy 2020, 11, 74. [Google Scholar] [CrossRef]
- Lutts, S.; Majerus, V.; Kinet, J.M. NaCl effects on proline metabolism in rice (Oryza sativa) seedlings. Physiol. Plant. 1999, 105, 450–458. [Google Scholar] [CrossRef]
- De Lacerda, C.F.; Cambraia, J.; Oliva, M.A.; Ruiz, H.A.; Prisco, J.T. Solute accumulation and distribution during shoot and leaf development in two sorghum genotypes under salt stress. Environ. Exp. Bot. 2003, 49, 107–120. [Google Scholar] [CrossRef]
- Batool, S.; Khan, S.; Basra, S.M.A. Foliar application of moringa leaf extract improves the growth of moringa seedlings in winter. South Afr. J. Bot. 2020, 129, 347–353. [Google Scholar] [CrossRef]
- Helaly, M.; Farouk, S.; Arafa, S.; Amhimmid, N. Inducing salinity tolerance of rosemary (Rosmarinus officinalis L.) plants by chitosan or zeolite application. AJAAR 2018, 5, 1–20. [Google Scholar] [CrossRef]
- El-Gabiery, A.E.; Ata Allah, Y.F.A. Effect of foliar application with bentonite on growth and productivity of egyptian cotton. J. Plant Prod. 2017, 8, 1029–1035. [Google Scholar] [CrossRef][Green Version]
- Ferretti, G.; Di Giuseppe, D.; Faccini, B.; Coltorti, M. Mitigation of sodium risk in a sandy agricultural soil by the use of natural zeolites. Environ. Monit. Assess 2018, 190, 646. [Google Scholar] [CrossRef] [PubMed]
- Hazrati, S.; Tahmasebi-Sarvestani, Z.; Mokhtassi-Bidgoli, A.; Modarres-Sanavy, S.A.M.; Mohammadi, H.; Nicola, S. Effects of zeolite and water stress on growth, yield and chemical compositions of Aloe vera L. Agric. Water Manag. 2017, 181, 66–72. [Google Scholar] [CrossRef]
- Gholamhoseini, M.; AghaAlikhani, M.; Dolatabadian, A.; Khodaei-Joghan, A.; Zakikhani, H. Decreasing nitrogen leaching and increasing canola forage yield in a sandy soil by application of natural zeolite. Agron. J. 2012, 104, 1467–1475. [Google Scholar] [CrossRef]
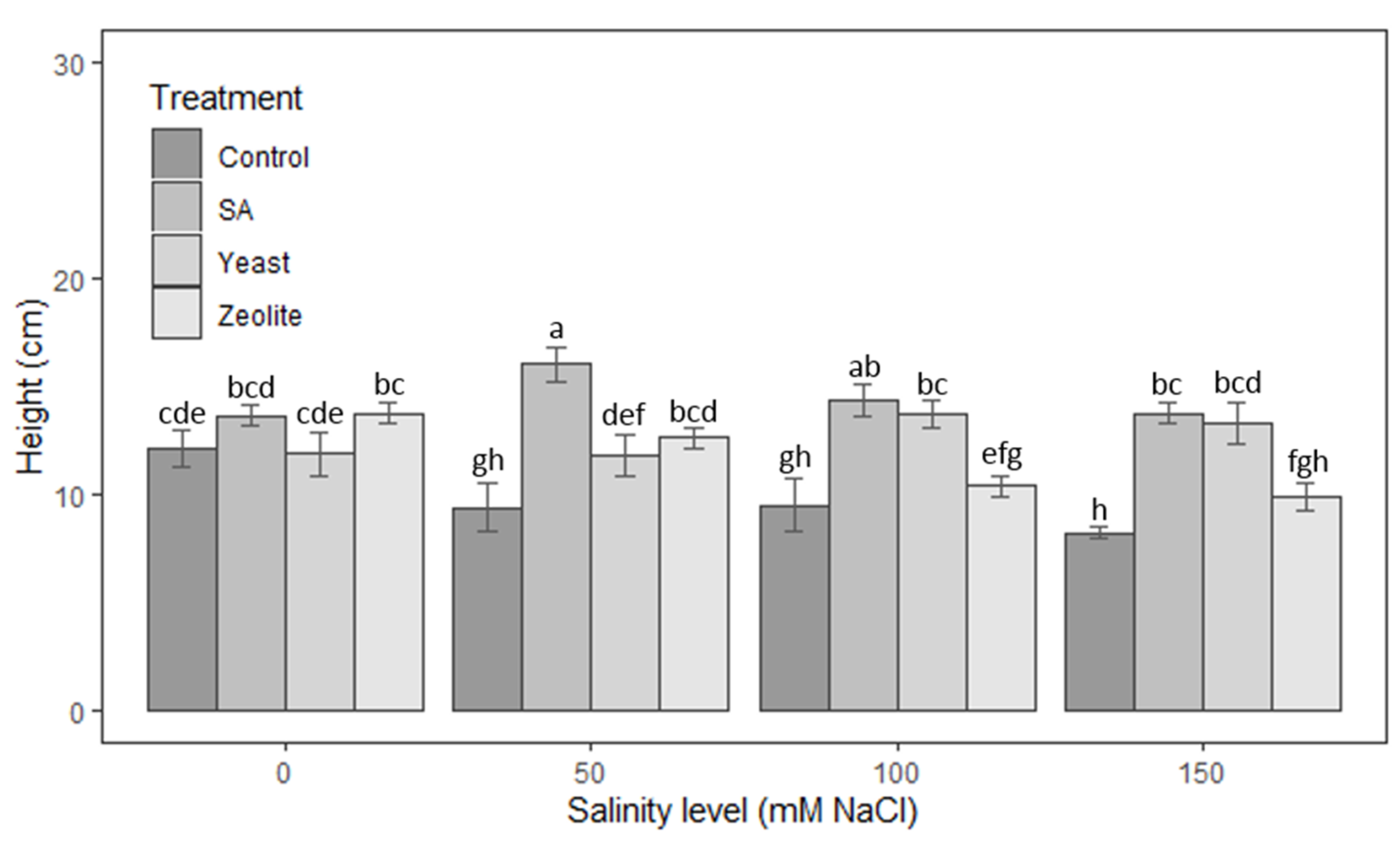
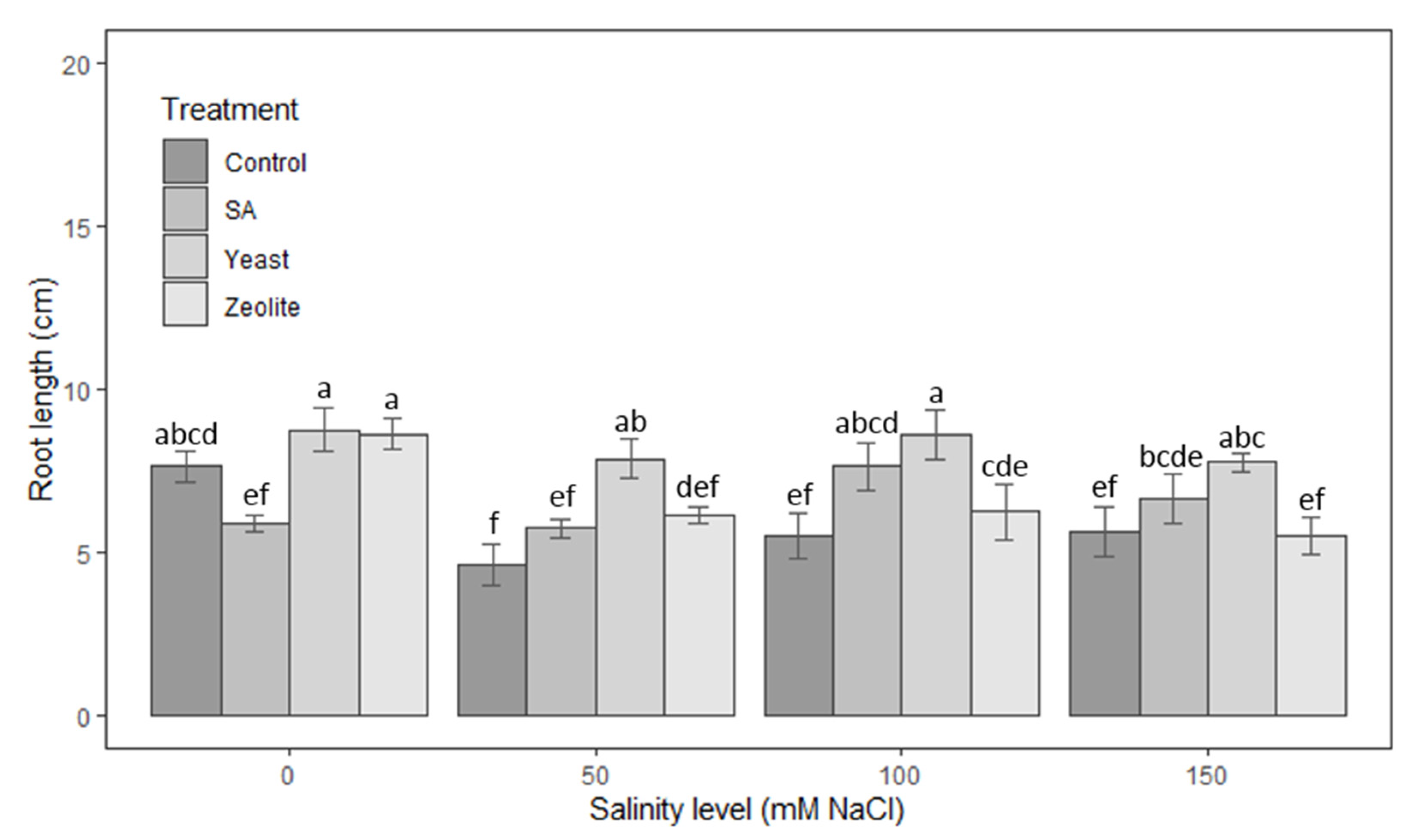
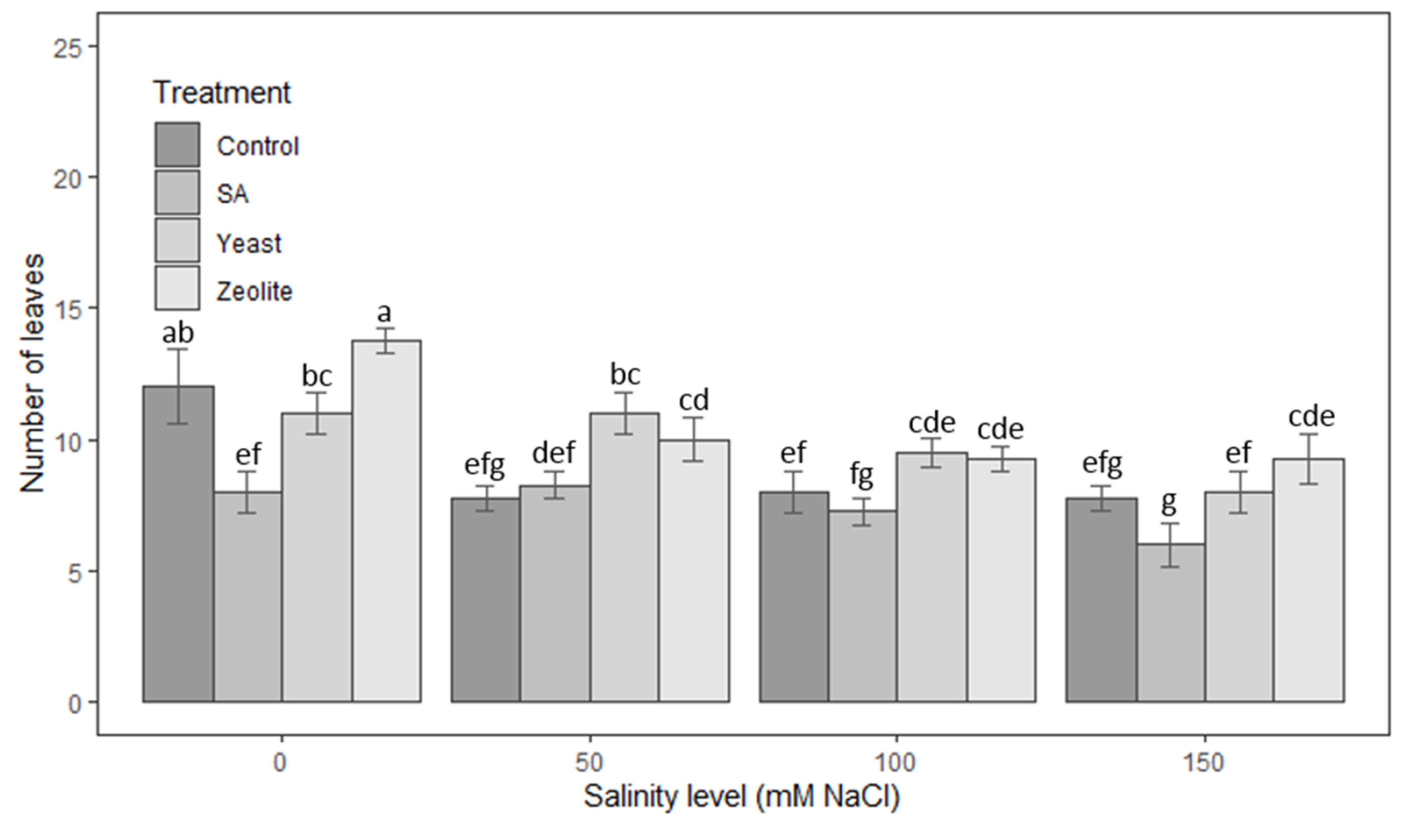
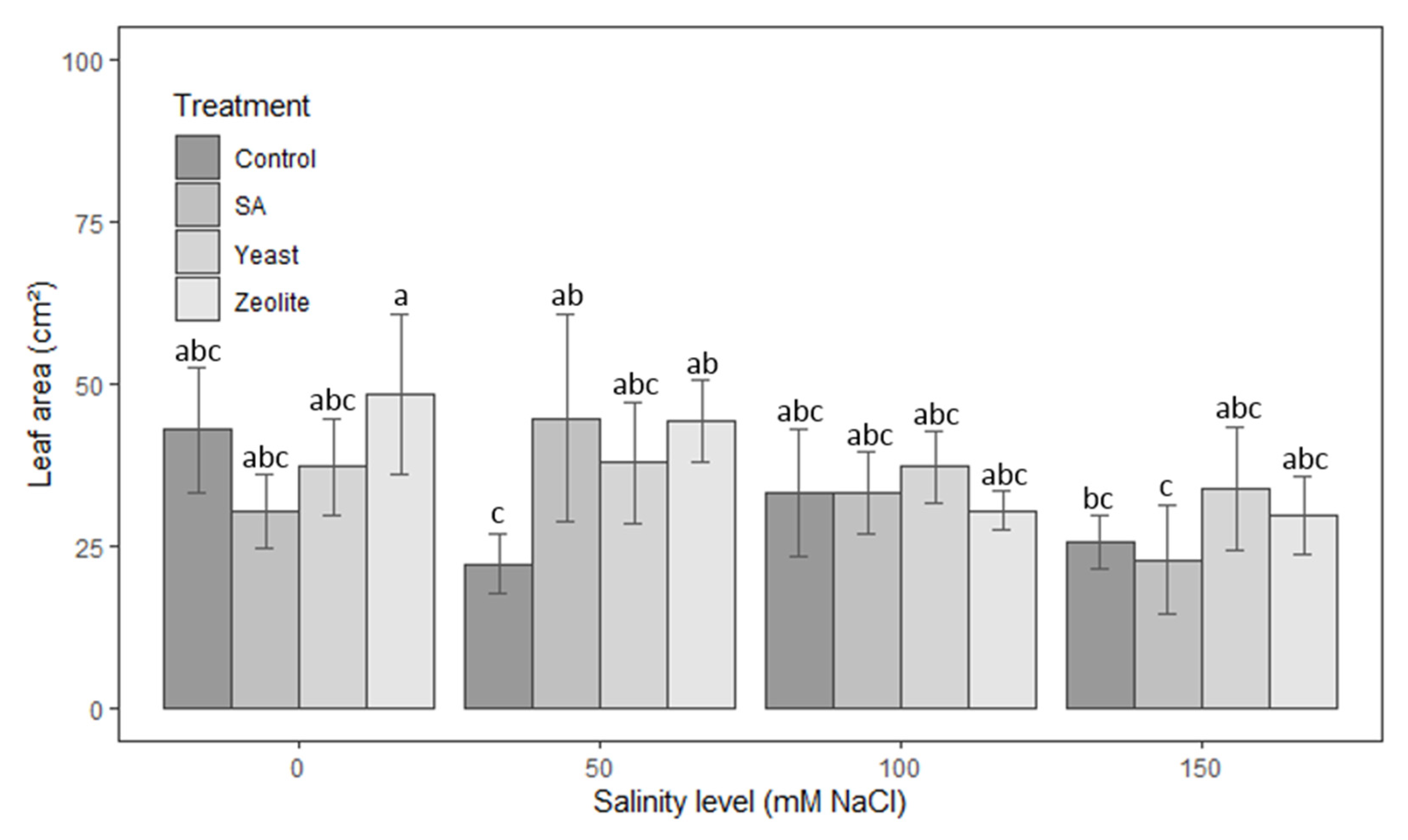
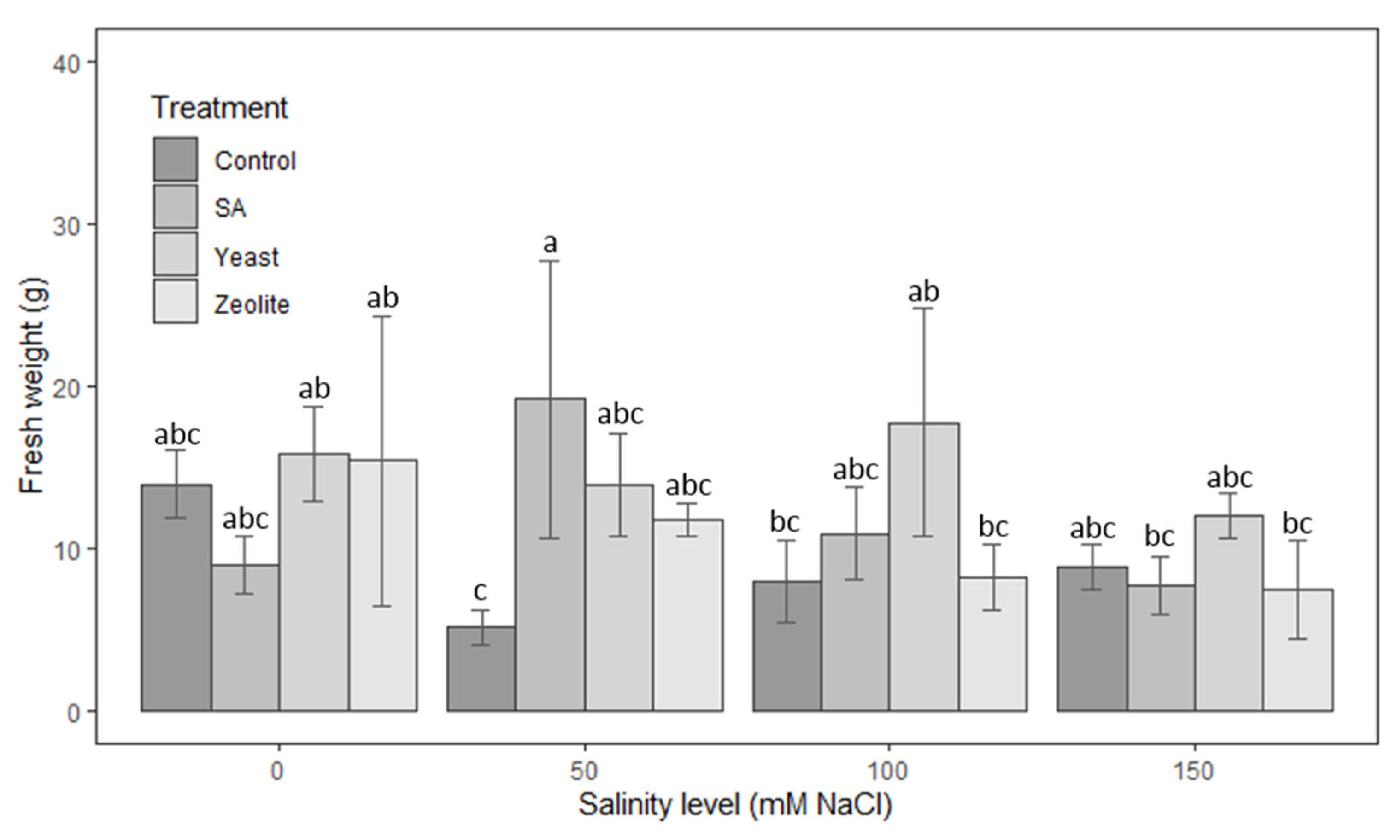
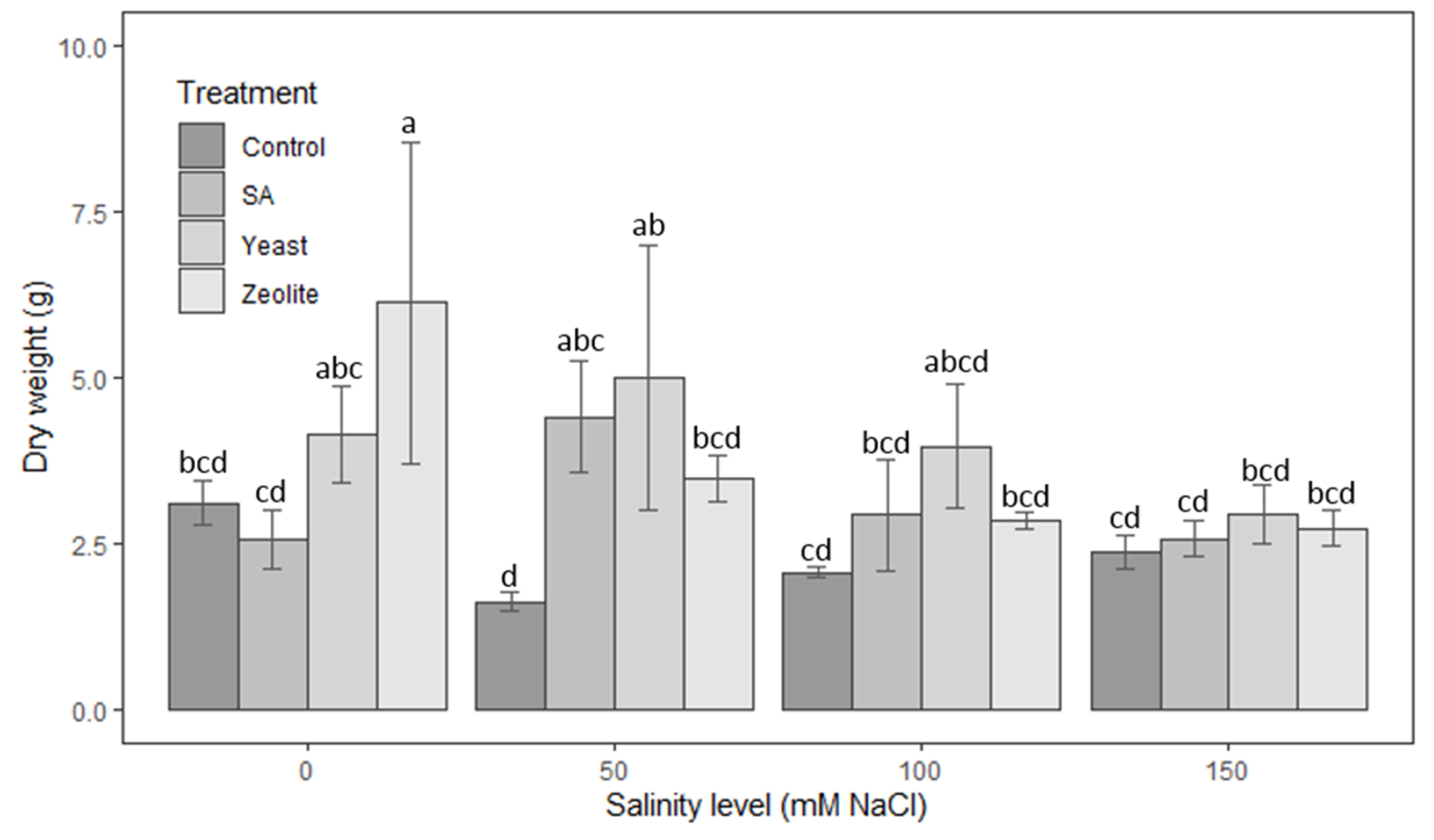
| Source | Df | Height of Shoots | Root Length | Number of Leaves | Leaf Area | Fresh Weight | Dry Weight |
|---|---|---|---|---|---|---|---|
| (cm) | (cm) | unit | (cm2) | (g) | (g) | ||
| Treatment | 3 | 59.87 *** | 16.839 *** | 30.64 *** | 175.2 ns | 97.88 ** | 9.582 *** |
| Salinity | 3 | 7.21 *** | 8.339 *** | 34.89 *** | 418.4 ** | 60.73 * | 5.953 *** |
| Treatment × Salinity | 9 | 8.64 *** | 3.217 *** | 4.47 *** | 207.3 ** | 58.59 ** | 3.860 *** |
| Df | Proline Content | Sugar Content | Chlorophyll a | Chlorophyll b | Total Chlorophyll | |
|---|---|---|---|---|---|---|
| (µg g−1 FW) | (mg 100 mg−1 FW) | (mg g−1 FW) | (mg g−1 FW) | (mg g−1 FW) | ||
| Treatment | 3 | 1786.0 *** | 0.03126 *** | 0.004967 *** | 0.011809 *** | 0.0019023 *** |
| Salinity | 3 | 100.7 *** | 0.00271 *** | 0.000149 *** | 0.002272 *** | 0.0028026 *** |
| Treatment × Salinity | 9 | 191.5 *** | 0.04481 *** | 0.000391 *** | 0.001427 *** | 0.0025205 *** |
| Treatment | Salinity Level (mM NaCl) | Content, as Mean ± S.E. | |
|---|---|---|---|
| Proline (µg g−1 FW) | Sugar (mg 100 mg−1 FW) | ||
| Control | 0 | 25,500 ± 0.064 g | 0.521 ± 0.011 a |
| 50 | 26,200 ± 0.065 g | 0.366 ± 0.011 bcd | |
| 100 | 28,800 ± 0.897 f | 0.363 ± 0.014 cd | |
| 150 | 31,900 ± 1.150 e | 0.235 ± 0.011 fg | |
| Salicylic acid | 0 | 60,300 ± 1.510 a | 0.346 ± 0.011 de |
| 50 | 54,700 ± 1.260 b | 0.318 ± 0.016 e | |
| 100 | 38,200 ± 0.293 c | 0.272 ± 0.008 f | |
| 150 | 37,500 ± 0.247 c | 0.138 ± 0.015 i | |
| Yeast | 0 | 34,400 ± 0.905 d | 0.380 ± 0.012 bcd |
| 50 | 25,100 ± 0.195 g | 0.363 ± 0.007 cd | |
| 100 | 22,500 ± 0.225 h | 0.185 ± 0.015 h | |
| 150 | 15,800 ± 0.497 j | 0.126 ± 0.016 i | |
| Zeolite | 0 | 13,800 ± 1.110 j | 0.403 ± 0.017 b |
| 50 | 19,800 ± 0.462 i | 0.393 ± 0.009 bc | |
| 100 | 20,900 ± 0.816 hi | 0.272 ± 0.006 f | |
| 150 | 25,700 ± 0.362 g | 0.231 ± 0.017 g | |
| Treatment | Salinity Level (mM NaCl) | Content, as Mean ± S.E. (in mg g−1 FW) | ||
|---|---|---|---|---|
| Chlorophyll a | Chlorophyll b | Total Chlorophyll | ||
| Control | 0 | 0.229 ± 0.003 cd | 0.136 ± 0.004 a | 0.364 ± 0.007 bc |
| 50 | 0.224 ± 0.004 cde | 0.125 ± 0.003 bc | 0.349 ± 0.007 cd | |
| 100 | 0.223 ± 0.004 cde | 0.116 ± 0.002 cd | 0.339 ± 0.006 d | |
| 150 | 0.215 ± 0.005 e | 0.104 ± 0.004 e | 0.320 ± 0.008 e | |
| Salicylic acid | 0 | 0.228 ± 0.004 cd | 0.122 ± 0.004 bcd | 0.368 ± 0.005 b |
| 50 | 0.232 ± 0.002 c | 0.121 ± 0.003 bcd | 0.356 ± 0.005 bcd | |
| 100 | 0.232 ± 0.003 c | 0.125 ± 0.003 bc | 0.353 ± 0.005 bcd | |
| 150 | 0.229 ± 0.002 cd | 0.138 ± 0.003 a | 0.350 ± 0.007 cd | |
| Yeast | 0 | 0.283 ± 0.004 a | 0.123 ± 0.002 bc | 0.406 ± 0.005 a |
| 50 | 0.264 ± 0.004 b | 0.053 ± 0.002 f | 0.317 ± 0.006 e | |
| 100 | 0.262 ± 0.002 b | 0.043 ± 0.003 g | 0.305 ± 0.004 e | |
| 150 | 0.262 ± 0.003 b | 0.022 ± 0.002 h | 0.285 ± 0.004 f | |
| Zeolite | 0 | 0.224 ± 0.003 cde | 0.126 ± 0.002 b | 0.370 ± 0.007 b |
| 50 | 0.220 ± 0.001 de | 0.124 ± 0.003 bc | 0.350 ± 0.004 cd | |
| 100 | 0.222 ± 0.003 de | 0.121 ± 0.002 bcd | 0.344 ± 0.004 d | |
| 150 | 0.256 ± 0.003 b | 0.114 ± 0.003 d | 0.343 ± 0.006 d | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Babaousmail, M.; Nili, M.S.; Brik, R.; Saadouni, M.; Yousif, S.K.M.; Omer, R.M.; Osman, N.A.; Alsahli, A.A.; Ashour, H.; El-Taher, A.M. Improving the Tolerance to Salinity Stress in Lettuce Plants (Lactuca sativa L.) Using Exogenous Application of Salicylic Acid, Yeast, and Zeolite. Life 2022, 12, 1538. https://doi.org/10.3390/life12101538
Babaousmail M, Nili MS, Brik R, Saadouni M, Yousif SKM, Omer RM, Osman NA, Alsahli AA, Ashour H, El-Taher AM. Improving the Tolerance to Salinity Stress in Lettuce Plants (Lactuca sativa L.) Using Exogenous Application of Salicylic Acid, Yeast, and Zeolite. Life. 2022; 12(10):1538. https://doi.org/10.3390/life12101538
Chicago/Turabian StyleBabaousmail, Mahfoud, Mohammed S. Nili, Rania Brik, Mohammed Saadouni, Sawsan K. M. Yousif, Rihab M. Omer, Nahid A. Osman, Abdulaziz A. Alsahli, Hatem Ashour, and Ahmed M. El-Taher. 2022. "Improving the Tolerance to Salinity Stress in Lettuce Plants (Lactuca sativa L.) Using Exogenous Application of Salicylic Acid, Yeast, and Zeolite" Life 12, no. 10: 1538. https://doi.org/10.3390/life12101538
APA StyleBabaousmail, M., Nili, M. S., Brik, R., Saadouni, M., Yousif, S. K. M., Omer, R. M., Osman, N. A., Alsahli, A. A., Ashour, H., & El-Taher, A. M. (2022). Improving the Tolerance to Salinity Stress in Lettuce Plants (Lactuca sativa L.) Using Exogenous Application of Salicylic Acid, Yeast, and Zeolite. Life, 12(10), 1538. https://doi.org/10.3390/life12101538







