Manipulation of Senescence of Plants to Improve Biotic Stress Resistance
Abstract
1. Introduction
2. Factors of Plant Senescence That Can Influence Plant–Pathogen Interactions
3. Effect of Pathogen Infection on the Physiological State of Plants
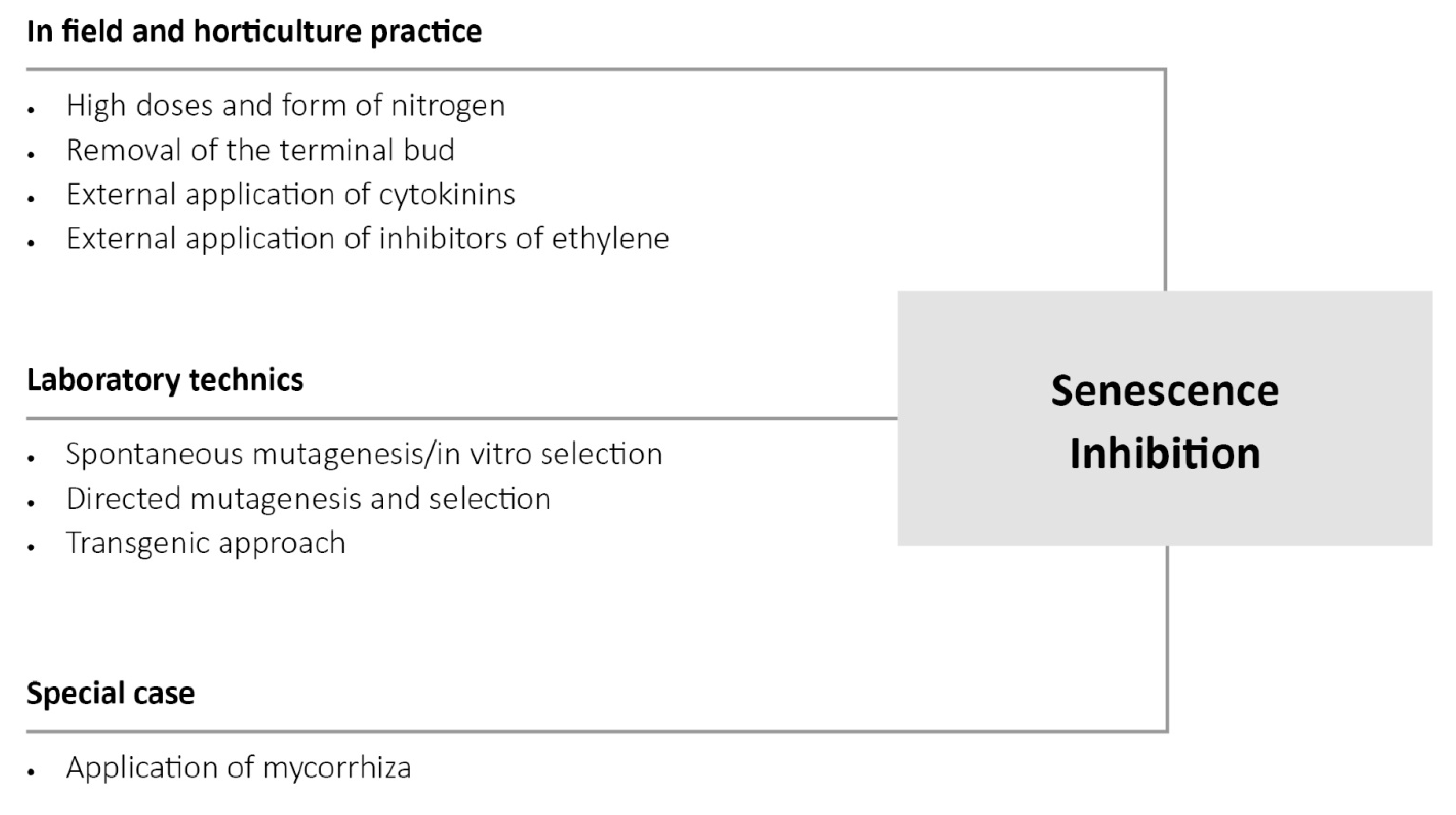
4. Manipulation of the Physiological State of Plants in Order to Improve Biotic and Abiotic Stress Tolerance
5. Concluding Remarks
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Stavely, J.R.; Slana, L.J. Relation of leaf age to reaction of tobacco to Alternaria altemata. Phytopathology 1971, 61, 73–78. [Google Scholar] [CrossRef]
- Lloyd, H.L. Therapeutic effect of kinetin on tobacco alternariosis. Nat. N. Biol. 1972, 240, 94–95. [Google Scholar] [CrossRef] [PubMed]
- Barna, B.; Györgyi, B. Resistance of young versus old tobacco leaves to necrotophs, fusaric acid, cell-wall degrading enzymes and autolysis of membrane lipids. Physiol. Mol. Plant Pathol. 1992, 40, 247–257. [Google Scholar] [CrossRef]
- Barna, B.; Ádám, A.; Király, Z. Juvenility and resistance of a superoxide-tolerant plant to disease and other stresses. Naturwissenschaften 1993, 80, 420–422. [Google Scholar] [CrossRef]
- Häffner, E.; Konietzki, S.; Diederichsen, E. Keeping control: The role of senescence and development in plant pathogenesis and defense. Plants 2015, 4, 449–488. [Google Scholar] [CrossRef] [PubMed]
- Durian, G.; Jeschke, V.; Rahikainen, M.; Vuorinen, K.; Gollan, P.J.; Brosché, M.; Salojärvi, J.; Glawischnig, E.; Winter, Z.; Li, S.; et al. PROTEIN PHOSPHATASE 2A-B’ controls Botrytis cinerea resistance and developmental leaf senescence. Plant Physiol. 2019, 182, 1161–1181. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, H.-L.; Li, Z.; Guo, H. Genetic Network between Leaf Senescence and Plant Immunity: Crucial Regulatory Nodes and New Insights. Plants 2020, 9, 495. [Google Scholar] [CrossRef]
- Marla, S.R.; Chu, K.; Chintamanani, S.; Multani, D.-S.; Klempien, A.; DeLeon, A.; Bong-Suk, K.; Dunkle, L.D.; Dilkes, B.P.; Johal, G.S. Adult plant resistance in maize to northern leaf spot is a feature of partial loss-of-function alleles of Hm1. PLoS Pathog. 2018, 14, e1007356. [Google Scholar] [CrossRef]
- Bostock, R.M.; Pye, M.F.; Roubtsova, T.V. Predisposition in plant disease: Exploiting the nexus in abiotic and biotic stress perception and response. Annu. Rev. Phytopathol. 2014, 52, 517–549. [Google Scholar] [CrossRef]
- Glazebrook, J. Contrasting Mechanisms of Defense against biotrophic and necrotrophic pathogens. Annu. Rev. Phytopathol. 2005, 43, 205–227. [Google Scholar] [CrossRef]
- Barna, B.; Fodor, J.; Harrach, B.D.; Pogány, M.; Király, Z. The Janus face of reactive oxygen species in resistance and susceptibility of plants to necrotrophic and biotrophic pathogens. Plant Physiol. Biochem. 2012, 59, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; He, Y.; Zhang, Z.; Shi, Y.; Zhang, X.; Xu, X.; Wu, J.L.; Tang, S. OsNAC109 regulates senescence, growth and development by altering the expression of senescence- and phytohormone-associated genes in rice. Plant Mol. Biol. 2021, 105, 637–654. [Google Scholar] [CrossRef] [PubMed]
- Mashaal, S.F.; Barna, B.; Király, Z. Effect of photosynthesis inhibitors on wheat stem rust development. Acta Phytopath. Acad. Sci. Hung. 1981, 16, 45–48. [Google Scholar]
- Levine, A.; Tenhaken, R.; Dixon, R.; Lamb, C. H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell 1994, 79, 583–593. [Google Scholar] [CrossRef]
- Baker, C.J.; Orlandi, E.W. Active oxygen in plant pathogenesis. Annu. Rev. Phytopathol. 1995, 33, 299–321. [Google Scholar] [CrossRef]
- Van Breusegem, F.; Dat, J.F. Reactive oxygen species in plant cell death. Plant Physiol. 2006, 141, 384–390. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive Oxygen Species: Metabolism, Oxidative Stress, and Signal Transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Waszczak, C.; Carmody, M.; Kangasjärvi, J. Reactive Oxygen Species in Plant Signaling. Annu. Rev. Plant Biol. 2018, 69, 209–236. [Google Scholar] [CrossRef]
- Bolwell, G.P.; Wojtaszek, P. Mechanisms for the generation of reactive oxygen species in plant defence—A broad perspective. Physiol. Mol. Plant Pathol. 1997, 51, 347–366. [Google Scholar] [CrossRef]
- Torres, M.Á.; Jones, J.D.G.; Dangl, J.L. Reactive oxygen species signalling in response to pathogens. Plant Physiol. 2006, 141, 373–378. [Google Scholar] [CrossRef]
- Gullner, G.; Juhász, C.; Németh, A.; Barna, B. Reactions of tobacco genotypes with different antioxidant capacities to powdery mildew and Tobacco mosaic virus infections. Plant Physiol. Biochem. 2017, 119, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Leshem, Y.Y. Plant senescence processes and free radicals. Free Radic. Biol. Med. 1988, 5, 39–49. [Google Scholar] [CrossRef]
- Harrach, B.D.; Fodor, J.; Pogány, M.; Preuss, J.; Barna, B. Antioxidant, ethylene and membrane leakage responses to powdery mildew infection of near-isogenic barley lines with various types of resistance. Eur. J. Plant Pathol. 2008, 121, 21–33. [Google Scholar] [CrossRef]
- Kim, J.; Hee, J.; Jae, K.; Lyu, I.; Woo, H.R.; Lim, P.O. New insights into the regulation of leaf senescence in Arabidopsis. J. Exp. Bot. 2018, 69, 787–799. [Google Scholar] [CrossRef] [PubMed]
- Gepstein, S.; Sabehi, G.; Carp, M.J.; Hajouj, T.; Nesher, M.F.; Yariv, I.; Dor, C.; Bassani, M. Large-scale identification of leaf senescence-associated genes. Plant J. 2003, 36, 629–642. [Google Scholar] [CrossRef] [PubMed]
- Butt, A.; Mousley, C.; Morris, K.; Beynon, J.; Can, C.; Holub, E.; Greenberg, J.T.; Buchanan-Wollaston, V. Differential expression of a senescence-enhanced metallothionein gene in Arabidopsis in response to isolates of Peronospora parasitica and Pseudomonas syringae. Plant J. 1998, 16, 209–221. [Google Scholar] [CrossRef]
- Pontier, D.; Gan, S.; Amasino, R.M.; Roby, D.; Lam, E. Markers for hypersensitive response and senescence show distinct patterns of expression. Plant Mol. Biol. 1999, 39, 1243–1255. [Google Scholar] [CrossRef]
- Espinoza, C.; Medina, C.; Somerville, S.; Arce-Johnson, P. Senescence-associated genes induced during compatible viral interactions with grapevine and Arabidopsis. J. Exp. Bot. 2007, 58, 3197–3212. [Google Scholar] [CrossRef]
- Huysmans, M.; Lema, A.S.; Coll, N.S.; Nowack, M.K. Dying two deaths—Programmed cell death regulation in development and disease. Curr. Opin. Plant Biol. 2017, 35, 37–44. [Google Scholar] [CrossRef]
- Barna, B.; Gémes, K.; Domoki, M.; Bernula, D.; Ferenc, G.; Bálint, B.; Nagy, I.; Fehér, A. Arabidopsis NAP-related proteins (NRPs) contribute to the coordination of plant growth, developmental rate, and age-related pathogen resistance under short days. Plant Sci. 2018, 267, 124–134. [Google Scholar] [CrossRef]
- Mueller-Roeber, B.; Balazadeh, S. Auxin and Its Role in Plant Senescence. J. Plant Growth Regul. 2014, 33, 21–33. [Google Scholar] [CrossRef]
- Zhang, Z.; Guo, Y. Hormone Treatments in Studying Leaf Senescence. Methods Mol. Biol. 2018, 1744, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Aldesuquy, H.S.; Abdel-Fattah, G.M.; Baka, Z.A. Changes in chlorophyll, polyamines and chloroplast ultrastructure of Puccinia striiformis induced ‘green islands’ on detached leaves of Triticum aestivum. Plant Physiol. Biochem. 2000, 38, 613–620. [Google Scholar] [CrossRef]
- Gelvin, S.B. Agrobacterium-mediated plant transformation: The biology behind the “gene-jockeying” tool. Microbiol. Mol. Biol. Rev. 2003, 67, 16–37. [Google Scholar] [CrossRef] [PubMed]
- Beneventi, M.; Sá, M.; Amorim, R.M.S.; Grossi-de-Sá, M. Role of gibberellins in plant pathogen interaction. In Gibberellins and Gibberellic Acid: Biosynthesis, Regulation and Physiological Effects; Nova Science Publishers, Incorporated: New York, NY, USA, 2015; pp. 47–57. [Google Scholar]
- Zhang, Y.; Liu, Z.; Wang, X.; Wang, J.; Fan, K.; Li, Z.; Lin, W. DELLA proteins negatively regulate dark-induced senescence and chlorophyll degradation in Arabidopsis through interaction with the transcription factor WRKY6. Plant Cell Rep. 2018, 37, 981–992. [Google Scholar] [CrossRef]
- Van Loon, L.; Geraats, B.; Linthorst, H. Ethylene as a modulator of disease resistance in plants. Trends Plant Sci. 2006, 11, 184–191. [Google Scholar] [CrossRef]
- Helliwell, E.E.; Wang, Q.; Yang, Y. Transgenic rice with inducible ethylene production exhibits broad-spectrum disease resistance to the fungal pathogens Magnaporthe oryzae and Rhizoctonia solani. Plant Biotechnol. J. 2013, 11, 33–42. [Google Scholar] [CrossRef]
- Guan, R.; Su, J.; Meng, X.; Li, S.; Liu, Y.; Xu, J.; Zhang, S. Multilayered regulation of ethylene induction plays a positive role in Arabidopsis resistance against Pseudomonas syringae. Plant Physiol. 2015, 169, 299–312. [Google Scholar] [CrossRef]
- Huang, P.Y.; Catinot, J.; Zimmerli, L. Ethylene response factors in Arabidopsis immunity. J. Exp. Bot. 2015, 67, 1231–1241. [Google Scholar] [CrossRef]
- Thomma, B.P.; Eggermont, K.; Penninckx, I.A.; Mauch-Mani, B.; Vogelsang, R.; Cammue, B.P.; Broekaert, W.F. Separate jasmonate-dependent and salicylate-dependent defense-response pathways in Arabidopsis are essential for resistance to distinct microbial pathogens. Proc. Natl. Acad. Sci. USA 1998, 95, 15107–15111. [Google Scholar] [CrossRef]
- Dong, X. SA, JA, ethylene, and disease resistance in plants. Curr. Opin. Plant Biol. 1998, 1, 316–323. [Google Scholar] [CrossRef]
- Zipfel, C. Combined roles of ethylene and endogenous peptides in regulating plant immunity and growth. Proc. Natl. Acad. Sci. USA 2013, 110, 5748–5749. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, X. Salicylic acid: Biosynthesis, perception, and contributions to plant immunity. Curr. Opin. Plant Biol. 2019, 50, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Navarro, L.; Bari, R.; Achard, P.; Lisón, P.; Nemri, A.; Harberd, N.P.; Jones, J.D.G. DELLAs control plant immune responses by modulating the balance of jasmonic acid and salicylic acid signaling. Curr. Biol. 2008, 18, 650–655. [Google Scholar] [CrossRef]
- Fu, Z.Q.; Dong, X. Systemic acquired resistance: Turning local infection into global defense. Annu. Rev. Plant Biol. 2013, 64, 839–863. [Google Scholar] [CrossRef]
- Fodor, J.; Gullner, G.; Ádám, A.L.; Barna, B.; Kőmíves, T.; Király, Z. Local and systemic responses of antioxidants to tobacco mosaic virus infection and to salicylic acid in tobacco: Role in systemic acquired resistance. Plant Physiol. 1997, 114, 1443–1451. [Google Scholar] [CrossRef]
- Király, Z.; Barna, B.; Kecskés, A.; Fodor, J. Down-regulation of antioxidative capacity in a transgenic tobacco which fails to develop acquired resistance to necrotization caused by TMV. Free Radic. Res. 2002, 36, 981–991. [Google Scholar] [CrossRef]
- Morris, K.; MacKerness, S.A.; Page, T.; John, C.F.; Murphy, A.M.; Carr, J.P.; Buchanan-Wollaston, V. Salicylic acid has a role in regulating gene expression during leaf senescence. Plant J. 2000, 23, 677–685. [Google Scholar] [CrossRef]
- He, Y.; Fukushige, H.; Hildebrand, D.F.; Gan, S. Evidence supporting a role of jasmonic acid in Arabidopsis leaf senescence. Plant Physiol. 2002, 128, 876–884. [Google Scholar] [CrossRef]
- Ueda, H.; Kusaba, M. Strigolactone Regulates Leaf Senescence in Concert with Ethylene in Arabidopsis. Plant Physiol. 2015, 169, 138–147. [Google Scholar] [CrossRef]
- Sasse, J.M. Physiological Actions of Brassinosteroids: An Update. J. Plant Growth Regul. 2003, 22, 276–288. [Google Scholar] [CrossRef] [PubMed]
- Sağlam Çağ, S. Brassinosteroids and Senescence. In Brassinosteroids: Plant Growth and Development; Hayat, S., Yusuf, M., Bhardwaj, R., Bajguz, A., Eds.; Springer: Singapore, 2019. [Google Scholar]
- Bajguz, A.; Hayat, S. Effects of brassinosteroids on the plant responses to environmental stresses. Plant Physiol. Biochem. 2009, 47, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.H.; Zhao, Z.Z.; He, J.X. Brassinosteroid Signaling in Plant⁻Microbe Interactions. Int. J. Mol. Sci. 2018, 19, 4091. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.E.; Froese, C.D.; Madey, E.; Smith, M.D.; Hong, Y. Lipid metabolism during plant senescence. Prog. Lipid Res. 1998, 37, 119–141. [Google Scholar] [CrossRef]
- Troncoso-Ponce, M.A.; Cao, X.; Yang, Z.; Ohlrogge, J.B. Lipid turnover during senescence. Plant Sci. 2013, 205–206, 13–19. [Google Scholar] [CrossRef]
- Millner, A.; Atilla-Gokcumen, G.E. Lipid Players of Cellular Senescence. Metabolites 2020, 10, 339. [Google Scholar] [CrossRef]
- Vigh, L.; Horváth, I.; Horváth, L.I.; Dudits, D.; Farkas, T. Protoplast plasmalemma fluidity of hardiness wheats correlates with frost resistance. FEBS Lett. 1979, 107, 291–294. [Google Scholar] [CrossRef]
- Ádám, A.; Barna, B.; Farkas, T.; Király, Z. Effect of TMV induced systemic acquired resistance and removal of the terminal bud on membrane lipids of tobacco leaves. Plant Sci. 1990, 66, 173–179. [Google Scholar] [CrossRef]
- Barna, B.; Pogány, M. Antioxidant enzymes and membrane lipid composition of disease resistant tomato plants regenerated from crown galls. Acta Physiol. Plant. 2001, 23, 273–277. [Google Scholar] [CrossRef]
- Barna, B.; Sarhan, A.R.T.; Király, Z. The effect of age of tomato and maize leaves on resistance to a non-specific and a host specific toxin. Physiol. Plant Pathol. 1985, 27, 159–165. [Google Scholar] [CrossRef]
- Ten Have, A.; Tenberge, K.B.; Benen, J.A.; Tudzynski, P.; Visser, J.; van Kan, J.A. The Contribution of cell Wall Degrading Enzymes to Pathogenesis of Fungal Plant Pathogens. In Agricultural Applications; Springer: Berlin/Heidelberg, Germany, 2002. [Google Scholar]
- Van Vu, B.; Itoh, K.; Nguyen, Q.B.; Tosa, Y.; Nakayashiki, H. Cellulases belonging to glycoside hydrolase families 6 and 7 contribute to the virulence of Magnaporthe oryzae. Mol. Plant Microbe Interact. 2012, 25, 1135–1141. [Google Scholar] [CrossRef] [PubMed]
- Deising, H.; Jungblut, P.R.; Mendgen, K. Differentiation-related proteins of the broad bean rust fungus Uromyces viciae-fabae, as revealed by high resolution two-dimensional polyacrylamide gel electrophoresis. Arch. Microbiol. 1991, 155, 191–198. [Google Scholar] [CrossRef]
- Schmidt, C.S.; Wolf, G.A. Cellulase in the Host–parasite System Phaseolus vulgaris (L.)–Uromyces appendiculatus [Pers.] Link. Eur. J. Plant Pathol. 1999, 105, 285–295. [Google Scholar] [CrossRef]
- Jones, J.D.G.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Loake, G.J. Molecular aspects of plant disease resistance. Annual Plant Reviews, Volume 34. Ann. Bot. 2009, 104, v. [Google Scholar] [CrossRef]
- Andersen, E.J.; Ali, S.; Byamukama, E.; Yen, Y.; Nepal, M.P. Disease Resistance Mechanisms in Plants. Genes 2018, 9, 339. [Google Scholar] [CrossRef]
- Ku, Y.-S.; Sintaha, M.; Cheung, M.-Y.; Lam, H.M. Plant hormone signaling crosstalks between biotic and abiotic stress responses. Int. J. Mol. Sci. 2018, 19, 3206. [Google Scholar] [CrossRef]
- Denancé, N.; Sánchez-Vallet, A.; Goner, D.; Molina, A. Disease resistance or growth: The role of plant hormones in balancing immune responses and fitness costs. Front. Plant Sci. 2013, 4, 155. [Google Scholar] [CrossRef]
- Han, X.; Kahmann, R. Manipulation of Phytohormone Pathways by effectors of Filamentous Plant Pathogens. Front. Plant Sci. 2019, 10, 822. [Google Scholar] [CrossRef]
- Dobrev, P.I.; Kamínek, M. Fast and efficient separation of cytokinins from auxin and abscisic acid and their purification using mixed-mode solid-phase extraction. J. Chromatogr. A 2002, 950, 21–29. [Google Scholar] [CrossRef]
- Dziurka, M.; Janeczko, A.; Juhász, C.; Gullner, G.; Oklestkova, J.; Novak, O.; Saja, D.; Skoczowski, A.; Tóbiás, I.; Barna, B. Local and systemic hormonal responses in pepper leaves during compatible and incompatible pepper-tobamovirus interactions. Plant Physiol. Biochem. 2016, 109, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Saja, D.; Janeczko, A.; Barna, B.; Skoczowski, A.; Dziurka, M.; Kornaś, A.; Gullner, G. Powdery Mildew-Induced Hormonal and Photosynthetic Changes in Barley Near Isogenic Lines Carrying Various Resistant Genes. Int. J. Mol. Sci. 2020, 21, 4536. [Google Scholar] [CrossRef] [PubMed]
- Kasote, D.M.; Jayaprakasha, G.K.; Ong, K.; Crosby, K.M.; Patil, B.S. Hormonal and metabolites responses in Fusarium wilt-susceptible and -resistant watermelon plants during plant-pathogen interactions. BMC Plant Biol. 2020, 20, 481. [Google Scholar] [CrossRef] [PubMed]
- Dermastia, M. Plant Hormones in Phytoplasma Infected Plants. Front. Plant Sci. 2019, 10, 477. [Google Scholar] [CrossRef]
- Alazem, M.; Lin, N.-S. Roles of plant hormones in the regulation of host-virus interactions. Mol. Plant Pathol. 2015, 16, 529–540. [Google Scholar] [CrossRef]
- Chanclud, E.; Morel, J.-B. Plant hormones: A fungal point of view. Mol. Plant Pathol. 2016, 17, 1289–1297. [Google Scholar] [CrossRef]
- Huber, D.M.; Watson, R.D. Nitrogen Form and Plant Disease. Annu. Rev. Phytopathol. 1974, 12, 139–165. [Google Scholar] [CrossRef]
- Dietrich, R.; Ploss, K.; Heil, M. Constitutive and induced resistance to pathogens in Arabidopsis thaliana depends on nitrogen supply. Plant Cell Environ. 2004, 27, 896–906. [Google Scholar] [CrossRef]
- Mur, L.A.; Simpson, C.; Kumari, A.; Gupta, A.K.; Gupta, K.J. Moving nitrogen to the centre of plant defence against pathogens. Ann. Bot. 2017, 119, 703–709. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, M.; Mur, L.A.J.; Shen, Q.; Guo, S. Unravelling the Roles of Nitrogen Nutrition in Plant Disease Defences. Int. J. Mol. Sci. 2020, 21, 572. [Google Scholar] [CrossRef]
- Darrall, N.M.; Wareing, P.F. The Effect of Nitrogen Nutrition on Cytokinin Activity and Free Amino Acids in Betula pendula Roth, and Acer pseudoplatanus L. J. Exp. Bot. 1981, 32, 369–379. [Google Scholar] [CrossRef]
- Sarhan, A.R.T.; Barna, B.; Király, Z. Effect of nitrogen nutrition on Fusarium wilt of tomato plants. Ann. Appl. Biol. 1982, 101, 242–250. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, M.; Sun, Y.; Gu, Z.; Wang, R.; Saydin, A.; Shen, Q.; Guo, S. Nitrate Increased Cucumber Tolerance to Fusarium Wilt by Regulating Fungal Toxin Production and Distribution. Toxins 2017, 9, 100. [Google Scholar] [CrossRef] [PubMed]
- Mashaal, S.F.; Barna, B.; Király, Z. Effect of nitrogen supply and peroxidase enzyme activity on susceptibility of wheat to stem rust. Acta Phytopath. Acad. Sci. Hung. 1976, 11, 161–166. [Google Scholar]
- Chen, Y.; Zhang, F.; Tang, L.; Zheng, Y.; Li, Y.; Christie, P.; Li, L. Wheat powdery mildew and foliar N concentrations as influenced by N fertilization and belowground interactions with intercropped faba bean. Plant Soil 2007, 291, 1–13. [Google Scholar] [CrossRef]
- Devadas, R.; Simpfendorfer, S.; Backhouse, D.; Lamb, D.W. Effect of stripe rust on the yield response of wheat to nitrogen. Crop J. 2014, 2, 201–206. [Google Scholar] [CrossRef]
- Balázs, E.; Barna, B.; Király, Z. Effect of kinetin on lesion development and infection sites in Xanthi-nc tobacco infected by TMV: Single-cell local lesions. Acta Phytopath. Acad. Sci. Hung. 1976, 11, 1–9. [Google Scholar]
- Choi, J.; Choi, D.; Lee, S.; Ryu, C.-M.; Hwang, I. Cytokinins and plant immunity: Old foes or new friends? Trends Plant Sci. 2011, 16, 389–394. [Google Scholar] [CrossRef]
- Grosskinsky, D.B.; Edelsbrunner, K.; Pfeifhofer, H.; van der Graaff, E.; Roitsch, T. Cis- and trans-zeatin differentially modulate plant immunity. Plant Signal. Behav. 2013, 8, e24798. [Google Scholar] [CrossRef]
- Babosha, A.V. Regulation of resistance and susceptibility in wheat- powdery mildew pathosystem with exogenous cytokinins. J. Plant Physiol. 2009, 166, 1892–1903. [Google Scholar] [CrossRef]
- Smigocki, A.C.; Neal, J.W., Jr.; McCanna, I.; Douglass, L. Cytokinin-mediated insect resistance in Nicotiana plants transformed with the ipt gene. Plant Mol. Biol. 1993, 23, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, S.S.; Mekureyaw, M.F.; Pandey, C.; Roitsch, T. Role of Cytokinins for Interactions of Plants with Microbial Pathogens and Pest Insects. Front. Plant Sci. 2020, 10, 1777. [Google Scholar] [CrossRef]
- Ohtsubo, N.; Mitsuhara, I.; Koga, M.; Seo, S.; Ohashi, Y. Ethylene promotes the necrotic lesion formation and basic PR gene expression in TMV-infected tobacco. Plant Cell Physiol. 1999, 40, 808–817. [Google Scholar] [CrossRef]
- Barna, B.; Pogány, M.; Koehl, J.; Heiser, I.; Elstner, E.F. Induction of ethylene synthesis and lipid peroxidation in damaged or TMV infected tobacco leaf tissues by light. Acta Physiol. Plant. 2012, 34, 1905–1914. [Google Scholar] [CrossRef]
- Khan, A.S.; Ali, S. Chapter 9—Preharvest Sprays Affecting Shelf Life and Storage Potential of Fruits. In Preharvest Modulation of Postharvest Fruit and Vegetable Quality; Siddiqui, M.W., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 209–255. ISBN 9780128098073. [Google Scholar]
- Schaller, G.E.; Binder, B.M. Inhibitors of Ethylene Biosynthesis and Signaling. Methods Mol. Biol. 2017, 1573, 223–235. [Google Scholar] [CrossRef]
- Ali, S.; Masud, T.; Ali, A.; Abbasi, K.S.; Hussain, S. Influence of packaging material and ethylene scavenger on biochemical composition and enzyme activity of apricot cv. Habi at ambient storage. Food Sci. Qual. Manag. 2015, 35, 73–82. [Google Scholar]
- Székács, A.; Hegedüs, G.; Tobiás, I.; Pogány, M.; Barna, B. Immunoassays for plant cytokinins as tools for the assessment of environmental stress and disease resistance. Anal. Chim. Acta 2000, 421, 135–146. [Google Scholar] [CrossRef]
- Barna, B.; Fodor, J.; Pogány, M.; Király, Z. Role of reactive oxygen species and antioxidants in plant disease resistance. Pest Manag. Sci. 2003, 59, 459–464. [Google Scholar] [CrossRef]
- Hirsch, J.; Deslandes, L.; Feng, D.X.; Balagué, C.; Marco, Y. Delayed symptom development in ein2-1, an Arabidopsis ethylene-insensitive mutant, in response to bacterial wilt caused by Ralstonia solanacearum. Phytopathology 2002, 92, 1142–1148. [Google Scholar] [CrossRef]
- Woo, H.R.; Kim, J.H.; Nam, H.G.; Lim, P.O. The Delayed Leaf Senescence Mutants of Arabidopsis, ore1, ore3, and ore9 are Tolerant to Oxidative Stress. Plant Cell Physiol. 2004, 45, 923–932. [Google Scholar] [CrossRef]
- Kim, Y.S.; Sakuraba, Y.; Han, S.H.; Yoo, S.C.; Paek, N.C. Mutation of the Arabidopsis NAC016 transcription factor delays leaf senescence. Plant Cell Physiol. 2013, 54, 1660–1672. [Google Scholar] [CrossRef] [PubMed]
- Lund, S.T.; Stall, R.E.; Klee, H.J. Ethylene Regulates the Susceptible Response to Pathogen Infection in Tomato. Plant Cell 1998, 10, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Knoestera, M.; Linthorsta, H.J.M.; Bola, J.F.; van Loon, L.C. Modulation of stress-inducible ethylene biosynthesis by sense and antisense gene expression in tobacco. Plant Sci. 1997, 126, 173–183. [Google Scholar] [CrossRef]
- Barna, B.; Smigocki, A.C.; Baker, J.C. Transgenic Production of Cytokinin Suppresses Bacterially Induced HR Symptoms and Increases Antioxidative Enzyme Levels in Nicotiana. Phytopathology 2008, 98, 1242–1247. [Google Scholar] [CrossRef] [PubMed]
- Smigocki, A.C. Expression of a wound-inducible cytokinin biosynthesis gene in transgenic tobacco: Correlation of root expression with induction of cytokinin effects. Plant Sci. 1995, 109, 153–163. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Al Mahmud, J.; Fujita, M.; Fotopoulos, V. Reactive Oxygen Species and Antioxidant Defense in Plants under Abiotic Stress: Revisiting the Crucial Role of a Universal Defense Regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Allen, R.D.; Webb, R.P.; Schake, S.A. Use of Transgenic Plants to Study Antioxidant Defenses. Free Radic. Biol. Med. 1997, 23, 473–479. [Google Scholar] [CrossRef]
- Bruggeman, Q.; Raynaud, C.; Benhamed, M.; Delarue, M. To die or not to die? Lessons from lesion mimic mutants. Front. Plant Sci. 2015, 6, 24. [Google Scholar] [CrossRef]
- Zheng, Y.; Xu, J.; Wang, F.; Tang, Y.; Wei, Z.; Ji, Z.; Wang, C.; Zhao, K. Mutation Types of CYP71P1 Cause Different Phenotypes of Mosaic Spot Lesion and Premature Leaf Senescence in Rice. Front. Plant Sci. 2021, 12, 641300. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Liu, L.; Liu, Q.; Bi, Z.; Yu, N.; Cheng, S.; Cao, L. Fine mapping of the lesion mimic and early senescence 1 (lmes1) in rice (Oryza sativa). Plant Physiol. Biochem. 2014, 80, 300–307. [Google Scholar] [CrossRef] [PubMed]
- McGrann, G.R.D.; Steed, A.; Burt, C.; Nicholson, P.; Brown, J.K.M. Differential effects of lesion mimic mutants in barley on disease development by facultative pathogens. J. Exp. Bot. 2015, 66, 3417–3428. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.C.; Martinez-Medina, A.; Lopez-Raez, J.A.; Pozo, M.J. Mycorrhiza-induced resistance and priming of plant defenses. J. Chem. Ecol. 2012, 38, 651–664. [Google Scholar] [CrossRef] [PubMed]
- Fiorilli, V.; Vannini, C.; Ortolani, F.; Garcia-Seco, D.; Chiapello, M.; Novero, M.; Domingo, G.; Terzi, V.; Morcia, C.; Bagnaresi, P.; et al. Omics approaches revealed how arbuscular mycorrhizal symbiosis enhances yield and resistance to leaf pathogen in wheat. Sci. Rep. 2018, 8, 9625. [Google Scholar] [CrossRef]
- Waller, F.; Achatz, B.; Baltruschat, H.; Fodor, J.; Becker, K.; Fischer, M.; Heier, T.; Hückelhoven, R.; Neumann, C.; von Wettstein, D.; et al. The endophytic fungus Piriformospora indica reprograms barley to salt-stress tolerance, disease resistance, and higher yield. Proc. Natl. Acad. Sci. USA 2005, 102, 13386–13391. [Google Scholar] [CrossRef]
- Baltruschat, H.; Fodor, J.; Harrach, B.D.; Niemczyk, E.; Barna, B.; Gullner, G.; Janeczko, A.; Kogel, K.H.; Schäfer, P.; Schwarczinger, I.; et al. Salt tolerance of barley induced by the root endophyte Piriformospora indica is associated with a strong increase in antioxidants. New Phytol. 2008, 180, 501–510. [Google Scholar] [CrossRef]
- Harrach, B.D.; Baltruschat, H.; Barna, B.; Fodor, J.; Kogel, K.-H. The mutualistic fungus Piriformospora indica protects barley roots from a loss of antioxidant capacity caused by the necrotrophic pathogen Fusarium culmorum. Mol. Plant-Microbe Interact. 2013, 26, 599–605. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barna, B. Manipulation of Senescence of Plants to Improve Biotic Stress Resistance. Life 2022, 12, 1496. https://doi.org/10.3390/life12101496
Barna B. Manipulation of Senescence of Plants to Improve Biotic Stress Resistance. Life. 2022; 12(10):1496. https://doi.org/10.3390/life12101496
Chicago/Turabian StyleBarna, Balázs. 2022. "Manipulation of Senescence of Plants to Improve Biotic Stress Resistance" Life 12, no. 10: 1496. https://doi.org/10.3390/life12101496
APA StyleBarna, B. (2022). Manipulation of Senescence of Plants to Improve Biotic Stress Resistance. Life, 12(10), 1496. https://doi.org/10.3390/life12101496




