Light-Driven Synthetic Biology: Progress in Research and Industrialization of Cyanobacterial Cell Factory
Abstract
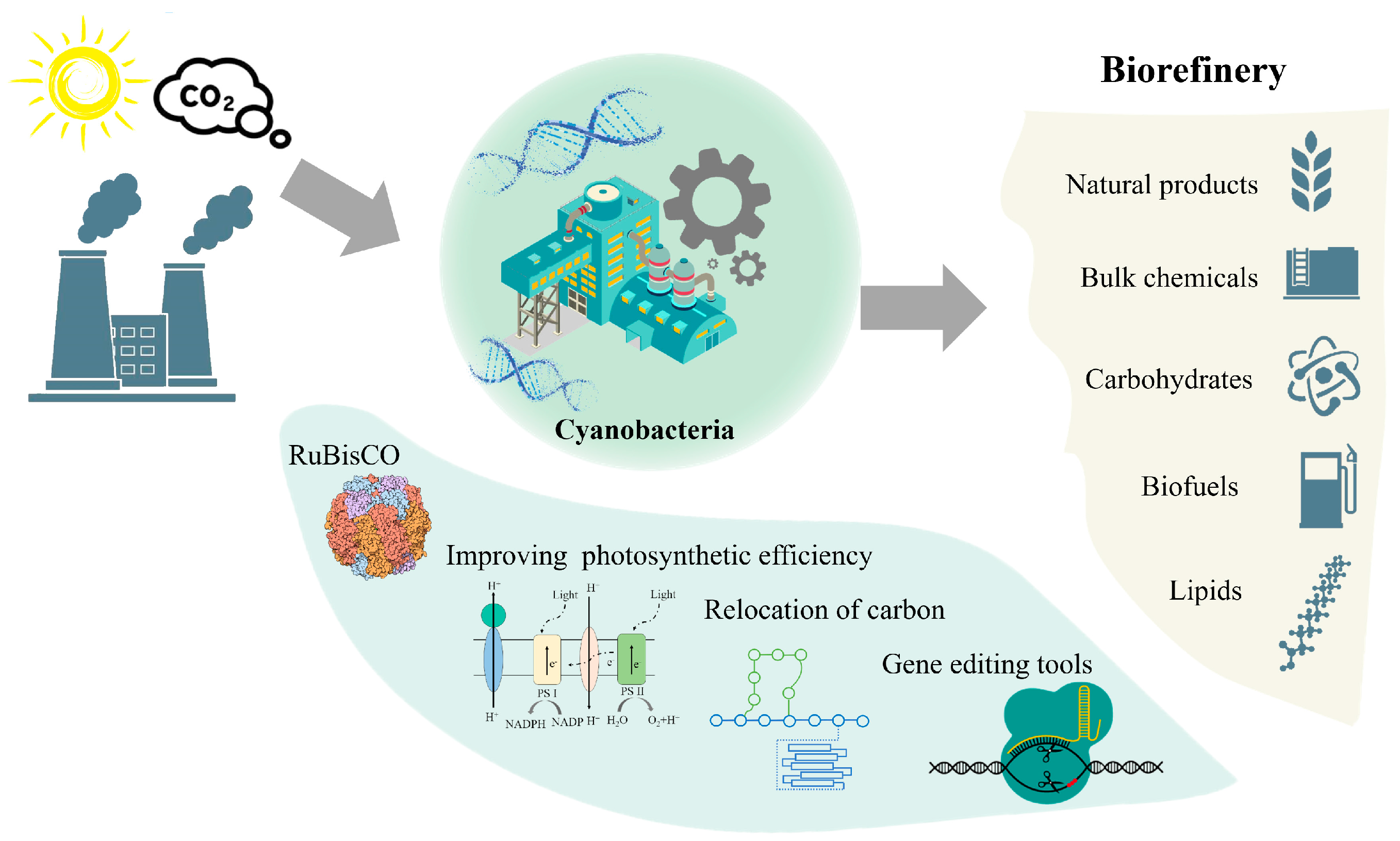
1. Introduction
2. Chassis Selection of Engineered Cyanobacteria
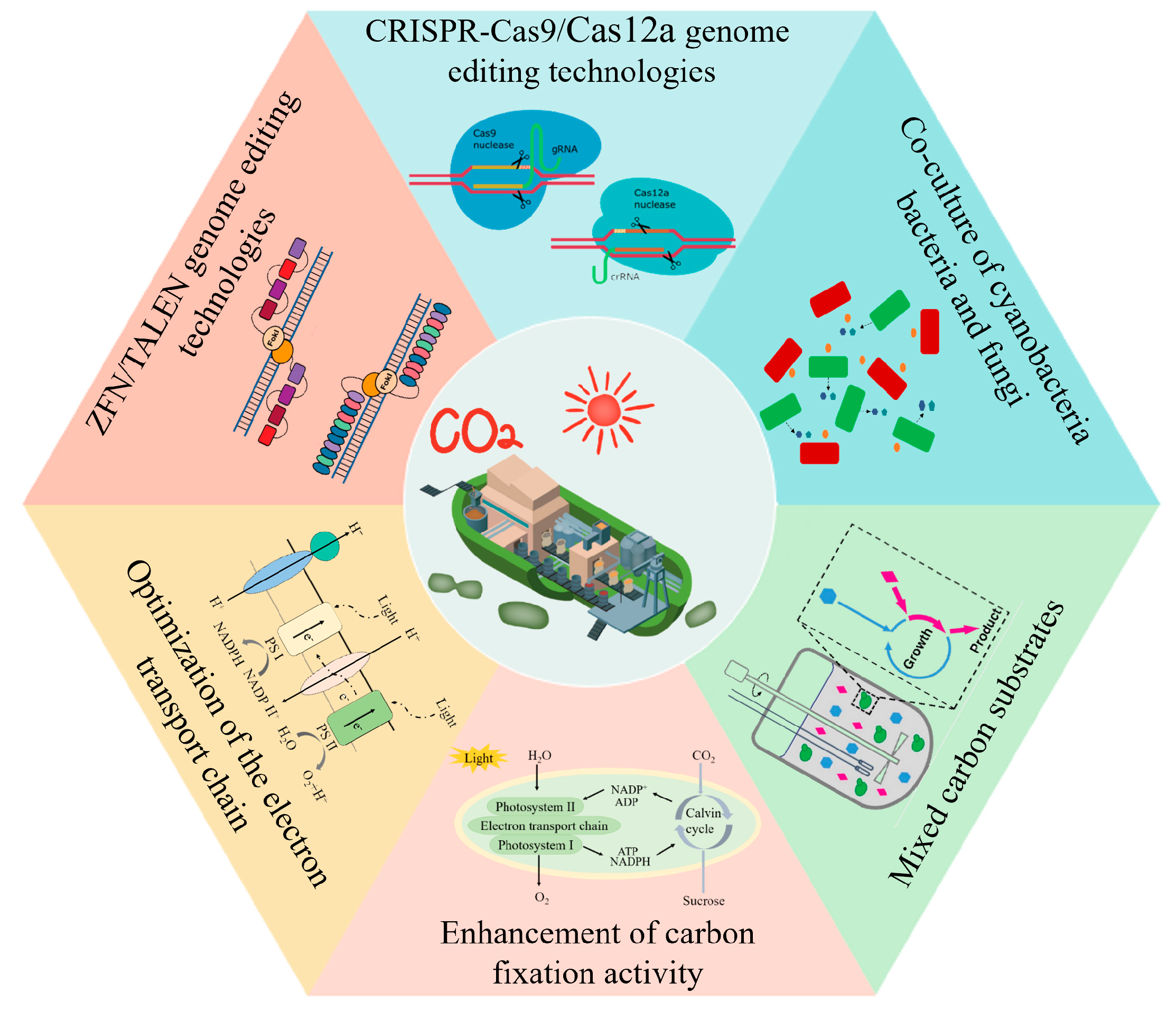
3. Enabling Technologies for Light-Driven Synthetic Biology
3.1. Gene-Editing Technology
3.1.1. ZFNs and TALENs
3.1.2. CRISPR-Cas9 and CRISPR-Cas12a
3.2. Enhancement of Photosynthetic Efficiency
3.3. Enhancement of Carbon-Fixation Efficiency
3.4. “Autotrophic–Heterotrophic” Symbiosis Platform
4. Research Progresses in the Application of Light-Driven Cyanobacterial Cell Factory
4.1. Biofuels
4.2. Bulk Chemicals
4.3. Carbohydrates
4.4. High-Value Natural Products
5. The Industrialization of Light-Driven Cyanobacterial Cell Factory
6. Remaining Challenges
7. Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sakimoto, K.K.; Wong, A.B.; Yang, P. Self-Photosensitization of Nonphotosynthetic Bacteria for Solar-to-Chemical Production. Science 2016, 351, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.C.; Mi, L.; Pontrelli, S.; Luo, S. Fuelling the Future: Microbial Engineering for the Production of Sustainable Biofuels. Nat. Rev. Microbiol. 2016, 14, 288–304. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Kishore, G.M.; Pakrasi, H.B. Emerging Platforms for Co-Utilization of One-Carbon Substrates by Photosynthetic Organisms. Curr. Opin. Biotechnol. 2018, 53, 201–208. [Google Scholar] [CrossRef]
- Case, A.E.; Atsumi, S. Cyanobacterial Chemical Production. J. Biotechnol. 2016, 231, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Kanno, M.; Carroll, A.L.; Atsumi, S. Global Metabolic Rewiring for Improved CO2 Fixation and Chemical Production in Cyanobacteria. Nat. Commun. 2017, 8, 14724. [Google Scholar] [CrossRef]
- Ruffing, A.M.; Jensen, T.J.; Strickland, L.M. Genetic Tools for Advancement of Synechococcus Sp. PCC 7002 as a Cyanobacterial Chassis. Microb. Cell Factories 2016, 15, 190. [Google Scholar] [CrossRef] [PubMed]
- Demay, J.; Bernard, C.; Reinhardt, A.; Marie, B. Natural Products from Cyanobacteria: Focus on Beneficial Activities. Mar. Drugs 2019, 17, E320. [Google Scholar] [CrossRef]
- Knoot, C.J.; Ungerer, J.; Wangikar, P.P.; Pakrasi, H.B. Cyanobacteria: Promising Biocatalysts for Sustainable Chemical Production. J. Biol. Chem. 2018, 293, 5044–5052. [Google Scholar] [CrossRef]
- Li, Z.; He, D.; Yan, X.; Dai, S.; Younan, S.; Ke, Z.; Pan, X.; Xiao, X.; Wu, H.; Gu, J. Size-Dependent Nickel-Based Electrocatalysts for Selective CO2 Reduction. Angew. Chem. Int. Ed. 2020, 59, 18572–18577. [Google Scholar] [CrossRef]
- Hu, Q.; Sommerfeld, M.; Jarvis, E.; Ghirardi, M.; Posewitz, M.; Seibert, M.; Darzins, A. Microalgal Triacylglycerols as Feedstocks for Biofuel Production: Perspectives and Advances. Plant J. 2008, 54, 621–639. [Google Scholar] [CrossRef]
- Dorrell, R.G.; Smith, A.G. Do Red and Green Make Brown?: Perspectives on Plastid Acquisitions within Chromalveolates. Eukaryot. Cell 2011, 10, 856–868. [Google Scholar] [CrossRef] [PubMed]
- Grigorieva, G.; Shestakov, S. Transformation in the Cyanobacterium Synechocystis Sp. 6803. FEMS Microbiol. Lett. 1982, 13, 367–370. [Google Scholar] [CrossRef]
- Marraccini, P.; Bulteau, S.; Cassier-Chauvat, C.; Mermet-Bouvier, P.; Chauvat, F. A Conjugative Plasmid Vector for Promoter Analysis in Several Cyanobacteria of the Genera Synechococcus and Synechocystis. Plant Mol. Biol. 1993, 23, 905–909. [Google Scholar] [CrossRef] [PubMed]
- Zang, X.; Liu, B.; Liu, S.; Arunakumara, K.K.I.U.; Zhang, X. Optimum Conditions for Transformation of Synechocystis Sp. PCC 6803. J. Microbiol. Seoul Korea 2007, 45, 241–245. [Google Scholar]
- Yu, J.; Liberton, M.; Cliften, P.F.; Head, R.D.; Jacobs, J.M.; Smith, R.D.; Koppenaal, D.W.; Brand, J.J.; Pakrasi, H.B. Synechococcus Elongatus UTEX 2973, a Fast Growing Cyanobacterial Chassis for Biosynthesis Using Light and CO2. Sci. Rep. 2015, 5, 8132. [Google Scholar] [CrossRef] [PubMed]
- Dexter, J.; Fu, P. Metabolic Engineering of Cyanobacteria for Ethanol Production. Energy Environ. Sci. 2009, 2, 857–864. [Google Scholar] [CrossRef]
- Gao, Z.; Zhao, H.; Li, Z.; Tan, X.; Lu, X. Photosynthetic Production of Ethanol from Carbon Dioxide in Genetically Engineered Cyanobacteria. Energy Environ. Sci. 2012, 5, 9857–9865. [Google Scholar] [CrossRef]
- Angermayr, S.A.; Hellingwerf, K.J. On the Use of Metabolic Control Analysis in the Optimization of Cyanobacterial Biosolar Cell Factories. J. Phys. Chem. B 2013, 117, 11169–11175. [Google Scholar] [CrossRef]
- Bellefleur, M.P.A.; Wanda, S.-Y.; Curtiss, R. Characterizing Active Transportation Mechanisms for Free Fatty Acids and Antibiotics in Synechocystis Sp. PCC 6803. BMC Biotechnol. 2019, 19, 5. [Google Scholar] [CrossRef]
- Veetil, V.P.; Angermayr, S.A.; Hellingwerf, K.J. Ethylene Production with Engineered Synechocystis Sp PCC 6803 Strains. Microb. Cell Factories 2017, 16, 34. [Google Scholar] [CrossRef][Green Version]
- Shimada, N.; Okuda, Y.; Maeda, K.; Umeno, D.; Takaichi, S.; Ikeuchi, M. Astaxanthin Production in a Model Cyanobacterium Synechocystis Sp. PCC 6803. J. Gen. Appl. Microbiol. 2020, 66, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Brey, L.F.; Włodarczyk, A.J.; Bang Thøfner, J.F.; Burow, M.; Crocoll, C.; Nielsen, I.; Zygadlo Nielsen, A.J.; Jensen, P.E. Metabolic Engineering of Synechocystis Sp. PCC 6803 for the Production of Aromatic Amino Acids and Derived Phenylpropanoids. Metab. Eng. 2020, 57, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Tsinoremas, N.F.; Kutach, A.K.; Strayer, C.A.; Golden, S.S. Efficient Gene Transfer in Synechococcus Sp. Strains PCC 7942 and PCC 6301 by Interspecies Conjugation and Chromosomal Recombination. J. Bacteriol. 1994, 176, 6764–6768. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.-D.; Coleman, J.R. Ethanol Synthesis by Genetic Engineering in Cyanobacteria. Appl. Environ. Microbiol. 1999, 65, 523–528. [Google Scholar] [CrossRef]
- Lan, E.I.; Liao, J.C. ATP Drives Direct Photosynthetic Production of 1-Butanol in Cyanobacteria. Proc. Natl. Acad. Sci. USA 2012, 109, 6018–6023. [Google Scholar] [CrossRef]
- Atsumi, S.; Higashide, W.; Liao, J.C. Direct Photosynthetic Recycling of Carbon Dioxide to Isobutyraldehyde. Nat. Biotechnol. 2009, 27, 1177–1180. [Google Scholar] [CrossRef]
- Oliver, J.W.K.; Machado, I.M.P.; Yoneda, H.; Atsumi, S. Cyanobacterial Conversion of Carbon Dioxide to 2,3-Butanediol. Proc. Natl. Acad. Sci. 2013, 110, 1249–1254. [Google Scholar] [CrossRef]
- Ducat, D.C.; Avelar-Rivas, J.A.; Way, J.C.; Silver, P.A. Rerouting Carbon Flux To Enhance Photosynthetic Productivity. Appl. Environ. Microbiol. 2012, 78, 2660–2668. [Google Scholar] [CrossRef]
- Kusakabe, T.; Tatsuke, T.; Tsuruno, K.; Hirokawa, Y.; Atsumi, S.; Liao, J.C.; Hanai, T. Engineering a Synthetic Pathway in Cyanobacteria for Isopropanol Production Directly from Carbon Dioxide and Light. Metab. Eng. 2013, 20, 101–108. [Google Scholar] [CrossRef]
- Chwa, J.-W.; Kim, W.J.; Sim, S.J.; Um, Y.; Woo, H.M. Engineering of a Modular and Synthetic Phosphoketolase Pathway for Photosynthetic Production of Acetone from CO2 in Synechococcus Elongatus PCC 7942 under Light and Aerobic Condition. Plant Biotechnol. J. 2016, 14, 1768–1776. [Google Scholar] [CrossRef]
- Videau, P.; Cozy, L.M. Anabaena Sp. Strain PCC 7120: Laboratory Maintenance, Cultivation, and Heterocyst Induction. Curr. Protoc. Microbiol. 2019, 52, e71. [Google Scholar] [CrossRef]
- Wolk, C.P.; Vonshak, A.; Kehoe, P.; Elhai, J. Construction of Shuttle Vectors Capable of Conjugative Transfer from Escherichia Coli to Nitrogen-Fixing Filamentous Cyanobacteria. Proc. Natl. Acad. Sci. USA 1984, 81, 1561–1565. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Zhou, X.; Qi, R.; Dai, N.; Fu, X.; Zhao, H.; Peng, K.; Yuan, H.; Huang, Y.; Lv, F.; et al. Photoactive Conjugated Polymer-Based Hybrid Biosystems for Enhancing Cyanobacterial Photosynthesis and Regulating Redox State of Protein. Adv. Funct. Mater. 2021, 31, 2007814. [Google Scholar] [CrossRef]
- Thiel, T.; Poo, H. Transformation of a Filamentous Cyanobacterium by Electroporation. J. Bacteriol. 1989, 171, 5743–5746. [Google Scholar] [CrossRef] [PubMed]
- Santos-Merino, M.; Singh, A.K.; Ducat, D.C. New Applications of Synthetic Biology Tools for Cyanobacterial Metabolic Engineering. Front. Bioeng. Biotechnol. 2019, 7, 33. [Google Scholar] [CrossRef]
- Malatinszky, D.; Steuer, R.; Jones, P.R. A Comprehensively Curated Genome-Scale Two-Cell Model for the Heterocystous Cyanobacterium Anabaena Sp. PCC 7120. Plant Physiol. 2017, 173, 509–523. [Google Scholar] [CrossRef]
- Li, S.; Sun, T.; Xu, C.; Chen, L.; Zhang, W. Development and Optimization of Genetic Toolboxes for a Fast-Growing Cyanobacterium Synechococcus Elongatus UTEX 2973. Metab. Eng. 2018, 48, 163–174. [Google Scholar] [CrossRef]
- Song, K.; Tan, X.; Liang, Y.; Lu, X. The Potential of Synechococcus Elongatus UTEX 2973 for Sugar Feedstock Production. Appl. Microbiol. Biotechnol. 2016, 100, 7865–7875. [Google Scholar] [CrossRef]
- Tan, X.; Hou, S.; Song, K.; Georg, J.; Klähn, S.; Lu, X.; Hess, W.R. The Primary Transcriptome of the Fast-Growing Cyanobacterium Synechococcus Elongatus UTEX 2973. Biotechnol. Biofuels 2018, 11, 218. [Google Scholar] [CrossRef]
- Stevens, S.E.; Porter, R.D. Transformation in Agmenellum Quadruplicatum. Proc. Natl. Acad. Sci. USA 1980, 77, 6052–6056. [Google Scholar] [CrossRef]
- Kopka, J.; Schmidt, S.; Dethloff, F.; Pade, N.; Berendt, S.; Schottkowski, M.; Martin, N.; Dühring, U.; Kuchmina, E.; Enke, H.; et al. Systems Analysis of Ethanol Production in the Genetically Engineered Cyanobacterium Synechococcus Sp. PCC 7002. Biotechnol. Biofuels 2017, 10, 56. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Alvey, R.M.; Byrne, P.O.; Graham, J.E.; Shen, G.; Bryant, D.A. Expression of Genes in Cyanobacteria: Adaptation of Endogenous Plasmids as Platforms for High-Level Gene Expression in Synechococcus Sp. PCC 7002. Methods Mol. Biol. Clifton NJ 2011, 684, 273–293. [Google Scholar] [CrossRef]
- Aikawa, S.; Nishida, A.; Ho, S.-H.; Chang, J.-S.; Hasunuma, T.; Kondo, A. Glycogen Production for Biofuels by the Euryhaline Cyanobacteria Synechococcus Sp. Strain PCC 7002 from an Oceanic Environment. Biotechnol. Biofuels 2014, 7, 88. [Google Scholar] [CrossRef] [PubMed]
- Korosh, T.C.; Markley, A.L.; Clark, R.L.; McGinley, L.L.; McMahon, K.D.; Pfleger, B.F. Engineering Photosynthetic Production of L-Lysine. Metab. Eng. 2017, 44, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Nozzi, N.E.; Atsumi, S. Genome Engineering of the 2,3-Butanediol Biosynthetic Pathway for Tight Regulation in Cyanobacteria. ACS Synth. Biol. 2015, 4, 1197–1204. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, J.H.; Frigaard, N.-U. Engineering of Photosynthetic Mannitol Biosynthesis from CO2 in a Cyanobacterium. Metab. Eng. 2014, 21, 60–70. [Google Scholar] [CrossRef]
- Davies, F.K.; Work, V.H.; Beliaev, A.S.; Posewitz, M.C. Engineering Limonene and Bisabolene Production in Wild Type and a Glycogen-Deficient Mutant of Synechococcus Sp. PCC 7002. Front. Bioeng. Biotechnol. 2014, 2, 21. [Google Scholar] [CrossRef]
- Ruffing, A.M. Improved Free Fatty Acid Production in Cyanobacteria with Synechococcus Sp. PCC 7002 as Host. Front. Bioeng. Biotechnol. 2014, 2. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Y.; Bryant, D.A. Metabolic Engineering of Synechococcus Sp. PCC 7002 to Produce Poly-3-Hydroxybutyrate and Poly-3-Hydroxybutyrate-Co-4-Hydroxybutyrate. Metab. Eng. 2015, 32, 174–183. [Google Scholar] [CrossRef]
- Włodarczyk, A.; Selão, T.T.; Norling, B.; Nixon, P.J. Newly Discovered Synechococcus Sp. PCC 11901 Is a Robust Cyanobacterial Strain for High Biomass Production. Commun. Biol. 2020, 3, 1–14. [Google Scholar] [CrossRef]
- Kłodawska, K.; Bujas, A.; Turos-Cabal, M.; Żbik, P.; Fu, P.; Malec, P. Effect of Growth Temperature on Biosynthesis and Accumulation of Carotenoids in Cyanobacterium Anabaena Sp. PCC 7120 under Diazotrophic Conditions. Microbiol. Res. 2019, 226, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Gaj, T.; Gersbach, C.A.; Barbas, C.F. ZFN, TALEN, and CRISPR/Cas-Based Methods for Genome Engineering. Trends Biotechnol. 2013, 31, 397–405. [Google Scholar] [CrossRef]
- Daboussi, F.; Leduc, S.; Maréchal, A.; Dubois, G.; Guyot, V.; Perez-Michaut, C.; Amato, A.; Falciatore, A.; Juillerat, A.; Beurdeley, M.; et al. Genome Engineering Empowers the Diatom Phaeodactylum Tricornutum for Biotechnology. Nat. Commun. 2014, 5, 3831. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Brueggeman, A.J.; Horken, K.M.; Plucinak, T.M.; Weeks, D.P. Successful Transient Expression of Cas9 and Single Guide RNA Genes in Chlamydomonas Reinhardtii. Eukaryot. Cell 2014, 13, 1465–1469. [Google Scholar] [CrossRef]
- Moses, T.; Mehrshahi, P.; Smith, A.G.; Goossens, A. Synthetic Biology Approaches for the Production of Plant Metabolites in Unicellular Organisms. J. Exp. Bot. 2017, 68, 4057–4074. [Google Scholar] [CrossRef] [PubMed]
- Christian, M.; Cermak, T.; Doyle, E.L.; Schmidt, C.; Zhang, F.; Hummel, A.; Bogdanove, A.J.; Voytas, D.F. Targeting DNA Double-Strand Breaks with TAL Effector Nucleases. Genetics 2010, 186, 757–761. [Google Scholar] [CrossRef]
- Ochiai, H.; Yamamoto, T. Genome Editing Using Zinc-Finger Nucleases (ZFNs) and Transcription Activator-Like Effector Nucleases (TALENs). In Targeted Genome Editing Using Site-Specific Nucleases: ZFNs, TALENs, and the CRISPR/Cas9 System; Yamamoto, T., Ed.; Springer Japan: Tokyo, Japan, 2015; pp. 3–24. ISBN 978-4-431-55227-7. [Google Scholar]
- Sizova, I.; Greiner, A.; Awasthi, M.; Kateriya, S.; Hegemann, P. Nuclear Gene Targeting in Chlamydomonas Using Engineered Zinc-Finger Nucleases. Plant J. 2013, 73, 873–882. [Google Scholar] [CrossRef]
- Kroth, P.G.; Bones, A.M.; Daboussi, F.; Ferrante, M.I.; Jaubert, M.; Kolot, M.; Nymark, M.; Río Bártulos, C.; Ritter, A.; Russo, M.T.; et al. Genome Editing in Diatoms: Achievements and Goals. Plant Cell Rep. 2018, 37, 1401–1408. [Google Scholar] [CrossRef]
- Ng, I.-S.; Keskin, B.B.; Tan, S.-I. A Critical Review of Genome Editing and Synthetic Biology Applications in Metabolic Engineering of Microalgae and Cyanobacteria. Biotechnol. J. 2020, 15, e1900228. [Google Scholar] [CrossRef]
- Li, H.; Shen, C.R.; Huang, C.-H.; Sung, L.-Y.; Wu, M.-Y.; Hu, Y.-C. CRISPR-Cas9 for the Genome Engineering of Cyanobacteria and Succinate Production. Metab. Eng. 2016, 38, 293–302. [Google Scholar] [CrossRef]
- Wendt, K.E.; Ungerer, J.; Cobb, R.E.; Zhao, H.; Pakrasi, H.B. CRISPR/Cas9 Mediated Targeted Mutagenesis of the Fast Growing Cyanobacterium Synechococcus Elongatus UTEX 2973. Microb. Cell Factories 2016, 15, 115. [Google Scholar] [CrossRef] [PubMed]
- Ungerer, J.; Pakrasi, H.B. Cpf1 Is A Versatile Tool for CRISPR Genome Editing Across Diverse Species of Cyanobacteria. Sci. Rep. 2016, 6, 39681. [Google Scholar] [CrossRef] [PubMed]
- Niu, T.-C.; Lin, G.-M.; Xie, L.-R.; Wang, Z.-Q.; Xing, W.-Y.; Zhang, J.-Y.; Zhang, C.-C. Expanding the Potential of CRISPR-Cpf1-Based Genome Editing Technology in the Cyanobacterium Anabaena PCC 7120. ACS Synth. Biol. 2019, 8, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Gomaa, M.A.; Al-Haj, L.; Abed, R.M.M. Metabolic Engineering of Cyanobacteria and Microalgae for Enhanced Production of Biofuels and High-Value Products. J. Appl. Microbiol. 2016, 121, 919–931. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Sun, T.; Pei, G.; Chen, L.; Zhang, W. Cyanobacterial Chassis Engineering for Enhancing Production of Biofuels and Chemicals. Appl. Microbiol. Biotechnol. 2016, 100, 3401–3413. [Google Scholar] [CrossRef] [PubMed]
- Joseph, A.; Aikawa, S.; Sasaki, K.; Matsuda, F.; Hasunuma, T.; Kondo, A. Increased Biomass Production and Glycogen Accumulation in ApcE Gene Deleted Synechocystis Sp. PCC 6803. AMB Express 2014, 4, 17. [Google Scholar] [CrossRef]
- Hasunuma, T.; Matsuda, M.; Senga, Y.; Aikawa, S.; Toyoshima, M.; Shimakawa, G.; Miyake, C.; Kondo, A. Overexpression of Flv3 Improves Photosynthesis in the Cyanobacterium Synechocystis Sp. PCC6803 by Enhancement of Alternative Electron Flow. Biotechnol. Biofuels 2014, 7, 493. [Google Scholar] [CrossRef]
- Chen, M.; Schliep, M.; Willows, R.D.; Cai, Z.-L.; Neilan, B.A.; Scheer, H. A Red-Shifted Chlorophyll. Science 2010, 329, 1318–1319. [Google Scholar] [CrossRef]
- Nürnberg, D.J.; Morton, J.; Santabarbara, S.; Telfer, A.; Joliot, P.; Antonaru, L.A.; Ruban, A.V.; Cardona, T.; Krausz, E.; Boussac, A.; et al. Photochemistry beyond the Red Limit in Chlorophyll F-Containing Photosystems. Science 2018, 360, 1210–1213. [Google Scholar] [CrossRef]
- Noreña-Caro, D.; Benton, M.G. Cyanobacteria as Photoautotrophic Biofactories of High-Value Chemicals. J. CO2 Util. 2018, 28, 335–366. [Google Scholar] [CrossRef]
- Gudmundsson, S.; Nogales, J. Cyanobacteria as Photosynthetic Biocatalysts: A Systems Biology Perspective. Mol. Biosyst. 2014, 11, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Bogorad, I.W.; Lin, T.-S.; Liao, J.C. Synthetic Non-Oxidative Glycolysis Enables Complete Carbon Conservation. Nature 2013, 502, 693–697. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Li, X.; Duchoud, F.; Chuang, D.S.; Liao, J.C. Augmenting the Calvin–Benson–Bassham Cycle by a Synthetic Malyl-CoA-Glycerate Carbon Fixation Pathway. Nat. Commun. 2018, 9, 2008. [Google Scholar] [CrossRef]
- Hays, S.G.; Ducat, D.C. Engineering Cyanobacteria as Photosynthetic Feedstock Factories. Photosynth. Res. 2015, 123, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Wintermute, E.H.; Silver, P.A. Dynamics in the Mixed Microbial Concourse. Genes Dev. 2010, 24, 2603–2614. [Google Scholar] [CrossRef] [PubMed]
- Hays, S.G.; Yan, L.L.W.; Silver, P.A.; Ducat, D.C. Synthetic Photosynthetic Consortia Define Interactions Leading to Robustness and Photoproduction. J. Biol. Eng. 2017, 11, 4. [Google Scholar] [CrossRef]
- Argun, H.; Kargi, F.; Kapdan, I.K. Hydrogen Production by Combined Dark and Light Fermentation of Ground Wheat Solution. Int. J. Hydrog. Energy 2009, 34, 4305–4311. [Google Scholar] [CrossRef]
- Renuka, N.; Sood, A.; Prasanna, R.; Ahluwalia, A.S. Phycoremediation of Wastewaters: A Synergistic Approach Using Microalgae for Bioremediation and Biomass Generation. Int. J. Environ. Sci. Technol. 2015, 12, 1443–1460. [Google Scholar] [CrossRef]
- Liu, J.; Vyverman, W. Differences in Nutrient Uptake Capacity of the Benthic Filamentous Algae Cladophora Sp., Klebsormidium Sp. and Pseudanabaena Sp. under Varying N/P Conditions. Bioresour. Technol. 2015, 179, 234–242. [Google Scholar] [CrossRef]
- Manjunath, M.; Kanchan, A.; Ranjan, K.; Venkatachalam, S.; Prasanna, R.; Ramakrishnan, B.; Hossain, F.; Nain, L.; Shivay, Y.S.; Rai, A.B.; et al. Beneficial Cyanobacteria and Eubacteria Synergistically Enhance Bioavailability of Soil Nutrients and Yield of Okra. Heliyon 2016, 2, e00066. [Google Scholar] [CrossRef]
- Ryu, B.-G.; Kim, W.; Nam, K.; Kim, S.; Lee, B.; Park, M.S.; Yang, J.-W. A Comprehensive Study on Algal–Bacterial Communities Shift during Thiocyanate Degradation in a Microalga-Mediated Process. Bioresour. Technol. 2015, 191, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; He, L.Y.; Tao, X.Q.; Dang, Z.; Guo, C.L.; Lu, G.N.; Yi, X.Y. Construction of an Artificial Microalgal-Bacterial Consortium That Efficiently Degrades Crude Oil. J. Hazard. Mater. 2010, 181, 1158–1162. [Google Scholar] [CrossRef] [PubMed]
- Fradinho, J.C.; Domingos, J.M.B.; Carvalho, G.; Oehmen, A.; Reis, M.A.M. Polyhydroxyalkanoates Production by a Mixed Photosynthetic Consortium of Bacteria and Algae. Bioresour. Technol. 2013, 132, 146–153. [Google Scholar] [CrossRef]
- Angelis, S.; Novak, A.C.; Sydney, E.B.; Soccol, V.T.; Carvalho, J.C.; Pandey, A.; Noseda, M.D.; Tholozan, J.L.; Lorquin, J.; Soccol, C.R. Co-Culture of Microalgae, Cyanobacteria, and Macromycetes for Exopolysaccharides Production: Process Preliminary Optimization and Partial Characterization. Appl. Biochem. Biotechnol. 2012, 167, 1092–1106. [Google Scholar] [CrossRef]
- Xu, L.; Li, D.; Wang, Q.; Wu, S. Improved Hydrogen Production and Biomass through the Co-Cultivation of Chlamydomonas Reinhardtii and Bradyrhizobium Japonicum. Int. J. Hydrog. Energy 2016, 41, 9276–9283. [Google Scholar] [CrossRef]
- Xu, L.; Cheng, X.; Wu, S.; Wang, Q. Co-Cultivation of Chlamydomonas Reinhardtii with Azotobacter Chroococcum Improved H2 Production. Biotechnol. Lett. 2017, 39, 731–738. [Google Scholar] [CrossRef]
- Weiss, T.L.; Young, E.J.; Ducat, D.C. A Synthetic, Light-Driven Consortium of Cyanobacteria and Heterotrophic Bacteria Enables Stable Polyhydroxybutyrate Production. Metab. Eng. 2017, 44, 236–245. [Google Scholar] [CrossRef]
- Smith, M.J.; Francis, M.B. A Designed A. Vinelandii-S. Elongatus Coculture for Chemical Photoproduction from Air, Water, Phosphate, and Trace Metals. ACS Synth. Biol. 2016, 5, 955–961. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, L.; Diao, J.; Song, X.; Shi, M.; Zhang, W. Construction and Analysis of an Artificial Consortium Based on the Fast-Growing Cyanobacterium Synechococcus Elongatus UTEX 2973 to Produce the Platform Chemical 3-Hydroxypropionic Acid from CO2. Biotechnol. Biofuels 2020, 13, 82. [Google Scholar] [CrossRef]
- Zuñiga, C.; Li, T.; Guarnieri, M.T.; Jenkins, J.P.; Li, C.-T.; Bingol, K.; Kim, Y.-M.; Betenbaugh, M.J.; Zengler, K. Synthetic Microbial Communities of Heterotrophs and Phototrophs Facilitate Sustainable Growth. Nat. Commun. 2020, 11, 3803. [Google Scholar] [CrossRef]
- Velmurugan, R.; Incharoensakdi, A. Heterologous Expression of Ethanol Synthesis Pathway in Glycogen Deficient Synechococcus Elongatus PCC 7942 Resulted in Enhanced Production of Ethanol and Exopolysaccharides. Front. Plant Sci. 2020, 11, 74. [Google Scholar] [CrossRef] [PubMed]
- Khetkorn, W.; Rastogi, R.P.; Incharoensakdi, A.; Lindblad, P.; Madamwar, D.; Pandey, A.; Larroche, C. Microalgal Hydrogen Production—A Review. Bioresour. Technol. 2017, 243, 1194–1206. [Google Scholar] [CrossRef] [PubMed]
- Savakis, P.E.; Angermayr, S.A.; Hellingwerf, K.J. Synthesis of 2,3-Butanediol by Synechocystis Sp. PCC6803 via Heterologous Expression of a Catabolic Pathway from Lactic Acid- and Enterobacteria. Metab. Eng. 2013, 20, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Ni, J.; Xu, P.; Tao, F. Enhancing Light-Driven 1,3-Propanediol Production by Using Natural Compartmentalization of Differentiated Cells. ACS Synth. Biol. 2018, 7, 2436–2446. [Google Scholar] [CrossRef]
- Joseph, A.; Aikawa, S.; Sasaki, K.; Tsuge, Y.; Matsuda, F.; Tanaka, T.; Kondo, A. Utilization of Lactic Acid Bacterial Genes in Synechocystis Sp. PCC 6803 in the Production of Lactic Acid. Biosci. Biotechnol. Biochem. 2013, 77, 966–970. [Google Scholar] [CrossRef]
- Angermayr, S.A.; Paszota, M.; Hellingwerf, K.J. Engineering a Cyanobacterial Cell Factory for Production of Lactic Acid. Appl. Environ. Microbiol. 2012, 78, 7098–7106. [Google Scholar] [CrossRef]
- Li, C.; Tao, F.; Ni, J.; Wang, Y.; Yao, F.; Xu, P. Enhancing the Light-Driven Production of D-Lactate by Engineering Cyanobacterium Using a Combinational Strategy. Sci. Rep. 2015, 5, 9777. [Google Scholar] [CrossRef]
- Wang, Y.; Tao, F.; Ni, J.; Li, C.; Xu, P. Production of C3 Platform Chemicals from CO2 by Genetically Engineered Cyanobacteria. Green Chem. 2015, 17, 3100–3110. [Google Scholar] [CrossRef]
- Lin, P.-C.; Zhang, F.; Pakrasi, H.B. Enhanced Production of Sucrose in the Fast-Growing Cyanobacterium Synechococcus Elongatus UTEX 2973. Sci. Rep. 2020, 10, 390. [Google Scholar] [CrossRef]
- Niederholtmeyer, H.; Wolfstädter, B.T.; Savage, D.F.; Silver, P.A.; Way, J.C. Engineering Cyanobacteria To Synthesize and Export Hydrophilic Products. Appl. Environ. Microbiol. 2010, 76, 3462–3466. [Google Scholar] [CrossRef]
- Fan, E.S.; Lu, K.W.; Wen, R.C.; Shen, C.R. Photosynthetic Reduction of Xylose to Xylitol Using Cyanobacteria. Biotechnol. J. 2020, 15, e1900354. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Tao, F.; Wang, Y.; Yao, F.; Xu, P. A Photoautotrophic Platform for the Sustainable Production of Valuable Plant Natural Products from CO2. Green Chem. 2016, 18, 3537–3548. [Google Scholar] [CrossRef]
- Ni, J.; Liu, H.-Y.; Tao, F.; Wu, Y.-T.; Xu, P. Remodeling of the Photosynthetic Chain Promotes Direct CO2 Conversion into Valuable Aromatic Compounds. Angew. Chem. Int. Ed Engl. 2018, 57, 15990–15994. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, T.D.; Wiberley, A.E.; Donohue, A.R. Isoprene Emission from Plants: Why and How. Ann. Bot. 2008, 101, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Gao, F.; Liu, D.; Zhang, H.; Nie, X.; Yang, C. Engineering the Methylerythritol Phosphate Pathway in Cyanobacteria for Photosynthetic Isoprene Production from CO2. Energy Environ. Sci. 2016, 9, 1400–1411. [Google Scholar] [CrossRef]
- Zhu, Q. Developments on CO2-Utilization Technologies. Clean Energy 2019, 3, 85–100. [Google Scholar] [CrossRef]
- Bekker, M.; Teixeira De, M.M.J.; Hellingwerf, K.J. L-Lactate Production in Cyanobacteria 2013. EP2563927B1, 14 December 2016. [Google Scholar]
- Hellingwerf, K.J.; Teixeira De, M.M.J. Process for Producing 1,3-Propanediol Compound 2021. US7056439B2, 6 June 2006. [Google Scholar]
- Van Der, W.A.D.; Hellingwerf, K.J.; Mulder, K. A Process for the Bioproduction of Glycolate 2022. US20220098627A1, 24 January 2020. [Google Scholar]
- Isabel, D.-P.; Fatima, A.; Manel, G. Engineered Microorganism for the Production of Cannabinoids 2021. PCT/CA2020/051452, 29 October 2020. [Google Scholar]
- Jester, B.W.; Zhao, H.; Gewe, M.; Adame, T.; Perruzza, L.; Bolick, D.T.; Agosti, J.; Khuong, N.; Kuestner, R.; Gamble, C.; et al. Development of Spirulina for the Manufacture and Oral Delivery of Protein Therapeutics. Nat. Biotechnol. 2022, 40, 956–964. [Google Scholar] [CrossRef]
- Wang, F.; Gao, Y.; Yang, G. Recent Advances in Synthetic Biology of Cyanobacteria for Improved Chemicals Production. Bioengineered 2020, 11, 1208–1220. [Google Scholar] [CrossRef]
- Abed, R.M.M.; Dobretsov, S.; Sudesh, K. Applications of Cyanobacteria in Biotechnology. J. Appl. Microbiol. 2009, 106, 1–12. [Google Scholar] [CrossRef]
- Hitchcock, A.; Hunter, C.N.; Canniffe, D.P. Progress and Challenges in Engineering Cyanobacteria as Chassis for Light-Driven Biotechnology. Microb. Biotechnol. 2020, 13, 363–367. [Google Scholar] [CrossRef]
- Zahra, Z.; Choo, D.H.; Lee, H.; Parveen, A. Cyanobacteria: Review of Current Potentials and Applications. Environments 2020, 7, 13. [Google Scholar] [CrossRef]
- Chávez, M.N.; Moellhoff, N.; Schenck, T.L.; Egaña, J.T.; Nickelsen, J. Photosymbiosis for Biomedical Applications. Front. Bioeng. Biotechnol. 2020, 8, 577204. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, S.A.M.; Shedid, E.S.; Saied, E.M.; Jassbi, A.R.; Jamebozorgi, F.H.; Rateb, M.E.; Du, M.; Abdel-Daim, M.M.; Kai, G.-Y.; Al-Hammady, M.A.M.; et al. Cyanobacteria-From the Oceans to the Potential Biotechnological and Biomedical Applications. Mar. Drugs 2021, 19, 241. [Google Scholar] [CrossRef] [PubMed]
- Chittora, D.; Meena, M.; Barupal, T.; Swapnil, P.; Sharma, K. Cyanobacteria as a Source of Biofertilizers for Sustainable Agriculture. Biochem. Biophys. Rep. 2020, 22, 100737. [Google Scholar] [CrossRef]

| Host | Delivery Approach | Genome Size | Doubling Time | Optimum Temperature | References |
|---|---|---|---|---|---|
| Synechocystis sp. PCC6803 | Natural transformation | 3.6 Mbp | 6.6 h | 30 °C | [15] |
| Synechococcus sp. PCC7002 | Natural transformation/Conjugation | 3.01 Mbp | 2.27 h | 38 °C | [50] |
| S. elongatus PCC7942 | Natural transformation | 2.7 Mbp | 4.9 h | 38 °C | [15] |
| S. elongatus UTEX2973 | Conjugation | 2.7 Mbp | 1.93 h | 38 °C | [50] |
| Anabaena sp. PCC7120 | Conjugation | 6.4 Mbp | 14 h | 23 °C | [36,51] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Zheng, J.; Wu, Y.; Wang, X.; Shao, H.; Yan, D. Light-Driven Synthetic Biology: Progress in Research and Industrialization of Cyanobacterial Cell Factory. Life 2022, 12, 1537. https://doi.org/10.3390/life12101537
Li C, Zheng J, Wu Y, Wang X, Shao H, Yan D. Light-Driven Synthetic Biology: Progress in Research and Industrialization of Cyanobacterial Cell Factory. Life. 2022; 12(10):1537. https://doi.org/10.3390/life12101537
Chicago/Turabian StyleLi, Chaofeng, Jiyang Zheng, Yushuang Wu, Xiaotong Wang, Hui Shao, and Dong Yan. 2022. "Light-Driven Synthetic Biology: Progress in Research and Industrialization of Cyanobacterial Cell Factory" Life 12, no. 10: 1537. https://doi.org/10.3390/life12101537
APA StyleLi, C., Zheng, J., Wu, Y., Wang, X., Shao, H., & Yan, D. (2022). Light-Driven Synthetic Biology: Progress in Research and Industrialization of Cyanobacterial Cell Factory. Life, 12(10), 1537. https://doi.org/10.3390/life12101537






