Abstract
The effect of γ-Aminobutyrate (GABA) on maize seedlings under saline stress conditions has not been well tested in previous literature. Maize seedlings were subjected to two saline water concentrations (50 and 100 mM NaCl), with distilled water as the control. Maize seedlings under saline and control conditions were sprayed with GABA at two concentrations (0.5 and 1 mM). Our results indicated that GABA application (1 mM) significantly enhanced plant growth parameters (fresh shoots and fresh roots by 80.43% and 47.13%, respectively) and leaf pigments (chlorophyll a, b, and total chlorophyll by 22.88%, 56.80%, and 36.21%, respectively) compared to untreated seedlings under the highest saline level. Additionally, under 100 mM NaCl, methylglyoxal (MG), malondialdehyde (MDA), and hydrogen peroxidase (H2O2) were reduced by 1 mM GABA application by 43.66%, 33.40%, and 35.98%, respectively. Moreover, maize seedlings that were treated with 1 mM GABA contained a lower Na content (22.04%) and a higher K content (60.06%), compared to the control under 100 mM NaCl. Peroxidase, catalase, ascorbate peroxidase, and superoxide dismutase activities were improved (24.62%, 15.98%, 62.13%, and 70.07%, respectively) by the highest GABA rate, under the highest stress level. Seedlings treated with GABA under saline conditions showed higher levels of expression of the potassium transporter protein (ZmHKT1) gene, and lower expression of the ZmSOS1 and ZmNHX1 genes, compared to untreated seedlings. In conclusion, GABA application as a foliar treatment could be a promising strategy to mitigate salinity stress in maize plants.
1. Introduction
Due to the increasing population of the world, and the demands for food, salinity is regarded as one of the most serious global issues confronting agricultural sectors in recent decades [1]. Salinity affects over 1125 million hectares worldwide, and that number is predicted to increase annually as a result of human activity [2]. It has been well known that plants exposed to abiotic stress, such as saline and drought conditions, produce an abundance of reactive oxygen species (ROS), including superoxide anion, hydrogen peroxide, and hydroxyl radical [3,4]. Consequently, the overproduction of reactive oxygen species causes lipid peroxidation, metabolic disturbances, and even cell death [5]. Plants suffer from major problems when grown under saline conditions, including water deficiency caused by osmotic stress, disruption of the plant’s ionic systems, and ion toxicity [6,7]. High Na+ and Cl ion concentrations in soil or water reduce net photosynthesis [8] and the concentration of several compounds, such as sugars, polyamines, and amino acids [9].
Plant salt tolerance is a multifaceted trait that involves numerous biochemical and physiological mechanisms. It is critical for breeding improved varieties to identify multiple genes whose expression allows plants to adapt to, or tolerate, different levels of salt [10]. There are some methods to control salinity, including installing new irrigation and drainage systems [11], breeding plants [12], root treatments by some types of fungi [13], and applying biostimulants to improve the plant’s defense system, such as salicylic acid [14].
One of the most important cereal crops grown around the world is maize (Zea mays), which is classified as a moderately sensitive crop to salinity [11]. Because of its high starch content, it is quickly becoming one of the main ingredients in foods, textiles, biofuels, silage, and many other industries. [15]. It has been well known that maize suffers from severe damage and significant losses in growth and productivity when grown in saline conditions [16]. More than 250 mM NaCl of salinity inhibits maize plant growth, causing severe wilting [17]. As a result, the aim of the current research was to find a method that could mitigate or minimize the negative impact of salts on maize plants.
Gamma-aminobutyric acid (GABA) is widely distributed in plants and is associated with stress responses, signaling, and storage [18]. Previous works indicated that GABA is involved in many plant processes and reactions, such as the oxidative stress defense [19], the balance of the C/N ratio [20], signaling function [21], and the control of osmotic pressure [22]. Under abiotic stresses such as salinity and drought, GABA is accumulated in the plant [23,24]. Previous reports indicated that the use of a GABA improved salinity stress tolerance by improving antioxidant enzyme activity and reducing reactive oxygen species accumulation in plants [25], maintaining the C/N ratio in balance, regulating cell osmosis [26], controlling Na+ and K+ absorption [27], and increasing some bioactive compounds such as phenolic compounds, proline, and amino acids [28]. In a previous work [29], treating seedling maize roots (root drenching) with a GABA application under saline conditions (150–300 Mm NaCl) led to an increase in seedling growth, proline and sugar content in shoots, and enzymatic antioxidant activity compared to untreated plants. To the best of our knowledge, the influence of GABA treatment via a foliar application on the growth, physiological, and molecular changes of maize plants has not been studied before. Thus, the current study was performed to investigate the morphological, physiological, and molecular responses of maize plants treated with GABA as a foliar application under saline conditions.
2. Materials and Methods
2.1. Seed Preparation and Materials
Maize grains hybrid (Hytech 2030) obtained from Misr Hytech Seed Int. (Cairo, Egypt) were soaked in NaOCl (0.5%) for 5 min for sterilization. The gains were washed 3 times with distilled water and left to dry for 2 h before germination.
2.2. Seed Germination
On wet filter paper, maize grains were allowed to germinate for 24 h at 25 °C. Five seedlings were placed in each of 8 Kg of pre-washed sand-filled black plastic pots (20 cm in diameter) after being chosen for their uniform size. After planting, the pots were kept in a growth chamber at 27/17 °C day/night with 170 µmol m−2 S−1 light intensity and 70–75% RH. The pots were irrigated every two days with a half-strength Hoagland’s solution.
2.3. Salt Treatments
After 14 days, the pots were split into two main groups: saline and control conditions. A half-strength Hoagland’s nutrient solution was used to irrigate seedlings from the saline group that contained NaCl salt with desired concentrations (50 and 100 mM NaCl), while those of the unstressed group (control, 0 mM NaCl) were irrigated with Hoagland’s nutrient solution (half strength).
2.4. GABA Foliar Application
To apply the GABA treatment, the pots (from every salinity level) were divided into three subgroups, and the following solutions were used: 0.5 mM GABA, 1 mM GABA, and control plants (sprayed with distilled water). The spraying solution was 25 mL/pot and was repeated five times at 15, 17, 19, 21, and 24 days after transplanting with Tween-20 (0.05%). The pots were arranged in a completely randomized design (CRD) with three replicates. After 7 days from the last GABA application (31 days), the seedlings were harvested for morphological, chemical, and molecular determination.
2.5. Seedlings Growth Parameters and Pigments
A digital balance was used to determine the fresh shoot and root. The methods described by Lichtenthaler and Wellburn [30] were followed to determine chlorophyll a, chlorophyll b, total chlorophyll, and carotenoids.
2.6. Determination of Cell Membrane Integrity
To determine the cell membrane stability index, the method of Abd Elbar et al. [31] was used. In brief, eight leaf discs were incubated in 10 mL of deionized water and kept for 24 h on a shaker. After that, EC meters were used to determine EC1 values, and then samples were autoclaved for 20 min at 120 °C to determine the values of EC2. The following equation was used to calculate the cell membrane stability index:
whereas MSI: Membrane stability index; EC1: Electrical conductivity after incubated and before autoclaved; EC2: Electrical conductivity after autoclaved.
2.7. Determination of Methylglyoxal Content, Hydrogen Peroxide, and Malondialdehyde
According to Hossain et al. [32], the methylglyoxal content (MG) was determined using a UV-spectrophotometer at 335 nm. Hydrogen peroxide (H2O2) content was determined calorimetrically by the K iodide method [33], as described previously by Nasser et al. [34]. The thiobarbituric acid method (TBA) was used to determine malondialdehyde (MDA), according to the method of [35].
2.8. Determination of Antioxidant Enzymes Activates
Ascorbate peroxidase (APX) activity was assessed as described previously by Doklega et al. [36], with some modifications. The decrease of absorbance at 290 nm was monitored for 3 min. The reaction mixture with a total volume of 3 mL included 100 µL crude enzyme, 50 mM phosphate buffer (pH 7), 0.1 mM EDTA, 0.5 mM ascorbic acid, and 0.1 mM H2O2. The addition of H2O2 initiated the reaction. One enzyme activity unit was defined as the amount of enzyme required for the oxidation of 1 µmol of ascorbate per minute. The rate of ascorbate oxidation was calculated using the extinction coefficient (ε = 2.8 mm−1 cm−1). Catalase (CAT) activity was measured according to a previous report [37]. The reaction mixture with a total volume of 3 mL contained 15 mM H2O2 in 50 mM phosphate buffer (pH = 7). The reaction was initiated by adding 50 μL crude enzyme. The activity was calculated from the extinction coefficient (ε = 40 mM−1 cm−1) for H2O2. One unit of enzyme activity was defined as the decomposition of 1 μmol of H2O2 per minute at 240 nm. Guaiacol peroxidase (G-POX) activity was assessed by tracking the rise in absorbance (470 nm) as guaiacol was transformed into tetraguaiacol [38]. The assay mixture (100 mL) contained 10 mL of 1% (v/v) guaiacol, 10 mL of 0.3% H2O2, and 80 mL of 50 mM phosphate buffer (pH = 6.6). The volume of 100 µL of the crude enzyme was added to 2.9 mL of the assay mixture to start the reaction. The absorbance was recorded every 30 s for 3 min at 470 nm. To determine superoxide dismutase (SOD), the method of Beyer and Fridovich [39] was followed. The reaction mixture with a total volume of 3 mL contained 100 μL crude enzyme, 50 mM phosphate buffer (pH 7.8), 75 μM NBT, 13 mM L-methionine, 0.1 mM EDTA, and 0.5 mM riboflavin. The addition of riboflavin initiated the reaction. Then, the reaction mixture was illuminated for 20 min with a 20 W fluorescent lamp. One enzyme activity unit was defined as the amount of enzyme required to result in a 50% inhibition in the rate of nitro blue tetrazolium (NBT) reduction at 560 nm.
2.9. Determination of Na and K
The flame photometric (Jenway, Leicestershire, UK) method described by Havre [40] was used to determine the Na and K mineral concentrations in the leaves.
2.10. Gene Expression
An RNA extraction kit (Sigma–Aldrich, St. Louis, MO, USA) was used to extract the total mRNA from different treatments according to the manufacturer’s protocol. To determine the RNA purification after the reverse transcription of RNA and cDNA formation, a NanoDrop™2000/2000c spectrophotometer was used according to the manufacturer’s protocol (Promega, Walldorf, Germany). Real-time PCR (Rotor-Gene 6000, Hilden, Germany) was used to run realtime quantitative reverse-transcription polymerase chain reaction (qRT-PCR) on diluted cDNA in triplicate, and the primer sequences used in qRT-PCR are provided in Table 1. The reference gene (β-Actin housekeeping gene) was utilized to analyse gene expression using SYBR® Green. The relative gene expression was determined using the 2∆DDCt method [41].

Table 1.
List of primers.
2.11. Statistical Analysis
All data were subjected to one way ANOVA. Tukey’s multiple range test (p ≤ 0.05) was used by using SAS software to analyse differences in means and ± SE from six replicates.
3. Results
3.1. Effect of GABA on Growth and Pigments under Salinity Condition
In Figure 1A,B there is a clear trend of decreased growth of maize seedlings that were exposed to saline stress compared to the control plants. Another important finding was that both rates of GABA application reduced the harmful effect of salinity stress on the growth of maize seedlings (fresh and dry weights) under either the saline condition or the control condition.

Figure 1.
Effect of GABA (0, 0.5, 1 mM) and salt stress (0, 50, 100 mM) interaction on shoot fresh weight (A), root fresh weight (B), chlorophyll a content (C), chlorophyll b (D) content, total chlorophyll (E), and carotenoid content (F) of leaves of maize seedlings under three level of salinity (0, 50, 100 mM). Different letters between all values are significantly different at p < 0.05 (n = 6 ± SE) according to the Tukey test.
From the data in Figure 1C,D, it is apparent that chlorophyll a, chlorophyll b, and total chlorophyll contents were decreased by increasing the salinity level. Further, GABA application at the highest rate (1 mM) showed a positive effect on the content of all chlorophyll parameters compared to the low GABA rate (0.5 mM) and untreated plants.
The results in Figure 1E indicated that carotenoid content in maize seedlings decreased with increasing salinity levels. The results did not detect any significant difference in carotenoid content by GABA application under normal or saline conditions.
3.2. Effect of GABA on the Membrane Stability Index (CMSI), Methylglyoxal Content (MG), H2O2, and Malondialdehyde Content (MDA) under Saline Condition
A significant decrease in CMSI was observed with increasing salinity levels (Figure 2A). There was no significant difference in CMSI for all rates of GABA application under 0 and 50 mM NaCl levels. However, both doses of GABA (0.5 and 1 mM) conserved CMSI compared to nontreated seedlings under a 100 mM NaCl level.
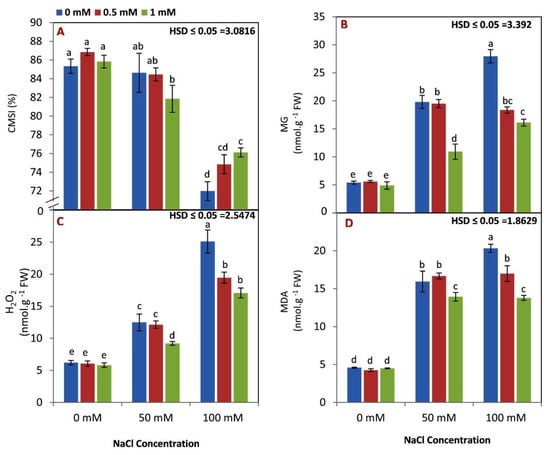
Figure 2.
Effect of GABA (0, 0.5, 1 mM) and salt stress (0, 50, 100 mM) interaction on CMSI (%) (A), MG (B), H2O2 (C), and MDA (D) content in the leaves of maize seedlings under two salinity levels. Different letters between all values are significantly different at p < 0.05 (n = 6 ± SE) according to the Tukey test.
As expected, MG, H2O2, and MDA were increased by increasing the level of salinity from 0 to 100 mM NaCl (Figure 2B–D). There were no differences between 0.5 and 1 mM of GABA application on MG, H2O2, and MDA under nonsaline conditions. However, the high rate of GABA was effective for reducing MG, H2O2, and MDA levels under 50 mM NaCl. Additionally, MG, H2O2, and MDA levels of GABA treated seedlings were lower than those of seedlings of control plants under 100 mM NaCl.
3.3. Effect of the GABA Application on the Activities of Antioxidant Enzymes
Under the nonsaline condition, no differences were observed in the activities of SOD, CAT, POX, and APX (Figure 3A–D). However, the activity of CAT and POX enzymes was increased by increasing the salinity level from 50 to 100 mM NaCl, while SOD and APX were decreased. The antioxidant activities of all enzymes were increased by increasing the rate of GBAB application from 0 to 1 mM.

Figure 3.
Effect of GABA application (0, 0.5, 1 mM) and salt stress (0, 50, 100 mM) interaction on the activity of SOD (A), CAT (B), POX (C), and APX (D) activities in the leaves of maize seedlings under two salinity levels. Different letters between all values are significantly different at p < 0.05 (n = 6 ± SE) according to the Tukey test.
3.4. Effect of GABA Application on Na, K, and Na/K Ratio
As expected, Na content and the Na/K ratio increased while the K content decreased with increasing salinity levels (Figure 4A–C). The most interesting result was that N content, and the Na/K ratio, were decreased (under salinity stress conditions) in seedling leaves by increasing the rate of GABA application from 0 to 1 mM. Additionally, K content was increased by increasing GABA levels under controlled or saline conditions. Under nonsaline conditions, GABA application didn’t affect the Na content and Na/K ratio in seedling leaves.
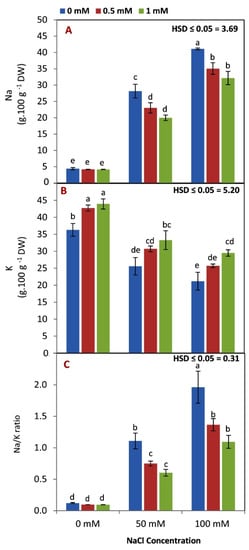
Figure 4.
Effect of GABA application (0, 0.5, 1 mM) and salt stress (0, 50, 100 mM) interaction on Na+ (A), K+ (B), and Na/K ratio (C) content in the leaves of maize seedlings under two salinity levels. Different letters between all values are significantly different at p < 0.05 (n = 6 ± SE) according to the Tukey test.
3.5. Effect of GABA Application on the Genes Expression
As shown in Figure 5A, the expression of the ZmHKT1 gene was higher in seedlings that were treated with both GABA concentrations compared to the nontreated seedlings under normal and nonsaline conditions. The expression of the ZmHKT1 gene was decreased by increasing salinity levels.
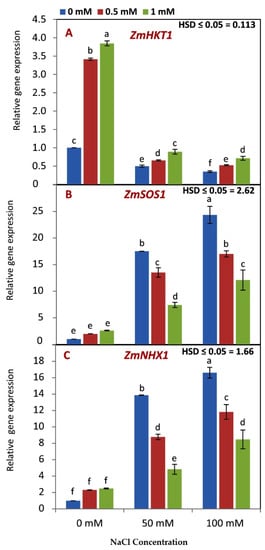
Figure 5.
Effect of GABA application (0, 0.5, 1 mM) and salt stress (0, 50, 100 mM) interaction on gene expression of ZmHKT1 (A), ZmSOS1 (B), and ZmNHX1 (C) in leaves of maize seedlings under two salinity levels. Different letters between all values are significantly different at p < 0.05 (n = 6 ± SE) according to the Tukey test.
The ZmSOS1 and ZmNHX1 genes showed a similar profile when seedlings treated with both GABA concentrations were compared to nontreated seedlings under nonsaline conditions (Figure 5B,C). The expression of both genes was increased by increasing salinity levels. Under 50 and 100 mM NaCl, increasing GABA rates resulted in the reduced expression of both the ZmSOS1 and ZmNHX1 genes.
4. Discussion
Plants exposed to salt stress, in this study, showed a decline in plant growth (shoot and root fresh weights, Figure 1A,B). These results seem to be consistent with previous research, which found that salinity reduces plant growth by several mechanisms, including osmotic stress [1], ionic toxicity [6], reduced photosynthesis [8], reduced cell division, and nutrient uptake [42,43]. Under normal and salt stress conditions, it was detected that plants treated with GABA showed a significant improvement in plant growth. The findings of the current study are consistent with those of Jin et al. [18], who found that exogenous GABA application enhanced the growth (fresh weight, dry weight, and leaf area) of watermelon seedlings under saline stress conditions. A possible explanation for these results may be due to the fact that GABA plays a role in reducing the reduction in net photosynthesis under saline conditions [44]. Additionally, previous work indicated that endogenous GABA could enhance the plant’s tolerance to stresses [45]. Accordingly, the exogenous GABA could, by foliar treatment, increase the level of GABA inside the plant tissue, resulting in higher tolerance to salinity stress [18]. The results of this study show that chlorophyll a, b, total chlorophyll, and carotenoids (Figure 1C–F) were significantly decreased by saline stress. This finding is in agreement with the Nasrallah et al. [46] findings, which showed that total chlorophyll and carotenoids in board bean plants were decreased by increasing the salinity level. This result might be due to the fact that salinity enhances membrane breakdown and the activation of chlorophyllase enzymes leads to an inhibition of chlorophyll synthesis [47].
Under abiotic stress conditions, some biochemical markers (such as ROS, OH, O2−, and H2O2) were triggered in plants and worked as signaling molecules to enhance the plant defense system [48]. However, the massive accumulation of these markers could negatively affect some physiological and chemical processes in plants [49]. GABA may have been an important factor in elevating the activity of antioxidant enzymes [50]. Additionally, it has been found that exogenous GABA applications protect plants from oxidative damage in some crops, such as peaches [44,51]. By influencing some physiological processes such as photosynthesis, stomatal movement, and root differentiation, methylglyoxal (MG) has been proven to be harmful at high concentrations [52]. Our results in Figure 2B show that MG was increased under saline conditions. Previous works also observed an increase in MG content in wheat and rice plants under NaCl stress [53,54]. In our study, the GABA application reduced MG content in maize seedlings. This result could be due to the role of GABA in reducing ROS. In this study, the GABA application reduced H2O2 content in seedlings irrigated with saline water (Figure 2C). Similar findings have been observed by [18]. Our results in Figure 2D indicated that MDA content was reduced by GABA application under saline conditions. This result is supported by previous studies [55,56].
Our results in Figure 3A–D show that antioxidant enzyme activity was increased by salinity stress. Many previous reports emphasize that antioxidant enzymes in plants are increased under stressful conditions to help plants resist unfavorable conditions [57,58]. GABA has been shown to play an important role in the regulation of reactive oxygen species (ROS) scavengers in plant systems [59]. It has been known that SOD is responsible for converting O2− to H2O2, while converting H2O2 to H2O and O2 is controlled by CAT, APX, and POX enzymes [60]. In this study, and a previous study [18], GABA treatment enhanced the activity of SOD under saline conditions. Additionally, GABA as a foliar application is also linked with controlling SOD and APX activity in some crops, such as tomatoes [31]. Redox balance in leaves is critical for maintaining the maximum efficiency of metabolic enzymes [18,61].
In many crops, such as barley and white cover, the ratio of Na/K can be utilized as a physiological index for salt sensitivity [27,62]. Additionally, the cytosolic Na/K ratio is maintained by the concentration of Na, which interacts with K, and is vital for the salinity tolerance mechanism given its involvement in multiple metabolic activities [63]. In this study, the data in Figure 3A–C indicate that GABA application reduced the Na level and Na/K ratio under saline stress. GABA application reduced the accumulation of N in roots under saline stress. The ability of GABA to reduce N content and Na/K ratio under saline conditions could be attributed to its role in mitigating oxidative damage by osmotic regulation [27]. Additionally, GABA could prevent lipid peroxidation and sustain hormone and mineral nutrition under a variety of environmental conditions [64]. GABA also has an impact on several physiological processes, including cell division, metabolic regulation, and detoxifying techniques, which enable plants to endure and grow in stressful environments, including salinity [61].
The expression of the ZmSOS1 and ZmNHX1 genes, as well as the opposite trend of the ZmHKT1 gene, can help plants survive under saline stress by excluding Na from the cytosol to the apoplast or vacuole, protecting cytosolic enzymes from Na ion toxicity. Conversely, GABA application led to over expression of ZmHKT1, and down expression of ZmSOS1 and ZmNHX1. A previous study found that the expression of the SOS1 salt gene was increased by salt stress [65,66]. These results suggested that GABA can decrease the Na/K ratio selectivity and photosynthetic capacity of maize plants under saline stress conditions. Our results in Figure 1 and Figure 4 confirmed our hypothesis that GABA application enhanced the photosynthesis process and reduced the Na/K ratio.
5. Conclusions
The current study shows that GABA as a foliar application could be useful to enhance the growth of maize seedlings and mitigate saline stress (Figure 6). GABA application is intended to regulate and control some measured parameters, including plant growth, chlorophyll pigments, the activity of antioxidant enzymes, and related gene expression under saline stress. Additionally, Na content in maize seedlings was decreased while K absorption was increased by GABA application under salinity conditions. This demonstrates that GABA reduces Na accumulation and isolates it in vacuoles in the leaves of maize seedlings under saline stress. Further investigations are required to investigate the effect of GABA on the production and quality of maize plants. The current work may also have agronomic significance for the use of GABA as a foliar application in the management of salt-sensitive crops in salinity-affected soils in arid and semiarid regions.

Figure 6.
Summarized chart of the effect of GABA application on the morphological, physiological, and molecular traits of maize seedlings under saline stress.
Author Contributions
Conceptualization, B.S.A. and H.A.; Data curation, B.S.A. and H.A.; Formal analysis, B.S.A. and H.A.; Funding acquisition, B.S.A.; Investigation, H.A.; Methodology, B.S.A. and H.A.; Project administration, B.S.A. and H.A.; Resources B.S.A. and H.A.; Software, B.S.A. and H.A.; Supervision, B.S.A. and H.A.; Validation, B.S.A. and H.A.; Visualization, B.S.A. and H.A.; Writing—original draft, B.S.A. and H.A.; Writing—review and editing, B.S.A. and H.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Faculty of Agriculture, Ain Shams University, Cairo, Egypt.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Taif University Researchers Supporting Project number (TURSP-2020/245), Taif University, P.O. Box 11099, Taif 21944, Saudi Arabia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Alzahrani, O.; Abouseadaa, H.; Abdelmoneim, T.K.; Alshehri, M.A.; El-beltagi, H.S.; El-Mogy, M.M.; Atia, M.A.M. Agronomical, Physiological and Molecular Evaluation Reveals Superior Salt-tolerance in Bread Wheat Through Salt-induced Priming Approach. Notulae Bot. Horti Agrobot. Cluj-Napoca 2021, 49, 12310. [Google Scholar] [CrossRef]
- Aboryia, M.S.; El-Dengawy, E.-R.F.A.; El-Banna, M.F.; El-Gobba, M.H.; Kasem, M.M.; Hegazy, A.A.; Hassan, H.M.; El-Yazied, A.A.; El-Gawad, H.G.A.; Al-Qahtani, S.M.; et al. Anatomical and Physiological Performance of Jojoba Treated with Proline under Salinity Stress Condition. Horticulturae 2022, 8, 716. [Google Scholar] [CrossRef]
- Akyol, T.Y.; Yilmaz, O.; Uzilday, B.; Uzilday, R.Ö.; Türkan, İ. Plant response to salinity: An analysis of ROS formation, signaling, and antioxidant defense. Turk. J. Bot. 2020, 44, 1–13. [Google Scholar]
- Helal, N.M.; Khattab, H.I.; Emam, M.M.; Niedbała, G.; Wojciechowski, T.; Hammami, I.; Alabdallah, N.M.; Darwish, D.B.E.; El-Mogy, M.M.; Hassan, H.M. Improving Yield Components and Desirable Eating Quality of Two Wheat Genotypes Using Si and NanoSi Particles under Heat Stress. Plants 2022, 11, 1819. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.M.; Rahman, M.A.; Skalicky, M.; Alabdallah, N.M.; Waseem, M.; Jahan, M.S.; Ahammed, G.J.; El-Mogy, M.M.; El-Yazied, A.A.; Ibrahim, M.F.M.; et al. Ozone Induced Stomatal Regulations, MAPK and Phytohormone Signaling in Plants. Int. J. Mol. Sci. 2021, 22, 6304. [Google Scholar] [CrossRef]
- El-Mogy, M.M.; Garchery, C.; Stevens, R. Irrigation with salt water affects growth, yield, fruit quality, storability and marker-gene expression in cherry tomato. Acta Agric. Scand. Sect. B Soil Plant Sci. 2018, 68, 727–737. [Google Scholar] [CrossRef]
- Paz, R.C.; Rocco, R.A.; Reinoso, H.; Menéndez, A.B.; Pieckenstain, F.L.; Ruiz, O.A. Comparative study of alkaline, saline, and mixed saline–alkaline stresses with regard to their effects on growth, nutrient accumulation, and root morphology of Lotus tenuis. J. Plant Growth Regul. 2012, 31, 448–459. [Google Scholar] [CrossRef]
- Abdelgawad, F.K.; El-Mogy, M.M.; Mohamed, M.I.A.; Garchery, C.; Stevens, R.G. Increasing Ascorbic Acid Content and Salinity Tolerance of Cherry Tomato Plants by Suppressed Expression of the Ascorbate Oxidase Gene. Agronomy 2019, 9, 51. [Google Scholar] [CrossRef]
- Youssef, M.H.M.; Raafat, A.; El-Yazied, A.A.; Selim, S.; Azab, E.; Khojah, E.; El Nahhas, N.; Ibrahim, M.F.M. Exogenous Application of Alpha-Lipoic Acid Mitigates Salt-Induced Oxidative Damage in Sorghum Plants through Regulation Growth, Leaf Pigments, Ionic Homeostasis, Antioxidant Enzymes, and Expression of Salt Stress Responsive Genes. Plants 2021, 10, 2519. [Google Scholar] [CrossRef]
- Hurkman, W.J. Effect of salt stress on plant gene expression: A review. Plant Soil 1992, 146, 145–151. [Google Scholar] [CrossRef]
- Cucci, G.; Lacolla, G.; Boari, F.; Mastro, M.A.; Cantore, V. Effect of water salinity and irrigation regime on maize (Zea mays L.) cultivated on clay loam soil and irrigated by furrow in Southern Italy. Agric. Water Manag. 2019, 222, 118–124. [Google Scholar] [CrossRef]
- Khan, A.A.; Rao, S.A.; McNeilly, T. Assessment of salinity tolerance based upon seedling root growth response functions in maize (Zea mays L.). Euphytica 2003, 131, 81–89. [Google Scholar] [CrossRef]
- Sheng, M.; Tang, M.; Chen, H.; Yang, B.; Zhang, F.; Huang, Y. Influence of arbuscular mycorrhizae on photosynthesis and water status of maize plants under salt stress. Mycorrhiza 2008, 18, 287–296. [Google Scholar] [CrossRef]
- Gunes, A.; Inal, A.; Alpaslan, M.; Eraslan, F.; Bagci, E.G.; Cicek, N. Salicylic acid induced changes on some physiological parameters symptomatic for oxidative stress and mineral nutrition in maize (Zea mays L.) grown under salinity. J. Plant Physiol. 2007, 164, 728–736. [Google Scholar] [CrossRef] [PubMed]
- Ostrander, B.M. Maize Starch for Industrial Applications. In Industrial Crops: Breeding for BioEnergy and Bioproducts; Cruz, V.M.V., Dierig, D.A., Eds.; Springer: New York, NY, USA, 2015; pp. 171–189. [Google Scholar]
- Farooq, M.; Hussain, M.; Wakeel, A.; Siddique, K.H. Salt stress in maize: Effects, resistance mechanisms, and management. A review. Agron. Sustain. Dev. 2015, 35, 461–481. [Google Scholar] [CrossRef]
- Menezes-Benavente, L.; Kernodle, S.P.; Margis-Pinheiro, M.; Scandalios, J.G. Salt-induced antioxidant metabolism defenses in maize (Zea mays L.) seedlings. Redox Rep. 2004, 9, 29–36. [Google Scholar] [CrossRef]
- Jin, X.; Liu, T.; Xu, J.; Gao, Z.; Hu, X. Exogenous GABA enhances muskmelon tolerance to salinity-alkalinity stress by regulating redox balance and chlorophyll biosynthesis. BMC Plant Biol. 2019, 19, 48. [Google Scholar] [CrossRef]
- Bown, A.W.; Shelp, B.J. Plant GABA: Not Just a Metabolite. Trends Plant Sci. 2016, 21, 811–813. [Google Scholar] [CrossRef]
- Fait, A.; Nesi, A.N.; Angelovici, R.; Lehmann, M.; Pham, P.A.; Song, L.; Haslam, R.P.; Napier, J.A.; Galili, G.; Fernie, A.R. Targeted Enhancement of Glutamate-to-γ-Aminobutyrate Conversion in Arabidopsis Seeds Affects Carbon-Nitrogen Balance and Storage Reserves in a Development-Dependent Manner. Plant Physiol. 2011, 157, 1026–1042. [Google Scholar] [CrossRef]
- Roberts, M.R. Does GABA Act as a Signal in Plants? Hints from Molecular Studies. Plant Signal. Behav. 2007, 2, 408–409. [Google Scholar] [CrossRef]
- Carroll, A.D.; Fox, G.G.; Laurie, S.; Phillips, R.; Ratcliffe, R.G.; Stewart, G.R. Ammonium Assimilation and the Role of [gamma]-Aminobutyric Acid in pH Homeostasis in Carrot Cell Suspensions. Plant Physiol. 1994, 106, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wang, P.; Chen, Z.; Gu, Z.; Yang, R. GABA enhances physio-biochemical metabolism and antioxidant capacity of germinated hulless barley under NaCl stress. J. Plant Physiol. 2018, 231, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Mekonnen, D.W.; Flügge, U.-I.; Ludewig, F. Gamma-aminobutyric acid depletion affects stomata closure and drought tolerance of Arabidopsis thaliana. Plant Sci. 2016, 245, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Jalil, S.U.; Ansari, M.I. Physiological Role of Gamma-Aminobutyric Acid in Salt Stress Tolerance. In Salt and Drought Stress Tolerance in Plants: Signaling Networks and Adaptive Mechanisms; Hasanuzzaman, M., Tanveer, M., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 337–350. [Google Scholar]
- Khan, M.I.R.; Jalil, S.U.; Chopra, P.; Chhillar, H.; Ferrante, A.; Khan, N.A.; Ansari, M.I. Role of GABA in plant growth, development and senescence. Plant Gene 2021, 26, 100283. [Google Scholar] [CrossRef]
- Cheng, B.; Li, Z.; Liang, L.; Cao, Y.; Zeng, W.; Zhang, X.; Ma, X.; Huang, L.; Nie, G.; Liu, W.; et al. The γ-Aminobutyric Acid (GABA) Alleviates Salt Stress Damage during Seeds Germination of White Clover Associated with Na+/K+ Transportation, Dehydrins Accumulation, and Stress-Related Genes Expression in White Clover. Int. J. Mol. Sci. 2018, 19, 2520. [Google Scholar] [CrossRef]
- Xu, J.; Liu, T.; Yang, S.; Jin, X.; Qu, F.; Huang, N.; Hu, X. Polyamines are involved in GABA-regulated salinity-alkalinity stress tolerance in muskmelon. Environ. Exp. Bot. 2019, 164, 181–189. [Google Scholar] [CrossRef]
- Wang, Y.; Gu, W.; Meng, Y.; Xie, T.; Li, L.; Li, J.; Wei, S. γ-Aminobutyric Acid Imparts Partial Protection from Salt Stress Injury to Maize Seedlings by Improving Photosynthesis and Upregulating Osmoprotectants and Antioxidants. Sci. Rep. 2017, 7, 43609. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Abd Elbar, O.H.; Elkelish, A.; Niedbała, G.; Farag, R.; Wojciechowski, T.; Mukherjee, S.; Abou-Hadid, A.F.; El-Hennawy, H.M.; Abou El-Yazied, A.; Abd El-Gawad, H.G. Protective Effect of γ-Aminobutyric Acid Against Chilling Stress during Reproductive Stage in Tomato Plants Through Modulation of Sugar Metabolism, Chloroplast Integrity, and Antioxidative Defense Systems. Front. Plant Sci. 2021, 12, 1917. [Google Scholar] [CrossRef]
- Hossain, M.A.; Hossain, M.Z.; Fujita, M. Stress-induced changes of methylglyoxal level and glyoxalase I activity in pumpkin seedlings and cDNA cloning of glyoxalase I gene. Aust. J. Crop Sci. 2009, 3, 53. [Google Scholar]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Nasser, M.A.; El-Mogy, M.M.; Samaan, M.S.F.; Hassan, K.M.; El-Sayed, S.M.; Alsubeie, M.S.; Darwish, D.B.; Mahmoud, S.F.; Al-Harbi, N.A.; Al-Qahtani, S.M.; et al. Postharvest Exogenous Melatonin Treatment of Table Grape Berry Enhances Quality and Maintains Bioactive Compounds during Refrigerated Storage. Horticulturae 2022, 8, 860. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Doklega, S.M.A.; El-Ezz, S.F.A.; Mostafa, N.A.; Dessoky, E.S.; Abdulmajeed, A.M.; Darwish, D.B.; Alzuaibr, F.M.; El-Yazied, A.A.; El-Mogy, M.M.; Mahmoud, S.F.; et al. Effect of Titanium and Vanadium on Antioxidants Content and Productivity of Red Cabbage. Horticulturae 2022, 8, 481. [Google Scholar] [CrossRef]
- Cakmak, I.; Strbac, D.; Marschner, H. Activities of hydrogen peroxide-scavenging enzymes in germinating wheat seeds. J. Exp. Bot. 1993, 44, 127–132. [Google Scholar] [CrossRef]
- Dias, M.A.; Costa, M.M. Effect of low salt concentrations on nitrate reductase and peroxidase of sugar beet leaves. J. Exp. Bot. 1983, 34, 537–543. [Google Scholar] [CrossRef]
- Beyer, W.F., Jr.; Fridovich, I. Assaying for superoxide dismutase activity: Some large consequences of minor changes in conditions. Anal. Biochem. 1987, 161, 559–566. [Google Scholar] [CrossRef]
- Havre, G.N. The flame photometric determination of sodium, potassium and calcium in plant extracts with special reference to interference effects. Anal. Chim. Acta 1961, 25, 557–566. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−∆∆CT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Abdeldym, E.A.; El-Mogy, M.M.; Abdellateaf, H.R.L.; Atia, M.A.M. Genetic Characterization, Agro-Morphological and Physiological Evaluation of Grafted Tomato under Salinity Stress Conditions. Agronomy 2020, 10, 1948. [Google Scholar] [CrossRef]
- Ferjani, A.; Mustardy, L.; Sulpice, R.; Marin, K.; Suzuki, I.; Hagemann, M.; Murata, N. Glucosylglycerol, a compatible solute, sustains cell division under salt stress. Plant Physiol. 2003, 131, 1628–1637. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Hu, L.; Xu, W.; Zhen, A.; Zhang, L.; Hu, X. Exogenous γ-Aminobutyric Acid Improves the Structure and Function of Photosystem II in Muskmelon Seedlings Exposed to Salinity-Alkalinity Stress. PLoS ONE 2016, 11, e0164847. [Google Scholar] [CrossRef] [PubMed]
- Zarei, A.; Chiu, G.Z.; Yu, G.; Trobacher, C.P.; Shelp, B.J. Salinity-regulated expression of genes involved in GABA metabolism and signaling. Botany 2017, 95, 621–627. [Google Scholar] [CrossRef]
- Nasrallah, A.K.; Kheder, A.A.; Kord, M.A.; Fouad, A.S.; El-Mogy, M.M.; Atia, M.A.M. Mitigation of Salinity Stress Effects on Broad Bean Productivity Using Calcium Phosphate Nanoparticles Application. Horticulturae 2022, 8, 75. [Google Scholar] [CrossRef]
- Parida, A.K.; Das, A.B. Salt tolerance and salinity effects on plants: A review. Ecotoxicol. Environ. Saf. 2005, 60, 324–349. [Google Scholar] [CrossRef] [PubMed]
- Pitzschke, A.; Forzani, C.; Hirt, H. Reactive oxygen species signaling in plants. Antioxid. Redox Signal. 2006, 8, 1757–1764. [Google Scholar] [CrossRef]
- Hameed, A.; Ahmed, M.Z.; Hussain, T.; Aziz, I.; Ahmad, N.; Gul, B.; Nielsen, B.L. Effects of Salinity Stress on Chloroplast Structure and Function. Cells 2021, 10, 2023. [Google Scholar] [CrossRef]
- Bouché, N.; Fait, A.; Bouchez, D.; Møller, S.G.; Fromm, H. Mitochondrial succinic-semialdehyde dehydrogenase of the γ-aminobutyrate shunt is required to restrict levels of reactive oxygen intermediates in plants. Proc. Natl. Acad. Sci. USA 2003, 100, 6843–6848. [Google Scholar] [CrossRef]
- Shang, H.; Cao, S.; Yang, Z.; Cai, Y.; Zheng, Y. Effect of Exogenous γ-Aminobutyric Acid Treatment on Proline Accumulation and Chilling Injury in Peach Fruit after Long-Term Cold Storage. J. Agric. Food Chem. 2011, 59, 1264–1268. [Google Scholar] [CrossRef]
- Ellis, E.M. Reactive carbonyls and oxidative stress: Potential for therapeutic intervention. Pharmacol. Ther. 2007, 115, 13–24. [Google Scholar] [CrossRef]
- Li, Z.-G.; Duan, X.-Q.; Min, X.; Zhou, Z.-H. Methylglyoxal as a novel signal molecule induces the salt tolerance of wheat by regulating the glyoxalase system, the antioxidant system, and osmolytes. Protoplasma 2017, 254, 1995–2006. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Nahar, K.; Hasanuzzaman, M.; Fujita, M. Calcium Supplementation Improves Na+/K+ Ratio, Antioxidant Defense and Glyoxalase Systems in Salt-Stressed Rice Seedlings. Front. Plant Sci. 2016, 7, 609. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Xu, L.; Zeng, X.; Li, Z.; Qin, B.; He, N. New perspective of GABA as an inhibitor of formation of advanced lipoxidation end-products: It’s interaction with malondiadehyde. J. Biomed. Nanotechnol. 2010, 6, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.; Li, J.; Xie, J.; Li, N.; Bakpa, E.P.; Han, K.; Yang, Y.; Wang, C. Exogenous Zeaxanthin Alleviates Low Temperature Combined with Low Light Induced Photosynthesis Inhibition and Oxidative Stress in Pepper (Capsicum annuum L.) Plants. Curr. Issues Mol. Biol. 2022, 44, 2453–2471. [Google Scholar] [CrossRef]
- Elkelish, A.; El-Mogy, M.M.; Niedbała, G.; Piekutowska, M.; Atia, M.A.; Hamada, M.M.; Shahin, M.; Mukherjee, S.; ElYazied, A.A.; Shebl, M. Roles of Exogenous α-Lipoic Acid and Cysteine in Mitigation of Drought Stress and Restoration of Grain Quality in Wheat. Plants 2021, 10, 2318. [Google Scholar] [CrossRef]
- Jahan, M.S.; Hasan, M.M.; Alotaibi, F.S.; Alabdallah, N.M.; Alharbi, B.M.; Ramadan, K.M.; Bendary, E.S.; Alshehri, D.; Jabborova, D.; Al-Balawi, D.A. Exogenous Putrescine Increases Heat Tolerance in Tomato Seedlings by Regulating Chlorophyll Metabolism and Enhancing Antioxidant Defense Efficiency. Plants 2022, 11, 1038. [Google Scholar] [CrossRef]
- Carillo, P. GABA Shunt in Durum Wheat. Front. Plant Sci. 2018, 9, 100. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Ascorbate and Glutathione: The Heart of the Redox Hub. Plant Physiol. 2011, 155, 2–18. [Google Scholar] [CrossRef]
- Hossain, M.S.; Dietz, K.-J. Tuning of redox regulatory mechanisms, reactive oxygen species and redox homeostasis under salinity stress. Front. Plant Sci. 2016, 7, 548. [Google Scholar] [CrossRef]
- Türkyilmaz, B.; AktaŞ, L.Y.; Güven, A. Salinity induced differences in growth and nutrient accumulation in five barley cultivars. Turk. J. Field Crops 2011, 16, 84–92. [Google Scholar]
- Assaha, D.V.M.; Ueda, A.; Saneoka, H.; Al-Yahyai, R.; Yaish, M.W. The Role of Na+ and K+ Transporters in Salt Stress Adaptation in Glycophytes. Front. Physiol. 2017, 8, 509. [Google Scholar] [CrossRef] [PubMed]
- Alqarawi, A.A.; Hashem, A.; AbdAllah, E.F.; Al-Huqail, A.A.; Alshahrani, T.S.; Alshalawi, S.A.R.; Egamberdieva, D. Protective role of gamma amminobutyric acid on Cassia italica Mill under salt stress. Legume Res. 2016, 39, 396–404. [Google Scholar] [CrossRef]
- Ma, X.; Gu, J.; Luo, Q.; Wen, M.; Li, H.; Wang, Z.-Y. The synergistic benefits of β-aminobutyric acid and γ-aminobutyrate on salt and drought tolerance in cassava. Plant Biotechnol. Rep. 2022, 16, 29–41. [Google Scholar] [CrossRef]
- Liu, W.; Liu, K.; Chen, D.; Zhang, Z.; Li, B.; El-Mogy, M.M.; Tian, S.; Chen, T. Solanum lycopersicum, a Model Plant for the Studies in Developmental Biology, Stress Biology and Food Science. Foods 2022, 11, 2402. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).