Seeding and Overseeding Native Hayseed Support Plant and Soil Arthropod Communities in Agriculture Areas
Abstract
1. Introduction
2. Materials and Methods
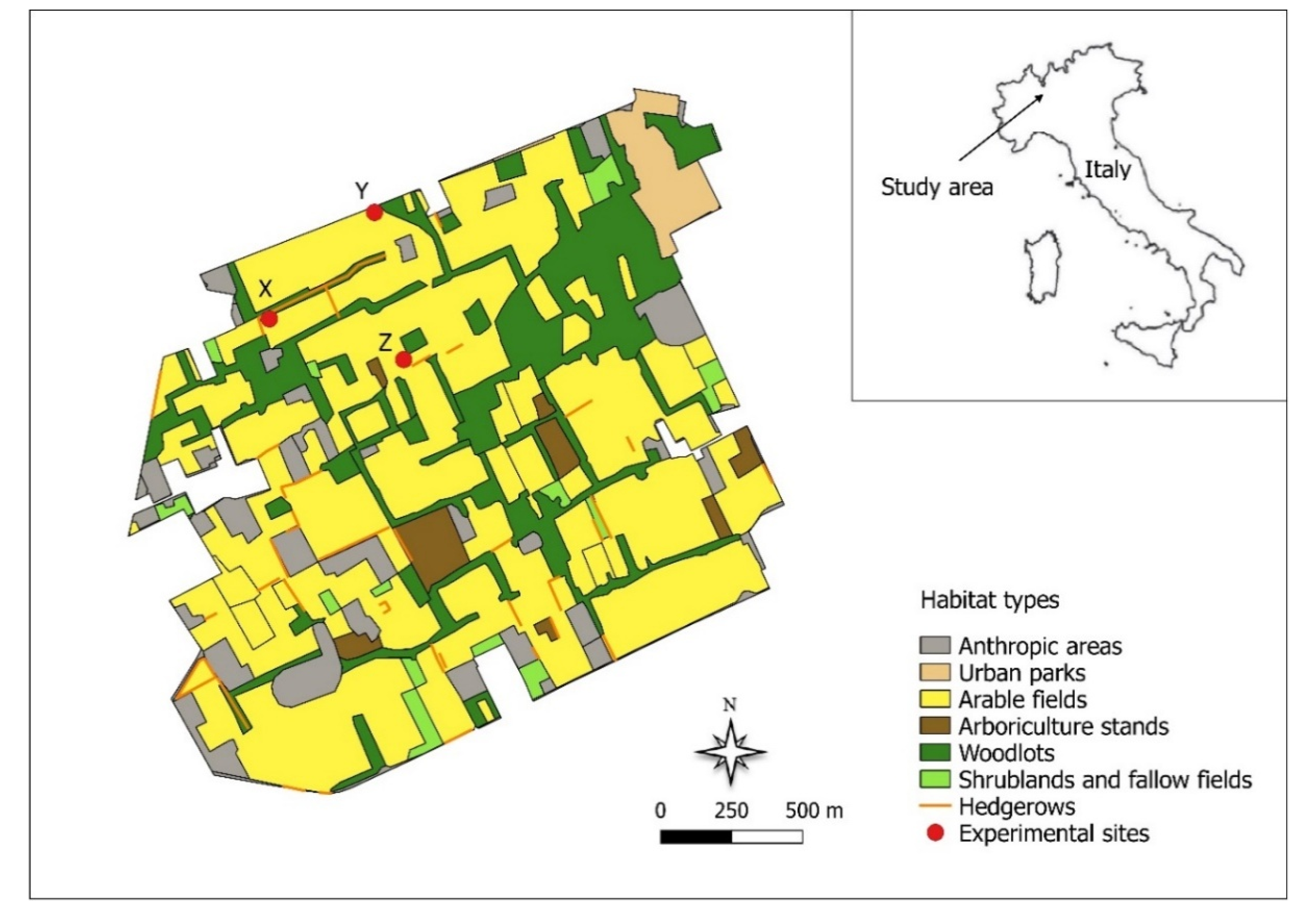
2.1. Study Area and Experimental Design
2.2. Data Collection
2.2.1. Vegetation
- (a)
- Vegetation cover (cumulative):
- Total vegetation percent cover;
- Percent cover of typical grassland species belonging to Molinio-Arrhenatheretea phytosociological class, based on the species composition of the donor grassland (Table S2);
- Percent cover of segetal (ruderal) weed species (only infesting crop fields);
- Percent cover of invasive alien species, listed according to the Lombardy region’s strategy for alien species [29]:
- (b)
- Ecological groups:
- (c)
- Species diversity:
2.2.2. Microarthropod Communities
2.3. Data Analysis
3. Results
3.1. Vegetation
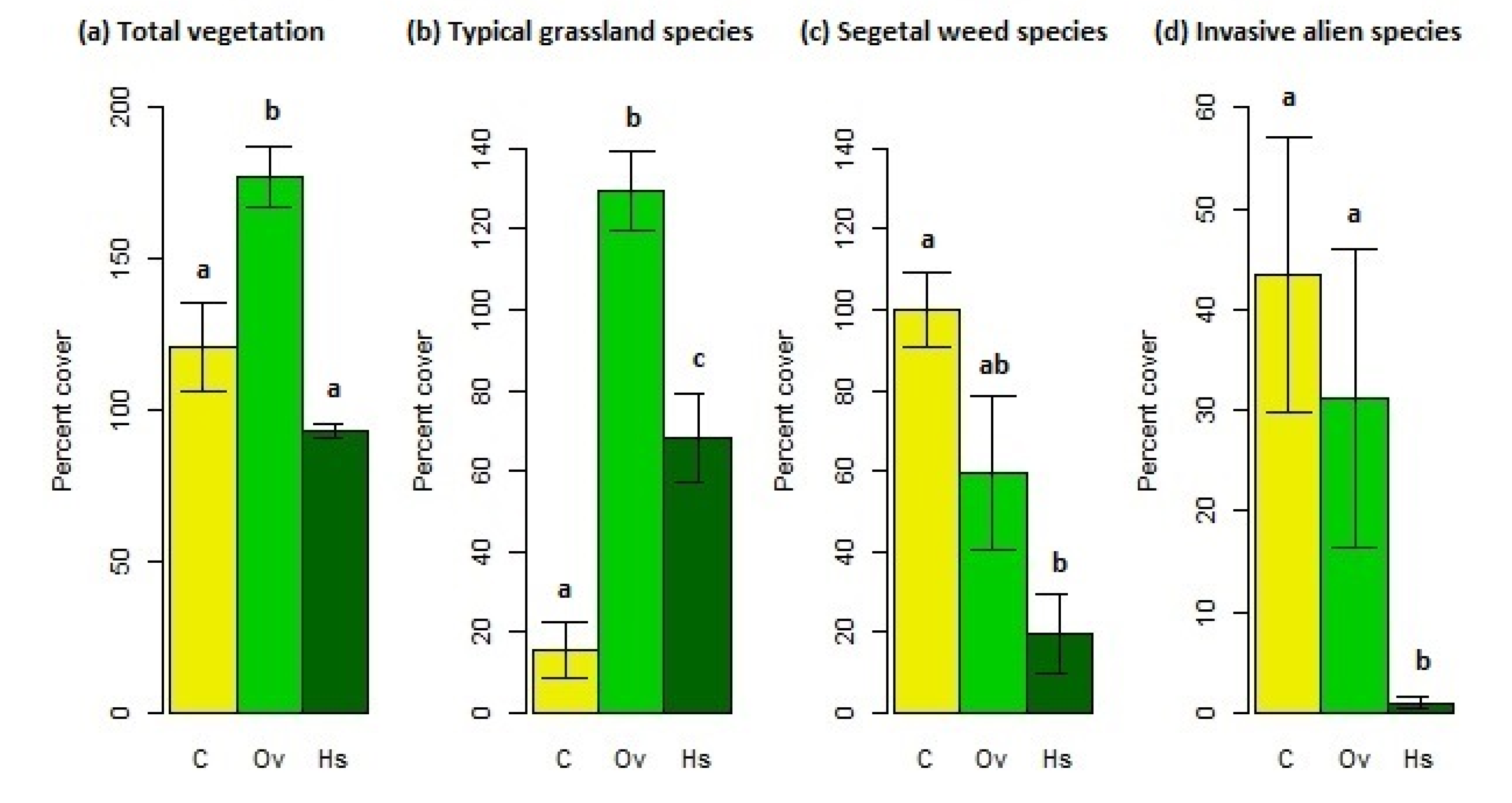
3.1.1. Vegetation Cover
3.1.2. Ecological Groups of Species
3.1.3. Plant Diversity
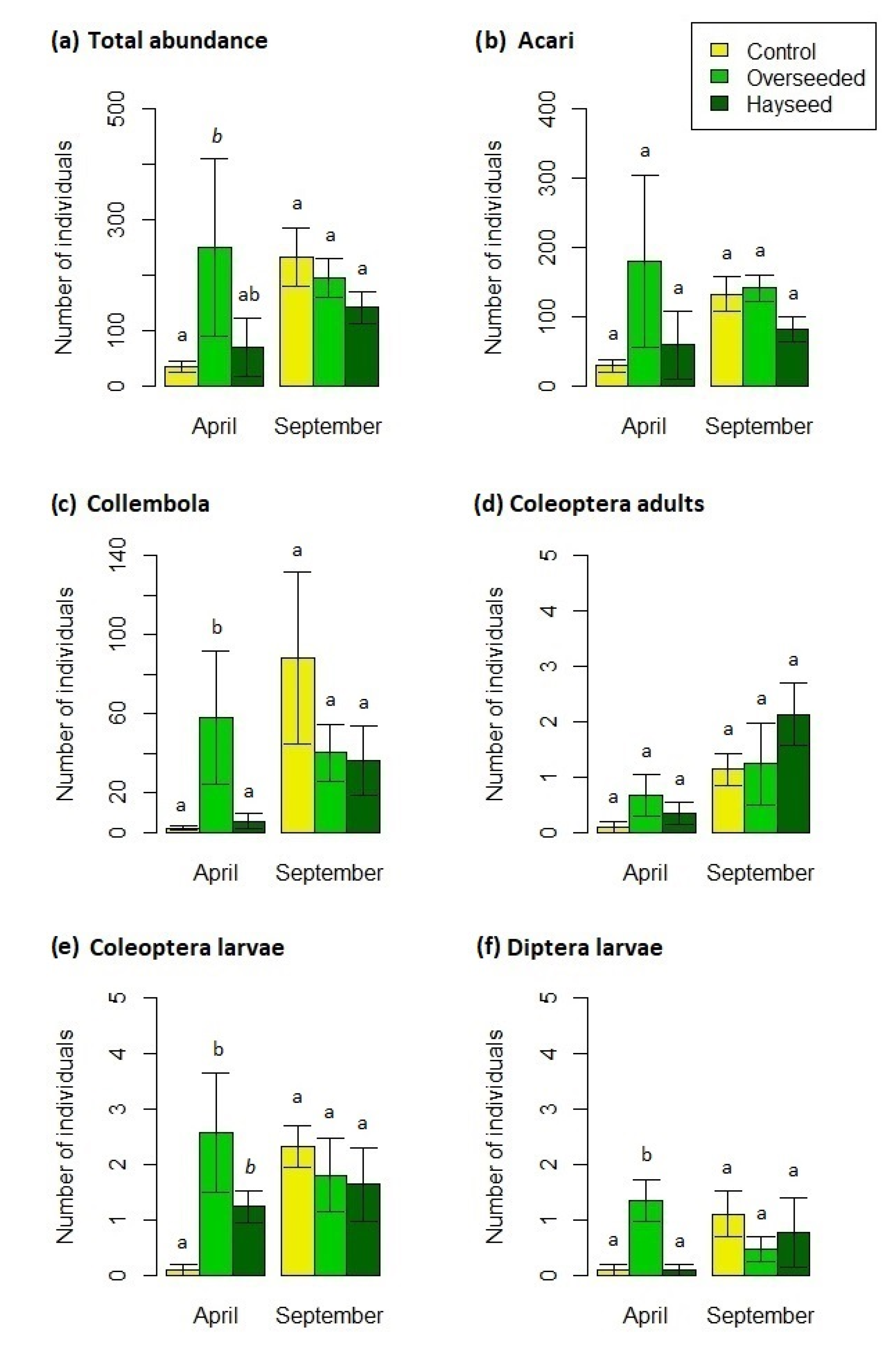
3.2. Microarthropod Communities
4. Discussion
4.1. Vegetation
4.2. Microarthropod Community
5. Management Implications
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Matson, P.A.; Parton, W.J.; Power, A.G.; Swift, M.J. Agricultural intensification and ecosystem properties. Science 1997, 277, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Garratt, M.P.D.; Senapathi, D.; Coston, D.J.; Mortimer, S.R.; Potts, S.G. The benefits of hedgerows for pollinators and natural enemies depends on hedge quality and landscape context. Agric. Ecosyst. Environ. 2017, 247, 363–370. [Google Scholar] [CrossRef]
- Gaba, S.; Fried, G.; Kazakou, E.; Chauvel, B.; Navas, M.-L. Agroecological weed control using a functional approach: A review of cropping systems diversity. Agron. Sustain. Dev. 2014, 34, 103–119. [Google Scholar] [CrossRef]
- Kuester, A.; Conner, J.K.; Culley, T.; Baucom, R.S. How weeds emerge: A taxonomic and trait-based examination using United States data. New Phytol. 2014, 202, 1055–1068. [Google Scholar] [CrossRef]
- Gentili, R.; Montagnani, C.; Gilardelli, F.; Guarino, M.F.; Citterio, S. Let native species take their course: Ambrosia artemisiifolia replacement during natural or “artificial” succession. Acta Oecol. 2017, 82, 32–40. [Google Scholar] [CrossRef]
- Tabaglio, V.; Gavazzi, C.; Menta, C. Physico-chemical indicators and microarthropod communities as influenced by no-till, conventional tillage and nitrogen fertilisation after four years of continuous maize. Soil Till. Res. 2009, 105, 135–142. [Google Scholar] [CrossRef]
- Menta, C.; Bonati, B.; Staffilani, F.; Conti, F.D. Agriculture management and soil fauna monitoring: The case of Emilia-Romagna region (Italy). Agric. Res. Technol. 2017, 4, 555649. [Google Scholar] [CrossRef]
- Menta, C.; Conti, F.D.; Pinto, S.; Bodini, A. Soil Biological Quality index (QBS-ar): 15 years of application at global scale. Ecol. Indic. 2018, 85, 773–780. [Google Scholar] [CrossRef]
- Menta, C.; Conti, F.D.; Pinto, S. Microarthropods biodiversity in natural, seminatural and cultivated soils—QBS-ar approach. Appl. Soil Ecol. 2018, 123, 740–743. [Google Scholar] [CrossRef]
- Huang, J.; Tichit, M.; Poulot, M.; Darly, S.; Li, S.; Petit, C.; Aubry, C. Comparative review of multifunctionality and ecosystem services in sustainable agriculture. J. Environ. Manag. 2015, 149, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Philpott, S.M.; Lin, B.B.; Jha, S.; Brines, S.J. A multi-scale assessment of hurricane impacts on agricultural landscapes based on land use and topographic features. Agric. Ecosyst. Environ. 2008, 128, 12–20. [Google Scholar] [CrossRef]
- Ryan, J.G.; McAlpine, C.A.; Ludwig, J.A. Integrated vegetation designs for enhancing water retention and recycling in agroecosystems. Landsc. Ecol. 2010, 25, 1277–1288. [Google Scholar] [CrossRef]
- Perego, A.; Rocca, A.; Cattivelli, V.; Tabaglio, V.; Fiorini, A.; Barbieri, S.; Schillaci, C.; Chiodini, M.E.; Brenna, S.; Acutis, M. Agro-environmental aspects of conservation agriculture compared to conventional systems: A 3-year experience on 20 farms in the Po valley (Northern Italy). Agric. Syst. 2019, 168, 73–87. [Google Scholar] [CrossRef]
- Ashworth, A.J.; Toler, H.D.; Allen, F.L.; Augé, R.M. Global meta-analysis reveals agro-grassland productivity varies based on species diversity over time. PLoS ONE 2018, 13, e0200274. [Google Scholar] [CrossRef]
- Bianchi, F.J.J.A.; Mikos, V.; Brussaard, L.; Delbaere, B.; Pulleman, M.M. Opportunities and limitations for functional agrobiodiversity in the European context. Environ. Sci. Policy 2013, 27, 223–231. [Google Scholar] [CrossRef]
- Duru, M.; Therond, O.; Martin, G.; Martin-Clouaire, R.; Magne, M.-A.; Justes, E.; Journet, E.-P.; Aubertot, J.-N.; Savary, S.; Bergez, J.-E.; et al. How to implement biodiversity-based agriculture to enhance ecosystem services: A review. Agron. Sustain. Dev. 2015, 35, 1259–1281. [Google Scholar] [CrossRef]
- Nicholls, C.I.; Altieri, M.A. Plant biodiversity enhances bees and other insect pollinators in agroecosystems. A review. Agron. Sustain. Dev. 2013, 33, 257–274. [Google Scholar] [CrossRef]
- Cardarelli, E.; Musacchio, A.; Montagnani, C.; Bogliani, G.; Citterio, S.; Gentili, R. Ambrosia artemisiifolia control in agricultural areas: Effect of grassland seeding and herbivory by the exotic leaf beetle Ophraella communa. NeoBiota 2018, 38, 1–22. [Google Scholar] [CrossRef]
- Rundlöf, M.; Lundin, O.; Bommarco, R. Annual flower strips support pollinators and potentially enhance red clover seed yield. Ecol. Evol. 2018, 8, 7974–7985. [Google Scholar] [CrossRef]
- Dechen Silva, A.; Roche, L.M.; Gornish, E.S. The use of strip-seeding for management of two late-season invasive plants. Heliyon 2019, 5, e01772. [Google Scholar] [CrossRef]
- Menta, C.; Leoni, A.; Gardi, C.; Conti, F.D. Are grasslands important habitats for soil microarthropod conservation? Biodivers. Conserv. 2011, 20, 1073–1087. [Google Scholar] [CrossRef]
- Cardarelli, E.; Bogliani, G. Effects of grass management intensity on ground beetle assemblages in rice field banks. Agric. Ecosyst. Environ. 2014, 195, 120–126. [Google Scholar] [CrossRef]
- Symondson, W.O.C.; Sunderland, K.D.; Greenstone, M.H. Can generalist predators be effective biocontrol agents? Annu. Rev. Entomol. 2002, 47, 561–594. [Google Scholar] [CrossRef] [PubMed]
- Kirmer, A.; Mahn, E. Spontaneous and initiated succession on unvegetated slopes in the abandoned lignite-mining area of Goitsche, Germany. Appl. Veg. Sci. 2001, 4, 19–27. [Google Scholar] [CrossRef]
- Ceriani, R.M.; Ferrario, A.; Villa, M. (Eds.) Il Fiorume: Una risorsa Per la Biodiversità. Regione Lombardia; Centro Flora Autoctona: Milano, Italy, 2011. [Google Scholar]
- Gentili, R.; Gilardelli, F.; Ciappetta, S.; Ghiani, A.; Citterio, S. Inducing competition: Intensive grassland seeding to control Ambrosia artemisiifolia L. Weed Res. 2015, 55, 278–288. [Google Scholar] [CrossRef]
- Lemasson, C.; Pierre, P.; Osson, B. Renovation of pastures and overseeding. How to understand, to study and to choose the method [Rénovation des prairies et sursemis. Comprendre, raisonner et choisir la méthode]. Fourrages 2008, 195, 315–330. [Google Scholar]
- Gentili, R.; Ferrè, C.; Cardarelli, E.; Montagnani, C.; Bogliani, G.; Citterio, S.; Comolli, R. Comparing Negative Impacts of Prunus serotina, Quercus rubra and Robinia pseudoacacia on Native Forest Ecosystems. Forests 2019, 10, 842. [Google Scholar] [CrossRef]
- LIFE GESTIRE 2020. Available online: http://www.naturachevale.it/specie-invasive/specie-aliene-invasive-in-lombardia/ (accessed on 8 April 2020).
- Ma, H.-K.; Pineda, A.; van der Wurff, A.W.G.; Raaijmakers, C.; Bezemer, T.M. Plant–Soil Feedback Effects on Growth, Defense and Susceptibility to a Soil-Borne Disease in a Cut Flower Crop: Species and Functional Group Effects. Front. Plant Sci. 2017, 8, 2127. [Google Scholar] [CrossRef]
- Raunkiær, C. The Life Forms of Plants and Statistical Plant Geography; Oxford University Press: Oxford, UK, 1934. [Google Scholar]
- Shannon, C. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Whittaker, R.H. Evolution and measurement of species diversity. Taxon 1972, 21, 213–251. [Google Scholar] [CrossRef]
- Parisi, V.; Menta, C.; Gardi, C.; Jacomini, C.; Mozzanica, E. Microarthropod communities as a tool to assess soil quality and biodiversity: A new approach in Italy. Agric. Ecosyst. Environ. 2005, 105, 323–333. [Google Scholar] [CrossRef]
- Menta, C.; Contia, F.D.; Pinto, S.; Leoni, A.; Lozano-Fondón, C. Monitoring soil restoration in an open-pit mine in northern Italy. Appl. Soil Ecol. 2014, 83, 22–29. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org/ (accessed on 20 December 2019).
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D.; R Core Team. Nlme: Linear and Nonlinear Mixed Effects Models. R Package Version 3.1-141. 2019. Available online: https://CRAN.R-project.org/package=nlme (accessed on 8 April 2020).
- Lenth, R.V. Least-Squares Means: The R Package lsmeans. J. Stat. Soft. 2016, 69, 1–33. [Google Scholar] [CrossRef]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous Inference in General Parametric Models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef] [PubMed]
- Gentili, R.; Gilardelli, F.; Bona, E.; Prosser, F.; Selvaggi, A.; Alessandrini, A.; Martini, F.; Nimis, P.L.; Wilhalm, T.; Adorni, M.; et al. Distribution map of Ambrosia artemisiifolia L. (Asteraceae) in Italy. Plant Biosyst. 2017, 151, 381–386. [Google Scholar] [CrossRef]
- Buffa, G.; Bracco, F.; Sburlino, G. Première contribution à l’analyse corologique des prairies à Arrhenatherum elatius (L.) Presl (Alliance Arrhenatherion elatioris W. Koch 1926) en Europe. Fitosociologia 1997, 34, 47–68. [Google Scholar]
- Jermini, M.; Schoenenberger, N. Neobiota nel sistema viticolo ticinese: Storia, diversità e impatti. Mem. Soc. Tic. Sci. Nat. Mus. Cant. St. Nat. 2017, 12, 125–140. [Google Scholar]
- Spehn, E.M.; Scherer-Lorenzen, M.; Schmid, B.; Hector, A.; Caldeira, M.C.; Dimitrakopoulos, P.G.; Finn, J.A.; Jumpponen, A.; O’Donnovan, G.; Pereira, J.S.; et al. The role of legumes as a component of biodiversity in a Cross-European study of grassland biomass nitrogen. Oikos 2002, 98, 205–218. [Google Scholar] [CrossRef]
- Huston, M.A.; Aarssen, L.W.; Austin, M.P.; Cade, B.S.; Friday, J.D.; Garnier, E.; Grime, J.P.; Hodgson, J.; Lauenroth, W.K.; Thompson, K.; et al. No consistent effect of plant diversity on productivity. Science 2000, 289, 1255. [Google Scholar] [CrossRef]
- Deng, L.; Shangguan, Z. Species Composition, Richness and Aboveground Biomass of Natural Grassland in Hilly-Gully Regions of the Loess Plateau, China. J. Integr. Agric. 2014, 13, 2527–2536. [Google Scholar] [CrossRef][Green Version]
- Gilardelli, F.; Sgorbati, S.; Citterio, S.; Gentili, R. Restoring limestone quarries: Hayseed, commercial seed mixture or spontaneous succession? Land Degrad. Dev. 2016, 27, 316–324. [Google Scholar] [CrossRef]
- Loucougaray, G.; Dobremez, L.; Gos, P.; Pauthenet, Y.; Nettier, B.; Lavorel, S. Assessing the effects of grassland management on forage production and environmental quality to identify paths to ecological intensification in mountain grasslands. Environ. Manag. 2015, 56, 1039–1052. [Google Scholar] [CrossRef]
- Tasser, E.; Tappeiner, U. Impact of land use changes on mountain vegetation. Appl. Veg. Sci. 2002, 5, 173–184. [Google Scholar] [CrossRef]
- Kampann, D.; Herzog, F.; Jeanneret, P.; Konold, W.; Peter, M.; Walter, T.; Wildi, O.; Lüscher, A. Mountain grassland biodiversity: Impact of site conditions versus management type. J. Nat. Conserv. 2008, 16, 12–25. [Google Scholar] [CrossRef]
- Farruggia, A.; Pomie`s, D.; Coppa, M.; Ferlay, A.; Verdier-Metz, I.; Le Morvan, A.; Bethier, A.; Pompanon, F.; Troquier, O.; Martina, B. Animal performances, pasture biodiversity and dairy product quality: How it works in contrasted mountain grazing systems. Agric. Ecosyst. Environ. 2014, 185, 231–244. [Google Scholar] [CrossRef]
- Rüdisser, J.; Tasser, E.; Peham, T.; Meyer, E.; Tappeiner, U. The dark side of biodiversity: Spatial application of the biological soil quality indicator (BSQ). Ecol. Indic. 2015, 53, 240–246. [Google Scholar] [CrossRef]
- Menta, C.; Leoni, A.; Tarasconi, K.; Affanni, P. Does compost use affect microarthropod soil communities? Fresenius Environ. Bull. 2010, 19, 2303–2311. [Google Scholar]
- Sapkota, T.B.; Mazzoncini, M.; Bàrberi, P.; Antichi, D.; Silvestri, N. Fifteen years of no till increase soil organic matter, microbial biomass and arthropod diversity in cover crop-based arable cropping systems. Agron. Sustain. Dev. 2012, 32, 853–863. [Google Scholar] [CrossRef]
- Neave, P.; Fox, C.A. Response of soil invertebrates to reduce tillage systems established on a clay loam soil. Appl. Soil Ecol. 1998, 9, 423–428. [Google Scholar] [CrossRef]
- UNIMIB; CFA. Il contrasto ad Ambrosia con la Semina di Specie Autoctone; Technical Report; Fondazione Cariplo: Bando Biodiversità, Milano, 2016. [Google Scholar]
- Litt, A.R.; Cord, E.E.; Fulbright, T.E.; Schuster, G.L. Effects of invasive plants on arthropods. Conserv. Biol. 2014, 28, 1532–1549. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Xie, J.F.; Quan, G.M.; Zhang, J.E.; Mao, D.J.; Wang, J.X. Changes in the soil meso-and micro-fauna community under the impacts of exotic Ambrosia artemisiifolia. Ecol. Res. 2019, 34, 265–276. [Google Scholar] [CrossRef]
- Pritekel, C.; Whittemore-Olson, A.; Snow, N.; Moore, J.C. Impacts from invasive plant species and their control on the plant community and belowground ecosystem at Rocky Mountain National Park, USA. Appl. Soil Ecol. 2006, 32, 132–141. [Google Scholar] [CrossRef]
- Wolkovich, E.M.; Bolger, D.T.; Holway, D.A. Complex responses to invasive grass litter by ground arthropods in a Mediterranean scrub ecosystem. Oecologia 2009, 161, 697–708. [Google Scholar] [CrossRef] [PubMed]
- Carlier, L.; De Vliegher, A.; Rotar, I. Importance and functions of European grasslands. Bull. USAMV-CN 2005, 61, 17–26. [Google Scholar]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cardarelli, E.; Gentili, R.; Della Rocca, F.; Zanella, M.; Caronni, S.; Bogliani, G.; Citterio, S. Seeding and Overseeding Native Hayseed Support Plant and Soil Arthropod Communities in Agriculture Areas. Life 2020, 10, 38. https://doi.org/10.3390/life10040038
Cardarelli E, Gentili R, Della Rocca F, Zanella M, Caronni S, Bogliani G, Citterio S. Seeding and Overseeding Native Hayseed Support Plant and Soil Arthropod Communities in Agriculture Areas. Life. 2020; 10(4):38. https://doi.org/10.3390/life10040038
Chicago/Turabian StyleCardarelli, Elisa, Rodolfo Gentili, Francesca Della Rocca, Marta Zanella, Sarah Caronni, Giuseppe Bogliani, and Sandra Citterio. 2020. "Seeding and Overseeding Native Hayseed Support Plant and Soil Arthropod Communities in Agriculture Areas" Life 10, no. 4: 38. https://doi.org/10.3390/life10040038
APA StyleCardarelli, E., Gentili, R., Della Rocca, F., Zanella, M., Caronni, S., Bogliani, G., & Citterio, S. (2020). Seeding and Overseeding Native Hayseed Support Plant and Soil Arthropod Communities in Agriculture Areas. Life, 10(4), 38. https://doi.org/10.3390/life10040038






