Abstract
Propanol isomers, which are oxygen-rich fuels, possess superior octane ratings and energy density in comparison to methanol and ethanol. Recently, due to advancements in fermentation techniques, these propanol isomers have garnered increased interest as additives for engines. They are being explored to decrease emissions and reduce the usage of conventional fossil fuels. This study delves into this emerging field. One of the alternatives is the use of alcohol fuels in their pure state or as additives to traditional fuels. Alcohols, due to their higher volumetric energy density, are better fuels for spark ignition engines than hydrogen and biogas. Alcohol-blended fuels or alcohol fuels in their pure state may be used in gasoline engines to reduce exhaust emissions. The current research emphasizes the effect of isopropanol gasoline blends on the performance and emissions characteristics of a gasoline direct injection (GDI) engine. This investigation was conducted with different blends of isopropanol and gasoline (by volume: 10% isopropanol [IP10], 20% isopropanol [IP10], 30% isopropanol [IP30], 40% isopropanol [IP40], and 50% isopropanol [IP50]). The reviewed results showed that with increasing isopropanol in the fuel blends, engine brake power increased while BSFC decreased. In terms of emissions, with the increase in isopropanol in the fuel blends, CO and HC emissions decreased while CO2 and NOx emissions increased.
1. Introduction
Despite the advent of renewable energy vehicles (powered by hydrogen, electric power, and a combination of different energy sources), internal combustion engines (ICE) are still the main source of energy for many vehicles [1]. GDI engines have not yet exhausted their potential as a source of energy for transportation. The combination of various technologies to reduce fuel consumption and emissions combined with various alternative fuels make these engines relevant in modern vehicles.
As the population increases, so does the number of vehicles, which leads to an increase in the consumption of fossil fuels [2]. This, in turn, is linked to an increase in pollution due to the burning of petroleum fuels, which leads to the greenhouse effect and acid rain [3,4]. All these problems are particularly sensitive in big cities and affect people’s health. Therefore, to deal with the global environmental and energy problems of internal combustion engines, it is necessary to use clean and renewable energy to replace the energy from petroleum fuels [5]. To be preferred, alternative fuels must be cheap, produced from local sources, must not require changes to the existing fuel system, and must not degrade engine power and increase harmful emissions.
With the use of oxygenated fuels or fuel mixtures (such as alcohols and their mixtures), more oxygen enters the combustion chamber and this leads to more complete combustion and reduced harmful emissions [6,7,8].
Some of the commonly used alcohols in spark ignition (SI) engines are ethanol, butanol, and methanol [9].
Properties of Isopropanol
Propanol (C3H7OH) is an alcohol with three carbons and two isomers, n-propanol (1-propaol) and iso-propanol (2-propanol). Isopropanol, also known as isopropyl alcohol (IPA), is a common type of alcohol used for various industrial, medical, and household purposes. Isopropyl alcohol is a transparent, odoriferous liquid similar to a blend of ethanol and acetone. It has a faint bitter taste and is highly flammable. Isopropyl alcohol mixes well with water, ethyl ether, and ethyl alcohol. However, it should not be combined with strong oxidizers, acetaldehyde, chlorine, ethylene oxide, acids, or isocyanates, as these substances are incompatible with it [10]. Isopropanol can be produced through several methods, with the most common being the indirect hydration of propylene. The primary raw material for isopropanol production is propylene (propene), which can be derived from petrochemical sources, such as crude oil or natural gas. It is important to note that isopropanol can also be produced through other methods, such as the fermentation of sugars or acetone hydrogenation. It can also be made from biomass using cyanobacteria [11] or metabolically engineered Escherichia coli [12]. These alternative methods are less common and are generally used for specific applications [13,14,15]. World production of isopropanol has reached around 1,500,000 tons per year [16].
Propanol isomers, which include isopropanol (isopropyl alcohol) and n-propanol (normal propyl alcohol), have not gained widespread use as automotive fuels mainly due to their higher production costs compared to ethanol. Additionally, justifying their use in place of more established fuels like ethanol and butanol is challenging. Consequently, there is limited research and attention focused on these propanol-based fuels when compared to ethanol and butanol alternatives in the automotive fuel industry. The improvement of the production technologies of isopropanol leads to a decrease in its cost, which makes it more affordable.
Ethanol and propanol isomers have higher octane numbers than regular motor gasoline’s (Table 1), while the octane number of butanol isomers is very close to that of conventional motor gasoline [10,17]. When it comes to larger linear alcohols, their octane ratings are lower than that of regular motor gasoline. Consequently, mixing these alcohols (like straight-chain alcohols with five or more carbon atoms or larger isoalcohols) with standard motor gasoline can actually reduce the likelihood of engine knocking in gasoline engines.

Table 1.
Properties of methanol, gasoline, isopropanol, ethanol, and n-butanol.
When using gasoline–alcohol blends, it can be easy to include some water in the mixture. A significant challenge when introducing alcohol–gasoline blends into the fuel system is ensuring that the mixture forms a stable, uniform liquid fuel to prevent issues like wet corrosion (due to alcohol absorption by the surrounding water). Ethanol and propanol isomers mix easily with water, but this mixing ability decreases as alcohols become larger. Regular gasoline and water do not naturally mix with each other. However, when blending alcohol into gasoline, some water can dissolve into the mixture and become measurable. The ability of alcohol blends to tolerate water increases significantly when moving from ethanol to propanol [24].
Isopropanol stands out as a safer option compared to methanol, as it rarely leads to blindness upon ingestion. It also has several advantages: it possesses a higher heating value and greater volumetric energy density than ethanol (Table 1). Isopropanol boasts a higher octane number, lower viscosity, superior evaporation properties, and a higher oxygen content compared to butanol. It effectively combines the benefits of ethanol, methanol, and butanol, making it well suited for large-scale applications in gasoline engines. However, it is essential to note that there are significantly fewer studies on the use of isopropanol in engines compared to ethanol, methanol, and butanol. Most of the research has concentrated on diesel engines, with less emphasis on exploring the use of isopropanol in gasoline engines.
In a study conducted by Gainey and colleagues [25], they examined how isopropanol behaves in a gasoline engine when running on a lean-burn mixture. The findings indicated that, under specific conditions of an inlet pressure of 1 bar and an inlet temperature of 320 K, the lean-burn limit was extended to an excess air ratio (λ) of 1.6. Additionally, the researchers harnessed isopropanol’s excellent resistance to knocking (its high anti-knock property) to advance the combustion phase, which, in turn, enhanced the stability of isopropanol combustion in the engine.
When it comes to estimating how oxygenates impact the vapor pressure of gasoline blends, you cannot rely on the assumption of ideal solution behavior. Researchers, including Andersen and colleagues [26], conducted a study on various alcohol gasoline blends, including some blends with two different alcohols. What they found is that at the low concentrations allowed, methanol, ethanol, n-propanol, and isopropanol actually increased the vapor pressure of a gasoline blend to levels higher than either of the individual components. Interestingly, when these alcohols are in their pure form, their vapor pressures are lower than that of gasoline. The authors of another study [27] investigated how the combustion and emissions behaved when using isopropanol–gasoline blends in a gasoline engine with a port fuel injection system. What they found was that when they increased the proportion of isopropanol in the blend (at ratios of 10%, 20%, and 30% by volume), it led to several effects: 1. Increased Cylinder Pressure: The engine experienced higher cylinder pressures as the isopropanol ratio went up; 2. Enhanced Thermal Efficiency: Higher isopropanol ratios resulted in improved thermal efficiency of the engine, which means it was able to convert more of the fuel’s energy into useful work; 3. Faster Heat Release: The rate at which heat was released during combustion also accelerated with a greater isopropanol ratio. However, there were trade-offs: 1. Increased NOx Emissions: Higher isopropanol ratios led to greater emissions of nitrogen oxides (NOx), which are harmful pollutants; 2. Reduced CO and HC Emissions: On the positive side, increasing the isopropanol ratio reduced emissions of carbon monoxide (CO) and hydrocarbons (HC), which are pollutants as well. So, while raising the proportion of isopropanol in the blend had some benefits in terms of engine performance, it also came with environmental trade-offs, notably increased NOx emissions. Other authors [28] have found that the addition of methanol, ethanol, and propanol to gasoline can make the gasoline less prone to engine knocking. Whether an alcohol–gasoline blend causes knocking or not depends on the specific alcohol used, rather than just the amount of oxygen it contains. Keskin and Gürü [29] found that when alcohols like ethanol and isopropanol were added to the fuel, it led to reduced emissions of CO and HC by 65.56% and 33.92%, respectively. However, there was an increase in emissions of NOx and carbon dioxide (CO2). Additionally, the blended fuels had the advantages of a higher octane number, lower sulfur content, and greater oxygen content. Gravalos et al. [30] found that when higher alcohols (C3-C5) were added to the fuel, it led to an increase in emissions of hydrocarbons, carbon dioxide, and nitrogen oxides, but it reduced carbon monoxide emissions. When comparing the emissions of CO, CO2, HC, and NO between fuel blends with higher-molecular-mass alcohols mixed with gasoline and those with only lower-molecular-mass alcohols mixed with gasoline, it became evident that the addition of higher-molecular-mass alcohols generally resulted in higher emissions, except for CO, which decreased.
In our review of the literature, there was not enough research about isopropanol gasoline blends on gasoline engines and their effect on engine performance and emissions. There were no studies conducted with a gasoline direct injection engine (at concentrations to 50% compared to net gasoline) without engine modifications. Most studies were conducted with small concentrations of isopropanol of 5%, 10%, 15%, and 20%. Comparing blended fuels with net gasoline in spark ignition engines is crucial to determine which type of blended fuel is better for reducing exhaust emissions in such engines. Additionally, it is essential to assess their influence on torque, power output, and fuel consumption. Hence, the aim of this study was to evaluate the performance and emissions of spark ignition engines when using isopropanol–gasoline blends (at concentrations not previously used by other researchers, 10%, 20%, 30%, 40%, and 50%) compared to net gasoline, all without making any modifications to the internal combustion engine.
2. Materials and Methods
The experiments were conducted on a four-cylinder, four-stroke, gasoline direct injection engine Toyota 1AZ-FSE. The specifications of the test engine are presented in Table 2.

Table 2.
Technical data of the engine Toyota 1AZ-FSE.
The experiments were conducted at medium loads (typical of most of the engine’s operating time in vehicle operation) for the engine speeds from 1400 to 5400 rpm. The following blends were used: 0% isopropanol (net gasoline) Gasoline, 10% isopropanol (90% gasoline) I10, 20% isopropanol (80% gasoline) I20, 30% isopropanol (70% gasoline) I30, 40% isopropanol (60% gasoline) I40, and 50% isopropanol (50% gasoline) I50 by volume.
The engine was mounted on a SCHENCK W230 eddy current dynamometer, on which engines up to 230 kW can be tested. The technical characteristics of the eddy current dynamometer are listed in Table 3.

Table 3.
Technical data of the eddy current dynamometer Schenck W230.
The accuracy of the eddy current dynamometer Schenck W230 is presented in Table 4.

Table 4.
Accuracy of eddy current dynamometer Schenck W230.
Ranges and measurement accuracy of the exhaust gas analyzer are presented in Table 5.

Table 5.
Ranges and measurement accuracy of the exhaust gas analyzer.
The research station is presented in Figure 1.

Figure 1.
The research station.
Figure 2 shows the schematic diagram of the system for determining environmental indicators. It consists of a dynamometer, a test engine, a Bosch 5-component gas analyzer type BEA 250, and a Hartridge smoke meter type YDA 309.
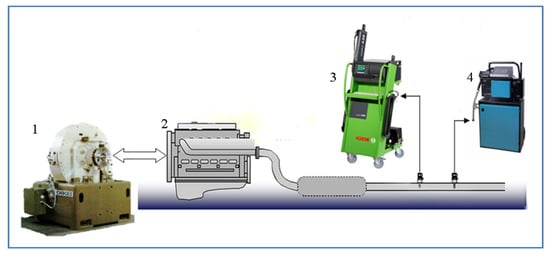
Figure 2.
System for measuring toxic indicators, engine and dynamometer: 1—Dynamometer; 2—engine; 3—gas analyzer; 4—dimometer.
The gas analyzer measures the content of the following components in the exhaust gases—HC, CO, CO2, O2, and NOx. The air–fuel ratio α is calculated based on the values of the measured components.
3. Results
The current study focused on investigating the performance and emission characteristics of a GDI engine when using combinations of isopropanol with net gasoline.
3.1. Performance Characteristics
Figure 3 displays the outcomes of brake power achieved using a combination of isopropanol and regular gasoline as fuel blends.
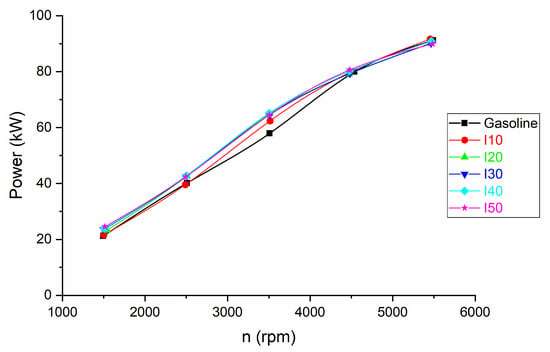
Figure 3.
The results for engine brake power obtained from blended isopropanol and net gasoline.
It can be observed that the engine brake power increased with increasing isopropanol in the fuel blends, with the increase being more significant up to mixtures with 20% isopropanol, after which the increase was insignificant. The reason for this phenomenon is due to the isopropanol having a greater oxygen content (26.6 compared to gasoline 0) and necessitates more energy, specifically heat, to transform one kilogram of liquid into one kilogram of vapor (758 kJ/kg) compared to gasoline (380 kJ/kg). In fact, this heat requirement is over twice that of gasoline, resulting in the absorption of more heat from the cylinder, consequently reducing heat loss through the cylinder walls while simultaneously cooling the incoming charge [31]. Despite isopropanol’s lower calorific value, its high oxygen content and greater latent heat of vaporization result in combustion that is more effective. Isopropanol has a charge-cooling effect in direct injection engines due to its high latent heat of vaporization [32]. This effect cools down the air–fuel mixture, making it denser, richer in oxygen, and less prone to knocking. These factors contribute to improved combustion efficiency, increased power output, and reduced emissions in modern engines. The calorific values for the I10 (42.64 MJ/kg), I20 (41.28 MJ/kg), I30 (39.92 MJ/kg), I40 (38.56 MJ/kg), and I50 (37.50 MJ/kg), respectively.
The increase in brake power is more pronounced at low and medium engine speeds and decreases at high engine speeds. The increase in brake power at low engine speeds is between 1.74% and 16.61%, while at medium engine speeds the increase in brake power is between 7.7% and 12.46%. The rationale behind diminishing the disparity in brake power between fuel blends and pure gasoline at higher speeds is that, at these elevated speeds, there is a restricted timeframe for the fuel to undergo complete combustion. This leads to a decreased occurrence of rapid burning. Conversely, at lower speeds, the situation is reversed; at lower engine speeds, there is more time available for the combustion process to occur. The engine’s pistons move at a slower rate, allowing the air and fuel mixture to spend a longer time in the combustion chamber before the next cycle begins. This extended duration provides a crucial advantage: it allows for more complete combustion of the air–fuel mixture [33]. During combustion, the air–fuel mixture needs time to mix thoroughly and burn efficiently. The slower piston movement allows for a more controlled ignition, ensuring that all the fuel particles have time to react with the available oxygen in the air. This extended timeframe permits a more thorough and even burning of the mixture.
Figure 4 and Figure 5 illustrate brake-specific fuel consumption (BSFC) when using a combination of isopropanol and regular gasoline as fuel blends. Due to the higher density of isopropanol and the low heat of combustion of isopropanol, the specific heat input to produce a unit of power is presented in MJ/kWh rather than the traditional g/kWh.
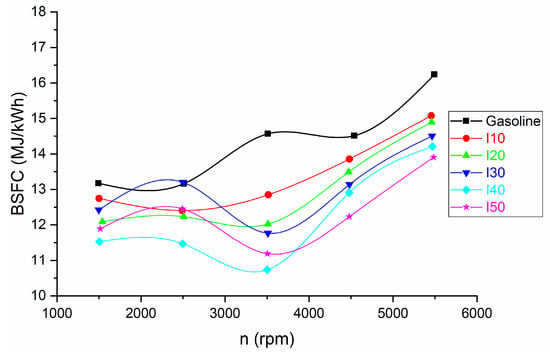
Figure 4.
The BSFC derived from the use of isopropanol blends and net gasoline.
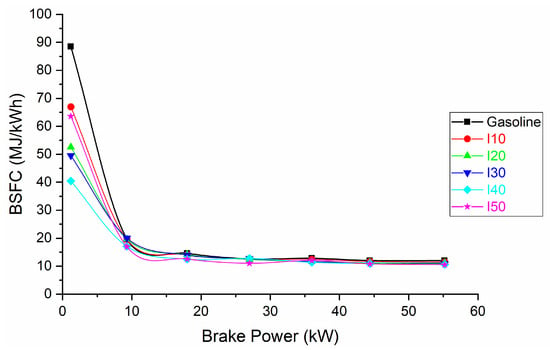
Figure 5.
The BSFC derived from the use of isopropanol blends and net gasoline.
It is noticeable that as the proportion of isopropanol in the fuel blends increased, there was a corresponding decrease in BSFC. The decrease in BSFC was more pronounced at low loads and less pronounced at high loads (Figure 5). The more pronounced decrease in BSFC at low loads when using isopropanol can be attributed to the fuel’s ability to enhance combustion efficiency, ensuring a higher percentage of the fuel is utilized for power generation. At high loads, the engine is already operating efficiently, so the impact of the fuel additive on BSFC is relatively smaller. This highlights how the effectiveness of isopropanol as a fuel additive varies based on the engine’s operating conditions. The decrease in BSFC at low loads is between 24.37% and 54.32%, while at high loads the decrease in BSFC is between 4.51% and 12.03%.
When mixing isopropanol and gasoline, several factors can influence fuel consumption, including the blend ratio and the specific characteristics of the fuel components. Here is how fuel consumption can change when blending isopropanol and gasoline:
- Blend Ratio: The proportion of isopropanol to gasoline in the blend is a crucial factor. If you increase the percentage of isopropanol in the blend, you may experience changes in fuel consumption. Isopropanol contains oxygen, which can promote combustion that is more complete and potentially improve fuel efficiency. However, if the blend has too much isopropanol, it could reduce energy density and lead to increased fuel consumption.
- Calorific Value: Isopropanol has a lower calorific value (energy content) compared to gasoline. This means that for the same volume of fuel, a higher percentage of isopropanol in the blend will provide less energy. As a result, you might need to burn more of the blend to generate the same amount of power, which can increase fuel consumption. This is in agreement with the results of other authors [31].
- Octane Rating: Isopropanol can increase the octane rating of gasoline, which can prevent engine knocking and allow for more advanced ignition timing. This can potentially improve engine efficiency and fuel consumption, especially in high-performance engines that require higher-octane fuels. This is in agreement with the results of other authors [28] who found that incorporating methanol, ethanol, and propanol into gasoline leads to an enhancement in its ability to withstand engine knocking.
- Engine Efficiency: The use of isopropanol in fuel blends can enhance combustion efficiency, reduce heat losses, and improve thermal efficiency. As a result, the engine becomes more efficient at converting the energy from fuel into mechanical work, leading to a decrease in BSFC and ultimately improving the overall fuel efficiency of the engine. This is in agreement with the results of other authors [27] who found that adding an isopropanol ratio 10% to 30% by volume into gasoline leads to the increased thermal efficiency of the engine.
- Cold-Weather Performance: Isopropanol has better cold-starting characteristics compared to ethanol, which is another common alcohol used in fuel blends. In cold weather, using isopropanol in the blend may improve fuel vaporization and combustion, reducing cold-start fuel consumption.
In summary, the impact of mixing isopropanol and gasoline on fuel consumption can vary depending on the blend ratio and engine characteristics. In the given case, at low loads and medium engine speed, a decrease in fuel consumption is observed with an increase in the percentage of isopropanol in the mixture.
3.2. Emissions Characteristics
Figure 6 illustrates the comparison of CO emissions between isopropanol–gasoline blends and pure gasoline.
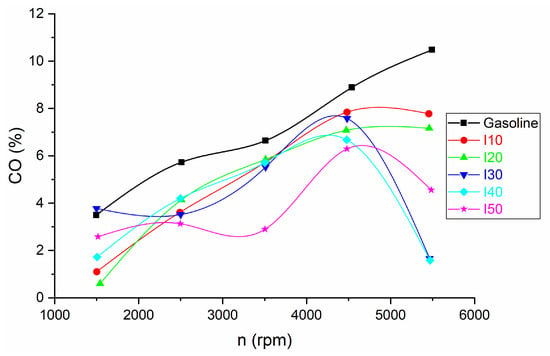
Figure 6.
The results for CO emissions obtained from blended isopropanol and net gasoline.
It can be observed that the CO emissions decrease with the increase in the isopropanol ratio at all engine speeds in comparison with net gasoline. The decrease in CO emissions at low engine speed is between 26.79% and 82.79%, while at high engine speed the decrease in CO emissions is between 25.75% and 84.9%.
CO formation occurs because combustion is incomplete, primarily due to insufficient oxygen in the fuel mixture and inadequate time for the combustion process to finish. Isopropanol contains oxygen, which can improve combustion efficiency when blended with gasoline. This increased oxygen content can lead to more complete combustion of the fuel mixture, reducing the production of carbon monoxide. Therefore, using an isopropanol–gasoline blend may result in lower CO emissions compared to using net gasoline.
It can be observed that the CO emission decreases with the increase of the isopropanol ratio at all engine speeds in comparison with net gasoline. CO formation occurs because combustion is incomplete, primarily due to insufficient oxygen in the fuel mixture and inadequate time for the combustion process to finish. Isopropanol contains oxygen, which can improve combustion efficiency when blended with gasoline. This increased oxygen content can lead to more complete combustion of the fuel mixture, reducing the production of carbon monoxide. Therefore, using an isopropanol–gasoline blend may result in lower CO emissions compared to using net gasoline. Another reason is that the stoichiometric air–fuel ratio (the ideal ratio for complete combustion) for isopropanol is different from that of gasoline. Blending the two fuels can alter the overall air–fuel ratio of the mixture. If the blend is closer to the ideal stoichiometric ratio, it can lead to combustion that is more efficient and reduced CO emissions. This is in agreement with the results of other authors [34], who found that the CO emissions of the engine decrease with an increase in the isopropanol ratio.
The comparison of CO2 emissions for blended isopropanol and net gasoline is shown in Figure 7.
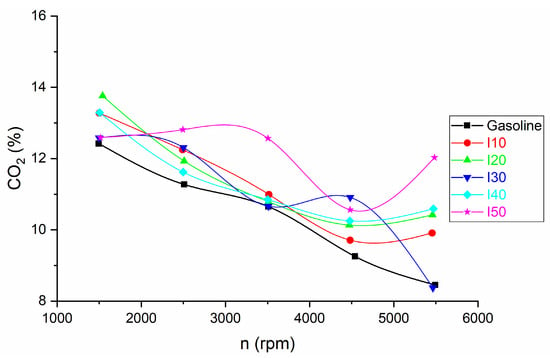
Figure 7.
The results for CO2 emissions obtained from blended isopropanol and net gasoline.
The change in CO2 emissions show the opposite behavior when compared to the CO emissions. Compared with net gasoline, CO2 emissions of the blends increase with increasing isopropanol in the blends. The increase in CO2 emissions at low engine speed is between 1.29% and 10.79%, while at high engine speed the increase in CO2 emissions is between 0.95% and 42.37%.
The impact of mixing isopropanol and gasoline on carbon dioxide (CO2) emissions is influenced by several factors:
- Carbon Content: Both isopropanol and gasoline are hydrocarbon-based fuels that contain carbon. When you blend them, the total carbon content of the fuel mixture remains relatively constant. Therefore, the CO2 emissions primarily depend on the total amount of carbon burned during combustion, which is related to the blend ratio and the overall efficiency of combustion.
- Oxygen Content: Isopropanol contains oxygen, which can promote combustion that is more complete. In some cases, this can lead to a more efficient burn and a reduction in CO emissions. However, it does not directly affect the total carbon dioxide emissions because the carbon content remains the same. This is in agreement with the results of other authors [27].
This is in agreement with the results of other authors [34] who found that the CO2 emissions of the engine increase with the increase of the isopropanol ratio.
The comparison of HC emissions for isopropanol blends and net gasoline is shown in Figure 8.
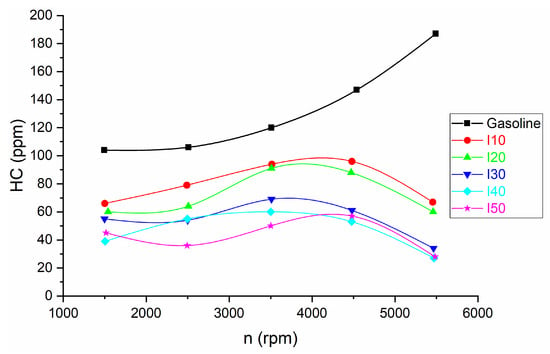
Figure 8.
The HC emissions resulting from the use of isopropanol blends and net gasoline.
It can be observed that as the as the isopropanol percentage in blends increased, the HC emissions decreased compared with that of net gasoline. The decrease in HC emissions at low engine speed was between 36.54% and 62.5%, while at high engine speed the decrease in BSFC was between 64.17% and 85.56%.
The reason for this effect is due to the same reason as those for the decrease in CO emissions described in the previous paragraph like oxygen content and stoichiometric ratio.
As the proportion of isopropanol in the fuel blends increased, a noticeable decrease in hydrocarbon (HC) emissions was observed when compared to pure gasoline. This reduction can be attributed to similar factors as those explained in the previous paragraph regarding the decrease in CO emissions, including oxygen content, stoichiometric ratio, and other relevant factors:
- Evaporative emissions: The vapor pressure and volatility of isopropanol and gasoline can differ. Blending the two can influence evaporative emissions, which contribute to HC emissions. Proper fuel system design and vapor recovery systems can help mitigate this effect.
- Blend Composition: The exact composition of the isopropanol–gasoline blend matters. Different percentages of isopropanol can have varying effects on combustion efficiency and HC emissions. Some blends may result in lower HC emissions due to improved combustion, while others may not show a significant difference compared to pure gasoline.
The comparison of NOx emissions for isopropanol blends and net gasoline is shown in Figure 9.
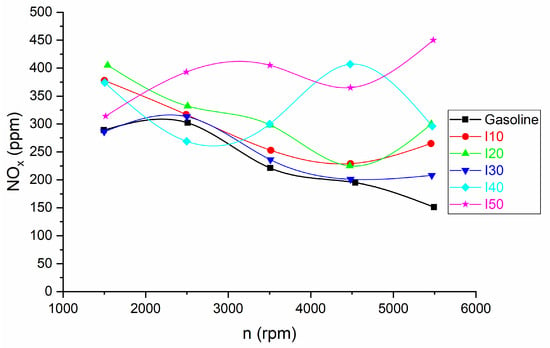
Figure 9.
The results for NOx emissions obtained from blended isopropanol and net gasoline.
As the percentage of isopropanol in the blends increased, there was an increase in NOx emissions when compared to pure gasoline. The increase in NOx emissions at low engine speed was between 1.04% and 40.14%, while at high engine speed the increase in NOx emissions was between 37.75% and 198.01%.
This effect can be attributed to the following reasons:
- Oxygen Content: Isopropanol contains oxygen, which can affect combustion dynamics. The presence of oxygen can lead to higher combustion temperatures and increased production of nitrogen oxides, particularly nitrogen oxide (NO) and nitrogen dioxide (NO2), which are collectively referred to as NOx. Therefore, in some cases, blending isopropanol with gasoline can result in increased NOx emissions due to the higher combustion temperatures. This is in agreement with the results of other authors [27].
- Blend Composition: The exact composition of the isopropanol–gasoline blend matters. Different percentages of isopropanol can have varying effects on combustion temperatures and NOx emissions. In some cases, the presence of isopropanol may lead to higher NOx emissions, while in others, it may not show a significant difference compared to pure gasoline.
- Engine speed: The rise in NOx emissions as engine speed increases is because at higher speeds, a larger quantity of fuel is injected into the cylinder. Consequently, the engine draws in more air, elevating the oxygen levels within. This heightened oxygen content promotes complete combustion, causing an increase in both in-cylinder and flame temperatures. As a result, thermal NOx is generated. This is in agreement with the results of other authors [27].
This is in agreement with the results of other authors [30], who found that the NO emissions of the engine decrease with the increase in the isopropanol ratio.
The comparison of exhaust gas temperature for isopropanol blends and net gasoline is shown in Figure 10.
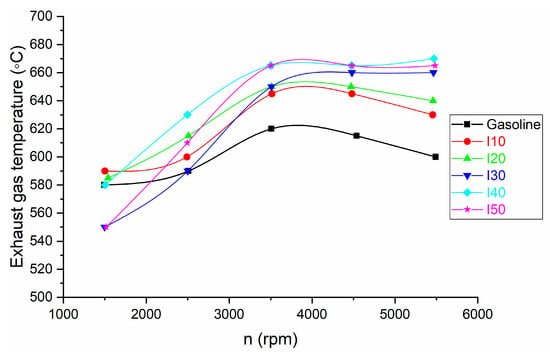
Figure 10.
The results for exhaust gas temperature obtained from blended isopropanol and net gasoline.
It can be observed that as the isopropanol percentage in blends increased, the exhaust gas temperature increased compared with that of net gasoline. The increase in the exhaust gas temperature at low engine speed is between 0.86% and 1.72%, while at high engine speed the exhaust gas temperature is between 5% and 11.67%.
The reason for this effect is due to:
- Increased Oxygen Content: Isopropanol contains oxygen in its molecular structure. When mixed with gasoline, this extra oxygen can enhance the combustion process. Oxygen is a key component for burning fuel, and a higher oxygen content can lead to combustion that is more complete. During complete combustion, a larger portion of the fuel’s energy is released as heat energy, resulting in higher exhaust gas temperatures. This is in agreement with the results of other authors [27].
- Improved Combustion Efficiency: The presence of isopropanol in the blend can promote better combustion efficiency. It helps ensure that more of the fuel is burned within the combustion chamber rather than being expelled as unburned hydrocarbons. Improved combustion efficiency means a greater conversion of chemical energy into thermal energy, raising the temperature of the exhaust gases.
- Altered Stoichiometry: The stoichiometric air–fuel ratio (the ideal ratio for complete combustion) for isopropanol is different from that of gasoline. Blending these fuels can lead to a change in the overall air–fuel ratio. Depending on the specific blend ratio and the adjustment of the engine’s fuel injection system, the mixture may be pushed closer to or farther from the stoichiometric point. An optimal air–fuel mixture can result in higher temperatures during combustion.
- Higher Latent Heat of Vaporization: Isopropanol has a higher latent heat of vaporization compared to gasoline. This means it requires more energy (heat) to vaporize and transition from a liquid to a vapor state. During the vaporization process, heat is absorbed from the combustion chamber, reducing the temperature of the air–fuel mixture and potentially leading to incomplete combustion. However, once the isopropanol is vaporized and ignites, it releases this stored heat energy, contributing to higher exhaust gas temperatures.
4. Conclusions
In this research, we explore how mixing gasoline with varying proportions of isopropanol (ranging from 0% to 50%, in increments of 10%, along with net gasoline) affects the functioning of a gasoline direct injection engine. We investigate how these blends impact both engine performance and emissions. The key findings from this investigation can be summarized as follows:
- The increase in the brake power of the engine is more pronounced at low and medium engine speeds, with the greatest increase at low engine speeds occurring with mixture IP50 (15.6%) and at medium engine speeds the greatest increase is with mixture IP40 (12.46%);
- The BSFC decreased with increasing isopropanol in the fuel blends. The decrease in BSFC was more pronounced at low loads and less pronounced at high loads, with the greatest decrease at low loads occurring in mixture IP40 (54.32%), and at high loads the greatest decrease was in mixture IP50 (12.03%);
- The CO emissions decrease with the increase in the isopropanol ratio at all engine speeds in comparison with net gasoline. The lowest CO emissions at low engine speeds were obtained with the IP20 mixture (0.602%), while at high engine speeds they were obtained with the IP40 mixture (1.583%);
- Compared with net gasoline, CO2 emissions of the blends increase with increasing isopropanol in the blends. The highest CO2 emissions at low engine speeds were obtained with the IP20 mixture (13.76%), while at high engine speeds they were obtained with the IP50 mixture (12.03%);
- As the proportion of isopropanol in the blends increased, there was a reduction in HC emissions in comparison to net gasoline. Specifically, when the engine operated at low speeds, the lowest HC emissions were achieved with the IP40 blend (39 ppm), whereas at high engine speeds, the IP40 blend (27 ppm) yielded the lowest HC emissions;
- When the percentage of isopropanol in blends increased, the NOx emissions increased compared with that of net gasoline. The highest NOx emissions (405 ppm) at low engine speeds were obtained with IP20 mixtures, while at high engine speeds they were obtained with IP50 mixtures (450 ppm);
- When the percentage of isopropanol in blends increased, the exhaust gas temperature increased compared with that of net gasoline. The highest exhaust gas temperature (590 °C) at low engine speeds were obtained with IP10 mixtures, while at high engine speeds they were obtained with IP40 mixtures (670 °C).
Author Contributions
Conceptualization, S.I.; Software, Z.I. and R.D.; Validation, S.I., Z.I., R.D. and V.M.; Formal analysis, Z.I., R.D., V.M. and D.I.; Investigation, S.I., Z.I., R.D., V.M., D.I., S.S. and S.A.; Data curation, Z.I., R.D., V.M., D.I. and S.A.; Writing—original draft, S.I.; Writing—review & editing, S.I.; Visualization, Z.I. and R.D.; Supervision, S.I.; Project administration, S.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The authors confirm that the data to support the findings of this study are available within the article or upon request.
Acknowledgments
The present document was produced with the financial assistance of the Project 2023-RU-03 “Development, research and optimization of the energy consumption of an electric car using the 3DExperience platform”.
Conflicts of Interest
The author declares no conflict of interest.
References
- Gao, J.; Huang, J.; Li, X.; Tian, G.; Wang, X.; Yang, C.; Ma, C. Challenges of the UK government and industries regarding emission control after ICE vehicle bans. Sci. Total Environ. 2022, 835, 155406. [Google Scholar] [CrossRef] [PubMed]
- Shuai, S.; Ma, X.; Li, Y.; Qi, Y.; Xu, H. Recent progress in automotive gasoline direct injection engine technology. Automot. Innov. 2018, 1, 95–113. [Google Scholar] [CrossRef]
- Iliev, S. A Comparison of Ethanol, Methanol, and Butanol Blending with Gasoline and Its Effect on Engine Performance and Emissions Using Engine Simulation. Processes 2021, 9, 1322. [Google Scholar] [CrossRef]
- Iliev, S. Investigation of the Gasoline Engine Performance and Emissions Working on Methanol-Gasoline Blends Using Engine Simulation. In Numerical and Experimental Studies on Combustion Engines and Vehicles; IntechOpen: London, UK, 2020. [Google Scholar]
- Sangeeta; Moka, S.; Pande, M.; Rani, M.; Gakhar, R.; Sharma, M.; Rani, J.; Bhaskarwar, A.N. Alternative fuels: An overview of current trends and scope for future. Renew. Sustain. Energy Rev. 2014, 32, 697–712. [Google Scholar] [CrossRef]
- Iliev, S. A comparison of ethanol and methanol blending with gasoline using a 1-D engine model. In Procedia Engineering; Elsevier: Viena, Austria, 2015; Volume 100, pp. 1013–1022. [Google Scholar]
- Iodice, P.; Cardone, M. Ethanol/gasoline blends as alternative fuel in last generation spark-ignition engines: A review on CO and HC engine out emissions. Energies 2021, 14, 4034. [Google Scholar] [CrossRef]
- Yusuf, A.; Inambao, F. Progress in alcohol-gasoline blends and their effects on the performance and emissions in SI engines under different operating conditions. Int. J. Ambient. Energy 2021, 42, 465–481. [Google Scholar] [CrossRef]
- Merola, S.; Valentino, G.; Tornatore, C.; Marchitto, L. In-cylinder spectroscopic measurements of knocking combustion in a SI engine fuelled with butanol–gasoline blend. Energy 2013, 62, 150–161. [Google Scholar] [CrossRef]
- Yanowitz, J.; Christensen, K.; McCormick, R. Utilization of Renewable Oxygenates as Gasoline Blending Components. Technical Report, NREL/TP-5400-50791, August 2011. Available online: https://www.nrel.gov/docs/fy11osti/50791.pdf (accessed on 20 November 2023).
- Wallner, T.; Ickes, A.; Lawyer, K. Analytical assessment of C2-C8 alcohols as spark-ignition engine fuels. In Proceedings of the FISITA 2012 World Automotive Congress; Springer: Berlin, Germany, 2013; pp. 15–26. [Google Scholar]
- Kusakabe, T.; Tatsuke, T.; Tsuruno, K.; Hirokawa, Y.; Atsumi, S.; Liao, J.C.; Hanai, T. Engineering a synthetic pathway in cyanobacteria for isopropanol production directly from carbon dioxide and light. Metab. Eng. 2013, 20, 101–108. [Google Scholar] [CrossRef]
- Balki, M.; Sayin, C. The effect of compression ratio on the performance, emissions and combustion of an SI (spark ignition) engine fueled with pure ethanol, methanol and unleaded gasoline. Energy 2014, 71, 194–201. [Google Scholar] [CrossRef]
- Rochón, E.; Cortizo, G.; Cabot, M.I.; Cubero, M.T.; Coca, M.; Ferrari, M.D.; Lareo, C. Bioprocess intensification for isopropanol, butanol and ethanol (IBE) production by fermentation from sugarcane and sweet sorghum juices through a gas stripping pervaporation recovery process. Fuel 2020, 281, 118593. [Google Scholar] [CrossRef]
- Pyrgakis, K.A.; de Vrije, T.; Budde, M.A.W.; Kyriakou, K.; Lopez-Contreras, A.M.; Kokossis, A.C. A process integration approach for the production of biological isopropanol, butanol and ethanol using gas stripping and adsorption as recovery methods. Biochem. Eng. J. 2016, 116, 176–194. [Google Scholar] [CrossRef]
- Riazi, M.; Chiaramonti, D. Biofuels Production and Processing Technology; CRC Press: London, UK, 2017. [Google Scholar]
- Dogan, O. The influence of n-butanol/diesel fuel blends utilization on a small diesel engine performance and emissions. Fuel 2011, 90, 2467–2472. [Google Scholar] [CrossRef]
- Graham, L.; Belisle, S.; Baas, C. Emissions from light duty gasoline vehicles operating on low blend ethanol gasoline and E85. Atmos. Environ. 2008, 42, 4498–4516. [Google Scholar] [CrossRef]
- Sileghem, L.; Alekseev, V.; Vancoillie, J.; Van Geem, K.; Nilsson, E.; Verhelst, S.; Konnov, A. Laminar burning velocity of gasoline and the gasoline surrogate components iso-octane, n-heptane and toluene. Fuel 2013, 112, 355–365. [Google Scholar] [CrossRef]
- Veloo, P.; Egolfopoulos, F. Studies of n-propanol, iso-propanol, and propane flames, Combust. Flame 2011, 158, 501–510. [Google Scholar] [CrossRef]
- MAN Diesel & Turbo. Using Methanol Fuel in the MAN B&W ME-LGI Series; MAN Group: Copenhagen, Denmark, 2014. [Google Scholar]
- Dutta, A. Forecasting Ethanol Market Volatility: New Evidence from the Corn Implied Volatility Index, Biofuels Bioprod; Biorefin: Janderup Vestj, Denmark, 2019; Volume 13, pp. 48–54. [Google Scholar]
- Veloo, P.; Wang, Y.; Egolfopoulos, F.; Westbrook, C. A comparative experimental and computational study of methanol, ethanol, and n-butanol flames, Combust. Flame 2010, 157, 1989–2004. [Google Scholar] [CrossRef]
- Christensen, E.; Yanowitz, J.; Ratcliff, M.; McCormick, R.L. Renewable oxygenate blending effects on gasoline properties. Energy Fuels 2011, 25, 4723–4733. [Google Scholar] [CrossRef]
- Gainey, B.; Yan, Z.; Moser, S.; Lawler, B. Lean flammability limit of high-dilution spark ignition with ethanol, propanol, and butanol. Int. J. Engine Res. 2021, 23, 638–648. [Google Scholar] [CrossRef]
- Andersen, V.; Anderson, J.; Wallington, T.; Mueller, S.; Nielsen, O. Vapor Pressures of Alcohol-Gasoline Blends. Energy Fuels 2010, 24, 3647–3654. [Google Scholar] [CrossRef]
- Sivasubramanian, H.; Pochareddy, Y.K.; Dhamodaran, G.; Esakkimuthu, G.S. Performance, emission and combustion characteristics of a branched higher mass, C3 alcohol (isopropanol) blends fuelled medium duty MPFI SI engine. Eng. Sci. Technol. Int. J. 2017, 20, 528–535. [Google Scholar]
- Yacoub, Y.; Bata, R.; Gautam, M. The performance and emission characteristics of C1-C5 alcohol-gasoline blends with matched oxygen content in a single-cylinder spark ignition engine. Proc. Inst. Mech. Eng. Part A J. Power Energy 1998, 212, 363–379. [Google Scholar] [CrossRef]
- Keskin, A.; Gürü, M. The Effects of Ethanol and Propanol Additions Into Unleaded Gasoline on Exhaust and Noise Emissions of a Spark Ignition Engine. Energy Sources Part A Recovery Util. Environ. Eff. 2011, 33, 2194–2205. [Google Scholar] [CrossRef]
- Gravalos, I.; Moshou, D.; Gialamas, T.; Xyradakis, P.; Kateris, D.; Tsiropoulos, Z. Emissions characteristics of spark ignition engine operating on lower–higher molecular mass alcohol blended gasoline fuels. Renew. Energy 2013, 50, 27–32. [Google Scholar] [CrossRef]
- Awad, O.I.; Mamat, R.; Ali, O.M.; Sidik, N.C.; Yusaf, T.; Kadirgama, K.; Kettner, M. Alcohol and ether as alternative fuels in spark ignition engine: A review. Renew. Sust. Energ. Rev. 2018, 82, 2586–2605. [Google Scholar] [CrossRef]
- Agarwal, A.; Karare, H.; Dhar, A. Combustion, performance, emissions and particulate characterization of a methanol-gasoline blend (gasohol) fuelled medium duty spark ignition transportation engine. Fuel Process. Technol. 2014, 121, 16–24. [Google Scholar] [CrossRef]
- Liu, S.; Cuty Clemente, E.; Hu, T.; Wei, Y. Study of spark ignition engine fueled with methanol/gasoline fuel blends. Appl. Therm. Eng. 2007, 27, 1904–1910. [Google Scholar] [CrossRef]
- Altun, S.; Oner, C.; Firat, M. Exhaust emissions from a spark-ignition engine operating on iso-propanol and unleaded gasoline blends. Technology 2010, 13, 183–188. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).